the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mechanistic insights into nitric acid-enhanced iodic acid particle nucleation in the upper troposphere and lower stratosphere
Jing Li
Fengyang Bai
Qishen Huang
Pai Liu
Xiucong Deng
Yunhong Zhang
In the upper troposphere and lower stratosphere (UTLS), new particles frequently form to seed cloud condensation nuclei (CCN), thereby affecting radiative forcing and global climate. Iodic acid (IA) particles have been widely detected in the UTLS; however, how they form is still largely unknown. Given the abundance of nitric acid (NA) and ammonia (NH3) in the UTLS and their nucleation potential, we explore the influence of NA and NH3 on IA nucleation by quantum chemical calculations and cluster dynamics simulations. The structural analysis indicates that NA and NH3 can cluster with IA via hydrogen bonds, halogen bonds, and electrostatic attractions between ions. The small-sized IA–NA–NH3 clusters have lower free energies than typical sulfuric acid (SA)–NA–NH3 clusters in the upper troposphere, exhibiting greater stability and higher nucleation efficiency. Moreover, the NA-enhanced effect on the established efficient IA–NH3 nucleation is more evident at lower temperatures, especially with richer NA and NH3. In the extremely low-temperature UTLS, the proposed IA–NA–NH3 ternary pathway dominates nucleation, while in the mid-troposphere with higher temperatures, the role of NA is minor due to its rapid evaporation. These findings underscore the important role of NA in iodine particle formation in the UTLS, offering mechanistic insights into the missing secondary particle sources.
- Article
(4364 KB) - Full-text XML
-
Supplement
(1265 KB) - BibTeX
- EndNote
Globally occurring new particle formation (NPF) events contribute significantly to the formation of cloud condensation nuclei (CCN) (Kerminen et al., 2018; Nieminen et al., 2018; Kulmala et al., 2022), which further affects climate and radiative force (Zhang et al., 2012). The first and key process in NPF is the clustering of gas-phase precursors into nanoparticles, known as the nucleation stage (Zhang et al., 2012; Kulmala et al., 2013). Despite the importance of particle nucleation, the origin of NPF is unclear due to limited understanding of its molecular mechanisms, especially in the upper troposphere and lower stratosphere (UTLS), which contributes over one-third of global CCN population (Williamson et al., 2019; Wang et al., 2022). However, due to the challenges of long-term field measurements in the UTLS, the lack of field data has led to some underlying nucleation mechanisms remaining undiscovered.
Prior studies primarily focused on particle nucleation driven by sulfuric acid (H2SO4, SA) (Kirkby et al., 2011; Almeida et al., 2013; Glasoe et al., 2015; Elm, 2019, 2021). Despite the involvement of water (H2O) and ammonia (NH3), the resulting SA–H2O/SA–NH3 nucleation cannot fully account for the observed high nucleation rates in most locations (Zhang et al., 2012; Elm, 2021). Even being further enhanced by nitric acid (HNO3, NA), the SA–NA–NH3 ternary nucleation can only explain nucleation in limited regions, e.g., the Asian monsoon zone (Wang et al., 2022). In fact, apart from SA particles, significant levels of iodic acid (HIO3, IA) particles have also been widely detected in the troposphere and even the stratosphere (Koenig et al., 2020; Frege et al., 2017; Beck et al., 2022; Salignat et al., 2024). Although the critical role of IA in NPF in the marine boundary layer (MBL) has been extensively identified (Rauch et al., 2002; Sipilä et al., 2016; Yu et al., 2019; Baccarini et al., 2020), its impacts on NPF in the high-altitude UTLS remain largely unknown. Crucially, the recent CERN CLOUD (Cosmics Leaving Outdoor Droplets) experiments provided solid evidence that IA-driven nucleation is highly temperature-dependent, showing a remarkable efficiency at low temperatures, even surpassing the well-established SA–NH3 system (He et al., 2021). Notably, the high-altitude atmosphere like UTLS is characterized by not only low temperatures but also sparse background particles (i.e., low condensation sinks CS: 10−4–10−5 s−1), both of which facilitate IA nucleation (He et al., 2021), but the underlying mechanisms in the UTLS are still poorly understood.
Given the base stabilization of NH3 on acidic IA (Rong et al., 2020; Xia et al., 2020) and its high levels in the upper troposphere (about 30 pptv to 1.4 ppbv) (Wang et al., 2022; Höpfner et al., 2016; Höpfner et al., 2019), our previous study found that NH3 can effectively promote IA nucleation via their acid–base reactions, especially at higher altitudes (Li et al., 2024a). However, the real atmosphere is complex, meaning that such IA–NH3 binary nucleation likely overlooks the impacts of other chemicals, particularly those with nucleation potential and high concentrations in the UTLS, e.g., NA. NA is abundant, accounting for up to 50 % of reactive nitrogen in the tropical upper troposphere, with its gas-phase mixing ratios ranging from 0.1 to 2 ppbv (Laaksonen et al., 1997; Popp et al., 2006). Moreover, as mentioned earlier, NA has been proven to work with NH3 to synergistically enhance SA nucleation, which plays a dominant role in NPF process in the upper troposphere over Asian monsoon regions (Wang et al., 2022, 2023; Liu et al., 2018). However, whether NA can enhance IA nucleation through its synergy with NH3 and the molecular mechanisms remain unknown despite the detection of NA and IA in the field-collected particles in the upper troposphere (Frege et al., 2017; Beck et al., 2022).
In this study, we performed both quantum chemical calculations and atmospheric cluster dynamic code (ACDC) simulations to investigate (IA)x(NA)y(NH3)z (1 6, x+y ≥z) nucleation. The molecular clustering process was explored through wave function analysis. Specifically, we analyzed the reactive binding sites as well as the type and strength of intermolecular interactions. The stability of the formed IA–NA–NH3 clusters was investigated through thermodynamic analysis. Additionally, a series of ACDC kinetic simulations were conducted to further calculate the cluster formation rate, steady-state concentration, and formation pathways of the IA–NA–NH3 system. To better evaluate the nucleation efficiency of the IA–NA–NH3 system, we further conducted a comparative analysis with the well-established SA–NA–NH3 system under UTLS conditions.
2.1 Quantum chemistry calculations
A multistep conformation search was employed to determine the global minima of (IA)x(NA)y(NH3)z (1 6, x+ y≥z clusters, and the details are shown in the Supplement. The final lowest-energy structures were optimized at the ωB97X-D/6-311G(3df,3pd) (for H, O, and N atoms) + aug-cc-pVTZ-PP (for I atom) level of theory (Francl et al., 1982; Peterson et al., 2003) using the Gaussian 09 package (Frisch et al., 2009). Further single-point energy calculations were performed on the identified lowest-free-energy conformations employing the Domain-based Local Pair Natural Orbital Coupled Cluster method (DLPNO-CCSD(T)) in combination with the aug-cc-pVTZ(-PP) basis set and TightPNO/TightSCF settings, using the ORCA 5.0.4 program (Neese, 2011). Moreover, given the heavy-atom nature of iodine, the spin–orbit coupling (SOC) corrections were taken into account in the Gibbs free formation energies (ΔG) of all iodine-containing clusters (Zaki et al., 2023; Verstraete et al., 2008; Engsvang et al., 2024). These corrections were calculated at the ωB97X-D/6-311G(3df,3pd) (for H, O, and N atoms) + dhf-TZVP-2c (for I atom) level of theory (Kuhn and Weigend, 2015; Sarr et al., 2021; Holzer et al., 2022; Chan and Yim, 2013) using the Gaussian 16 package (Frisch et al., 2016). Herein, we calculated ΔGref (kcal mol−1) of the IA–NA–NH3 systems at the reference pressure (Pref= 1 atm) as in Eq. (1):
where and ΔEDLPNO−CCSD(T) are the thermal contribution and electronic contribution to Gibbs free energy, respectively. ΔESOC is the spin–orbit coupling energies in the formation of iodine-containing clusters. The values at different temperatures (220–260 K) were calculated using the Shermo 2.0 package (Lu and Chen, 2021), and the final Gibbs formation free energies are presented in Table S1. When considering the effect of the concentrations of nucleation precursors, ΔGref can be further converted to ΔG using Eq. (2):
where ΔGref is the Gibbs formation free energy calculated at the reference pressure Pref (1 atm), kB is the Boltzmann constant, and T is the temperature. Ni denotes the number of molecules i within the cluster, and Pi represents the partial pressure of vapor i. Additional theoretical methods and computational details are provided in the Supplement.
2.2 Wave function analysis
The intrinsic stability of the cluster is essentially determined by the intermolecular interactions among the molecules forming the cluster. Therefore, we performed the wave function analysis on the IA–NA–NH3 clusters using Multiwfn 3.7 code (Lu and Chen, 2012) to further understand the interactions within the clusters. Initially, the electrostatic potential (ESP) mapped on the molecular van der Waals (vdW) surface was calculated to predict the potential interaction sites of the nucleation precursor molecules (i.e., IA, NA, and NH3). Subsequently, reduced density gradient (RDG) analysis was performed to reveal the nature and characteristics of noncovalent interactions. Finally, the atoms-in-molecule (AIM) theory (Lane et al., 2013) was applied to further quantify the strength of intermolecular interactions.
2.3 Atmospheric cluster dynamic simulations
The atmospheric cluster dynamic code (ACDC) (McGrath et al., 2012) was employed to investigate the cluster formation rates, formation pathways, and steady-state concentrations of the IA–NA–NH3 clusters by solving the birth–death equation (Eq. 3).
where βi,j denotes the collision rate coefficient between clusters i and j, Ci is the concentration of cluster i, and indicates the rate coefficient for the evaporation of cluster (i+j) into smaller clusters i and j. Qi and Si correspond to the external source and possible loss term of cluster i. Further details on the calculation of βi,j and can be found in the Supplement. Moreover, a cluster is considered stable when the collision frequency (β⋅C) exceeds the total evaporation rate coefficients (Σγ; Table S2). This criterion was used to establish the boundary conditions for the simulation system, which are detailed in Table S3.
In addition, we applied a collision factor of 2.3 to account for the enhancement of intermolecular van der Waals forces on βi,j in the ACDC simulation (Halonen et al., 2019) based on its application in previous research (Cai et al., 2021; Li et al., 2023; Stolzenburg et al., 2020; Ning et al., 2024). Furthermore, we employed a size-dependent coagulation sink (CoagSi) (Lehtipalo et al., 2016) in this study, which is calculated as follows:
where CSref is the condensation sink (CS) of the reference monomer (i.e., IA monomer) and d is the diameter of the monomer or cluster. The exponent m, with a value of 1.7, varies based on the background distribution and is within the common range observed for atmospheric aerosols (Lehtinen et al., 2007).
3.1 Cluster structure and stability
The formation of stable pre-nucleation clusters relies on robust intermolecular interactions such as hydrogen bonds (HB) and halogen bonds (XB). Here, we mainly probed the newly identified ternary IA–NA–NH3 clusters since the binary clusters (IA–NH3 and NA–NH3) and pure-IA clusters were discussed in previous studies (Rong et al., 2020; Liu et al., 2018). Furthermore, all clusters investigated in this study comprise an equal or greater number of acid molecules relative to base molecules as previous studies have demonstrated that such compositions generally exhibit enhanced thermodynamic stability (Xie and Elm, 2021). To reveal the clustering potential of IA, NA, and NH3, their electrostatic potential (ESP) mapped on van der Waals (vdW) surfaces was calculated and presented in Fig. S1 in the Supplement. And the results suggest that all these precursors possess donor or acceptor sites for HB and XB. Thus, NA is expected to jointly cluster with IA and NH3 molecules via HB and XB.
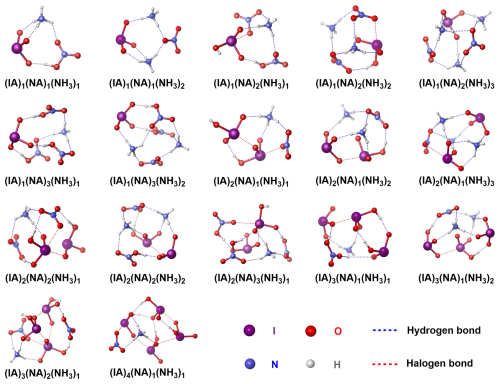
Figure 1The identified (IA)x(NA)y(NH3)z ( 6, ) cluster conformation with lowest Gibbs free energies at the ωB97X-D/6-311G(3df, 3pd) (for H, O, and N atoms) + aug-cc-pVTZ-PP (for I atom) level of theory.
As depicted in Fig. 1, in the identified stable IA–NA–NH3 clusters (Cartesian coordinates in Table S6), NA and NH3 interacts with IA via a network of HBs (blue dashed lines) and XBs (red dashed lines). NA molecules tend to localize at the periphery of the cluster, likely limited by their planar geometry. If positioned at the center of such sub-nanometer cluster, it may increase steric hindrance. More specifically, statistical analysis suggests that the prevalence of HBs (84 %) is inherently higher than that of XBs (Fig. S2), which arises from the ability of NH3 to form up to four HBs, resulting in more N–HO HBs. Despite most sites being involved in HB and XB, there remain vacant sites for further adsorption and growth, as shown in the ESP analysis of the large-size pre-nucleation (IA)2(NA)2(NH3)2 cluster (Fig. S3). During the IA–NA–NH3 clustering, acid–base reactions always occur. When acids outnumber or equal NH3, all NH3 molecules are fully protonated. Due to the stronger acidity of NA compared to IA, most NH3 (60 %) in ternary clusters is more prone to be protonated by NA first, yielding NO–NH ion pairs. Overall, the IA–NA–NH3 clusters are simultaneously stabilized by intermolecular HBs, XBs, and robust electrostatic interactions between the acid–base ions, which are similar to the features of other studied IA-containing clusters (Rong et al., 2020; Ning et al., 2022a, b; Li et al., 2024a, b).
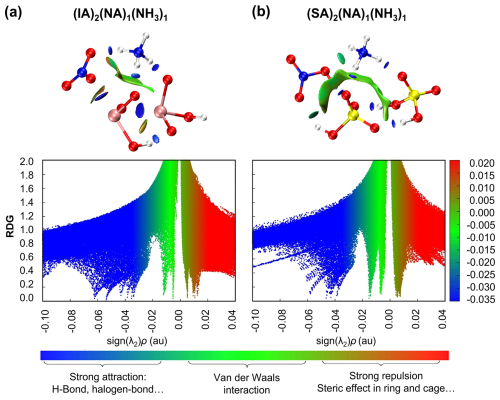
In the IA–NA–NH3 system, the presence of IA introduces XBs, allowing NA to be stabilized simultaneously by HBs, XBs, and electrostatic interactions. In contrast, in the SA–NA–NH3 clusters, a typical high-efficiency system in the UTLS (Liu et al., 2018; Wang et al., 2022), NA only participates in HB formation and the protonation of NH3. To further assess the cluster stability, we compared the Gibbs free energies (ΔG) of the IA–NA–NH3 and SA–NA–NH3 cluster formation at T= 220 K, as shown in Fig. S4. Overall, under the condition of same number of molecules, the ΔG values of the IA–NA–NH3 clusters are relatively lower than those of the SA–NA–NH3 clusters, especially for clusters with fewer molecules (Table S4). This suggests that like the well-established SA–NA–NH3 system, the IA–NA–NH3 system may also play an important role in particle nucleation in the UTLS. Specifically, as to the clusters with one NH3 molecule, the IA–NA–NH3 clusters exhibit lower energies compared to the SA–NA–NH3 clusters. For example, the ΔG of (IA)2(NA)1(NH3)1 cluster is even lower than that of (SA)2(NA)1(NH3)1 cluster by 4.03 kcal mol−1. To probe the cause of the energy difference, herein we employ reduced density gradient (RDG) analysis to uncover the bonding nature of the (IA)2(NA)1(NH3)1 and (SA)2(NA)1(NH3)1 clusters. As presented in Fig. 2, the blue regions indicate strong attractions like HB and XB, and the green regions signifies vdW interaction, while the red regions indicate repulsive interactions. The structure of (IA)2(NA)1(NH3)1 cluster (Fig. 2a) features four HBs (three N–HO and one O–HO) and two XBs (O–IO). The RDG analysis of (IA)2(NA)1 (NH3)1 cluster (bottom-left panel) presents six prominent blue spikes. Further combined with the AIM analysis, the spikes located at sign(λ2)ρ= −0.062 and −0.054 au are attributed to the two XBs, while the remaining four spikes correspond to HBs. In contrast, the (SA)2(NA)1(NH3)1 cluster contains only HBs, and all blue spike values in its RDG plot (bottom-right panel) are greater than −0.06 au. Generally, the smaller sign(λ2)ρ values indicate stronger interactions. Thus, the presence of XBs in the (IA)2(NA)1(NH3)1 cluster likely contributes to stronger attractions compared to (SA)2(NA)1(NH3)1 cluster, resulting in a lower free energy of the (IA)2(NA)1(NH3)1 cluster.
3.2 Cluster formation rate
To further evaluate the nucleation efficiency of the IA–NA–NH3 system, we performed ACDC simulations to calculate cluster formation rates (J, cm−3 s−1) under the UTLS conditions. The concentrations of the nucleation precursors (IA, NA, and NH3) used in this study were primarily based on currently available field observations and model simulation data.
3.2.1 Concentration of IA
Sipilä et al. (2016) reported that atmospheric IA concentrations range from 106 to 108 molec. cm−3 during new particle formation events at the Mace Head site in the boundary layer. Moreover, Salignat et al. (2024) reported an average IA concentration of 2.9 × 105 molec. cm−3 at the Maïdo Observatory (2150 m a.s.l.) over the Indian Ocean, with peak values reaching up to 3.3 × 106 molec. cm−3. Although, to our knowledge, gaseous IA levels at higher altitudes have not yet been reported, and vertical profiles of IO radicals, a key intermediate in IA formation, are available across different elevations. Importantly, CLOUD experimental evidence shows a strong correlation between IA concentrations ([IA]) and IO radical levels, and IA formation of in this process appears to be insensitive to variations in O3, H2O, and temperature (Finkenzeller et al., 2023). Notably, the mixing ratio of IO radicals exhibits minimal variation with altitude, particularly above 2 km (Saiz-Lopez et al., 2014; Karagodin-Doyennel et al., 2021). This suggests that at high altitudes, IA might exhibit similar concentrations at ∼ 2 km altitude (∼ 106 molec. cm−3), although lower IA levels are also possible. This issue warrants further investigation in future studies. Given the limited field observations of IA concentrations in the UTLS, this study adopts an IA concentration range of 105 to 106 molec. cm−3, which are comparable to or even lower than those observed at around 2 km altitude.
3.2.2 Concentration of NA
NA mixing ratios at an altitude of approximately 10 km range from 100 to 2500 pptv, with average values exceeding 1000 pptv (Singh et al., 1996). Meanwhile, theoretical model investigations indicated that NA mixing ratios in the upper troposphere range from 0.1 to 2 ppbv (Laaksonen et al., 1997). In addition, gas-phase NA concentrations observed in the tropical lower stratosphere were typically 0.1 ppbv or lower (Popp et al., 2006). Therefore, based on the environmental conditions such as temperature and pressure in the UTLS, the concentration of NA in this study was set to a broad range from 10 pptv to 10 ppbv (108–1011 molec. cm−3).
3.2.3 Concentration of NH3
Based on satellite observations and high-altitude aircraft measurements, NH3 mixing ratios in the upper troposphere over the Asian monsoon region can reach up to 30 pptv as a 3-month average and up to 1.4 ppbv in hotspots (Höpfner et al., 2016; Höpfner et al., 2019). Accordingly, the NH3 concentration was set to 30 pptv in this study based on the typical conditions of the UTLS. Under these conditions, specifically at a temperature of 220 K and a pressure of 0.2 atm, 30 pptv corresponds to 3 × 108 molec. cm−3.
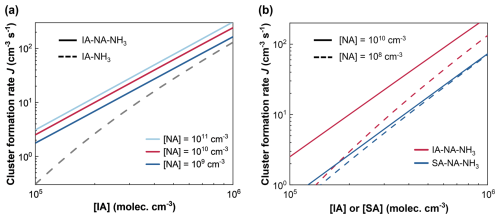
Figure 3(a) Cluster formation rate J (cm−3 s−1) as a function of [IA] of IA–NH3 and IA–NA–NH3 systems at the conditions of T= 220 K, CS = 10−4 s−1, [NA] = 109–1011 molec. cm−3, and [NH3] = 3 × 108 molec. cm−3. (b) J of IA–NA–NH3 and SA–NA–NH3 systems at T= 220 K, CS = 10−4 s−1, [IA] = 105–106 molec. cm−3, [NA] = 108 or 1010 molec. cm−3, and [NH3] = 3 × 108 molec. cm−3.
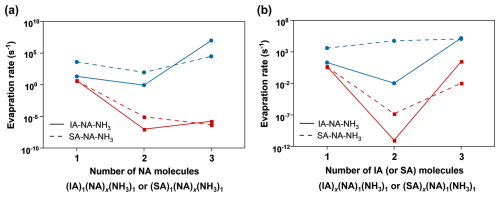
Figure 4Evaporation rates of NA (blue lines) and IA/SA (red lines) molecules from IA–NA–NH3 (solid lines) and SA–NA–NH3 (dashed lines) clusters. (a) The results for (IA)1(NA)x(NH3)1 and (SA)1(NA)x(NH3)1 clusters and (b) the results for (IA)x(NA)1(NH3)1 and (SA)x(NA)1(NH3)1 clusters.
As illustrated in Fig. 3a, at T= 220 K, CS = 10−4 s−1, [IA] = 105–106 molec. cm−3, and [NH3] = 3 × 108 molec. cm−3, NA exhibits a significant enhancing effect on IA–NH3 nucleation. Even when the NA concentration is as low as 109 molec. cm−3, it can enhance J(IA–NH3) by up to a factor of 6, increasing from 0.3 to 1.8 cm−3 s−1. Under the conditions of [IA] = 105 molec. cm−3 and [NA] = 1011 molec. cm−3 (light blue line), J(IA–NA–NH3) is approximately 10 times higher than J(IA–NH3). This suggests that the enhancement of NA on IA–NH3 nucleation is pronounced in high-altitude regions characterized by low [IA] and high [NA]. Moreover, we have calculated the enhancement strength R (Fig. S5) to quantify the rate enhancement of IA–NA–NH3 ternary nucleation compared to the established IA–NH3 and NA–NH3 binary nucleation in a broader array of atmospheric conditions (see details in the Supplement). The role of IA–NA–NH3 nucleation is stronger in regions with lower temperatures and higher concentrations of NA and NH3, which corresponds to the UTLS conditions. This suggests that IA–NA–NH3 nucleation may provide a plausible explanation for the unknown sources of CCN in the UTLS. In addition, considering the potential uncertainties in quantum chemical calculations, we also investigated the effect of the uncertainty in the calculated ΔG on the cluster formation rate J. As shown in Fig. S6, under NA concentrations ranging from 108 to 1010 molec. cm−3, adjusting the ΔG220 K of clusters by subtracting 1 kcal mol−1 results in a minor variation in J. Although an increase in ΔG220 K by adding 1 kcal mol−1 may cause a slight offset in J values, the DLPNO method tends to underestimate binding energies to some extent, indicating that the present results (JΔG 220 K) are at a low limit. Therefore, the scenario of adding 1 kcal mol−1 may be unlikely to occur. Taken together, the uncertainty of calculated quantum chemistry does not significantly impact the conclusions of this study.
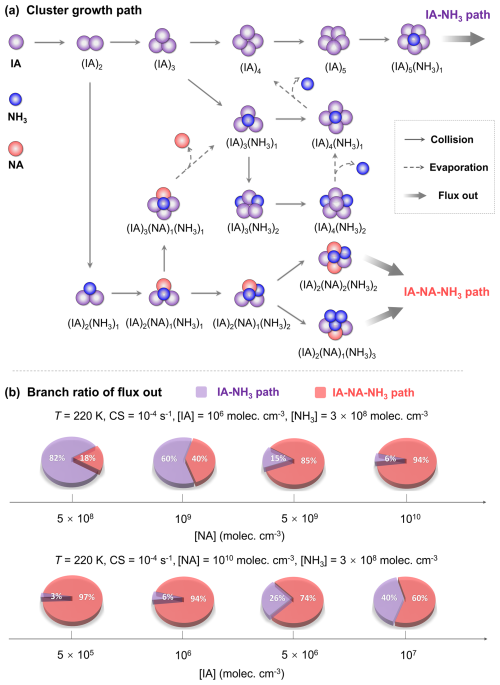
Figure 5(a) Main clustering pathways of the IA–NA–NH3 system. (b) Branch ratio of flux out at T= 220 K, CS = 10−4 s−1, [IA] = 5 × 105–107 molec. cm−3, [NA] = 5 × 108–1010 molec. cm−3, and [NH3] = 3 × 108 molec. cm−3.
Furthermore, under the conditions of T= 220 K, CS = 10−4 s−1, [IA] = 105–106 molec. cm−3, [NA] = 108 or 1010 molec. cm−3, and [NH3] = 3 × 108 molec. cm−3, we simulated J for the IA–NA–NH3 and SA–NA–NH3 systems and conducted a comparative analysis, as shown in Fig. 3b. The results reveal that, at the same [NA] levels, J(IA–NA–NH3) (red line) consistently surpasses J(SA–NA–NH3) (blue line), with this difference becoming more pronounced when [NA] reaches 1010 molec. cm−3 (closer to actual UTLS conditions). Considering that the [IA] in the actual UTLS atmospheric environment may be slightly lower than [SA], we also simulated the scenarios where [IA] is lower than [SA]. The results show that when [IA] = 50 %[SA], J(IA–NA–NH3) is already comparable to J(SA–NA–NH3) (Fig. S7). This observation indicates that, although the SA–NA–NH3 system is widely regarded as an efficient nucleation mechanism in the UTLS, the IA–NA–NH3 system achieves notably higher nucleation efficiency under similar conditions. To reveal the underlying reasons for higher J(IA–NA–NH3), we further analyzed the evaporation rates of the clusters in both systems. Figure 4 presents the evaporation rates of NA and IA (or SA) from the cluster versus clusters sizes for different clusters compositions. For both cluster types, the evaporation of the NA molecule (blue lines) is faster than that of the IA (or SA) molecule (red lines). For the clusters containing three–four molecules, NA and IA evaporate more slowly within the IA–NA–NH3 clusters, while for larger-size clusters with five–six molecules, the evaporation rates of NA and SA are lower in the SA–NA–NH3 cluster (see Tables S2 and S5). Based on the resulting J(IA–NA–NH3) and the corresponding results for evaporation, it can be inferred that smaller clusters containing two to four molecules formed early have a greater effect on the cluster formation rate. Similar behavior has also been observed in the SA–NH3–H2O system (Napari et al., 2002). Therefore, understanding the gas-to-cluster process at the molecular level, especially the formation of initial clusters that underpin subsequent growth but are challenging to reveal experimentally, is essential for atmospheric nucleation.
3.3 Cluster formation pathway
To gain deeper insights into the impact of NA and NH3 on IA nucleation, we have performed the ACDC simulations under the UTLS conditions to track the detailed dynamics of the NA–IA–NH3 clustering process. As shown in Fig. 5a, the nucleation process of IA–NA–NH3 system can proceed through two types of pathways: (i) IA–NH3 and (ii) IA–NA–NH3 pathways. The IA–NH3 pathway primarily involves the initial self-collision of IA molecules to form the (IA)5 cluster, which further collides with NH3 to grow into the (IA)5(NH3)1 cluster. Interestingly, NA and NH3 also participate in initial cluster formation and subsequently evaporate out, acting like “catalysts” to promote clustering. In the IA–NA–NH3 pathway, the clustering process primarily proceeds via the sequential addition of acid or base monomers. Furthermore, given the impacts of atmospheric conditions on nucleation pathways, we calculated the branch ratio of different pathways under UTLS conditions. As illustrated in Fig. 5b, the contribution of the IA–NA–NH3 pathway increases with rising [NA] and declining [IA]. Notably, when [NA] reaches 1010 molec. cm−3 or [IA] decreases to 5 × 105 molec. cm−3, the contribution of IA–NA–NH3 pathway can reach nearly 95 %. This finding indicates that the IA–NA–NH3 ternary pathway dominates in regions where IA level is limited, while NA is abundant, aligning well with the conditions in the focused UTLS. More broadly, such scenario characterized by scarce IA and rich NA also exists in higher atmosphere, such as ∼ 20 km (i.e., the bottom of near space). This NA-enhanced mechanism is likely a vital source of fresh particles in this region. Therefore, the IA–NA–NH3 mechanism may have significant implications for the formation of aerosols or ice particles at the high altitude, which provides deeper insights into the origin of global CCN and helps to improve atmospheric modeling.
In addition to the UTLS, here we also investigate the formation mechanism of IA–NA–NH3 clusters in the low–mid free troposphere with higher temperatures and precursor concentrations. However, in the mid free troposphere with T= 240 K (Fig. S8), the nucleation process proceeds mainly through the pure IA and IA–NH3 binary pathway. While NA contributes to the initial stages of cluster formation, it subsequently evaporates from the pre-nucleation cluster, ultimately functioning in a catalyst-like capacity. Moreover, at T= 260 K in the lower free troposphere, NA plays a negligible role in cluster formation due to its rapid evaporation at an elevated temperature. Consequently, we conclude that the IA–NA–NH3 ternary nucleation pathway can play a vital role in iodine particle nucleation under extremely low-temperature conditions, such as the high-altitude UTLS.
To provide a baseline for future experimental study, we have calculated the steady-state concentrations of clusters formed in the IA–NA–NH3 and SA–NA–NH3 system under the typical UTLS conditions in Fig. 6. The simulated results indicate that the concentrations of most IA–NA–NH3 clusters exceed 10 cm−3, while those of SA–NA–NH3 clusters remain below this threshold (dashed line). This suggests that under equivalent conditions, the number concentrations of IA–NA–NH3 cluster are generally higher, showing a potentially greater contribution to particle formation. Although [SA] may be higher than that of [IA] under the UTLS conditions, the resulting impact of the IA–NA–NH3 system is likely comparable to that of the SA–NA–NH3 system. Thus, these results further highlight that the IA–NA–NH3 nucleation mechanism is potentially significant in the UTLS.
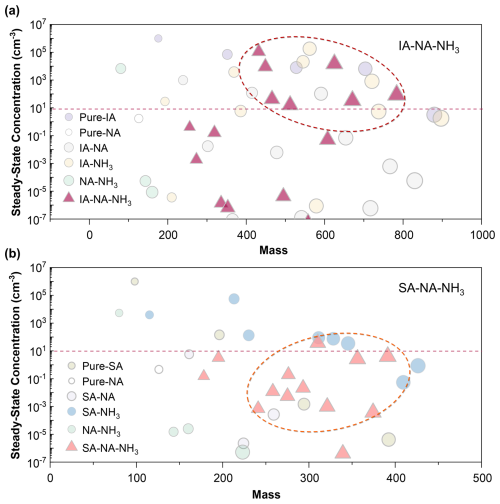
Figure 6Simulated steady-state concentrations (cm−3) of (a) IA–NA–NH3 and (b) SA–NA–NH3 clusters against cluster mass at the conditions of T= 220 K, CS = 10−4 s−1, [IA] = 106 molec. cm−3, [SA] = 106 molec. cm−3, [NA] = 109 molec. cm−3, and [NH3] = 3 × 108 molec. cm−3. For better clarify, the concentrations of ternary clusters in the IA–NA–NH3 and SA–NA–NH3 systems are highlighted with circles, respectively.
High-altitude NPF serves as an essential source of global CCN, thereby impacting climate. Iodic acid (IA) particles are widely found in the UTLS; however, their formation mechanism is yet unknown. Given the abundance of NA and NH3 in the UTLS and their nucleation potential, they may jointly affect IA nucleation. To probe their nucleation process, we performed quantum chemical calculations and cluster dynamics simulations to investigate the IA–NA–NH3 ternary system under the UTLS conditions. The structural analysis shows that NA, IA, and NH3 can bind together via HBs, XBs, and electrostatic attraction between the formed ion pairs (NO–NH and IO–NH) to form stable nanoscale clusters. In contrast to the role of NA in the well-established SA–NA–NH3 clusters in the UTLS, which only engages in HB formation, NA additionally forms XB with IA in the IA–NA–NH3 system. Notably, the resulting small-sized IA–NA–NH3 clusters (three–four molecules) exhibit lower free energies than SA–NA–NH3 clusters, with the reduced evaporation rates indicating greater stability. As a result, the simulated rate J(IA–NA–NH3) is even higher than J(SA–NA–NH3) at the same precursor concentrations, showing higher nucleation efficiency. Furthermore, we found that the enhancement strength is sensitive to temperature as well as NA and NH3 concentrations. In regions characterized by lower temperatures and high levels of NA and NH3, such as the UTLS, the impact of IA–NA–NH3 nucleation is more pronounced. At a lower temperature of 220 K in the UTLS, the ternary IA–NA–NH3 clustering pathway dominates during nucleation. However, as the temperature rises to 240 K, NA facilitates IA–NH3 nucleation through functioning as a quasi-catalyst precursor by initially participating in cluster formation and then evaporating out. However, in the lower free troposphere with a higher temperature of 260 K, NA evaporates rapidly, ceasing to participate in clustering process.
Our findings highlight the significant role of NA in IA nucleation, which potentially helps explain the missing source of the detected fresh IA particles in the UTLS. Even in the higher stratosphere (e.g., ∼ 20 km, the lower near space), NA (Orsolini et al., 2009; Fahey et al., 2001; Wespes et al., 2007), NH3 (Xenofontos et al., 2024; Johansson et al., 2024), and IA particles (Koenig et al., 2020) were also frequently detected (Wen-Qin, 2009), suggesting that the IA–NA–NH3 nucleation could also be a significant source of near-space iodine particles. Further, these ultrafine particles can participate in the formation of CCN or ice nuclei, which alters high-altitude cloud microphysical properties, thereby affecting radiation balance and global climate.
The data in this article are available from the corresponding author upon reasonable request (anning@bit.edu.cn and zhangxiuhui@bit.edu.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-14237-2025-supplement.
XZ designed and supervised the research. JL, XD, and AN performed the quantum chemical calculations and the ACDC simulations. JL, AN, FB, and LL analyzed data. JL, AN, and XZ wrote the paper. YZ, PL, and QH reviewed the paper. All authors commented on the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This work is funded by the National Science Fund for Distinguished Young Scholars (grant no. 22225607), the National Natural Science Foundation of China (grant nos. 22306011 and 22376013), and the China Postdoctoral Science Foundation (grant no. 2023M730236).
This work is supported by the National Science Fund for Distinguished Young Scholars (grant no. 22225607) and the National Natural Science Foundation of China (grant nos. 22306011 and 22376013). An Ning was also supported by the China Postdoctoral Science Foundation (grant no. 2023M730236).
This paper was edited by Ari Laaksonen and reviewed by Theo Kurtén and two anonymous referees.
Almeida, J., Schobesberger, S., Kürten, A., Ortega, I. K., Kupiainen-Määttä, O., Praplan, A. P., Adamov, A., Amorim, A., Bianchi, F., Breitenlechner, M., David, A., Dommen, J., Donahue, N. M., Downard, A., Dunne, E., Duplissy, J., Ehrhart, S., Flagan, R. C., Franchin, A., Guida, R., Hakala, J., Hansel, A., Heinritzi, M., Henschel, H., Jokinen, T., Junninen, H., Kajos, M., Kangasluoma, J., Keskinen, H., Kupc, A., Kurten, T., Kvashin, A. N., Laaksonen, A., Lehtipalo, K., Leiminger, M., Leppä, J., Loukonen, V., Makhmutov, V., Mathot, S., McGrath, M. J., Nieminen, T., Olenius, T., Onnela, A., Petäjä, T., Riccobono, F., Riipinen, I., Rissanen, M., Rondo, L., Ruuskanen, T., Santos, F. D., Sarnela, N., Schallhart, S., Schnitzhofer, R., Seinfeld, J. H., Simon, M., Sipilä, M., Stozhkov, Y., Stratmann, F., Tomé, A., Tröstl, J., Tsagkogeorgas, G., Vaattovaara, P., Viisanen, Y., Virtanen, A., Vrtala, A., Wagner, P. E., Weingartner, E., Wex, H., Williamson, C., Wimmer, D., Ye, P., Yli-Juuti, T., Carslaw, K. S., Kulmala, M., Curtius, J., Baltensperger, U., Worsnop, D. R., Vehkamäki, H., and Kirkby, J.: Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere, Nature, 502, 359–363, https://doi.org/10.1038/nature12663, 2013.
Baccarini, A., Karlsson, L., Dommen, J., Duplessis, P., Vullers, J., Brooks, I. M., Saiz-Lopez, A., Salter, M., Tjernstrom, M., Baltensperger, U., Zieger, P., and Schmale, J.: Frequent new particle formation over the high Arctic pack ice by enhanced iodine emissions, Nat. Commun., 11, 4924, https://doi.org/10.1038/s41467-020-18551-0, 2020.
Beck, L. J., Schobesberger, S., Junninen, H., Lampilahti, J., Manninen, A., Dada, L., Leino, K., He, X.-C., Pullinen, I., Quéléver, L. L. J., Franck, A., Poutanen, P., Wimmer, D., Korhonen, F., Sipilä, M., Ehn, M., Worsnop, D. R., Kerminen, V.-M., Petäjä, T., Kulmala, M., and Duplissy, J.: Diurnal evolution of negative atmospheric ions above the boreal forest: from ground level to the free troposphere, Atmos. Chem. Phys., 22, 8547–8577, https://doi.org/10.5194/acp-22-8547-2022, 2022.
Cai, R., Yan, C., Yang, D., Yin, R., Lu, Y., Deng, C., Fu, Y., Ruan, J., Li, X., Kontkanen, J., Zhang, Q., Kangasluoma, J., Ma, Y., Hao, J., Worsnop, D. R., Bianchi, F., Paasonen, P., Kerminen, V.-M., Liu, Y., Wang, L., Zheng, J., Kulmala, M., and Jiang, J.: Sulfuric acid–amine nucleation in urban Beijing, Atmos. Chem. Phys., 21, 2457–2468, https://doi.org/10.5194/acp-21-2457-2021, 2021.
Chan, B. and Yim, W. L.: Accurate Computation of Cohesive Energies for Small to Medium-Sized Gold Clusters, J. Chem. Theory Comput., 9, 1964–1970, https://doi.org/10.1021/ct400047y, 2013.
Elm, J.: An atmospheric cluster database consisting of sulfuric acid, bases, organics, and water, ACS Omega, 4, 10965–10974, https://doi.org/10.1021/acsomega.9b00860, 2019.
Elm, J.: Clusteromics I: Principles, protocols, and applications to sulfuric acid–base bluster formation, ACS Omega, 6, 7804–7814, https://doi.org/10.1021/acsomega.1c00306, 2021.
Engsvang, M., Wu, H., and Elm, J.: Iodine clusters in the atmosphere I: Computational benchmark and dimer formation of oxyacids and oxides, ACS Omega, 9, 31521–31532, https://doi.org/10.1021/acsomega.4c01235, 2024.
Fahey, D. W., Gao, R. S., Carslaw, K. S., Kettleborough, J., Popp, P. J., Northway, M. J., Holecek, J. C., Ciciora, S. C., McLaughlin, R. J., Thompson, T. L., Winkler, R. H., Baumgardner, D. G., Gandrud, B., Wennberg, P. O., Dhaniyala, S., McKinney, K., Peter, T., Salawitch, R. J., Bui, T. P., Elkins, J. W., Webster, C. R., Atlas, E. L., Jost, H., Wilson, J. C., Herman, R. L., Kleinböhl, A., and von König, M.: The Detection of Large HNO3-Containing Particles in the Winter Arctic Stratosphere, Science, 291, 1026–1031, https://doi.org/10.1126/science.1057265, 2001.
Finkenzeller, H., Iyer, S., He, X. C., Simon, M., Koenig, T. K., Lee, C. F., Valiev, R., Hofbauer, V., Amorim, A., Baalbaki, R., Baccarini, A., Beck, L., Bell, D. M., Caudillo, L., Chen, D., Chiu, R., Chu, B., Dada, L., Duplissy, J., Heinritzi, M., Kemppainen, D., Kim, C., Krechmer, J., Kurten, A., Kvashnin, A., Lamkaddam, H., Lee, C. P., Lehtipalo, K., Li, Z., Makhmutov, V., Manninen, H. E., Marie, G., Marten, R., Mauldin, R. L., Mentler, B., Muller, T., Petaja, T., Philippov, M., Ranjithkumar, A., Rorup, B., Shen, J., Stolzenburg, D., Tauber, C., Tham, Y. J., Tome, A., Vazquez-Pufleau, M., Wagner, A. C., Wang, D. S., Wang, M., Wang, Y., Weber, S. K., Nie, W., Wu, Y., Xiao, M., Ye, Q., Zauner-Wieczorek, M., Hansel, A., Baltensperger, U., Brioude, J., Curtius, J., Donahue, N. M., Haddad, I. E., Flagan, R. C., Kulmala, M., Kirkby, J., Sipila, M., Worsnop, D. R., Kurten, T., Rissanen, M., and Volkamer, R.: The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source, Nat. Commun., 15, 129–135, https://doi.org/10.1038/s41557-022-01067-z, 2023.
Francl, M. M., Pietro, W. J., Hehre, W. J., Binkley, J. S., Gordon, M. S., DeFrees, D. J., and Pople, J. A.: Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements, J. Chem. Phys., 77, 3654–3665, https://doi.org/10.1063/1.444267, 1982.
Frege, C., Bianchi, F., Molteni, U., Tröstl, J., Junninen, H., Henne, S., Sipilä, M., Herrmann, E., Rossi, M. J., Kulmala, M., Hoyle, C. R., Baltensperger, U., and Dommen, J.: Chemical characterization of atmospheric ions at the high altitude research station Jungfraujoch (Switzerland), Atmos. Chem. Phys., 17, 2613–2629, https://doi.org/10.5194/acp-17-2613-2017, 2017.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, O., Foresman, J. B., Ortiz, J. V., Cioslowski, J., and Fox, D. J.: Gaussian 09, Revision A.02, Gaussian Inc, Wallingford CT, https://gaussian.com/g09citation/ (last access: 7 May 2022), 2009.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, O., Foresman, J. B., Ortiz, J. V., Cioslowski, J., and Fox, D. J.: Gaussian 16, Revision A.03, Gaussian Inc, Wallingford CT, https://gaussian.com/gaussian16/ (last access: 20 November 2023), 2016.
Glasoe, W. A., Volz, K., Panta, B., Freshour, N., Bachman, R., Hanson, D. R., McMurry, P. H., and Jen, C.: Sulfuric acid nucleation: An experimental study of the effect of seven bases, J. Geophys. Res.-Atmos., 120, 1933–1950, https://doi.org/10.1002/2014jd022730, 2015.
Halonen, R., Zapadinsky, E., Kurtén, T., Vehkamäki, H., and Reischl, B.: Rate enhancement in collisions of sulfuric acid molecules due to long-range intermolecular forces, Atmos. Chem. Phys., 19, 13355–13366, https://doi.org/10.5194/acp-19-13355-2019, 2019.
He, X.-C., Tham, Y. J., Dada, L., Wang, M., Finkenzeller, H., Stolzenburg, D., Iyer, S., Simon, M., Kürten, A. K., Shen, J., Roerup, B., Rissanen, M., Schobesberger, S., Baalbaki, R., Wang, D. S., Koenig, T. K., Jokinen, T., Sarnela, N., Beck, L. J., Almeida, J., Amanatidis, S., Amorim, A., Ataei, F., Baccarini, A., Bertozzi, B., Bianchi, F., Brilke, S., Caudillo, L., Chen, D., Chiu, R., Chu, B., Dias, A., Ding, A., Dommen, J., Duplissy, J., Haddad, I. E., Carracedo, L. G., Granzin, M., Hansel, A., Heinritzi, M., Hofbauer, V., Junninen, H., Kangasluoma, J., Kemppainen, D., Kim, C., Kong, W., Krechmer, J. E., Kvashin, A., Laitinen, T., Lamkaddam, H., Lee, C. P., Lehtipalo, K., Leiminger, M., Li, Z., Makhmutov, V., Manninen, H. E., Marie, G., Marten, R., Mathot, S., Mauldin, R. L., Mentler, B., Moehler, O., Mueller, T., Nie, W., Onnela, A., Petaja, T., Pfeifer, J., Philippov, M., Ranjithkumar, A., Saiz-Lopez, A., Salma, I., Scholz, W., Schuchmann, S., Schulze, B., Steiner, G., Stozhkov, Y., Tauber, C., Tome, A., Thakur, R. C., Vaisanen, O., Vazquez-Pufleau, M., Wagner, A. C., Wang, Y., Weber, S. K., Winkler, P. M., Wu, Y., Xiao, M., Yan, C., Ye, Q., Ylisirnio, A., Zauner-Wieczorek, M., Zha, Q., Zhou, P., Flagan, R. C., Curtius, J., Baltensperger, U., Kulmala, M., Kerminen, V.-M., Kurten, T., Donahue, N. M., Volkamer, R., Kirkby, J., Worsnop, D. R., and Sipila, M.: Role of iodine oxoacids in atmospheric aerosol nucleation, Science, 371, 589–595, https://doi.org/10.1126/science.abe0298, 2021.
Holzer, C., Franzke, Y. J., and Pausch, A.: Current density functional framework for spin–orbit coupling, J. Chem. Phys., 157, 204102, https://doi.org/10.1063/5.0122394, 2022.
Höpfner, M., Volkamer, R., Grabowski, U., Grutter, M., Orphal, J., Stiller, G., von Clarmann, T., and Wetzel, G.: First detection of ammonia (NH3) in the Asian summer monsoon upper troposphere, Atmos. Chem. Phys., 16, 14357–14369, https://doi.org/10.5194/acp-16-14357-2016, 2016.
Höpfner, M., Ungermann, J., Borrmann, S., Wagner, R., Spang, R., Riese, M., Stiller, G., Appel, O., Batenburg, A. M., Bucci, S., Cairo, F., Dragoneas, A., Friedl-Vallon, F., Hünig, A., Johansson, S., Krasauskas, L., Legras, B., Leisner, T., Mahnke, C., Möhler, O., Molleker, S., Müller, R., Neubert, T., Orphal, J., Preusse, P., Rex, M., Saathoff, H., Stroh, F., Weigel, R., and Wohltmann, I.: Ammonium nitrate particles formed in upper troposphere from ground ammonia sources during Asian monsoons, Nat. Geosci., 12, 608–612, https://doi.org/10.1038/s41561-019-0385-8, 2019.
Johansson, S., Höpfner, M., Friedl-Vallon, F., Glatthor, N., Gulde, T., Huijnen, V., Kleinert, A., Kretschmer, E., Maucher, G., Neubert, T., Nordmeyer, H., Piesch, C., Preusse, P., Riese, M., Sinnhuber, B.-M., Ungermann, J., Wetzel, G., and Woiwode, W.: Ammonia in the upper troposphere–lower stratosphere (UTLS): GLORIA airborne measurements for CAMS model evaluation in the Asian monsoon and in biomass burning plumes above the South Atlantic, Atmos. Chem. Phys., 24, 8125–8138, https://doi.org/10.5194/acp-24-8125-2024, 2024.
Karagodin-Doyennel, A., Rozanov, E., Sukhodolov, T., Egorova, T., Saiz-Lopez, A., Cuevas, C. A., Fernandez, R. P., Sherwen, T., Volkamer, R., Koenig, T. K., Giroud, T., and Peter, T.: Iodine chemistry in the chemistry–climate model SOCOL-AERv2-I, Geosci. Model Dev., 14, 6623–6645, https://doi.org/10.5194/gmd-14-6623-2021, 2021.
Kerminen, V.-M., Chen, X., Vakkari, V., Petäjä, T., Kulmala, M., and Bianchi, F.: Atmospheric new particle formation and growth: review of field observations, Environ. Res. Lett., 13, 103003, https://doi.org/10.1088/1748-9326/aadf3c, 2018.
Kirkby, J., Curtius, J., Almeida, J., Dunne, E., Duplissy, J., Ehrhart, S., Franchin, A., Gagné, S., Ickes, L., Kürten, A., Kupc, A., Metzger, A., Riccobono, F., Rondo, L., Schobesberger, S., Tsagkogeorgas, G., Wimmer, D., Amorim, A., Bianchi, F., Breitenlechner, M., David, A., Dommen, J., Downard, A., Ehn, M., Flagan, R. C., Haider, S., Hansel, A., Hauser, D., Jud, W., Junninen, H., Kreissl, F., Kvashin, A., Laaksonen, A., Lehtipalo, K., Lima, J., Lovejoy, E. R., Makhmutov, V., Mathot, S., Mikkilä, J., Minginette, P., Mogo, S., Nieminen, T., Onnela, A., Pereira, P., Petäjä, T., Schnitzhofer, R., Seinfeld, J. H., Sipilä, M., Stozhkov, Y., Stratmann, F., Tomé, A., Vanhanen, J., Viisanen, Y., Vrtala, A., Wagner, P. E., Walther, H., Weingartner, E., Wex, H., Winkler, P. M., Carslaw, K. S., Worsnop, D. R., Baltensperger, U., and Kulmala, M.: Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation, Nature, 476, 429–433, https://doi.org/10.1038/nature10343, 2011.
Koenig, T. K., Baidar, S., Campuzano-Jost, P., Cuevas, C. A., Dix, B., Fernandez, R. P., Guo, H., Hall, S. R., Kinnison, D., Nault, B. A., Ullmann, K., Jimenez, J. L., Saiz-Lopez, A., and Volkamer, R.: Quantitative detection of iodine in the stratosphere, P. Natl. Acad. Sci. USA, 117, 1860–1866, https://doi.org/10.1073/pnas.1916828117, 2020.
Kuhn, M. and Weigend, F.: Two-component hybrid time-dependent density functional theory within the Tamm-Dancoff approximation, J. Chem. Phys., 142, 034116, https://doi.org/10.1063/1.4905829, 2015.
Kulmala, M., Kontkanen, J., Junninen, H., Lehtipalo, K., Manninen, H. E., Nieminen, T., Petäjä, T., Sipilä, M., Schobesberger, S., Rantala, P., Franchin, A., Jokinen, T., Järvinen, E., Äijälä, M., Kangasluoma, J., Hakala, J., Aalto, P. P., Paasonen, P., Mikkilä, J., Vanhanen, J., Aalto, J., Hakola, H., Makkonen, U., Ruuskanen, T., Mauldin, R. L., 3rd, Duplissy, J., Vehkamäki, H., Bäck, J., Kortelainen, A., Riipinen, I., Kurtén, T., Johnston, M. V., Smith, J. N., Ehn, M., Mentel, T. F., Lehtinen, K. E., Laaksonen, A., Kerminen, V. M., and Worsnop, D. R.: Direct observations of atmospheric aerosol nucleation, Science, 339, 943–946, https://doi.org/10.1126/science.1227385, 2013.
Kulmala, M., Junninen, H., Dada, L., Salma, I., Weidinger, T., Thén, W., Vörösmarty, M., Komsaare, K., Stolzenburg, D., Cai, R., Yan, C., Li, X., Deng, C., Jiang, J., Petäjä, T., Nieminen, T., and Kerminen, V.-M.: Quiet New Particle Formation in the Atmosphere, Front. Environ. Sci., 10, 912385, https://doi.org/10.3389/fenvs.2022.912385, 2022.
Laaksonen, A., Hienola, J., Kulmala, M., and Arnold, F.: Supercooled cirrus cloud formation modified by nitric acid pollution of the upper troposphere, Geophysical Research Letters, 24, 3009–3012, https://doi.org/10.1029/97gl02996, 1997.
Lane, J. R., Contreras-García, J., Piquemal, J. P., Miller, B. J., and Kjaergaard, H. G.: Are bond critical points really critical for hydrogen bonding?, J. Chem. Theory Comput., 9, 3263–3266, https://doi.org/10.1021/ct400420r, 2013.
Lehtinen, K. E. J., Dal Maso, M., Kulmala, M., and Kerminen, V.-M.: Estimating nucleation rates from apparent particle formation rates and vice versa: Revised formulation of the Kerminen–Kulmala equation, J. Aerosol Sci., 38, 988–994, https://doi.org/10.1016/j.jaerosci.2007.06.009, 2007.
Lehtipalo, K., Rondo, L., Kontkanen, J., Schobesberger, S., Jokinen, T., Sarnela, N., Kürten, A., Ehrhart, S., Franchin, A., Nieminen, T., Riccobono, F., Sipilä, M., Yli-Juuti, T., Duplissy, J., Adamov, A., Ahlm, L., Almeida, J., Amorim, A., Bianchi, F., Breitenlechner, M., Dommen, J., Downard, A. J., Dunne, E. M., Flagan, R. C., Guida, R., Hakala, J., Hansel, A., Jud, W., Kangasluoma, J., Kerminen, V.-M., Keskinen, H., Kim, J., Kirkby, J., Kupc, A., Kupiainen-Määttä, O., Laaksonen, A., Lawler, M. J., Leiminger, M., Mathot, S., Olenius, T., Ortega, I. K., Onnela, A., Petäjä, T., Praplan, A., Rissanen, M. P., Ruuskanen, T., Santos, F. D., Schallhart, S., Schnitzhofer, R., Simon, M., Smith, J. N., Tröstl, J., Tsagkogeorgas, G., Tomé, A., Vaattovaara, P., Vehkamäki, H., Vrtala, A. E., Wagner, P. E., Williamson, C., Wimmer, D., Winkler, P. M., Virtanen, A., Donahue, N. M., Carslaw, K. S., Baltensperger, U., Riipinen, I., Curtius, J., Worsnop, D. R., and Kulmala, M.: The effect of acid–base clustering and ions on the growth of atmospheric nano-particles, Nat. Commun., 7, 11594, https://doi.org/10.1038/ncomms11594, 2016.
Li, J., Ning, A., Liu, L., and Zhang, X.: Atmospheric Bases-Enhanced Iodic Acid Nucleation: Altitude-Dependent Characteristics and Molecular Mechanisms, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.4c06053, 2024a.
Li, J., Wu, N., Chu, B., Ning, A., and Zhang, X.: Molecular-level study on the role of methanesulfonic acid in iodine oxoacid nucleation, Atmos. Chem. Phys., 24, 3989–4000, https://doi.org/10.5194/acp-24-3989-2024, 2024b.
Li, Y., Shen, J., Zhao, B., Cai, R., Wang, S., Gao, Y., Shrivastava, M., Gao, D., Zheng, J., Kulmala, M., and Jiang, J.: A dynamic parameterization of sulfuric acid–dimethylamine nucleation and its application in three-dimensional modeling, Atmos. Chem. Phys., 23, 8789–8804, https://doi.org/10.5194/acp-23-8789-2023, 2023.
Liu, L., Li, H., Zhang, H., Zhong, J., Bai, Y., Ge, M., Li, Z., Chen, Y., and Zhang, X.: The role of nitric acid in atmospheric new particle formation, Phys. Chem. Chem. Phys., 20, 17406–17414, https://doi.org/10.1039/c8cp02719f, 2018.
Lu, T. and Chen, F.: Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 33, 580–592, https://doi.org/10.1002/jcc.22885, 2012.
Lu, T. and Chen, Q.: Shermo: A general code for calculating molecular thermochemistry properties, Comput. Theor. Chem., 1200, 113249, https://doi.org/10.1016/j.comptc.2021.113249, 2021.
McGrath, M. J., Olenius, T., Ortega, I. K., Loukonen, V., Paasonen, P., Kurtén, T., Kulmala, M., and Vehkamäki, H.: Atmospheric Cluster Dynamics Code: a flexible method for solution of the birth-death equations, Atmos. Chem. Phys., 12, 2345–2355, https://doi.org/10.5194/acp-12-2345-2012, 2012.
Napari, I., Noppel, M., Vehkamäki, H., and Kulmala, M.: An improved model for ternary nucleation of sulfuric acid–ammonia–water, J. Chem. Phys., 116, 4221–4227, https://doi.org/10.1063/1.1450557, 2002.
Neese, F.: The ORCA program system, WIREs Comput. Mol. Sci., 2, 73–78, https://doi.org/10.1002/wcms.81, 2011.
Nieminen, T., Kerminen, V.-M., Petäjä, T., Aalto, P. P., Arshinov, M., Asmi, E., Baltensperger, U., Beddows, D. C. S., Beukes, J. P., Collins, D., Ding, A., Harrison, R. M., Henzing, B., Hooda, R., Hu, M., Hõrrak, U., Kivekäs, N., Komsaare, K., Krejci, R., Kristensson, A., Laakso, L., Laaksonen, A., Leaitch, W. R., Lihavainen, H., Mihalopoulos, N., Németh, Z., Nie, W., O'Dowd, C., Salma, I., Sellegri, K., Svenningsson, B., Swietlicki, E., Tunved, P., Ulevicius, V., Vakkari, V., Vana, M., Wiedensohler, A., Wu, Z., Virtanen, A., and Kulmala, M.: Global analysis of continental boundary layer new particle formation based on long-term measurements, Atmos. Chem. Phys., 18, 14737–14756, https://doi.org/10.5194/acp-18-14737-2018, 2018.
Ning, A., Liu, L., Ji, L., and Zhang, X.: Molecular-level nucleation mechanism of iodic acid and methanesulfonic acid, Atmos. Chem. Phys., 22, 6103–6114, https://doi.org/10.5194/acp-22-6103-2022, 2022a.
Ning, A., Liu, L., Zhang, S., Yu, F., Du, L., Ge, M., and Zhang, X.: The critical role of dimethylamine in the rapid formation of iodic acid particles in marine areas, npj Clim. Atmos. Sci., 5, 92, https://doi.org/10.1038/s41612-022-00316-9, 2022b.
Ning, A., Shen, J., Zhao, B., Wang, S., Cai, R., Jiang, J., Yan, C., Fu, X., Zhang, Y., Li, J., Ouyang, D., Sun, Y., Saiz-Lopez, A., Francisco, J. S., and Zhang, X.: Overlooked significance of iodic acid in new particle formation in the continental atmosphere, P. Natl. Acad. Sci. USA, 121, e2404595121, https://doi.org/10.1073/pnas.2404595121, 2024.
Orsolini, Y. J., Urban, J., and Murtagh, D. P.: Nitric acid in the stratosphere based on Odin observations from 2001 to 2009 – Part 2: High-altitude polar enhancements, Atmos. Chem. Phys., 9, 7045–7052, https://doi.org/10.5194/acp-9-7045-2009, 2009.
Peterson, K. A., Figgen, D., Goll, E., Stoll, H., and Dolg, M.: Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16–18 elements, J. Chem. Phys., 119, 11113–11123, https://doi.org/10.1063/1.1622924, 2003.
Popp, P. J., Marcy, T. P., Jensen, E. J., Kärcher, B., Fahey, D. W., Gao, R. S., Thompson, T. L., Rosenlof, K. H., Richard, E. C., Herman, R. L., Weinstock, E. M., Smith, J. B., May, R. D., Vömel, H., Wilson, J. C., Heymsfield, A. J., Mahoney, M. J., and Thompson, A. M.: The observation of nitric acid-containing particles in the tropical lower stratosphere, Atmos. Chem. Phys., 6, 601–611, https://doi.org/10.5194/acp-6-601-2006, 2006.
Rauch, H., Lemmel, H., Baron, M., and Loidl, R.: Measurement of a confinement induced neutron phase, Nature, 417, 630–632, https://doi.org/10.1038/nature00773, 2002.
Rong, H., Liu, J., Zhang, Y., Du, L., Zhang, X., and Li, Z.: Nucleation mechanisms of iodic acid in clean and polluted coastal regions, Chemosphere, 253, 126743, https://doi.org/10.1016/j.chemosphere.2020.126743, 2020.
Saiz-Lopez, A., Fernandez, R. P., Ordóñez, C., Kinnison, D. E., Gómez Martín, J. C., Lamarque, J.-F., and Tilmes, S.: Iodine chemistry in the troposphere and its effect on ozone, Atmos. Chem. Phys., 14, 13119–13143, https://doi.org/10.5194/acp-14-13119-2014, 2014.
Salignat, R., Rissanen, M., Iyer, S., Baray, J.-L., Tulet, P., Metzger, J.-M., Brioude, J., Sellegri, K., and Rose, C.: Measurement report: Insights into the chemical composition and origin of molecular clusters and potential precursor molecules present in the free troposphere over the southern Indian Ocean: observations from the Maïdo Observatory (2150 m a.s.l., Réunion), Atmos. Chem. Phys., 24, 3785–3812, https://doi.org/10.5194/acp-24-3785-2024, 2024.
Sarr, S., Graton, J., Rahali, S., Montavon, G. F., and Galland, N.: Delocalized relativistic effects, from the viewpoint of halogen bonding, Phys. Chem. Chem. Phys., 23, 4064–4074, https://doi.org/10.1039/D0CP05840H, 2021.
Singh, H. B., Herlth, D., Kolyer, R., Salas, L., Bradshaw, J. D., Sandholm, S. T., Davis, D. D., Crawford, J., Kondo, Y., Koike, M., Talbot, R., Gregory, G. L., Sachse, G. W., Browell, E., Blake, D. R., Rowland, F. S., Newell, R., Merrill, J., Heikes, B., Liu, S. C., Crutzen, P. J., and Kanakidou, M.: Reactive nitrogen and ozone over the western Pacific: Distribution, partitioning, and sources, Journal of Geophysical Research: Atmospheres, 101, 1793–1808, https://doi.org/10.1029/95jd01029, 1996.
Sipilä, M., Sarnela, N., Jokinen, T., Henschel, H., Junninen, H., Kontkanen, J., Richters, S., Kangasluoma, J., Franchin, A., peräkylä, O., Rissanen, M. P., Ehn, M., Vehkamäki, H., Kurten, T., Berndt, T., Petäjä, T., Worsnop, D., Ceburnis, D., Kerminen, V. M., Kulmala, M., and O'Dowd, C.: Molecular-scale evidence of aerosol particle formation via sequential addition of HIO3, Nature, 537, 532–534, https://doi.org/10.1038/nature19314, 2016.
Stolzenburg, D., Simon, M., Ranjithkumar, A., Kürten, A., Lehtipalo, K., Gordon, H., Ehrhart, S., Finkenzeller, H., Pichelstorfer, L., Nieminen, T., He, X.-C., Brilke, S., Xiao, M., Amorim, A., Baalbaki, R., Baccarini, A., Beck, L., Bräkling, S., Caudillo Murillo, L., Chen, D., Chu, B., Dada, L., Dias, A., Dommen, J., Duplissy, J., El Haddad, I., Fischer, L., Gonzalez Carracedo, L., Heinritzi, M., Kim, C., Koenig, T. K., Kong, W., Lamkaddam, H., Lee, C. P., Leiminger, M., Li, Z., Makhmutov, V., Manninen, H. E., Marie, G., Marten, R., Müller, T., Nie, W., Partoll, E., Petäjä, T., Pfeifer, J., Philippov, M., Rissanen, M. P., Rörup, B., Schobesberger, S., Schuchmann, S., Shen, J., Sipilä, M., Steiner, G., Stozhkov, Y., Tauber, C., Tham, Y. J., Tomé, A., Vazquez-Pufleau, M., Wagner, A. C., Wang, M., Wang, Y., Weber, S. K., Wimmer, D., Wlasits, P. J., Wu, Y., Ye, Q., Zauner-Wieczorek, M., Baltensperger, U., Carslaw, K. S., Curtius, J., Donahue, N. M., Flagan, R. C., Hansel, A., Kulmala, M., Lelieveld, J., Volkamer, R., Kirkby, J., and Winkler, P. M.: Enhanced growth rate of atmospheric particles from sulfuric acid, Atmos. Chem. Phys., 20, 7359–7372, https://doi.org/10.5194/acp-20-7359-2020, 2020.
Verstraete, M. J., Torrent, M., Jollet, F. C., Zérah, G., and Gonze, X.: Density functional perturbation theory with spin-orbit coupling: Phonon band structure of lead, Phys. Rev. B, 78, 045119, https://doi.org/10.1103/PhysRevB.78.045119, 2008.
Wang, M., Xiao, M., Bertozzi, B., Marie, G., Rorup, B., Schulze, B., Bardakov, R., He, X. C., Shen, J., Scholz, W., Marten, R., Dada, L., Baalbaki, R., Lopez, B., Lamkaddam, H., Manninen, H. E., Amorim, A., Ataei, F., Bogert, P., Brasseur, Z., Caudillo, L., De Menezes, L. P., Duplissy, J., Ekman, A. M. L., Finkenzeller, H., Carracedo, L. G., Granzin, M., Guida, R., Heinritzi, M., Hofbauer, V., Hohler, K., Korhonen, K., Krechmer, J. E., Kurten, A., Lehtipalo, K., Mahfouz, N. G. A., Makhmutov, V., Massabo, D., Mathot, S., Mauldin, R. L., Mentler, B., Muller, T., Onnela, A., Petaja, T., Philippov, M., Piedehierro, A. A., Pozzer, A., Ranjithkumar, A., Schervish, M., Schobesberger, S., Simon, M., Stozhkov, Y., Tome, A., Umo, N. S., Vogel, F., Wagner, R., Wang, D. S., Weber, S. K., Welti, A., Wu, Y., Zauner-Wieczorek, M., Sipila, M., Winkler, P. M., Hansel, A., Baltensperger, U., Kulmala, M., Flagan, R. C., Curtius, J., Riipinen, I., Gordon, H., Lelieveld, J., El-Haddad, I., Volkamer, R., Worsnop, D. R., Christoudias, T., Kirkby, J., Mohler, O., and Donahue, N. M.: Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation, Nature, 605, 483–489, https://doi.org/10.1038/s41586-022-04605-4, 2022.
Wang, S., Peng, Y., Zhang, Q., Wang, W., and Wang, Q.: Mechanistic understanding of rapid H2SO4-HNO3-NH3 nucleation in the upper troposphere, Sci. Total Environ., 883, 163477, https://doi.org/10.1016/j.scitotenv.2023.163477, 2023.
Wen-Qin, W.: Near-Space Wide-Swath Radar Imaging With Multiaperture Antenna, IEEE Antennas Wirel. Propag. Lett., 8, 461–464, https://doi.org/10.1109/lawp.2009.2016584, 2009.
Wespes, C., Hurtmans, D., Herbin, H., Barret, B., Turquety, S., Hadji-Lazaro, J., Clerbaux, C., and Coheur, P. F.: First global distributions of nitric acid in the troposphere and the stratosphere derived from infrared satellite measurements, J. Geophys. Res.-Atmos., 112, D13311, https://doi.org/10.1029/2006jd008202, 2007.
Williamson, C. J., Kupc, A., Axisa, D., Bilsback, K. R., Bui, T., Campuzano-Jost, P., Dollner, M., Froyd, K. D., Hodshire, A. L., Jimenez, J. L., Kodros, J. K., Luo, G., Murphy, D. M., Nault, B. A., Ray, E. A., Weinzierl, B., Wilson, J. C., Yu, F., Yu, P., Pierce, J. R., and Brock, C. A.: A large source of cloud condensation nuclei from new particle formation in the tropics, Nature, 574, 399–403, https://doi.org/10.1038/s41586-019-1638-9, 2019.
Xenofontos, C., Kohl, M., Ruhl, S., Almeida, J., Beckmann, H. M., Caudillo-Plath, L., Ehrhart, S., Höhler, K., Kaniyodical Sebastian, M., Kong, W., Kunkler, F., Onnela, A., Rato, P., Russell, D. M., Simon, M., Stark, L., Umo, N. S., Unfer, G. R., Yang, B., Yu, W., Zauner-Wieczorek, M., Zgheib, I., Zheng, Z., Curtius, J., Donahue, N. M., El Haddad, I., Flagan, R. C., Gordon, H., Harder, H., He, X.-C., Kirkby, J., Kulmala, M., Möhler, O., Pöhlker, M. L., Schobesberger, S., Volkamer, R., Wang, M., Borrmann, S., Pozzer, A., Lelieveld, J., and Christoudias, T.: The impact of ammonia on particle formation in the Asian Tropopause Aerosol Layer, npj Clim. Atmos. Sci., 7, https://doi.org/10.1038/s41612-024-00758-3, 2024.
Xia, D., Chen, J., Yu, H., Xie, H. B., Wang, Y., Wang, Z., Xu, T., and Allen, D. T.: Formation Mechanisms of Iodine-Ammonia Clusters in Polluted Coastal Areas Unveiled by Thermodynamics and Kinetic Simulations, Environ. Sci. Technol., 54, 9235–9242, https://doi.org/10.1021/acs.est.9b07476, 2020.
Xie, H.-B. and Elm, J.: Tri-Base Synergy in Sulfuric Acid-Base Clusters, Atmosphere, 12, 1260, https://doi.org/10.3390/atmos12101260, 2021.
Yu, H., Ren, L., Huang, X., Xie, M., He, J., and Xiao, H.: Iodine speciation and size distribution in ambient aerosols at a coastal new particle formation hotspot in China, Atmos. Chem. Phys., 19, 4025–4039, https://doi.org/10.5194/acp-19-4025-2019, 2019.
Zaki, N. H. M., Ali, A. M. M., Mohamad Taib, M. F., Wan Ismail, W. I. N., Sepeai, S., and Ramli, A.: Dispersion-correction density functional theory (DFT+D) and spin-orbit coupling (SOC) method into the structural, electronic, optical and mechanical properties of CH3NH3PbI3, Comput. Condens. Matte., 34, e00777, https://doi.org/10.1016/j.cocom.2022.e00777, 2023.
Zhang, R., Khalizov, A., Wang, L., Hu, M., and Xu, W.: Nucleation and growth of nanoparticles in the atmosphere, Chem. Rev., 112, 1957–2011, https://doi.org/10.1021/cr2001756, 2012.