the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Vertical and seasonal variations in airborne endotoxins in a coastal megacity of North China: insights from 3-hydroxy fatty acids
Wenxin Zhang
Mutong Niu
Quanfei Zhu
Na An
Qiang Zhang
Rui Jin
Xiaoli Fu
Jian Hao
Jianbo Yang
Jingle Liu
Jing Shi
Suqin Han
Junjun Deng
Libin Wu
Yuqi Feng
Kimitaka Kawamura
Endotoxins, integral components of Gram-negative bacteria, are released into the atmosphere during bacterial fragmentation and pose health risks. This study investigated 3-hydroxy fatty acids (3-OH-FAs, C8–C18) in inhalable particles (PM10) from urban Tianjin, a coastal megacity in northern China, to estimate endotoxin levels utilizing ultra-high-performance liquid chromatography mass spectrometry (UHPLC-MS). Results revealed seasonal and altitudinal variations in 3-OH-FAs and endotoxin levels. Total endotoxin concentrations averaged 21.5 ng m−3 at near ground (2 m) and 16.1 ng m−3 at a higher altitude (220 m), corresponding to total 3-OH-FAs (C10–C18) concentrations of 2.8 and 2.0 ng m−3, respectively. Maximum endotoxin level (26.5 ng m−3) occurred near ground during winter, attributed to enhanced near-surface emissions. Bioactive endotoxins peaked at 12.4 ng m−3 near ground in winter, exceeding the exposure threshold, while averaging 8.1 ng m−3 in other seasons. Short- and mid-chain 3-OH-FAs (C8–C13) exhibited significant correlations with meteorological factors (e.g., temperature, humidity, and wind speed) at both altitudes, indicating regulation through microbial growth dynamics and photochemical processes. Long-chain homologues (C14–C18) were affected by both meteorological conditions and particulate pollutants (e.g., organic carbon, K+, Ca2+), reflecting mixed influences from natural sources (e.g., soils) and anthropogenic activities (e.g., combustion). These findings advance understanding of dynamic variations in airborne endotoxins within complex urban environments, providing critical data for assessing health risks associated with particulate pollution and informing urban air quality management strategies.
- Article
(3087 KB) - Full-text XML
-
Supplement
(1078 KB) - BibTeX
- EndNote
Inhalable particles with aerodynamic diameters less than 10 µm (PM10) may originate from both natural and anthropogenic sources (Yin et al., 2022; Xue et al., 2024), and could further result in various respiratory and cardiovascular diseases (Makkonen et al., 2010; Mukherjee and Agrawal, 2017). Among the biological components of PM10, endotoxins are of extreme importance due to strong chronic health effects (Mahapatra et al., 2018; Mueller-Anneling et al., 2004).
Endotoxin, also known as lipopolysaccharide (LPS), is an integral component of the outer membrane of Gram-negative bacteria (GNB) (Rylander, 2002). An endotoxin contains three regions, consisting of a core polysaccharide, a long-chain polysaccharide, and a nonpolar lipid named lipid A, with lipid A being the most associated with toxicity (Schneier et al., 2020; Rylander, 2002; Spaan et al., 2006). Endotoxins may transport via adhering to the surface of fine inorganic particles (Zhang et al., 2024; Hwang et al., 2021). As a potent inflammagen, an endotoxin can cause fever, shaking chills, septic shock, toxic pneumonitis and respiratory symptoms, through inhalation, dermal and/or eye contact or ingestion (Rylander, 2002; Farokhi et al., 2018). LPS can be oxidized by ozone and the resulting reactant greatly enhanced inflammatory anemia (Liu et al., 2025). The health risks of endotoxins to humans have been investigated (Farokhi et al., 2018; Laboha et al., 2023; Liebers et al., 2008). Their harmful effects depend on both the composition of the inhaled particles and the degree and duration of exposure (Liebers et al., 2020; Lundin and Checkoway, 2009). The quantification and characterization of LPS in environmental samples are critical to understanding the biological effects of environmental endotoxin exposure (Park et al., 2004).
Previous studies have investigated endotoxin levels in various environments, including occupational settings (Heederik and Douwes, 1997; Liebers et al., 2020, 2006), indoor environments (Amin et al., 2023; Gabriel et al., 2021; Phiri et al., 2023), and ambient aerosols and dust (Cheng et al., 2012; Hwang and Park, 2019; Lang-Yona et al., 2014; Hines et al., 2003; Park et al., 2000). Airborne endotoxin levels exhibited spatial and temporal variations, influenced by geographical location (Moretti et al., 2018), season (Makkonen et al., 2010; Hwang and Park, 2019), emission source (Mueller-Anneling et al., 2004; Mahapatra et al., 2018), meteorological condition (Guan et al., 2014; Rolph et al., 2018), and pollution level (Guo et al., 2018). These variations might reveal the potential sources, atmospheric processes, and the survival mechanisms of airborne microbes (Hu et al., 2020a). However, the factors influencing endotoxin levels are not fully understood (Hwang and Park, 2019; Mahapatra et al., 2018). To gain further clarity, outdoor sampling coupled with simultaneous monitoring of meteorological and air quality parameters is essential (Amin et al., 2023). Additionally, the sources of endotoxins in ambient environment, whether from long-range transport or local emissions, have yet to be definitively identified.
Previous studies on endotoxins have primarily focused on occupational exposure and the health effects of excessive endotoxin exposure (Liebers et al., 2006; Amin et al., 2023). However, research on endotoxin levels in the ambient atmosphere remains limited. The vertical distribution, spatiotemporal patterns of airborne endotoxins in the urban boundary layer, and their possible influencing factors have yet to be reported.
Multiple factors including sample types (personal or stationary), sampling method (active or passive), extraction procedure, and storage condition, can affect the determination of endotoxins (Liebers et al., 2020). One mainstream detection method of endotoxins is the Limulus amebocyte lysate (LAL) test (Rylander, 2002). This enzyme activation-based method provides direct information on bioactive endotoxins. Despite its sensitivity, the LAL test may still underestimate endotoxin levels, because many endotoxins, including bioactive ones, may remain concealed within the intact bacterial structure, making them unavailable to react with the Limulus enzymes (Mattsby-Baltzer et al., 1991). False-negative results may occur when endotoxins are masked by buffer constituents, product formulation, cell culture medium compositions, and surfactants (Schwarz et al., 2017; Schneier et al., 2020). Whilst, false-positive results can be caused by β-1,3-glucan, which might as well trigger the enzymatic coagulation of the blood (Cheng et al., 2012; Uhlig et al., 2016).
The alternative approach most commonly applied to detect endotoxins in environmental samples is to measure 3-hydroxy fatty acids (3-OH-FAs) with carbon chain lengths from 10 to 18 as endotoxin biomarkers utilizing chemical analytical methods such as gas chromatography-mass spectrometry (GC-MS) (Bikkina et al., 2021a; Cheng et al., 2012). In contrast to the LAL test, 3-OH-FAs measured by GC-MS do not reflect the biologically active endotoxin, but rather total concentration (Liebers et al., 2008). In addition, the sample pretreatment for GC-MS analysis with strong alkaline hydrolysis may result in 3-OH-FAs turning into unsaturated fatty acids while eliminating water during the derivatization process (trifluoracetamide) (Binding et al., 2004; Wollenweber and Rietschel, 1990). Liquid chromatography-mass spectrometry (LC-MS) with its high sensitivity and selectivity, and broad adaptability, is regarded as a powerful analytical tool for small molecules (Zhang et al., 2019; Paba et al., 2019). Recently, ultra-high-performance liquid chromatography mass spectrometry (UHPLC-MS) combined with isotope labeling has been used to effectively separate, identify and quantify positional isomers of hydroxy fatty acids, demonstrating exceptional accuracy and precision in complex environmental matrices including aerosols (Niu et al., 2024; Zhu et al., 2020).
The coastal zone is a transition area between the ocean and the land, which is affected by natural continental processes (e.g., dust storms) and marine emissions, as well as intense human activities due to a large population. In addition, sea-land breeze is one of the most common circulations over coastal areas and has a great impact on the local meteorology and atmospheric environment (Xiao et al., 2023). These interactions make the atmospheric environment of coastal zones more complex, providing unique conditions for investigating the effects of local emissions and regional or long-distance transport on atmospheric environment. Meteorological tower-based studies complement ground-based observations (Yang et al., 2023), offering new insights into the formation, transformation, and transport of organic matter under different atmospheric environments (Lei et al., 2021). Comprehensive vertical measurements of the physical and chemical properties of aerosol particles in the lower boundary layer have been conducted to evaluate the roles of local emissions and regional and long-distance transport in air pollution (Fan et al., 2022; Li et al., 2022). However, the levels, sources, and health impacts of airborne endotoxins in coastal zones remain largely unknown.
Herein, diurnal PM10 samples were collected at two altitudes using a 255 m meteorological tower in urban Tianjin, the largest coastal megacity in North China, enabling the investigation of the vertical distribution of airborne endotoxins. The UHPLC-MS system, combined with isotope labeling, was applied for accurate determination of 3-OH-FAs (C8–C18) in PM10. The amounts of endotoxins and GNB dry mass in PM10 were estimated using the concentrations of 3-OH-FAs (C10–C18) according to previous studies (Bikkina et al., 2021a; Tyagi et al., 2015b, 2016). This study aims to provide: (1) the quantification and distribution patterns of the bacterial marker 3-OH-FAs in ambient PM10 within the urban boundary layer in Tianjin; (2) insights into the vertical, seasonal and diurnal variations in airborne endotoxins, along with estimates of the bioactive portion of 3-OH-FAs and the mass loading of GNB for assessing the potential allergenic impact of airborne endotoxin levels, and (3) the preliminary identification of influencing factors of endotoxin levels, in combination with key environmental parameters, including meteorological and air quality data.
2.1 Samples collection
Tianjin is a coastal megacity located in northeast part of the North China Plain (NCP), bordering the Bohai Sea in the east, leaning against the Yanshan Mountain in the north (Fig. S1 in the Supplement). It is 120 km from Beijing, the capital of China, in the northwest. The sampling was conducted by utilizing the 255 m meteorological tower at the Tianjin Atmospheric Boundary Layer Observatory of the China Meteorological Administration, located at the southern area of urban Tianjin. Two automatic high-volume aerosol samplers (DIGITEL DHA-80) were set at two floors in the meteorological tower, i.e., the near-ground (2 m) and a higher altitude (220 m), respectively. PM10 samples were collected on pre-combusted (450 °C for 6 h) quartz fiber filter (Φ 150 mm, Pall, USA) during the autumn (18–24 September) and winter (8–14 December) of 2020, and the spring (26–31 May) and summer (2–8 August) of 2021. After collection, the samples were stored at −20 °C in a freezer until analysis.
2.2 Samples pretreatment
The extraction and detection methods of 3-OH-FAs are available in our previous study (Niu et al., 2024). Briefly, aliquots of aerosol samples (Φ 28 mm) were cut and placed in a centrifuge tube, followed by adding 4 mL of ethyl acetate (EtOAc), 1 min of vortex, and 15 min of sonicating at room temperature. Then, 4 mL of Milli-Q water was added, and the resulting solution was vortexed for remixing. Centrifugation was performed for 5 min at 5000 rpm, and the supernatant was extracted afterward, and then dried under nitrogen. Subsequently, 100 µL of acetonitrile (ACN) was added to the dried centrifuge tube and vortexed for 2 min, then derivatization reagents (10 µL of 20 µmol mL−1 2-chloro-1-methylpyridinium iodide (CMPI), 20 µL of 20 µmol mL−1 triethylamine (TEA), and 20 µL of 20 µmol mL−1 2-dimethylaminoethylamine (DMED)) were added and vortexed for 2 min, and the mixture was incubated for 1 h at 40 °C and 1500 rpm, then dried under nitrogen. Milli-Q water and internal standards (d4-DMED-labeled hydroxy fatty acid standard) (, ) were added to each dried centrifuge tube, vortexed to redissolve, and then centrifuged for 5 min at 4 °C and 12 000 rpm to extract the supernatant. Details of the chemicals, reagents and method validation are described in Sect. S1 in the Supplement.
2.3 UHPLC-MS analysis
The UHPLC-MS system comprised an ACQUITY UPLC I-Class LC from Waters (Milford, MA, USA) coupled with an AB SCIEX 6500+ triple quadrupole MS (Framingham, MA, USA). Separation of the target compounds was achieved using ACQUITY HPLC HSS T3 (1.8 µm, 2.1 mm × 50 mm) column (Waters, Milford, MA, USA). The mobile phases consisted of solvent A (0.1 % formic acid in water) and solvent B (ACN), delivered at a flow rate of 0.4 mL min−1. The gradient program was set as follows: 0–3 min at 5 % B, 3–15 min from 22 % to 60 % B, 15–17 min at 60 % B, 17–24 min at 95 % B, 24–40 min at 5 % B. Mass spectrometric analysis was performed in positive ion mode using electrospray ionization (ESI) and operated in multiple reaction monitoring (MRM) mode. The ESI conditions were set as follows: curtain gas, 35 L min−1; collision gas, 8 L min−1; ion spray voltage, 5500 V; temperature, 500 °C; ion source gas 1, 50 L min−1; ion source gas 2, 45 L min−1. Data acquisition and quantification were conducted using SCIEX OS version 2.0.1 software. The mass concentrations of 3-OH-FAs (C8–C18) were quantified using internal standard method, employing d4-DMED-labeled 3-OH-FA standards as internal standards. The detailed analytical procedure is described in Niu et al. (2024).
2.4 Endotoxin and GNB dry mass estimation
The amounts of endotoxins and GNB dry mass were estimated using the following formulas:
where 4 means each lipid A carries 4 mol 3-OH-FAs (C10–C18) (Laitinen et al., 2001; Rietschel et al., 1984), while the multiplication factor 8000 represents the average molecular weight of endotoxins (Laitinen et al., 2001).
where 15 refers to the biomarker-to-microbial mass conversion factor of 15 nmol of 3-OH-FAs per mg dry cell weight (Lee et al., 2004; Balkwill et al., 1988).
2.5 Other measurements
The concentrations of organic carbon (OC) and elemental carbon (EC) were determined using a Thermal/Optical Carbon Analyzer (Model RT-4, Sunset Laboratory Inc., Oregon, USA). Analytical errors were controlled within ±10 % via a duplicate analysis of each filter. The concentrations of water-soluble organic carbon (WSOC) were measured using Total Organic Carbon (TOC) Analyzer (Shimadzu 5000A, Japan). Water-soluble inorganic anions and cations (Cl−, SO, NO, Na+, NH, K+, Mg2+, Ca2+) were analyzed using an Ion Chromatography system (Dionex Aquion, Thermo Scientific, Waltham, MA, USA). Gradient meteorological parameters were continuously recorded by automatic weather stations installed on the 255 m high meteorological tower, while both gaseous and particulate pollutants were collected simultaneously at the ground platform.
2.6 Concentration-weighted trajectory (CWT) analysis
Concentration-weighted trajectories (CWTs) were calculated per hour, and starting height of 220 m, based on the 3 d (72 h) backward trajectories of air masses with the Hybrid Single Particle Lagrangian Integrated Trajectory (HYSPLIT) model (Stein et al., 2015) in conjunction with the measured concentrations of 3-OH-FAs as follows:
where l is the number of the trajectory; Cl is the average concentration of 3-OH-FAs, and nijl is the number of trajectory endpoints that lie in the grid cell (i,j). The CWT value indicates the potential distant sources impacting the sampling site. The analyses were performed for 1° × 1° grids covering the area encompassed by 20–80° N and 60–150° E. A weight function (Wij) in Eq. (4) was applied in the CWT analysis to increase statistical stability, and Nij is the number of trajectory endpoints that lie in the grid cell (i,j).
2.7 Positive matrix factorization (PMF)
Source apportionment of 3-OH-FAs was carried out with U.S. EPA PMF 5.0. PMF solves X≈GF by weighted least-squares minimization of:
where X is the observed concentration matrix, u the corresponding uncertainty matrix, G the factor-contribution matrix, and F the factor-profile matrix (Paatero and Tapper, 1994; Bhandari et al., 2022). Uncertainties (u) were calculated as recommended by the EPA guidance:
If the concentration is less than or equal to the species-specific method detection limit (MDL) provided, the uncertainty is calculated using a fixed fraction of the MDL (Eq. 6).
If the concentration is greater than the MDL provided, the calculation is based on a user provided fraction of the concentration and MDL (Eq. 7).
An error fraction of 0.2 was adopted for most species; values up to 0.6 were assigned to constituents near the detection limit.
A total of 19 measured species in 87 samples were used, including bulk carbonaceous fractions (OC, EC), major inorganic ions (K+, Na+, Ca2+, Mg2+, NH, NO, SO, Cl−) and nine 3-OH-FA homologues (C10–C18). Species selection followed three criteria: (1) Signal-to-noise ratio () – species with were excluded; were down-weighted (“weak”) (Paatero and Hopke, 2003). Accordingly, C12 3-OH-FA was removed. (2) Goodness-of-fit (R2) – after trial runs, variables with persistently low R2 between modelled and observed concentrations were discarded. (3) Q evaluation – models with 4–9 factors (p) were explored; the lowest Q value at five factors when moving from four to nine factors. The solution giving ratio closest to 1 was selected (Bhandari et al., 2022). The final configuration (five factors) yielded stable and physically interpretable profiles.
2.8 Statistical analysis
Spearman rank analysis was conducted to evaluate the correlation between airborne endotoxins and air pollutants. Data were analyzed using unpaired t-tests, two-tailed for single comparisons. Graphs were generated using RStudio software version 2023.09.1+494, R version 4.3.2 and Origin 2021. Mantel test was performed between 3-OH-FA homologues and those of meteorological/pollution variables using Spearman's correlation coefficient (Spearman's r) with 999 permutations, computed with the vegan package (v2.6-4) in R (v4.3.2). Significance was accepted at p<0.05. Hierarchical-cluster analysis was performed via Ward's minimum-variance method on Euclidean distance using OriginPro. The distribution characteristics of 3-OH-FAs and airborne endotoxins were described in range (minimum–maximum) and arithmetic mean.
3.1 Molecular distribution of 3-hydroxy fatty acids
The molecular distribution of 3-OH-FAs with carbon numbers from 8 to 18 (C8–C18) is illustrated in Fig. 1. In terms of diurnal variations, the mass concentrations of most 3-OH-FAs were higher at night than during the daytime (Fig. 1a). This feature, observed predominantly at ground level may be partly due to the lower boundary layer height at night, which contributes to the accumulation of pollutants (Li et al., 2017; Qiu et al., 2019).
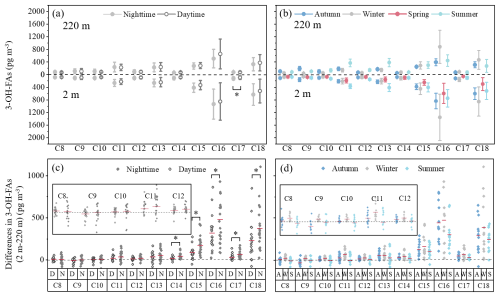
Figure 1The vertical, diurnal and seasonal variations in molecular distribution of 3-OH-FAs (C8–C18). (a–b) The mean values (indicated by points) and standard deviations (error bars) of 3-OH-FAs concentrations. (c–d) The differences in 3-OH-FAs concentrations between 2 m and 220 m. The short lines indicate the mean values of the differences. The small figures in (c) and (d) are the enlarged view of species with low concentration differences (C8–C12). * The difference is significant (p<0.05).
The molecular distribution of 3-OH-FAs also showed apparent seasonal variations (Fig. 1b). Even-carbon homologues (C16, C18) were predominant in all seasons, especially in winter, with average mass concentrations of 1355.2 ± 737.6 pg m−3 (C16) and 855.4 ± 461.8 pg m−3 (C18) at near ground, 882.6 ± 523.2 pg m−3 (C16) and 467.7 ± 299.8 pg m−3 (C18) at 220 m. C11, C13, and C15 3-OH-FAs were the predominant odd-carbon 3-OH-FAs, particularly in summer, with average mass concentrations of 367.4 ± 70.5 pg m−3 (C11), 412.7 ± 126.6 pg m−3 (C13), and 405.8 ± 143.8 pg m−3 (C15) at near ground, respectively. In terms of 3-OH-FAs with different carbon numbers, most 3-OH-FAs were more abundant at near ground, suggesting that these homologues were mainly influenced by near-surface emissions. In contrast, C9 at 220 m remained higher than at 2 m in autumn and summer, regardless of diurnal changes (Fig. 1c, d). Other short-chain homologues (C8, C10, C11) also presented higher concentrations at 220 m during summer, indicating that they were possibly contributed by regional transport or photochemical oxidation processes (Lei et al., 2021).
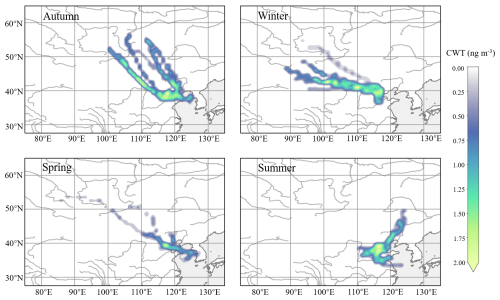
Figure 2Seasonal concentration-weighted trajectory (CWT) maps for 3-OH-FAs measured at the Atmospheric Boundary Layer Observatory of Tianjin. This analysis is based on air mass backward trajectories during the observation periods.
The result of concentration-weighted trajectory (CWT) analysis revealed that the mass concentration of 3-OH-FAs at 220 m was influenced by marine sources to varying extents in spring, autumn, and summer (Fig. 2). During autumn and winter, the major source areas were the Beijing-Tianjin-Hebei region, with transport pathways originating from the northwest of Tianjin, including Mongolia, Inner Mongolia and Beijing, before reaching the sampling site. In summer, predominant origin areas included Beijing-Tianjin-Hebei region, Heilongjiang province, and the Bohai Sea in the northeast. Photochemical oxidation of organic matter is prone to occur in summer due to high temperatures and intense solar radiation. The elevated concentrations of short-chain 3-OH-FAs (C8–C11) at 220 m may result from secondary transformation processes during long-range transport or photochemical oxidation of the local emissions. Bikkina et al. (2019) reported that photochemical oxidation of marine organic matter likely accounts for the predominance of odd-carbon and short-chain 3-OH-FAs in sea-spray aerosols.
3.2 Vertical-temporal patterns of airborne endotoxin levels
Total mass concentrations of endotoxins in PM10 from urban Tianjin during 2020–2021 were estimated based on the measured 3-OH-FAs (C10–C18) (Table 1, Fig. 3a). There was no obvious diurnal difference in total endotoxin levels (Fig. 3a). The endotoxin level was significantly higher (p<0.05) at 2 m than at 220 m during each season (Fig. 4a), indicating that endotoxin emissions were mainly dominated by near-ground sources. It was suggested that the majority of airborne endotoxins may originate from soils (Brooks et al., 2006), as aliphatic 3-OH-FAs (< C20) originate from soil microorganisms (Zelles, 1999; Bikkina et al., 2021a). 3-OH-FAs are also detected in marine ultrafiltered dissolved organic matter (Wakeham et al., 2003), groundwater sediments and estuarine sediments (Parker et al., 1982). Marine source could also be a dominant contributor to elevated endotoxin levels in coastal areas. Lang-Yona et al. (2014) observed high endotoxins content correlated with cyanobacteria at a coastal site of the eastern Mediterranean Sea, assumed that higher wind speeds with low pressure system led to increased sea spray and the consequent primary aerosol emission. Therefore, the endotoxins at 220 m in urban Tianjin might originate from the vertical transport of ground emissions, long-range transport from northwest of Tianjin or from marine emissions (e.g., Bohai Sea) as depicted in CWT results (refer to Sect. 3.1).
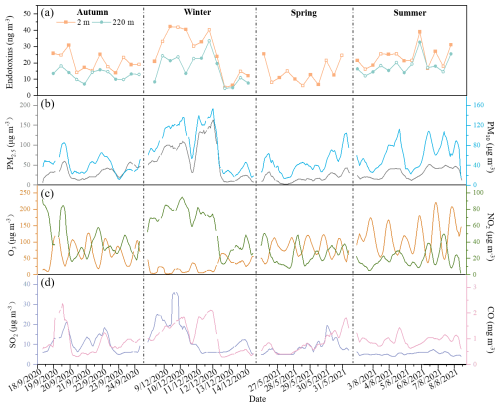
Figure 3Time series of concentrations of total endotoxins (a), particulate matter (b), and gaseous pollutants (c, d) during the observation periods.
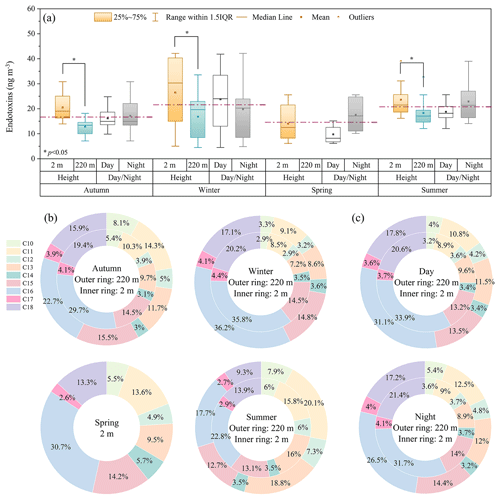
Figure 4Concentrations of total endotoxins and relative contributions of 3-OH-FAs in urban aerosols from Tianjin. (a) The vertical, diurnal and seasonal variations in mass concentrations of endotoxins. The horizontal dashed line denotes the seasonally averaged PM10 endotoxin level. Seasonal (b) and diurnal (c) variations in the relative contributions of 3-OH-FAs with different carbon number to total endotoxins at different altitudes.
Table 1Seasonal variations in airborne endotoxin concentrations in PM10 from urban Tianjin at different attitudes during 2020–2021.
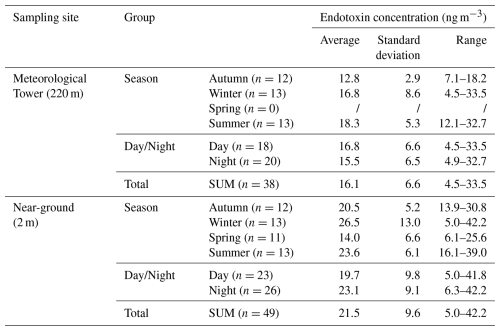
In the near-ground (2 m) air, the average endotoxin mass concentrations were the highest (26.5 ± 13.0 ng m−3) in winter, followed by 23.7 ± 6.1 ng m−3 in summer, 20.5 ± 7.1 ng m−3 in fall, and the lowest 14.0 ± 6.6 ng m−3 in spring (Table 1, Fig. 4a). While at a higher altitude (220 m), the average endotoxin level was the highest in summer (18.3 ± 5.3 ng m−3), followed by winter (16.8 ± 8.7 ng m−3) and fall (12.8 ± 2.9 ng m−3). The endotoxin concentration ratio between 220 and 2 m during summer was 0.8 ± 0.1, higher than those during autumn (0.6 ± 0.2) and winter (0.7 ± 0.2), suggesting an increased contribution of possible regional transport or photochemical oxidation processes during summer. Most airborne bacteria could not survive during long-range transport due to ultraviolet radiation, which further lead to photodegradation of proteinaceous matter and significant losses of microbial viability (Hu et al., 2021; Yin et al., 2021). LPS is then released into the air during cell death (Petsch and Anspach, 2000), cause the rising in higher endotoxin level. Moreover, the ratio of endotoxin concentrations measured at 220 and 2 m was higher during the daytime (0.8 ± 0.1) than nighttime (0.6 ± 0.1). The average endotoxin mass concentration was slightly higher at night than during the daytime in spring, summer and autumn, but lower at night during winter (Fig. 4a). The increasing concentration of certain bacteria at night may also attribute to the diurnal changes in endotoxins. Hu et al. (2020b) observed richer operational taxonomic units per PM2.5 sample in Hangzhou at night during spring, with higher relative abundance of Thermomicrobia, a Gram-negative bacterial class, generally distributed away from anthropogenic regions. Abdel Hameed et al. (2009) found the diurnal variations in number concentration of airborne bacteria peaked in the evening at Helwan, Egypt.
The relative contributions of different carbon-numbered 3-OH-FAs to total endotoxin mass concentrations in PM10 of Tianjin presented seasonal and altitudinal variations (Fig. 4b). Overall, the even-carbon long-chain C16 and C18 3-OH-FAs made up the largest proportion in all seasons, with higher relative contributions (46.5 ± 10.1 %) to total endotoxins at near-ground level than at 220 m (39.7 ± 13.7 %), suggesting their local emissions. The relative contribution of the even-carbon C16 and C18 homologues were the highest in winter (56.0 ± 6.9 % at near ground and 53.3 ± 8.1 % at 220 m), followed by autumn (49.1 ± 5.4 %, 38.6 ± 6.2 %), and the lowest in summer (36.7 ± 7.4 %, 27.0 ± 9.8 %). This finding is consistent with those obtained in suburban Tokyo (Bikkina et al., 2021a), Chichijima island (Bikkina et al., 2021b), and Jeju island (Tyagi et al., 2017), in which 3-OH-FAs were also characterized by a strong even-carbon predominance. Hydroxy fatty acids exhibiting a strong even-carbon preference are usually derived from biological pathways (Bikkina et al., 2019) or indicative of biogenic sources (Tyagi et al., 2015a), such as the lipid fractions of microorganisms (bacteria, algae, fungi, etc.) and vascular plant surface waxes (Kawamura et al., 2003; Simoneit et al., 2004).
However, in this study, the odd-carbon homologues (C11, C13 and C15) accounts for 36.7 % and 41.9 % of total endotoxins at near ground and at 220 m, respectively. Their relative contribution was maximum in summer (45.0 % at near ground and 51.6 % at 220 m), followed by autumn (34.5 % at near ground and 41.4 % at 220 m), and the lowest in winter (30.2 % at near ground and 32.5 % at 220 m). Compared with remote islands dominated by natural emissions such as microorganisms and vegetation (Bikkina et al., 2021b; Tyagi et al., 2015a, 2017), the urban aerosols in Tianjin are largely affected by both anthropogenic emissions and secondary formation (Li et al., 2022). These odd-carbon 3-OH-FAs (C11, C13 and C15) may originate from various sources, such as anthropogenic activities (e.g., fossil fuel combustion, biomass emissions), or from the photochemical oxidation of long-chain fatty acids or hydroxy fatty acids during atmospheric transport (Bikkina et al., 2019).
In addition, long-chain C16, C18 and odd-carbon homologues (C11, C13 and C15) 3-OH-FAs exhibited similar variation trend at the two heights. Long-chain C16, C18 3-OH-FAs contributed more at 2 m, while odd-carbon homologues (C11, C13 and C15) 3-OH-FAs contributed more at 220 m, regardless of diurnal changes (Fig. 4c).
3.3 Comparison of the endotoxin level with literature results
The airborne endotoxin level at near-ground observed in Tianjin is compared with results in literatures (Fig. 5). Different sampling methods and endotoxin assay methodologies may affect the results of the endotoxin assessment (de Rooij et al., 2017). Herein only the endotoxin level in aerosol samples assessed by chemical analytical method with 3-OH-FAs as biomarkers are compared.
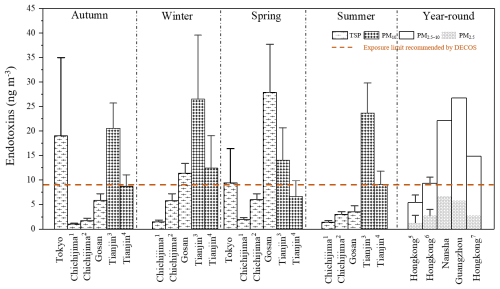
Figure 5Comparisons of average endotoxin concentrations in aerosols with various size ranges during different seasons. All data are estimated from 3-OH-FAs measured as biomarkers by chemical analytical method. The error bars are the standard derivations. Note: (1) 1990–1993; (2) 2001–2003; (3) total endotoxins in this study (at ground); (4) bioactive endotoxins in this study (at ground); (5) rural region, Hongkong University of Science and Technology; (6) urban region, Hongkong Science Museum; (7) urban region, Hong Kong. TSP, total suspended particle; DECOS, Dutch Expert Committee on Occupational Safety.
During all seasons, the mean mass concentration of airborne endotoxins in Tianjin was much higher than that in remote islands such as Gosan (except for spring) (Tyagi et al., 2017), Chichijima (Bikkina et al., 2021b; Tyagi et al., 2015a), but close to the endotoxin levels measured in developed areas such as Tokyo (Bikkina et al., 2021a), and the Pearl River Delta in China (Cheng et al., 2012). These results imply that high endotoxin mass concentrations in urban areas may be caused by enhanced human activities (Lee et al., 2004). Guan et al. (2014) discovered a higher endotoxin level in a high-traffic urban setting than in a low-traffic residential area, and similarly Madsen (2006) reported that the endotoxin concentrations on congested streets were higher than in residential areas.
Different from higher endotoxin levels observed in winter and summer in Tianjin, the endotoxin level was much higher in spring at Gosan, Korea, which was probably due to the enhanced mobilization of mineral dust through high altitudinal long-range atmospheric transport (Tyagi et al., 2017). Dutch Expert Committee on Occupational Safety (DECOS), a Committee of the Health Council of the Netherlands stated that exposure to endotoxin above 9 ng m−3 for 8 h or more increases the risk of respiratory diseases (DECOS, 2010). The endotoxin concentrations reported in this study and in previous studies exceeded this value, except for remote islands such as Chichijima (Fig. 5), raising an environmental health issue caused by airborne endotoxins.
It should be noted that the endotoxin concentration proposed by DECOS refers to the mass concentration of bioactive endotoxin rather than total mass concentration (DECOS, 2010). In light of the multiple studies, bioactive endotoxin appears to be correlated with C10, C14, C16 3-OH-FAs in coarse mode particles (PM2.5−10) in the Pearl River Delta region (Cheng et al., 2012); C10, C12, C14 3-OH-FAs in house dust (Saraf et al., 1997, 1999; Park et al., 2004; Uhlig et al., 2016); C14, C16 3-OH-FAs in airplane seat dust (Hines et al., 2003); and C12, C14 3-OH-FAs in agricultural dust (Reynolds et al., 2005). In this regard, C10, C12, C14, C16 3-OH-FAs were selected to estimate the bioactive endotoxin potential in this study. Correspondingly, the estimated concentrations of bioactive endotoxins were substantially lower compared to total endotoxins (Fig. 5). The level of bioactive endotoxins at 2 m was 6.6 ng m−3 in spring, 8.7 ng m−3 in autumn, lower than the endotoxin exposure threshold (9.0 ng m−3). While the level of bioactive endotoxins exceeded the threshold in summer (9.1 ng m−3) and winter (12.4 ng m−3), implying possible health risks of airborne endotoxin exposure.
3.4 Mass loading of airborne Gram-negative bacteria
Based on the empirical equations, the dry mass loading of airborne GNB was estimated from the endotoxin mass concentration. The mass concentrations of airborne GNB measured in this study and previous literatures measured in urban, marine and alpine aerosols are listed in Table 2. The dry mass concentration of airborne GNB showed obvious regional variations. The airborne GNB dry mass concentration in Tianjin was the highest in winter (882.5 ± 167.4 ng m−3), followed by summer (787.6 ± 538.0 ng m−3), autumn (684.2 ± 464.6 ng m−3), and the lowest in spring (467.8 ± 220.4 ng m−3) at 2 m. Dry-mass concentrations of GNB in Tianjin PM10 are markedly higher than those reported for PM10 from Hong Kong in 2002–2003 (Lee et al., 2004) and even exceed GNB levels measured in TSP at Chichijima (Japan) (Bikkina et al., 2021b), Tokyo (Japan) (Bikkina et al., 2021a), and Gosan (Korea) (Tyagi et al., 2017). These findings underscore the exceptionally heavy bioaerosol burden in urban Tianjin and highlight the urgency of mitigating biological, as well as chemical, particulate pollution.
Table 2Dry mass concentrations of GNB estimated in this study and previous studies.
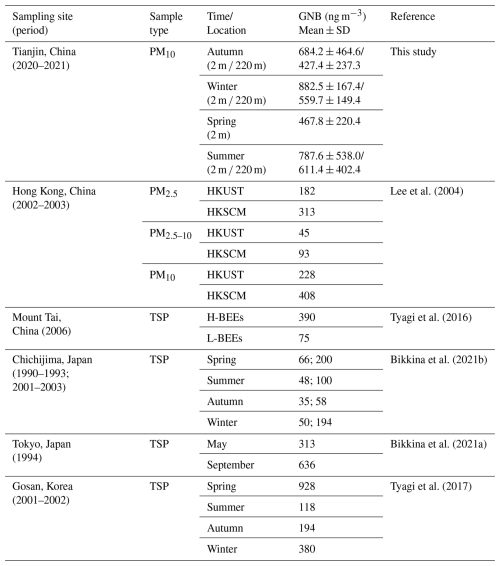
Note: TSP, total suspended particulate; SD, standard derivation; HKUST, Hongkong University of Science and Technology; HKSCM, Hongkong Science Museum; H-BEEs, high biomass-burning samples; L-BEEs, low biomass-burning samples.
Amato et al. (2007) reported that cultivable airborne GNB in cloud water were more abundant in summer than in winter. The phenomenon was further explained by the preferential development of microbial populations on vegetation in summer, coupled with the relative resistance of GNB to ultraviolet (UV) damage. Heavy biomass burning activities may also result in higher GNB mass concentration. GNB mass concentration measured at Mount Tai, China during the high biomass burning events, were 5 times higher than that during the low biomass burning period (Tyagi et al., 2016). During biomass burning events (i.e., wildfires and prescribed fires), microbes can be aerosolized from terrestrial sources into the atmosphere (Bonfantine et al., 2024; Kobziar et al., 2024). It was previously assumed that pyrogenic carbon or smoke produced by biomass burning provides temporary habitat for microbes aerosolized from soils and vegetations (Bonfantine et al., 2024; Kobziar and Thompson, 2020). Meanwhile, particulate matter in smoke confers attenuation of UV-B and UV-A radiation, further leading to increasing bioaerosol viability and higher microbial cell concentration (Mims et al., 1997; Moore et al., 2021). Thus, the high GNB dry mass loading in Tianjin, extremely during summer and winter, could be resulted from the contributions of specific strains of GNB and/or microbial emissions during biomass burning. There are few descriptions of the atmospheric GNB mass concentrations in Tianjin in the literatures, and the distribution of different GNB community, namely the species distribution and their effects on human health remain to be further investigated.
3.5 Influencing factors of airborne endotoxins
3.5.1 Effects of meteorological conditions and gaseous pollutants
As components of GNB cell membranes, endotoxins are released during bacterial growth or death, meaning their mass concentration is determined not only by the abundance of GNB but also by factors such as GNB sources, meteorological conditions, surface types, and gaseous pollutants (Guan et al., 2014; Rolph et al., 2018).
Previous studies have demonstrated that air temperature plays a more predominant role in influencing endotoxin levels than other environmental factors (Traversi et al., 2010; Carty et al., 2003). Higher endotoxin level was found in winter and autumn in Korea, and a negative association was observed between endotoxins and air temperature (Hwang and Park, 2019). Guan et al. (2014) observed high endotoxin level at 4–10 °C, and assumed this temperature range was suitable for bacterial growth. The endotoxin concentration was higher under very dry or wet conditions compared to relative humidity (RH) was 40 %–80 %. Cheng et al. (2012) suggested the differences in bacterial fauna and growth rates can cause different endotoxin level under similar climatic conditions. In this study, during the winter period in Tianjin, the RH was the lowest (46 %) compared to other seasons, while wind speed reached the highest at 220 m (4.8 m s−1) and the temperature was −7 to 5 °C, which might be suitable for the survival of specific GNB species, resulting the higher endotoxin level. In winter when air pollution is frequent, pollutants and other elements originating from anthropogenic sources act as toxins to GNB, thereby increasing the concentration of endotoxins as a result of cell death level (Guan et al., 2014; Mahapatra et al., 2018), providing a possible explanation for the highest endotoxin level in winter. Moreover, meteorological conditions characterized by stable weather patterns, such as high atmospheric pressure (Ormanova et al., 2020), reduced mixing layer height (MLH) and temperature inversions create unfavorable conditions for pollutant dispersion (Li et al., 2022; Yao et al., 2022). These conditions can lead to the accumulation of pollutants, exacerbating air pollution levels, particularly during the winter season in Tianjin.
Hierarchical cluster analysis classified the mass concentration of 3-OH-FAs (C8–C18) into 3 groups: short-chain (C8, C9), mid-chain (C10–C13), and long-chain (C14–C18) 3-OH-FAs. Each group likely shares similar sources or influencing factors. Spearman correlation analysis (Fig. S2) and Mantel test (Fig. 6) were used to evaluate the relationships between 3-OH-FAs, endotoxins, and environmental factors (meteorological parameters, carbonaceous fraction, anions and cations, air pollutants). Among meteorological parameters, RH and air temperature mainly accounted for observed variations in 3-OH-FAs concentrations (Fig. 6) (Mantel's p<0.01). Most short/mid-chain (C8–C13) 3-OH-FAs are positively correlated with air temperature and relative humidity, and negatively correlated with wind speed at both ground level and 220 m (Fig. S2). Long-chain (C14–C18) 3-OH-FAs were negatively correlated with air temperature and wind speed at near ground. These findings align with previous study (Guan et al., 2014), which reported significant correlations between endotoxin levels and air temperature and RH. Moderate air temperature and RH are considered conducive to bacterial growth (Allen et al., 2011; Guan et al., 2014). During summer, the average air temperature (27.8 °C at 2 m, 25.7 °C at 220 m) and relative humidity (72 % at 2 m, 81 % at 220 m) were the highest, with relatively intense solar radiation (156 W m−2). These meteorological conditions were favorable for photochemical reactions to occur (Huo et al., 2024; Pavuluri et al., 2015). While the data alone cannot prove a direct photochemical production route, the coincidence between intense photochemistry and the increase in these short/mid-chain (C8–C13) 3-OH-FAs may point to photochemically driven secondary pathways of fatty acids that have been hypothesized in previous studies (Bikkina et al., 2019; Wakeham, 1999; Tyagi et al., 2015a).
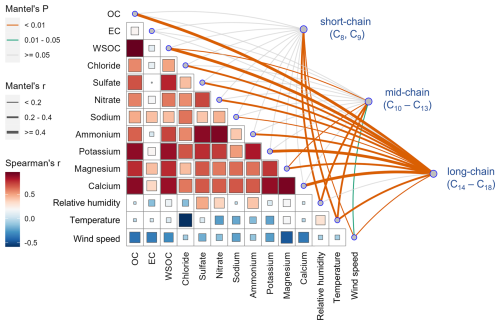
Figure 6Mantel test of 3-OH-FAs with meteorological conditions and chemical components of PM10. The square size scales with , and larger (smaller) squares indicate stronger (weaker) correlation.
Previous studies have shown that gaseous pollutants (O3, SO2, NO2, and CO) can affect bacterial diversity and richness (Yan et al., 2018; Qi et al., 2020; Dong et al., 2016), potentially leading to varying distributions of 3-OH-FAs and endotoxins. The endotoxin mass concentrations exhibited a similar trend with PM2.5, PM10, NO2 and CO (Fig. 3). Spearman's correlation analysis revealed significant positive correlations (p<0.01) between endotoxin mass concentrations, NO2, and CO near the ground (2 m) (Figs. S2, 7c, d), along with a significant negative correlation (p<0.05) with O3 (Figs. S2, 7b). These results are consistent with previous studies (Guan et al., 2014; Hwang and Park, 2019), suggesting that gaseous pollutants, including SO2, NOx and O3, may kill GNB, thereby increasing endotoxin levels as result of cell death. Exposure to high concentration of SO2 and NO2 significantly increases the fragility and disruption of bioaerosols such as pollen, and may possibly increase the incidence of allergic airway disease in sensitized individuals by facilitating the bioavailability of airborne bioaerosols (Ouyang et al., 2016). When exposed to higher O3, oxidation of the hydroxyl group in the 3-OH-FAs may turn fatty acid chain into carbonyl group, thus cause the decrease in detected 3-OH-FAs and estimated endotoxins (Liu et al., 2025). Atmospheric photodegradation of proteinaceous matter may also occur through reactions with O3, NO2, and the corresponding radicals, causing the damage of the main components of cell membranes (Green et al., 2013; Wang et al., 2019b; Xu et al., 2020).
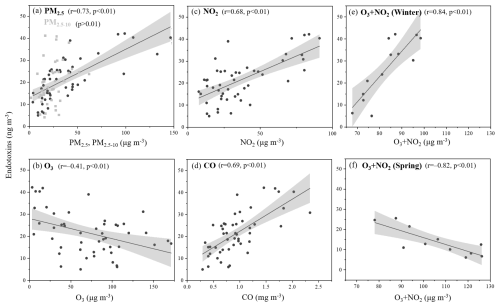
Figure 7Spearman's correlation analyses between mass concentrations of endotoxins and air pollutants. (a) PM2.5 and PM2.5–10; (b) O3; (c) NO2; (d) CO; (e–f) Total oxidants [O3 + NO2] in winter and spring.
Additionally, the combined concentration of O3 and NO2, defined as the total oxidants in previous studies, has been used to estimate atmospheric oxidizing capacity (Hu et al., 2016, 2017). Endotoxin mass concentrations were positively associated with total oxidants in winter but negatively associated in spring (Fig. 7e, f). These opposite signs most likely reflect season-specific controlling factors rather than a single universal mechanism. During winter the boundary layer experienced moderate oxidizing conditions (Ox = 68–98 µg m−3); such environments have been linked to oxidative stress and lysis of Gram-negative bacteria, which could increase aerosol-phase lipopolysaccharide (Hwang and Park, 2019; Mahapatra et al., 2018). In contrast, springtime Ox levels were even higher (78–126 µg m−3), yet endotoxin decreased. One plausible explanation is a shift in microbial community composition: oxidant-tolerant taxa may dominate, while overall richness declines (Yin et al., 2021), leading to lower net release of endotoxin. No significant correlations were found between endotoxins and total oxidants in summer (Ox = 92–199 µg m−3) or autumn (Ox = 69–140 µg m−3).
3.5.2 Effects of particulate pollutants
The endotoxin levels generally increased significantly under high PM pollution, showing a strong correlation with PM10 concentrations (Shen et al., 2019; Zhong et al., 2019). Previous studies demonstrated that 60 % of biological active endotoxins (measured by LAL method) were associated with coarse-mode particles (PM2.5–10) emitted from natural sources, including soil dust and vegetation. Meanwhile, 80 % of total endotoxins (measured by GC-MS method) were associated with fine particles (PM2.5) originating from anthropogenic combustion processes (Cheng et al., 2012; Seinfeld et al., 1998). The endotoxin mass concentrations measured by LC-MS in this study showed a similar temporal trend to PM2.5 and PM10 (Fig. 3a, b). Here, airborne endotoxin levels were significantly and positively correlated with PM2.5 (p<0.01), while no significant correlation with PM2.5–10 was observed (Fig. 7a). This result indicates that total airborne endotoxins are most likely related to anthropogenic sources.
Spearman correlation analysis (Fig. S2) and Mantel test (Fig. 6) revealed that short-chain 3-OH-FAs (C10–C13) at 220 m were mainly associated with meteorological conditions, WSOC, and Mg2+, reflecting their emissions from natural sources and secondary transformation during transport. According to Fig. S2, endotoxins at the near ground level (2 m) attributed by long-chain 3-OH-FAs (C15–C18) are correlated (p≤0.05) with chloride (Cl−) and sodium (Na+). Na+ is generally used to characterize the impact of marine aerosols, as their usual source is sea salt, with mass ratio of 1.8 (Martens et al., 1973; Wang and Shooter, 2001). The mass ratio of (2.9 ± 3.5) at the near ground level was much higher than that of sea salt aerosols. This result indicated the impact of anthropogenic emissions, especially combustion emissions (Wang et al., 2005, 2012), on those 3-OH-FAs. Moreover, the positive correlation (p≤0.05) of endotoxins with secondary inorganic species (SO, NO, NH) and crustal species (Ca2+, Mg2+) separately, suggesting the possible sources of 3-OH-FAs (C10–C18) from atmospheric secondary processes (Jung et al., 2009) and soil dust (Huang et al., 2006). This finding suggests that the emissions of long-chain 3-OH-FAs are influenced by multiple factors, including natural sources such as soil and anthropogenic sources such as combustion (Zhong et al., 2019). Composting facilities, intensive farming (e.g., cattle, swine and poultry), and wastewater treatment facilities are all potential anthropogenic sources of airborne endotoxins (Hu et al., 2020a; Rolph et al., 2018). According to Mahapatra et al. (2018), certain species of GNB may release more endotoxins when disrupted by anthropogenic contamination, or the source of higher pollution may themselves be significant sources of endotoxins.
3.6 Sources of 3-OH-FAs in Tianjin urban aerosols
Based on the PMF factor profiles, we identified 5 source factors (microorganisms, secondary nitrate formation process, secondary sulfate formation process, biomass burning, and dust) of PM10 from Tianjin.
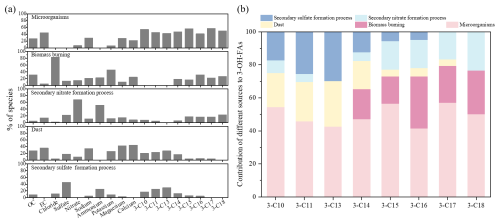
Figure 8Source apportionment of 3-OH-FAs in Tianjin urban aerosols. (a) Factor profiles for the 5-factor solution from PMF analysis. (b) Contribution of different sources to 3-OH-FAs.
Factor 1 was highly loaded on 3-OH-FAs, thus was mainly identified as microbial sources. Factor 2 was characterized by high loading of K+ and Cl−, which were mainly emitted from biomass burning (Srivastava et al., 2021; Hays et al., 2005). Factor 3 was heavily weighted by NH and NO, which was typical of secondary nitrate formation process (Wang et al., 2019a). Factor 4 presented high loaded of two crustal elements Ca2+ and Mg2+, mainly emitted from dust sources (Huang et al., 2016). Factor 5 indicated secondary sulfate formation process, which was identified by high concentrations of NH and SO.
The contributions of different sources to 3-OH-FAs in PM10 were estimated (Fig. 8). Mid-chain 3-OH-FAs (C10, C11, C13) were predominantly contributed by microorganisms (42.6 %–54.4 %), and also associated with secondary sulfate formation process (17.3 %–29.7 %) and dust sources (20.6 %–27.7 %). This result indicates substantial inputs from microorganisms associated with soil dust resuspension and aging of primary aerosols. Long-chain 3-OH-FAs (C14–C18) were likewise primarily originated from microorganisms (41.5 %–57.1 %), with notable contribution from biomass burning (16.5 %–31.4 %) and associated with secondary nitrate formation process (5.2 %–23.2 %). Overall, microorganisms represent the primary biological source of both mid- and long-chain 3-OH-FAs (C10–C18), with additional contributions from secondary processes and biomass burning.
The different behaviors of sulfate and nitrate further highlight source-specific processes. Sulfate-related processes are mainly driven by photochemical oxidation, aqueous phase or cloud chemistry at a regional scale (Slowik et al., 2010; Zheng et al., 2015), whereas nitrate-related processes are typically controlled by gas-particle partitioning, rapid secondary photochemical formation at a local scale (Vispute et al., 2025; Guo et al., 2010; Hu et al., 2016). Furthermore, the higher relative contributions of mid-chain 3-OH-FAs at 220 m (Fig. 4b) support their attribution to regional transport processes, similar to sulfate. Conversely, the enrichment of long-chain homologues at 2 m (Fig. 4b), together with their association with nitrate formation process, suggests a stronger influence from local combustion and secondary formation. In general, the PMF results, consistent with the results of Mantel test, indicate that mid-chain 3-OH-FAs are mainly influenced by natural sources and secondary processes, representing aged, regionally transported aerosols. In contrast, long-chain homologues show stronger links to anthropogenic emissions, particularly biomass burning and local secondary formation, reflecting fresher and more locally derived contributions.
This study obtained the seasonal, altitudinal, and diurnal dynamics of airborne endotoxins in urban Tianjin through comprehensive quantification of 3-OH-FAs (C10–C18) in PM10. Airborne endotoxins exhibited clear altitude-dependent gradients (2 m vs. 220 m) and distinct temporal variability, with winter maxima reflecting combined contributions of near-ground natural emissions (e.g., soil microbial activity) and anthropogenic inputs (e.g., biomass burning). Source apportionment further revealed a chain-length-specific patterns: mid-chain homologues (C10–C13) were primarily linked to microorganisms and regional secondary formation, while long-chain species (C14–C18) showed stronger associations with biomass burning and local secondary processes. This differentiation was consistent with the vertical distribution of 3-OH-FAs, as the higher relative abundance of mid-chain homologues at 220 m suggested regional transport, whereas the enrichment of long-chain species at the ground level reflected stronger local influences.
Overall, total endotoxin levels in urban Tianjin were comparable to those reported in industrialized regions. The pronounced vertical attenuation underscores the localized impact of ground-level emissions. Bioactive endotoxin concentrations generally remained below DECOS thresholds, except during winter peaks, indicating a seasonal pattern of potential health risks. The opposing correlation between endotoxin levels and total oxidants (O3 + NO2) in winter and spring highlights atmospheric oxidative capacity as a critical modulator of endotoxin activity. This study emphasizes the dominant role of microbial and secondary sources for mid-chain homologues, contrasted with the stronger influence of anthropogenic combustion on long-chain species. Future work should focus on quantifying these contributions, clarifying the dynamics between total and bioactive endotoxins, and identifying the bacterial taxa responsible for airborne endotoxins in typical atmospheric environments.
The dataset for this paper is available upon request from the corresponding authors (huwei@tju.edu.cn; fupingqing@tju.edu.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-14513-2025-supplement.
PF and WH designed the entire study and the experiments. WZ visualized the data, and determined the structure of the article. WZ, MN, QZ, and NA executed experiments. WZ analyzed the data. WZ and WH wrote the draft manuscript with input from all the authors. All the authors discussed, edited, and approved the final manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We thank Yajie Wang and Donghuan Zhang for their help in sampling.
This research has been supported by the National Natural Science Foundation of China (grant no. 42130513).
This paper was edited by Theodora Nah and reviewed by three anonymous referees.
Abdel Hameed, A. A., Khoder, M. I., Yuosra, S., Osman, A. M., and Ghanem, S.: Diurnal distribution of airborne bacteria and fungi in the atmosphere of Helwan area, Egypt, Sci. Total Environ., 407, 6217–6222, https://doi.org/10.1016/j.scitotenv.2009.08.028, 2009.
Allen, J., Bartlett, K., Graham, M., and Jackson, P.: Ambient concentrations of airborne endotoxin in two cities in the interior of British Columbia, Canada, J. Environ. Monit., 13, 631–640, https://doi.org/10.1039/c0em00235f, 2011.
Amato, P., Parazols, M., Sancelme, M., Laj, P., Mailhot, G., and Delort, A. M.: Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dome: major groups and growth abilities at low temperatures, FEMS Microbiol. Ecol., 59, 242–254, https://doi.org/10.1111/j.1574-6941.2006.00199.x, 2007.
Amin, H., Santl-Temkiv, T., Cramer, C., Finster, K., Real, F. G., Gislason, T., Holm, M., Janson, C., Jogi, N. O., Jogi, R., Malinovschi, A., Marshall, I. P. G., Modig, L., Norback, D., Shigdel, R., Sigsgaard, T., Svanes, C., Thorarinsdottir, H., Wouters, I. M., Schlunssen, V., and Bertelsen, R. J.: Indoor Airborne Microbiome and Endotoxin: Meteorological Events and Occupant Characteristics Are Important Determinants, Environ. Sci. Technol., 57, 11750–11766, https://doi.org/10.1021/acs.est.3c01616, 2023.
Balkwill, D. L., Leach, F. R., Wilson, J. T., McNabb, J. F., and White, D. C.: Equivalence of microbial biomass measures based on membrane lipid and cell wall components, adenosine triphosphate, and direct counts in subsurface aquifer sediments, Microb. Ecol., 16, 73–84, https://doi.org/10.1007/BF02097406, 1988.
Bhandari, S., Arub, Z., Habib, G., Apte, J. S., and Hildebrandt Ruiz, L.: Source apportionment resolved by time of day for improved deconvolution of primary source contributions to air pollution, Atmos. Meas. Tech., 15, 6051–6074, https://doi.org/10.5194/amt-15-6051-2022, 2022.
Bikkina, P., Kawamura, K., Bikkina, S., and Tanaka, N.: Hydroxy Fatty Acids in Rainwater and Aerosols from Suburban Tokyo in Central Japan: The Impact of Long-Range Transport of Soil Microbes and Plant Waxes, ACS Earth Space Chem., 5, 257–267, https://doi.org/10.1021/acsearthspacechem.0c00275, 2021a.
Bikkina, P., Kawamura, K., Bikkina, S., and Yamaguchi, H.: Decadal Variations in Hydroxy Fatty Acids Over Chichijima Island in the North Pacific: Long-Term Seasonal Variability in Plant and Microbial Markers, J. Geophys. Res. Atmos., 126, e2020JD033347, https://doi.org/10.1029/2020JD033347, 2021b.
Bikkina, P., Kawamura, K., Bikkina, S., Kunwar, B., Tanaka, K., and Suzuki, K.: Hydroxy Fatty Acids in Remote Marine Aerosols over the Pacific Ocean: Impact of Biological Activity and Wind Speed, ACS Earth Space Chem., 3, 366–379, https://doi.org/10.1021/acsearthspacechem.8b00161, 2019.
Binding, N., Jaschinski, S., Werlich, S., Bletz, S., and Witting, U.: Quantification of bacterial lipopolysaccharides (endotoxin) by GC-MS determination of 3-hydroxy fatty acids, J. Environ. Monit., 6, 65–70, https://doi.org/10.1039/b309237b, 2004.
Bonfantine, K., Vuono, D. C., Christner, B. C., Moore, R., Fox, S., Dean, T., Betancourt, D., Watts, A., and Kobziar, L. N.: Evidence for Wildland Fire Smoke Transport of Microbes From Terrestrial Sources to the Atmosphere and Back, J. Geophys. Res. Biogeosci., 129, e2024JG008236, https://doi.org/10.1029/2024JG008236, 2024.
Brooks, J. P., Tanner, B. D., Gerba, C. P., and Pepper, I. L.: The measurement of aerosolized endotoxin from land application of Class B biosolids in Southeast Arizona, Can. J. Microbiol., 52, 150–156, https://doi.org/10.1139/w05-115, 2006.
Carty, C. L., Gehring, U., Cyrys, J., Bischof, W., and Heinrich, J.: Seasonal variability of endotoxin in ambient fine particulate matter, J. Environ. Monit., 5, 953–958, https://doi.org/10.1039/b308488d, 2003.
Cheng, J. Y. W., Hui, E. L. C., and Lau, A. P. S.: Bioactive and total endotoxins in atmospheric aerosols in the Pearl River Delta region, China, Atmos. Environ., 47, 3–11, https://doi.org/10.1016/j.atmosenv.2011.11.055, 2012.
DECOS: Endotoxins. Health-based recommended occupational exposure limit, The Hague: Health Council of the Netherlands, 18, publication no. 2010/2004OSH, 2010.
de Rooij, M. M., Heederik, D. J., Borlee, F., Hoek, G., and Wouters, I. M.: Spatial and temporal variation in endotoxin and PM10 concentrations in ambient air in a livestock dense area, Environ. Res., 153, 161–170, https://doi.org/10.1016/j.envres.2016.12.004, 2017.
Dong, L., Qi, J., Shao, C., Zhong, X., Gao, D., Cao, W., Gao, J., Bai, R., Long, G., and Chu, C.: Concentration and size distribution of total airborne microbes in hazy and foggy weather, Sci. Total Environ., 541, 1011–1018, https://doi.org/10.1016/j.scitotenv.2015.10.001, 2016.
Gabriel, M. F., Paciência, I., Felgueiras, F., Cavaleiro Rufo, J., Castro Mendes, F., Farraia, M., Mourão, Z., Moreira, A., and de Oliveira Fernandes, E.: Environmental quality in primary schools and related health effects in children. An overview of assessments conducted in the Northern Portugal, Energy Build., 250, 111305, https://doi.org/10.1016/j.enbuild.2021.111305, 2021.
Fan, M. Y., Zhang, Y. L., Hong, Y. H., Lin, Y. C., Zhao, Z. Y., Cao, F., Sun, Y. L., Guo, H., and Fu, P. Q.: Vertical Differences of Nitrate Sources in Urban Boundary Layer Based on Tower Measurements, Environ. Sci. Technol. Lett., 9, 906–912, https://doi.org/10.1021/acs.estlett.2c00600, 2022.
Farokhi, A., Heederik, D., and Smit, L. A. M.: Respiratory health effects of exposure to low levels of airborne endotoxin – a systematic review, Environ. Health, 17, 14, https://doi.org/10.1186/s12940-018-0360-7, 2018.
Green, M. C., Fedorov, D. G., Kitaura, K., Francisco, J. S., and Slipchenko, L. V.: Open-shell pair interaction energy decomposition analysis (PIEDA): formulation and application to the hydrogen abstraction in tripeptides, J. Chem. Phys., 138, 074111, https://doi.org/10.1063/1.4790616, 2013.
Guan, T. J., Yao, M. S., Wang, J. X., Fang, Y. H., Hu, S. H., Wang, Y., Dutta, A., Yang, J. N., Wu, Y. S., Hu, M., and Zhu, T.: Airborne endotoxin in fine particulate matter in Beijing, Atmos. Environ., 97, 35–42, https://doi.org/10.1016/j.atmosenv.2014.08.005, 2014.
Guo, S., Hu, M., Wang, Z. B., Slanina, J., and Zhao, Y. L.: Size-resolved aerosol water-soluble ionic compositions in the summer of Beijing: implication of regional secondary formation, Atmos. Chem. Phys., 10, 947–959, https://doi.org/10.5194/acp-10-947-2010, 2010.
Guo, Z., Wang, Z., Qian, L., Zhao, Z., Zhang, C., Fu, Y., Li, J., Zhang, C., Lu, B., and Qian, J.: Biological and chemical compositions of atmospheric particulate matter during hazardous haze days in Beijing, Environ. Sci. Pollut. Res. Int., 25, 34540–34549, https://doi.org/10.1007/s11356-018-3355-6, 2018.
Hays, M. D., Fine, P. M., Geron, C. D., Kleeman, M. J., and Gullett, B. K.: Open burning of agricultural biomass: Physical and chemical properties of particle-phase emissions, Atmos. Environ., 39, 6747–6764, https://doi.org/10.1016/j.atmosenv.2005.07.072, 2005.
Heederik, D. and Douwes, J.: Towards an occupational exposure limit for endotoxins, Ann. Agric. Environ. Med., 4, 17–19, 1997.
Hines, C. J., Waters, M. A., Larsson, L., Petersen, M. R., Saraf, A., and Milton, D. K.: Characterization of endotoxin and 3-hydroxy fatty acid levels in air and settled dust from commercial aircraft cabins, Indoor Air, 13, 166–173, https://doi.org/10.1034/j.1600-0668.2003.00175.x, 2003.
Hu, W., Hu, M., Hu, W., Jimenez, J. L., Yuan, B., Chen, W., Wang, M., Wu, Y., Chen, C., Wang, Z., Peng, J., Zeng, L., and Shao, M.: Chemical composition, sources, and aging process of submicron aerosols in Beijing: Contrast between summer and winter, J. Geophys. Res. Atmos., 121, 1955–1977, https://doi.org/10.1002/2015JD024020, 2016.
Hu, W., Hu, M., Hu, W.-W., Zheng, J., Chen, C., Wu, Y., and Guo, S.: Seasonal variations in high time-resolved chemical compositions, sources, and evolution of atmospheric submicron aerosols in the megacity Beijing, Atmos. Chem. Phys., 17, 9979–10000, https://doi.org/10.5194/acp-17-9979-2017, 2017.
Hu, W., Wang, Z. H., Huang, S., Ren, L. J., Yue, S. Y., Li, P., Xie, Q. R., Zhao, W. Y., Wei, L. F., Ren, H., Wu, L. B., Deng, J. J., and Fu, P. Q.: Biological Aerosol Particles in Polluted Regions, Curr. Pollut. Rep., 6, 65–89, https://doi.org/10.1007/s40726-020-00138-4, 2020a.
Hu, W., Liu, D. D., Su, S. H., Ren, L. J., Ren, H., Wei, L. F., Yue, S. Y., Xie, Q. R., Zhang, Z. M., Wang, Z. H., Yang, N., Wu, L. B., Deng, J. J., Qi, Y. L., and Fu, P. Q.: Photochemical Degradation of Organic Matter in the Atmosphere, Adv. Sustain. Syst., 5, 2100027, https://doi.org/10.1002/adsu.202100027, 2021.
Hu, Z., Liu, H., Zhang, H., Zhang, X., Zhou, M., Lou, L., Zheng, P., Xi, C., and Hu, B.: Temporal discrepancy of airborne total bacteria and pathogenic bacteria between day and night, Environ. Res., 186, 109540, https://doi.org/10.1016/j.envres.2020.109540, 2020b.
Huang, X., Liu, Z., Zhang, J., Wen, T., Ji, D., and Wang, Y.: Seasonal variation and secondary formation of size-segregated aerosol water-soluble inorganic ions during pollution episodes in Beijing, Atmos. Res., 168, 70–79, https://doi.org/10.1016/j.atmosres.2015.08.021, 2016.
Huang, X.-F., Yu, J. Z., He, L.-Y., and Hu, M.: Size distribution characteristics of elemental carbon emitted from Chinese vehicles: results of a tunnel study and atmospheric implications, Environ. Sci. Technol., 40, 5355–5360, https://doi.org/10.1021/es0607281, 2006.
Huo, Y. X., Lyu, X., Yao, D. W., Zhou, B. N., Yuan, Q., Lee, S. C., and Guo, H.: Exploring the Formation of High Levels of Hydroxyl Dicarboxylic Acids at an Urban Background Site in South China, J. Geophys. Res. Atmos., 129, e2023JD040096, https://doi.org/10.1029/2023JD040096, 2024.
Hwang, S., Kim, S.-Y., Choi, S., Lee, S., and Park, D.-U.: Correlation between levels of airborne endotoxin and heavy metals in subway environments in South Korea, Sci. Rep., 11, 17086, https://doi.org/10.1038/s41598-021-95860-4, 2021.
Hwang, S. H. and Park, D. U.: Ambient Endotoxin and Chemical Pollutant (PM2.5, PM10, and O3) Levels in South Korea, Aerosol Air Qual. Res., 19, 786–793, https://doi.org/10.4209/aaqr.2018.06.0235, 2019.
Jung, J., Lee, H., Kim, Y. J., Liu, X., Zhang, Y., Hu, M., and Sugimoto, N.: Optical properties of atmospheric aerosols obtained by in situ and remote measurements during 2006 Campaign of Air Quality Research in Beijing (CAREBeijing-2006), J. Geophys. Res. Atmos., 114, https://doi.org/10.1029/2008JD010337, 2009.
Kawamura, K., Ishimura, Y., and Yamazaki, K.: Four years' observations of terrestrial lipid class compounds in marine aerosols from the western North Pacific, Global Biogeochem. Cy., 17, 1003, https://doi.org/10.1029/2001gb001810, 2003.
Kobziar, L. N. and Thompson, G. R., 3rd: Wildfire smoke, a potential infectious agent, Science, 370, 1408–1410, https://doi.org/10.1126/science.abe8116, 2020.
Kobziar, L. N., Lampman, P., Tohidi, A., Kochanski, A. K., Cervantes, A., Hudak, A. T., McCarley, R., Gullett, B., Aurell, J., Moore, R., Vuono, D. C., Christner, B. C., Watts, A. C., Cronan, J., and Ottmar, R.: Bacterial Emission Factors: A Foundation for the Terrestrial-Atmospheric Modeling of Bacteria Aerosolized by Wildland Fires, Environ. Sci. Technol., 58, 2413–2422, https://doi.org/10.1021/acs.est.3c05142, 2024.
Laboha, P., Sychrova, E., Brozman, O., Sovadinova, I., Blahova, L., Prokes, R., Ondracek, J., and Babica, P.: Cyanobacteria, cyanotoxins and lipopolysaccharides in aerosols from inland freshwater bodies and their effects on human bronchial cells, Environ. Toxicol. Pharmacol., 98, 104073, https://doi.org/10.1016/j.etap.2023.104073, 2023.
Laitinen, S., Kangas, J., Husman, K., and Susitaival, P.: Evaluation of exposure to airborne bacterial endotoxins and peptidoglycans in selected work environments, Ann. Agric. Environ. Med., 8, 213–219, https://doi.org/10.1016/S0749-3797(00)00253-1, 2001.
Lang-Yona, N., Lehahn, Y., Herut, B., Burshtein, N., and Rudich, Y.: Marine aerosol as a possible source for endotoxins in coastal areas, Sci. Total Environ., 499, 311–318, https://doi.org/10.1016/j.scitotenv.2014.08.054, 2014.
Lee, A. K. Y., Chan, C. K., Fang, M., and Lau, A. P. S.: The 3-hydroxy fatty acids as biomarkers for quantification and characterization of endotoxins and Gram-negative bacteria in atmospheric aerosols in Hong Kong, Atmos. Environ., 38, 6307–6317, https://doi.org/10.1016/j.atmosenv.2004.08.013, 2004.
Lei, L., Sun, Y., Ouyang, B., Qiu, Y., Xie, C., Tang, G., Zhou, W., He, Y., Wang, Q., Cheng, X., Fu, P., and Wang, Z.: Vertical Distributions of Primary and Secondary Aerosols in Urban Boundary Layer: Insights into Sources, Chemistry, and Interaction with Meteorology, Environ. Sci. Technol., 55, 4542–4552, https://doi.org/10.1021/acs.est.1c00479, 2021.
Li, Y., Du, A., Lei, L., Sun, J., Li, Z., Zhang, Z., Wang, Q., Tang, G., Song, S., Wang, Z., Wang, Z., and Sun, Y.: Vertically Resolved Aerosol Chemistry in the Low Boundary Layer of Beijing in Summer, Environ. Sci. Technol., 56, 9312–9324, https://doi.org/10.1021/acs.est.2c02861, 2022.
Li, Z. Q., Guo, J. P., Ding, A. J., Liao, H., Liu, J. J., Sun, Y. L., Wang, T. J., Xue, H. W., Zhang, H. S., and Zhu, B.: Aerosol and boundary-layer interactions and impact on air quality, Natl. Sci. Rev., 4, 810–833, https://doi.org/10.1093/nsr/nwx117, 2017.
Liebers, V., Bruning, T., and Raulf-Heimsoth, M.: Occupational endotoxin-exposure and possible health effects on humans, Am. J. Ind. Med., 49, 474–491, https://doi.org/10.1002/ajim.20310, 2006.
Liebers, V., Raulf-Heimsoth, M., and Bruning, T.: Health effects due to endotoxin inhalation (review), Arch. Toxicol., 82, 203–210, https://doi.org/10.1007/s00204-008-0290-1, 2008.
Liebers, V., Bruning, T., and Raulf, M.: Occupational endotoxin exposure and health effects, Arch. Toxicol., 94, 3629–3644, https://doi.org/10.1007/s00204-020-02905-0, 2020.
Liu, H., Xing, Q., Zhu, C., Wang, Q., Lu, K., Guo, S., Wu, Z., Hu, M., Li, S.-M., and Yao, M.: Exposure to Endotoxin Oxidized by Atmospheric Ozone Greatly Enhances Anemia, Environ. Sci. Technol., 59, 7015–7027, https://doi.org/10.1021/acs.est.4c14589, 2025.
Lundin, J. I. and Checkoway, H.: Endotoxin and cancer, Environ. Health Perspect., 117, 1344–1350, https://doi.org/10.1289/ehp.0800439, 2009.
Madsen, A. M.: Airborne endotoxin in different background environments and seasons, Ann. Agric. Environ. Med., 13, 81–86, https://doi.org/10.1016/j.ijheh.2010.03.001, 2006.
Mahapatra, P. S., Jain, S., Shrestha, S., Senapati, S., and Puppala, S. P.: Ambient endotoxin in PM10 and association with inflammatory activity, air pollutants, and meteorology, in Chitwan, Nepal, Sci. Total Environ., 618, 1331–1342, https://doi.org/10.1016/j.scitotenv.2017.09.249, 2018.
Makkonen, U., Hellen, H., Anttila, P., and Ferm, M.: Size distribution and chemical composition of airborne particles in south-eastern Finland during different seasons and wildfire episodes in 2006, Sci. Total Environ., 408, 644–651, https://doi.org/10.1016/j.scitotenv.2009.10.050, 2010.
Martens, C. S., Wesolowski, J. J., Harriss, R. C., and Kaifer, R.: Chlorine loss from Puerto Rican and San Francisco Bay area marine aerosols, J. Geophys. Res., 78, 8778–8792, https://doi.org/10.1029/JC078i036p08778, 1973.
Mattsby-Baltzer, I., Lindgren, K., Lindholm, B., and Edebo, L.: Endotoxin shedding by enterobacteria: free and cell-bound endotoxin differ in Limulus activity, Infect. Immun., 59, 689–695, https://doi.org/10.1128/iai.59.2.689-695.1991, 1991.
Mims, F. M., Holben, B. N., Eck, T. F., Montgomery, B. C., and Grant, W. B.: Smoky skies, mosquitoes, and disease, Science, 276, 1774–1775, https://doi.org/10.1126/science.276.5320.1773c, 1997.
Moore, R. A., Bomar, C., Kobziar, L. N., and Christner, B. C.: Wildland fire as an atmospheric source of viable microbial aerosols and biological ice nucleating particles, ISME J., 15, 461–472, https://doi.org/10.1038/s41396-020-00788-8, 2021.
Moretti, S., Smets, W., Oerlemans, E., Blust, R., and Lebeer, S.: The abundance of urban endotoxins as measured with an impinger-based sampling strategy, Aerobiologia, 34, 487–496, https://doi.org/10.1007/s10453-018-9525-7, 2018.
Mueller-Anneling, L., Avol, E., Peters, J. M., and Thorne, P. S.: Ambient endotoxin concentrations in PM10 from Southern California, Environ. Health Perspect., 112, 583–588, https://doi.org/10.1289/ehp.6552, 2004.
Mukherjee, A. and Agrawal, M.: World air particulate matter: sources, distribution and health effects, Environ. Chem. Lett., 15, 283–309, https://doi.org/10.1007/s10311-017-0611-9, 2017.
Niu, M. T., An, N., Zhang, W. X., Hu, W., Fu, X. L., Kawamura, K., Feng, Y. Q., Zhu, Q. F., and Fu, P. Q.: Position-specific isomers of monohydroxy fatty acids in the land-atmosphere interface: identification and quantification, npj Clim. Atmos. Sci., 7, 75, https://doi.org/10.1038/s41612-024-00621-5, 2024.
Ormanova, G., Karaca, F., and Kononova, N.: Analysis of the impacts of atmospheric circulation patterns on the regional air quality over the geographical center of the Eurasian continent, Atmos. Res., 237, 104858, https://doi.org/10.1016/j.atmosres.2020.104858, 2020.
Ouyang, Y., Xu, Z., Fan, E., Li, Y., and Zhang, L.: Effect of nitrogen dioxide and sulfur dioxide on viability and morphology of oak pollen, Int. Forum Allergy Rhinol., 95–100, https://doi.org/10.1002/alr.21632, 2016.
Paatero, P. and Hopke, P. K.: Discarding or downweighting high-noise variables in factor analytic models, Anal. Chim. Acta, 490, 277–289, https://doi.org/10.1016/S0003-2670(02)01643-4, 2003.
Paatero, P. and Tapper, U.: Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values, Environmetrics, 5, 111–126, https://doi.org/10.1002/env.3170050203, 1994.
Paba, E., Chiominto, A., Marcelloni, A. M., Tombolini, F., Paci, E., and Tranfo, G.: Endotoxin Analysis: Correlation Between Biological and Chemical Methods, Biomed. J. Sci. Tech. Res., 19, https://doi.org/10.26717/BJSTR.2019.19.003339, 2019.
Park, J. H., Spiegelman, D. L., Burge, H. A., Gold, D. R., Chew, G. L., and Milton, D. K.: Longitudinal study of dust and airborne endotoxin in the home, Environ. Health Perspect., 108, 1023–1028, https://doi.org/10.1289/ehp.001081023, 2000.
Park, J. H., Szponar, B., Larsson, L., Gold, D. R., and Milton, D. K.: Characterization of lipopolysaccharides present in settled house dust, Appl. Environ. Microbiol., 70, 262–267, https://doi.org/10.1128/AEM.70.1.262-267.2004, 2004.
Parker, J. H., Smith, G. A., Fredrickson, H. L., Vestal, J. R., and White, D. C.: Sensitive assay, based on hydroxy fatty acids from lipopolysaccharide lipid A, for Gram-negative bacteria in sediments, Appl. Environ. Microbiol., 44, 1170–1177, https://doi.org/10.1128/aem.44.5.1170-1177.1982, 1982.
Pavuluri, C. M., Kawamura, K., Mihalopoulos, N., and Swaminathan, T.: Laboratory photochemical processing of aqueous aerosols: formation and degradation of dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls, Atmos. Chem. Phys., 15, 7999–8012, https://doi.org/10.5194/acp-15-7999-2015, 2015.
Petsch, D. and Anspach, F. B.: Endotoxin removal from protein solutions, J. Biotechnol., 76, 97–119, https://doi.org/10.1016/s0168-1656(99)00185-6, 2000.
Phiri, Y. V. A., Wu, M. M., Chen, Y. H., Zou, M. L., Jiang, C. B., Wu, C. D., Huang, H. C., Lung, S. C. C., Chien, L. C., Lo, Y. C., Lee, F. Y., and Chao, H. J.: Environmental determinants of household microbial and allergen levels in the Greater Taipei Area, Build. Environ., 230, 110003, https://doi.org/10.1016/j.buildenv.2023.110003, 2023.
Qi, Y., Li, Y., Xie, W., Lu, R., Mu, F., Bai, W., and Du, S.: Temporal-spatial variations of fungal composition in PM2.5 and source tracking of airborne fungi in mountainous and urban regions, Sci. Total Environ., 708, 135027, https://doi.org/10.1016/j.scitotenv.2019.135027, 2020.
Qiu, Y. M., Xie, Q. R., Wang, J. F., Xu, W. Q., Li, L. J., Wang, Q. Q., Zhao, J., Chen, Y. T., Chen, Y. F., Wu, Y. Z., Du, W., Zhou, W., Lee, J., Zhao, C. F., Ge, X. L., Fu, P. Q., Wang, Z. F., Worsnop, D. R., and Sun, Y. L.: Vertical Characterization and Source Apportionment of Water-Soluble Organic Aerosol with High-resolution Aerosol Mass Spectrometry in Beijing, China, ACS Earth Space Chem., 3, 273–284, https://doi.org/10.1021/acsearthspacechem.8b00155, 2019.
Reynolds, S. J., Milton, D. K., Heederik, D., Thorne, P. S., Donham, K. J., Croteau, E. A., Kelly, K. M., Douwes, J., Lewis, D., Whitmer, M., Connaughton, I., Koch, S., Malmberg, P., Larsson, B. M., Deddens, J., Saraf, A., and Larsson, L.: Interlaboratory evaluation of endotoxin analyses in agricultural dusts – comparison of LAL assay and mass spectrometry, J. Environ. Monit., 7, 1371–1377, https://doi.org/10.1039/b509256f, 2005.
Rietschel, E. T., Zähringer, U., Wollenweber, H. W., Miragliotta, G., Musehold, J., Lüderitz, T., and Schade, U.: Bacterial endotoxins: chemical structure and biologic activity, Am. J. Emerg. Med., 2, 60–69, https://doi.org/10.1016/0735-6757(84)90110-4, 1984.
Rolph, C. A., Gwyther, C. L., Tyrrel, S. F., Nasir, Z. A., Drew, G. H., Jackson, S. K., Khera, S., Hayes, E. T., Williams, B., Bennett, A., Collins, S., Walsh, K., Kinnersley, R., and Gladding, T. L.: Sources of Airborne Endotoxins in Ambient Air and Exposure of Nearby Communities – A Review, Atmosphere, 9, 375, https://doi.org/10.3390/atmos9100375, 2018.
Rylander, R.: Endotoxin in the environment – exposure and effects, J. Endotoxin Res., 8, 241–252, https://doi.org/10.1179/096805102125000452, 2002.
Saraf, A., Larsson, L., Burge, H., and Milton, D.: Quantification of ergosterol and 3-hydroxy fatty acids in settled house dust by gas chromatography-mass spectrometry: comparison with fungal culture and determination of endotoxin by a Limulus amebocyte lysate assay, Appl. Environ. Microbiol., 63, 2554–2559, https://doi.org/10.1128/aem.63.7.2554-2559.1997, 1997.
Saraf, A., Park, J. H., Milton, D. K., and Larsson, L.: Use of quadrupole GC-MS and ion trap GC-MS-MS for determining 3-hydroxy fatty acids in settled house dust: relation to endotoxin activity, J. Environ. Monit., 1, 163–168, https://doi.org/10.1039/a809019j, 1999.
Schneier, M., Razdan, S., Miller, A. M., Briceno, M. E., and Barua, S.: Current technologies to endotoxin detection and removal for biopharmaceutical purification, Biotechnol. Bioeng., 117, 2588–2609, https://doi.org/10.1002/bit.27362, 2020.
Schwarz, H., Gornicec, J., Neuper, T., Parigiani, M. A., Wallner, M., Duschl, A., and Horejs-Hoeck, J.: Biological Activity of Masked Endotoxin, Sci. Rep., 7, 44750, https://doi.org/10.1038/srep44750, 2017.
Seinfeld, J. H., Pandis, S. N., and Noone, K. J.: Atmospheric Chemistry and Physics, Physics Today, John Wiley & Sons, Inc, The United States of America, ISBN 0-471-17815-2, 1998.
Shen, F., Niu, M., Zhou, F., Wu, Y., and Zhu, T.: Culturability, metabolic activity and composition of ambient bacterial aerosols in a surrogate lung fluid, Sci. Total Environ., 690, 76–84, https://doi.org/10.1016/j.scitotenv.2019.07.005, 2019.
Simoneit, B. R. T., Kobayashi, M., Mochida, M., Kawamura, K., Lee, M., Lim, H. J., Turpin, B. J., and Komazaki, Y.: Composition and major sources of organic compounds of aerosol particulate matter sampled during the ACE-Asia campaign, J. Geophys. Res. Atmos., 109, D19S10, https://doi.org/10.1029/2004jd004598, 2004.
Slowik, J. G., Stroud, C., Bottenheim, J. W., Brickell, P. C., Chang, R. Y.-W., Liggio, J., Makar, P. A., Martin, R. V., Moran, M. D., Shantz, N. C., Sjostedt, S. J., van Donkelaar, A., Vlasenko, A., Wiebe, H. A., Xia, A. G., Zhang, J., Leaitch, W. R., and Abbatt, J. P. D.: Characterization of a large biogenic secondary organic aerosol event from eastern Canadian forests, Atmos. Chem. Phys., 10, 2825–2845, https://doi.org/10.5194/acp-10-2825-2010, 2010.
Spaan, S., Wouters, I. M., Oosting, I., Doekes, G., and Heederik, D.: Exposure to inhalable dust and endotoxins in agricultural industries, J. Environ. Monit., 8, 63–72, https://doi.org/10.1039/b509838f, 2006.
Srivastava, D., Xu, J., Vu, T. V., Liu, D., Li, L., Fu, P., Hou, S., Moreno Palmerola, N., Shi, Z., and Harrison, R. M.: Insight into PM2.5 sources by applying positive matrix factorization (PMF) at urban and rural sites of Beijing, Atmos. Chem. Phys., 21, 14703–14724, https://doi.org/10.5194/acp-21-14703-2021, 2021.
Stein, A. F., Draxler, R. R., Rolph, G. D., Stunder, B. J. B., Cohen, M. D., and Ngan, F.: NOAA's HYSPLIT Atmospheric Transport and Dispersion Modeling System, Bull. Am. Meteorol. Soc., 96, 2059–2077, https://doi.org/10.1175/BAMS-D-14-00110.1, 2015.
Traversi, D., Alessandria, L., Schiliro, T., Chiado Piat, S., and Gilli, G.: Meteo-climatic conditions influence the contribution of endotoxins to PM10 in an urban polluted environment, J. Environ. Monit., 12, 484–490, https://doi.org/10.1039/b913314c, 2010.
Tyagi, P., Ishimura, Y., and Kawamura, K.: Hydroxy fatty acids in marine aerosols as microbial tracers: 4-year study on β- and ω-hydroxy fatty acids from remote Chichijima Island in the western North Pacific, Atmos. Environ., 115, 89–100, https://doi.org/10.1016/j.atmosenv.2015.05.038, 2015a.
Tyagi, P., Yamamoto, S., and Kawamura, K.: Hydroxy fatty acids in fresh snow samples from northern Japan: long-range atmospheric transport of Gram-negative bacteria by Asian winter monsoon, Biogeosciences, 12, 7071–7080, https://doi.org/10.5194/bg-12-7071-2015, 2015b.
Tyagi, P., Kawamura, K., Fu, P. Q., Bikkina, S., Kanaya, Y., and Wang, Z. F.: Impact of biomass burning on soil microorganisms and plant metabolites: A view from molecular distributions of atmospheric hydroxy fatty acids over Mount Tai, J. Geophys. Res. Biogeosci., 121, 2684–2699, https://doi.org/10.1002/2016jg003324, 2016.
Tyagi, P., Kawamura, K., Kariya, T., Bikkina, S., Fu, P. Q., and Lee, M.: Tracing atmospheric transport of soil microorganisms and higher plant waxes in the East Asian outflow to the North Pacific Rim by using hydroxy fatty acids: Year-round observations at Gosan, Jeju Island, J. Geophys. Res. Atmos., 122, 4112–4131, https://doi.org/10.1002/2016jd025496, 2017.
Uhlig, S., Negard, M., Heldal, K. K., Straumfors, A., Madso, L., Bakke, B., and Eduard, W.: Profiling of 3-hydroxy fatty acids as environmental markers of endotoxin using liquid chromatography coupled to tandem mass spectrometry, J. Chromatogr. A, 1434, 119–126, https://doi.org/10.1016/j.chroma.2016.01.038, 2016.
Vispute, A. S., Acharja, P., Gosavi, S. W., Govardhan, G., Ruge, V., Patil, M. N., Dharmaraj, T., and Ghude, S. D.: Source characteristics of non-refractory particulate matter (NR-PM1) using high-resolution time-of-flight aerosol mass spectrometric (HR-ToF-AMS) measurements in the urban industrial city in India, Atmos. Environ., 351, 121186, https://doi.org/10.1016/j.atmosenv.2025.121186, 2025.
Wakeham, S. G.: Monocarboxylic, dicarboxylic and hydroxy acids released by sequential treatments of suspended particles and sediments of the Black Sea, Org. Geochem., 30, 1059–1074, https://doi.org/10.1016/S0146-6380(99)00084-4, 1999.
Wakeham, S. G., Pease, T. K., and Benner, R.: Hydroxy fatty acids in marine dissolved organic matter as indicators of bacterial membrane material, Org. Geochem., 34, 857–868, https://doi.org/10.1016/S0146-6380(02)00189-4, 2003.
Wang, H. and Shooter, D.: Water soluble ions of atmospheric aerosols in three New Zealand cities: seasonal changes and sources, Atmos. Environ., 35, 6031–6040, https://doi.org/10.1016/S1352-2310(01)00437-X, 2001.
Wang, H., Zhu, B., Shen, L., and Kang, H.: Size distributions of aerosol and water-soluble ions in Nanjing during a crop residual burning event, J. Environ. Sci., 24, 1457–1465, https://doi.org/10.1016/S1001-0742(11)60968-6, 2012.
Wang, Q., Huang, X. H. H., Tam, F. C. V., Zhang, X., Liu, K. M., Yeung, C., Feng, Y., Cheng, Y. Y., Wong, Y. K., Ng, W. M., Wu, C., Zhang, Q., Zhang, T., Lau, N. T., Yuan, Z., Lau, A. K. H., and Yu, J. Z.: Source apportionment of fine particulate matter in Macao, China with and without organic tracers: A comparative study using positive matrix factorization, Atmos. Environ., 198, 183–193, https://doi.org/10.1016/j.atmosenv.2018.10.057, 2019a.
Wang, S., Song, T., Shiraiwa, M., Song, J., Ren, H., Ren, L., Wei, L., Sun, Y., Zhang, Y., Fu, P., and Lai, S.: Occurrence of Aerosol Proteinaceous Matter in Urban Beijing: An Investigation on Composition, Sources, and Atmospheric Processes During the “APEC Blue” Period, Environ. Sci. Technol., 53, 7380–7390, https://doi.org/10.1021/acs.est.9b00726, 2019b.
Wang, Y., Zhuang, G., Tang, A., Yuan, H., Sun, Y., Chen, S., and Zheng, A.: The ion chemistry and the source of PM2.5 aerosol in Beijing, Atmos. Environ., 39, 3771–3784, https://doi.org/10.1016/j.atmosenv.2005.03.013, 2005.
Wollenweber, H.-W. and Rietschel, E. T.: Analysis of lipopolysaccharide (lipid A) fatty acids, J. Microbiol. Meth., 11, 195–211, https://doi.org/10.1016/0167-7012(90)90056-c, 1990.
Xiao, Y. T., Yang, J. B., Cui, L. H., Deng, J. J., Fu, P. Q., and Zhu, J. L.: Weakened Sea-Land Breeze in a Coastal Megacity Driven by Urbanization and Ocean Warming, Earths Future, 11, e2022EF003341, https://doi.org/10.1029/2022EF003341, 2023.
Xu, Y., Xiao, H., Wu, D., and Long, C.: Abiotic and Biological Degradation of Atmospheric Proteinaceous Matter Can Contribute Significantly to Dissolved Amino Acids in Wet Deposition, Environ. Sci. Technol., 54, 6551–6561, https://doi.org/10.1021/acs.est.0c00421, 2020.
Xue, T., Wang, R., Wang, M., Wang, Y., Tong, D., Meng, X., Huang, C., Ai, S., Li, F., Cao, J., Tong, M., Ni, X., Liu, H., Deng, J., Lu, H., Wan, W., Gong, J., Zhang, S., and Zhu, T.: Health benefits from the rapid reduction in ambient exposure to air pollutants after China's clean air actions: progress in efficacy and geographic equality, Natl. Sci. Rev., 11, nwad263, https://doi.org/10.1093/nsr/nwad263, 2024.
Yan, D., Zhang, T., Su, J., Zhao, L. L., Wang, H., Fang, X. M., Zhang, Y. Q., Liu, H. Y., and Yu, L. Y.: Structural Variation in the Bacterial Community Associated with Airborne Particulate Matter in Beijing, China, during Hazy and Nonhazy Days, Appl. Environ. Microbiol., 84, e00004-00018, https://doi.org/10.1128/AEM.00004-18, 2018.
Yang, T., Li, H., Xu, W., Song, Y., Xu, L., Wang, H., Wang, F., Sun, Y., Wang, Z., and Fu, P.: Strong Impacts of Regional Atmospheric Transport on the Vertical Distribution of Aerosol Ammonium over Beijing, Environ. Sci. Technol. Lett., 11, 29–34, https://doi.org/10.1021/acs.estlett.3c00791, 2023.
Yao, Q., Ma, Z., Liu, J., Qiu, Y., Hao, T., Yao, L., Ding, J., Tang, Y., Cai, Z., and Han, S.: Effects of Meteorological Factors on the Vertical Distribution of Peroxyacetyl Nitrate in Autumn in Tianjin, Aerosol Air Qual. Res., 22, 220226, https://doi.org/10.4209/aaqr.220226, 2022.
Yin, Y., Qi, J., Gong, J., and Gao, D.: Distribution of bacterial concentration and viability in atmospheric aerosols under various weather conditions in the coastal region of China, Sci. Total Environ., 795, 148713, https://doi.org/10.1016/j.scitotenv.2021.148713, 2021.
Yin, Z., Wan, Y., Zhang, Y., and Wang, H.: Why super sandstorm 2021 in North China?, Natl. Sci. Rev., 9, nwab165, https://doi.org/10.1093/nsr/nwab165, 2022.
Zelles, L.: Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review, Biol. Fertil. Soils, 29, 111–129, https://doi.org/10.1007/s003740050533, 1999.
Zhang, T., Lui, K. H., Ho, S. S. H., Chen, J., Chuang, H.-C., and Ho, K. F.: Characterization of airborne endotoxin in personal exposure to fine particulate matter (PM2.5) and bioreactivity for elderly residents in Hong Kong, Ecotoxicol. Environ. Saf., 280, 116530, https://doi.org/10.1016/j.ecoenv.2024.116530, 2024.
Zhang, T. Y., Li, S., Zhu, Q. F., Wang, Q., Hussain, D., and Feng, Y. Q.: Derivatization for liquid chromatography-electrospray ionization-mass spectrometry analysis of small-molecular weight compounds, TrAC Trends Anal. Chem., 119, 115608, https://doi.org/10.1016/j.trac.2019.07.019, 2019.
Zheng, B., Zhang, Q., Zhang, Y., He, K. B., Wang, K., Zheng, G. J., Duan, F. K., Ma, Y. L., and Kimoto, T.: Heterogeneous chemistry: a mechanism missing in current models to explain secondary inorganic aerosol formation during the January 2013 haze episode in North China, Atmos. Chem. Phys., 15, 2031–2049, https://doi.org/10.5194/acp-15-2031-2015, 2015.
Zhong, S., Zhang, L., Jiang, X., and Gao, P.: Comparison of chemical composition and airborne bacterial community structure in PM2.5 during haze and non-haze days in the winter in Guilin, China, Sci. Total Environ., 655, 202–210, https://doi.org/10.1016/j.scitotenv.2018.11.268, 2019.
Zhu, Q. F., An, N., and Feng, Y. Q.: In-Depth Annotation Strategy of Saturated Hydroxy Fatty Acids Based on Their Chromatographic Retention Behaviors and MS Fragmentation Patterns, Anal. Chem., 92, 14528–14535, https://doi.org/10.1021/acs.analchem.0c02719, 2020.




