the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Fast particulate nitrate formation via N2O5 uptake aloft in winter in Beijing
Haichao Wang
Xiaorui Chen
Qindan Zhu
Zhijun Wu
Yusheng Wu
Kang Sun
Particulate nitrate () is an important component of secondary aerosols in urban areas. Therefore, it is critical to explore its formation mechanism to assist with the planning of haze abatement strategies. Here we report vertical measurements of NOx and O3 by in situ instruments on a movable carriage on a tower during a winter heavy-haze episode (18 to 20 December 2016) in urban Beijing, China. Based on the box model simulation at different heights, we found that formation via N2O5 heterogeneous uptake was negligible at ground level due to N2O5 concentrations of near zero controlled by high NO emissions and NO concentration. In contrast, the contribution from N2O5 uptake was large at high altitudes (e.g., > 150 m), which was supported by the lower total oxidant (NO2 + O3) level at high altitudes than at ground level. Modeling results show the specific case that the nighttime integrated production of for the high-altitude air mass above urban Beijing was estimated to be 50 µg m−3 and enhanced the surface-layer the next morning by 28 µg m−3 through vertical mixing. Sensitivity tests suggested that the nocturnal NOx loss by NO3–N2O5 chemistry was maximized once the N2O5 uptake coefficient was over 2 × 10−3 on polluted days with Sa at 3000 µm2 cm−3 in wintertime. The case study provided a chance to highlight the fact that formation via N2O5 heterogeneous hydrolysis may be an important source of particulate nitrate in the urban airshed during wintertime.
- Article
(2097 KB) - Full-text XML
-
Supplement
(425 KB) - BibTeX
- EndNote
Winter particulate matter (PM) pollution events occur frequently in China and have drawn widespread and sustained attention in recent years (Guo et al., 2014; Zhang et al., 2015; Huang et al., 2014; Wang et al., 2016). PM pollution reduces visibility (Lei and Wuebbles, 2013) and has harmful effects on public health (Cao et al., 2012). Particulate nitrate () is an important component of secondary inorganic aerosols and contributes 15–40 % of the PM2.5 mass concentration in China (Sun et al., 2013, 2015a, b; Chen et al., 2015; Zheng et al., 2015; Wen et al., 2015). The main atmospheric pathways of nitrate formation are (1) the reaction of OH with NO2 and (2) N2O5 heterogeneous hydrolysis (Seinfeld and Pandis, 2006). The reaction of OH with NO2 is a daytime pathway, as OH is very low in concentration at night, and N2O5 uptake is a nighttime pathway, as NO3 and N2O5 are easily photolabile.
Particulate nitrate formation via N2O5 heterogeneous hydrolysis in summer was proved efficient by ground-based observation in North China (Wang et al., 2017b; Z. Wang et al., 2017) and found comparable to or even higher than the daytime formation. Several studies showed that N2O5 hydrolysis is responsible for nocturnal enhancement in summer in Beijing (Pathak et al., 2009, 2011; Wang et al., 2017a). Although formation via N2O5 uptake is significant in summertime, the importance of this pathway in wintertime is not well characterized. Many differences in N2O5 chemistry exist between winter and summer. First, as the key precursor of NO3 and N2O5, O3 has a much lower concentration in winter than in summer owing to the short daytime length and weak solar radiation. Second, colder temperatures and high NO2 levels favor partitioning towards N2O5. Third, longer nighttime length in winter makes N2O5 heterogeneous hydrolysis potentially more important in formation. Finally, the N2O5 uptake coefficient, as an important parameter in N2O5 heterogeneous hydrolysis, is likely very different from that in summer. This is because the properties of aerosol particles (e.g., organic compounds, particulate nitrate, liquid water contents, solubility, and viscosity) and meteorological conditions (e.g., temperature and relative humidity) differ between summer and winter (Chen et al., 2015; Zhang et al., 2007). These effects would result in large variations in the N2O5 uptake coefficient (Wahner et al., 1998; Mentel et al., 1999; Kane et al., 2001; Hallquist et al., 2003; Thornton et al., 2003; Bertram and Thornton, 2009; Tang et al., 2012; Wagner et al., 2013; Grzinic et al., 2015). Several parameterization methods did not have good performance in predicting N2O5 uptake coefficient accurately (Chang et al., 2011; Chang et al., 2016).
In addition to the seasonal differences in formation via N2O5 uptake, modeling and field studies showed high levels of NO3 and N2O5 at high altitudes within the nocturnal boundary layer (NBL) owing to the stratification of surface NO and volatile organic compound (VOC) emissions, which lead to gradients in the loss rates for these compounds as a function of altitude (e.g., Brown et al., 2007; Geyer and Stutz, 2004; Stutz et al., 2004). The formation via N2O5 uptake contributes to the gradients in the compound percentage and size distribution of the particle (Ferrero et al., 2010, 2012). On nights when NO3 cannot accumulate in the surface layer owing to high NO emissions, N2O5 uptake can still be active aloft without NO titration. The N2O5 uptake aloft leads to elevated formation in the upper layer as well as effective NOx removal (Watson and Chow, 2002; S. G. Brown et al., 2006; Lurmann et al., 2006; Pusede et al., 2016; Baasandorj et al., 2017). Field observations at high-altitude sites in Kleiner Feldberg, Germany (Crowley et al., 2010a), the London British Telecommunications tower, UK (Benton et al., 2010), and Boulder, CO, USA (Wagner et al., 2013) showed elevated N2O5 concentrations aloft. Model studies showed that varied at different heights and stressed the importance of the heterogeneous formation mechanism (Kim et al., 2014; Ying, 2011; Su et al., 2017). The mass fraction and concentration of in Beijing was reported higher aloft (260 m) than at the ground level (Chan et al., 2005; Sun et al., 2015b), which was explained by favorable gas–particle partitioning aloft under lower temperature conditions. Overall, the active nighttime chemistry in the upper level plays an important role in surface PM pollution through mixing and dispersing within the planet boundary layer (PBL; Prabhakar et al., 2017), and the pollution was even worse in valley terrain regions coupled with adverse meteorological processes (Baasandorj et al., 2017; Green et al., 2015).
To explore the possible sources of and the dependence of its formation on altitude in wintertime in Beijing, we conducted vertical profile measurements of NO, NO2, and O3 with a moving cabin at a tower platform in combination with simultaneous ground measurements of more comprehensive parameters in urban Beijing. A box model was used to investigate the reaction rate of N2O5 heterogeneous hydrolysis and its impact on formation at different altitudes during a heavy-haze episode over urban Beijing. Additionally, the dependence of NOx removal and formation on the N2O5 uptake coefficient was investigated.
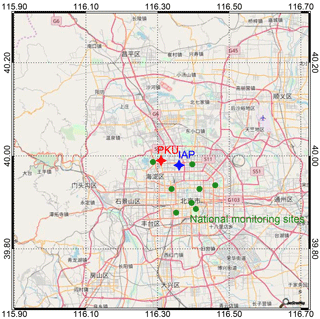
Figure 1Location of the monitoring sites used in this study, including PKU (red diamond), IAP (blue diamond), and other national monitoring sites (green circles). Vertical profiles of NOx and O3 were collected at a tower at the IAP. Measurements of particle number and size distribution (used to calculate N2O5 and particle nitrate formation) were collected from a ground site at PKU. Additional measurements of PM2.5 concentrations were continuously taken at national monitoring sites throughout Beijing.
2.1 Field measurement
Ground measurements (15 m above the ground) were carried out on the campus of Peking University (PKU; 39∘59′21 N, 116∘18′25 E) in Beijing, China. The vertical measurements were conducted at the Institute of Atmospheric Physics (IAP), Chinese Academy of Sciences (39∘58′28 N, 116∘22′16 E). The IAP site is within 4 km of the PKU site. The locations of the PKU and IAP sites are shown in Fig. 1. At the PKU site, the dry-state mass concentration of PM2.5 was measured using a TEOM 1400A analyzer. NOx was measured via a chemiluminescence analyzer (Thermo Scientific, TE-42i-TR), and O3 was measured with a UV photometric O3 analyzer (Thermo Scientific, TE-49i). Dry-state particle number and size distribution (PNSD) were measured from 0.01 to 0.7 µm with a scanning mobility particle sizer (SMPS; TSI Inc. 3010). The instrumental parameters are summarized in Table S1 in the Supplement. The data were collected from 16 to 22 December 2016. Additionally, relative humidity (RH), temperature (T), and wind direction and speed data were available during the measurement period.
Vertical profile measurements were conducted from 18 to 20 December 2016 from the tower-based platform (maximum height 325 m) on the IAP campus. The NOx and O3 instruments were installed aboard a movable cabin on the tower. NOx and O3 were measured with two low-power, lightweight instruments (model 405 nm and model 106-L, 2B Technologies, USA). The model 405 nm instrument measures NO2 directly based on the absorbance at 405 nm, and NO is measured by adding excess O3 (conversion efficiency ∼ 100 %). The limit of detection of both NO and NO2 is 1 part per billion volume (ppbv), with an accuracy of 2 ppbv or 2 % of the reading, and the time resolution is 10 s (Birks et al., 2018). The model 106-L instrument measures O3 based on the absorbance at 254 nm, with a precision of 1 ppbv, or 2 % of the reading, and a limit of detection of 3 ppbv. NOx calibration was performed in the lab using a gas calibrator (TE-146i, Thermo Electron, USA) associated with an NO standard (9.8 ppmv). The O3 calibration was done with an O3 calibrator (TE 49i-PS), which was traceable to NIST (National Institute of Standards and Technology) standards annually. Before the campaign, the NOx monitor was compared with a cavity attenuated phase shift (CAPS) particle light extinction monitor, and the O3 monitor was compared to a commercial O3 analyzer (TE-49i, Thermo Electron, USA). Good agreement was found between the portable instruments and the conventional monitors. Height information was retrieved via the observed atmospheric pressure measured by the model 405 nm instrument. The cabin ascended and descended at a rate of 10 m min−1, with a height limit of 260 m during the daytime and 240 m at night. The cabin stopped after reaching the peak, and parameters were measured continually during the last 10 min of each cycle. One vertical cycle lasted for approximately 1 h. We measured two cycles per day, one in the morning and the other in the evening. Six cycles were measured in total during the campaign.
2.2 Box model simulation
A simple chemical mechanism (see Reactions R1–R5) was used in a box model to simulate the nighttime NO3 and N2O5 chemistry under NO free-air-mass conditions. Physical mixing, dilution, deposition, or interruption during the transport of the air mass was not considered in the base case, and the physical influence on the model result will be discussed in Sect. 3.4. Here, f represents the ClNO2 yield from N2O5 uptake. Homogeneous hydrolysis of N2O5 and NO3 heterogeneous uptake reaction were neglected in this analysis because of the low level of absolute humidity and the extremely low NO3 concentration during wintertime (Brown and Stutz, 2012). The corresponding rate constants of Reactions (R1)–(R3) are those reported by Sander et al. (2011).
Following the work of Wagner et al. (2013), the box model can be solved using six equations (Eqs. 1–6). In the framework, O3 is only lost via the reaction of NO2 + O3 and the change in the O3 concentration can be expressed as Eq. (1). Equation (2) can express the losses of NO2. The s(t) is between 0 and 1 and is expressed as Eq. (5), and the physical meaning of s(t) is the ratio of NO3 production, which goes through N2O5 (either as N2O5 or lost through uptake) to the total NO3 production (Wagner et al., 2013). The s(t) favors 0 when direct loss of NO3 dominates and favors 1 when N2O5 uptake dominates NO3 loss. The model calculation has two steps. The first step is to calculate the mixing ratio of NO2 and O3 at time zero (herein designated as sunset). According to Eqs. (1) and (2), the initial NO2 (t=0) and O3 (t=0) concentrations can then be integrated backward in time starting with the measured concentrations of NO2 and O3 at each height. During the pollution period in winter in Beijing (NO2 = 45 ppbv, temperature = 273 K, Sa= 3000 µm2 cm−3), the ratio of N2O5 to NO3 is large enough, i.e., 450. The pseudo-first-order loss rate of N2O5 heterogeneous uptake will be 1 × 10−3 s−1, with an N2O5 uptake coefficient of 5 × 10−3. N2O5 uptake contributes to the NO3 loss rate of 0.4 s−1, which is much higher than the direct NO3 loss through the reaction of NO3 with VOCs. Therefore, N2O5 uptake was proposed to be dominantly responsible for the NO3 loss and the initial s(t) was set to 1. Equation (3) can describe the sum concentration of NO3 and N2O5. Assuming that equilibrium between NO3 and N2O5 is maintained after a certain period, based on the temperature-dependent equilibrium rate constant (keq) and the modeled NO2 at a certain time, Eq. (4) can be used to determine the ratio of N2O5 to NO3. Combined, Eqs. (1)–(4) allow for the calculation of NO3 and N2O5 concentrations considering stable NO3 and N2O5 loss rate constants ( and , respectively). In the second step, a new s(t) was calculated using the data from the first step (Eq. 5); new initial NO2 and O3 concentrations were then approximated, and NO3 and N2O5 values were derived using the same method as in the first step. This process was repeated until the difference between the two s(t) values was less than 0.005. The number of adjustments to a new s(t) could not be calculated more than 10 times. Otherwise, the calculating process would become non-convergent.
The modeled N2O5 concentrations and given were then used to estimate formation. The HNO3 produced in Reaction (R4) was not considered because many of the products are organic nitrates (Brown and Stutz, 2012). Here, k and k denote the pseudo-first-order reaction rate constants of the total NO3 reactivity caused by ambient VOCs and N2O5 heterogeneous uptake, respectively. is given in Eq. (6). Sa is the aerosol surface area, C is the mean molecular speed of N2O5, and is the N2O5 uptake coefficient. Sunset and sunrise times during the measurements were 16:55 and 07:30 (Chinese National Standard Time, CNST), and the running time of the model was set to 14.5 h from sunset to sunrise.
Dry-state Sa at the PKU site was calculated based on the PNSD measurement, which was corrected to ambient (wet) Sa for particle hygroscopicity via a growth factor (Liu et al., 2013). The uncertainty of the wet Sa was estimated to be ∼ 30 %, which was associated with the error from dry PNSD measurement (∼ 20 %) and the growth factor (∼ 20 %). Nighttime averaged Sa on the night of 19 December was about 3000 µm2 cm−3. PM measurements at the national monitoring sites proved that this heavy-haze pollution episode was a typical regional event (Fig. S1 in the Supplement). Furthermore, a synchronous study on the night of 19 December 2016 showed small variation in the vertical particle number concentration, with a boundary layer height of 340 m (Zhong et al., 2017). Overall, the Sa measured at the PKU site can represent the urban Beijing conditions on a horizontal and vertical scale (< 340 m). Although the PNSD information for particles larger than 0.7 µm was not valid during the study period, the particles smaller than 0.7 µm dominated more than 95 % of the aerosol surface area in a subsequent pollution episode (1 January to 1 July 2017), and similar results also were reported in other studies (e.g., Crowley et al., 2010a; Wang et al., 2018). The possible lower bias of Sa (5 %) only led to a small overestimation of N2O5, i.e., 3.6–4.2 %, and an underestimation of of 0.2–2.5 % when varied from 1 × 10−3 to 0.05.
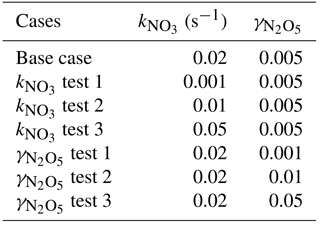
The N2O5 uptake coefficient and ClNO2 yield are key parameters in the estimation of formation (Thornton et al., 2010; Riedel et al., 2013; Wagner et al., 2013; Phillips et al., 2016). Wagner et al. (2013) show the significant suppression of N2O5 uptake aloft in the wintertime in Denver, CO, USA; the uptake coefficient is 0.005 when the percentage of in the PM2.5 mass concentration is 40 %. As the proportion of nitrate in the particle mass concentration is similarly high in North China during wintertime (Sun et al., 2013, 2015a; Chen et al., 2015; Zheng et al., 2015; Wen et al., 2015), herein we fixed the uptake coefficient to 0.005 for the base model initial input. Because the model input of ClNO2 yield only affects the value of produced concentration and would not change the modeled N2O5 concentration, we set the initial to zero. Previous work showed the averaged was 0.01–0.02 s−1 in summer in Beijing, with BVOCs contributing significantly (Wang et al., 2017a; Wang et al., 2018). The intensity of BVOC emissions decreased in wintertime owing to the lower temperature and weak solar radiation, and thus the should be smaller than it is in summer. In this work, the model input was set to an arbitrary and relatively high value of 0.02 s−1 (equivalent to 0.2 ppbv isoprene +40 parts per trillion volume (pptv) monoterpene +1.0 ppbv cis-2-butene) to constrain the impact of N2O5 uptake in the model. A series of sensitivity tests was conducted to study the uncertainties of the model simulation, and the detailed test sets are listed in Table 1, including the test of N2O5 uptake coefficient and . The sensitivity tests were set from 0.001 to 0.05, and the tests were set to 0.001, 0.01, and 0.1 s−1.
In the calculation of particulate nitration formation by N2O5 uptake, an assumption is that all soluble nitrate formed from N2O5 uptake goes to the particle phase rather than the gas phase. The assumption would lead to an upper bias due to the degassing of gas-phase HNO3 from particulate nitrate. While in winter in Beijing, the mixing ratio of NH3 was rich to tens of ppbv and always much higher than the nocturnal gas-phase HNO3 (e.g., Liu et al., 2017). The high NH3 suppressed the degassing of particulate nitrate effectively. The measurement of gas-phase HNO3 and in the surface layer of Beijing showed that soluble nitrate favors the particle phase in winter, especially on polluted days. For example, the nocturnal ratio of to total soluble nitrate was larger than 0.95 on average (Liu et al., 2017). Due to the low temperature and high RH at high altitude, the ratio would increase and the degassing of particulate nitrate is negligible.
3.1 Ground-based observations
A severe winter PM pollution event lasted from 16 to 22 December 2016 in Beijing. Figure 2a shows the time series of PM2.5 and other relevant parameters based on ground measurements at the PKU site. The mass concentration of PM2.5 began to increase from 16 December, reaching 480 µg m−3 on 20 December. A fast PM growth event was captured, with an overall increment of 100 µg m−3 on the night of 19 December (Fig. 2a). Throughout the pollution episode, the meteorological conditions included high RH (50 % ± 16 %) and low temperature (2 ± 3 ∘C). The slow surface wind speed (< 3 m s−1) implied the atmosphere was stable (Fig. 2c, d). The daytime O3 concentration was low owing to high NO emission and weak solar radiation. After sunset, O3 at the surface layer was rapidly titrated to zero by the elevated NO. The presence of high NO concentrations would have strongly suppressed the concentration of NO3, further suppressing N2O5 near the ground. Figure 2b depicts the high amounts of NO and NO2 that were observed at ground level during the PM pollution episode, suggesting that production via N2O5 uptake was not important near the ground during the winter haze episode.
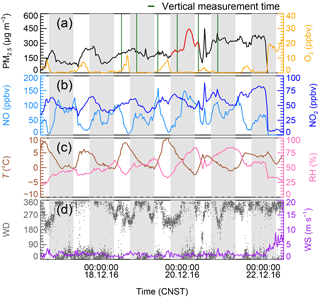
Figure 2Time series of (a) PM2.5 and O3, (b) NO and NO2, (c) temperature (T) and relative humidity (RH), and (d) wind direction (WD) and wind speed (WS) from 16 to 22 December 2016 at the PKU site in Beijing, China. The shaded region represents the nighttime periods. The red line in (a) shows an example of fast PM2.5 enhancement on the night of 19 December and the green lines are the time periods when the vertical measurements were conducted at the IAP site.
3.2 Tower observations
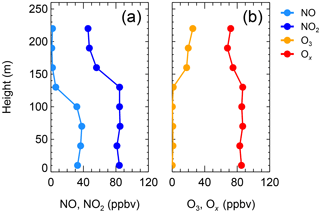
Six vertical measurements of the total oxidants () below 50 m were consistent with those measured at ground level and are shown in Fig. S2, confirming that the two sites are comparable. On the night of 20 December (Fig. 3a), the NO2 and NO from 0–240 m were abundant and conservative around 21:00, with concentrations of 80 and 100 ppbv, respectively. The O3 concentrations remained zero during the nighttime (Fig. 3b). The vertical profile on 20 December suggests that at least below 240 m, the N2O5 chemistry was not important, which is consistent with the results at ground level as mentioned above. The vertical profile on 19 December was different from that on 20 December. Figure 4a shows the vertical profiles around 21:00 on 19 December; NO was abundant from the ground to 100 m, then gradually decreased to zero from 100 to 150 m and remained at zero above 150 m. The observed NO2 concentration was 85 ± 2 ppbv below 100 m, which gradually decreased from 100 to 150 m and was 50 ± 2 ppbv from 150 to 240 m. The observed O3 concentrations below 150 m were below the instrumental limit of detection (Fig. 4b). Above 150 m, the O3 concentration was 20 ± 2 ppbv, corresponding to zero NO concentration. With respect to Ox, the mixing ratio of was 85 ± 2 ppbv at lower altitudes, whereas the concentration at higher altitudes was 15 ppbv lower than at lower altitudes (Fig. 4b). The Ox missing from the higher-altitude air mass indicated an additional nocturnal removal of Ox aloft.
Figure 5 depicts the vertical profiles of NOx, O3, and Ox at 09:30 on the morning of 20 December, which have similar features to those observed at 21:00 on 19 December. The vertical profiles suggest that stratification still existed at that time. The amount of Ox missing aloft in the morning increased to 25 ppbv at 240–260 m, demonstrating that an additional 25 ppbv of Ox was removed or converted to other compounds at higher altitudes than at the surface layer during the night from 19 to 20 December. Figure S3 shows the vertical profiles of NO, NO2, O3, and Ox at ∼ 12:00 on 18 December, when solar radiation was strong enough to mix the trace gases well in the vertical direction. NOx and O3 were found to be well mixed indeed, with small variation from the ground level to 260 m.
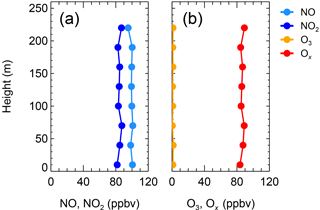
Figure 3Vertical profiles of NO and NO2 (a) and of O3 and Ox (b) at 20:38–21:06 on the night of 20 December 2016.
3.3 Particulate nitrate formation aloft
N2O5 uptake is one of the two most important pathways of ambient NOx loss and formation (Wagner et al., 2013; Stutz et al., 2010; Tsai et al., 2014). At high altitudes (e.g., > 150 m), NO3 and N2O5 chemistry can be initiated in the co-presence of high NO2 and significant O3 levels. Therefore, N2O5 uptake could represent a plausible explanation for the Ox observed to be missing at high altitude on the night of 19 December. To explore this phenomenon, a time-step box model was used to simulate the NO3 and N2O5 chemistry based on the observed vertical profiles of NO2 and O3 on the night of 19 December.
In the base case, the average initial NO2 and O3 levels above 150 m at sunset were 61 ± 3 and 27 ± 6 ppbv, respectively. The measured NO2 concentration at the PKU site at sunset (local time 16:55) was 61 ppbv and showed good consistency with the model result. The modeled N2O5 concentration was zero below 150 m, as the high level of NO made for rapid consumption of the formed NO3. In contrast, the modeled N2O5 concentrations at 21:00 above 150 m were in the range of 400–600 pptv (Fig. 6a). The formation by N2O5 heterogeneous uptake from sunset to the measurement time can be calculated using Eq. (7), which was significant at 24 µg m−3 after sunset above 150 m. The formed in 4.5 h was equivalent to 13 ppbv Ox loss and consistent with the observation (15 ppbv; Fig. 6b). The 1.5:1 relationship between Ox and was used to calculate the Ox equivalence (S. S. Brown et al., 2006a).
The box model enabled the analysis of the integrated and ClNO2 via N2O5 uptake throughout the night. As shown in Fig. 6c, the modeled integrated went as high as 50 µg m−3. The integrated at sunrise was equal to the loss of 27 ppbv Ox, showing a good agreement with the observed Ox missing (25 ppbv) aloft in the morning hours. During the nighttime, the formed aloft via N2O5 uptake led to a much higher particle nitrate concentration than in the surface layer, which has been reported in many field observations (Watson and Chow, 2002; S. G. Brown et al., 2006; Lurmann et al., 2006; Ferrero et al., 2012; Sun et al., 2015b). The elevated aloft was well dispersed through vertical mixing and enhanced the surface-layer PM concentration; this phenomenon was also observed in previous studies (Watson and Chow, 2002; S. G. Brown et al., 2006; Lurmann et al., 2006; Prabhakar et al., 2017). Zhong et al. (2017) showed that the NBL and PBL both were at 340 m from 19 to 20 December 2016 in Beijing. Daytime vertical downward transportation was helpful in mixing the air mass within the PBL. Assuming the newly formed aloft from 150 to 340 m is 50 µg m−3 during the nighttime and is well mixed within the PBL the next morning, the enhancement at the surface layer (Δ) can be simplified to the calculation in Eq. (8) as follows.
Here, P() is the integral production of and H represents height. Owing to high NO below 150 m, the formation via N2O5 uptake was zero. The enhancement of from 150 to 340 m was calculated as 28 µg m−3, which is in good agreement with the observed PM peak in the morning on 20 December, with PM enhancement of ∼ 60 µg m−3. The result demonstrated that the nocturnal N2O5 uptake aloft and downward transportation were critical for understanding the PM growth process.
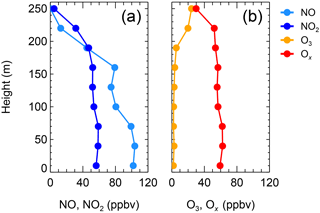
Figure 5Vertical profiles of (a) NO and NO2 and of (b) O3 and Ox at 09:06–09:34 on the morning of 20 December 2016.
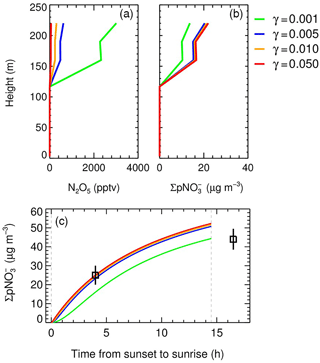
Figure 6Base case (γ= 0.005) and sensitivity tests of the vertical profile on the night of 19 December at different N2O5 uptake coefficients, including (a) the mixing ratio of N2O5 at 21:00, (b) the integral production from sunset to 21:00, and (c) the time series of the integral formed at 240 m via N2O5 uptake from sunset (17:00) to sunrise (07:30; nighttime length 14.5 h). The squares represent the equivalent weight from the observed Ox missing in the two vertical measurements at ∼ 21:00 and ∼ 09:30 on the following morning.
3.4 Sensitivity studies
Previous studies have emphasized that the N2O5 uptake coefficient varies greatly (0.001–0.1) in different ambient conditions (Chang et al., 2011; Brown and Stutz, 2012; Wang and Lu, 2016), which is the main source of uncertainties in this model. In the present research, sensitivity studies showed the modeled N2O5 concentration dropping from 3 ppbv to 60 pptv when the N2O5 uptake coefficients increased from 0.001 to 0.05 (Fig. 6a), as the N2O5 concentration is very sensitive to loss from heterogeneous reactions. Compared to the base case, the accumulated was evidently lower at γ= 0.001 (44 µg m−3). Low N2O5 uptake coefficients correspond to several types of aerosols, such as secondary organic aerosols (Gross et al., 2009), humic acids (Badger et al., 2006), and certain solid aerosols (Gross and Bertram, 2008). When the N2O5 uptake coefficient increased from 0.005 to 0.05 (Fig. 6b, c), the increase in integral was negligible. The conversion capacity of N2O5 uptake to is maximized for a given fixed value of the ClNO2 yield. The conversion of NOx to was not limited by the N2O5 heterogeneous reaction rate, but limited by the formation of NO3 via the reaction of NO2 with O3 during the polluted night.
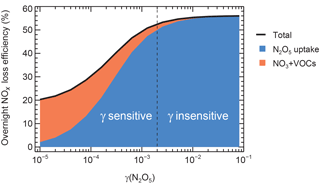
Figure 7The dependence of overnight NOx loss via N2O5 uptake on in typical winter pollution conditions. The initial NO2 and O3 were set to 60 and 30 ppbv, respectively; Sa was set to 3000 µm2 cm−3, the ClNO2 yield was zero, and was 0.02 s−1. The reaction time was set to 14.5 h. The blue and orange zones represent the contribution by NO3+VOCs and N2O5 uptake, and the dashed line (γ= 0.002 when N2O5 uptake contributes to 90 % of the maximum NOx loss) divides the loss into γ-sensitive and γ-insensitive regions. The maximum nocturnal NOx loss by NO3–N2O5 chemistry is 56 %.
For describing the nocturnal NOx removal capacity and formation via NO3 and N2O5 chemistry, the overnight NOx loss efficiency (ε) was calculated using Eq. (9).
The case modeled typical winter haze pollution conditions in Beijing from sunset to sunrise, with the initial model values of NO2 and O3 set to 60 and 30 ppbv, respectively. Sa was set to 3000 µm2 cm−3, the ClNO2 yield was zero, and was 0.02 s−1. The reaction time was set to 14.5 h to represent an overnight period in wintertime. The consumed NO3 by the reaction with VOCs and N2O5 by uptake reaction were regarded as NOx removal. Figure 7 shows the dependence of the overnight NOx loss efficiency on the N2O5 uptake coefficient, as it varied from 1 × 10−5 to 0.1. This is an increase from 20 to 56 % with increasing , which is similar to the result addressed by Chang et al. (2011). The ceiling of overnight NOx loss via NO3–N2O5 chemistry was fixed when all the NOx loss was through N2O5 uptake on polluted days, which is limited by the reaction time and the formation rate of NO3 (Reaction R1). In this case, the N2O5 uptake contributed about 90 % of the overnight NOx loss (50.4 %) when was equal to 2 × 10−3. When was less than 2 × 10−3, NOx removal increased rapidly with increasing , which was defined as the -sensitive region. When 2 × 10−3, the contribution of N2O5 uptake to NOx loss was over 90 % and became insensitive. This region was defined as the -insensitive region. According to Eqs. (3) and (5), high Sa, high NOx, low , or low temperature allow the N2O5 uptake to more easily be located in the -insensitive region. Here, the critical value of the N2O5 uptake coefficient (2 × 10−3) was relatively low compared with that recommended for the surface of mineral dust (0.013, 290–300 K; Crowley et al., 2010b; Tang et al., 2017) or determined in many field experiments (e.g., S. S. Brown et al., 2006b, 2009; Wagner et al., 2013; Morgan et al., 2015; Phillips et al., 2016; Z. Wang et al., 2017; Brown et al., 2016; Wang et al., 2017b; X. F. Wang et al., 2017). This suggests that the NOx loss and formation by N2O5 uptake were easily maximized in the pollution episode and further worsened the PM pollution.
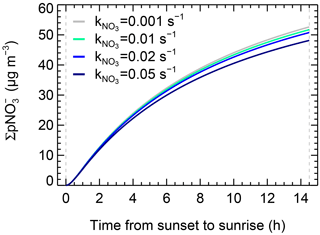
Figure 8Base case ( 0.02 s−1) and sensitivity tests of the integral formed at 240 m via N2O5 uptake at different NO3 reactivity (0.001, 0.01, 0.05 s−1) on the whole night of 19 December 2016.
In the base case, the modeled formation via N2O5 uptake was an upper limit result, as the ClNO2 yield was set to zero. High coal combustion emitted chloride into the atmosphere of Beijing during the heating period (Sun et al., 2013), like the emissions from power plants in North China. This enhanced anthropogenic chloride provides abundant chloride-containing aerosols to form ClNO2 via N2O5 uptake aloft, implying that significant ClNO2 formed in the upper layer of the NBL (Tham et al., 2016; Z. Wang et al., 2017). Assuming the ClNO2 yield is the average value of 0.28 determined at high altitude in North China (Z. Wang et al., 2017), the produced throughout the night decreased 7 µg m−3. The modeled formation of ClNO2 aloft throughout the night was 2.5 ppbv, which is comparable with the observation in North China (Tham et al., 2016; Z. Wang et al., 2017; X. F. Wang et al., 2017). Since the modeled formation is sensitive to the ClNO2 yield, a higher yield would increase the model uncertainty directly, and hence investigating the ClNO2 yield is warranted in future studies. As for NO3 reactivity, Fig. 8 shows the sensitivity tests of the integral formation for the whole night at values of 0.001, 0.01, 0.02, and 0.05 s−1. The integral formation decreased when varied from 0.001 to 0.1 s−1, but the variation ratio to the base case was within 5 %. The result shows that the NO3–N2O5 loss via NO3 reaction with VOCs during the polluted wintertime was not important, which may only lead to relatively small uncertainties in the integral formation calculation. Nevertheless, if N2O5 uptake was extremely low (e.g., < 10−4), the uncertainty of NO3 oxidation would increase significantly.
The uncertainty caused by the physical changes in the air masses were analyzed from two angles: one is the dilution and the other is the mixing and exchange of the air mass. With respect to the impact of the dilution process, it would decrease the mixing ratio of NO2, O3, NO3, and N2O5 and leads to a lower contribution to particulate nitrate formation. An additional loss process for trace gases with a lifetime of 24 h was assumed for calculated species in the sensitivity test (Lu et al., 2012). The result shows that the integrated production of particulate nitrate decreased 28 % compared with the base case. With respect to the exchange and mixing of the air mass at high altitude during nighttime in polluted winter, the stable atmospheric stratification was featured with strong inversion (Zhong et al., 2017). The nocturnal atmosphere is stable and layered, the upward mixing from the surface is minimized, and air masses above the surface are less affected by nocturnal emissions (Wagner et al., 2013). Nevertheless, the injection by warm combustion sources or the clean air mass can affect the air mass. If the warm combustion source emitted NOx into the air mass after sunset, which would increase the mixing ratio of Ox, and restart the zero time of the model. Accounting for the uncertainties from the mixing and sensitivity tests of the box model to shortening the duration by 25 %, the bias of the integrated throughout the night was small within 12 % relative to the base case. If the air mass was affected by the clean air mass from the north, it would be featured with very low NOx and about 40 ppbv O3 (background condition), which was not consistent with our observation.
During the wintertime, ambient O3 is often fully titrated at the ground level in urban Beijing owing to its fast reaction with NO emissions. Consequently, the near-surface air masses are chemically inert. Nevertheless, the chemical information of the air masses at higher altitudes was indicative of a reactive layer above urban Beijing, which potentially drives fast production via N2O5 uptake and contributes to the surface PM mass concentration. In this study, we found a case to show evidence for additional Ox missing (25 ppbv) aloft throughout the night. Based on model simulation, we found that the particulate nitrate formed above 150 m reached 50 µg m−3 and enhanced the surface level PM concentration significantly by 28 µg m−3 with downward mixing after the breakup of the NBL in the morning.
Our result emphasized the importance of the heterogeneous chemistry aloft over the city through a case study. The model simulation also demonstrated that during the heavy PM pollution period, the particulate nitrate formation capacity via N2O5 uptake was easily maximized in the high altitudes above urban Beijing, even with a low N2O5 uptake coefficient. This indicates that the mixing ratio of NO2 aloft was directly linked to nitrate formation, and reduction of NOx is helpful in decreasing nocturnal nitrate formation. Overall, this study highlights the importance of the interplay between chemical formation aloft and dynamic processes for investigating the ground-level PM pollution problem. In the future, direct observations of N2O5 and the associated parameters should be performed to explore the physical and chemical properties of this overhead nighttime reaction layer and to reach a better understanding of winter haze formation.
All the observational data used in this study are available from the corresponding author upon request (k.lu@pku.edu.cn).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-18-10483-2018-supplement.
KL and ZW organized the field contributions from the Peking University group for the APHH-Beijing project. KL and HW designed the experiments presented in this study. HW, QZ, and KL analyzed the data. HW wrote the paper and KL revised it. All authors contributed to measurements, discussing results, and commenting on the paper.
The authors declare that they have no conflict of interest.
This article is part of the special issue “In-depth study of air pollution sources and processes within Beijing and its surrounding region (APHH-Beijing) (ACP/AMT inter-journal SI)”. It is not associated with a conference.
This work was supported by the National Natural Science Foundation of China
(grant nos. 91544225, 41375124, 21522701, 41571130021) and the National Key
Technology Research and Development Program of the Ministry of Science and
Technology of China (grant no. 2014BAC21B01). The authors gratefully
acknowledge the science team of Peking University for their general support,
as well as the team running the tower platform, which enabled the vertical
profile observations.
Edited by: Mei Zheng
Reviewed by: four anonymous referees
Baasandorj, M., Hoch, S. W., Bares, R., Lin, J. C., Brown, S. S., Millet, D. B., Martin, R., Kelly, K., Zarzana, K. J., Whiteman, C. D., Dube, W. P., Tonnesen, G., Jaramillo, I. C., and Sohl, J.: Coupling between Chemical and Meteorological Processes under Persistent Cold-Air Pool Conditions: Evolution of Wintertime PM2.5 Pollution Events and N2O5 Observations in Utah's Salt Lake Valley, Environ. Sci. Technol., 51, 5941–5950, 2017.
Badger, C. L., Griffiths, P. T., George, I., Abbatt, J. P. D., and Cox, R. A.: Reactive uptake of N2O5 by aerosol particles containing mixtures of humic acid and ammonium sulfate, J. Phys. Chem. A, 110, 6986–6994, 2006.
Benton, A. K., Langridge, J. M., Ball, S. M., Bloss, W. J., Dall'Osto, M., Nemitz, E., Harrison, R. M., and Jones, R. L.: Night-time chemistry above London: measurements of NO3 and N2O5 from the BT Tower, Atmos. Chem. Phys., 10, 9781–9795, https://doi.org/10.5194/acp-10-9781-2010, 2010.
Bertram, T. H. and Thornton, J. A.: Toward a general parameterization of N2O5 reactivity on aqueous particles: the competing effects of particle liquid water, nitrate and chloride, Atmos. Chem. Phys., 9, 8351–8363, https://doi.org/10.5194/acp-9-8351-2009, 2009.
Birks, J. W., Andersen, P. C., Williford, C. J., Turnipseed, A. A., Strunk, S. E., Ennis, C. A., and Mattson, E.: Folded tubular photometer for atmospheric measurements of NO2 and NO, Atmos. Meas. Tech., 11, 2821–2835, https://doi.org/10.5194/amt-11-2821-2018, 2018.
Brown, S. G., Roberts, P. T., McCarthy, M. C., Lurmann, F. W., and Hyslop, N. P.: Wintertime vertical variations in particulate matter (PM) and precursor concentrations in the San Joaquin Valley during the California Regional coarse PM/fine PM Air Quality Study, J. Air Waste Manage., 56, 1267–1277, 2006.
Brown, S. S. and Stutz, J.: Nighttime radical observations and chemistry, Chem. Soc. Rev., 41, 6405–6447, 2012.
Brown, S. S., Neuman, J. A., Ryerson, T. B., Trainer, M., Dube, W. P., Holloway, J. S., Warneke, C., de Gouw, J. A., Donnelly, S. G., Atlas, E., Matthew, B., Middlebrook, A. M., Peltier, R., Weber, R. J., Stohl, A., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. R.: Nocturnal odd-oxygen budget and its implications for ozone loss in the lower troposphere, Geophys. Res. Lett., 33, L08801, https://doi.org/10.1029/2006gl025900, 2006a.
Brown, S. S., Ryerson, T. B., Wollny, A. G., Brock, C. A., Peltier, R., Sullivan, A. P., Weber, R. J., Dube, W. P., Trainer, M., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. R.: Variability in nocturnal nitrogen oxide processing and its role in regional air quality, Science, 311, 67–70, 2006b.
Brown, S. S., Dubé, W. P., Osthoff, H. D., Wolfe, D. E., Angevine, W. M., and Ravishankara, A. R.: High resolution vertical distributions of NO3 and N2O5 through the nocturnal boundary layer, Atmos. Chem. Phys., 7, 139–149, https://doi.org/10.5194/acp-7-139-2007, 2007.
Brown, S. S., Dube, W. P., Fuchs, H., Ryerson, T. B., Wollny, A. G., Brock, C. A., Bahreini, R., Middlebrook, A. M., Neuman, J. A., Atlas, E., Roberts, J. M., Osthoff, H. D., Trainer, M., Fehsenfeld, F. C., and Ravishankara, A. R.: Reactive uptake coefficients for N2O5 determined from aircraft measurements during the Second Texas Air Quality Study: Comparison to current model parameterizations, J. Geophys. Res.-Atmos., 114, D00F10, 2009.
Brown, S. S., Dube, W. P., Tham, Y. J., Zha, Q. Z., Xue, L. K., Poon, S., Wang, Z., Blake, D. R., Tsui, W., Parrish, D. D., and Wang, T.: Nighttime chemistry at a high altitude site above Hong Kong, J. Geophys. Res.-Atmos., 121, 2457–2475, 2016.
Cao, J. J., Xu, H. M., Xu, Q., Chen, B. H., and Kan, H. D.: Fine Particulate Matter Constituents and Cardiopulmonary Mortality in a Heavily Polluted Chinese City, Environ. Health Persp., 120, 373–378, 2012.
Chan, C. Y., Xu, X. D., Li, Y. S., Wong, K. H., Ding, G. A., Chan, L. Y., and Cheng, X. H.: Characteristics of vertical profiles and sources of PM2.5, PM10 and carbonaceous species in Beijing, Atmos. Environ., 39, 5113–5124, 2005.
Chang, W. L., Bhave, P. V., Brown, S. S., Riemer, N., Stutz, J., and Dabdub, D.: Heterogeneous Atmospheric Chemistry, Ambient Measurements, and Model Calculations of N2O5: A Review, Aerosol Sci. Tech., 45, 665–695, https://doi.org/10.1080/02786826.2010.551672, 2011.
Chang, W. L., Brown, S. S., Stutz, J., Middlebrook, A. M., Bahreini, R., Wagner, N. L., Dube, W. P., Pollack, I. B., Ryerson, T. B., and Riemer, N.: Evaluating N2O5 heterogeneous hydrolysis parameterizations for CalNex 2010, J. Geophys. Res.-Atmos., 121, 5051–5070, https://doi.org/10.1002/2015jd024737, 2016.
Chen, C., Sun, Y. L., Xu, W. Q., Du, W., Zhou, L. B., Han, T. T., Wang, Q. Q., Fu, P. Q., Wang, Z. F., Gao, Z. Q., Zhang, Q., and Worsnop, D. R.: Characteristics and sources of submicron aerosols above the urban canopy (260 m) in Beijing, China, during the 2014 APEC summit, Atmos. Chem. Phys., 15, 12879–12895, https://doi.org/10.5194/acp-15-12879-2015, 2015.
Crowley, J. N., Schuster, G., Pouvesle, N., Parchatka, U., Fischer, H., Bonn, B., Bingemer, H., and Lelieveld, J.: Nocturnal nitrogen oxides at a rural mountain-site in south-western Germany, Atmos. Chem. Phys., 10, 2795–2812, https://doi.org/10.5194/acp-10-2795-2010, 2010a.
Crowley, J. N., Ammann, M., Cox, R. A., Hynes, R. G., Jenkin, M. E., Mellouki, A., Rossi, M. J., Troe, J., and Wallington, T. J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume V – heterogeneous reactions on solid substrates, Atmos. Chem. Phys., 10, 9059–9223, https://doi.org/10.5194/acp-10-9059-2010, 2010b.
Ferrero, L., Perrone, M. G., Petraccone, S., Sangiorgi, G., Ferrini, B. S., Lo Porto, C., Lazzati, Z., Cocchi, D., Bruno, F., Greco, F., Riccio, A., and Bolzacchini, E.: Vertically-resolved particle size distribution within and above the mixing layer over the Milan metropolitan area, Atmos. Chem. Phys., 10, 3915–3932, https://doi.org/10.5194/acp-10-3915-2010, 2010.
Ferrero, L., Cappelletti, D., Moroni, B., Sangiorgi, G., Perrone, M. G., Crocchianti, S., and Bolzacchini, E.: Wintertime aerosol dynamics and chemical composition across the mixing layer over basin valleys, Atmos. Environ., 56, 143–153, 2012.
Geyer, A. and Stutz, J.: Vertical profiles of NO3, N2O5, O-3, and NOx in the nocturnal boundary layer: 2. Model studies on the altitude dependence of composition and chemistry, J. Geophys. Res.-Atmos., 109, D12307, https://doi.org/10.1029/2003jd004211, 2004.
Green, M. C., Chow, J. C., Watson, J. G., Dick, K., and Inouye, D.: Effects of snow cover and atmospheric stability on winter PM2.5 concentrations in Western U.S. valleys, J. Appl. Meteor. Climatol., 54, 1191–1201, https://doi.org/10.1175/JAMC-D-14-0191.1, 2015.
Gross, S. and Bertram, A. K.: Reactive uptake of NO3, N2O5, NO2, HNO3, and O3 on three types of polycyclic aromatic hydrocarbon surfaces, J. Phys. Chem. A, 112, 3104–3113, 2008.
Gross, S., Iannone, R., Xiao, S., and Bertram, A. K.: Reactive uptake studies of NO3 and N2O5 on alkenoic acid, alkanoate, and polyalcohol substrates to probe nighttime aerosol chemistry, Phys. Chem. Chem. Phys., 11, 7792–7803, 2009.
Gržinic, G., Bartels-Rausch, T., Berkemeier, T., Türler, A., and Ammann, M.: Viscosity controls humidity dependence of N2O5 uptake to citric acid aerosol, Atmos. Chem. Phys., 15, 13615–13625, https://doi.org/10.5194/acp-15-13615-2015, 2015.
Guo, S., Hu, M., Zamora, M. L., Peng, J. F., Shang, D. J., Zheng, J., Du, Z. F., Wu, Z., Shao, M., Zeng, L. M., Molina, M. J., and Zhang, R. Y.: Elucidating severe urban haze formation in China, P. Natl. Acad. Sci. USA, 111, 17373–17378, 2014.
Hallquist, M., Stewart, D. J., Stephenson, S. K., and Cox, R. A.: Hydrolysis of N2O5 on sub-micron sulfate aerosols, Phys. Chem. Chem. Phys., 5, 3453–3463, 2003.
Huang, R. J., Zhang, Y. L., Bozzetti, C., Ho, K. F., Cao, J. J., Han, Y. M., Daellenbach, K. R., Slowik, J. G., Platt, S. M., Canonaco, F., Zotter, P., Wolf, R., Pieber, S. M., Bruns, E. A., Crippa, M., Ciarelli, G., Piazzalunga, A., Schwikowski, M., Abbaszade, G., Schnelle-Kreis, J., Zimmermann, R., An, Z. S., Szidat, S., Baltensperger, U., El Haddad, I., and Prevot, A. S. H.: High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 514, 218–222, 2014.
Kane, S. M., Caloz, F., and Leu, M. T.: Heterogeneous uptake of gaseous N2O5 by (NH4)2SO4, NH4HSO4, and H2SO4 aerosols, J. Phys. Chem. A, 105, 6465–6470, 2001.
Kim, Y. J., Spak, S. N., Carmichael, G. R., Riemer, N., and Stanier, C. O.: Modeled aerosol nitrate formation pathways during wintertime in the Great Lakes region of North America, J. Geophys. Res.-Atmos., 119, 12420–12445, 2014.
Lei, H. and Wuebbles, D. J.: Chemical competition in nitrate and sulfate formations and its effect on air quality, Atmos. Environ., 80, 472–477, 2013.
Liu, M. X., Song, Y., Zhou, T., Xu, Z. Y., Yan, C. Q., Zheng, M., Wu, Z. J., Hu, M., Wu, Y. S., and Zhu, T.: Fine particle pH during severe haze episodes in northern China, Geophys. Res. Lett., 44, 5213–5221, https://doi.org/10.1002/2017gl073210, 2017.
Liu, X. G., Gu, J. W., Li, Y. P., Cheng, Y. F., Qu, Y., Han, T. T., Wang, J. L., Tian, H. Z., Chen, J., and Zhang, Y. H.: Increase of aerosol scattering by hygroscopic growth: Observation, modeling, and implications on visibility, Atmos. Res., 132, 91–101, https://doi.org/10.1016/j.atmosres.2013.04.007, 2013.
Lu, K. D., Rohrer, F., Holland, F., Fuchs, H., Bohn, B., Brauers, T., Chang, C. C., Haseler, R., Hu, M., Kita, K., Kondo, Y., Li, X., Lou, S. R., Nehr, S., Shao, M., Zeng, L. M., Wahner, A., Zhang, Y. H., and Hofzumahaus, A.: Observation and modelling of OH and HO2 concentrations in the Pearl River Delta 2006: a missing OH source in a VOC rich atmosphere, Atmos. Chem. Phys., 12, 1541–1569, https://doi.org/10.5194/acp-12-1541-2012, 2012.
Lurmann, F. W., Brown, S. G., McCarthy, M. C., and Roberts, P. T.: Processes influencing secondary aerosol formation in the San Joaquin Valley during winter, J. Air Waste Manage., 56, 1679–1693, 2006.
Mentel, T. F., Sohn, M., and Wahner, A.: Nitrate effect in the heterogeneous hydrolysis of dinitrogen pentoxide on aqueous aerosols, Phys. Chem. Chem. Phys., 1, 5451–5457, 1999.
Morgan, W. T., Ouyang, B., Allan, J. D., Aruffo, E., Di Carlo, P., Kennedy, O. J., Lowe, D., Flynn, M. J., Rosenberg, P. D., Williams, P. I., Jones, R., McFiggans, G. B., and Coe, H.: Influence of aerosol chemical composition on N2O5 uptake: airborne regional measurements in northwestern Europe, Atmos. Chem. Phys., 15, 973–990, https://doi.org/10.5194/acp-15-973-2015, 2015.
Pathak, R. K., Wu, W. S., and Wang, T.: Summertime PM2.5 ionic species in four major cities of China: nitrate formation in an ammonia-deficient atmosphere, Atmos. Chem. Phys., 9, 1711–1722, https://doi.org/10.5194/acp-9-1711-2009, 2009.
Pathak, R. K., Wang, T., and Wu, W. S.: Nighttime enhancement of PM2.5 nitrate in ammonia-poor atmospheric conditions in Beijing and Shanghai: Plausible contributions of heterogeneous hydrolysis of N2O5 and HNO3 partitioning, Atmos. Environ., 45, 1183–1191, 2011.
Phillips, G. J., Thieser, J., Tang, M., Sobanski, N., Schuster, G., Fachinger, J., Drewnick, F., Borrmann, S., Bingemer, H., Lelieveld, J., and Crowley, J. N.: Estimating N2O5 uptake coefficients using ambient measurements of NO3, N2O5, ClNO2 and particle-phase nitrate, Atmos. Chem. Phys., 16, 13231–13249, https://doi.org/10.5194/acp-16-13231-2016, 2016.
Prabhakar, G., Parworth, C. L., Zhang, X., Kim, H., Young, D. E., Beyersdorf, A. J., Ziemba, L. D., Nowak, J. B., Bertram, T. H., Faloona, I. C., Zhang, Q., and Cappa, C. D.: Observational assessment of the role of nocturnal residual-layer chemistry in determining daytime surface particulate nitrate concentrations, Atmos. Chem. Phys., 17, 14747–14770, https://doi.org/10.5194/acp-17-14747-2017, 2017.
Pusede, S. E., Duffey, K. C., Shusterman, A. A., Saleh, A., Laughner, J. L., Wooldridge, P. J., Zhang, Q., Parworth, C. L., Kim, H., Capps, S. L., Valin, L. C., Cappa, C. D., Fried, A., Walega, J., Nowak, J. B., Weinheimer, A. J., Hoff, R. M., Berkoff, T. A., Beyersdorf, A. J., Olson, J., Crawford, J. H., and Cohen, R. C.: On the effectiveness of nitrogen oxide reductions as a control over ammonium nitrate aerosol, Atmos. Chem. Phys., 16, 2575–2596, https://doi.org/10.5194/acp-16-2575-2016, 2016.
Riedel, T. P., Wagner, N. L., Dube, W. P., Middlebrook, A. M., Young, C. J., Ozturk, F., Bahreini, R., VandenBoer, T. C., Wolfe, D. E., Williams, E. J., Roberts, J. M., Brown, S. S., and Thornton, J. A.: Chlorine activation within urban or power plant plumes: Vertically resolved ClNO2 and Cl2 measurements from a tall tower in a polluted continental setting, J. Geophys. Res.-Atmos., 118, 8702–8715, 2013.
Sander, S. P., Friedl, R. R., Barker, J. R., Golden, D. M., Kurylo, M. J., Wine, P. H., Abbatt, J. P. D., Burkholder, J. B., Kolb, C. E., Moortgat, G. K., Huie, R. E., and Orkin, V. L.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies Evaluation Number 17, JPL Publication 10–6 Rep., NASA Jet Propul. Lab, Pasadena, California, 2011.
Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics: from Air Pollution to Climate Change (Second edition), John Wiley & Sons, Inc., Hoboken, New Jersey, 2006.
Stutz, J., Alicke, B., Ackermann, R., Geyer, A., White, A., and Williams, E.: Vertical profiles of NO3, N2O5, O-3, and NOx in the nocturnal boundary layer: 1. Observations during the Texas Air Quality Study 2000, J. Geophys. Res.-Atmos., 109, D12306, https://doi.org/10.1029/2003jd004209, 2004.
Stutz, J., Wong, K. W., Lawrence, L., Ziemba, L., Flynn, J. H., Rappengluck, B., and Lefer, B.: Nocturnal NO3 radical chemistry in Houston, TX, Atmos. Environ., 44, 4099–4106, 2010.
Su, X., Tie, X. X., Li, G. H., Cao, J. J., Huang, R. J., Feng, T., Long, X., and Xu, R. G.: Effect of hydrolysis of N2O5 on nitrate and ammonium formation in Beijing China: WRF-Chem model simulation, Sci. Total Environ., 579, 221–229, 2017.
Sun, Y. L., Wang, Z. F., Fu, P. Q., Yang, T., Jiang, Q., Dong, H. B., Li, J., and Jia, J. J.: Aerosol composition, sources and processes during wintertime in Beijing, China, Atmos. Chem. Phys., 13, 4577–4592, https://doi.org/10.5194/acp-13-4577-2013, 2013.
Sun, Y. L., Wang, Z. F., Du, W., Zhang, Q., Wang, Q. Q., Fu, P. Q., Pan, X. L., Li, J., Jayne, J., and Worsnop, D. R.: Long-term real-time measurements of aerosol particle composition in Beijing, China: seasonal variations, meteorological effects, and source analysis, Atmos. Chem. Phys., 15, 10149–10165, https://doi.org/10.5194/acp-15-10149-2015, 2015a.
Sun, Y. L., Du, W., Wan, Q. Q., Zhang, Q., Chen, C., Chen, Y., Chen, Z. Y., Fu, P. Q., Wang, Z. F., Gao, Z. Q., and Worsnop, D. R.: Real-Time Characterization of Aerosol Particle Composition above the Urban Canopy in Beijing: Insights into the Interactions between the Atmospheric Boundary Layer and Aerosol Chemistry, Environ. Sci. Technol., 49, 11340–11347, 2015b.
Tang, M., Huang, X., Lu, K., Ge, M., Li, Y., Cheng, P., Zhu, T., Ding, A., Zhang, Y., Gligorovski, S., Song, W., Ding, X., Bi, X., and Wang, X.: Heterogeneous reactions of mineral dust aerosol: implications for tropospheric oxidation capacity, Atmos. Chem. Phys., 17, 11727–11777, https://doi.org/10.5194/acp-17-11727-2017, 2017.
Tang, M. J., Thieser, J., Schuster, G., and Crowley, J. N.: Kinetics and Mechanism of the Heterogeneous Reaction of N2O5 with Mineral Dust Particles, Phys. Chem. Chem. Phys., 14, 8551–8561, 2012.
Tham, Y. J., Wang, Z., Li, Q., Yun, H., Wang, W., Wang, X., Xue, L., Lu, K., Ma, N., Bohn, B., Li, X., Kecorius, S., Größ, J., Shao, M., Wiedensohler, A., Zhang, Y., and Wang, T.: Significant concentrations of nitryl chloride sustained in the morning: investigations of the causes and impacts on ozone production in a polluted region of northern China, Atmos. Chem. Phys., 16, 14959–14977, https://doi.org/10.5194/acp-16-14959-2016, 2016.
Thornton, J. A., Braban, C. F., and Abbatt, J. P. D.: N2O5 hydrolysis on sub-micron organic aerosols: the effect of relative humidity, particle phase, and particle size, Phys. Chem. Chem. Phys., 5, 4593–4603, 2003.
Thornton, J. A., Kercher, J. P., Riedel, T. P., Wagner, N. L., Cozic, J., Holloway, J. S., Dube, W. P., Wolfe, G. M., Quinn, P. K., Middlebrook, A. M., Alexander, B., and Brown, S. S.: A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry, Nature, 464, 271–274, 2010.
Tsai, C., Wong, C., Hurlock, S., Pikelnaya, O., Mielke, L. H., Osthoff, H. D., Flynn, J. H., Haman, C., Lefer, B., Gilman, J., de Gouw, J., and Stutz, J.: Nocturnal loss of NOx during the 2010 CalNex-LA study in the Los Angeles Basin, J. Geophys. Res.-Atmos., 119, 13004–13025, 2014.
Wagner, N. L., Riedel, T. P., Young, C. J., Bahreini, R., Brock, C. A., Dube, W. P., Kim, S., Middlebrook, A. M., Ozturk, F., Roberts, J. M., Russo, R., Sive, B., Swarthout, R., Thornton, J. A., VandenBoer, T. C., Zhou, Y., and Brown, S. S.: N2O5 uptake coefficients and nocturnal NO2 removal rates determined from ambient wintertime measurements, J. Geophys. Res.-Atmos., 118, 9331–9350, 2013.
Wahner, A., Mentel, T. F., and Sohn, M.: Gas-phase reaction of N2O5 with water vapor: Importance of heterogeneous hydrolysis of N2O5 and surface desorption of HNO3 in a large teflon chamber, Geophys. Res. Lett., 25, 2169–2172, 1998.
Wang, G. H., Zhang, R. Y., Gomez, M. E., Yang, L. X., Zamora, M. L., Hu, M., Lin, Y., Peng, J. F., Guo, S., Meng, J. J., Li, J. J., Cheng, C. L., Hu, T. F., Ren, Y. Q., Wang, Y. S., Gao, J., Cao, J. J., An, Z. S., Zhou, W. J., Li, G. H., Wang, J. Y., Tian, P. F., Marrero-Ortiz, W., Secrest, J., Du, Z. F., Zheng, J., Shang, D. J., Zeng, L. M., Shao, M., Wang, W. G., Huang, Y., Wang, Y., Zhu, Y. J., Li, Y. X., Hu, J. X., Pan, B., Cai, L., Cheng, Y. T., Ji, Y. M., Zhang, F., Rosenfeld, D., Liss, P. S., Duce, R. A., Kolb, C. E., and Molina, M. J.: Persistent sulfate formation from London Fog to Chinese haze, P. Natl. Acad. Sci. USA, 113, 13630–13635, https://doi.org/10.1073/pnas.1616540113, 2016.
Wang, H., Lu, K., Guo, S., Wu, Z., Shang, D., Tan, Z., Wang, Y., Le Breton, M., Lou, S., Tang, M., Wu, Y., Zhu, W., Zheng, J., Zeng, L., Hallquist, M., Hu, M., and Zhang, Y.: Efficient N2O5 uptake and NO3 oxidation in the outflow of urban Beijing, Atmos. Chem. Phys., 18, 9705–9721, https://doi.org/10.5194/acp-18-9705-2018, 2018.
Wang, H. C. and Lu, K. D.: Determination and Parameterization of the Heterogeneous Uptake Coefficient of Dinitrogen Pentoxide (N2O5), Prog. Chem., 28, 917–933, 2016.
Wang, H. C., Lu, K. D., Tan, Z. F., Sun, K., Li, X., Hu, M., Shao, M., Zeng, L. M., Zhu, T., and Zhang, Y. H.: Model simulation of NO3, N2O5 and ClNO2 at a rural site in Beijing during CAREBeijing-2006, Atmos. Res., 196, 97–107, 2017a.
Wang, H. C., Lu, K. D., Chen, X. R., Zhu, Q. D., Chen, Q., Guo, S., Jiang, M. Q., Li, X., Shang, D. J., Tan, Z. F., Wu, Y. S., Wu, Z. J., Zou, Q., Zheng, Y., Zeng, L. M., Zhu, T., Hu, M., and Zhang, Y. H.: High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway, Environ. Sci. Tech. Lett., 4, 416–420, 2017b.
Wang, X. F., Wang, H., Xue, L. K., Wang, T., Wang, L. W., Gu, R. R., Wang, W. H., Tham, Y. J., Wang, Z., Yang, L. X., Chen, J. M., and Wang, W. X.: Observations of N2O5 and ClNO2 at a polluted urban surface site in North China: High N2O5 uptake coefficients and low ClNO2 product yields, Atmos. Environ., 156, 125–134, 2017.
Wang, Z., Wang, W., Tham, Y. J., Li, Q., Wang, H., Wen, L., Wang, X., and Wang, T.: Fast heterogeneous N2O5 uptake and ClNO2 production in power plant and industrial plumes observed in the nocturnal residual layer over the North China Plain, Atmos. Chem. Phys., 17, 12361–12378, https://doi.org/10.5194/acp-17-12361-2017, 2017.
Watson, J. G. and Chow, J. C.: A wintertime PM2.5 episode at the fresno, CA, supersite, Atmos. Environ., 36, 465–475, 2002.
Wen, L. A., Chen, J. M., Yang, L. X., Wang, X. F., Xu, C. H., Sui, X. A., Yao, L., Zhu, Y. H., Zhang, J. M., Zhu, T., and Wang, W. X.: Enhanced formation of fine particulate nitrate at a rural site on the North China Plain in summer: The important roles of ammonia and ozone, Atmos. Environ., 101, 294–302, 2015.
Ying, Q.: Physical and chemical processes of wintertime secondary nitrate aerosol formation, Front. Environ. Sci. En., 5, 348–361, 2011.
Zhang, Q., Jimenez, J. L., Canagaratna, M. R., Allan, J. D., Coe, H., Ulbrich, I., Alfarra, M. R., Takami, A., Middlebrook, A. M., Sun, Y. L., Dzepina, K., Dunlea, E., Docherty, K., DeCarlo, P. F., Salcedo, D., Onasch, T., Jayne, J. T., Miyoshi, T., Shimono, A., Hatakeyama, S., Takegawa, N., Kondo, Y., Schneider, J., Drewnick, F., Borrmann, S., Weimer, S., Demerjian, K., Williams, P., Bower, K., Bahreini, R., Cottrell, L., Griffin, R. J., Rautiainen, J., Sun, J. Y., Zhang, Y. M., and Worsnop, D. R.: Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes, Geophys. Res. Lett., 34, L13801, https://doi.org/10.1029/2007GL029979, 2007.
Zhang, R. Y., Wang, G. H., Guo, S., Zarnora, M. L., Ying, Q., Lin, Y., Wang, W. G., Hu, M., and Wang, Y.: Formation of Urban Fine Particulate Matter, Chem. Rev., 115, 3803–3855, 2015.
Zheng, G. J., Duan, F. K., Su, H., Ma, Y. L., Cheng, Y., Zheng, B., Zhang, Q., Huang, T., Kimoto, T., Chang, D., Pöschl, U., Cheng, Y. F., and He, K. B.: Exploring the severe winter haze in Beijing: the impact of synoptic weather, regional transport and heterogeneous reactions, Atmos. Chem. Phys., 15, 2969–2983, https://doi.org/10.5194/acp-15-2969-2015, 2015.
Zhong, J. T., Zhang, X. Y., Wang, Y. Q., Sun, J. Y., Zhang, Y. M., Wang, J. Z., Tan, K. Y., Shen, X. J., Che, H. C., Zhang, L., Zhang, Z. X., Qi, X. F., Zhao, H. R., Ren, S. X., and Li, Y.: Relative Contributions of Boundary-Layer Meteorological Factors to the Explosive Growth of PM2.5 during the Red-Alert Heavy Pollution Episodes in Beijing in December 2016, J. Meteorol. Res.-PRC, 31, 809–819, 2017.