the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Comparison of six approaches to predicting droplet activation of surface active aerosol – Part 2: Strong surfactants
Sampo Vepsäläinen
Silvia M. Calderón
Surfactants have been a focus of investigation in atmospheric sciences for decades due to their ability to modify the water uptake and cloud formation potential of aerosols. Surfactants adsorb at the surface and can decrease the surface tension of aqueous solutions. In microscopic aqueous droplets with finite amounts of solute, surface adsorption may simultaneously deplete the droplet bulk of the surfactant. While this mechanism is now broadly accepted, the representation in atmospheric and cloud droplet models is still not well constrained. We compare the predictions of five bulk–surface partitioning models documented in the literature to represent aerosol surface activity in Köhler calculations of cloud droplet activation. The models are applied to common aerosol systems, consisting of strong atmospheric surfactants (sodium myristate or myristic acid) and sodium chloride in a wide range of relative mixing ratios. For the same particles, the partitioning models predict similar critical droplet properties at small surfactant mass fractions, but differences between the model predictions increase significantly with the surfactant mass fraction in the particles. Furthermore, significantly different surface tensions are predicted for growing droplets at given ambient conditions along the Köhler curves. The inter-model variation for these strong surfactant particles is different than previously observed for moderately surface active atmospheric aerosol components. Our results highlight the importance of establishing bulk–surface partitioning effects in Köhler calculations for a wide range of conditions and aerosol types relevant to the atmosphere. In particular, conclusions made for a single type of surface active aerosol and surface activity model may not be immediately generalized.
- Article
(2371 KB) - Full-text XML
- Companion paper
-
Supplement
(5320 KB) - BibTeX
- EndNote
The global climate is affected by atmospheric aerosols both directly through interaction with solar radiation and indirectly through their ability to serve as cloud condensation nuclei (CCN). The indirect effect from aerosol–cloud interactions still remains one of the largest sources of uncertainty in global radiative forcing estimates (IPCC, 2013, 2021). Surface active species (surfactants) are commonly found in atmospheric aerosols (e.g., Gérard et al., 2016; Petters and Petters, 2016; Nozière et al., 2017; Kroflič et al., 2018; Gérard et al., 2019). Aerosol surface activity has been shown to affect the critical point of cloud droplet activation, but a clear consensus has not yet been reached on the extent and specific mechanisms (e.g., Hänel, 1976; Shulman et al., 1996; Facchini et al., 1999, 2000; Li et al., 1998; Sorjamaa et al., 2004; Prisle et al., 2008, 2010, 2011; Topping, 2010; Raatikainen and Laaksonen, 2011; Ruehl and Wilson, 2014; Nozière et al., 2014; Ruehl et al., 2016; Petters and Petters, 2016; Ovadnevaite et al., 2017; Malila and Prisle, 2018; Lin et al., 2018; Prisle et al., 2019; Davies et al., 2019; Lowe et al., 2019; Lin et al., 2020; Bzdek et al., 2020; Prisle, 2021; Vepsäläinen et al., 2022).
Surfactants adsorb at the surface of a solution, resulting in enhanced surface concentrations compared to the interior (bulk) solution. The presence of surfactants in aqueous solutions can significantly reduce the surface tension, compared to pure water, which typically decreases with the concentration of surface active compounds (e.g., Wen et al., 2000; Hyvärinen et al., 2006; Vanhanen et al., 2008; Bzdek et al., 2020). The surface tension of a solution can be described in terms of either the surface or bulk composition, which are related via the bulk-to-surface concentration gradient for a given surface-active substance. The relation between surface tension and surface-specific composition is often unknown (Prisle et al., 2012b; Werner et al., 2014, 2018; Toribio et al., 2018), and experimentally based surface tension relations are therefore typically expressed in terms of the solution bulk composition. Measurements of surface tension are commonly performed for macroscopic solutions, where the bulk phase contains a sufficiently large amount of solute that the adsorption to the surface phase has negligible effect on the composition of the bulk. The surface tension of a macroscopic solution is therefore readily described in terms of the total solution composition.
In microscopic droplets, the surface adsorption of surfactants can significantly alter the bulk composition and therefore the composition-dependent droplet properties, due to the finite total amount of solute comprised in such small droplets (e.g., Prisle et al., 2010; Lin et al., 2018, 2020; Bzdek et al., 2020; Prisle, 2021). The distribution of surfactant mass between the surface and bulk phases is referred to as bulk–surface partitioning (Prisle et al., 2010; Vepsäläinen et al., 2022). Surface tension measurements for microscopic droplets (i.e., with diameters in the micrometer range or smaller) have only recently been achieved and for only a few droplet systems, including aqueous sodium chloride (NaCl), aqueous malonic or glutaric acid (Morris et al., 2015), aqueous NaCl or glutaric acid (Bzdek et al., 2016), and aqueous mixtures of strong surfactant Triton X-100 with glutaric acid or NaCl (Bzdek et al., 2020). Bzdek et al. (2020) showed that surface tension of aqueous Triton X-100 microscopic droplets (7–9 µm radius) suspended in air was significantly higher than for a macroscopic solution with identical composition. Their measurements provided the first direct experimental evidence of the influence of bulk-phase depletion due to bulk–surface partitioning in finite sized droplets.
Experimental data for composition-dependent properties of microscopic droplets are rare. Therefore, composition–property relations based on macroscopic data are used for estimating microscopic droplet properties, by accounting for the effect of bulk–surface partitioning on bulk composition with a partitioning model (e.g., Sorjamaa et al., 2004; Prisle et al., 2008, 2010; Malila and Prisle, 2018; Lin et al., 2018, 2020; Bzdek et al., 2020; Prisle, 2021). Several models have been developed to describe surfactant partitioning effects in aqueous droplets of atmospheric relevance. Most models either employ Gibbs surface thermodynamics, where the surface phase is approximated as a two-dimensional interface (e.g., Sorjamaa et al., 2004; Prisle et al., 2008, 2010; Topping, 2010; Raatikainen and Laaksonen, 2011; Petters and Petters, 2016; McGraw and Wang, 2021; Prisle, 2021), or assume a physical surface layer in the form of a molecular monolayer (e.g., Malila and Prisle, 2018), liquid–liquid phase separation (e.g., Ovadnevaite et al., 2017), a compressed film (e.g., Ruehl et al., 2016), or complete phase separation (e.g., Prisle et al., 2011; Ovadnevaite et al., 2017). Each surface partitioning model is based on specific assumptions and requirements for application. An overview of the most widely used partitioning models is given by Malila and Prisle (2018).
We have previously compared predictions with five bulk–surface partitioning models (Prisle et al., 2010, 2011; Ruehl et al., 2016; Ovadnevaite et al., 2017; Malila and Prisle, 2018) and a general bulk solution model (Prisle et al., 2010) for droplet activation of moderately surface active organic aerosol, comprised of malonic, succinic, or glutaric acid mixed with ammonium sulfate across a range of compositions (Vepsäläinen et al., 2022). Surfactant strength is here considered in terms of the ability to reduce the surface tension of aqueous solutions at a given concentration. We found that, for the same moderately surface active aerosol, the different models predict significantly different CCN activity, droplet surface tension, and degree of bulk–surface partitioning. However, these results cannot be immediately generalized to aerosols with significantly different surface activity. Here, we therefore compare predictions of cloud droplet activation with different surface activity models for strongly surface active aerosol in common conditions. Strong surfactants have different surface adsorption properties than moderately surface active compounds and a pronounced ability to reduce surface tension in macroscopic solutions (e.g., Campbell and Lakshminarayanan, 1965; Wen et al., 2000; Álvarez Silva et al., 2010; Petters and Petters, 2016). The mutual agreement between different surface activity models may therefore also be different than previously observed.
Strongly surface active aerosols are here represented by the atmospheric fatty acid myristic acid and its sodium salt (sodium myristate). Fatty acids have been found in atmospheric aerosol samples and are a major component of sea spray aerosol (SSA) (e.g., Mochida et al., 2002, 2007; Cheng et al., 2004; Wang et al., 2015; Cochran et al., 2016; Kirpes et al., 2019) and part of particle compositions associated with ice nucleation by SSA (e.g., DeMott et al., 2018; Perkins et al., 2020). Experiments by Wang et al. (2015) indicate that long-chain fatty acids are the dominant contributor to submicron organic SSA (aerodynamic diameter 0.56–1 µm). Cochran et al. (2016) tentatively identified over 280 organic compounds in nascent SSA, including saturated and unsaturated fatty acids and derivatives of fatty acids. Kirpes et al. (2019) observed thick organic coatings, consisting of marine saccharides, amino acids, fatty acids, and divalent cations, on Alaskan Arctic winter SSA, where 40 % of the particles containing surfactants matched only long-chain fatty acids, while the rest also contained short-chain fatty acids or saccharides. Fatty acid salts were the first atmospheric surfactants to be investigated in comprehensive experimental and partitioning modeling studies (Prisle et al., 2008, 2010, 2011) and have been subject of several subsequent similar studies (e.g., Nguyen et al., 2017; Forestieri et al., 2018).
We perform Köhler predictions of cloud droplet activation for surface active aerosol using six different models to describe possible surfactant effects during droplet growth, including five bulk–surface partitioning models and one bulk solution model. As a reference, we also include a classical Köhler model that does not consider any effects of surface activity. In addition, we estimate the relative change in cloud droplet number concentration from Köhler predictions with the surface activity models in comparison to the classical Köhler model.
Brief descriptions of the six surface activity models are given in the following sections. A conceptual figure of the models is shown in Fig. 1 of Vepsäläinen et al. (2022). For the most detailed documentation of each model, we refer the reader to the original publications.
2.1 Köhler theory
Cloud droplet activation is predicted with equilibrium Köhler theory (Köhler, 1936) in the form (Prisle et al., 2010)
where S is the equilibrium water vapor saturation ratio, pw is the equilibrium partial pressure of water over the solution droplet, is the saturation vapor pressure over a flat surface of pure water, aw is the droplet solution water activity, is the molar volume of water, σ is the droplet surface tension, R is the universal gas constant, T is the temperature in Kelvin, and d is the spherical droplet diameter. Droplet activation is determined in terms of the critical saturation ratio (Sc) or the critical supersaturation (SS %), both corresponding to the maximum value of the Köhler curve described by Eq. (1).
We calculate Köhler growth curves for particles comprising surfactants (abbreviated sft) sodium myristate (abbreviated NaC14) or myristic acid mixed with NaCl in mass fractions of –0.95 in dry particles with diameter Dp=50 nm. Compound properties used in the calculations are presented in Table 1 for water, NaC14, myristic acid, and NaCl. The total amount of surfactant and NaCl in each particle is calculated from the respective solid-phase densities and relative mass fractions in the particle, which in turn determine the total amount of solute present in the growing aqueous droplet. All models assume spherical particles and droplets. Droplet solutions are described as ternary water–surfactant–inorganic salt mixtures, but the specific treatment of bulk–surface partitioning and the resulting droplet water activity and surface tension vary between the different models. Table 2 summarizes the surface tension and water activity calculation methods, as well as components considered in the droplet bulk and surface phases for the different models. For more details on the calculations, we refer the reader to Vepsäläinen et al. (2022).
2.2 Partitioning models
2.2.1 Gibbs adsorption model
In the Gibbs model of Prisle et al. (2010), the Gibbs adsorption equation,
is solved iteratively for the bulk composition with the boundary condition that the molar ratio of water and NaCl is the same in both the bulk and surface phases. In Eq. (2), is the total amount of species j in the droplet solution, k is the Boltzmann constant, is the amount of surfactant in the droplet bulk, is the activity of j in the droplet bulk solution, A is the spherical droplet surface area, and σ is the droplet surface tension, given as a function of bulk-phase composition. The position of the Gibbs diving surface is selected so that the droplet bulk-phase volume equals the total equimolar droplet volume and mass conservation () is assumed for all components in the droplet (water, surfactant, NaCl).
Table 1Molar mass (M), densities of the liquid (ρl) and solid (ρs) phases, and surface tension (σ) of the pure compounds.
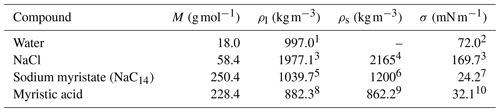
1 Pátek et al. (2009). 2 International Association for the Properties of Water and Steam (IAPWS) (2014). 3 Vanhanen et al. (2008) and Janz (1980). 4 Prisle et al. (2010). 5 Extended from binary aqueous density estimated via method of Calderón and Prisle (2021). 6 Estimate Prisle et al. (2011). 7 Value at CMC. 8 (Noureddini et al., 1992). 9 At 54 ∘C (CRC Handbook, 2020). 10 The method of Zhang et al. (2018), using data from Di Nicola et al. (2016).
2.2.2 Simple complete partitioning model
The simple partitioning model of Prisle et al. (2011) assumes that all surfactants in the droplet are completely partitioned into an insoluble surface layer of pure surfactant and the remaining bulk phase is a binary mixture of water and NaCl. The surface tension of the droplet solution is assumed to be equal to that of pure water, representing that the surface coverage of the surfactant is insufficient to form a full monolayer.
2.2.3 Compressed film surface model
The compressed film model of Ruehl et al. (2016) divides the droplet into an organic surface and a ternary solution droplet bulk of water, surfactant, and NaCl. The surface tension is calculated as
where σw is the surface tension of water, A0 is the critical molecular area, Am is the molecular area (Eq. 6), mσ accounts for the interaction between surfactants at the interface, and σmin is a lower limit imposed on the surface tension. The isotherm for the equation of state (EoS) of the compressed film is
where C0 represents the surfactant bulk concentration at the phase transition, Cbulk represents the surfactant bulk concentration (Eq. 5), and NA is Avogadro’s number, which is used to calculate the fraction of surfactant partitioned to the surface (fsurf). The parameters Cbulk and Am can be expressed as functions of the NaCl seed (Dseed), dry particle (Dp) and droplet (d) diameters, and fsurf as
and
The model-specific parameters A0, C0, mσ, and σmin are assumed to be compound-specific physical constants. The values used for parameters A0=29.2 Å2, log 10 , and mσ=1.28 mJ m−2 Å−2 were obtained from Forestieri et al. (2018) for myristic acid. The minimum surface tension is assumed to be σmin=0, as a conservative estimate, because it could not be determined experimentally (Forestieri et al., 2018). We use the same parameters for NaC14 due to the lack of suitable data to fit parameters specifically for NaC14.
2.2.4 Monolayer surface model
In the molecular monolayer model of Malila and Prisle (2018), the partitioning between the bulk and surface phases for each compound j is calculated iteratively from the semi-empirical relation
where vj is the liquid-phase molecular volume, is the droplet surface mole fraction of compound j, and σj is the surface tension of pure j. The vector xB contains the bulk mole fractions for water, surfactant, and NaCl. The thickness of the surface monolayer is calculated as
and the condition of mass conservation () is imposed for each compound j.
2.2.5 Partial organic film model
In the partial organic film model of Ovadnevaite et al. (2017), all surfactants are assumed to reside in a NaCl- and water-free surface film, similar to the simple partitioning model of Prisle et al. (2011). The surface film is assumed to completely coat the droplet bulk until a minimum surface thickness is reached (δsft), where the surface film breaks, resulting in partial coverage of the droplet. The effective surface tension of the droplet is calculated as the surface-area-weighted mean of the surface tensions from the surface and bulk phases as
where
is the surface coverage, VS is the volume of the surface phase, and Vδ is the corresponding volume of a spherical shell of thickness δsft. Here, the surface thickness δsft is set equal to the corresponding values calculated with Eq. (8) of the monolayer model of Malila and Prisle (2018). A comparison between using a constant δsft vs. the values calculated with the monolayer model is presented in Sect. S1.3 in the Supplement. The surface tensions of individual liquid bulk (σB) and surface (σS) phases are calculated as volume fraction-weighted means of the pure-component surface tensions (Table 1).
2.3 Bulk surface activity model
In the bulk solution model (e.g., Prisle et al., 2010; Prisle, 2021), droplet properties are assumed to be equivalent to those for a macroscopic solution with the same total composition. The droplet bulk-phase composition is determined directly as the total composition of the droplet and the droplet surface tension is estimated from a macroscopic ternary solution surface tension parametrization (Sect. S2.2) using this composition.
2.4 Classical Köhler model
In the classical Köhler model (e.g., Prisle et al., 2010; Prisle, 2021), surface active aerosol components are treated as regular soluble solutes and specific effects of surface activity are not considered. The droplet surface tension is assumed to be constant and equal to that of pure water.
Table 2Methods of calculating the droplet water activity (aw) and surface tension (σ), and the composition of the droplet surface and bulk phases used with the different models. For more details on the calculations, we refer the reader to Vepsäläinen et al. (2022).
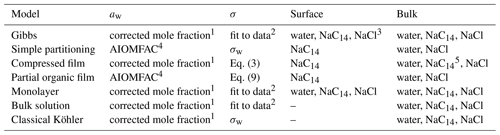
1 Corresponding to the bulk-phase composition; see Sect. S2.1. For the bulk solution and classical Köhler models this calculated from the total composition of the droplet. 2 Fit into the data of Wen et al. (2000) (Sect. S2.2 of the Supplement). 3 NaCl and water depletion from the surface balance the surfactant partitioning. 4 Fit into AIOMFAC-web (2023) calculations (Zuend et al., 2008, 2011); see Sect. S2.1. 5 Typically, all surfactants have partitioned to the surface at activation.
2.5 Critical micelle concentration
Above the so-called critical micelle concentration (CMC), some surfactants can self-aggregate to form structures here collectively referred to as micelles. The micellization process is highly dependent on the surfactant species (Langevin, 1992). In ternary water–surfactant–inorganic salt solutions, both the surfactant CMC and solution surface tension at the CMC can vary with inorganic salt concentration and may be further affected for ionic surfactants that share a common counterion with the inorganic salt. Accounting for these effects requires complex modeling (e.g., Kralchevsky et al., 1999; Calderón et al., 2020) with parameters obtained from experiments. The surfactant CMC often decreases with increasing inorganic salt concentration compared to a binary water–surfactant solution (Calderón and Prisle, 2021). However, for most ternary surfactant solutions, and in particular for atmospheric surfactants, the relevant interaction parameters are not known or readily accessible with existing experimental techniques.
In this work, the CMC of NaC14 is estimated from binary water–NaC14 surface tension data (Wen et al., 2000) and taken into account in the composition-dependent ternary surface tension function (Sect. S2.2) used for the Gibbs (Sect. 2.2.1), monolayer (Sect. 2.2.4), and bulk solution (Sect. 2.3) models. The surface tension at this concentration, σCMC=24.2 mN m−1, is set as a lower limit for the droplet surface tension and assumed to be constant, such that NaCl has no effect on σCMC. For calculations with the Gibbs and monolayer models, the predicted droplet surface tension must be larger than σCMC for the Köhler curves to be calculated, because neither model explicitly treats micelle formation. With the bulk solution model, the CMC limits the maximum amount of surfactant dissolved in the bulk when the droplet surface tension is equal to σCMC. Any additional surfactant present in the droplets, as micelles or undissolved, is assumed to have negligible impact on the droplet volume and solution state. For the monolayer and partial organic film models, the surface tension of pure NaC14 (in a hypothetical supercooled liquid state) is assumed to be equal to σCMC, due to lack of available data (Malila and Prisle, 2018). More information is given in Sect. S2.4.
2.6 Relative change in the cloud droplet number concentration
We estimate the relative change in cloud droplet number concentration caused by the differences in critical supersaturation (SSc) predicted for the different partitioning models, as well as the bulk solution model, with respect to the classical Köhler model (SS), using the method outlined by Bzdek et al. (2020). The cloud droplet number concentration N is assumed to depend on the supersaturation SS as N∝SSk, where k≈0.5 (Facchini et al., 1999), and the relative change in cloud droplet number concentration is calculated as
In the following sections, we present the results of Köhler calculations with the different models described above for common aerosol systems with dry diameters of Dp=50 nm and comprising NaC14 and NaCl in various mixing ratios. Results for particles comprising myristic acid are presented in Sect. S1.1 in the Supplement. Furthermore, critical supersaturations and droplet diameters predicted for particles across the size range Dp=50–200 nm are presented in Sect. S1.4. With a given mass fraction of NaC14, the inter-model variation between the different partitioning models is similar for particles of all sizes. The absolute differences in predicted SSc between the models are larger for small particles, and therefore we here focus on the results for Dp=50 nm.
3.1 Köhler curves and droplet activation
Figure 1 shows the Köhler curves predicted with the different models in terms of supersaturation (SS) as a function of droplet size (d) for particles with initial dry diameter Dp=50 nm and NaC14 mass fractions (wp,sft) of 0.2, 0.5, 0.8, and 0.95 relative to NaCl. The critical points (dc, SSc) corresponding to the Köhler curves in Fig. 1 are given in Table 3. Figure 1 immediately highlights that the different models can predict significantly different Köhler growth curves and activation properties for the same dry particles. The differences in both SSc and dc predicted with the different bulk–surface partitioning models increase with wp,sft. The simple partitioning and compressed film models consistently predict the highest SSc and the smallest dc for all wp,sft. For the Gibbs model, predicted dc are larger and SSc are lower than for the other partitioning models. The monolayer and partial organic film models agree well and the predicted critical points are between the extremes for all models at each wp,sft. There are generally large differences between the SSc of the partitioning models and the bulk solution model, whereas the critical points of the classical Köhler model fall between the extremes of the different surface activity models.
3.1.1 Critical supersaturations
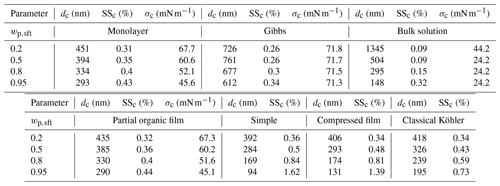
For particles with in Fig. 1a, the Köhler curves and SSc values of the different models are comparable, except for the bulk solution model. The SSc predicted with the monolayer and partial organic film models agree even as wp,sft increases in Fig. 1b–d. We used the surface thickness predicted by the monolayer model (δML) as input for the partial organic film model calculations, but this only slightly enhances the similarities of results from the two models. Using a constant surface thickness of 0.5 nm (δ0.5) for the partial organic film model does not drastically change the SSc. The largest differences were seen for and 0.8, where . A detailed comparison is provided in Sect. S1.3.
The Gibbs model predicts lower SSc than the monolayer and partial organic film models for all wp,sft in Fig. 1. However, the critical points for the Gibbs model correspond to droplet sizes immediately after the droplet surface tension increases from the minimum value at the NaC14 CMC (see Fig. 2), suggesting that predictions could be sensitive to the assumed value of the CMC. Experimentally determined CMC values can vary significantly for a given solution, depending on the measurement technique used (Álvarez Silva et al., 2010).
The SSc of the simple partitioning and compressed film models increase more with wp,sft than for the other models (Table 3). For particles with –0.8 in Fig. 1a–c, the SSc is only slightly higher for the simple partitioning model than for the compressed film model, but the difference between models increases for in Fig. 1d. Köhler calculations for myristic acid with the simple partitioning, compressed film, and partial film models (Sect. S1.1) predict higher SSc and smaller dc than for NaC14, but the inter-model variation between the three models for myristic acid is similar to that for NaC14 in Fig. 1. We assessed the sensitivity of the compressed film model predictions to the assumed molar volume of myristic acid, and found that the critical point is only significantly affected at (Sect. S1.2).
In Fig. 1, the SSc of the classical Köhler model is lower than for the simple and compressed film models but larger than for the monolayer, Gibbs, and partial film models. The bulk solution model predicts significantly lower SSc than the partitioning models, analogously to several other results for strong surfactants (e.g., Sorjamaa et al., 2004; Prisle et al., 2008, 2010; Topping, 2010; Prisle et al., 2019; Prisle, 2021). For moderately surface active malonic, succinic, or glutaric acid and ammonium sulfate particles, we previously did not predict significantly lower SSc with the bulk solution model than with the bulk–surface partitioning models (Vepsäläinen et al., 2022). Due to the moderate surface activity of these di- and polycarboxylic acids, the monolayer and Gibbs partitioning models predicted only a moderate extent of surface partitioning and consequently droplet bulk-phase compositions, surface tensions, and Köhler curves similar to the bulk solution model. The mutual agreement between the different models and in particular the significance of bulk–surface partitioning can therefore vary significantly with the surface active properties of the investigated aerosol.
For comparison, a critical supersaturation of SS % calculated from a fit to experimental SSc data of Prisle et al. (2008) for and Dp=50 nm is included in Fig. 1d. The simple partitioning and compressed film models predict larger SSc than this experimental value and the monolayer, Gibbs, partial organic film, bulk solution, and classical Köhler models predict smaller SSc than this experimental value. For , the SSc is higher than with , but the difference is minor compared to the difference between the SSc for the monolayer, Gibbs, and partial organic film models and SS in Fig. 1d. The simple model has previously been observed to overestimate SSc at high wp,sft and for less surface active aerosols (Prisle et al., 2011; Vepsäläinen et al., 2022).
Average supersaturations in low-level clouds range from 0.1 % to 0.4 % (e.g., Politovich and Cooper, 1988) but higher supersaturations of 0.7 % to 1.3 % can be reached during turbulent fluctuations in temperature and water vapor concentration (e.g., Siebert and Shaw, 2017). Only the simple partitioning, compressed film, and classical Köhler models predict SSc above 0.7 % at high wp,sft, while the other models predict considerably lower SSc (Table 3). Therefore, the inter-model variation for the strongly surface active aerosol studied here is sufficiently pronounced to affect predictions of activation for the ambient conditions in low level clouds. The absolute differences between the highest and lowest SSc in Fig. 1 are ΔSSc=0.27 %, 0.41 %, 0.69 %, and 1.30 % for , 0.5, 0.8, and 0.95, respectively. However, excluding the bulk solution model, maximum differences in SSc between the bulk–surface partitioning models are ΔSSc=0.10 %, 0.24 %, 0.54 %, and 1.28 % for , 0.5, 0.8, and 0.95, respectively. This range of ΔSSc for strongly surface active NaC14 particles is similar to predictions for malonic acid by Vepsäläinen et al. (2022). The large differences between the SSc of the different models for both strongly and moderately surface active aerosol means that representation of surface activity during Köhler calculations could cause significant changes in predictions of cloud droplet number concentrations (Fig. 4), with corresponding uncertainty in estimations of the cloud radiative effect.
3.1.2 Critical droplet diameters
In Fig. 1, the critical droplet diameters (dc) predicted with the different models generally decrease as wp,sft increases, except for the Gibbs model where dc increases between and 0.5. The Gibbs model predicts the largest dc of the bulk–surface partitioning models, and also larger dc than for the bulk solution model, except for particles with . However, as the critical point of cloud droplet activation predicted with the Gibbs model occurs close to the droplet size where the surface tension increases from the minimum value σCMC (Fig. 2), predictions may vary depending on the value used for the CMC of aqueous sodium myristate. For in Fig. 1a, the dc values agree fairly well between the different bulk–surface partitioning models, except the Gibbs model. The monolayer and partial film models furthermore agree well for each wp,sft value in Fig. 1 (see also Table 3). The simple partitioning and compressed film models predict similar dc, only showing a significant difference for in Fig. 1d.
The Köhler curves predicted with the bulk solution model have a distinct shape with two local maxima, because the droplet surface tension is constant and equal to σCMC for the concentrated small droplet sizes before eventually increasing as the droplet grows and dilutes beyond the myristate CMC (Fig. 2). For in Fig. 1a, the critical point is reached at the maximum corresponding to the larger droplet size, where surface tension is higher than σCMC. For the classical Köhler model, predicted dc are lower than for the monolayer and partial film models but higher than the simple and compressed film models.
The absolute differences between the largest and smallest dc in Fig. 1 are , and 518 nm for particle compositions , 0.5, 0.8, and 0.95, respectively. Differences between the bulk–surface partitioning models are however smaller, Δdc=334 nm, for . The size of activating droplets will affect their liquid water content and in turn the optical properties of droplets and the cloud albedo (Twohy et al., 2013). Variations in dc between the different models may therefore contribute to the uncertainty in estimations for radiative properties of clouds.
3.1.3 Inter-model variation
For particles containing strong surfactant NaC14 with NaCl in Fig. 1, the simple partitioning and compressed film models predict the highest SSc at high surfactant fractions wp,sft. The monolayer and partial film models agree well for the entire range of particle compositions, while the Gibbs model predicts comparable SSc. In previous Köhler calculations for particles containing moderately surface active malonic, succinic, or glutaric acid with ammonium sulfate (Vepsäläinen et al., 2022), the highest SSc were predicted with the simple partitioning model, while the Gibbs, monolayer, and bulk solution models agreed well and predicted similar SSc to the compressed film and partial film models for most wp,sft. The inter-comparison of surface activity and partitioning models therefore shows several differences between strongly and moderately surface active aerosols. This suggests that effects of aerosol surface activity on cloud droplet formation captured by the different partitioning models cannot be robustly understood based on any single model or a few aerosol systems with similar surface activity. Therefore, assessments of bulk–surface partitioning effects in Köhler calculations should be based on predictions for a wide range of conditions and types of surface active aerosol relevant to the atmosphere.
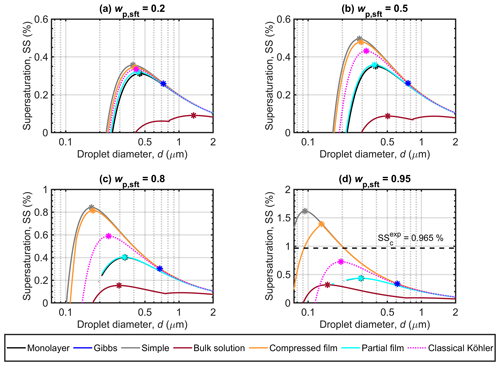
Figure 1Köhler curves calculated with the different models for dry NaC14–NaCl particles with Dp=50 nm. Each panel shows curves for droplets with different NaC14 mass fractions (wp,sft) in the particles. Critical points are also marked on each curve, and the experimental critical supersaturation reported by Prisle et al. (2008) corresponding to is included in panel (d). Note that the vertical-axis scaling changes between the panels.
3.2 Droplet surface tension
Figure 2 shows the droplet surface tensions predicted along the corresponding Köhler curves in Fig. 1. The simple partitioning and classical Köhler models both assume a constant surface tension equal to that of pure water (σw). The surface tensions predicted during droplet growth vary significantly between the other models, but the surface tension curves for each model show similar characteristics between different wp,sft.
Surface tensions at the critical point of cloud droplet activation (σc) predicted with the different models for each wp,sft are given in Table 3. The σc differ significantly between the models and the differences increase with surfactant fraction wp,sft in the particles. For in Fig. 2d, σc spans the extremes from σCMC to σw between the different models. The monolayer and partial organic film models predict σc that significantly decreases with wp,sft, while σc for the Gibbs model is close to σw. For the compressed film model, σc=σw for the investigated wp,sft. The bulk solution model predicts σc=σCMC for –0.95 and also the lowest σc among the models for .
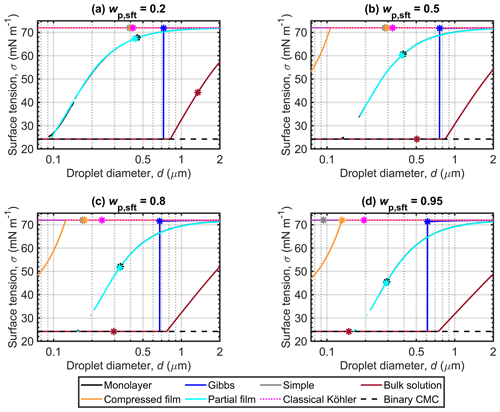
Figure 2Droplet surface tensions along the Köhler curves, predicted with the different models for dry particles of Dp=50 nm with varying NaC14 mass fractions (wp,sft). The critical points evaluated for the Köhler curves in Fig. 1 are also marked. The surface tension at the CMC (σCMC) for NaC14 in binary aqueous solution was estimated from the measurements of Wen et al. (2000) and is indicated as a lower limit for the droplet surface tension.
Droplet surface tensions predicted with the Gibbs model show a step increase from σCMC to values near σw for each wp,sft in Fig. 2. The droplet diameter at (or immediately after) the step increase corresponds to the critical point, as discussed in connection with the Köhler curves. The surface tension curves of the monolayer and partial organic film models are similar for all wp,sft in Fig. 2. The partial organic film model calculations were also performed with a constant surface thickness δ=0.5 nm, but changes to the results were minor (Sect. S1.3).
The droplet surface tension curves for the compressed film model behave differently for NaC14 particles in Fig. 2 than for previous applications to other surface active aerosol systems (e.g., Ruehl et al., 2016; Forestieri et al., 2018; Vepsäläinen et al., 2022). Surface tension depression from σw before droplet activation is visible in Fig. 2b–d, but only for the highest NaC14 fractions in Fig. 2d is dc close to droplet size where the surface tension reaches σw, as was the typical observation of Ruehl et al. (2016) for dicarboxylic acids. Vepsäläinen et al. (2022) observed that the critical point and surface tension reaching σw occurred at different droplet sizes for particles with Dp=50 nm comprising moderately surface active di- and tricarboxylic acids and ammonium sulfate at . Forestieri et al. (2018) made analogous observations as Vepsäläinen et al. (2022) for 80 nm NaCl particles coated with strongly surface active oleic acid at an organic volume fraction of 0.8. Predictions for myristic acid–NaCl particles (Sect. S1.1) show that the critical point and the surface tension reaching σw do not happen for similar droplet sizes at all wp,sft values. Forestieri et al. (2018) used the same compressed film model parameters fitted for myristic acid as used here for both NaC14 and myristic acid, at organic volume fractions of 0.40–0.98 for Dseed=180 and 200 nm and RH =99.83 %–99.93 %. The compressed film model parameters are fitted assuming pseudo-ideal droplet solutions, but aqueous fatty acids and their sodium salts can show significant deviations from an ideal solution (Michailoudi et al., 2020; Calderón et al., 2020). The present results suggest that the compressed film model is sensitive to the fitting conditions of the model parameters. The underlying assumption that the model parameters are compound-specific physical constants across varying surfactant mass fractions and dry particle sizes may not hold true for real droplet solutions.
The σc predicted for the monolayer, Gibbs, partial organic film, and bulk solution models in Fig. 2 decreases with increasing wp,sft. For the monolayer, Gibbs, and partial organic film models, the mutual variations in σc are within 4.7, 11.8, 20.4, and 26.9 mN m−1 of σw for particles with , 0.5, 0.8, and 0.95, respectively (Table 3). For the monolayer and partial organic film models, σc shows large depressions from σw, in agreement with previous applications of the models (Ovadnevaite et al., 2017; Malila and Prisle, 2018; Lin et al., 2018, 2020; Vepsäläinen et al., 2022). For sodium myristate particles in Fig. 2, the Gibbs model predicts σc close to σw and σc=σw for –0.95 with the simple partitioning, compressed film, and classical Köhler models. The small or absent surface tension depression predicted with the Gibbs and compressed film models for NaC14 is in agreement with other surface active aerosols (Prisle et al., 2008, 2010; Ruehl et al., 2016; Forestieri et al., 2018; Lin et al., 2018; Prisle, 2021; Vepsäläinen et al., 2022). The Gibbs model has been observed to predict significantly higher σc than the monolayer model for strongly surface active SDS–NaCl and moderately surface active ragweed pollenkitt–ammonium sulfate aerosol, as well as somewhat lower σc than the monolayer model for even more moderately surface active succinic acid–NaCl aerosol (Lin et al., 2018). The droplet σc for the bulk solution model shows a 27.8 mN m−1 reduction from σw for (Fig. 2a) and a 47.7 mN m−1 reduction from σw for –0.95 (Fig. 2b–d), where σc=σCMC.
Droplet surface tension impacts the conditions for growth and activation via the Kelvin term of the Köhler Eq. (1). The importance of surface tension in cloud droplet formation has been a topic of debate for decades (e.g., Li et al., 1998; Sorjamaa et al., 2004; Booth et al., 2009; Prisle et al., 2008, 2010; Nozière et al., 2014; Gérard et al., 2016; Ruehl et al., 2016; Ovadnevaite et al., 2017; Davies et al., 2019; Lowe et al., 2019; Bzdek et al., 2020). Here, we observe that different models predict diverging droplet surface tensions for strongly surface active aerosol, similarly to predictions for moderately surface active aerosol by Vepsäläinen et al. (2022). The σc of the monolayer and bulk solution models for strongly surface active aerosol is significantly lower than for moderately surface active aerosol, while the other partitioning models predict similar σc for both strongly and moderately surface active aerosol. The predicted droplet surface tensions reflect the underlying assumptions of the different models (Table 2), which do not account for all surfactant properties to the same degree. This suggests that varying conclusions about the importance of surface tension in cloud droplet activation could partly be due to differences between the various surface activity models applied. These model differences show that caution should be taken when interpreting the role of surface tension based on the predictions of any given model.
3.3 Bulk–surface partitioning of surfactant
Figure 3 shows the surface partitioning factors, in terms of the fraction of the total amount of NaC14 that is partitioned to the surface (), for growing droplets along the Köhler curves in Fig. 1. Both the simple partitioning and partial organic film models assume that all surfactant content is partitioned to the droplet surface throughout the droplet growth, such that . The bulk solution and classical Köhler models do not consider bulk–surface partitioning, and therefore . The constant partitioning factors for these models are not shown in Fig. 3.
Figure 3 shows that the compressed film model predicts the strongest bulk–surface partitioning of NaC14 in growing droplets, followed by the Gibbs and monolayer models. For these models, the predicted partitioning factors are close, or equal, to unity throughout the respective Köhler curves, but the predicted droplet activation properties can vary significantly between the models (Table 3). For moderately surface active malonic, succinic, and glutaric acid particles, we previously found that the compressed film model predicted considerably larger partitioning factors than the monolayer and Gibbs models, whereas the different models could still predict similar droplet activation properties (Vepsäläinen et al., 2022). The strong partitioning predicted (or assumed) with the different partitioning models for NaC14 means that inter-model differences in predicted droplet activation in Fig. 1 are mainly caused by differences in the droplet surface tension (Fig. 2). Surface activity could also have important implications for a variety of processes related to cloud microphysics, including aqueous droplet chemistry (Prisle, 2021). Chemical reactions in aqueous aerosols can be accelerated relative to macroscopic solutions (Marsh et al., 2019). Strong partitioning of surface-active species and the simultaneous depletion of the droplet bulk phase can affect the chemical environment in the droplet, in particular in the submicron range (Prisle et al., 2010), changing possible reaction pathways and rates in the surface (Prisle et al., 2012b; Öhrwall et al., 2015; Werner et al., 2018) and bulk (Prisle, 2021) phases of the droplet. Interfacial reactivity could be pronounced due to the large surface-area-to-volume ratios of finite volume droplets (Prisle et al., 2012b; Bzdek et al., 2020; Prisle, 2021).
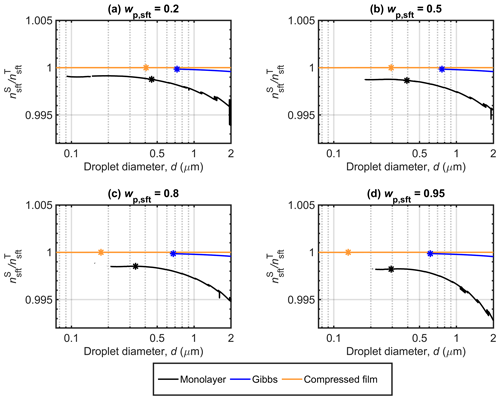
Figure 3Surface partitioning factors () predicted with the monolayer, Gibbs, and compressed film bulk–surface partitioning models along the Köhler curves for NaC14 particles with Dp=50 nm at different NaC14 mass fractions (wp,sft). The critical points of the corresponding Köhler curves in Fig. 1 are also marked.
Table 3Critical droplet diameters (dc), supersaturations (SSc), and surface tensions (σc) predicted with the different models for NaC14–NaCl particles with Dp=50 nm at 298.15 K. The simple partitioning, compressed film, and classical Köhler models all predict that σc = σw = 72.0 mN m−1 for each wp,sft, which is not shown in the table.
3.4 Relative change in the cloud droplet number concentration
Figure 4 shows the relative change in cloud droplet number concentration predicted with the different bulk–surface partitioning models and the bulk solution model to account for aerosol surface activity, with respect to predictions of the classical Köhler model (ΔNN−1, Eq. 11), for NaC14 particles in the size range Dp=50–200 nm. Implementations corresponding to the classical Köhler model are used in most larger-scale simulations, with a few exceptions (e.g., Prisle et al., 2012a; Lowe et al., 2019). The magnitude of ΔNN−1 therefore highlights how variations in critical supersaturations predicted with the different surface activity models may translate into uncertainty in estimates of atmospheric cloud droplet number concentrations based on Köhler theory.
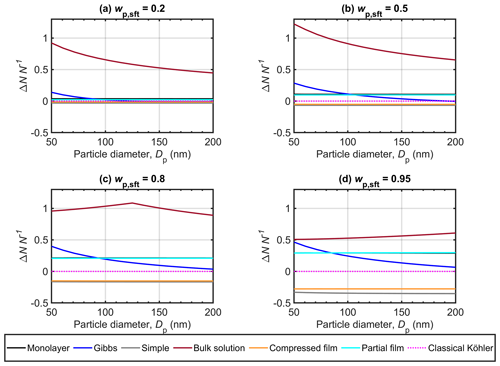
Figure 4Relative change in cloud droplet number concentration (ΔNN−1) predicted the different surface activity models, for Dp=50–200 nm at different NaC14 mass fractions (wp,sft) in the particles. The relative change is calculated with respect to predictions of the classical Köhler model.
In Fig. 4, the monolayer, simple partitioning, compressed film, and partial film models all show very little variation in ΔNN−1 with particle size Dp for each wp,sft. The differences in ΔNN−1 between the surface activity model predictions increase with wp,sft, as also seen with the SSc in Fig. 1. For , the monolayer, simple partitioning, compressed film, and partial film models each yield ΔNN−1 within ±5 %. For , we predict 29 % more cloud droplets with the monolayer and partial film models than with the classical Köhler model, on average over the investigated particle size range. This is in reasonable agreement with results of Lowe et al. (2019), who used a partial film partitioning model combined with a cloud parcel model to predict an increase of 13 % in cloud droplet number concentrations for marine and an increase of 26 % for continental boreal aerosol populations compared to classical Köhler predictions. For , we however predict a 28 % decrease in cloud droplet number concentrations with the compressed film model and a decrease of 35 % with the simple model compared to the classical Köhler model. This shows that, for these strongly surface active particles, even the direction of the effects of surface activity as significant positive or negative ΔNN−1 can vary with the choice of partitioning model.
With the Gibbs model, we here predict ΔNN−1 of 2–21 % averaged over the particle size range for the surfactant fractions –0.95. The bulk solution model predicts the lowest SSc for the cases studied, resulting in the largest positive ΔNN−1 of all the surface activity models. This is similar to the results of Prisle et al. (2012a), who implemented surfactant effects in the global circulation model (GCM) ECHAM5.5-HAM2. The bulk solution model here yields ΔNN−1 which decreases (for and 0.5), increases (for ), or changes non-monotonically (for ) with increasing Dp. This variation is due to the constraint on the droplet water activity imposed by the CMC, as well as the critical point of droplet activation moving between the two maxima of the Köhler curve, located at droplet sizes before and after the droplet surface tension increases from the minimum value σCMC (see Figs. 1 and 2). On overage over the investigated particle size range, the bulk solution model predicts ΔNN−1 of 55 %–99 % for particles with different surfactant mass fractions. However, these very large ΔNN−1 should be considered with some caution, as large relative changes in SSc may conflict with the assumption of simple exponential dependence in Eq. (11). The bulk–surface partitioning models are considered to give more comprehensive and realistic representations of the droplet activation behavior, whereas the bulk solution model has been included in this comparison mainly for reference.
Results in Fig. 4 support previous estimates, showing that the surface activity of organic aerosol and its representation in calculations of cloud droplet activation have the potential to significantly influence global-scale predictions, at least for specific regions (Prisle et al., 2012a). Increased cloud droplet number concentrations would ultimately have a negative radiative effect and, therefore, a cooling effect on the climate. Decreased cloud droplet number concentrations would conversely lead to a warming effect. Facchini et al. (1999) estimated that a 20 % increase in the cloud droplet number concentration from consideration of aerosol surface activity would correspond to a change in cloud radiative forcing of −1 Wm−2, in good agreement with the later full GCM predictions of Prisle et al. (2012a) for similar conditions. In the present work, the variation in ΔNN−1 between the different model predictions for particles with high NaC14 mass fractions suggests that the representation of surfactant effects could translate to significant uncertainty in larger-scale predictions of cloud radiative effects for regions where strongly surface active aerosol are prevalent. Consideration of surface activity could result in both significant warming or cooling effects, compared to the conventional predictions of a classical Köhler model, depending on the specific partitioning model used. Therefore, conclusions regarding bulk–surface partitioning effects on cloud droplet number concentrations and aerosol–cloud–climate effects based on any one model should be considered and extrapolated with caution.
We have applied five bulk–surface partitioning models (Prisle et al., 2010, 2011; Ruehl et al., 2016; Ovadnevaite et al., 2017; Malila and Prisle, 2018) currently in use in the atmospheric aerosol research community, together with a general bulk solution model and a classical Köhler model, in predictive Köhler modeling of droplet growth and activation for particles comprising strong surfactants sodium myristate or myristic acid mixed with NaCl across a range of particle compositions.
The different models can predict significantly different droplet activation properties (SSc, dc, and σc) for the same strongly surface active particles. Differences between the predictions of the bulk–surface partitioning models increase with the surfactant mass fraction in the particles. Each partitioning model predicts or assumes strong partitioning of surfactant to the droplet surface, leading to a small overall Raoult effect and small variations in the droplet bulk water activity between the different partitioning model predictions. Predicted differences in the critical droplet properties for the investigated strongly surface active aerosols mainly stem from the droplet surface tension and ensuing Kelvin effect. Our results further show that cloud droplet number concentrations predicted for strongly surface active aerosol can vary significantly between the different models. Relative changes in cloud droplet number concentrations with respect to a classical Köhler model range from −35 % to 29 % between the five partitioning models, corresponding to both considerable warming and cooling climate effects. These differences represent a significant uncertainty in estimating the cloud radiative effects for regions where strongly surface active aerosol are prevalent. Therefore, conclusions regarding aerosol–cloud–climate effects of aerosol surface activity and bulk–surface partitioning based on any one surface activity model should not be immediately generalized.
Comparison of inter-model variation for strongly and moderately (Vepsäläinen et al., 2022) surface active aerosol shows how the mutual agreement between the different surface activity and partitioning models varies with aerosol surface activity. Therefore, conclusions about the robustness of our understanding of the effects of aerosol surface activity, as captured by the inter-model variation, do not immediately translate between different surface active aerosol systems. This emphasizes the need to validate aerosol surface activity models for a range of surface active aerosol types and ambient conditions before establishing their broad applicability in atmospheric modeling. Generalization of Köhler predictions for only a few surface active aerosol systems and conditions could introduce significant bias in modeling larger-scale atmospheric processes.
Output data of the different models are available at https://doi.org/10.5281/zenodo.10006607 (Vepsäläinen et al., 2023).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-23-15149-2023-supplement.
SV performed the simulations and the analysis of model results with assistance from SMC and NLP. SV and NLP wrote the original and revised article drafts, responded to the reviewers, and made the visualizations, with assistance from SMC. NLP conceived the project and methodology, was responsible for supervision and project management, and secured funding for the work.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme, Project SURFACE (grant agreement no. 717022). The authors also gratefully acknowledge the financial contribution from the Academy of Finland (grant nos. 308238, 314175, and 335649).
This paper was edited by Luis A. Ladino and reviewed by three anonymous referees.
AIOMFAC-web: version 3.04, https://aiomfac.lab.mcgill.ca (last access: 13 April 2023), 2023. a
Álvarez Silva, E., García-Abuín, A., Gómez-Díaz, D., Navaza, J. M., and Vidal-Tato, I.: Density, Speed of Sound, Surface Tension, and Electrical Conductivity of Sodium Dodecanoate Aqueous Solutions from T = (293.15 to 323.15) K, J. Chem. Eng. Data, 55, 4058–4061, https://doi.org/10.1021/je100186x, 2010. a, b
Booth, A. M., Topping, D. O., McFiggans, G., and Percival, C. J.: Surface tension of mixed inorganic and dicarboxylic acid aqueous solutions at 298.15 K and their importance for cloud activation predictions, Phys. Chem. Chem. Phys., 11, 8021–8028, https://doi.org/10.1039/B906849J, 2009. a
Bzdek, B. R., Power, R. M., Simpson, S. H., Reid, J. P., and Royall, C. P.: Precise, contactless measurements of the surface tension of picolitre aerosol droplets, Chem. Sci., 7, 274–285, https://doi.org/10.1039/C5SC03184B, 2016. a
Bzdek, B. R., Reid, J. P., Malila, J., and Prisle, N. L.: The surface tension of surfactant-containing, finite volume droplets, P. Natl. Acad. Sci. USA, 117, 8335–8343, https://doi.org/10.1073/pnas.1915660117, 2020. a, b, c, d, e, f, g, h, i
Calderón, S. M. and Prisle, N. L.: Composition dependent density of ternary aqueous solutions of ionic surfactants and salts, J. Atmos. Chem, 78, 99–123, https://doi.org/10.1007/s10874-020-09411-8, 2021. a, b
Calderón, S. M., Malila, J., and Prisle, N. L.: Model for estimating activity coefficients in binary and ternary ionic surfactant solutions, J. Atmos. Chem., 77, 141–168, https://doi.org/10.1007/s10874-020-09407-4, 2020. a, b
Campbell, A. N. and Lakshminarayanan, G. R.: CONDUCTANCES AND SURFACE TENSIONS OF AQUEOUS SOLUTIONS OF SODIUM DECANOATE, SODIUM LAURATE, AND SODIUM MYRISTATE, AT 25∘ AND 35∘, Can. J. Chem., 43, 1729–1737, https://doi.org/10.1139/v65-228, 1965. a
Cheng, Y., Li, S.-M., Leithead, A., Brickell, P. C., and Leaitch, W. R.: Characterizations of cis-pinonic acid and n-fatty acids on fine aerosols in the Lower Fraser Valley during Pacific 2001 Air Quality Study, Atmos. Environ., 38, 5789–5800, https://doi.org/10.1016/j.atmosenv.2004.01.051, 2004. a
Cochran, R. E., Laskina, O., Jayarathne, T., Laskin, A., Laskin, J., Lin, P., Sultana, C., Lee, C., Moore, K. A., Cappa, C. D., Bertram, T. H., Prather, K. A., Grassian, V. H., and Stone, E. A.: Analysis of Organic Anionic Surfactants in Fine and Coarse Fractions of Freshly Emitted Sea Spray Aerosol, Environ. Sci. Technol., 50, 2477–2486, https://doi.org/10.1021/acs.est.5b04053, 2016. a, b
CRC Handbook: CRC Handbook of Chemistry and Physics, 101st edition, edited by: Rumble, J., CRC Press, Boca Raton, FL, 1572 pp., ISBN-13 978-0-367-41724-6, 2020. a
Davies, J. F., Zuend, A., and Wilson, K. R.: Technical note: The role of evolving surface tension in the formation of cloud droplets, Atmos. Chem. Phys., 19, 2933–2946, https://doi.org/10.5194/acp-19-2933-2019, 2019. a, b
DeMott, P. J., Mason, R. H., McCluskey, C. S., Hill, T. C. J., Perkins, R. J., Desyaterik, Y., Bertram, A. K., Trueblood, J. V., Grassian, V. H., Qiu, Y., Molinero, V., Tobo, Y., Sultana, C. M., Lee, C., and Prather, K. A.: Ice nucleation by particles containing long-chain fatty acids of relevance to freezing by sea spray aerosols, Environ. Sci.-Process. Impact., 20, 1559–1569, https://doi.org/10.1039/C8EM00386F, 2018. a
Di Nicola, G., Coccia, G., and Pierantozzi, M.: A new equation for the surface tension of carboxylic acids, Fluid Phase Equilib., 417, 229–236, https://doi.org/10.1016/j.fluid.2016.03.001, 2016. a
Facchini, M. C., Mircea, M., Fuzzi, S., and Charlson, R. J.: Cloud albedo enhancement by surface-active organic solutes in growing droplets, Nature, 401, 257–259, https://doi.org/10.1038/45758, 1999. a, b, c
Facchini, M. C., Decesari, S., Mircea, M., Fuzzi, S., and Loglio, G.: Surface tension of atmospheric wet aerosol and cloud/fog droplets in relation to their organic carbon content and chemical composition, Atmos. Environ., 34, 4853–4857, https://doi.org/10.1016/S1352-2310(00)00237-5, 2000. a
Forestieri, S. D., Staudt, S. M., Kuborn, T. M., Faber, K., Ruehl, C. R., Bertram, T. H., and Cappa, C. D.: Establishing the impact of model surfactants on cloud condensation nuclei activity of sea spray aerosol mimics, Atmos. Chem. Phys., 18, 10985–11005, https://doi.org/10.5194/acp-18-10985-2018, 2018. a, b, c, d, e, f, g
Gérard, V., Nozière, B., Baduel, C., Fine, L., Frossard, A. A., and Cohen, R. C.: Anionic, Cationic, and Nonionic Surfactants in Atmospheric Aerosols from the Baltic Coast at Askö, Sweden: Implications for Cloud Droplet Activation, Environ. Sci. Technol., 50, 2974–2982, https://doi.org/10.1021/acs.est.5b05809, 2016. a, b
Gérard, V., Noziere, B., Fine, L., Ferronato, C., Singh, D. K., Frossard, A., Cohen, R. C., Asmi, E., Lihavainen, H., Kivekäs, N., Aurela, M., Brus, D., Frka, S., and Cvitešić Kušan, A.: Concentrations and Adsorption Isotherms for Amphiphilic Surfactants in PM1 Aerosols from Different Regions of Europe, Environ. Sci. Technol., 53, 12379–12388, https://doi.org/10.1021/acs.est.9b03386, 2019. a
Hyvärinen, A.-P., Lihavainen, H., Gaman, A., Vairila, L., Ojala, H., Kulmala, M., and Viisanen, Y.: Surface Tensions and Densities of Oxalic, Malonic, Succinic, Maleic, Malic, and cis-Pinonic Acids, J. Chem. Eng. Data, 51, 255–260, https://doi.org/10.1021/je050366x, 2006. a
Hänel, G.: The Properties of Atmospheric Aerosol Particles as Functions of the Relative Humidity at Thermodynamic Equilibrium with the Surrounding Moist Air, Adv. Geophys., 19, 73–188, https://doi.org/10.1016/S0065-2687(08)60142-9, 1976. a
International Association for the Properties of Water and Steam (IAPWS): Revised Release on Surface Tension of Ordinary Water Substance: IAPWS R1-76 (2014), Moscow, http://www.iapws.org/relguide/Surf-H2O.html (last access: 18 March 2020), 2014. a
IPCC: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia,, Y., Bex, V., and Midgley, P. M., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, ISBN 978-1-107-66182-0, https://doi.org/10.1017/CBO9781107415324, 2013. a
IPCC: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change e, edited by: Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I., Huang, M., Leitzell, K., Lonnoy, E., Matthews, J. B. R., Maycock, T. K., Waterfield, T., Yelekçi, O., Yu, R., and Zhou, B., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, https://doi.org/10.1017/9781009157896, 2021. a
Janz, G. J.: Molten Salts Data as Reference Standards for Density, Surface Tension, Viscosity, and Electrical Conductance: KNO3 and NaCl, J. Phys. Chem. Ref. Data, 9, 791–830, https://doi.org/10.1063/1.555634, 1980. a
Kirpes, R. M., Bonanno, D., May, N. W., Fraund, M., Barget, A. J., Moffet, R. C., Ault, A. P., and Pratt, K. A.: Wintertime Arctic Sea Spray Aerosol Composition Controlled by Sea Ice Lead Microbiology, ACS Cent. Sci., 5, 1760–1767, https://doi.org/10.1021/acscentsci.9b00541, 2019. a, b
Kralchevsky, P. A., Danov, K. D., Broze, G., and Mehreteab, A.: Thermodynamics of Ionic Surfactant Adsorption with Account for the Counterion Binding: Effect of Salts of Various Valency, Langmuir, 15, 2351–2365, https://doi.org/10.1021/LA981127T, 1999. a
Kroflič, A., Frka, S., Simmel, M., Wex, H., and Grgić, I.: Size-Resolved Surface-Active Substances of Atmospheric Aerosol: Reconsideration of the Impact on Cloud Droplet Formation, Environ. Sci. Technol., 52, 9179–9187, https://doi.org/10.1021/acs.est.8b02381, 2018. a
Köhler, H.: The nucleus in and the growth of hygroscopic droplets, Trans. Faraday Soc., 32, 1152–1161, https://doi.org/10.1039/TF9363201152, 1936. a
Langevin, D.: Micelles and Microemulsions, Annu. Rev. Phys. Chem., 43, 341–369, https://doi.org/10.1146/annurev.pc.43.100192.002013, 1992. a
Li, Z., Williams, A. L., and Rood, M. J.: Influence of Soluble Surfactant Properties on the Activation of Aerosol Particles Containing Inorganic Solute, J. Atmos. Sci., 55, 1859–1866, https://doi.org/10.1175/1520-0469(1998)055<1859:IOSSPO>2.0.CO;2, 1998. a, b
Lin, J. J., Malila, J., and Prisle, N. L.: Cloud droplet activation of organic-salt mixtures predicted from two model treatments of the droplet surface, Environ. Sci.-Process. Impact., 20, 1611–1629, https://doi.org/10.1039/c8em00345a, 2018. a, b, c, d, e, f
Lin, J. J., Kristensen, T. B., Calderón, S. M., Malila, J., and Prisle, N. L.: Effects of surface tension time-evolution for CCN activation of a complex organic surfactant, Environ. Sci.-Process. Impact., 22, 271–284, https://doi.org/10.1039/C9EM00426B, 2020. a, b, c, d
Lowe, S. J., Partridge, D. G., Davies, J. F., Wilson, K. R., Topping, D., and Riipinen, I.: Key drivers of cloud response to surface-active organics, Nat. Commun., 10, 5214, https://doi.org/10.1038/s41467-019-12982-0, 2019. a, b, c, d
Malila, J. and Prisle, N. L.: A Monolayer Partitioning Scheme for Droplets of Surfactant Solutions, J. Adv. Model. Earth Syst., 10, 3233–3251, https://doi.org/10.1029/2018MS001456, 2018. a, b, c, d, e, f, g, h, i, j
Marsh, B. M., Iyer, K., and Cooks, R. G.: Reaction Acceleration in Electrospray Droplets: Size, Distance, and Surfactant Effects, J. Am. Soc. Mass Spectrom., 30, 2022–2030, https://doi.org/10.1007/s13361-019-02264-w, 2019. a
McGraw, R. and Wang, J.: Surfactants and cloud droplet activation: A systematic extension of Köhler theory based on analysis of droplet stability, J. Chem. Phys., 154, 024707, https://doi.org/10.1063/5.0031436, 2021. a
Michailoudi, G., Hyttinen, N., Kurtén, T., and Prisle, N. L.: Solubility and Activity Coefficients of Atmospheric Surfactants in Aqueous Solution Evaluated Using COSMOtherm, J. Phys. Chem. A, 124, 430–443, https://doi.org/10.1021/acs.jpca.9b09780, 2020. a
Mochida, M., Kitamori, Y., Kawamura, K., Nojiri, Y., and Suzuki, K.: Fatty acids in the marine atmosphere: Factors governing their concentrations and evaluation of organic films on sea-salt particles, J. Geophys. Res.-Atmos., 107 (D17), AAC1-1–AAC1-10, https://doi.org/10.1029/2001JD001278, 2002. a
Mochida, M., Umemoto, N., Kawamura, K., Lim, H.-J., and Turpin, B. J.: Bimodal size distributions of various organic acids and fatty acids in the marine atmosphere: Influence of anthropogenic aerosols, Asian dusts, and sea spray off the coast of East Asia, J. Geophys. Res.-Atmos., 112, D15209, https://doi.org/10.1029/2006JD007773, 2007. a
Morris, H. S., Grassian, V. H., and Tivanski, A. V.: Humidity-dependent surface tension measurements of individual inorganic and organic submicrometre liquid particles, Chem. Sci., 6, 3242–3247, https://doi.org/10.1039/C4SC03716B, 2015. a
Nguyen, Q. T., Kjær, K. H., Kling, K. I., Boesen, T., and Bilde, M.: Impact of fatty acid coating on the CCN activity of sea salt particles, Tellus B: Chem. Phys. Meteorol., 69, 1304064, https://doi.org/10.1080/16000889.2017.1304064, 2017. a
Noureddini, H., Teoh, B. C., and Davis Clements, L.: Densities of vegetable oils and fatty acids, J. Am. Oil Chem. Soc., 69, 1184–1188, https://doi.org/10.1007/BF02637677, 1992. a
Nozière, B., Baduel, C., and Jaffrezo, J.-L.: The dynamic surface tension of atmospheric aerosol surfactants reveals new aspects of cloud activation, Nat. Commun., 5, 3335, https://doi.org/10.1038/ncomms4335, 2014. a, b
Nozière, B., Gérard, V., Baduel, C., and Ferronato, C.: Extraction and Characterization of Surfactants from Atmospheric Aerosols., J. Visualized Exp., 122, e55622, https://doi.org/10.3791/55622, 2017. a
Öhrwall, G., Prisle, N. L., Ottosson, N., Werner, J., Ekholm, V., Walz, M.-M., and Björneholm, O.: Acid–Base Speciation of Carboxylate Ions in the Surface Region of Aqueous Solutions in the Presence of Ammonium and Aminium Ions, J. Phys. Chem. B, 119, 4033–4040, https://doi.org/10.1021/jp509945g, 2015. a
Ovadnevaite, J., Zuend, A., Laaksonen, A., Sanchez, K. J., Roberts, G., Ceburnis, D., Decesari, S., Rinaldi, M., Hodas, N., Facchini, M. C., Seinfeld, J. H., and O'Dowd, C.: Surface tension prevails over solute effect in organic-influenced cloud droplet activation, Nature, 546, 637–641, https://doi.org/10.1038/nature22806, 2017. a, b, c, d, e, f, g, h
Perkins, R. J., Vazquez de Vasquez, M. G., Beasley, E. E., Hill, T. C. J., Stone, E. A., Allen, H. C., and DeMott, P. J.: Relating Structure and Ice Nucleation of Mixed Surfactant Systems Relevant to Sea Spray Aerosol, J. Phys. Chem. A, 124, 8806–8821, https://doi.org/10.1021/acs.jpca.0c05849, 2020. a
Petters, S. S. and Petters, M. D.: Surfactant effect on cloud condensation nuclei for two-component internally mixed aerosols, J. Geophys. Res.-Atmos., 121, 1878–1895, https://doi.org/10.1002/2015JD024090, 2016. a, b, c, d
Politovich, M. K. and Cooper, W. A.: Variability of the Supersaturation in Cumulus Clouds, J. Atmos. Sci., 45, 1651–1664, https://doi.org/10.1175/1520-0469(1988)045<1651:VOTSIC>2.0.CO;2, 1988. a
Prisle, N. L.: A predictive thermodynamic framework of cloud droplet activation for chemically unresolved aerosol mixtures, including surface tension, non-ideality, and bulk–surface partitioning, Atmos. Chem. Phys., 21, 16387–16411, https://doi.org/10.5194/acp-21-16387-2021, 2021. a, b, c, d, e, f, g, h, i, j, k
Prisle, N. L., Raatikainen, T., Sorjamaa, R., Svenningsson, B., Laaksonen, A., and Bilde, M.: Surfactant partitioning in cloud droplet activation: a study of C8, C10, C12 and C14 normal fatty acid sodium salts, Tellus B, 60, 416–431, https://doi.org/10.1111/j.1600-0889.2008.00352.x, 2008. a, b, c, d, e, f, g, h, i
Prisle, N. L., Raatikainen, T., Laaksonen, A., and Bilde, M.: Surfactants in cloud droplet activation: mixed organic-inorganic particles, Atmos. Chem. Phys., 10, 5663–5683, https://doi.org/10.5194/acp-10-5663-2010, 2010. a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r
Prisle, N. L., Dal Maso, M., and Kokkola, H.: A simple representation of surface active organic aerosol in cloud droplet formation, Atmos. Chem. Phys., 11, 4073–4083, https://doi.org/10.5194/acp-11-4073-2011, 2011. a, b, c, d, e, f, g, h, i
Prisle, N. L., Asmi, A., Topping, D., Partanen, A., Romakkaniemi, S., Dal Maso, M., Kulmala, M., Laaksonen, A., Lehtinen, K. E. J., McFiggans, G., and Kokkola, H.: Surfactant effects in global simulations of cloud droplet activation, Geophys. Res. Lett., 39, L05802, https://doi.org/10.1029/2011GL050467, 2012a. a, b, c, d
Prisle, N. L., Ottosson, N., Öhrwall, G., Söderström, J., Dal Maso, M., and Björneholm, O.: Surface/bulk partitioning and acid/base speciation of aqueous decanoate: direct observations and atmospheric implications, Atmos. Chem. Phys., 12, 12227–12242, https://doi.org/10.5194/acp-12-12227-2012, 2012. a, b, c
Prisle, N. L., Lin, J. J., Purdue, S., Lin, H., Meredith, J. C., and Nenes, A.: Cloud condensation nuclei activity of six pollenkitts and the influence of their surface activity, Atmos. Chem. Phys., 19, 4741–4761, https://doi.org/10.5194/acp-19-4741-2019, 2019. a, b
Pátek, J., Hrubý, J., Klomfar, J., Součková, M., and Harvey, A. H.: Reference Correlations for Thermophysical Properties of Liquid Water at 0.1 MPa, J. Phys. Chem. Ref. Data, 38, 21–29, https://doi.org/10.1063/1.3043575, 2009. a
Raatikainen, T. and Laaksonen, A.: A simplified treatment of surfactant effects on cloud drop activation, Geosci. Model Dev., 4, 107–116, https://doi.org/10.5194/gmd-4-107-2011, 2011. a, b
Ruehl, C. R. and Wilson, K. R.: Surface organic monolayers control the hygroscopic growth of submicrometer particles at high relative humidity, J. Phys. Chem. A, 118, 3952–3966, https://doi.org/10.1021/jp502844g, 2014. a
Ruehl, C. R., Davies, J. F., and Wilson, K. R.: An interfacial mechanism for cloud droplet formation on organic aerosols, Science, 351, 1447–1450, https://doi.org/10.1126/science.aad4889, 2016. a, b, c, d, e, f, g, h, i
Shulman, M. L., Jacobson, M. C., Carlson, R. J., Synovec, R. E., and Young, T. E.: Dissolution behavior and surface tension effects of organic compounds in nucleating cloud droplets, Geophys. Res. Lett., 23, 277–280, https://doi.org/10.1029/95GL03810, 1996. a
Siebert, H. and Shaw, R. A.: Supersaturation Fluctuations during the Early Stage of Cumulus Formation, J. Atmos. Sci., 74, 975–988, https://doi.org/10.1175/JAS-D-16-0115.1, 2017. a
Sorjamaa, R., Svenningsson, B., Raatikainen, T., Henning, S., Bilde, M., and Laaksonen, A.: The role of surfactants in Köhler theory reconsidered, Atmos. Chem. Phys., 4, 2107–2117, https://doi.org/10.5194/acp-4-2107-2004, 2004. a, b, c, d, e
Topping, D.: An analytical solution to calculate bulk mole fractions for any number of components in aerosol droplets after considering partitioning to a surface layer, Geosci. Model Dev., 3, 635–642, https://doi.org/10.5194/gmd-3-635-2010, 2010. a, b, c
Toribio, A. R., Prisle, N. L., and Wexler, A. S.: Statistical Mechanics of Multilayer Sorption: Surface Concentration Modeling and XPS Measurement, J. Phys. Chem. Lett., 9, 1461–1464, https://doi.org/10.1021/acs.jpclett.8b00332, 2018. a
Twohy, C. H., Anderson, J. R., Toohey, D. W., Andrejczuk, M., Adams, A., Lytle, M., George, R. C., Wood, R., Saide, P., Spak, S., Zuidema, P., and Leon, D.: Impacts of aerosol particles on the microphysical and radiative properties of stratocumulus clouds over the southeast Pacific Ocean, Atmos. Chem. Phys., 13, 2541–2562, https://doi.org/10.5194/acp-13-2541-2013, 2013. a
Vanhanen, J., Hyvärinen, A.-P., Anttila, T., Raatikainen, T., Viisanen, Y., and Lihavainen, H.: Ternary solution of sodium chloride, succinic acid and water; surface tension and its influence on cloud droplet activation, Atmos. Chem. Phys., 8, 4595–4604, https://doi.org/10.5194/acp-8-4595-2008, 2008. a, b
Vepsäläinen, S., Calderón, S. M., Malila, J., and Prisle, N. L.: Comparison of six approaches to predicting droplet activation of surface active aerosol – Part 1: moderately surface active organics, Atmos. Chem. Phys., 22, 2669–2687, https://doi.org/10.5194/acp-22-2669-2022, 2022. a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r
Vepsäläinen, S., Calderón, S. M., and Prisle, N. L.: Output data for “Comparison of six approaches to predicting droplet activation of surface active aerosol. Part 2: strong surfactants” by Vepsäläinen et al. (2023), Zenodo [data set], https://doi.org/10.5281/zenodo.10006607, 2023. a
Wang, X., Sultana, C. M., Trueblood, J., Hill, T. C. J., Malfatti, F., Lee, C., Laskina, O., Moore, K. A., Beall, C. M., McCluskey, C. S., Cornwell, G. C., Zhou, Y., Cox, J. L., Pendergraft, M. A., Santander, M. V., Bertram, T. H., Cappa, C. D., Azam, F., DeMott, P. J., Grassian, V. H., and Prather, K. A.: Microbial Control of Sea Spray Aerosol Composition: A Tale of Two Blooms, ACS Cent. Sci., 1, 124–131, https://doi.org/10.1021/acscentsci.5b00148, 2015. a, b
Wen, X., Lauterbach, J., and Franses, E. I.: Surface Densities of Adsorbed Layers of Aqueous Sodium Myristate Inferred from Surface Tension and Infrared Reflection Absorption Spectroscopy, Langmuir, 16, 6987–6994, https://doi.org/10.1021/la991326s, 2000. a, b, c, d, e
Werner, J., Julin, J., Dalirian, M., Prisle, N. L., Öhrwall, G., Persson, I., Björneholm, O., and Riipinen, I.: Succinic acid in aqueous solution: connecting microscopic surface composition and macroscopic surface tension, Phys. Chem. Chem. Phys., 16, 21486–21495, https://doi.org/10.1039/C4CP02776K, 2014. a
Werner, J., Persson, I., Björneholm, O., Kawecki, D., Saak, C.-M., Walz, M.-M., Ekholm, V., Unger, I., Valtl, C., Caleman, C., Öhrwall, G., and Prisle, N. L.: Shifted equilibria of organic acids and bases in the aqueous surface region, Phys. Chem. Chem. Phys., 20, 23281–23293, https://doi.org/10.1039/C8CP01898G, 2018. a, b
Zhang, C., Tian, J., Zheng, M., Yi, H., Zhang, L., and Liu, S.: A new corresponding state-based correlation for the surface tension of organic fatty acids, Mod. Phys. Lett. B, 32, 1750361, https://doi.org/10.1142/S0217984917503614, 2018. a
Zuend, A., Marcolli, C., Luo, B. P., and Peter, T.: A thermodynamic model of mixed organic-inorganic aerosols to predict activity coefficients, Atmos. Chem. Phys., 8, 4559–4593, https://doi.org/10.5194/acp-8-4559-2008, 2008. a
Zuend, A., Marcolli, C., Booth, A. M., Lienhard, D. M., Soonsin, V., Krieger, U. K., Topping, D. O., McFiggans, G., Peter, T., and Seinfeld, J. H.: New and extended parameterization of the thermodynamic model AIOMFAC: calculation of activity coefficients for organic-inorganic mixtures containing carboxyl, hydroxyl, carbonyl, ether, ester, alkenyl, alkyl, and aromatic functional groups, Atmos. Chem. Phys., 11, 9155–9206, https://doi.org/10.5194/acp-11-9155-2011, 2011. a





