the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Direct measurement of N2O5 heterogeneous uptake coefficients on atmospheric aerosols in southwestern China and evaluation of current parameterizations
Jiayin Li
Tianyu Zhai
Haichao Wang
Shuyang Xie
Shiyi Chen
Chunmeng Li
Yuanjun Gong
Huabin Dong
The heterogeneous hydrolysis of dinitrogen pentoxide (N2O5) is a critical process in assessing NOx fate and secondary pollutant formation. However, accurate quantification of the N2O5 uptake coefficient (γ (N2O5)) in ambient conditions is a challenging problem that can cause unpredictable uncertainties in the predictions of air quality models. Here, the γ (N2O5) values were directly measured using an improved in situ aerosol flow tube system at a site located in a highland region in southwestern China to investigate influencing factors and the performance of current γ (N2O5) parameterizations under this typical environmental condition. The nocturnal mean γ (N2O5) value ranged from 0.0018 to 0.12 with an average of 0.023±0.021. The relationship between the measured γ (N2O5) and impacting factors was consistent with previous laboratory results, except for aerosol chloride. The aerosol water significantly promoted N2O5 uptake, while particulate organics and nitrate showed suppression effects. We found that several parameterizations can capture the median of measured values, whereas none of the 10 parameterizations were able to reproduce the variabilities and showed poor correlations (R2=0.00–0.09). Elevated biases of predictions specifically occurred at high aerosol liquid water content (ALWC) (> 35 M) and low ALWC (< 25 M) levels with an underestimation of −37 % to −1 % and an overestimation of 34 % to 189 %, respectively. Such differences between the measured and parameterized γ (N2O5) would lead to a biased estimation (−77 % to 74 %) of the particulate nitrate production potential. Our findings suggest the need for more direct field quantifications of γ (N2O5) and laboratory measurements under extreme ALWC conditions to re-evaluate the response coefficients between γ (N2O5) and aerosol chemical compositions in parameterizations.
- Article
(4166 KB) - Full-text XML
-
Supplement
(1138 KB) - BibTeX
- EndNote
Nitrate radical (NO3) and dinitrogen pentoxide (N2O5) are dominant in nocturnal atmospheric chemistry as reactive nitrogen species that can strongly influence the concentration and distribution of ozone (O3) and nitrogen oxides (NOx=NO+NO2) and the air quality (Brown et al., 2006; Wang et al., 2023; Decker et al., 2019; Dentener and Crutzen, 1993). NO3 is produced by the reaction of NO2 and O3 (Reaction R1), and there is a thermodynamic equilibrium between NO3 and N2O5 (Reaction R2), which is the source of N2O5 (Brown and Stutz, 2012). There are two main pathways for NO3 removal: the direct one is the reactions of NO3 and VOCs (volatile organic compounds; Reaction R3), especially alkenes, and the indirect way is the heterogeneous hydrolysis of N2O5 (Asaf et al., 2009; Ng et al., 2017). N2O5 can react with H2O and chloride (Cl−) in the particle phase and form soluble nitrate and nitryl chloride (ClNO2) (Reaction R4) (Osthoff et al., 2008; Chang et al., 2011). The uptake of N2O5 is the main pathway for the formation of particulate nitrate at night, which contributes to PM2.5 (particulate matter < 2.5 µm in diameter) pollution. Meanwhile, chlorine radical is produced by ClNO2 photodecomposition in the daytime and further regulates the O3 pollution production by promoting the oxidation of VOCs (Finlaysonpitts et al., 1989; Riedel et al., 2014). Thus, it is important to quantify the rate of the N2O5 heterogeneous hydrolysis reaction in ambient conditions.
γ (N2O5) is defined as the net probability of N2O5 irreversibly taken up onto an aerosol surface upon collision (McDuffie et al., 2018). According to the previous study, the process of the N2O5 heterogeneous hydrolysis reaction on aerosols was treated as a resistor model including three steps: gas diffusion (Reaction R5), surface accommodation, and aqueous reaction (Reactions R6–R8) (Abbatt et al., 2012; Fang et al., 2024). This process can be influenced by aerosol chemical compositions (e.g., aerosol liquid water content (ALWC), nitrate (NO) concentration, Cl− concentration, and organics), morphology, and ambient meteorological factors (Bertram and Thornton, 2009; Mozurkewich and Calvert, 1988; Roberts et al., 2009; Thornton et al., 2003). High concentrations of aerosol water and Cl− can promote the uptake reaction (Reactions R6–R8), and NO suppresses the reaction (Reaction R6). Organics also can suppress the reaction by forming a coating on the surface of the particles and regulating the ALWC and the passage rate of N2O5 molecules (Folkers et al., 2003; Gaston et al., 2014; Anttila et al., 2006). However, the above results are mainly based on laboratory studies. In ambient conditions, the correlations between γ (N2O5) and aerosol chemical compositions were generally weak mainly due to the coupling effects of particle morphology, size, the mixing state, and meteorological parameters (e.g., temperature and relative humidity) (Phillips et al., 2016; Wang et al., 2020b; Riedel et al., 2012).
In order to accurately quantify the contribution of N2O5 heterogeneous hydrolysis to nitrate formation and NOx regulation, a variety of parameterizations of γ (N2O5) have been established based on laboratory and field studies (Evans and Jacob, 2005; Davis et al., 2008; Yu et al., 2020; Bertram and Thornton, 2009). The parameters in parameterizations mainly include the meteorological parameters, concentrations of aerosol chemical compositions, and particle physicochemical parameters. However, these parameterizations usually exhibit low correlations with observed γ (N2O5) in varying environments (Brown et al., 2009; Ryder et al., 2014; McDuffie et al., 2018). Moreover, the overestimation or underestimation of the parameterized γ (N2O5) can lead to unpredictable biases in the simulations of the chemical transport models (Murray et al., 2021; Chen et al., 2018; Ryder et al., 2014).
Until now, only a few studies have quantified γ (N2O5) values in ambient conditions (< 10−4 to 0.1) mostly by indirect quantification methods (Brown et al., 2016; Wang et al., 2018; X. R. Chen et al., 2020; Morgan et al., 2015; Tham et al., 2018), while some were quantified by direct measurements (Yu et al., 2020; Riedel et al., 2012; Bertram et al., 2009a). The N2O5 heterogeneous uptake process has been reported to be active in China. The γ (N2O5) values in the North China Plain, Yangtze River Delta, and Pearl River Delta in China (10−2–10−1) were generally about 1 to 2 orders of magnitude larger than those in Europe and North America (10−3–10−2) (Yan et al., 2023; H. C. Wang et al., 2017; X. F. Wang et al., 2017; Z. Wang et al., 2017; Niu et al., 2022). To further investigate the N2O5 heterogeneous chemistry in China, the γ (N2O5) values were directly measured in a typical highland city in China, Kunming, using an improved in situ aerosol flow tube system from 15 April to 20 May 2021. The relationship between the γ (N2O5) values and impacting factors was determined. We then examine the performance of current γ (N2O5) parameterizations by comparing them to the observed values and analyze the causes of discrepancies in extreme ALWC conditions. We further observe significant biases when estimating particulate nitrate formation potential based on current γ (N2O5) parameterization.
2.1 Site description
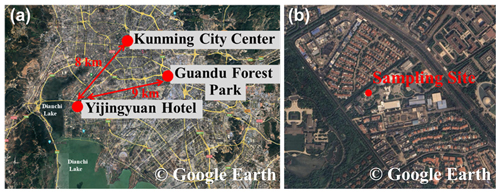
The field campaign was conducted in Kunming, China, from 15 April to 20 May 2021. The main sampling site was on the roof of the Yihe Building (Yijingyuan Hotel; 24°59 N, 102°39 E), about 20 m above the ground. As shown in Fig. 1, the measurement site was located approximately 1890 m above sea level, 8 km away from the city center, and 1 km from Dianchi Lake to the west. The site receives traffic emissions from two roads within a radius of 500 m. The site was mainly surrounded by residential areas, and there was no major industrial source around. Besides this, the particle composition was measured at the Guandu Forest Park (25°00 N, 102°45 E), which was about 9 km away from the Yijingyuan Hotel, 5.2 km from the city center, and also mainly surrounded by residential living area. Sunrise was around 06:30 CNST (China national standard time; UTC + 8 h), and sunset was at 19:30 CNST.
2.2 Instrument setup
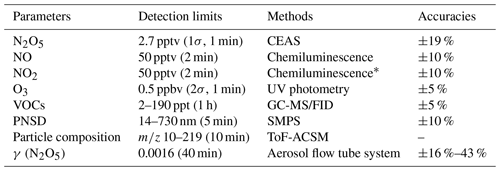
Multiple gas phase and particulate parameters were measured during the campaign, including N2O5, NO, NO2, O3, VOCs, PM2.5, particle number size distribution (PNSD), particle composition, and meteorological parameters. Detailed information about the instruments is listed in Table 1.
N2O5 concentration was measured by a cavity-enhanced absorption spectrometer (CEAS) developed by H. Wang et al. (2017), which has been used in several field campaigns. N2O5 in the sampling gas was thermally decomposed to NO3 in a preheated perfluoroalkoxy alkane (PFA) tube (130 °C) and then detected in a resonator cavity maintained at 110 °C to avoid the reversible reaction of N2O5 and NO3. Excess NO was injected into the cavity every 5 min to obtain the reference spectrum by eliminating the influence of water vapor. The N2O5 loss in the sampling system and detection system were also calibrated and corrected during data processing. The limit of detection (LOD) of CEAS was 2.7 pptv (1σ), and the uncertainty was 19 %.
NO, NO2, and O3 were monitored by commercial instruments (Thermo Fisher 42i and 49i). A total of 117 kinds of volatile organic compound (VOC) species were measured by an automated gas chromatograph equipped with a mass spectrometer and flame ionization detector (GC-MS/FID). The particle composition was measured by a time-of-flight aerosol chemical speciation monitor (ToF-ACSM), including sulfate, nitrate, ammonium, chloride, and organics. The ALWC was calculated by the ISORROPA-II model and did not consider the hygroscopicity of organic compounds (Fountoukis and Nenes, 2007). PNSD were measured by a scanning mobility particle sizer (SMPS; TSI model 3938), including an electrostatic classifier (model 3082) and a condensation particle counter (CPC; model 3776). Meteorological parameters, including relative humidity (RH), temperature (T), pressure, wind speed, and wind direction, were available during the campaign.
2.3 Measurement and calculation of γ (N2O5)
The γ (N2O5) was directly measured by an aerosol flow tube system (AFTS) coupled with a detailed box model developed by Chen et al. (2022). The detection limit and accuracy of the AFTS are listed in Table 1. Briefly, the AFTS mainly consists of an N2O5 generator; an aerosol flow tube; and detection instruments for N2O5, NOx, O3, and aerosol surface area (Sa) (Fig. S1 in the Supplement). N2O5 generated by O3 and NO2 (in excess) was added to the sampling gas in the front of the aerosol flow tube. The aerosol flow tube consists of two cones at both ends with a vertex angle of 15° and a straight cylinder in the middle with an inner diameter of 140 mm and a length of 343 mm. The total flow rate in the tube was 2.08 L min−1, and the residence time was 259 s. The detection instruments used for measurements of N2O5, NOx, O3, and Sa are CEAS PKU, Thermo 42i-TL, Teledyne T265, and SMPS (TSI model 3938). Additionally, an RH–T sensor (Rotronic model HC2A-S) was utilized to monitor relative humidity and temperature inside the flow tube. During each duty cycle, N2O5 concentrations were recorded at both the inlet and the exit of the flow tube under conditions with and without aerosols to derive the wall loss of N2O5. NO, NO2, and O3 concentrations were consistently measured at the inlet of the flow tube, and Sa concentrations were consistently measured at the exit of the flow tube. The loss rate coefficients of N2O5 were calculated by a time-dependent box model coupled with NO3–N2O5 chemistry under the constraint of the measurement of N2O5 concentrations and other auxiliary parameters to overcome the influence of homogeneous reactions (e.g., NO2, O3, and NO) and variations in air mass on γ (N2O5) retrieval. The N2O5 loss rate in the absence of aerosols was expected as wall loss rate coefficients () of N2O5, and the loss rate in the presence of aerosols was expected as the loss rate on both the wall and aerosols () of N2O5. Therefore, γ (N2O5) could be calculated by Eq. (1). Among them, the loss of Sa concentration in the aerosol flow tube was corrected by the penetration efficiency derived in our previous study (Chen et al., 2022), and the dry-state Sa was corrected to ambient (wet) Sa by a hygroscopic growth factor (Liu et al., 2013). A stringent data QA/QC (quality assurance/quality control) procedure is applied before model calculation based on the above-measured variables to retrieve robust γ (N2O5) values. Other detailed information about this system can be found in the Supplement and Chen et al. (2022).
2.4 Calculation of NO3 and N2O5 reactivity
NO3 production rate (P(NO3)) was calculated by the measured NO2 and O3 concentrations via Eq. (2); represents the reaction rate constant of NO2 and O3 (Atkinson et al., 2004). NO3 concentration can be calculated by the measured N2O5 concentration with the temperature-dependent equilibrium relationship (Eq. 3). The steady-state lifetime of N2O5 (τ(N2O5)) and NO3 (τ (NO3)) was calculated by concentrations and P(NO3) as shown in Eqs. (4) and (5) (Brown and Stutz, 2012). The NO3 reactivity with VOCs (k(NO3)) can be calculated by Eq. (6); among them, ki represents the bimolecular rate coefficients.
2.5 Calculation of the nitrate production rate
The N2O5 uptake for nighttime particulate nitrate production is regarded as a pseudo-first-order reaction, the rate constant (kN2O5) of which can be calculated from Eq. (7) with measured or parameterized γ (N2O5), where C is the mean molecular speed of N2O5. The yield ratio of ClNO2 (φ) was set as a constant of 0.5 in all calculations, which is consistent with the previously observed yield range of 0.3–0.73 in northern China (Z. Wang et al., 2017; Wang et al., 2018). The nitrate production rate can be calculated by Eq. (8), where [N2O5] is the concentration of N2O5.
3.1 γ (N2O5) measurement overview and comparison
The mean diurnal variation in the measured N2O5 concentration; γ (N2O5) values; RH; T; and concentrations of NO2, O3, NO, and PM2.5 from 15 April to 20 May 2021 are shown in Fig. 2a, and the time series are shown in Fig. S2 in the Supplement. A higher PM2.5 concentration was observed at night (average of 27.8±14.3 µg m−3, peak of 81.0 µg m−3) than during the day (Figs. 2a and S2). The NO2 (average of 6.5±8.4 ppbv) and O3 (average of 45.5±19.7 ppbv) concentrations in Kunming are lower than in other regions in China (Z. Wang et al., 2017; Wang et al., 2020a; Niu et al., 2022; Li et al., 2020), indicating a lower atmospheric oxidation capacity. The mean nocturnal NO3 production rate (P(NO3)) was 0.6±0.8 ppbv h−1, which is also lower than previous reports in China (Tham et al., 2016; Zhai et al., 2023; Wang et al., 2022). During this campaign, a significant N2O5 concentration (at a maximum of 395.1 pptv) was only observed within 16–27 April, mainly with low humidity and high precursor concentrations, while the concentrations fluctuated around the detection limit during other periods. The nocturnal mean concentration of N2O5 was 33.4±75.2 pptv, which is lower than reported concentrations in other regions of China (Wang et al., 2018; Brown et al., 2016; Zhai et al., 2023). During the field measurement, high temperature (∼ 20°C) favors the equilibrium shifting from N2O5 towards NO3 and the site mainly received the emissions from vegetation in the surrounding parks. In that case, the major removal of NO3–N2O5 at night was the reaction of NO3 with VOCs represented by monoterpene (67 %) and isoprene (4 %), followed by N2O5 uptake (15 %) shown in Fig. 2c. Rapid depletion of daytime-emitted isoprene by NO3 led to a low contribution of isoprene to NO3 reactivity after sunset (Fig. S3). The steady-state lifetime of N2O5 (τ(N2O5)) was 185±294 s on average, and its diel pattern was similar to the N2O5 concentration. The τ (N2O5) in Kunming was higher than that in most other cities in China (Wang et al., 2020a; Li et al., 2020; Yan et al., 2019). Comparisons of NO3 and N2O5 concentrations, P(NO3), and other parameters from various regions around the world in recent years are summarized in Table S1.
The nocturnal mean γ (N2O5) value ranged from 0.0018 to 0.12 with an average of 0.023±0.021. The diurnal profiles showed that the γ (N2O5) value decreased after sunset and then sharply increased with relative humidity after midnight, peaking at 05:00 CNST (Fig. 2a). The mean γ (N2O5) was lower than that in the North China Plain and eastern China and similar to that in the Pearl River Delta, China; Europe; and North America (Fig. 2b) (Yan et al., 2023; H. C. Wang et al., 2017; X. F. Wang et al., 2017; Z. Wang et al., 2017; Niu et al., 2022; Morgan et al., 2015; Phillips et al., 2016; Bertram et al., 2009a; McDuffie et al., 2018). The detailed comparisons of field-derived γ (N2O5) are summarized in Table S2.
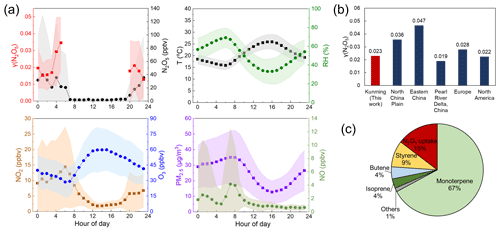
Figure 2Overview of γ (N2O5); gas phase and particulate parameters; meteorological parameters; and NO3 loss pathways. (a) Mean diurnal profiles of measured γ (N2O5), N2O5, T, RH, NO2, O3, PM2.5, and NO. (b) Comparison of γ (N2O5) values in China, Europe, and North America calculated from previous work with measured values in this work. (c) Percentage of the NO3 loss pathway via VOCs and N2O5 uptake at night.
3.2 Dependence of γ (N2O5) on impacting factors
The dependence of measured γ (N2O5) values on organics, ALWC, and NO and Cl− concentration in the particle phase in this study are shown in Fig. 3. The organic wet mass fraction showed a significant negative correlation (R2=0.83) with measured γ (N2O5) values (Fig. 3a), indicating that organics in the aerosol significantly inhibited the uptake of N2O5 during the measuring period in Kunming. While a large number of studies have observed evident suppression of particulate organics on N2O5 uptake of lab-generated aerosols (Escoreia et al., 2010; Cosman and Bertram, 2008; Gaston et al., 2014), the negative correlation of particulate organics and γ (N2O5) was usually weak as derived from field measurements (Brown et al., 2009; McDuffie et al., 2018; Chen et al., 2018; Wang et al., 2020b). The organic wet mass fraction in this study varies between 0.3 and 0.8, while other previous studies have reported a variation range of 0.1 to 0.5 (McDuffie et al., 2018; Wang et al., 2020b; Brown et al., 2009). The large proportion and variation range of organics in the aerosols may lead to a more significant inhibition effect on γ (N2O5). Additionally, we found that both the dry and wet mass fractions of organics in this study showed significant negative correlations with ALWC, with Pearson coefficients of −0.66 and −0.79 (Table S3), respectively. Therefore, organics might decrease γ (N2O5) by forming an organic coating to limit the penetration of liquid water into the particle phase and hinder the reaction of N2O5 with the liquid phase.
Aerosol liquid water also exhibited a controlling role in the heterogeneous uptake of N2O5 in this study as demonstrated by the evidently positive correlation (R2=0.74) of ALWC and γ (N2O5) (Fig. 3b). A weak correlation was observed with ALWC below 25 M, and a significant correlation was observed with ALWC higher than 25 M. A similar trend has been reported by previous laboratory studies (Mozurkewich and Calvert, 1988; Bertram and Thornton, 2009; Folkers et al., 2003; Hallquist et al., 2003). When RH is low, the aerosols mainly exist in the solid state with low ALWC, limiting the uptake reaction, whereas the aerosols become deliquesced as the RH (also ALWC) increases, which greatly promotes the uptake reaction. Previous field studies also found good correlations of γ (N2O5) values with ALWC or RH in most regions in China, indicating that ALWC may be one of the rate-limiting steps of a heterogeneous reaction in China (McDuffie et al., 2018; Yu et al., 2020; Tham et al., 2018; Wang et al., 2022).
Figure 3c shows the negative dependence of measured γ (N2O5) values on aerosol nitrate concentration, similar to the results of previous laboratory studies and most field observations (Tham et al., 2018; Bertram et al., 2009b; Morgan et al., 2015; Yu et al., 2020). The suppression effect of NO on the N2O5 heterogeneous uptake is mainly caused by the competition of aerosol nitrate with chloride and H2O for the H2ONO intermediate (Reactions R5–R8) (Bertram and Thornton, 2009). The positive correlation (R2=0.48) between γ (N2O5) and the molar ratio of values was weaker than that of ALWC (Fig. 3d), which indicates that Cl− may promote the N2O5 uptake reaction instead of playing a critical role during our observation. The particulate Cl− concentration also contributes to a weaker enhancement of γ (N2O5) compared to ALWC in other field observations (Wang et al., 2020b; Yu et al., 2020; McDuffie et al., 2018).
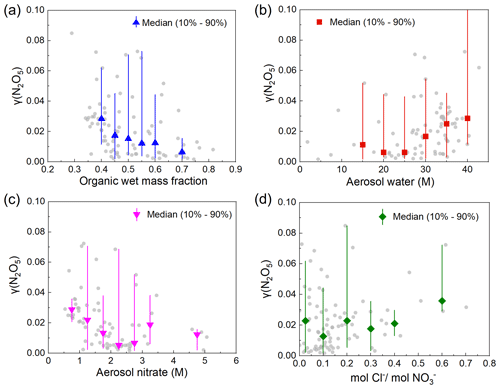
Figure 3Correlations of measured γ (N2O5) values between the influencing factors. Variation in γ (N2O5) with (a) the organic wet mass fraction, (b) the aerosol water content, (c) the aerosol nitrate content, and (d) the molar ratio of chloride to nitrate. The gray points represent the measured values. The symbols in different colors represent the median in each bin, with a range from the 10th to 90th percentile in each bin denoted as lines.
3.3 Comparison of parameterized γ (N2O5) values
The γ (N2O5) values were predicted using 10 widely used parameterizations and compared with the measured results. The details of the parameterizations are summarized in Table S4. Parameterizations were categorized into inorganic-only and inorganic kernels with organic coating or organic mass (inorganic + organic).
The γ (N2O5) values predicted by inorganic-only parameterizations were generally larger than the measurements. Among these inorganic-only parameterizations, RIE03, BT09 w/o Cl, and Yu20 exhibited relatively low deviation in predicted median values from the measurements (Fig. 4a). However, the correlations of the predictions and measurements were bad for these three parameterizations (R2=0–0.09, Fig. 4b). The empirical parameterization Yu20, derived from several field campaigns in China, showed the best performance with a median difference of 4 %, the lowest RMSE (0.0200), and the highest correlation coefficient (R2=0.09) in Kunming, indicating the effectiveness of the improvement by the localized field results. The overestimation of DAV08, BT09, and GRI09 was also reported by previous studies (Bertram et al., 2009b; Brown et al., 2009; Chang et al., 2016; Griffiths et al., 2009; McDuffie et al., 2018). All parameterizations had difficulties in predicting the low and high values of measured γ (N2O5). For the parameterizations with a median deviation less than 10 %, the parameterized γ (N2O5) values mainly fell in the range of 0.0036–0.035, while the measured values varied from 0.0018 to 0.12, indicating that the relevant parameters in the parameterizations were still inappropriate and cannot reproduce the range of the measurements.
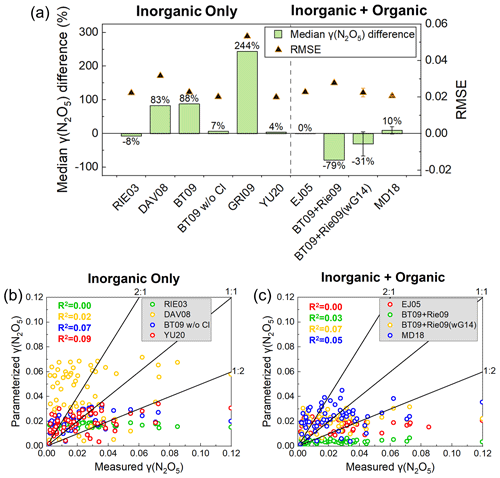
Figure 4Comparison of parameterized and measured γ (N2O5). (a) Comparison of the median difference and root mean square error (RMSE) between measured γ (N2O5) and parameterized γ (N2O5) values. The error bars of BT09 + Rie09wG14 and MD18 show the range of the results of the atomic ratio between 0.5 and 0.8. The distribution of parameterized γ (N2O5) values, including (b) inorganic-only parameterizations (RIE03, DAV08, BT09 w/o Cl−, YU20) and (c) inorganic + organic parameterizations (EJ05, BT09 + Rie09, BT09 + Rie09wG14, MD18) ( = 0.8). The solid black line represents the 1:1, 1:2, and 2:1 lines, respectively. The R2 displayed in different colors corresponds to the parameterization of the same color.
The inorganic + organic parameterizations tend to underestimate the measured γ (N2O5) due to the suppression effects of organics. Worse agreement and larger scatter were found for the parameterized γ (N2O5) (R2=0–0.07, Fig. 4c) when the organic part was added into inorganic part. BT09 + Rie09(wG14) showed the best correlation with an R2 of 0.07 but a relatively large median deviation (−66 %–5 %). EJ05 and MD18 showed the lowest deviations among the four parameterizations, while EJ05 showed the worst correlation (R2=0.00). Among them, the empirical parameterization MD18, derived from field observations, exhibited the best performance with a deviation of −1 %–20 % and the lowest RMSE (0.0207), which also indicates that parameterization can be improved by fitting to field observations, similar to the results of inorganic-only parameterizations.
The commonly used parameterizations mainly consist of the inorganic and inorganic + organic framework, such as BT09 w/o Cl, YU20, and MD18. In this study, among all parameterizations, YU20 demonstrated the best performance, most likely because YU20 was optimized based on datasets observed in four rural regions in China. BT09 w/o Cl also performed well in this study, overestimating the median by only 7 %. However, the poor performance of BT09 w/o Cl was still reported in the Pearl River Delta and North China Plain (Wang et al., 2022, 2020b). Conversely, BT09 w/o Cl performed well in northwestern Europe, mainly because γ (N2O5) in Europe is predominantly controlled by the ions in the bulk phase (Morgan et al., 2015; Chen et al., 2018; Phillips et al., 2016). In North America, γ (N2O5) is significantly inhibited by organic effects (Chang et al., 2016). The parameterizations considering organic effects, like MD18, might be more suitable for the conditions in North America. However, in this study, MD18 showed an overestimation of up to 20 %, suggesting that this parameterization is not suitable for China but more applicable to North American regions.
Hence, most regions in China, where γ (N2O5) is controlled by aerosol liquid water content, are more suited to YU20. European regions, where gamma is controlled by and less influenced by organics, are better served by BT09 w/o Cl. Meanwhile, MD18 is more appropriate for North American regions. Localized parameterizations established on the basis of local measurements can exhibit superior performance within the respective regions. Parameterizations incorporating organic effects generally exhibit larger errors than others, underscoring the importance of further improving the consideration of organic effects in parameterizations.
3.4 Impact of ALWC on parameterized γ (N2O5)
Although some parameterizations performed relatively well in reproducing the median values of γ (N2O5), none of the 10 parameterizations were able to reproduce the range of measured γ (N2O5) values, as indicated by poor correlations and a large RMSE. This phenomenon was possibly caused by several aspects, including the inaccurate estimation of response coefficients of aerosol compositions (representing the quantitative relationship between γ (N2O5) and aerosol chemical compositions), relative rates of competitive reactions, and the missing parameters. The missing influencing factors in current parameterizations include parameters such as particle morphology, the phase state, and the mixing state (You et al., 2014; Shiraiwa et al., 2017; Ng et al., 2010). These parameters, which are difficult to measure in field conditions with current methodologies, have been proven to affect γ (N2O5) and can contribute to the discrepancy between parameterized and measured values.
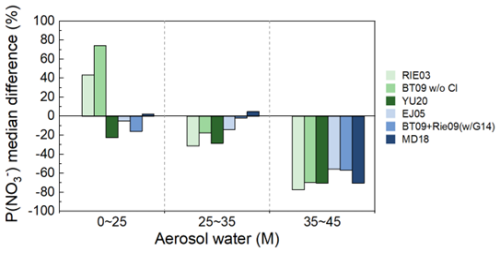
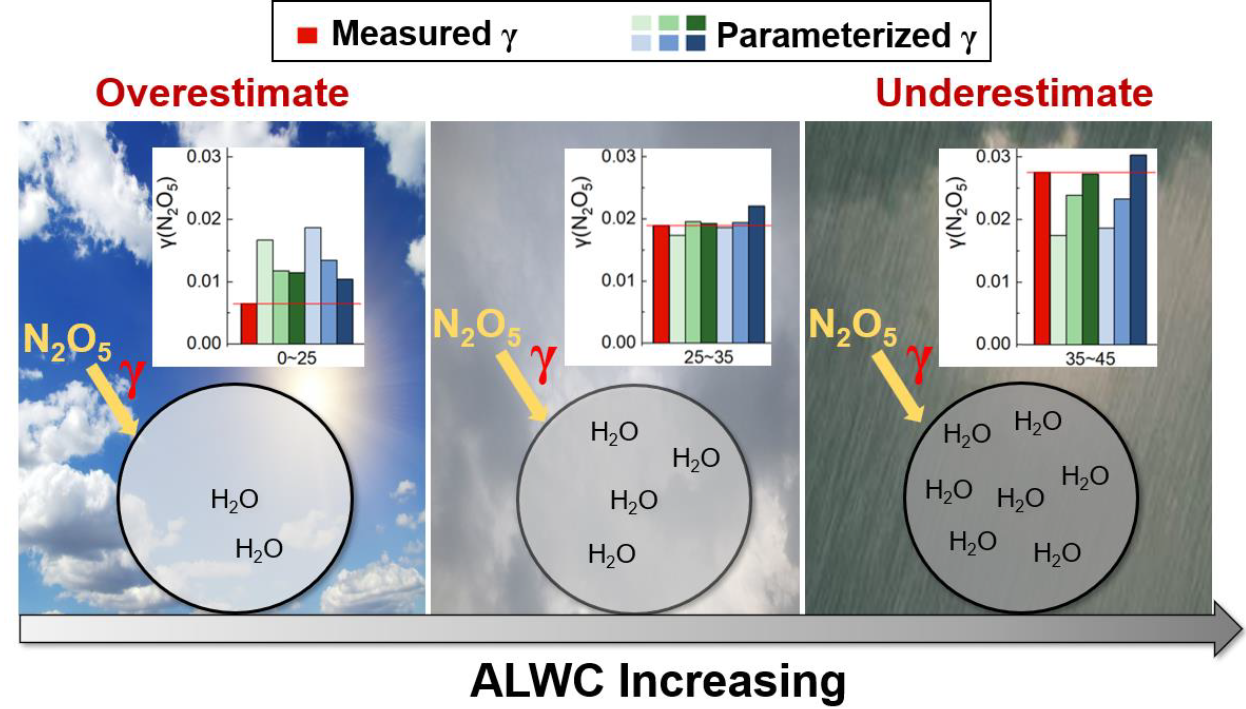
ALWC is one of the factors controlling N2O5 uptake during our observation, and the coefficients related to ALWC should play a critical role in reproducing the varying range of γ (N2O5). To investigate the accuracy of the ALWC-related response coefficients in γ (N2O5) parameterizations, we compared the parameterized and measured γ (N2O5) values at three ALWC levels: low concentration (0–25 M), medium concentration (25–35 M), and high concentration (35–45 M). Six parameterizations were selected for the comparison at different ALWC levels due to their low deviations (below 10 % of median values) over the entire observation (Fig. 5). At low ALWC, all six parameterizations showed an overestimation with the maximum difference for EJ05 (189 %) and the minimum for MD18 (34 %). At medium ALWC, the deviation of parameterized γ (N2O5) reduced to −8 %–4 %. At high ALWC, the parameterizations tend to underestimate the measured γ (N2O5) with the difference ranging from −37 % to −1 %. The treatment of ALWC-related effects on γ (N2O5) following the BT09 and Rie09 parameterization framework was generally better than that following RIE03 and EJ05. YU20 and MD18 showed the best performance across all three ALWC levels among inorganic-only parameterizations and inorganic + organic parameterizations, respectively. The overestimation at low ALWC and underestimation at high ALWC suggest that the treatment of coefficients related to ALWC in most parameterizations can hardly capture the response of γ (N2O5) to largely varied ALWC.
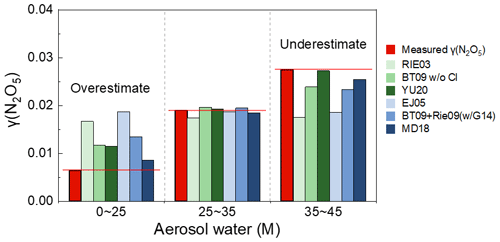
Figure 5Comparison of the median values of measured and parameterized γ (N2O5) at low, medium, and high ALWC levels. The settings of BT09 + Rie09wG14 and MD18 were 0.8 and 0.5, respectively.
The biased prediction of γ (N2O5) at low and high ALWC levels might cause considerable uncertainties in estimating the impacts of N2O5 uptake when ALWC varies largely in ambient conditions. We calculated the particulate nitrate production potential contributed by N2O5 uptake based on measured γ (N2O5) and six selected parameterizations at low and high ALWC levels, respectively. The maximum deviations of median nitrate production rates were 74 % and −77 % at low and high ALWC levels, respectively (Fig. 6). Our results indicate that current parameterizations may lead to large deviations in nitrate production potential predictions. The contribution of the N2O5 heterogeneous reaction to nitrate production is important in some regions (Wang et al., 2021; S. Chen et al., 2020; Fan et al., 2020; Wagner et al., 2013) and can be comparable with that of OH + NO2 pathway (Alexander et al., 2020; Fan et al., 2022; Zhai et al., 2023). Therefore, we suggest that future studies should conduct more γ (N2O5) measurements under extreme ALWC levels, which helps to improve the reliability of response coefficients between γ (N2O5) and ALWC in ambient conditions.
The γ (N2O5) values of ambient aerosols were directly measured by an improved in situ aerosol flow tube system in Kunming, which represents a typical highland environment. The relationship between the measured γ (N2O5) and impacting factors was consistent with previous laboratory results, except for aerosol chloride. The median of γ (N2O5) predicted by inorganic-only and inorganic + organic parameterizations generally overestimate and underestimate the measurements, respectively. While some parameterizations agreed well with the measurements on median values, they failed to reproduce the variabilities and showed low correlations. In particular, parameterizations overestimate γ (N2O5) by 34 %–189 % at low ALWC and underestimate it by −37 %–−1 % at high ALWC, respectively. Among the 10 parameterizations, the empirical parameterizations YU20 and MD18 performed relatively well with lower deviations in median values and RMSE. The suggestions on how to choose the different parameterization scenarios under various conditions were given. Our result reveals that using ambient measurements can effectively improve parameterizations derived from laboratory experiments. Therefore, we call for the need to conduct more field observations of γ (N2O5) directly on ambient aerosols to improve the performance of parameterizations and better elucidate the environmental impacts of the N2O5 uptake reaction. Meanwhile, further studies on the mechanism of N2O5 uptake under extreme ALWC conditions would help to improve the accuracy of its response coefficients in parameterizations.
The code and datasets used in this study are available from the corresponding authors upon request (chenxr95@mail.sysu.edu.cn, k.lu@pku.edu.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-6395-2025-supplement.
XC and KL designed the study. KL organized the field campaign with the help from YG. TZ and XC measured the γ (N2O5) data. CL, SX, HD, and SC provided the field data of normal gases, particulate components, and other supporting parameters. JL, TZ, XC, and HW analyzed the data. JL, TZ, and XC wrote the paper with input from KL.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This work was supported by the National Natural Science Foundation of China (grants nos. 22221004 and 22406204).
This research has been supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant no. 22221004) and the National Natural Science Foundation of China (grant no. 22406204).
This paper was edited by Guangjie Zheng and reviewed by two anonymous referees.
Abbatt, J. P. D., Lee, A. K. Y., and Thornton, J. A.: Quantifying trace gas uptake to tropospheric aerosol: recent advances and remaining challenges, Chem. Soc. Rev., 41, 6555–6581, https://doi.org/10.1039/c2cs35052a, 2012.
Alexander, B., Sherwen, T., Holmes, C. D., Fisher, J. A., Chen, Q., Evans, M. J., and Kasibhatla, P.: Global inorganic nitrate production mechanisms: comparison of a global model with nitrate isotope observations, Atmos. Chem. Phys., 20, 3859–3877, https://doi.org/10.5194/acp-20-3859-2020, 2020.
Anttila, T., Kiendler-Scharr, A., Tillmann, R., and Mentel, T. F.: On the reactive uptake of gaseous compounds by organic-coated aqueous aerosols: Theoretical analysis and application to the heterogeneous hydrolysis of N2O5, J. Phys. Chem. A, 110, 10435–10443, https://doi.org/10.1021/jp062403c, 2006.
Asaf, D., Pedersen, D., Matveev, V., Peleg, M., Kern, C., Zingler, J., Platt, U., and Luria, M.: Long-Term Measurements of NO3 Radical at a Semiarid Urban Site: 1. Extreme Concentration Events and Their Oxidation Capacity, Environ. Sci. Technol., 43, 9117–9123, https://doi.org/10.1021/es900798b, 2009.
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., and Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I – gas phase reactions of Ox, HOx, NOx and SOx species, Atmos. Chem. Phys., 4, 1461–1738, https://doi.org/10.5194/acp-4-1461-2004, 2004.
Bertram, T. H. and Thornton, J. A.: Toward a general parameterization of N2O5 reactivity on aqueous particles: the competing effects of particle liquid water, nitrate and chloride, Atmos. Chem. Phys., 9, 8351–8363, https://doi.org/10.5194/acp-9-8351-2009, 2009.
Bertram, T. H., Thornton, J. A., and Riedel, T. P.: An experimental technique for the direct measurement of N2O5 reactivity on ambient particles, Atmos. Meas. Tech., 2, 231–242, https://doi.org/10.5194/amt-2-231-2009, 2009a.
Bertram, T. H., Thornton, J. A., Riedel, T. P., Middlebrook, A. M., Bahreini, R., Bates, T. S., Quinn, P. K., and Coffman, D. J.: Direct observations of N2O5 reactivity on ambient aerosol particles, Geophys. Res. Lett., 36, L19803, https://doi.org/10.1029/2009gl040248, 2009b.
Brown, S. S. and Stutz, J.: Nighttime radical observations and chemistry, Chem. Soc. Rev., 41, 6405–6447, https://doi.org/10.1039/c2cs35181a, 2012.
Brown, S. S., Ryerson, T. B., Wollny, A. G., Brock, C. A., Peltier, R., Sullivan, A. P., Weber, R. J., Dube, W. P., Trainer, M., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. R.: Variability in nocturnal nitrogen oxide processing and its role in regional air quality, Science, 311, 67–70, https://doi.org/10.1126/science.1120120, 2006.
Brown, S. S., Dube, W. P., Fuchs, H., Ryerson, T. B., Wollny, A. G., Brock, C. A., Bahreini, R., Middlebrook, A. M., Neuman, J. A., Atlas, E., Roberts, J. M., Osthoff, H. D., Trainer, M., Fehsenfeld, F. C., and Ravishankara, A. R.: Reactive uptake coefficients for N2O5 determined from aircraft measurements during the Second Texas Air Quality Study: Comparison to current model parameterizations, J. Geophys. Res.-Atmos., 114, D00F10, https://doi.org/10.1029/2008jd011679, 2009.
Brown, S. S., Dube, W. P., Tham, Y. J., Zha, Q. Z., Xue, L. K., Poon, S., Wang, Z., Blake, D. R., Tsui, W., Parrish, D. D., and Wang, T.: Nighttime chemistry at a high altitude site above Hong Kong, J. Geophys. Res.-Atmos., 121, 2457–2475, https://doi.org/10.1002/2015jd024566, 2016.
Chang, W. L., Bhave, P. V., Brown, S. S., Riemer, N., Stutz, J., and Dabdub, D.: Heterogeneous Atmospheric Chemistry, Ambient Measurements, and Model Calculations of N2O5: A Review, Aerosol Sci. Technol., 45, 665–695, https://doi.org/10.1080/02786826.2010.551672, 2011.
Chang, W. L., Brown, S. S., Stutz, J., Middlebrook, A. M., Bahreini, R., Wagner, N. L., Dube, W. P., Pollack, I. B., Ryerson, T. B., and Riemer, N.: Evaluating N2O5 heterogeneous hydrolysis parameterizations for CalNex 2010, J. Geophys. Res.-Atmos., 121, 5051–5070, https://doi.org/10.1002/2015jd024737, 2016.
Chen, S., Wang, H., Lu, K., Zeng, L., Hu, M., and Zhang, Y.: The trend of surface ozone in Beijing from 2013 to 2019: Indications of the persisting strong atmospheric oxidation capacity, Atmos. Environ., 242, 117801, https://doi.org/10.1016/j.atmosenv.2020.117801, 2020.
Chen, X., Wang, H., Zhai, T., Li, C., and Lu, K.: Direct measurement of N2O5 heterogeneous uptake coefficients on ambient aerosols via an aerosol flow tube system: design, characterization and performance, Atmos. Meas. Tech., 15, 7019–7037, https://doi.org/10.5194/amt-15-7019-2022, 2022.
Chen, X. R., Wang, H. C., Lu, K. D., Li, C. M., Zhai, T. Y., Tan, Z. F., Ma, X. F., Yang, X. P., Liu, Y. H., Chen, S. Y., Dong, H. B., Li, X., Wu, Z. J., Hu, M., Zeng, L. M., and Zhang, Y. H.: Field Determination of Nitrate Formation Pathway in Winter Beijing, Environ. Sci. Technol., 54, 9243–9253, https://doi.org/10.1021/acs.est.0c00972, 2020.
Chen, Y., Wolke, R., Ran, L., Birmili, W., Spindler, G., Schröder, W., Su, H., Cheng, Y., Tegen, I., and Wiedensohler, A.: A parameterization of the heterogeneous hydrolysis of N2O5 for mass-based aerosol models: improvement of particulate nitrate prediction, Atmos. Chem. Phys., 18, 673–689, https://doi.org/10.5194/acp-18-673-2018, 2018.
Cosman, L. M. and Bertram, A. K.: Reactive uptake of N2O5 on aqueous H2SO4 solutions coated with 1-component and 2-component monolayers, J. Phys. Chem. A, 112, 4625–4635, https://doi.org/10.1021/jp8005469, 2008.
Davis, J. M., Bhave, P. V., and Foley, K. M.: Parameterization of N2O5 reaction probabilities on the surface of particles containing ammonium, sulfate, and nitrate, Atmos. Chem. Phys., 8, 5295–5311, https://doi.org/10.5194/acp-8-5295-2008, 2008.
Decker, Z. C. J., Zarzana, K. J., Coggon, M., Min, K. E., Pollack, I., Ryerson, T. B., Peischl, J., Edwards, P., Dubé, W. P., Markovic, M. Z., Roberts, J. M., Veres, P. R., Graus, M., Warneke, C., de Gouw, J., Hatch, L. E., Barsanti, K. C., and Brown, S. S.: Nighttime Chemical Transformation in Biomass Burning Plumes: A Box Model Analysis Initialized with Aircraft Observations, Environ. Sci. Technol., 53, 2529–2538, https://doi.org/10.1021/acs.est.8b05359, 2019.
Dentener, F. J. and Crutzen, P. J.: Reaction of N2O5 on Tropospheric Aerosols – Impact on the Global Distributions of NOx, O3, and OH, J. Geophys. Res.-Atmos., 98, 7149–7163, https://doi.org/10.1029/92jd02979, 1993.
Escoreia, E. N., Sjostedt, S. J., and Abbatt, J. P. D.: Kinetics of N2O5 Hydrolysis on Secondary Organic Aerosol and Mixed Ammonium Bisulfate-Secondary Organic Aerosol Particles, J. Phys. Chem. A, 114, 13113–13121, https://doi.org/10.1021/jp107721v, 2010.
Evans, M. J. and Jacob, D. J.: Impact of new laboratory studies of N2O5 hydrolysis on global model budgets of tropospheric nitrogen oxides, ozone, and OH, Geophys. Res. Lett., 32, L09813, https://doi.org/10.1029/2005gl022469, 2005.
Fan, M.-Y., Zhang, Y.-L., Lin, Y.-C., Cao, F., Zhao, Z.-Y., Sun, Y., Qiu, Y., Fu, P., and Wang, Y.: Changes of Emission Sources to Nitrate Aerosols in Beijing After the Clean Air Actions: Evidence From Dual Isotope Compositions, J. Geophys. Res.-Atmos., 125, 1–15, https://doi.org/10.1029/2019jd031998, 2020.
Fan, M. Y., Zhang, Y. L., Lin, Y. C., Hong, Y. H., Zhao, Z. Y., Xie, F., Du, W., Cao, F., Sun, Y. L., and Fu, P. Q.: Important Role of NO3 Radical to Nitrate Formation Aloft in Urban Beijing: Insights from Triple Oxygen Isotopes Measured at the Tower, Environ. Sci. Technol., 56, 6870–6879, https://doi.org/10.1021/acs.est.1c02843, 2022.
Fang, Y.-G., Tang, B., Yuan, C., Wan, Z., Zhao, L., Zhu, S., Francisco, J. S., Zhu, C., and Fang, W.-H.: Mechanistic insight into the competition between interfacial and bulk reactions in microdroplets through N2O5 ammonolysis and hydrolysis, Nat. Commun., 15, 2347, https://doi.org/10.1038/s41467-024-46674-1, 2024.
Finlaysonpitts, B. J., Ezell, M. J., and Pitts, J. N.: Formation of chemically active chlorine compounds by reactions of atmospheric nacl particles with gaseous N2O5 and ClONO2, Nature, 337, 241–244, https://doi.org/10.1038/337241a0, 1989.
Folkers, M., Mentel, T. F., and Wahner, A.: Influence of an organic coating on the reactivity of aqueous aerosols probed by the heterogeneous hydrolysis of N2O5, Geophys. Res. Lett., 30, 12, https://doi.org/10.1029/2003gl017168, 2003.
Fountoukis, C. and Nenes, A.: ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+–Ca–Mg2+–NH–Na+–SO–NO–Cl—H2O aerosols, Atmos. Chem. Phys., 7, 4639–4659, https://doi.org/10.5194/acp-7-4639-2007, 2007.
Gaston, C. J., Thornton, J. A., and Ng, N. L.: Reactive uptake of N2O5 to internally mixed inorganic and organic particles: the role of organic carbon oxidation state and inferred organic phase separations, Atmos. Chem. Phys., 14, 5693–5707, https://doi.org/10.5194/acp-14-5693-2014, 2014.
Griffiths, P. T., Badger, C. L., Cox, R. A., Folkers, M., Henk, H. H., and Mentel, T. F.: Reactive Uptake of N2O5 by Aerosols Containing Dicarboxylic Acids. Effect of Particle Phase, Composition, and Nitrate Content, J. Phys. Chem. A, 113, 5082–5090, https://doi.org/10.1021/jp8096814, 2009.
Hallquist, M., Stewart, D. J., Stephenson, S. K., and Cox, R. A.: Hydrolysis of N2O5 on sub-micron sulfate aerosols, Phys. Chem. Chem. Phys., 5, 3453–3463, https://doi.org/10.1039/b301827j, 2003.
Li, Z. Y., Xie, P. H., Hu, R. Z., Wang, D., Jin, H. W., Chen, H., Lin, C., and Liu, W. Q.: Observations of N2O5 and NO3 at a suburban environment in Yangtze river delta in China: Estimating heterogeneous N2O5 uptake coefficients, J. Environ. Sci., 95, 248–255, https://doi.org/10.1016/j.jes.2020.04.041, 2020.
Liu, X. G., Gu, J. W., Li, Y. P., Cheng, Y. F., Qu, Y., Han, T. T., Wang, J. L., Tian, H. Z., Chen, J., and Zhang, Y. H.: Increase of aerosol scattering by hygroscopic growth: Observation, modeling, and implications on visibility, Atmos. Res., 132, 91–101, https://doi.org/10.1016/j.atmosres.2013.04.007, 2013.
McDuffie, E. E., Fibiger, D. L., Dube, W. P., Lopez-Hilfiker, F., Lee, B. H., Thornton, J. A., Shah, V., Jaegle, L., Guo, H. Y., Weber, R. J., Reeves, J. M., Weinheimer, A. J., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Dibb, J. E., Veres, P., Ebben, C., Sparks, T. L., Wooldridge, P. J., Cohen, R. C., Hornbrook, R. S., Apel, E. C., Campos, T., Hall, S. R., Ullmann, K., and Brown, S. S.: Heterogeneous N2O5 Uptake During Winter: Aircraft Measurements During the 2015 WINTER Campaign and Critical Evaluation of Current Parameterizations, J. Geophys. Res.-Atmos., 123, 4345–4372, https://doi.org/10.1002/2018jd028336, 2018.
Morgan, W. T., Ouyang, B., Allan, J. D., Aruffo, E., Di Carlo, P., Kennedy, O. J., Lowe, D., Flynn, M. J., Rosenberg, P. D., Williams, P. I., Jones, R., McFiggans, G. B., and Coe, H.: Influence of aerosol chemical composition on N2O5 uptake: airborne regional measurements in northwestern Europe, Atmos. Chem. Phys., 15, 973–990, https://doi.org/10.5194/acp-15-973-2015, 2015.
Mozurkewich, M. and Calvert, J. G.: Reaction probability of N2O5 on Aqueous Aerosols, J. Geophys. Res.-Atmos., 93, 15889–15896, https://doi.org/10.1029/JD093iD12p15889, 1988.
Murray, L. T., Fiore, A. M., Shindell, D. T., Naik, V., and Horowitz, L. W.: Large uncertainties in global hydroxyl projections tied to fate of reactive nitrogen and carbon, P. Natl. Acad. Sci. USA, 118, e2115204118, https://doi.org/10.1073/pnas.2115204118, 2021.
Ng, N. L., Canagaratna, M. R., Zhang, Q., Jimenez, J. L., Tian, J., Ulbrich, I. M., Kroll, J. H., Docherty, K. S., Chhabra, P. S., Bahreini, R., Murphy, S. M., Seinfeld, J. H., Hildebrandt, L., Donahue, N. M., DeCarlo, P. F., Lanz, V. A., Prévôt, A. S. H., Dinar, E., Rudich, Y., and Worsnop, D. R.: Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry, Atmos. Chem. Phys., 10, 4625–4641, https://doi.org/10.5194/acp-10-4625-2010, 2010.
Ng, N. L., Brown, S. S., Archibald, A. T., Atlas, E., Cohen, R. C., Crowley, J. N., Day, D. A., Donahue, N. M., Fry, J. L., Fuchs, H., Griffin, R. J., Guzman, M. I., Herrmann, H., Hodzic, A., Iinuma, Y., Jimenez, J. L., Kiendler-Scharr, A., Lee, B. H., Luecken, D. J., Mao, J., McLaren, R., Mutzel, A., Osthoff, H. D., Ouyang, B., Picquet-Varrault, B., Platt, U., Pye, H. O. T., Rudich, Y., Schwantes, R. H., Shiraiwa, M., Stutz, J., Thornton, J. A., Tilgner, A., Williams, B. J., and Zaveri, R. A.: Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol, Atmos. Chem. Phys., 17, 2103–2162, https://doi.org/10.5194/acp-17-2103-2017, 2017.
Niu, Y. B., Zhu, B., He, L. Y., Wang, Z., Lin, X. Y., Tang, M. X., and Huang, X. F.: Fast Nocturnal Heterogeneous Chemistry in a Coastal Background Atmosphere and Its Implications for Daytime Photochemistry, J. Geophys. Res.-Atmos., 127, 1–14, https://doi.org/10.1029/2022jd036716, 2022.
Osthoff, H. D., Roberts, J. M., Ravishankara, A. R., Williams, E. J., Lerner, B. M., Sommariva, R., Bates, T. S., Coffman, D., Quinn, P. K., Dibb, J. E., Stark, H., Burkholder, J. B., Talukdar, R. K., Meagher, J., Fehsenfeld, F. C., and Brown, S. S.: High levels of nitryl chloride in the polluted subtropical marine boundary layer, Nat. Geosci., 1, 324–328, https://doi.org/10.1038/ngeo177, 2008.
Phillips, G. J., Thieser, J., Tang, M., Sobanski, N., Schuster, G., Fachinger, J., Drewnick, F., Borrmann, S., Bingemer, H., Lelieveld, J., and Crowley, J. N.: Estimating N2O5 uptake coefficients using ambient measurements of NO3, N2O5, ClNO2 and particle-phase nitrate, Atmos. Chem. Phys., 16, 13231–13249, https://doi.org/10.5194/acp-16-13231-2016, 2016.
Riedel, T. P., Bertram, T. H., Ryder, O. S., Liu, S., Day, D. A., Russell, L. M., Gaston, C. J., Prather, K. A., and Thornton, J. A.: Direct N2O5 reactivity measurements at a polluted coastal site, Atmos. Chem. Phys., 12, 2959–2968, https://doi.org/10.5194/acp-12-2959-2012, 2012.
Riedel, T. P., Wolfe, G. M., Danas, K. T., Gilman, J. B., Kuster, W. C., Bon, D. M., Vlasenko, A., Li, S.-M., Williams, E. J., Lerner, B. M., Veres, P. R., Roberts, J. M., Holloway, J. S., Lefer, B., Brown, S. S., and Thornton, J. A.: An MCM modeling study of nitryl chloride (ClNO2) impacts on oxidation, ozone production and nitrogen oxide partitioning in polluted continental outflow, Atmos. Chem. Phys., 14, 3789–3800, https://doi.org/10.5194/acp-14-3789-2014, 2014.
Roberts, J. M., Osthoff, H. D., Brown, S. S., Ravishankara, A. R., Coffman, D., Quinn, P., and Bates, T.: Laboratory studies of products of N2O5 uptake on Cl- containing substrates, Geophys. Res. Lett., 36, L20808, https://doi.org/10.1029/2009gl040448, 2009.
Ryder, O. S., Ault, A. P., Cahill, J. F., Guasco, T. L., Riedel, T. P., Cuadra-Rodriguez, L. A., Gaston, C. J., Fitzgerald, E., Lee, C., Prather, K. A., and Bertram, T. H.: On the Role of Particle Inorganic Mixing State in the Reactive Uptake of N2O5 to Ambient Aerosol Particles, Environ. Sci. Technol., 48, 1618–1627, https://doi.org/10.1021/es4042622, 2014.
Shiraiwa, M., Li, Y., Tsimpidi, A. P., Karydis, V. A., Berkemeier, T., Pandis, S. N., Lelieveld, J., Koop, T., and Poschl, U.: Global distribution of particle phase state in atmospheric secondary organic aerosols, Nat. Commun., 8, 1–7, https://doi.org/10.1038/ncomms15002, 2017.
Tham, Y. J., Wang, Z., Li, Q., Yun, H., Wang, W., Wang, X., Xue, L., Lu, K., Ma, N., Bohn, B., Li, X., Kecorius, S., Größ, J., Shao, M., Wiedensohler, A., Zhang, Y., and Wang, T.: Significant concentrations of nitryl chloride sustained in the morning: investigations of the causes and impacts on ozone production in a polluted region of northern China, Atmos. Chem. Phys., 16, 14959–14977, https://doi.org/10.5194/acp-16-14959-2016, 2016.
Tham, Y. J., Wang, Z., Li, Q., Wang, W., Wang, X., Lu, K., Ma, N., Yan, C., Kecorius, S., Wiedensohler, A., Zhang, Y., and Wang, T.: Heterogeneous N2O5 uptake coefficient and production yield of ClNO2 in polluted northern China: roles of aerosol water content and chemical composition, Atmos. Chem. Phys., 18, 13155–13171, https://doi.org/10.5194/acp-18-13155-2018, 2018.
Thornton, J. A., Braban, C. F., and Abbatt, J. P. D.: N2O5 hydrolysis on sub-micron organic aerosols: the effect of relative humidity, particle phase, and particle size, Phys. Chem. Chem. Phys., 5, 4593–4603, https://doi.org/10.1039/b307498f, 2003.
Wagner, N. L., Riedel, T. P., Young, C. J., Bahreini, R., Brock, C. A., Dube, W. P., Kim, S., Middlebrook, A. M., Ozturk, F., Roberts, J. M., Russo, R., Sive, B., Swarthout, R., Thornton, J. A., VandenBoer, T. C., Zhou, Y., and Brown, S. S.: N2O5 uptake coefficients and nocturnal NO2 removal rates determined from ambient wintertime measurements, J. Geophys. Res.-Atmos., 118, 9331–9350, https://doi.org/10.1002/jgrd.50653, 2013.
Wang, H., Chen, J., and Lu, K.: Development of a portable cavity-enhanced absorption spectrometer for the measurement of ambient NO3 and N2O5: experimental setup, lab characterizations, and field applications in a polluted urban environment, Atmos. Meas. Tech., 10, 1465–1479, https://doi.org/10.5194/amt-10-1465-2017, 2017.
Wang, H., Lu, K., Guo, S., Wu, Z., Shang, D., Tan, Z., Wang, Y., Le Breton, M., Lou, S., Tang, M., Wu, Y., Zhu, W., Zheng, J., Zeng, L., Hallquist, M., Hu, M., and Zhang, Y.: Efficient N2O5 uptake and NO3 oxidation in the outflow of urban Beijing, Atmos. Chem. Phys., 18, 9705–9721, https://doi.org/10.5194/acp-18-9705-2018, 2018.
Wang, H., Yuan, B., Zheng, E., Zhang, X., Wang, J., Lu, K., Ye, C., Yang, L., Huang, S., Hu, W., Yang, S., Peng, Y., Qi, J., Wang, S., He, X., Chen, Y., Li, T., Wang, W., Huangfu, Y., Li, X., Cai, M., Wang, X., and Shao, M.: Formation and impacts of nitryl chloride in Pearl River Delta, Atmos. Chem. Phys., 22, 14837–14858, https://doi.org/10.5194/acp-22-14837-2022, 2022.
Wang, H. C., Lu, K. D., Chen, X. R., Zhu, Q. D., Chen, Q., Guo, S., Jiang, M. Q., Li, X., Shang, D. J., Tan, Z. F., Wu, Y. S., Wu, Z. J., Zou, Q., Zheng, Y., Zeng, L. M., Zhu, T., Hu, M., and Zhang, Y. H.: High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway, Environ. Sci. Technol. Lett., 4, 416–420, https://doi.org/10.1021/acs.estlett.7b00341, 2017.
Wang, H. C., Chen, X. R., Lu, K. D., Hu, R. Z., Li, Z. Y., Wang, H. L., Ma, X. F., Yang, X. P., Chen, S. Y., Dong, H. B., Liu, Y., Fang, X., Zeng, L. M., Hu, M., and Zhang, Y. H.: NO3 and N2O5 chemistry at a suburban site during the EXPLORE-YRD campaign in 2018, Atmos. Environ., 224, 117180, https://doi.org/10.1016/j.atmosenv.2019.117180, 2020a.
Wang, H. C., Chen, X. R., Lu, K. D., Tan, Z. F., Ma, X. F., Wu, Z. J., Li, X., Liu, Y. H., Shang, D. J., Wu, Y. S., Zeng, L. M., Hu, M., Schmitt, S., Kiendler-Scharr, A., Wahner, A., and Zhang, Y. H.: Wintertime N2O5 uptake coefficients over the North China Plain, Sci. Bull., 65, 765–774, https://doi.org/10.1016/j.scib.2020.02.006, 2020b.
Wang, H. C., Lu, K. D., Chen, S. Y., Li, X., Zeng, L. M., Hu, M., and Zhang, Y. H.: Characterizing nitrate radical budget trends in Beijing during 2013–2019, Sci. Total Environ., 795, https://doi.org/10.1016/j.scitotenv.2021.148869, 2021.
Wang, H. C., Wang, H. L., Lu, X., Lu, K. D., Zhang, L., Tham, Y. J., Shi, Z. B., Aikin, K., Fan, S. J., Brown, S. S., and Zhang, Y. H.: Increased night-time oxidation over China despite widespread decrease across the globe, Nat. Geosci., 16, 217–223, https://doi.org/10.1038/s41561-022-01122-x, 2023.
Wang, X. F., Wang, H., Xue, L. K., Wang, T., Wang, L. W., Gu, R. R., Wang, W. H., Tham, Y. J., Wang, Z., Yang, L. X., Chen, J. M., and Wang, W. X.: Observations of N2O5 and ClNO2 at a polluted urban surface site in North China: High N2O5 uptake coefficients and low ClNO2 product yields, Atmos. Environ., 156, 125–134, https://doi.org/10.1016/j.atmosenv.2017.02.035, 2017.
Wang, Z., Wang, W., Tham, Y. J., Li, Q., Wang, H., Wen, L., Wang, X., and Wang, T.: Fast heterogeneous N2O5 uptake and ClNO2 production in power plant and industrial plumes observed in the nocturnal residual layer over the North China Plain, Atmos. Chem. Phys., 17, 12361–12378, https://doi.org/10.5194/acp-17-12361-2017, 2017.
Yan, C., Tham, Y. J., Zha, Q., Wang, X., Xue, L., Dai, J., Wang, Z., and Wang, T.: Fast heterogeneous loss of N2O5 leads to significant nighttime NOx removal and nitrate aerosol formation at a coastal background environment of southern China, Sci. Total Environ., 677, 637–647, https://doi.org/10.1016/j.scitotenv.2019.04.389, 2019.
Yan, C., Tham, Y. J., Nie, W., Xia, M., Wang, H. C., Guo, Y. S., Ma, W., Zhan, J. L., Hua, C. J., Li, Y. Y., Deng, C. J., Li, Y. R., Zheng, F. X., Chen, X., Li, Q. Y., Zhang, G., Mahajan, A. S., Cuevas, C. A., Huang, D. D., Wang, Z., Sun, Y. L., Saiz-Lopez, A., Bianchi, F., Kerminen, V. M., Worsnop, D. R., Donahue, N. M., Jiang, J. K., Liu, Y. C., Ding, A. J., and Kulmala, M.: Increasing contribution of nighttime nitrogen chemistry to wintertime haze formation in Beijing observed during COVID-19 lockdowns, Nat. Geosci., 16, 975, https://doi.org/10.1038/s41561-023-01285-1, 2023.
You, Y., Smith, M. L., Song, M. J., Martin, S. T., and Bertram, A. K.: Liquid-liquid phase separation in atmospherically relevant particles consisting of organic species and inorganic salts, Int. Rev. Phys. Chem., 33, 43–77, https://doi.org/10.1080/0144235x.2014.890786, 2014.
Yu, C., Wang, Z., Xia, M., Fu, X., Wang, W., Tham, Y. J., Chen, T., Zheng, P., Li, H., Shan, Y., Wang, X., Xue, L., Zhou, Y., Yue, D., Ou, Y., Gao, J., Lu, K., Brown, S. S., Zhang, Y., and Wang, T.: Heterogeneous N2O5 reactions on atmospheric aerosols at four Chinese sites: improving model representation of uptake parameters, Atmos. Chem. Phys., 20, 4367–4378, https://doi.org/10.5194/acp-20-4367-2020, 2020.
Zhai, T., Lu, K., Wang, H., Lou, S., Chen, X., Hu, R., and Zhang, Y.: Elucidate the formation mechanism of particulate nitrate based on direct radical observations in the Yangtze River Delta summer 2019, Atmos. Chem. Phys., 23, 2379–2391, https://doi.org/10.5194/acp-23-2379-2023, 2023.