the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A possible unaccounted source of nitrogen-containing compound formation in aerosols: amines reacting with secondary ozonides
Junting Qiu
Xinlin Shen
Jiangyao Chen
Guiying Li
Taicheng An
Nitrogen (N)-containing compounds have a significant impact on the optical and toxicological properties of aerosols. 1,2,4-Trioxolanes, known as secondary ozonides (SOZs), i.e., key products from the ozonolysis of biogenic terpenoids, are readily taken up into atmospheric aerosols and act as oxidants, potentially interacting with amines in the atmosphere. In the present work, we carefully investigated the component of the particles produced by the ozonolysis of β-caryophyllene (β-C) in the presence of ethylamine (EA), methylamine (MA), dimethylamine (DMA), or ammonia. The mass spectrometric results show that SOZ is the dominant product from the ozonolysis of β-C. It readily reacts with EA and MA but has inert reactivities toward DMA and ammonia. Similar experimental results were achieved with α-humulene (α-H), an isomer of β-C, was used in place of β-C. Additionally, D2O and HO solvents were used for the characterization of products. The results revealed an intriguing phenomenon where the products from β-C SOZ and α-H SOZ reacting with the same amine (EA or MA) possessed different functional groups, despite the fact that they are isomerized species with identical chemical structure (1,2,4-trioxolane). This indicates that the chemical conformation of SOZs has a strong influence on how they react with amines. For the first time, SOZs derived from β-C and α-H reacting with amines are reported in this study; this may represent a hitherto unrecognized source of N-containing compound production in atmospheric aerosols.
- Article
(1855 KB) - Full-text XML
-
Supplement
(958 KB) - BibTeX
- EndNote
Nitrogen (N)-containing compounds are ubiquitous in atmospheric aerosols. The compounds possessing some N-containing functional groups, such as imidazole, pyrazine, and organonitrate groups, are recognized as acting as chromophores, which are closely associated with optical properties of the aerosols (Laskin et al., 2015; Moise et al., 2015). Meanwhile, some N-containing compounds are considered to be hazardous to human health. For instance, nitration of polycyclic aromatic compounds (PAHs) typically contributes more to the toxicity of ambient PM than parent PAHs (Albinet et al., 2008). Another study revealed that the compounds in some pollens will have a higher allergenic potential after being converted into N-containing species (Cuinica et al., 2014). A main contributor to the formation of N-containing compounds is the process where NO and/or NO2 are transferred to terminal products in the NOx radical cycle, including peroxy nitrates (RO2NO2), alkyl nitrates (RONO2), and nitric acid (HNO3) (Perring et al., 2013). An additional route for N-containing species formation in the atmosphere is the oxidation of volatile organic compounds (VOCs) by the nitrate radical (NO3) (Ng et al., 2017; Fry et al., 2014). On the other hand, atmospheric amine chemistry involving new particle formation (NPF) events is another important contributor to N-containing compounds in aerosols. The substitution by one or more organic functional groups leads to stronger basicity of amines than ammonia, indicating that amines are ready to participate in NPF though acid–base reactions, which have been confirmed in numerous field research studies (Yao et al., 2016; Yu et al., 2012) and laboratory work (Almeida et al., 2013; Erupe et al., 2011; Glasoe et al., 2015; Kurten et al., 2014; Tong et al., 2020).
Amines are extensively emitted from various biogenic and anthropogenic sources, such as biomass burning, animal husbandry, ocean organisms, automobiles, and industries (Ge et al., 2011). Aliphatic amines with low molecular weight, including methylamine (MA), dimethylamine (DMA), trimethylamine (TMA), or ethylamine (EA), are the dominant species of amines in the atmosphere. Yao et al. (2016) reported a high concentration of total DMA and EA up to 130 pptv in Shanghai, China. In a boreal forest, the concentration of total DMA and EA were detected to be around 150 pptv (Kieloaho et al., 2013). Amines involving atmospheric chemistry regarding the formation of N-containing compounds and growth of secondary organic aerosols (SOAs) have been investigated in cohort work. For instance, amines are found to be fairly reactive toward important atmospheric aldehydes in the condensed phase, such as glyoxal, methylglyoxal, glycoaldehyde, or acetaldehyde, which significantly affect the physiochemical properties of aerosols and contribute to SOA growth (De Haan et al., 2011; Galloway et al., 2014). A recent study showed that alkylaminium carboxylates formed from the reactions of amines with organic acids have lower vapor pressures than original organic acids, implying that alkylaminium carboxylates could enhance SOAs formation (Lavi et al., 2015). Another study carried out by the same group reinforced the crucial role of alkylaminium carboxylates in determining the characteristics of aerosols for the reason that alkylaminium carboxylates are capable of enhancing the particle hygroscopicity and the cloud condensation nuclei activity (Gomez-Hernandez et al., 2016). In addition, Duporte et al. (2017) reported a systematic study on the ozonolysis of α-pinene in the presence of DMA, and they found that DMA was able to react with aldehydes or carboxylate acids generated from the ozonolysis of α-pinene, enhancing SOAs formation.
Amines can also be directly oxidized by major atmospheric oxidants, such as OH, O3, and NO3, in gas phase or in the condensed phase (Ge et al., 2011; Qiu and Zhang, 2013; Tang et al., 2013). Recently, the interactions of Criegee intermediates with amines have been investigated in some studies; however, the actual effect of Criegee intermediates on oxidizing amines is by now unclear (Chhantyal-Pun et al., 2019b; Kumar and Francisco, 2019; Ma et al., 2020; Mull et al., 2020). 1,2,4-Trioxolanes, known as secondary ozonides (SOZs), are formed by the intramolecular reactions of the Criegee moieties with the carbonyl endo groups, as well as bimolecular reaction of Criegee intermediates with carbonyl species such as formaldehyde and acetone (Chhantyal-Pun et al., 2019a, 2020; Cornwell et al., 2021; Wang et al., 2022). SOZs are major products from the ozonolysis of important biogenic terpenoids, such as limonene, carene, β-pinene, β-caryophyllene, and α-humulene (Winterhalter et al., 2000; Vibenholt et al., 2009; Nguyen et al., 2009; Winterhalter et al., 2009; Beck et al., 2011), and they are readily taken up into atmospheric aerosols (Yao et al., 2014). The formation of SOZs occurs not only in the gas phase but also in bulk liquid phases (Griesbaum et al., 1996) and at gas–liquid/solid interfaces (Enami et al., 2008; Karagulian et al., 2008; Coffaro and Weisel, 2022). Additionally, SOZs can also be formed via OH reactions of lipid molecules (Zeng et al., 2020; Zhang et al., 2018). Since SOZs are categorized as both organic peroxides and reactive oxygen species (Sanchez and Myers, 2000), they potentially function as oxidants and interact with amines in the atmospheric condensed phase. Therefore, the aim of this study is to determine whether the interaction of SOZs with amines results in the formation of N-containing compounds.
β-Caryophyllene (β-C) and α-humulene (α-H) are representative sesquiterpenes (Arey et al., 1995; Helmig et al., 2007), and their chemical structures are listed in Fig. S1 in the Supplement. Albeit not predominant terpene species like isoprene or α-pinene, β-C and α-H are of special significance as powerful SOA makers, due to their rapid degradation in the atmosphere and the low volatility of the degradation products. SOZs are dominant products from the ozonolysis of both β-C and α-H (Nguyen et al., 2009; Winterhalter et al., 2009; Beck et al., 2011); thus, in this study, we choose the reactions of β-C and α-H with O3 to produce SOZs and investigate the reactivities of SOZs toward amines. Firstly, we carefully carry out the ozonolysis experiments of β-C in the absence/presence of EA in a smog chamber. The particles generated inside the smog chamber are monitored, and the chemical components of the particles are detected by mass spectrometry. D2O and HO isotope labelling experiments were performed for the identification of the products detected. Next, chemical structures of the products formed from EA reacting with β-C SOZ and α-H SOZ are compared to understand the effect of molecular conformation on the reaction mechanism of SOZs. In addition, the reactivities of SOZs toward MA, EA, DMA, or ammonia are also comparably investigated.
2.1 Ozonolysis experiment
All the ozonolysis experiments were carried out in a smog chamber. Considering that the details of the smog chamber have been reported in other articles (Luo et al., 2020, 2021), we just make a brief description herein. Two extremely similar pillow-shaped Teflon reactors (2.5 m × 2.0 m) were mounted inside the smog chamber, and each reactor was surrounded by three high-efficiency ionizing blowers (varying from 0 to a maximum of 2000 rpm), in order to mix the air inside the reactors evenly. In this study, each experiment needs to be repeated at least three times. To avoid unexpected errors in the experiment processes, all the experiments were conducted in the same reactor. Before the operation of each experiment, the reactor was filled with zero air at a volume of 1000 L, with no detectable particles; <0.5 ppb non-methane hydrocarbon (NMHC); and <1 ppb NOx, O3, and carbonyl compounds. The relative humidity (RH) inside the reactor was ≤5 %, and the temperature was kept at 295±3 K.
The schematic experimental procedure for the ozonolysis of β-C in the presence of EA is presented in Fig. S2. β-C is well mixed with EA before the addition of O3, and the initial concentrations of chemical species inside a reactor are β-C 200 ppb, EA 80 ppb, and O3 50 ppb. Other experiments (such as 200 ppb β-C + 80 ppb MA + 50 ppb O3, or 200 ppb α-H + 80 ppb ammonia + 50 ppb O3) are carried out with the same method. Because the reaction rate of O3 toward β-C ( cm3 molec.−1 s−1) or α-H ( cm3 molec.−1 s−1) (Atkinson and Arey, 2003) is much faster than amines or ammonia (10−18–10−21 cm3 molec.−1 s−1) (Ge et al., 2011) and because the initial concentration of β-C or α-H (200 ppb) is many times that of O3 (50 ppb), O3 will almost be consumed intermediately via the reaction with β-C or α-H after being injected inside the reactor. The products from ozonolysis of β-C or α-H may subsequently participate in the reactions with amines or ammonia. Ozonolysis leads to the generation of particle matter via condensation of oxidized low-volatility species, which are able to be monitored by a scanning mobility particle sizer (SMPS, TSI). All experiments were performed in dark conditions and without an OH scavenger.
2.2 Particle collection and analysis
Particles with considerable sizes were collected on 47 mm quartz filters at a timing of 3 h after the injection of ozone, and quartz filters were pretreated via 8 h baking inside a muffle furnace at a temperature of 450 ∘C. All the filter samples were wrapped in aluminum foil and stored in a freezer at −18 ∘C until extraction. Particle-phase compounds were extracted by soaking filter samples in a 5 mL mixture of acetonitrile ultrapure water (AN W, ) for 30 min at room temperature. D2O and HO were also used in extraction instead of ultrapure water for a detailed characterization of products. A high-resolution electrospray ionization mass spectrometer (ESI-MS, Thermo Fisher Q Extract quadrupole-Orbitrap) was used in the detection of chemical compounds extracted in solutions.
2.3 Materials
Gas-phase chemicals, such as O3 and MA, were directly injected into the reactor, while liquid phase chemicals, such as EA and DMA, were injected slowly through a T-junction connected to a fluorinated ethylene propylene line and spread with the flow of purified dry air, by using airtight syringes (Shanghai Anting). O3 was generated by a commercial ozone generator, and the amount of O3 was carefully calculated according to the injection time and the power of the ozone generator. Before the operation of each experiment, O3 was injected into the reactor filled with 1000 L zero air, and its concentration was confirmed by an O3 analyzer (model 49i, Thermo Scientific). Ultrapure water was obtained from a Millipore Milli-Q water purification system (Xiamen Research Water Purification Technology, Unique-R20, resistivity ≥18.2 MΩ cm at 298 K). Chemicals β-caryophyllene (Tokyo Chemical Industrial, >95 %), α-humulene (Tokyo Chemical Industrial, >93 %), methylamine (Wuhan Newradar, 98.6 ppm mixed in N2 gas), ethylamine (Aladdin Industrial, 70 wt % in H2O), dimethylamine (Aladdin Industrial, 40 wt % in H2O), ammonia solution (Aladdin Industrial, 25 wt % in H2O), acetonitrile (Aladdin Industrial, ≥99.9 %), D2O (J&K, >99.8 at. % D), and HO (Macklin, >97 at. % 18O) were used as received.
3.1 Reaction of β-C SOZ with EA
The particle formation was monitored by SMPS from the ozonolysis of β-C in the absence/presence of EA, and the chemical components of the particles were analyzed, as shown in Fig. 1. Because β-C is fairly reactive toward O3 and because the products generated in situ have extremely low volatility, the formation of particles can be observed intermediately after O3 being injected inside the Teflon reactor. The number of particles decreased rapidly as presented in Fig. 1a due to their coagulation to form larger particles or due to deposition on the wall. According to Fig. 1b, the total particle volume produced by the ozonolysis of β-C grew initially before beginning to decline about 10 min after the particle loss rate surpassed the creation rate. With the addition of EA, no discernible change in the number concentration of particles was seen in Fig. 1a; however, the volume of total particles slightly increased as shown in Fig. 1b.
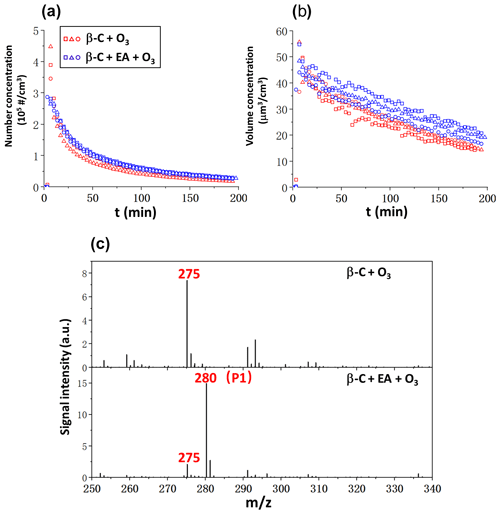
Figure 1Effects of EA on size and chemical composition of particles produced by the ozonolysis of β-C. (a) Number concentrations of particles, (b) Volume concentrations of total particles, (c) Positive-ion ESI mass spectra of the chemical components of particles extracted in AN W () solutions. Blue dots and red dots represent three independent experiments.
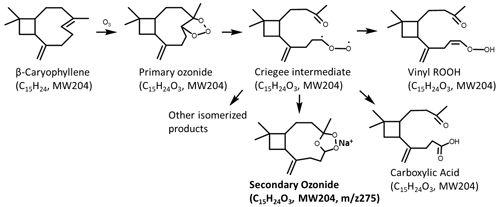
The addition of EA has limited effect on promoting particle formation, because the volatility of the products from the ozonolysis of β-C is sufficiently low. Nevertheless, the reaction of EA with the products from the ozonolysis of β-C considerably altered the chemical components of particles as shown in Fig. 1c that contrasts the positive-ion ESI mass spectra of products from the ozonolysis of β-C in the absence/presence of EA, respectively. In the β-C + O3 experiment, the prominent signal rise at is assigned to Na+-adducted C15H24O3 species, . Both experimental and theoretical research have explicitly explored the mechanisms on the ozonolysis of β-C (Nguyen et al., 2009; Winterhalter et al., 2009). Major species of C15H24O3 are SOZ, vinyl ROOH, and carboxylic acid, which are isomerized products of Criegee intermediates as demonstrated in Scheme 1. Furthermore, the product appearing at was identified by the method of replacing the AN W mixture with AN D2O () in the extraction process. D2O was used to test if a molecule contains an active H atom. When a molecule possessing an active H atom is dissolved in D2O solution, the active H atom readily exchanges with the D atom of D2O, increasing its molecular weight by 1 unit. The results in Fig. S3 show that the product appearing at possesses no exchangeable H atom, implying it should be . In contrast, the H atoms of vinyl ROOH and carboxylic acid are exchangeable with the D atom. Na+ was leached into the solution from trace metals in the laboratory glassware (Greaves and Roboz, 2014). Na+ has an affinity toward an O atom of a species possessing R–O–R′ such as ethers (Sugimura et al., 2015). In addition, a previous study reported that SOZ originated from α-terpineol was detectable as Na+-adducted species (Qiu et al., 2022).
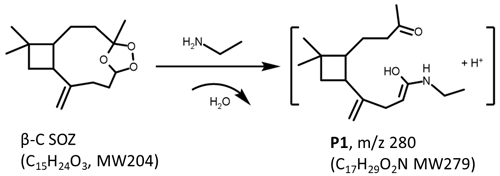
The observation that the intensity of clearly decreased in the experiment of β-C + EA + O3 indicates that β-C SOZ readily reacts with EA. The intense peaks appear at (P1), which is assigned to the H+-adducted products from β-C SOZ reacting with EA, and . Because the aim of this study is to investigate the interactions of SOZs with amines, we only present a part of the products related to this study in mass spectra. The larger products from the ozonolysis of β-C as well as their potential interactions with amines will not be discussed in this work. For the same reason, all the experiments were carried out at an extremely low humidity (RH ≤5 %) to avoid the generation of unwanted products in the presence of high-concentration water vapor (Kundu et al., 2017).
The vast majority of P1 is formed from the heterogeneous reaction of EA with SOZ in the condensed phase. The evidence can be found in Fig. S4, that is even when EA is added in the reactor 30 min after the start of ozonolysis (a situation that SOZ is almost in the condensed phase), more than half of the P1 is still produced compared to the situation that EA is well mixed. The results in Fig. S4 provides another information that P1 is not the product from EA reacting with Criegee intermediate, the precursor of SOZ, which is a highly reactive species, because Criegee intermediate could not have survived for so long.
Moreover, the particles generated from the ozonolysis of β-C in the absence of EA were sampled and dissolved in AN W solution. EA was directly added into the solution, and 30 min later the solution was analyzed by the mass spectrometer. As a result, no signal appeared at in mass spectra, indicating that β-C SOZ reacting with EA in liquid phase is not available. Through this experiment, the possibility that EA vapor condensed onto the particles first and then reacted with β-C SOZ in the extraction process was ruled out, and it was determined that the reaction of β-C SOZ with EA occurred in the smog chamber.
To the best of our knowledge, the phenomenon that β-C SOZ reacting with EA leads to the production of N-containing compounds is reported for the first time in this work. Next, chemical analysis of products appearing at was conducted to better comprehend the chemical structures of previously unreported N-containing compounds.
3.2 Chemical identification of P1
D2O and HO isotope labelling experiments were performed for the chemical identification of P1 in this work. As mentioned before, D2O was used to confirm if a molecule contains an active H atom, while HO was used to test if a molecule is carrying a carbonyl group. After being dissolved in HO solution, the compound possessing a carbonyl group forms a gem-diol via the addition of HO, which is a reversible process. Since the concentration of HO is overwhelming, 16O of the compound will be almost replaced by 18O of HO, resulting in a rise in molecular weight by 2 units. This method was proven to be beneficial for examining the chemical structure of unidentified species in previous studies (Qiu et al., 2019, 2020a, b). Figure 2 shows high-resolution positive-ion ESI mass spectra of products extracted in AN W (), AN D2O (), and AN HO () solutions, from the reaction of β-C SOZ with EA, where P1 appeared at 280.226 in the AN W experiment. P1 shifted by +3 mass units in the AN D2O experiment and +2 mass units in the AN HO experiment, showing that P1 possess two exchangeable H atoms ( contributed another +1 mass unit) and one exchangeable O atom, respectively.
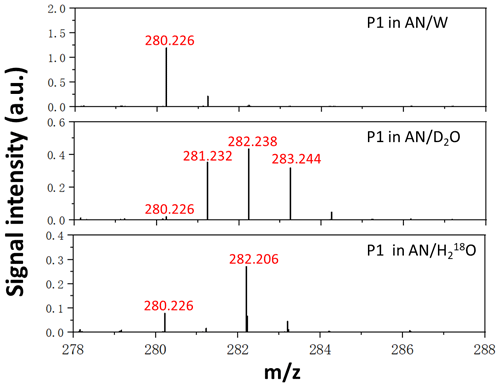
Figure 2High-resolution positive-ion ESI mass spectra of P1 extracted in AN W (), AN D2O (), and AN HO () solutions.
The mechanism of β-C SOZ reacting with EA can be explained as follows. The electronegativity of the neighboring oxygens induced a net positive charge on the α-carbon of β-C SOZ. EA acting as a nucleophile may add to α-carbon and cleave β-C SOZ. This theory is supported by a previously reported study by Na et al. (2006), which revealed that ammonia reacts with styrene SOZ via a nucleophilic attack at the α-carbon of styrene SOZ, producing benzaldehyde, hydrogen peroxide, and phenylmethanimine in the process. Despite the attack of EA that opened the cyclic structure of β-C SOZ, we did not detect cleavage products as Na et al. (2006) reported. Instead, P1 detected in this work is the product from the addition reaction between β-C SOZ and EA, and a water molecule was removed in the process. Based on the molecular weight of P1 and the results of D2O and HO isotope labelling experiments, a potential structure of P1 is illustrated in Scheme 2. It has two active H atoms in the –NH and –OH moieties, and C=16O can be transferred into C=18O via an HO addition reaction (see Scheme S1 in the Supplement).
3.3 Reactions of β-C SOZ with MA, DMA and ammonia
By replacing EA with MA, DMA, or ammonia, another three smog chamber experiments were carried out. The particles were sampled and analyzed by electrospray mass spectrometry as shown in Fig. 3. Obviously, in the β-C + MA + O3 experiment, the intensity of clearly diminished and an intense peak appeared at (P2), which is assigned to the H+-adducted product from β-C SOZ reacting with MA; . In sharp contrast, the intensities of in the other two experiments are essentially identical to that in the β-C + O3 experiment, suggesting that DMA and ammonia have inert reactivities toward β-C SOZ. The results of D2O and HO isotope labelling experiments of P2 are shown in Fig. S5a. P2 shifted by +3 mass units in the AN D2O experiment and +2 mass units in the AN HO experiment, which is identical to P1. A possible structure of P2 that is similar to P1 is presented in Scheme S2.
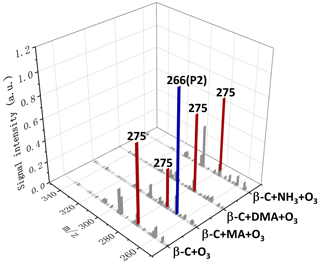
Figure 3Positive-ion ESI mass spectra of the products extracted in AN W () from ozonolysis of β-C in the absence/presence of MA, DMA, or ammonia.
Substituted by one alkyl moiety, EA or MA are considered more basic than ammonia, which potentially increased the reactivity of EA or MA toward β-C SOZ. On the other hand, the reason why DMA is less reactive than EA and MA can be explained by the fact that DMA possesses two alkyl moieties, resulting in a steric hinderance that would limit the accessibility of DMA to β-C SOZ. Na et al. (2006) also pointed out that α-methylstyrene SOZ is less reactive than styrene SOZ toward ammonia, due to it being sterically hindered by the methyl group attached to the α-carbon of 1,2,4-trioxolane. Moreover, in order to obtain more information about the mechanism of SOZs reacting with amines, in the following section we mainly report the reactions of another SOZ produced by the ozonolysis of α-humulene (α-H), an isomer of β-C, with EA, MA, DMA, and ammonia.
3.4 Reactions of α-H SOZ with EA, MA, DMA, and ammonia
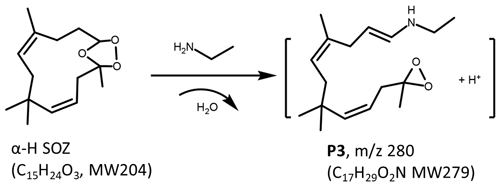
The smog chamber experiments of α-H + amines/ammonia + O3 were conducted by using the same procedure, and the chemical composition of particles generated in the reactor was analyzed by the mass spectrometer as shown in Fig. 4. SOZ (, ) is the dominant product from the ozonolysis of α-H, which is consistent with the previous study (Beck et al., 2011). As can be seen, the behavior of α-H SOZ is comparable to that observed in the experiments of β-C, exhibiting inert reactivities toward DMA and ammonia and selectively reacting with EA and MA. The products from α-H SOZ reacting with EA and MA appeared at (P3) and 266 (P4). Since α-H SOZ and β-C SOZ are isomerized species, molecular formula of P3 and P4 should be the same as P1 and P2, respectively, which are and .
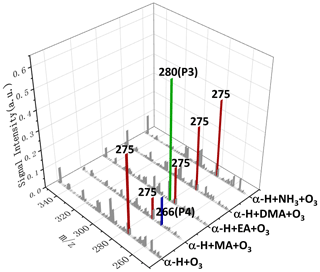
Figure 4Positive-ion ESI mass spectra of the products extracted in AN W () from ozonolysis of α-H in the absence/presence of MA, EA, DMA, or ammonia.
D2O and HO isotope labelling experiments were also performed for chemical identification of P3 and P4. High-resolution positive-ion ESI mass spectra of P3 extracted in AN W (), AN D2O (), and AN HO () solutions are demonstrated in Fig. 5. The observation that P3 shifted by +2 mass units in AN D2O experiment and has no mass-shift in the AN HO experiment indicates that P3 generated from α-H SOZ reacting with EA possesses only one exchangeable H atom and no exchangeable O atom. A probable contributor to the exchangeable H atom is the –NH moiety. Given that P3 possesses no carbonyl or hydroxyl moieties, a dioxirane structure generated by the breaking of C–O bonds appears to be plausible for P3, and a potential structure of P3 is deducted and displayed in Scheme 3. The results from isotope labelling experiments of P4 are presented in Fig. S5B, and they point out a production mechanism of P4 that is similar to that of P3 as illustrated in Scheme S2. What should be mentioned is that α-H contains three endocyclic double bonds which are able to be attacked by ozone to generate different SOZs. As a result, P3 and P4 may have multiple conformations; however, to simplify the representations, just one kind is provided here as an example of each. In addition, it is worth noting that the signal intensity of P4 in the α-H + MA + O3 experiment is rather weak, even though the majority of α-H SOZ has been consumed via its reaction with MA and contribute to the formation of P4. This phenomenon can be explained by the fact that dioxirane compounds are active species and their stabilities are highly dependent on their molecular structures (El-Assaad et al., 2022). In other words, P4 dissipated rapidly after it formed because it is less stable than P3.
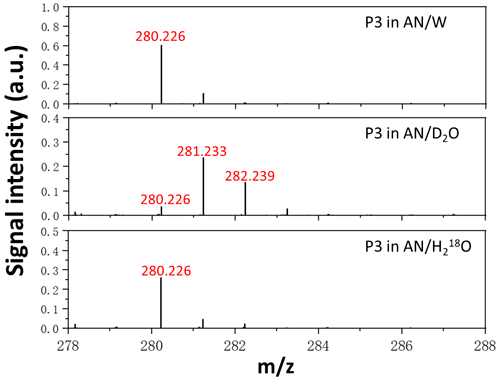
Figure 5High-resolution positive-ion ESI mass spectra of P3 extracted in AN W (), AN D2O (), and AN HO () solutions.
An astonishing phenomenon revealed here is that the product of α-H SOZ reacting with EA (P3) bears no resemblance to that of β-C SOZ reacting with EA (P1), in spite of the fact that α-H SOZ is an isomerized species of β-C SOZ and they share the same chemical structure of 1,2,4-trioxolane, which suggests that the molecular conformations of SOZs have a substantial impact on their reaction mechanism, resulting in the formation of N-containing products processing various functional groups.
The predominant source of SOZs is biogenic terpenoids, which are emitted to the atmosphere at a rate of 1014 g yr−1 (Guenther et al., 1995); SOZs originating from terpenoids are less volatile and hence more easily taken up into aerosols (Yao et al., 2014) Multiphase ozonolysis and OH oxidations of unsaturated organic compounds possessing C=C bond(s) also produce SOZs, which causes an accumulation of SOZs in condensed phases (Heine et al., 2017; Enami et al., 2008; Karagulian et al., 2008; Coffaro and Weisel, 2022; Zhou et al., 2022; Zhang et al., 2018). Terpenoid-derived SOZs are comparatively stable organic peroxides in the condensed phase. For instance, a recent study revealed that C10 and C13 SOZs derived from terpineol can persist in water for weeks (Qiu et al., 2022). In addition, products from the ozonolysis of terpenoids, including SOZs, are usually surface active in aerosols (Qiu et al., 2018a, b), which facilitated their reactions toward gas-phase amines. Thus, SOZs reacting with amines are probably a non-negligible source of N-containing compound formation in aerosols.
Moreover, Na et al. (2006) reported that SOZs derived from styrene and α-methylstyrene can react with ammonia. In sharp contrast, our research suggests that both β-C SOZ and α-H SOZ exhibit inert reactivities toward ammonia but readily react with EA and MA. Additionally, we discovered that SOZs in different conformations reacting with EA or MA produce N-containing compounds with various functional groups. The aforementioned studies indicate that the interaction of SOZs with amines or ammonia is a complicated process in the real atmosphere, leading to the formation of various N-containing compounds. Due to the distinct roles that N-containing compounds with different functional groups play in the properties of aerosols (Laskin et al., 2015), a thorough investigation on the mechanism of SOZs reacting with amines is still urgently required. Nevertheless, the information obtained in the present study is just the tip of the iceberg, and detailed laboratory work combined with field research is necessary toward a full picture of N-containing compounds originating from SOZs reacting with amines or ammonia.
Apart from SOZs, other organic peroxides like ROOH or ROOR′ play more significant roles in atmospheric chemistry (Wang et al., 2023). For example, the oxidation of dissolved SO2 by organic peroxides has been considered a main source of sulfate formation in aerosols (Dovrou et al., 2019, 2021; Wang et al., 2019, 2021; Yao et al., 2019). In addition, organic peroxides can directly interact with transitional metal ions via Fenton-like reaction mechanisms (Fang et al., 2020; Hu et al., 2021; Tong et al., 2016; Wei et al., 2022). However, the interaction of organic peroxides with amines has rarely been reported in previous studies. The present study recommends extensive research on organic peroxides including SOZs reacting with amines, which will deepen our understanding of the source of N-containing compounds and benefit the studies on precisely evaluating the effects of atmospheric aerosols on human health and climate (Seinfeld et al., 2016; Shiraiwa et al., 2017; Shrivastava et al., 2017).
In this study, chamber experiments showed that the component of particles produced by the ozonolysis of both β-C and α-H was dramatically altered in addition of EA or MA, which originated from the reactions of SOZs with EA or MA. However, both β-C SOZ and α-H SOZ were found to have inert reactivities toward DMA and ammonia. Additionally, D2O and HO isotope labelling experiments revealed that the products from β-C SOZ and α-H SOZ reacting with the same amine (EA or MA) possessed different functional groups, despite β-C SOZ and α-H SOZ being isomerized species that share the same chemical structure of 1,2,4-trioxolane. The experimental results obtained in this study indicate that a variety of N-containing compounds can be generated via the interaction of SOZs with amines, which may constitute a hitherto unaccounted for source of N-containing compound formation in atmospheric aerosols.
The data that support the results are available upon request. Please email Junting Qiu (paziqjt@gamil.com).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-24-155-2024-supplement.
TA and JQ designed research. JQ and XS performed experiments. JQ analyzed the data. All the authors participated in writing the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This research has been supported by the National Natural Science Foundation of China (grant nos. 42020104001, 42107118, and 42177354), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (grant no. 2017BT01Z032), and China Postdoctoral Science Foundation (grant no. 2021M700881).
This paper was edited by Jianping Huang and reviewed by two anonymous referees.
Albinet, A., Leoz-Garziandia, E., Budzinski, H., Villenave, E., and Jaffrezo, J.-L.: Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys: Part 1: Concentrations, sources and gas/particle partitioning, Atmos. Environ., 42, 43–54, https://doi.org/10.1016/j.atmosenv.2007.10.009, 2008.
Almeida, J., Schobesberger, S., Kurten, A., Ortega, I. K., Kupiainen-Maatta, O., Praplan, A. P., Adamov, A., Amorim, A., Bianchi, F., Breitenlechner, M., David, A., Dommen, J., Donahue, N. M., Downard, A., Dunne, E., Duplissy, J., Ehrhart, S., Flagan, R. C., Franchin, A., Guida, R., Hakala, J., Hansel, A., Heinritzi, M., Henschel, H., Jokinen, T., Junninen, H., Kajos, M., Kangasluoma, J., Keskinen, H., Kupc, A., Kurten, T., Kvashin, A. N., Laaksonen, A., Lehtipalo, K., Leiminger, M., Leppa, J., Loukonen, V., Makhmutov, V., Mathot, S., McGrath, M. J., Nieminen, T., Olenius, T., Onnela, A., Petaja, T., Riccobono, F., Riipinen, I., Rissanen, M., Rondo, L., Ruuskanen, T., Santos, F. D., Sarnela, N., Schallhart, S., Schnitzhofer, R., Seinfeld, J. H., Simon, M., Sipila, M., Stozhkov, Y., Stratmann, F., Tome, A., Trostl, J., Tsagkogeorgas, G., Vaattovaara, P., Viisanen, Y., Virtanen, A., Vrtala, A., Wagner, P. E., Weingartner, E., Wex, H., Williamson, C., Wimmer, D., Ye, P., Yli-Juuti, T., Carslaw, K. S., Kulmala, M., Curtius, J., Baltensperger, U., Worsnop, D. R., Vehkamaki, H., and Kirkby, J.: Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere, Nature, 502, 359–363, https://doi.org/10.1038/nature12663, 2013.
Arey, J., Crowley, D. E., Crowley, M., Resketo, M., and Lester, J.: Hydrocarbon emissions from natural vegetation in California's South Coast Air Basin, Atmos. Environ., 29, 2977–2988, https://doi.org/10.1016/1352-2310(95)00137-N, 1995.
Atkinson, R. and Arey, J.: Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review, Atmos. Environ., 37, 197–219, https://doi.org/10.1016/s1352-2310(03)00391-1, 2003.
Beck, M., Winterhalter, R., Herrmann, F., and Moortgat, G. K.: The gas-phase ozonolysis of α-humulene, Phys. Chem. Chem. Phys., 13, 10970, https://doi.org/10.1039/c0cp02379e, 2011.
Chhantyal-Pun, R., Khan, M. A. H., Martin, R., Zachhuber, N., Buras, Z. J., Percival, C. J., Shallcross, D. E., and Orr-Ewing, A. J.: Direct Kinetic and Atmospheric Modeling Studies of Criegee Intermediate Reactions with Acetone, ACS Earth Space Chem., 3, 2363–2371, https://doi.org/10.1021/acsearthspacechem.9b00213, 2019a.
Chhantyal-Pun, R., Shannon, R. J., Tew, D. P., Caravan, R. L., Duchi, M., Wong, C., Ingham, A., Feldman, C., McGillen, M. R., Khan, M. A. H., Antonov, I. O., Rotavera, B., Ramasesha, K., Osborn, D. L., Taatjes, C. A., Percival, C. J., Shallcross, D. E., and Orr-Ewing, A. J.: Experimental and computational studies of Criegee intermediate reactions with NH3 and CH3NH2, Phys. Chem. Chem. Phys., 21, 14042–14052, https://doi.org/10.1039/c8cp06810k, 2019b.
Chhantyal-Pun, R., Khan, M. A. H., Taatjes, C. A., Percival, C. J., Orr-Ewing, A. J., and Shallcross, D. E.: Criegee intermediates: production, detection and reactivity, Int. Rev. Phys. Chem., 39, 383–422, https://doi.org/10.1080/0144235x.2020.1792104, 2020.
Coffaro, B. and Weisel, C. P.: Reactions and Products of Squalene and Ozone: A Review, Environ. Sci. Technol., 56, 7396–7411, https://doi.org/10.1021/acs.est.1c07611, 2022.
Cornwell, Z. A., Harrison, A. W., and Murray, C.: Kinetics of the Reactions of CH2OO with Acetone, α-Diketones, and β-Diketones, J. Phys. Chem. A, 125, 8557-8571, https://doi.org/10.1021/acs.jpca.1c05280, 2021.
Cuinica, L. G., Abreu, I., and Esteves da Silva, J.: Effect of air pollutant NO2 on Betula pendula, Ostrya carpinifolia and Carpinus betulus pollen fertility and human allergenicity, Environ. Pollut., 186, 50–55, https://doi.org/10.1016/j.envpol.2013.12.001, 2014.
De Haan, D. O., Hawkins, L. N., Kononenko, J. A., Turley, J. J., Corrigan, A. L., Tolbert, M. A., and Jimenez, J. L.: Formation of Nitrogen-Containing Oligomers by Methylglyoxal and Amines in Simulated Evaporating Cloud Droplets, Environ. Sci. Technol., 45, 984–991, https://doi.org/10.1021/es102933x, 2011.
Dovrou, E., Rivera-Rios, J. C., Bates, K. H., and Keutsch, F. N.: Sulfate Formation via Cloud Processing from Isoprene Hydroxyl Hydroperoxides (ISOPOOH), Environ. Sci. Technol., 53, 12476–12484, https://doi.org/10.1021/acs.est.9b04645, 2019.
Dovrou, E., Bates, K. H., Rivera-Rios, J. C., Cox, J. L., Shutter, J. D., and Keutsch, F. N.: Towards a chemical mechanism of the oxidation of aqueous sulfur dioxide via isoprene hydroxyl hydroperoxides (ISOPOOH), Atmos. Chem. Phys., 21, 8999–9008, https://doi.org/10.5194/acp-21-8999-2021, 2021.
Duporte, G., Riva, M., Parshintsev, J., Heikkinen, E., Barreira, L. M. F., Myllys, N., Heikkinen, L., Hartonen, K., Kulmala, M., Ehn, M., and Riekkola, M. L.: Chemical Characterization of Gas- and Particle-Phase Products from the Ozonolysis of alpha-Pinene in the Presence of Dimethylamine, Environ. Sci. Technol., 51, 5602–5610, https://doi.org/10.1021/acs.est.6b06231, 2017.
El-Assaad, T. H., Zhu, J., Sebastian, A., McGrath, D. V., Neogi, I., and Parida, K. N.: Dioxiranes: a half-century journey, Org. Chem. Front., 9, 5675–5725, https://doi.org/10.1039/D2QO01005D, 2022.
Enami, S., Hoffmann, M. R., and Colussi, A. J.: Acidity enhances the formation of a persistent ozonide at aqueous ascorbate/ozone gas interfaces, P. Natl. Acad. Sci. USA, 105, 7365–7369, https://doi.org/10.1073/pnas.0710791105, 2008.
Erupe, M. E., Viggiano, A. A., and Lee, S.-H.: The effect of trimethylamine on atmospheric nucleation involving H2SO4, Atmos. Chem. Phys., 11, 4767–4775, https://doi.org/10.5194/acp-11-4767-2011, 2011.
Fang, T., Lakey, P. S. J., Rivera-Rios, J. C., Keutsch, F. N., and Shiraiwa, M.: Aqueous-Phase Decomposition of Isoprene Hydroxy Hydroperoxide and Hydroxyl Radical Formation by Fenton-like Reactions with Iron Ions, J. Phys. Chem. A, 124, 5230–5236, https://doi.org/10.1021/acs.jpca.0c02094, 2020.
Fry, J. L., Draper, D. C., Barsanti, K. C., Smith, J. N., Ortega, J., Winkle, P. M., Lawler, M. J., Brown, S. S., Edwards, P. M., Cohen, R. C., and Lee, L.: Secondary Organic Aerosol Formation and Organic Nitrate Yield from NO3 Oxidation of Biogenic Hydrocarbons, Environ. Sci. Technol., 48, 11944–11953, https://doi.org/10.1021/es502204x, 2014.
Galloway, M. M., Powelson, M. H., Sedehi, N., Wood, S. E., Millage, K. D., Kononenko, J. A., Rynaski, A. D., and De Haan, D. O.: Secondary Organic Aerosol Formation during Evaporation of Droplets Containing Atmospheric Aldehydes, Amines, and Ammonium Sulfate, Environ. Sci. Technol., 48, 14417–14425, https://doi.org/10.1021/es5044479, 2014.
Ge, X., Wexler, A. S., and Clegg, S. L.: Atmospheric amines – Part I. A review, Atmos. Environ., 45, 524–546, https://doi.org/10.1016/j.atmosenv.2010.10.012, 2011.
Glasoe, W. A., Volz, K., Panta, B., Freshour, N., Bachman, R., Hanson, D. R., McMurry, P. H., and Jen, C.: Sulfuric acid nucleation: An experimental study of the effect of seven bases, J. Geophys. Res.-Atmos., 120, 1933–1950, https://doi.org/10.1002/2014jd022730, 2015.
Gomez-Hernandez, M., Mckeown, M., Secrest, J., Marrero-Ortiz, W., Lavi, A., Rudich, Y., Collins, D. R., and Zhang, R.: Hygroscopic Characteristics of Alkylaminium Carboxylate Aerosols, Environ. Sci. Technol., 50, 2292–2300, https://doi.org/10.1021/acs.est.5b04691, 2016.
Greaves, J. and Roboz, J.: Mass spectrometry for the novice, CRC Press, Boca Raton, xxxii, 275 pp., ISBN 9781420094183, 2014.
Griesbaum, K., Hilß, M., and Bosch, J.: Ozonides of mono-, bi- and tricyclic terpenes, Tetrahedron, 52, 14813–14826, https://doi.org/10.1016/0040-4020(96)00936-2, 1996.
Guenther, A., Hewitt, C. N., Erickson, D., Fall, R., Geron, C., Graedel, T., Harley, P., Klinger, L., Lerdau, M., McKay, W. A., Pierce, T., Scholes, B., Steinbrecher, R., Tallamraju, R., Taylor, J., and Zimmerman, P.: A global-model of natural volatile organic-compound emissions, J. Geophys. Res.-Atmos., 100, 8873–8892, https://doi.org/10.1029/94jd02950, 1995.
Heine, N., Houle, F. A., and Wilson, K. R.: Connecting the Elementary Reaction Pathways of Criegee Intermediates to the Chemical Erosion of Squalene Interfaces during Ozonolysis, Environ. Sci. Technol., 51, 13740–13748, https://doi.org/10.1021/acs.est.7b04197, 2017.
Helmig, D., Ortega, J., Duhl, T., Tanner, D., Guenther, A., Harley, P., Wiedinmyer, C., Milford, J., and Sakulyanontvittaya, T.: Sesquiterpene Emissions from Pine Trees - Identifications, Emission Rates and Flux Estimates for the Contiguous United States, Environ. Sci. Technol., 41, 1545–1553, https://doi.org/10.1021/es0618907, 2007.
Hu, M., Tonokura, K., Morino, Y., Sato, K., and Enami, S.: Effects of Metal Ions on Aqueous-Phase Decomposition of α-Hydroxyalkyl-Hydroperoxides Derived from Terpene Alcohols, Environ. Sci. Technol., 55, 12893–12901, https://doi.org/10.1021/acs.est.1c04635, 2021.
Karagulian, F., Lea, A. S., Dilbeck, C. W., and Finlayson-Pitts, B. J.: A new mechanism for ozonolysis of unsaturated organics on solids: phosphocholines on NaCl as a model for sea salt particles, Phys. Chem. Chem. Phys., 10, 528–541, https://doi.org/10.1039/b712715d, 2008.
Kieloaho, A. J., Hellen, H., Hakola, H., Manninen, H. E., Nieminen, T., Kulmala, M., and Pihlatie, M.: Gas-phase alkylamines in a boreal Scots pine forest air, Atmos. Environ., 80, 369–377, https://doi.org/10.1016/j.atmosenv.2013.08.019, 2013.
Kumar, M. and Francisco, J. S.: Elucidating the molecular mechanisms of Criegee-amine chemistry in the gas phase and aqueous surface environments, Chem. Sci., 10, 743–751, https://doi.org/10.1039/c8sc03514h, 2019.
Kundu, S., Fisseha, R., Putman, A. L., Rahn, T. A., and Mazzoleni, L. R.: Molecular formula composition of β-caryophyllene ozonolysis SOA formed in humid and dry conditions, Atmos. Environ., 154, 70–81, https://doi.org/10.1016/j.atmosenv.2016.12.031, 2017.
Kurten, A., Jokinen, T., Simon, M., Sipila, M., Sarnela, N., Junninen, H., Adamov, A., Almeida, J., Amorim, A., Bianchi, F., Breitenlechner, M., Dommen, J., Donahue, N. M., Duplissy, J., Ehrhart, S., Flagan, R. C., Franchin, A., Hakala, J., Hansel, A., Heinritzi, M., Hutterli, M., Kangasluoma, J., Kirkby, J., Laaksonen, A., Lehtipalo, K., Leiminger, M., Makhmutov, V., Mathot, S., Onnela, A., Petaja, T., Praplan, A. P., Riccobono, F., Rissanen, M. P., Rondo, L., Schobesberger, S., Seinfeld, J. H., Steiner, G., Tome, A., Trostl, J., Winkler, P. M., Williamson, C., Wimmer, D., Ye, P. L., Baltensperger, U., Carslaw, K. S., Kulmala, M., Worsnop, D. R., and Curtius, J.: Neutral molecular cluster formation of sulfuric acid-dimethylamine observed in real time under atmospheric conditions, P. Natl. Acad. Sci. USA, 111, 15019–15024, https://doi.org/10.1073/pnas.1404853111, 2014.
Laskin, A., Laskin, J., and Nizkorodov, S. A.: Chemistry of Atmospheric Brown Carbon, Chem. Rev., 115, 4335–4382, https://doi.org/10.1021/cr5006167, 2015.
Lavi, A., Segre, E., Gomez-Hernandez, M., Zhang, R., and Rudich, Y.: Volatility of Atmospherically Relevant Alkylaminium Carboxylate Salts, The J. Phys. Chem. A, 119, 4336–4346, https://doi.org/10.1021/jp507320v, 2015.
Luo, H., Li, G., Chen, J., Wang, Y., and An, T.: Reactor characterization and primary application of a state of art dual-reactor chamber in the investigation of atmospheric photochemical processes, J. Environ. Sci., 98, 161–168, https://doi.org/10.1016/j.jes.2020.05.021, 2020.
Luo, H., Chen, J., Li, G., and An, T.: Formation kinetics and mechanisms of ozone and secondary organic aerosols from photochemical oxidation of different aromatic hydrocarbons: dependence on NOx and organic substituents, Atmos. Chem. Phys., 21, 7567–7578, https://doi.org/10.5194/acp-21-7567-2021, 2021.
Ma, X., Zhao, X., Wei, Y., Wang, W., Xu, F., Zhang, Q., and Wang, W.: Effect of multifunctional compound monoethanolamine on Criegee intermediates reactions and its atmospheric implications, Sci. Total Environ., 715, 136812, https://doi.org/10.1016/j.scitotenv.2020.136812, 2020.
Moise, T., Flores, J. M., and Rudich, Y.: Optical Properties of Secondary Organic Aerosols and Their Changes by Chemical Processes, Chem. Rev., 115, 4400–4439, https://doi.org/10.1021/cr5005259, 2015.
Mull, H. F., Aroeira, G. J. R., Turney, J. M., and Schaefer, H. F.: The atmospheric importance of methylamine additions to Criegee intermediates, Phys. Chem. Chem. Phys., 22, 22555–22566, https://doi.org/10.1039/d0cp03781h, 2020.
Na, K., Song, C., and Cockeriii, D.: Formation of secondary organic aerosol from the reaction of styrene with ozone in the presence and absence of ammonia and water, Atmos. Environ., 40, 1889–1900, https://doi.org/10.1016/j.atmosenv.2005.10.063, 2006.
Ng, N. L., Brown, S. S., Archibald, A. T., Atlas, E., Cohen, R. C., Crowley, J. N., Day, D. A., Donahue, N. M., Fry, J. L., Fuchs, H., Griffin, R. J., Guzman, M. I., Herrmann, H., Hodzic, A., Iinuma, Y., Jimenez, J. L., Kiendler-Scharr, A., Lee, B. H., Luecken, D. J., Mao, J., McLaren, R., Mutzel, A., Osthoff, H. D., Ouyang, B., Picquet-Varrault, B., Platt, U., Pye, H. O. T., Rudich, Y., Schwantes, R. H., Shiraiwa, M., Stutz, J., Thornton, J. A., Tilgner, A., Williams, B. J., and Zaveri, R. A.: Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol, Atmos. Chem. Phys., 17, 2103–2162, https://doi.org/10.5194/acp-17-2103-2017, 2017.
Nguyen, T. L., Winterhalter, R., Moortgat, G., Kanawati, B., Peeters, J., and Vereecken, L.: The gas-phase ozonolysis of β-caryophyllene (C15H24). Part II: A theoretical study, Phys. Chem. Chem. Phys., 11, 4173–4183, https://doi.org/10.1039/b817913a, 2009.
Perring, A. E., Pusede, S. E., and Cohen, R. C.: An observational perspective on the atmospheric impacts of alkyl and multifunctional nitrates on ozone and secondary organic aerosol, Chem. Rev., 113, 5848–5870, https://doi.org/10.1021/cr300520x, 2013.
Qiu, C. and Zhang, R. Y.: Multiphase chemistry of atmospheric amines, Phys. Chem. Chem. Phys., 15, 5738–5752, https://doi.org/10.1039/c3cp43446j, 2013.
Qiu, J., Ishizuka, S., Tonokura, K., and Enami, S.: Reactions of Criegee Intermediates with Benzoic Acid at the Gas/Liquid Interface, J. Phys. Chem. A, 122, 6303–6310, https://doi.org/10.1021/acs.jpca.8b04995, 2018a.
Qiu, J., Ishizuka, S., Tonokura, K., Colussi, A. J., and Enami, S.: Reactivity of Monoterpene Criegee Intermediates at Gas-Liquid Interfaces, J. Phys. Chem. A, 122, 7910–7917, https://doi.org/10.1021/acs.jpca.8b06914, 2018b.
Qiu, J., Ishizuka, S., Tonokura, K., Colussi, A. J., and Enami, S.: Water Dramatically Accelerates the Decomposition of α-Hydroxyalkyl-Hydroperoxides in Aerosol Particles, J. Phys. Chem. Lett., 10, 5748–5755, https://doi.org/10.1021/acs.jpclett.9b01953, 2019.
Qiu, J., Liang, Z., Tonokura, K., Colussi, A. J., and Enami, S.: Stability of Monoterpene-Derived alpha-Hydroxyalkyl-Hydroperoxides in Aqueous Organic Media: Relevance to the Fate of Hydroperoxides in Aerosol Particle Phases, Environ. Sci. Technol., 54, 3890–3899, https://doi.org/10.1021/acs.est.9b07497, 2020b.
Qiu, J., Tonokura, K., and Enami, S.: Proton-Catalyzed Decomposition of alpha-Hydroxyalkyl-Hydroperoxides in Water, Environ. Sci. Technol., 54, 10561–10569, https://doi.org/10.1021/acs.est.0c03438, 2020a.
Qiu, J., Fujita, M., Tonokura, K., and Enami, S.: Stability of Terpenoid-Derived Secondary Ozonides in Aqueous Organic Media, J. Phys. Chem. A, 126, 5386–5397, https://doi.org/10.1021/acs.jpca.2c04077, 2022.
Sanchez, J. and Myers, T. N.: Peroxides and peroxide compounds, organic peroxides, Kirk-Othmer Encyclopedia of Chemical Technology, https://doi.org/10.1002/0471238961.1518070119011403.a01, 2000.
Seinfeld, J. H., Bretherton, C., Carslaw, K. S., Coe, H., DeMott, P. J., Dunlea, E. J., Feingold, G., Ghan, S., Guenther, A. B., Kahn, R., Kraucunas, I., Kreidenweis, S. M., Molina, M. J., Nenes, A., Penner, J. E., Prather, K. A., Ramanathan, V., Ramaswamy, V., Rasch, P. J., Ravishankara, A. R., Rosenfeld, D., Stephens, G., and Wood, R.: Improving our fundamental understanding of the role of aerosol-cloud interactions in the climate system, P. Natl. Acad. Sci. USA, 113, 5781–5790, https://doi.org/10.1073/pnas.1514043113, 2016.
Shiraiwa, M., Ueda, K., Pozzer, A., Lammel, G., Kampf, C. J., Fushimi, A., Enami, S., Arangio, A. M., Fröhlich-Nowoisky, J., Fujitani, Y., Furuyama, A., Lakey, P. S. J., Lelieveld, J., Lucas, K., Morino, Y., Pöschl, U., Takahama, S., Takami, A., Tong, H., Weber, B., Yoshino, A., and Sato, K.: Aerosol Health Effects from Molecular to Global Scales, Environ. Sci. Technol., 51, 13545–13567, https://doi.org/10.1021/acs.est.7b04417, 2017.
Shrivastava, M., Cappa, C. D., Fan, J. W., Goldstein, A. H., Guenther, A. B., Jimenez, J. L., Kuang, C., Laskin, A., Martin, S. T., Ng, N. L., Petaja, T., Pierce, J. R., Rasch, P. J., Roldin, P., Seinfeld, J. H., Shilling, J., Smith, J. N., Thornton, J. A., Volkamer, R., Wang, J., Worsnop, D. R., Zaveri, R. A., Zelenyuk, A., and Zhang, Q.: Recent advances in understanding secondary organic aerosol: Implications for global climate forcing, Rev. Geophys., 55, 509–559, https://doi.org/10.1002/2016rg000540, 2017.
Sugimura, N., Furuya, A., Yatsu, T., and Shibue, T.: Prediction of adducts on positive mode electrospray ionization mass spectrometry: proton/sodium selectivity in methanol solutions, Eur. J. Mass Spectrom., 21, 725–731, https://doi.org/10.1255/ejms.1389, 2015.
Tang, X., Price, D., Praske, E., Lee, S. A., Shattuck, M. A., Purvis-Roberts, K., Silva, P. J., Asa-Awuku, A., and Cocker, D. R.: NO3 radical, OH radical and O3-initiated secondary aerosol formation from aliphatic amines, Atmos. Environ., 72, 105–112, https://doi.org/10.1016/j.atmosenv.2013.02.024, 2013.
Tong, D., Chen, J., Qin, D., Ji, Y., Li, G., and An, T.: Mechanism of atmospheric organic amines reacted with ozone and implications for the formation of secondary organic aerosols, Sci. Total Environ., 737, 139830, https://doi.org/10.1016/j.scitotenv.2020.139830, 2020.
Tong, H., Arangio, A. M., Lakey, P. S. J., Berkemeier, T., Liu, F., Kampf, C. J., Brune, W. H., Pöschl, U., and Shiraiwa, M.: Hydroxyl radicals from secondary organic aerosol decomposition in water, Atmos. Chem. Phys., 16, 1761–1771, https://doi.org/10.5194/acp-16-1761-2016, 2016.
Vibenholt, A., Norgaard, A. W., Clausen, P. A., and Wolkoff, P.: Formation and stability of secondary ozonides from monoterpenes studied by mass spectrometry, Chemosphere, 76, 572–577, https://doi.org/10.1016/j.chemosphere.2009.02.060, 2009.
Wang, P.-B., Truhlar, D. G., Xia, Y., and Long, B.: Temperature-dependent kinetics of the atmospheric reaction between CH2OO and acetone, Phys. Chem. Chem. Phys., 24, 13066–13073, https://doi.org/10.1039/D2CP01118B, 2022.
Wang, S., Zhou, S., Tao, Y., Tsui, W. G., Ye, J., Yu, J. Z., Murphy, J. G., McNeill, V. F., Abbatt, J. P. D., and Chan, A. W. H.: Organic Peroxides and Sulfur Dioxide in Aerosol: Source of Particulate Sulfate, Environ. Sci. Technol., 53, 10695–10704, https://doi.org/10.1021/acs.est.9b02591, 2019.
Wang, S., Liu, T., Jang, J., Abbatt, J. P. D., and Chan, A. W. H.: Heterogeneous interactions between SO2 and organic peroxides in submicron aerosol, Atmos. Chem. Phys., 21, 6647–6661, https://doi.org/10.5194/acp-21-6647-2021, 2021.
Wang, S., Zhao, Y., Chan, A. W. H., Yao, M., Chen, Z., and Abbatt, J. P. D.: Organic Peroxides in Aerosol: Key Reactive Intermediates for Multiphase Processes in the Atmosphere, Chem. Rev., 123, 1635–1679, https://doi.org/10.1021/acs.chemrev.2c00430, 2023.
Wei, J., Fang, T., Lakey, P. S. J., and Shiraiwa, M.: Iron-Facilitated Organic Radical Formation from Secondary Organic Aerosols in Surrogate Lung Fluid, Environ. Sci. Technol., 56, 7234–7243, https://doi.org/10.1021/acs.est.1c04334, 2022.
Winterhalter, R., Neeb, P., Grossmann, D., Kolloff, A., Horie, O., and Moortgat, G.: Products and mechanism of the gas phase reaction of ozone with beta-pinene, J. Atmos. Chem., 35, 165–197, https://doi.org/10.1023/A:1006257800929, 2000.
Winterhalter, R., Herrmann, F., Kanawati, B., Nguyen, T. L., Peeters, J., Vereecken, L., and Moortgat, G. K.: The gas-phase ozonolysis of β-caryophyllene (C15H24). Part I: an experimental study, Phys. Chem. Chem. Phys., 11, 4152–4172, https://doi.org/10.1039/b817824k, 2009.
Yao, L., Ma, Y., Wang, L., Zheng, J., Khalizov, A., Chen, M. D., Zhou, Y. Y., Qi, L., and Cui, F. P.: Role of stabilized Criegee Intermediate in secondary organic aerosol formation from the ozonolysis of α-cedrene, Atmos. Environ., 94, 448–457, https://doi.org/10.1016/j.atmosenv.2014.05.063, 2014.
Yao, L., Wang, M.-Y., Wang, X.-K., Liu, Y.-J., Chen, H.-F., Zheng, J., Nie, W., Ding, A.-J., Geng, F.-H., Wang, D.-F., Chen, J.-M., Worsnop, D. R., and Wang, L.: Detection of atmospheric gaseous amines and amides by a high-resolution time-of-flight chemical ionization mass spectrometer with protonated ethanol reagent ions, Atmos. Chem. Phys., 16, 14527–14543, https://doi.org/10.5194/acp-16-14527-2016, 2016.
Yao, M., Zhao, Y., Hu, M., Huang, D., Wang, Y., Yu, J. Z., and Yan, N.: Multiphase Reactions between Secondary Organic Aerosol and Sulfur Dioxide: Kinetics and Contributions to Sulfate Formation and Aerosol Aging, Environ. Sci. Tech. Let., 6, 768–774, https://doi.org/10.1021/acs.estlett.9b00657, 2019.
Yu, H., Mcgraw, R., and Lee, S.-H.: Effects of amines on formation of sub-3 nm particles and their subsequent growth, Geophys. Res. Lett., 39, L02807, https://doi.org/10.1029/2011gl050099, 2012.
Zeng, M., Heine, N., and Wilson, K. R.: Evidence that Criegee intermediates drive autoxidation in unsaturated lipids, P. Natl. Acad. Sci. USA, 117, 4486–4490, https://doi.org/10.1073/pnas.1920765117, 2020.
Zhang, X., Barraza, K. M., and Beauchamp, J. L.: Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air-water interface, P. Natl. Acad. Sci. USA, 115 3255–3260, https://doi.org/10.1073/pnas.1722323115, 2018.
Zhou, Z., Lakey, P. S. J., von Domaros, M., Wise, N., Tobias, D. J., Shiraiwa, M., and Abbatt, J. P. D.: Multiphase Ozonolysis of Oleic Acid-Based Lipids: Quantitation of Major Products and Kinetic Multilayer Modeling, Environ. Sci. Technol., 56, 7716–7728, https://doi.org/10.1021/acs.est.2c01163, 2022.