the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Accurate elucidation of oxidation under heavy ozone pollution: a full suite of radical measurements in the chemically complex atmosphere
Renzhi Hu
Guoxian Zhang
Haotian Cai
Jingyi Guo
Keding Lu
Shengrong Lou
Zhaofeng Tan
Changjin Hu
Pinhua Xie
Wenqing Liu
The Yangze River Delta (YRD) in China encountered prolonged ozone pollution in September 2020. To accurately elucidate the limitations of oxidation processes in the chemically complex atmosphere, a full suite of radical measurements (OH, HO2, RO2, and kOH) was established in the YRD region for the first time. The diurnal peaks of radicals exhibited considerable variation due to environmental factors, showing ranges of 3.6 to 27.1 × 106 cm−3 for OH, 2.1 to 33.2 × 108 cm−3 for HO2, and 4.9 to 30.5 × 108 cm−3 for RO2. The simulated results provided by RACM2-LIM1 failed to adequately match the observed data in both radical concentration and experimental budget for a heavy ozone pollution episode. Sensitivity tests utilizing a comprehensive set of radical measurements revealed that the Higher Aldehyde Mechanism (HAM) effectively complements the regeneration of OH radicals, yielding enhancements of 4.4 %–6.0 % compared to the base scenario, while the concentrations of HO2 and RO2 radicals have shown increments of about 7.4 % and 12.5 %, respectively. It is noteworthy that under the constraints of kOH measurement, the inclusion of oxygenated volatile organic compounds (OVOCs) and larger alkoxy radicals derived from monoterpenes improved the model–measurement consistency for ozone formation, reducing the discrepancy under high NO conditions from 4.17 to 2.39. This outcome corroborates the hypothesis of sensitivity analysis as it pertains to ozone formation. Moving forward, by implementing a comprehensive radical detection approach, further investigations should concentrate on a broader range of OVOCs to rectify the imbalance associated with RO2 radicals, thereby providing a more precise understanding of oxidation processes during severe ozone pollution episodes.
- Article
(5356 KB) - Full-text XML
-
Supplement
(1588 KB) - BibTeX
- EndNote
In recent years, China's rapid economic development has led to severe environmental pollution problems, which has significantly impacted the respiratory, dermatological, and ocular health of local residents (Wang et al., 2022c; Huang et al., 2018). This has raised concerns about the coexistence of regional primary and secondary pollution, making air quality improvement efforts a focal point (Liu et al., 2021; Wang et al., 2022a). In the complex atmosphere, near-surface ozone (O3) is formed through continuous photochemical reactions between nitrogen oxides (NOx ≡ NO + NO2) and volatile organic compounds (VOCs) under light conditions, while hydroxyl radicals (OH) serve as the main oxidant in the troposphere, converting VOCs into hydroperoxy (HO2) and organic peroxy (RO2) radicals (Rohrer et al., 2014; Hofzumahaus et al., 2009). Additionally, the oxidation of nitric oxide (NO) and peroxy radicals produces nitrogen dioxide (NO2), which is the sole photochemical source of ozone (Lu et al., 2012; Stone et al., 2012).
Despite numerous experimental and theoretical explorations to establish the radical-cored photooxidation mechanism in the troposphere, field observations were primarily focused on HOx (HOx ≡ OH + HO2) radicals due to the limitations of detection technology (Kanaya et al., 2012; Lu et al., 2012; Hofzumahaus et al., 2009; Kanaya et al., 2007a; Ren et al., 2008; Stone et al., 2012; Levy, 1971). Recent advancements in detection technology, such as the application of a new LIF technique (ROxLIF), have made the detection of RO2 radicals possible (Whalley et al., 2013; Tan et al., 2017a). Moreover, the union of comprehensive field campaigns and box models has proven to be an effective method for verifying the integrity of radical chemistry at local to global scales (Lu et al., 2019; Tan et al., 2018). Several experiments have indicated that the existing atmospheric chemical mechanism posed challenges in deepening the understanding of the regional pollution explosion (Whalley et al., 2021; Slater et al., 2020; Tan et al., 2017a; Woodward-Massey et al., 2023). For instance, the observation of up to 4 × 109 cm−3 of the RO2 radical in the center of Beijing in 2017 (APHH) was significantly underestimated by MCM v3.3.1 (Whalley et al., 2021). Further exploring the unreproducible concentration and the oxidation process in the chemically complex atmosphere is deemed necessary (Whalley et al., 2021; Woodward-Massey et al., 2023).
The Yangze River Delta (YRD) region, situated between the North China Plain (NCP) and Pearl River Delta (PRD), is highly prone to regional transport interactions and aerosol–boundary layer feedback (Jia et al., 2021; Huang et al., 2020). In an effort to gain a better understanding between the complex radical chemistry and the intensive oxidation level in the Yangze River Delta, TROPSTECT-YRD (The experiment on Radical chemistry and Ozone Pollution perSpectively: long-Term Elucidation of the photochemiCal oxidaTion in the Yangze River Delta) was conducted in Hefei during September 2020. Accurate elucidation of the oxidation process under heavy ozone pollution was provided by a full suite of radical measurements (OH, HO2, RO2, and kOH) in the chemically complex atmosphere.
2.1 Site description and instrumentation
The TROPSTECT observation was conducted from 1 to 20 September 2020 at the Science Island background station (31.9° N, 117.2° E) in Hefei, a typical megacity located in the central region of Anhui Province within the Yangtze River Delta. The station is situated on a peninsula with abundant vegetation to the northwest of urban areas and is in close proximity to Dongpu Lake, which is only 200 m away, and the main road, positioned 250 m southward (Fig. 1). Consequently, the relatively enclosed environment exhibits typical suburban characteristics of anthropogenic emissions. The station is located in the transition region between urban and suburban areas, reflecting the regional transport of pollution in Hefei and its surrounding areas.
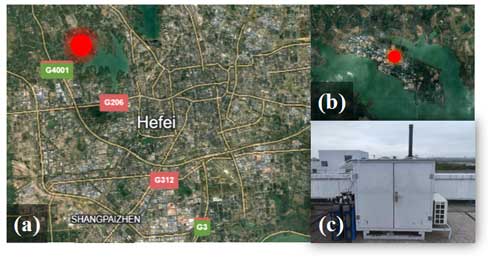
Figure 1(a) The location of the measurement site (source: © Google Earth). (b) A close shot of the measurement site location (source: © Google Earth). (c) The actual image for the LIF box.
Regarding the instrumentation, a group of oxidation-related instruments were installed on the seventh floor of the Comprehensive Building at the Anhui Institute of Optics and Fine Mechanics (AIOFM), with all sampling outlets positioned more than 20 m above the ground. The details of the instruments measuring various parameters such as meteorological factors (WS, WD, T, RH, P, J values), gas pollutants (O3, CO, SO2, NO, NO2, HONO, HCHO, PAN), and non-methane hydrocarbons (NMHCs) are provided in Table S1 in the Supplement.
The measurement of NO, NO2, O3, CO, and SO2 was carried out using commercial Thermo Electron model series instruments. Thereof, NO and NO2 were measured using a chemical luminescence method (CL) with an enhanced trace-level NO–NO2–NOx analyzer (PKU-PL), which achieved a detection limit of 50 ppt (Tan et al., 2017a). The detection of O3 and SO2 was conducted through Thermo Electron model 49i and 43i, respectively, while Thermo Electron model 48i was utilized for CO detection. Cavity ring-down spectroscopy (CRDS, Picarro-G2401) was employed for CO detection in parallel, and another ultraviolet absorption instrument (Ecotech EC9810B) was for ozone detection. The instrument inlets were placed within 5 m of each other for comparison.
All Thermo series gas analyzers were equipped with individual sampling inlet tubing, which was made of polyfluoroalkyl (PFA; with 6.35 mm outer diameter), and the flow rate was operated at 1.5 L min−1. The total length of the inlet tubing from the sampling tip to the instrument inlet was 2.5 m, with the entire line wrapped with insulation material to prevent condensation of water vapor. To ensure measurement accuracy, the instruments in the campaign underwent zero-point calibration procedures during the early (31 August) and late (21 September) observation periods. Furthermore, additional zero calibration for Thermo 48i CO detection was undertaken daily from 00:00–00:30 UTC+8 (this time zone applies to all times mentioned in the paper) to minimize shift correction. Zero-point calibration includes the instrument and the complete inlet system. Cross-calibrations for O3 and CO measurements were carried out during the middle (9 September). The comparison results revealed high consistency within the instrument accuracy range for both CO and O3 measurements (Fig. S1a, b in the Supplement).
HONO was detected using a home-built instrument by cavity-enhanced absorption spectroscopy (CEAS), while formaldehyde was determined by the Hantzsch method (SDL model 4050) (Duan et al., 2018; Yang et al., 2021a). An automated gas chromatograph equipped with a mass spectrometer and flame ionization detector (GC-FID/MS) was employed for the online measurement of 99 VOC species. Information for parts of the VOC monitoring species by online GC-MS/FID is listed in Table S2.
The eight crucial photolysis frequencies (j(NO2), j(H2O2), j(HCHO_M), j(HCHO_R), j(HONO), j(NO3_M), j(NO3_R), j(O1D)) were directly measured by a photolysis spectrometer (Metcon, Germany). The unmeasured photolysis frequencies of the remaining active species were computed using Eq. (1):
The variations in photolysis frequency due to solar zenith angle (χ) were adjusted based on the ratio of observed and simulated j(NO2). The optimal values for parameters (l, m, and n) for different photolysis frequencies were extensively detailed by MCM v3.3.1 (https://mcm.york.ac.uk/MCM/rates/photolysis, last access: 12 March 2025) (Jenkin et al., 2003, 1997).
2.2 Radical measurement
2.2.1 OH, HO2, RO2 concentrations
The laser-induced fluorescence instrument developed by the Anhui Institute of Optics and Fine Mechanics (AIOFM-LIF) was used to simultaneously detect the concentrations of OH, HO2, and RO2 radicals, along with OH reactivity (kOH). The OH radical was directly measured by detecting on-resonance fluorescence excited by a 308 nm laser. An indirect measurement for HO2 was carried out after converting it to OH at a fixed efficiency (Heard and Pilling, 2003).
The laser utilized for fluorescence excitation is a high-frequency tunable dye laser that emits a 308 nm laser, with the laser power divided into a ratio of 0.45 : 0.45 : 0.08 : 0.02. Of this power, 90 % is directed towards fluorescence cells for concentration and reactivity detection via optical fibers, respectively, and 8 % of the laser power is directed to a reference cell, while the remaining 2 % is used to monitor real-time power fluctuations. The laser is transmitted through HO2, OH, and RO2 cells in turn via a coaxial optical path. Two photodiodes are set up at the end of the reference cell and RO2 detection cell, respectively. The voltage signals and power fluctuations are compared synchronously to diagnose the laser stability. To maintain detection efficiency, the power inside the measurement cells should not be less than 10 mW. Sampling nozzles of 0.4 mm are deployed above OH and HO2 cells for efficient sampling at a flow rate of approximately 1.1 slpm, and the pressure for all fluorescence cells is maintained at 400 Pa. The micro-channel plate (MCP) detects the weak fluorescence signal collected by lens systems with low noise and high gain. Additionally, a digital delay generator (DG645) optimizes the timing control between the laser output, signal detection, and data acquisition. All of these modules are integrated into a sampling box with constant air conditioning, except for the laser.
The detection of RO2 radicals is more complex compared to the integrated detection of OH and HO2 radicals (Whalley et al., 2013). To achieve the complete chemical conversion from ROx to HO2, a crucial role is played by a 66 mm × 830 mm aluminum flow tube, whose performance has been confirmed through the CHOOSE-2019 field campaign (Li et al., 2020). A mixture of 0.17 % CO and 0.7 ppm NO injected into the flow tube facilitates the reduction of heterogeneous radical loss and enhancement of conversion efficiency. The sampling flow is limited to 7 slpm by a 1 mm nozzle, and the tube pressure is maintained at 25 hPa. In contrast to the HOx cells, the large-diameter nozzle (4 mm) is equipped above the cell, and a high concentration of NO (∼ 300 ppm) facilitates the full-magnitude HO2 → OH conversion.
The observation data (H2O, O3) are combined with experimental characterization to eliminate ozone photolysis interference, and most interference signals are excluded by utilizing wavelength modulation (Zhang et al., 2022a). A comparison experiment with PKU-LIF demonstrated the consistency of OH measurement in the complex atmosphere (Zhang et al., 2022b). An additional atmospheric oxidation observation was conducted in the same location and season in 2022 with a chemical modulation method to determine the chemical background of OH radicals (Fig. S2). During the ozone pollution (29 September 2022–2 October 2022), the daytime ozone concentration peaks above 75 ppb, accompanied by alkene species approaching ∼ 10 ppb. The diurnal concentration of isoprene was also at a high level (> 1 ppb). The chemical conditions are more favorable to induce OH interference than in the TROPSTECT campaign, while the OH concentrations achieved by chemical modulation (OHchem) and wavelength modulation (OHwav) were in good agreement. No obvious chemical background was observed by deploying an inlet pre-injector. While it was not anticipated that OH measurements would be influenced by internal interference, the possibility of unknown interferences cannot be excluded since titration tests were not employed during the campaign. Consequently, the OH measurements represent an upper bound to the actual values.
For HO2 measurement, lower NO concentrations (∼ 1.6 × 1012 cm−3, corresponding to ∼ 15 % conversion efficiency) are selected to limit the RO2 → HO2 interference to less than 5 % (Wang et al., 2021). Since only the total-RO2 mode is used for the campaign, the additional uncertainty introduced by RO2 R(OH)O2 classification is negligible (Tan et al., 2017b). The observed maximum daily PAN (11:00–14:00) is only 1.15 ± 0.67 ppb, resulting in a calculated PAN-pyrolytic interference for RO2 measurement of less than 1 ppt (Fuchs et al., 2008). The general applicability of AIOFM-LIF in the complex atmosphere has been demonstrated through various campaigns (Zhang et al., 2022b; Wang et al., 2021; Wang et al., 2019a).
To complete the calibration task, a standard source stably generates equal amounts of OH and HO2 radicals (Wang et al., 2020). The radical source is also capable of yielding specific RO2 by titrating hydrocarbon with OH. It is noteworthy that CH3O2 has the highest mixing ratio in the RO2 species, thus it was chosen to represent sensitivity calibration. The instrument is calibrated every 2 d, except during rainy weather. The limit of detection (LOD) for OH, HO2, and RO2 in different cells with a typical laser power of 10 mW is measured at 3.3 × 105, 1.1 × 106, and 2.5 × 106 cm−3, respectively (60 s, 1σ). Measurement accuracy for OH, HO2, and RO2 radicals is reported to be 13 %, 17 %, and 21 %, respectively.
2.2.2 OH reactivity (kOH)
The detection of kOH in the atmosphere, defined as the reciprocal of OH lifetime, was conducted using a laser flash photolysis laser-induced fluorescence (LP-LIF) instrument (Lou et al., 2010). The configuration structure for kOH measurement has been detailed in a previous study (Liu et al., 2019). The flow tube in the OH production–reaction unit is at ambient pressure, with a gas flow rate of 17 slpm. A pulsed laser beam (266 nm with an average power of 15 mJ) is output from a frequency-quadrupled Nd:YAG laser, which generates stable OH radical through flash photolysis of ambient ozone in the flow tube. Consistent and stable production of OH radicals is ensured by maintaining a stable concentration of reactants, flow field, and laser energy. Under conditions of 80 ppb O3 and 8000 ppm water vapor concentration, OH radicals produced in the flow tube remain at the concentration order of 109 cm−3. Subsequently, the OH radicals are sampled through a nozzle into a fluorescence cell. The OH fluorescence signal is then detected using laser pump and probe techniques and is fitted to calculate the slope of OH decay (kOH). The detection accuracy, achieved with an integration time of 180 s, is 0.3 s−1 (1σ).
2.3 Observation-based model
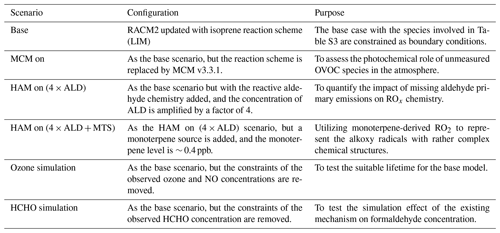
The Regional Atmospheric Chemical Mechanism version 2 (RACM2) incorporating the latest Leuven isoprene mechanism (LIM) was utilized to simulate the concentrations and reactions of OH, HO2, and RO2 radicals (Stockwell et al., 1997; Griffith et al., 2013; Peeters et al., 2014). RACM2-LIM1 was specifically involved with fewer species compared to the explicit MCM, thus ensuring higher operational efficiency (Liu et al., 2022). The comprehensive list of model constraints is provided in Table S3. The measured NMHCs include 29 alkanes, 11 alkenes, and 15 aromatics, as well as acetylene and isoprene. For the base scenario, boundary conditions were established using the observed species, with assumed concentrations of hydrogen (H2) and methane (CH4) at 550 and 1900 ppb, respectively. An ozone-simulation test was conducted to determine the suitable depositional lifetime (τD) for the species involved in the base model. At the lifetime of 24 h, with a corresponding first-order loss rate of 1.1 cm s−1 (assuming a boundary layer height of 1 km), the simulated ozone concentration closely matched the observed values (Fig. S3). The time resolution of all constraints was uniformly set to 15 min through averaging or linear interpolation. To reinitialize unconstrained species to a steady-state, 3 d of data was input in advance as the spin-up time.
To improve the model–measurement consistency between OH, HO2, and RO2 radicals, a series of sensitivity analyses were performed to evaluate the impacts of potential mechanisms, as detailed in Table 1. The Higher Aldehyde Mechanism (HAM) was introduced to discuss the influence of reactive aldehyde chemistry, and additional monoterpene species (MTS) was employed to represent the complex isomerization steps experienced by RO2 radicals derived from other unmeasured VOCs. Reactions involving monoterpene and HAM are listed in Tables S4 and S5. Furthermore, under the framework of the base model, another investigation on the photochemical role of unmeasured oxygenated volatile organic compound (OVOC) species in the atmosphere was conducted by MCM v3.3.1.
Table 1The sensitive test scenarios utilized to improve the model–measurement consistency between OH, HO2, and RO2 radicals.
The local formation of ozone can be accurately quantified through the online measurement of ROx radicals (Tan et al., 2018). To overcome the interference from NO, the total oxidant (Ox), which is defined as the sum of NO2 and O3, can serve as a reliable parameter to indicate the level of oxidation. Equation (2) shows that the rate of NO oxidation by peroxy radicals is equivalent to the production of O3, denoted as F(Ox):
The major loss pathways for Ox encompass ozone photolysis, ozonolysis reactions, and radical-related reactions (OH HO2 + O3, OH + NO2) are represented as D(Ox) in Eq. (3):
Here, the OH yields from ozone photolysis and ozonolysis reactions are denoted as φOH and , respectively.
The net photochemical Ox production rate in the troposphere, denoted as P(Ox) in Eq. (4), can therefore be calculated as the difference between Eqs. (2) and (3):
2.4 Experimental budget analysis
In this study, an experimental radical budget analysis was also conducted (Eqs. 5–12). Unlike model studies, this method relies solely on field measurements (concentrations and photolysis rates) and chemical kinetic data, without depending on concentrations calculated by models (Whalley et al., 2021; Tan et al., 2019b). Given the short-lived characteristics of OH, HO2, and RO2 radicals, it is expected that the concentrations are in a steady state, with total production and loss rates being balanced (Lu et al., 2019). By comparing the known sources and sinks for radicals, unknown processes for initiation, transformation, and termination can be determined.
where j(HONO) and j(O1D) are the measured photolysis rates of HONO and O3, respectively, and j(HCHO_R) is the measured photolysis rate for the channel of formaldehyde photolysis generating HO2. φOH represents the OH yield in the O3 photolysis reaction. , , and are the yields for the ozonolysis reaction producing OH, HO2, and RO2, respectively. α is the proportion of RO2 radicals reacting with NO that are converted to HO2, and β is the proportion of alkyl nitrate formation, which are set to 1 and 0.05, respectively (Tan et al., 2019b).
3.1 Overview of measurement
The meteorological parameters and trace gas concentrations during the observation period are plotted in Fig. S4. The time series revealed that the peak temperature exceeded 30 °C, and the humidity levels remained between 30 %–50 % during the daytime. The photolysis rates were observed to peak at noon (11:00–13:00), with j(O1D) and j(NO2) reaching approximately 3 × 10−5 s−1 and 8 × 10−3 s−1, respectively. Brief rainfall events temporarily happened on 10, 15, and 17 September, but very favorable meteorology induced the prolonged ozone pollution. The daily maximum 8 h average ozone concentration (MDA8), as depicted in Fig. 2, consistently exceeded the Chinese Grade I national air quality standard (GB3095-2012) throughout the observation, with 9 d exceeding the Grade II standard.
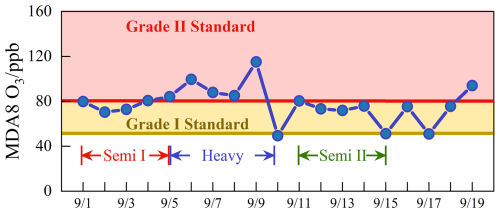
Figure 2The daily maximum 8 h average O3 during the campaign. The yellow and red lines denote the Grade I and Grade II national standards for O3, respectively. Brief rainfall events temporarily happened on 10, 15, and 17 September.
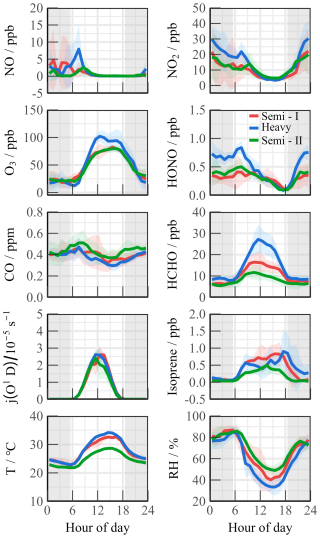
Figure 3Mean diurnal profiles of observed meteorological and chemical parameters during the campaign. Three periods were divided for subsequent study (Semi I, Heavy, and Semi II).
The ozone pollution can be categorized into three continuous periods based on pollution levels, which display transitional “Semi–Heavy–Semi” pollution characteristics. Figure 3 depicts daily variations in meteorological and trace gas concentrations for different periods. During Semi I (1 to 5 September) and Semi II (11 to 14 September) periods, the MDA8 levels exceeded the Grade I standard, with an average value of 75.92 ± 5.14 and 75.45 ± 3.73 ppb, respectively. Notably, NO levels peaked around 09:00 and rapidly decreased to a few hundred parts per trillion (ppt) due to photochemistry. In addition, HONO and NO2 exhibited bimodal variations, with diurnal concentration ranges of 0.09–0.50 and 3.35–13.77 ppb, respectively. The HONO NO2 ratios during both Semi periods were consistent with previous urban/suburban observations, with daytime values of 0.049 ± 0.014 and 0.035 ± 0.012, respectively (Yang et al., 2021b; Shi et al., 2020; Hu et al., 2022). Isoprene levels accumulated during the day and decreased at night during both Semi pollution episodes, with a diurnal average concentration in Semi II only 49.3 % of that in Semi I (0.71 ± 0.087 ppb vs 0.35 ± 0.073 ppb). Formaldehyde, as the key oxidation species, exhibited a concentration profile mirroring that of isoprene, with significantly higher concentrations ranging from 1.20 to 36.34 ppb compared to other urban regions (Ma et al., 2022; Yang et al., 2022; Tan et al., 2017b; Yang et al., 2021a). Heavy pollution episodes from 5 to 9 September resulted in daytime ozone concentration as high as 129.9 ppb, and oxidation-related species such as HCHO, HONO, NOx, and VOCs increased synchronously compared to other days.
3.2 ROx radical concentrations and budgets
The observed and modeled time series for OH, HO2, RO2, and kOH during the observation time are depicted in Fig. 4. The diurnal peaks of radicals exhibited a wide span due to changes in environmental conditions, with ranges of 3.6–27.1 × 106 cm−3 for OH, 2.1–33.2 × 108 cm−3 for HO2, and 4.9–30.5 × 108 cm−3 for RO2. Continuous data for kOH observation were acquired within a range of 8.6–30.2 s−1. Figure S5 presents the diurnal profiles of the observed and modeled values during different episodes. The diurnal maximum of OH radical at noon differed between Semi I and Semi II, with 9.28 × 106 and 5.08 × 106 cm−3, respectively, while total peroxy radicals (HO2 + RO2) remained at similar levels with 19.43 × 108 and 18.38 × 108 cm−3. Additionally, the distribution of peroxy radicals is not similar in the two Semi periods, with HO2 RO2 ratios of 1.69 : 1 and 0.76 : 1, respectively, which reflects the uneven oxidation levels between Semi I and Semi II. During the Heavy ozone pollution, the averaged OH, HO2, and RO2 concentrations were 1.90, 2.15, and 1.98 times higher than those in the Semi periods, suggesting a stronger oxidation capacity, with kOH in Heavy being 26.43 % and 9.56 % higher than in Semi I and Semi II, respectively. Limited anthropogenic emissions in the suburban environment reduced the oxidation contribution by NOx and CO (27.59 %). During the heavy pollution, organic species exhibited dominant behavior regarding diurnal reactivity (9.22 s−1 for 69.79 %), and anthropogenic hydrocarbons were not major kOH sources. With an abundant level (∼ 1 ppb), isoprene contributed more than 10 % of the reactivity in the diurnal cycle. Therefore, the effect of biogenic VOC (BVOC) species (such as monoterpenes and limonene) on radical chemistry cannot be ignored (Ma et al., 2022; Wang et al., 2022b). kOVOCs are categorized into three groups: kOVOCs(Obs), kOVOCs(Model), and kHCHO. Given the significance of formaldehyde photolysis, the contribution of HCHO to kOVOCs is established. kOVOCs(Obs) encompasses species observed in addition to formaldehyde, such as acetaldehyde (ACD) and the oxidation products of isoprene (MACR and MVK). Intermediates generated by the model, including glyoxal (GLY), methylglyoxal (MGLY), higher aldehydes (ALD), ketones (KET), methyl ethyl ketone (MEK), and methanol (MOH), are classified as kOVOCs(Model). Upon considering kOVOCs(Model) calculated by RACM2-LIM1, the reactivity calculated prior to 10 September aligns quite well with the observed OH reactivity.
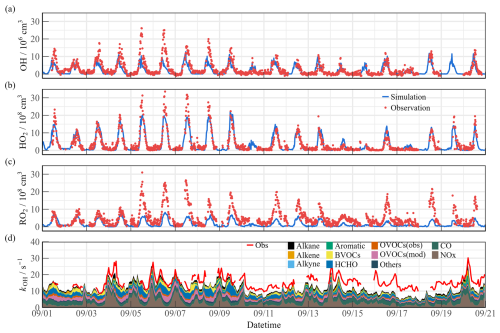
Figure 4Time series of the observed and modeled parameters for (a) OH, (b) HO2, (c) RO2, and (d) kOH during the observation period.
The significant variations in oxidation can be inferred from the disparities during different pollution periods (Fig. S5). During Semi I, there was good agreement between the measurement and model data for peroxy radicals during the daytime. RACM2-LIM1 effectively replicated the morning OH radical concentration. However, following 10:00, NO gradually declined, and the increasing OH concentration could not be accounted for by the HO2 + NO formation channel, resulting in a maximum underestimation of 5.85 × 106 cm−3 (Hofzumahaus et al., 2009; Lu et al., 2012). In the Semi II episode, OH was not underestimated in the low NO regime, with a slight overestimation of HO2 concentration. However, the simulated RO2 concentration was only 3.78 × 108 cm−3, whereas observations were 2.77 times larger than the simulation, indicating the existence of additional reaction pathways that likely propagated the OH → RO2 conversion efficiency. A significant discrepancy of radicals existed in the heavy ozone concentration, with OH, HO2, and RO2 radicals concurrently underestimated at noon by 8.23 × 106, 3.94 × 108, and 11.59 × 108 cm−3, respectively. The observed HO2 RO2 ratio approached 1 : 1, while the model reflected an unreasonable ratio of 3 : 1, indicating deficiencies in both primary sources and secondary propagation. The calculated reactivity seems to compare well with the observed OH reactivity at the start of the measurement period, but then there is evidence of missing OH reactivity after 10 September (Fig. 4d). Due to the limitations of available instruments, this observation only measured a limited number of OVOC species, making it difficult to accurately quantify the contribution of larger aldehydes and ketones, carboxylic acids, nitrophenols, and other multifunctional species to kOH (Wang et al., 2024). Since the MCM considers more secondary formation reactions than RACM2, it can qualitatively assess the photochemical role of unmeasured OVOC species in the atmosphere (Wang et al., 2022d). The additional modeled OVOCs by MCM v3.3.1 contributed ∼ 2.4 s−1 to the missing OH reactivity (Fig. S6). During the Heavy period, the reactivity of more model oxidation products increased the daytime kOH by about 5.1 s−1. Therefore, the observed kOH can serve as an upper limit for sensitivity tests, and the full suite of radical measurements can be performed to explore the missing oxidation properties and ozone formation (Sect. 4.1).
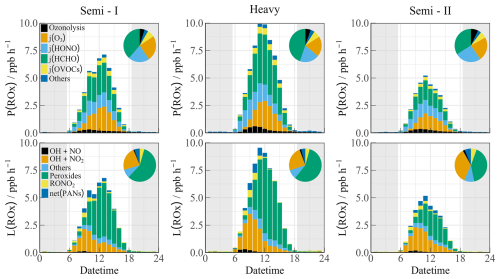
Figure 5The diurnal profiles of ROx budget during different polluted episodes (Semi I, Heavy, and Semi II). The pie chart denotes proportions in different parts during the daytime (10:00–15:00). The grey areas denote nighttime.
Figure 5 displays the diurnal profiles of the ROx budget during different episodes. In Semi I, formaldehyde photolysis showed a higher contribution (38.6 %), while HONO photolysis (21.0 %) and ozone photolysis (24.7 %) accounted for similar proportions in primary sources. The contribution of photolysis from other OVOCs was comparable to that of ozonolysis reactions (7.2 % vs. 4.8 %). However, in Semi II, the decreased oxidation level was attributed to lower ROx sources, despite the similar proportions. During the Heavy period, the primary sources dramatically increased (up to ∼ 10 ppb h−1), with HCHO photolysis contributing the most, alongside other sources at common levels (ranging between 1.74–2.66 ppb h−1) in the YRD region (Ma et al., 2022). Fast HCHO oxidation dominated the radical primary source during heavy ozone pollution, which contrasts with the dominant role of HONO O3 in other megacities (Yang et al., 2022; Tan et al., 2017b; Yang et al., 2021a).
The radical removal rate during the daytime was generally balanced with production contributions. In the morning, owing to high NOx concentrations, radical termination was mainly dominated by OH + NO2, OH + NO, RO2 + NO, and RO2 + NO2. Furthermore, the formation of peroxy nitrate accounted for a certain proportion (∼ 5 %). As NOx concentrations decreased after 10:00, self-reactions in peroxy radicals became significant.
By comparing the known sources and sinks for radicals, unknown processes for initiation, transformation, and termination can be determined in the experimental budget analysis (Fig. S7). During the Semi I period, the production and destruction rates of HO2, RO2, and total ROx radicals were very consistent, but a significant lack of a source term for OH radicals existed after 10:00. This missing source became more pronounced during the Heavy period, reaching 16 ppb h−1 at noon, which is close to the results observed by APHH but 3 times that observed by Heshan in the PRD region (Tan et al., 2019b; Whalley et al., 2021). The ratio of OH production-to-destruction rate during the Semi II period was close to 1, indicating consistency between the observed results of OH, HO2, kOH, and other precursors (Whalley et al., 2018). However, the generation of HO2 radicals in the morning was about twice as high as the removal rate, suggesting that there are contributions from unconsidered HO2 radical removal channels (such as heterogeneous reactions) (Song et al., 2021). During the Heavy period, there was a rapid total removal rate of RO2 radicals, reflecting the dominated HO2 generation by the reaction of RO2 radicals with NO. Although the P(HO2) and D(HO2) were quite in balance, the removal rate of RO2 radicals far exceeded the known production rate (especially before 12:00). Previous work has shown that halogen chemistry (such as photolysis of nitryl chloride (ClNO2)) could be an important source in the morning time, but this was not included in the calculation of ROx or RO2 budget in this campaign (Tan et al., 2017b). The steady-state analysis for HO2 radical in the London campaign emphasized that only by significantly reducing the observed RO2-to-HO2 propagation rate to just 15 % could balance both P(HO2) and D(HO2), indicating that the RO2-related mechanism for propagation to other radical species may not be fully understood (Whalley et al., 2018). Therefore, the current knowledge seems unlikely to explain the required source–sink difference of nearly 25 ppb h−1 in the RO2 budget. Sensitivity analysis is needed to further infer the causes of the difference for the experimental budget analysis.
3.3 Oxidation comparison
The concentration of OH radicals during the daytime is a crucial indicator of atmospheric oxidation levels (Liu et al., 2021). Table 2 summarized radicals and related parameters for regions with similar latitudes (32.0° ± 2° N, j(O1D) ≈ 2.5 ± 0.5 × 10−5 s−1). The joint influence of solar radiation and local photochemistry resulted in megacities exhibiting intense oxidation levels in summer/autumn, characterized by OH radicals being maintained at approximately 10.0 × 106 cm−3 at noon. Notably, an observation in Houston revealed an OH concentration of nearly 20.0 × 106 cm−3, with kOH of 10 s−1 (Mao et al., 2010). In areas such as Los Angeles, Pasadena, and Tokyo, the propagation efficiency of radicals was restricted due to fresh anthropogenic emissions. OH concentrations were only half of those observed in other megacities, with higher inorganic-dominated kOH recorded (Pasadena, ∼ 20 s−1) (George et al., 1999; Griffith et al., 2016; Kanaya et al., 2007b). In the TROPSTECT observation, the observed kOH exceeded the mean value at the same latitude (> 15 s−1). Additionally, during the Heavy episode, higher OH concentration (13.5 × 106 cm−3) was found, comparable to the highest level at regions with similar latitude (Houston 2000/2006, (Mao et al., 2010)). Synchronous elevation in radical concentration and reactivity indicated a strong oxidation level in the YRD region.
Table 2Summary of radical concentrations and related species concentrations at regions with similar latitude and megapolitan areas in China. All data are listed as the average in noontime (11:00–13:00).
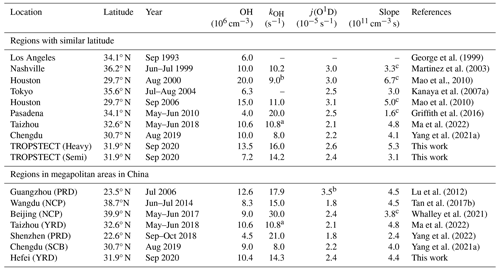
a The modeled kOH. b Value only in the afternoon. c Using the ratio of OH j(O1D).
The observations in the YRD region showed a stable conversion factor (OH-j(O1D)) of 4 ± 1 × 1011 cm−3 s, which was comparable to other megacities in the PRD, NCP, and SCB regions (Ma et al., 2022; Tan et al., 2019a). The corresponding slope between OH concentration and solar radiation was used to quantify the oxidation efficiency from photolysis, and it was observed that a higher slope of 5.3 × 1011 cm−3 s during the Heavy period indicated an active radical chemistry. This implies that there is a strong oxidation efficiency from photolysis in the YRD region.
During summer and autumn seasons, photochemical pollution is a common occurrence, as noted by Tan et al. (2021). Analysis of radical concentration across different regions reveals that the YRD region exhibited concentrations higher than 107 cm−3, slightly lower than in Guangzhou in 2006 but consistent with observations in other megacities (Ma et al., 2022; Tan et al., 2017a; Lu et al., 2012; Yang et al., 2021a). Conversely, winter is characterized by haze pollution (Ma et al., 2019). An urban site in Shanghai reported a peak OH concentration of 2.6 × 106 cm−3, closely resembling the 1.7–3.1 × 106 cm−3 range found in the polluted winter atmosphere (Zhang et al., 2022a). Although no significant regional disparities in oxidation levels were detected in agglomerations, attention should be directed to the YRD region due to its elevated radical concentration, reactivity, and photolysis efficiency, signaling the need to investigate its role in radical chemistry.
4.1 Measurement–model reconciliation for radicals
4.1.1 OH underestimation
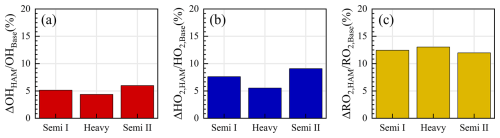
A full suite of OH, HO2, RO2, and kOH measurements was utilized in the TROPSTECT campaign to untangle a thorough understanding of oxidation mechanisms where base model failed. One specific phenomenon was the absence of an OH source in situations where NO levels gradually decreased after 10:00. Missing OH sources are closely related to the chemistry of OVOCs (Yang et al., 2024a; Qu et al., 2021). Reactive aldehyde chemistry, particularly the autoxidation of carbonyl organic peroxy radicals (R(CO)O2) derived from higher aldehydes, is a significant OH regeneration mechanism that has been shown to contribute importantly to OH sources in regions with abundant natural and anthropogenic emissions during warm seasons (Yang et al., 2024b). In this study, the Higher Aldehyde Mechanism (HAM) by Yang et al. (2024b) was parameterized into the base model to test new insights into the potential missing radical chemistry (Fig. 6). The results indicate that the contribution of HAM to OH radicals in different episodes ranged between 4.4 %–6.0 % (Fig. 6a). The addition of HAM seems to have a small effect on the measured OH radical concentration. Thus, an empirical hypothesis is proposed in the “HAM+4ALD on” scenario to increase the concentration of higher-order aldehydes by approximately 4-fold, thereby replicating the effect of missing OVOC sources on radicals. Detailed description is presented in Sect. 4.3.
4.1.2 RO2 underestimation
The base scenario in Semi II is capable of accurately reproducing the concentrations of OH and HO2 radicals within the data uncertainty. However, the simulated RO2 concentration by the base model is only 3.78 × 108 cm−3, which does not align with the observed oxidation levels in YRD, indicating a clear discrepancy. This underestimation is similarly evident in the APHH observation in Beijing, as the highest observed concentration of RO2 radicals reached 5.5 × 109 cm−3, far exceeding the level predicted by MCM v3.3.1 (Whalley et al., 2021). The failure to reproduce the RO2 concentration reflects the inadequacy of the mechanisms related to RO2 radicals due to diverse oxidation reactions. This issue is further elucidated by previous studies, which highlighted the possibility of certain VOCs undergoing more intricate isomerization or fragmentation steps to sustain the long lifetime of RO2 radicals (Whalley et al., 2018; Whalley et al., 2021). Higher aldehyde chemistry is a concrete manifestation of verifying the aforementioned hypothesis for RO2 sources (Yang et al., 2024b). The autoxidation process of R(CO)O2 encompasses a hydrogen migration process that transforms it into the •OOR(CO)OOH radical (Wang et al., 2019b). This radical subsequently reacts with NO to yield the •OR(CO)OOH radical. The •OR(CO)OOH radical predominantly undergoes two successive rapid hydrogen migration reactions, ultimately resulting in the formation of HO2 radicals and hydroperoxy carbonyl (HPC). Consequently, HAM extends the lifetime of the RO2 radical, providing a valuable complement to the unaccounted sources of RO2 radicals. As depicted in Fig. 6, the incorporation of HAM results in an approximate 7.4 % and 12.5 % increase in the concentrations of HO2 and RO2 radicals, respectively.
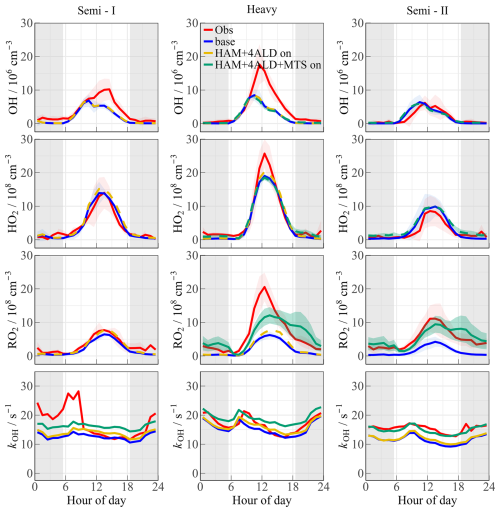
Figure 7The mean diurnal profiles of measured and modeled OH, HO2, RO2, and kOH at different scenarios. Among them, the red, blue, and dotted yellow lines represent the observed values, simulated values under base model and “HAM+4ALD” scenario, respectively. The green line and its shaded area represent the results under the HAM+4ALD+MTS scenario. The grey areas denote nighttime.
It is important to note that the total concentrations of primary emitted aldehydes and the HPC group may be underestimated, which could lead to the aforementioned analysis being conservative in nature. The union of kOH and RO2 measurement can help reveal the magnitude of missing RO2 as a hypothesis of sensitivity analysis. Discrepancy of OH reactivity (∼ 3–5 s−1) between measurement and model suggested that an additional driving force was necessary to complete the OH to RO2 step. Additional monoterpene species (MTS) were employed to represent the complex isomerization steps experienced by RO2 radicals derived from other unmeasured VOCs. In the “HAM+4ALD+MTS” scenario, approximately 0.4 ppb of monoterpenes is introduced to represent the reactive BVOCs. The 0.4 ppb monoterpene level aligns with another observation from the EXPLORE-2018 campaign conducted in a suburban environment of the YRD region and can better reconcile the missing kOH between observation and simulation (Fig. 7) (Wang et al., 2022b). RACM2 identified α-pinene (API) and limonene (LIM) as being representative of monoterpenes, and the mean of the species was considered the average effect of monoterpenes chemistry (the green line in Fig. 7). The “HAM+4ALD+MTS on” scenario can reasonably reproduce the measured reactivity, and the chemistry of peroxy radicals in Semi II was reasonably described by introducing the source of complex alkoxy radicals, decreasing the observed-to-modeled ratio from 2.2 to 1.3. Furthermore, the introduction of additional complex alkoxy radicals had a minimal impact on HOx chemistry, with changes in daytime OH and HO2 concentrations of less than 5 × 105 and 2.5 × 107 cm−3, respectively. This demonstrates the robustness of the HOx radical in response to potential monoterpene.
4.2 Effect of mechanism reconciliation on oxidation
Upon completing the hypothetical investigation into the radical underestimation, both radical concentration and oxidation coordinating deficiency are worth being examined (Fig. S8). To eliminate the influence of non-photolytic processes, only the daytime concentration range with j(O1D) greater than 5 × 10−6 s−1 was selected. The box plots illustrate the ratio of observation to simulation (base model), with the circles representing the average values after integrating different mechanisms into the base scenario. In the low NO regime (NO < 1 ppb), the OH underestimation was consistently prominent as NO concentration decreased, and the base model was able to reasonably reflect the HO2 distribution. As NO levels increased, the simulated OH concentration aligned well with the observation, but both HO2 and RO2 concentrations exhibited underprediction. RO2 underestimation extended across the entire NO range and could rise to over 10 times when NO levels reached about 10 ppb. Sensitivity tests based on the full suite of radical measurements revealed that the introduction of larger RO2 alleviated the absence of certain sources by 2 to 4 times.
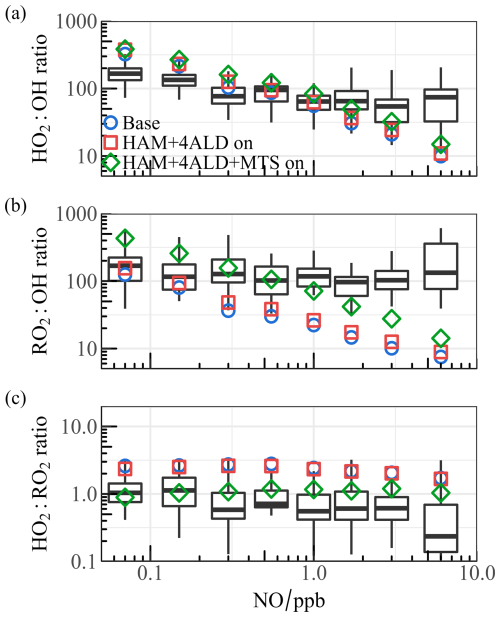
Figure 8The ratios for (a) HO2 OH, (b) RO2 OH, and (c) HO2 RO2 show a correlation with NO levels. Box plot diagrams are used to illustrate the minimum, 25th percentile, median, 75th percentile, and maximum values of the observed dataset. The point styles (circular, square, diamond-shaped) represent the median values for the base model as well as for different mechanisms added to the model within various ranges.
The coordinate ratios of radical serves as another test for ROx propagation (Fig. 8). The observed HO2 OH ratio is approximately 100, declining to some extent as the concentration of NO increases, which is consistent with previous studies (Griffith et al., 2016; Griffith et al., 2013). However, the base model does not accurately replicate the curve depicting the change in HO2 OH ratio, as shown in Fig. 8a. At low NO levels, the ratio is significantly overestimated and shows a steeper decline compared to the base scenario as NO levels increase. Furthermore, the observed RO2 OH ratios remain around 100, whereas the predicted values are significantly underestimated when NO exceeds 1 ppb (refer to Fig. 8b). In terms of the observed HO2 RO2 ratio, it maintains a relatively constant trend within the range of 0.5–1.5, while the model overestimated by more than 2 times, highlighting an inconsistency between the conversion of RO2 → HO2. The incorporation of HAM has proven to slightly balance the HO2 OH ratio as illustrated in Fig. 8a and altered the coordination between RO2 and OH across the entire NO range (Fig. 8b). The larger RO2 isomerization associated with HAM in chemically complex environments is key to fully understanding tropospheric chemistry, and a better coordination of HO2 OH, RO2 OH, and HO2 RO2 ratios is established by incorporating additional mechanisms.
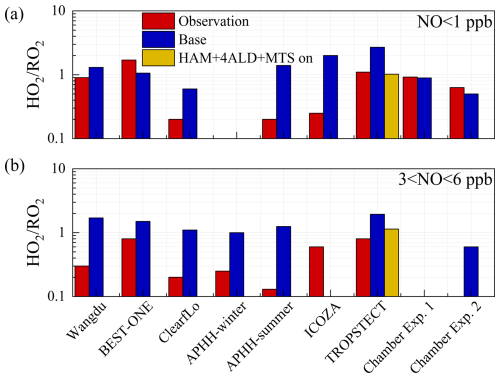
Figure 9Summary of the HO2 RO2 ratios derived from radical concentrations measured by laser-induced fluorescence instruments and calculated using the MCM or RACM models under (a) low NO and (b) high NO conditions. Chamber Exp. 1 and Chamber Exp. 2 denote the parameters by single-step HO2 formation and multi-step HO2 formation determined in the chamber by Färber et al. (2024).
The HO2 RO2 parameter was utilized to explore the transformation relationship between HO2 and RO2 radicals. If HO2 is formed from an RO2 radical, it would result in an HO2 RO2 radical concentration ratio of approximately 1. The HO2 RO2 ratios derived from radical concentrations measured by laser-induced fluorescence instruments and calculated using the MCM or RACM models were summarized in Fig. 9. In field studies, the observed HO2 RO2 ratios were between 0.2–1.7 under low NO conditions (NO < 1 ppb) and only 0.1–0.8 under high NO conditions (3 < NO < 6 ppb). From the perspective of model–observation matching, except for three measurements in ClearfLo, ICOZA, and APHH-summer campaigns, the HO2 RO2 ratios in other regions could be reasonably reflected by the MCM or RACM2 (Woodward-Massey et al., 2023; Whalley et al., 2021; Whalley et al., 2018; Färber et al., 2024). However, the ratio is generally underestimated under high NO conditions, reaching up to 5 times in ClearfLo. According to the latest chamber experiments, the HO2 RO2 radical concentration ratios for VOCs forming HO2 are 0.6 for both one-step and two-step reactions. Therefore, the extremely low HO2 RO2 ratios observed in field campaigns can only be explained if almost all RO2 radicals undergo multiple-step reactions before forming HO2. During the TROPSTECT campaign, the observed HO2 RO2 remains at 1.1 and 0.8 under low NO and high NO conditions, respectively. After considering the sources of complex alkoxy radicals in the HAM+4ALD+MTS on scenario, the simulated values of HO2 RO2 in both low NO and high NO regions match the observed values well.
4.3 Missing OVOC sources influence ozone production
The consistency between model predictions and observed measurements for ozone production, akin to the concentration ratio of HO2 RO2, is depicted in Fig. 10a and b. In areas with low NO levels, the ratio of modeled to actual ozone production ranges from 0.5 to 2, with the exception of the ClearfLo and APHH-summer datasets (Woodward-Massey et al., 2023; Whalley et al., 2021). Conversely, under high NO conditions (with NO concentrations between 3 and 6 ppbv), the ozone production rate (P(Ox)) derived from measured radical concentrations typically exceeds that of the base model's predictions by more than threefold. Laboratory experiments focusing on the oxidation of representative VOCs suggest that ozone production can be enhanced by approximately 25 % for the anthropogenic VOCs under investigation (Färber et al., 2024).
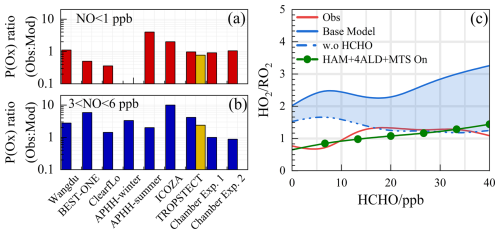
Figure 10Summary of the P(Ox)Obs P(Ox)Mod under (a) low NO and (b) high NO conditions. The yellow bar chart represents the simulation scenario of HAM+4ALD+MTS on. (c) The ratios for HO2 RO2 show a correlation with HCHO levels. The blue shading represents the range of variation from constrained to unconstrained formaldehyde conditions. Chamber Exp. 1 and Chamber Exp. 2 denote the parameters by single-step HO2 formation and multi-step HO2 formation determined in the chamber by Färber et al. (2024).
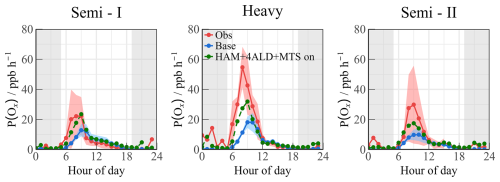
Figure 11The P(Ox) values that calculated by radical values under different scenarios. The grey areas denote nighttime.
The reasons for the discrepancy between simulated and observed values for ozone production deserve further investigation. As depicted in Fig. 10c, the simulated HO2 RO2 ratios display a robust positive correlation with photochemical activity, fluctuating between 2 and 4. A notable feature during severe ozone pollution is the intense distribution of formaldehyde, with an average concentration of 21.81 ± 4.57 ppb (11:00–13:00). While formaldehyde acts as a precursor for HO2 radicals, it does not directly generate RO2 radicals. The contributions of OVOCs to the ROx radical do not exhibit the same intensity as formaldehyde, and the current mechanism encounters difficulties in replicating formaldehyde concentrations (Fig. S9). The simulation of formaldehyde concentrations using MCM v3.3.1 has shown improvement, indicating that the secondary formation of unmeasured species, such as OVOCs, will feed back on RO2 radical levels. When formaldehyde levels are unconstrained, the simulated HO2 RO2 ratios align with observations, suggesting that under the prevailing chemical mechanism, the photochemical efficiency of formaldehyde and other OVOCs is similar. Therefore, an empirical hypothesis is proposed to amplify the concentration of higher-order aldehydes by a factor of about 4, which is the proportion of formaldehyde concentration underestimated by the model. The qualitative assessment of the impact of missing aldehyde primary emissions on RO2 radical concentrations was combined with HAM across the entire photochemical spectrum (Fig. S10). An enhanced impact of aldehyde autoxidation in the presence of weak photochemical conditions could alter the simulated levels of OH and HO2 radicals by approximately 13.9 % and 18.1 %, respectively. However, higher ALD concentrations will be achieved under intensive photochemical conditions, leading to the gradual dominance of the sink channels for OH + OVOCs, with the effect of autoxidation mechanisms gradually decreasing. RO2 radical concentrations are notably more sensitive to HAM, where additional incorporated OVOCs can enhance the simulation of RO2 radical concentrations by 20 %–40 %.
On the basis of HAM, the HAM+4ALD+MTS on scenario represents an effort to enhance the congruence between modeled and measured radical concentrations. In Fig. S11, with increasing NO concentration, the overall P(Ox) was amplified, reaching a maximum of approximately 30 ppb h−1. However, the imperfect understanding of the mechanisms related to peroxy radicals ultimately leads to misjudgment of the ozone production process in high NO regimes, with a degree of underestimation close to 10 times, as illustrated in Fig. S11b. Notably, the deficiency in the ozone generation mechanism was adequately explained within a certain range in the HAM +4ALD+MTS on scenario, leading to an enhancement in the simulation performance of P(Ox) in the high NOx region. The incorporation of OVOCs and larger alkoxy radicals derived from monoterpenes has refined the model–measurement agreement for ozone formation under high NO conditions, reducing the discrepancy from 4.17 to 2.39 (Fig. 11).
Therefore, reasonable simulation of the concentration of peroxy radicals is key to accurately quantifying the process of ozone generation. Although limiting formaldehyde can partially offset the HO2 radical cycle and enhance the precision of HOx radical chemistry studies, additional measurements should be undertaken for other OVOCs, coupled with the deployment of full-chain radical detection systems, to accurately elucidate the oxidation processes under severe ozone pollution conditions.
The full suite of radical measurements of OH, HO2, RO2, and kOH was first deployed in the YRD region (TROPSTECT) and encountered with a prolonged ozone pollution in September 2020. The diurnal peaks of radicals exhibited considerable variation due to environmental factors, showing ranges of 3.6 to 27.1 × 106 cm−3 for OH, 2.1 to 33.2 × 108 cm−3 for HO2, and 4.9 to 30.5 × 108 cm−3 for RO2. Continuous kOH data fell within a range of 8.6–30.2 s−1, demonstrating the dominant behavior of organic species in diurnal reactivity. Furthermore, observations in the YRD region were found to be similar to those in other megacities, suggesting no significant regional differences in oxidation levels were observed in agglomerations overall.
In a heavy ozone pollution episode, the oxidation reached an intensive level compared with other sites, and the simulated OH, HO2, and RO2 radicals provided by RACM2-LIM1 failed to adequately match the observed data in terms of both radical concentration and experimental radical budget. Sensitivity tests based on the full suite of radical measurement revealed that HAM effectively complements the non-traditional regeneration of OH radicals, improving by 4.4 %–6.0 % compared to the base scenario, while the concentrations of HO2 and RO2 radicals increased by approximately 7.4 % and 12.5 %, respectively. Under the constraints of kOH measurement, the inclusion of OVOCs and larger alkoxy radicals derived from monoterpenes enabled better coordination of HO2 OH, RO2 OH, and HO2 RO2 ratios and adequately improved the model–measurement consistency for ozone formation, reducing the discrepancy under high NO conditions from 4.17 to 2.39. This study enabled a deeper understanding of the tropospheric radical chemistry at play. The following two points were notable:
-
A full suite of radical measurements can bridge the gap as the base model in more chemically complex environments as a hypothesis of sensitivity tests.
-
Additional measurements targeting more OVOCs should also be conducted to fulfill the RO2-related imbalance and then accurately elucidate the oxidation under severe ozone pollution.
The data used in this study are available upon request (rzhu@aiofm.ac.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-3011-2025-supplement.
WQL, PHX, and RZH contributed to the conception of this study. RZH and GXZ performed the data analyses and manuscript writing. All authors contributed to measurements, discussed results, and commented on the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors appreciate the valuable comments from three anonymous reviewers.
This work was supported by the National Key R&D Program of China (grant no. 2022YFC3700301), the National Natural Science Foundation of China (grant nos. 62275250, U19A2044, 42030609), the Natural Science Foundation of Anhui Province (grant no. 2008085J20), the Anhui Provincial Key R&D Program (grant no. 2022l07020022), and the Distinguished Program of Jianghuai Talents Program of Excellence (HYRCSTZ202401).
This paper was edited by Eleanor Browne and reviewed by three anonymous referees.
Duan, J., Qin, M., Ouyang, B., Fang, W., Li, X., Lu, K., Tang, K., Liang, S., Meng, F., Hu, Z., Xie, P., Liu, W., and Häsler, R.: Development of an incoherent broadband cavity-enhanced absorption spectrometer for in situ measurements of HONO and NO2, Atmos. Meas. Tech., 11, 4531–4543, https://doi.org/10.5194/amt-11-4531-2018, 2018.
Färber, M., Fuchs, H., Bohn, B., Carlsson, P. T. M., Gkatzelis, G. I., Marcillo Lara, A. C., Rohrer, F., Vereecken, L., Wedel, S., Wahner, A., and Novelli, A.: Effect of the Alkoxy Radical Chemistry on the Ozone Formation from Anthropogenic Organic Compounds Investigated in Chamber Experiments, ACS ES&T Air, 1, 1096–1111, https://doi.org/10.1021/acsestair.4c00064, 2024.
Fuchs, H., Holland, F., and Hofzumahaus, A.: Measurement of tropospheric RO2 and HO2 radicals by a laser-induced fluorescence instrument, Rev. Sci. Instrum., 79, 084104, https://doi.org/10.1063/1.2968712, 2008.
George, L. A., Hard, T. M., and O'Brien, R. J.: Measurement of free radicals OH and HO2 in Los Angeles smog, J. Geophys. Res.-Atmos., 104, 11643–11655, 1999.
Griffith, S. M., Hansen, R. F., Dusanter, S., Stevens, P. S., Alaghmand, M., Bertman, S. B., Carroll, M. A., Erickson, M., Galloway, M., Grossberg, N., Hottle, J., Hou, J., Jobson, B. T., Kammrath, A., Keutsch, F. N., Lefer, B. L., Mielke, L. H., O'Brien, A., Shepson, P. B., Thurlow, M., Wallace, W., Zhang, N., and Zhou, X. L.: OH and HO2 radical chemistry during PROPHET 2008 and CABINEX 2009 – Part 1: Measurements and model comparison, Atmos. Chem. Phys., 13, 5403–5423, https://doi.org/10.5194/acp-13-5403-2013, 2013.
Griffith, S. M., Hansen, R. F., Dusanter, S., Michoud, V., Gilman, J. B., Kuster, W. C., Veres, P. R., Graus, M., de Gouw, J. A., Roberts, J., Young, C., Washenfelder, R., Brown, S. S., Thalman, R., Waxman, E., Volkamer, R., Tsai, C., Stutz, J., Flynn, J. H., Grossberg, N., Lefer, B., Alvarez, S. L., Rappenglueck, B., Mielke, L. H., Osthoff, H. D., and Stevens, P. S.: Measurements of hydroxyl and hydroperoxy radicals during CalNex-LA: Model comparisons and radical budgets, J. Geophys. Res.-Atmos., 121, 4211–4232, https://doi.org/10.1002/2015jd024358, 2016.
Heard, D. E. and Pilling, M. J.: Measurement of OH and HO2 in the troposphere, Chem. Rev., 103, 5163–5198, https://doi.org/10.1021/cr020522s, 2003.
Hofzumahaus, A., Rohrer, F., Lu, K., Bohn, B., Brauers, T., Chang, C.-C., Fuchs, H., Holland, F., Kita, K., Kondo, Y., Li, X., Lou, S., Shao, M., Zeng, L., Wahner, A., and Zhang, Y.: Amplified Trace Gas Removal in the Troposphere, Science, 324, 1702–1704, https://doi.org/10.1126/science.1164566, 2009.
Hu, B., Duan, J., Hong, Y., Xu, L., Li, M., Bian, Y., Qin, M., Fang, W., Xie, P., and Chen, J.: Exploration of the atmospheric chemistry of nitrous acid in a coastal city of southeastern China: results from measurements across four seasons, Atmos. Chem. Phys., 22, 371–393, https://doi.org/10.5194/acp-22-371-2022, 2022.
Huang, J., Pan, X., Guo, X., and Li, G.: Health impact of China's Air Pollution Prevention and Control Action Plan: an analysis of national air quality monitoring and mortality data, Lancet Planet Health, 2, e313–e323, https://doi.org/10.1016/S2542-5196(18)30141-4, 2018.
Huang, X., Ding, A., Wang, Z., Ding, K., Gao, J., Chai, F., and Fu, C.: Amplified transboundary transport of haze by aerosol–boundary layer interaction in China, Nat. Geosci., 13, 428–434, https://doi.org/10.1038/s41561-020-0583-4, 2020.
Jenkin, M. E., Saunders, S. M., and Pilling, M. J.: The tropospheric degradation of volatile organic compounds: A protocol for mechanism development, Atmos. Environ., 31, 81–104, https://doi.org/10.1016/s1352-2310(96)00105-7, 1997.
Jenkin, M. E., Saunders, S. M., Wagner, V., and Pilling, M. J.: Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic compounds, Atmos. Chem. Phys., 3, 181–193, https://doi.org/10.5194/acp-3-181-2003, 2003.
Jia, W., Zhang, X., and Wang, Y.: Assessing the pollutant evolution mechanisms of heavy pollution episodes in the Yangtze-Huaihe valley: A multiscale perspective, Atmos. Environ., 244, 117986, https://doi.org/10.1016/j.atmosenv.2020.117986, 2021.
Kanaya, Y., Cao, R. Q., Akimoto, H., Fukuda, M., Komazaki, Y., Yokouchi, Y., Koike, M., Tanimoto, H., Takegawa, N., and Kondo, Y.: Urban photochemistry in central Tokyo: 1. Observed and modeled OH and HO2 radical concentrations during the winter and summer of 2004, J. Geophys. Res.-Atmos., 112, D21312, https://doi.org/10.1029/2007jd008670, 2007a.
Kanaya, Y. G., Cao, R. Q., Kato, S. G., Miyakawa, Y. K., Kajii, Y., Tanimoto, H., Yokouchi, Y., Mochida, M., Kawamura, K., and Akimoto, H.: Chemistry of OH and HO2 radicals observed at Rishiri Island, Japan, in September 2003: Missing daytime sink of HO2 and positive nighttime correlations with monoterpenes, J. Geophys. Res.-Atmos., 112, D11308, https://doi.org/10.1029/2006jd007987, 2007b.
Kanaya, Y., Hofzumahaus, A., Dorn, H.-P., Brauers, T., Fuchs, H., Holland, F., Rohrer, F., Bohn, B., Tillmann, R., Wegener, R., Wahner, A., Kajii, Y., Miyamoto, K., Nishida, S., Watanabe, K., Yoshino, A., Kubistin, D., Martinez, M., Rudolf, M., Harder, H., Berresheim, H., Elste, T., Plass-Dülmer, C., Stange, G., Kleffmann, J., Elshorbany, Y., and Schurath, U.: Comparisons of observed and modeled OH and HO2 concentrations during the ambient measurement period of the HOxComp field campaign, Atmos. Chem. Phys., 12, 2567–2585, https://doi.org/10.5194/acp-12-2567-2012, 2012.
Levy, H.: Normal Atmosphere: Large Radical and Formaldehyde Concentrations Predicted, Science, 173, 141–143, https://doi.org/10.1126/science.173.3992.141, 1971.
Li, S., Lu, K., Ma, X., Yang, X., Chen, S., and Zhang, Y.: Field measurement of the organic peroxy radicals by the low-pressure reactor plus laser-induced fluorescence spectroscopy, Chinese Chem. Lett., 31, 2799–2802, https://doi.org/10.1016/j.cclet.2020.07.051, 2020.
Liu, S., Li, X., Shen, X., Zeng, L., Huang, X., Zhu, B., Lin, L., and Lou, S.: Measurement and partition analysis of atmospheric OH reactivity in autumn in Shenzhen, Acta Scientiae Circumstantiae, 39, 3600–3610, 2019.
Liu, Y., Li, J., Ma, Y., Zhou, M., Tan, Z., Zeng, L., Lu, K., and Zhang, Y.: A review of gas-phase chemical mechanisms commonly used in atmospheric chemistry modelling, J. Environ. Sci., 123, 522–534, https://doi.org/10.1016/j.jes.2022.10.031, 2022.
Liu, Z., Wang, Y., Hu, B., Lu, K., Tang, G., Ji, D., Yang, X., Gao, W., Xie, Y., Liu, J., Yao, D., Yang, Y., and Zhang, Y.: Elucidating the quantitative characterization of atmospheric oxidation capacity in Beijing, China, Sci. Total Environ., 771, 145306, https://doi.org/10.1016/j.scitotenv.2021.145306, 2021.
Lou, S., Holland, F., Rohrer, F., Lu, K., Bohn, B., Brauers, T., Chang, C. C., Fuchs, H., Häseler, R., Kita, K., Kondo, Y., Li, X., Shao, M., Zeng, L., Wahner, A., Zhang, Y., Wang, W., and Hofzumahaus, A.: Atmospheric OH reactivities in the Pearl River Delta – China in summer 2006: measurement and model results, Atmos. Chem. Phys., 10, 11243–11260, https://doi.org/10.5194/acp-10-11243-2010, 2010.
Lu, K., Guo, S., Tan, Z., Wang, H., Shang, D., Liu, Y., Li, X., Wu, Z., Hu, M., and Zhang, Y.: Exploring atmospheric free-radical chemistry in China: the self-cleansing capacity and the formation of secondary air pollution, Nat. Sci. Rev., 6, 579–594, https://doi.org/10.1093/nsr/nwy073, 2019.
Lu, K. D., Rohrer, F., Holland, F., Fuchs, H., Bohn, B., Brauers, T., Chang, C. C., Häseler, R., Hu, M., Kita, K., Kondo, Y., Li, X., Lou, S. R., Nehr, S., Shao, M., Zeng, L. M., Wahner, A., Zhang, Y. H., and Hofzumahaus, A.: Observation and modelling of OH and HO2 concentrations in the Pearl River Delta 2006: a missing OH source in a VOC rich atmosphere, Atmos. Chem. Phys., 12, 1541–1569, https://doi.org/10.5194/acp-12-1541-2012, 2012.
Ma, X., Tan, Z., Lu, K., Yang, X., Chen, X., Wang, H., Chen, S., Fang, X., Li, S., Li, X., Liu, J., Liu, Y., Lou, S., Qiu, W., Wang, H., Zeng, L., and Zhang, Y.: OH and HO2 radical chemistry at a suburban site during the EXPLORE-YRD campaign in 2018, Atmos. Chem. Phys., 22, 7005–7028, https://doi.org/10.5194/acp-22-7005-2022, 2022.
Ma, X. F., Tan, Z. F., Lu, K. D., Yang, X. P., Liu, Y. H., Li, S. L., Li, X., Chen, S. Y., Novelli, A., Cho, C. M., Zeng, L. M., Wahner, A., and Zhang, Y. H.: Winter photochemistry in Beijing: Observation and model simulation of OH and HO2 radicals at an urban site, Sci. Total Environ., 685, 85–95, https://doi.org/10.1016/j.scitotenv.2019.05.329, 2019.
Mao, J., Ren, X., Chen, S., Brune, W. H., Chen, Z., Martinez, M., Harder, H., Lefer, B., Rappenglück, B., Flynn, J., and Leuchner, M.: Atmospheric oxidation capacity in the summer of Houston 2006: Comparison with summer measurements in other metropolitan studies, Atmos. Environ., 44, 4107–4115, https://doi.org/10.1016/j.atmosenv.2009.01.013, 2010.
Martinez, M., Harder, H., Kovacs, T. A., Simpas, J. B., Bassis, J., Lesher, R., Brune, W. H., Frost, G. J., Williams, E. J., Stroud, C. A., Jobson, B. T., Roberts, J. M., Hall, S. R., Shetter, R. E., Wert, B., Fried, A., Alicke, B., Stutz, J., Young, V. L., White, A. B., and Zamora, R. J.: OH and HO2 concentrations, sources, and loss rates during the Southern Oxidants Study in Nashville, Tennessee, summer 1999, J. Geophys. Res.-Atmos., 108, 4617, https://doi.org/10.1029/2003jd003551, 2003.
Peeters, J., Muller, J. F., Stavrakou, T., and Nguyen, V. S.: Hydroxyl Radical Recycling in Isoprene Oxidation Driven by Hydrogen Bonding and Hydrogen Tunneling: The Upgraded LIM1 Mechanism, J. Phys. Chem. A, 118, 8625–8643, https://doi.org/10.1021/jp5033146, 2014.
Qu, H., Wang, Y., Zhang, R., Liu, X., Huey, L. G., Sjostedt, S., Zeng, L., Lu, K., Wu, Y., Shao, M., Hu, M., Tan, Z., Fuchs, H., Broch, S., Wahner, A., Zhu, T., and Zhang, Y.: Chemical Production of Oxygenated Volatile Organic Compounds Strongly Enhances Boundary-Layer Oxidation Chemistry and Ozone Production, Environ. Sci. Technol., 55, 13718–13727, https://doi.org/10.1021/acs.est.1c04489, 2021.
Ren, X., Olson, J. R., Crawford, J. H., Brune, W. H., Mao, J., Long, R. B., Chen, Z., Chen, G., Avery, M. A., Sachse, G. W., Barrick, J. D., Diskin, G. S., Huey, L. G., Fried, A., Cohen, R. C., Heikes, B., Wennberg, P. O., Singh, H. B., Blake, D. R., and Shetter, R. E.: HOx chemistry during INTEX-A 2004: Observation, model calculation, and comparison with previous studies, J. Geophys. Res.-Atmos., 113, D05310, https://doi.org/10.1029/2007jd009166, 2008.
Rohrer, F., Lu, K., Hofzumahaus, A., Bohn, B., Brauers, T., Chang, C.-C., Fuchs, H., Haeseler, R., Holland, F., Hu, M., Kita, K., Kondo, Y., Li, X., Lou, S., Oebel, A., Shao, M., Zeng, L., Zhu, T., Zhang, Y., and Wahner, A.: Maximum efficiency in the hydroxyl-radical-based self-cleansing of the troposphere, Nat. Geosci., 7, 559–563, https://doi.org/10.1038/ngeo2199, 2014.
Shi, X., Ge, Y., Zheng, J., Ma, Y., Ren, X., and Zhang, Y.: Budget of nitrous acid and its impacts on atmospheric oxidative capacity at an urban site in the central Yangtze River Delta region of China, Atmos. Environ., 238, 117725, https://doi.org/10.1016/j.atmosenv.2020.117725, 2020.
Slater, E. J., Whalley, L. K., Woodward-Massey, R., Ye, C., Lee, J. D., Squires, F., Hopkins, J. R., Dunmore, R. E., Shaw, M., Hamilton, J. F., Lewis, A. C., Crilley, L. R., Kramer, L., Bloss, W., Vu, T., Sun, Y., Xu, W., Yue, S., Ren, L., Acton, W. J. F., Hewitt, C. N., Wang, X., Fu, P., and Heard, D. E.: Elevated levels of OH observed in haze events during wintertime in central Beijing, Atmos. Chem. Phys., 20, 14847–14871, https://doi.org/10.5194/acp-20-14847-2020, 2020.
Song, H., Lu, K., Dong, H., Tan, Z., Chen, S., Zeng, L., and Zhang, Y.: Reduced Aerosol Uptake of Hydroperoxyl Radical May Increase the Sensitivity of Ozone Production to Volatile Organic Compounds, Environ. Sci. Tech. Lett., 9, 22–29, https://doi.org/10.1021/acs.estlett.1c00893, 2021.
Stockwell, W. R., Kirchner, F., Kuhn, M., and Seefeld, S.: A new mechanism for regional atmospheric chemistry modeling, J. Geophys. Res.-Atmos., 102, 25847–25879, https://doi.org/10.1029/97jd00849, 1997.
Stone, D., Whalley, L. K., and Heard, D. E.: Tropospheric OH and HO2 radicals: field measurements and model comparisons, Chem. Soc. Rev., 41, 6348–6404, https://doi.org/10.1039/c2cs35140d, 2012.
Tan, Z., Fuchs, H., Lu, K., Hofzumahaus, A., Bohn, B., Broch, S., Dong, H., Gomm, S., Häseler, R., He, L., Holland, F., Li, X., Liu, Y., Lu, S., Rohrer, F., Shao, M., Wang, B., Wang, M., Wu, Y., Zeng, L., Zhang, Y., Wahner, A., and Zhang, Y.: Radical chemistry at a rural site (Wangdu) in the North China Plain: observation and model calculations of OH, HO2 and RO2 radicals, Atmos. Chem. Phys., 17, 663–690, https://doi.org/10.5194/acp-17-663-2017, 2017a.
Tan, Z., Fuchs, H., Lu, K., Hofzumahaus, A., Bohn, B., Broch, S., Dong, H., Gomm, S., Häseler, R., He, L., Holland, F., Li, X., Liu, Y., Lu, S., Rohrer, F., Shao, M., Wang, B., Wang, M., Wu, Y., Zeng, L., Zhang, Y., Wahner, A., and Zhang, Y.: Radical chemistry at a rural site (Wangdu) in the North China Plain: observation and model calculations of OH, HO2 and RO2 radicals, Atmos. Chem. Phys., 17, 663–690, https://doi.org/10.5194/acp-17-663-2017, 2017b.
Tan, Z., Lu, K., Jiang, M., Su, R., Wang, H., Lou, S., Fu, Q., Zhai, C., Tan, Q., Yue, D., Chen, D., Wang, Z., Xie, S., Zeng, L., and Zhang, Y.: Daytime atmospheric oxidation capacity in four Chinese megacities during the photochemically polluted season: a case study based on box model simulation, Atmos. Chem. Phys., 19, 3493–3513, https://doi.org/10.5194/acp-19-3493-2019, 2019a.
Tan, Z., Lu, K., Hofzumahaus, A., Fuchs, H., Bohn, B., Holland, F., Liu, Y., Rohrer, F., Shao, M., Sun, K., Wu, Y., Zeng, L., Zhang, Y., Zou, Q., Kiendler-Scharr, A., Wahner, A., and Zhang, Y.: Experimental budgets of OH, HO2, and RO2 radicals and implications for ozone formation in the Pearl River Delta in China 2014, Atmos. Chem. Phys., 19, 7129–7150, https://doi.org/10.5194/acp-19-7129-2019, 2019b.
Tan, Z., Ma, X., Lu, K., Jiang, M., Zou, Q., Wang, H., Zeng, L., and Zhang, Y.: Direct evidence of local photochemical production driven ozone episode in Beijing: A case study, Sci. Total Environ., 800, 148868, https://doi.org/10.1016/j.scitotenv.2021.148868, 2021.
Tan, Z. F., Lu, K. D., Dong, H. B., Hu, M., Li, X., Liu, Y. H., Lu, S. H., Shao, M., Su, R., Wang, H. C., Wu, Y. S., Wahner, A., and Zhang, Y. H.: Explicit diagnosis of the local ozone production rate and the ozone-NOx-VOC sensitivities, Sci. Bull., 63, 1067–1076, https://doi.org/10.1016/j.scib.2018.07.001, 2018.
Wang, F., Hu, R., Xie, P., Wang, Y., Chen, H., Zhang, G., and Liu, W.: Calibration source for OH radical based on synchronous photolysis, Acta Phys. Sin.-Ch. Ed., 69, 090701, https://doi.org/10.7498/aps.69.20200153, 2020.
Wang, F. Y., Hu, R. Z., Chen, H., Xie, P. H., Wang, Y. H., Li, Z. Y., Jin, H. W., Liu, J. G., and Liu, W. Q.: Development of a field system for measurement of tropospheric OH radical using laser-induced fluorescence technique, Opt. Express, 27, A419–A435, https://doi.org/10.1364/oe.27.00a419, 2019a.
Wang, H., Lu, K., Tan, Z., Chen, X., Liu, Y., and Zhang, Y.: Formation mechanism and control strategy for particulate nitrate in China, J. Environ. Sci., 123, 476–486, https://doi.org/10.1016/j.jes.2022.09.019, 2022a.
Wang, H., Ma, X., Tan, Z., Wang, H., Chen, X., Chen, S., Gao, Y., Liu, Y., Liu, Y., Yang, X., Yuan, B., Zeng, L., Huang, C., Lu, K., and Zhang, Y.: Anthropogenic monoterpenes aggravating ozone pollution, Nat. Sci. Rev., 9, nwac103, https://doi.org/10.1093/nsr/nwac103, 2022b.
Wang, S.-n., Wu, R.-r., and Wang, L.-m.: Role of Hydrogen Migrations in Carbonyl Peroxy Radicals in the Atmosphere, Chinese J. Chem. Phys., 32, 457–466, https://doi.org/10.1063/1674-0068/cjcp1811265, 2019b.
Wang, T., Xue, L., Feng, Z., Dai, J., Zhang, Y., and Tan, Y.: Ground-level ozone pollution in China: a synthesis of recent findings on influencing factors and impacts, Environ. Res. Lett., 17, 063003, https://doi.org/10.1088/1748-9326/ac69fe, 2022c.
Wang, W., Yuan, B., Peng, Y., Su, H., Cheng, Y., Yang, S., Wu, C., Qi, J., Bao, F., Huangfu, Y., Wang, C., Ye, C., Wang, Z., Wang, B., Wang, X., Song, W., Hu, W., Cheng, P., Zhu, M., Zheng, J., and Shao, M.: Direct observations indicate photodegradable oxygenated volatile organic compounds (OVOCs) as larger contributors to radicals and ozone production in the atmosphere, Atmos. Chem. Phys., 22, 4117–4128, https://doi.org/10.5194/acp-22-4117-2022, 2022d.
Wang, W., Yuan, B., Su, H., Cheng, Y., Qi, J., Wang, S., Song, W., Wang, X., Xue, C., Ma, C., Bao, F., Wang, H., Lou, S., and Shao, M.: A large role of missing volatile organic compound reactivity from anthropogenic emissions in ozone pollution regulation, Atmos. Chem. Phys., 24, 4017–4027, https://doi.org/10.5194/acp-24-4017-2024, 2024.
Wang, Y., Hu, R., Xie, P., Chen, H., Wang, F., Liu, X., Liu, J., and Liu, W.: Measurement of tropospheric HO2 radical using fluorescence assay by gas expansion with low interferences, J. Environ. Sci. (China), 99, 40–50, https://doi.org/10.1016/j.jes.2020.06.010, 2021.
Whalley, L. K., Blitz, M. A., Desservettaz, M., Seakins, P. W., and Heard, D. E.: Reporting the sensitivity of laser-induced fluorescence instruments used for HO2 detection to an interference from RO2 radicals and introducing a novel approach that enables HO2 and certain RO2 types to be selectively measured, Atmos. Meas. Tech., 6, 3425–3440, https://doi.org/10.5194/amt-6-3425-2013, 2013.
Whalley, L. K., Stone, D., Dunmore, R., Hamilton, J., Hopkins, J. R., Lee, J. D., Lewis, A. C., Williams, P., Kleffmann, J., Laufs, S., Woodward-Massey, R., and Heard, D. E.: Understanding in situ ozone production in the summertime through radical observations and modelling studies during the Clean air for London project (ClearfLo), Atmos. Chem. Phys., 18, 2547–2571, https://doi.org/10.5194/acp-18-2547-2018, 2018.
Whalley, L. K., Slater, E. J., Woodward-Massey, R., Ye, C., Lee, J. D., Squires, F., Hopkins, J. R., Dunmore, R. E., Shaw, M., Hamilton, J. F., Lewis, A. C., Mehra, A., Worrall, S. D., Bacak, A., Bannan, T. J., Coe, H., Percival, C. J., Ouyang, B., Jones, R. L., Crilley, L. R., Kramer, L. J., Bloss, W. J., Vu, T., Kotthaus, S., Grimmond, S., Sun, Y., Xu, W., Yue, S., Ren, L., Acton, W. J. F., Hewitt, C. N., Wang, X., Fu, P., and Heard, D. E.: Evaluating the sensitivity of radical chemistry and ozone formation to ambient VOCs and NOx in Beijing, Atmos. Chem. Phys., 21, 2125–2147, https://doi.org/10.5194/acp-21-2125-2021, 2021.
Woodward-Massey, R., Sommariva, R., Whalley, L. K., Cryer, D. R., Ingham, T., Bloss, W. J., Ball, S. M., Cox, S., Lee, J. D., Reed, C. P., Crilley, L. R., Kramer, L. J., Bandy, B. J., Forster, G. L., Reeves, C. E., Monks, P. S., and Heard, D. E.: Radical chemistry and ozone production at a UK coastal receptor site, Atmos. Chem. Phys., 23, 14393–14424, https://doi.org/10.5194/acp-23-14393-2023, 2023.
Yang, X., Lu, K., Ma, X., Liu, Y., Wang, H., Hu, R., Li, X., Lou, S., Chen, S., Dong, H., Wang, F., Wang, Y., Zhang, G., Li, S., Yang, S., Yang, Y., Kuang, C., Tan, Z., Chen, X., Qiu, P., Zeng, L., Xie, P., and Zhang, Y.: Observations and modeling of OH and HO2 radicals in Chengdu, China in summer 2019, Sci. Total Environ., 772, 144829–144829, https://doi.org/10.1016/j.scitotenv.2020.144829, 2021a.
Yang, Y., Li, X., Zu, K., Lian, C., Chen, S., Dong, H., Feng, M., Liu, H., Liu, J., Lu, K., Lu, S., Ma, X., Song, D., Wang, W., Yang, S., Yang, X., Yu, X., Zhu, Y., Zeng, L., Tan, Q., and Zhang, Y.: Elucidating the effect of HONO on O3 pollution by a case study in southwest China, Sci. Total Environ., 756, 144127, https://doi.org/10.1016/j.scitotenv.2020.144127, 2021b.
Yang, X., Lu, K., Ma, X., Gao, Y., Tan, Z., Wang, H., Chen, X., Li, X., Huang, X., He, L., Tang, M., Zhu, B., Chen, S., Dong, H., Zeng, L., and Zhang, Y.: Radical chemistry in the Pearl River Delta: observations and modeling of OH and HO2 radicals in Shenzhen in 2018, Atmos. Chem. Phys., 22, 12525–12542, https://doi.org/10.5194/acp-22-12525-2022, 2022.
Yang, X., Li, Y., Ma, X., Tan, Z., Lu, K., and Zhang, Y.: Unclassical Radical Generation Mechanisms in the Troposphere: A Review, Environ. Sci. Technol., 58, 15888–15909, https://doi.org/10.1021/acs.est.4c00742, 2024a.
Yang, X., Wang, H., Lu, K., Ma, X., Tan, Z., Long, B., Chen, X., Li, C., Zhai, T., Li, Y., Qu, K., Xia, Y., Zhang, Y., Li, X., Chen, S., Dong, H., Zeng, L., and Zhang, Y.: Reactive aldehyde chemistry explains the missing source of hydroxyl radicals, Nat. Commun., 15, 1648, https://doi.org/10.1038/s41467-024-45885-w, 2024b.
Zhang, G., Hu, R., Xie, P., Lou, S., Wang, F., Wang, Y., Qin, M., Li, X., Liu, X., Wang, Y., and Liu, W.: Observation and simulation of HOx radicals in an urban area in Shanghai, China, Sci. Total Environ., 810, 152275, https://doi.org/10.1016/j.scitotenv.2021.152275, 2022a.
Zhang, G., Hu, R., Xie, P., Lu, K., Lou, S., Liu, X., Li, X., Wang, F., Wang, Y., Yang, X., Cai, H., Wang, Y., and Liu, W.: Intercomparison of OH radical measurement in a complex atmosphere in Chengdu, China, Sci. Total Environ., 838, 155924, https://doi.org/10.1016/j.scitotenv.2022.155924, 2022b.