the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Measurement report: Per- and polyfluoroalkyl substances (PFAS) in particulate matter (PM10) from activated sludge aeration
Jishnu Pandamkulangara Kizhakkethil
Zongbo Shi
Anna Bogush
Ivan Kourtchev
Environmental pollution with per- and polyfluoroalkyl substances (PFAS), commonly referred to as “forever chemicals”, received significant attention due to their environmental persistence and bioaccumulation tendencies. Effluents from wastewater treatment plants (WWTPs) have been reported to contain significant levels of PFAS. Wastewater treatment processes such as aeration have the potential to transfer PFAS into the atmosphere. However, understanding their fate during sewage treatment remains challenging. This study aims to assess aerosolisation of PFAS during a WWTP process. Special emphasis is given to new-generation and legacy PFAS (e.g. perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA)) as they are still observed in sewage after years of restrictions. Particulate matter with aerodynamic diameter ≤10 µm (PM10) collected above a scaled-down activated sludge tank treating domestic sewage for a population of >10 000 people in the UK was analysed for a range of short-, medium-, and long-chain PFAS. Eight PFAS including perfluorobutanoic acid (PFBA), perfluorobutanesulfonic acid (PFBS), perfluoroheptanoic acid (PFHpA), perfluorohexanesulfonic acid (PFHxS), PFOA, perfluorononanoic acid (PFNA), PFOS, and perfluorodecanoic acid (PFDA) were detected in the PM10. The presence of legacy PFOA and PFOS in the PM10 samples, despite being restricted for over a decade, raises concerns about their movement through domestic and industrial sewage cycles. The total PFAS concentrations in PM10 were 15.49 and 4.25 pg m−3 during autumn and spring campaigns, respectively. PFBA was the most abundant of the PFAS, suggesting a shift towards short-chain PFAS use. Our results suggest that wastewater treatment (WWT) processes such as activated sludge aeration could aerosolise PFAS into airborne PM.
- Article
(983 KB) - Full-text XML
-
Supplement
(458 KB) - BibTeX
- EndNote
Particulate matter (PM) is a critical component of air pollution and has significant implications for the environment (Boucher et al., 2013; Chen et al., 2021; Taylor and Penner, 1994; Zhang et al., 2023) and human health (Pope et al., 2020; Vohra et al., 2021; Zhou et al., 2024). PM with an aerodynamic diameter ≤10 µm (PM10) is of particular concern because it is known to penetrate into the human respiratory system and cause severe health effects (Abbey et al., 1995; Pope et al., 1992). The chemical composition of PM is very complex, and it can contain thousands of organic compounds (Goldstein and Galbally, 2007) including persistent organic pollutants (POPs) and new and emerging pollutants (NEPs) such as per- and polyfluoroalkyl substances (PFAS) (Kourtchev et al., 2022; Zhou et al., 2021, 2022).
PFAS, commonly referred to as “forever chemicals”, are a large group of synthetic organic compounds. PFAS are thermally and chemically inert due to the strong carbon–fluorine bonds (Buck et al., 2011), and therefore they are widely used in the production of numerous consumer goods such as water- and thermal-resistant apparel, engine oil, and cooking wares (Glüge et al., 2020). PFAS are known for their environmental persistence and bioaccumulation potential (Buck et al., 2011; Lesmeister et al., 2021). Several PFAS are shown to have negative health effects, e.g. endocrine disruption, cancer including kidney and testicular cancer, and liver disease (Fenton et al., 2021; Sunderland et al., 2019).
Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) are the most scrutinised PFAS due to their environmental persistence and human health effects (Beach et al., 2006; Saikat et al., 2013; US EPA, 2024b; Zareitalabad et al., 2013). In 2009, the Stockholm Convention on POPs included PFOS and its salts in its Annex B of restricted compounds. Further, in 2019 and 2022, PFOA and perfluorohexanesulfonic acid (PFHxS) were added to its Annex A of compounds for elimination. Despite being restricted for more than a decade, these compounds are still observed in various environmental matrices (Li et al., 2022; Nguyen et al., 2017; Xiao et al., 2015; Zhou et al., 2022). Shortly after the introduction of restrictions for several PFAS, they were replaced with short-chain and other new-generation PFAS that were thought to be less hazardous (Brendel et al., 2018; Wang et al., 2013, 2015). These include perfluorobutanesulfonic acid (PFBS), fluorotelomer sulfonates (FTS), and hexafluoropropylene dimer acid (HFPO-DA, more commonly known as GenX) (Wang et al., 2013, 2015). Recent studies have indicated that numerous replacement PFAS could potentially have similar health effects to those of the legacy ones (Gomis et al., 2018; Liu et al., 2020; Solan and Lavado, 2022).
The majority of reports on PFAS pollution have predominantly focused on drinking water (Domingo and Nadal, 2019), surface water (Podder et al., 2021), sewage (Lenka et al., 2021), and soil matrices (Brusseau et al., 2020). Therefore, most of the current regulations on PFAS are focused on water matrices (European Union, 2020; US EPA, 2024a). There is growing evidence that PFAS can transfer from contaminated waters via aerosolisation/volatilisation into the atmosphere (Ahrens et al., 2011; Johansson et al., 2019; Shoeib et al., 2016; Qiao et al., 2024).
Laboratory simulation experiments have shown that the aeration of PFAS-contaminated water leads to formation of aerosolised PFAS (Nguyen et al., 2024; Pandamkulangara Kizhakkethil et al., 2024). The extent of PFAS aerosolisation has a clear dependence on the PFAS carbon chain length and functional groups (Johansson et al., 2019; Pandamkulangara Kizhakkethil et al., 2024; Reth et al., 2011).
Wastewater treatment techniques such as activated sludge (AS) and secondary extended aeration which involve vigorous aeration/mechanical turbulence could lead to the aerosolisation/volatilisation of PFAS from contaminated wastewater effluents (Ahrens et al., 2011; Shoeib et al., 2016). PFAS were detected in the gas phase and total suspended particles (TSP) near the aeration tanks and secondary clarifier in a wastewater treatment plant (WWTP) in Canada (Vierke et al., 2011). Airborne PFAS were also observed at WWTPs that employ treatment techniques such as AS, secondary extended aeration, and facultative lagoons in Canada (Shoeib et al., 2016). PFAS, including restricted PFOS, were identified in the TSP and gas phase above aeration tanks in a WWTP in northern Germany (Weinberg et al., 2011). A more recent study by Qiao et al. (2024) identified PFAS in both gas and particle phases above the influent and aeration tanks at a WWTP in China.
Limited studies have assessed the PFAS emission associated with the inhalable PM fraction (e.g. PM10) during wastewater treatment (WWT) processes. For example, a recent study identified PFAS in the 11 PM size fractions between 0.1 to 18 µm, collected from three WWTPs in Hong Kong SAR, China (Lin et al., 2022). These WWTPs (the largest in Hong Kong SAR) utilised treatment techniques such as AS and chemically enhanced primary treatment (CEPT) to treat sewage from industrialised areas. The study reported that atmospheric PFAS in WWTPs (e.g. PFOS, PFOA, PFBS, and perfluorobutanoic acid (PFBA)) are primarily distributed in aerosol particles with an aerodynamic diameter ≤10 µm. Additionally, the distribution of PFAS depends on the type of WWT process, nature of sewage, and aerosol properties (organic content, presence of microbes, etc.) (Lin et al., 2022). This suggests that PFAS levels in inhalable PM, and thus the associated health risks, will vary based on the location and the type of sewage being treated. European countries have restricted the production and use of several PFAS such as PFOS, PFOA, PFHxS, and C9–C14 perfluorocarboxylic acids (PFCA) (European Union, 2020; ECHA, 2022a, b). Nevertheless, the restricted PFOA and PFOS are still observed in wastewater effluents (Eriksson et al., 2017; Gobelius et al., 2023; Moneta et al., 2023; Müller et al., 2023; Semerád et al., 2020), raising the question of whether these chemicals could be aerosolised during open-air aeration WWT processes. To the best of our knowledge, there are no studies assessing the PFAS levels in PM10 at European WWTPs. Furthermore, PM10-associated emissions of PFAS from WWTPs have been assessed only for a limited number of PFAS.
As highlighted in the reviews by Phong Vo et al. (2020) and O'Connor et al. (2022), domestic wastewater has been reported to contain significant levels of PFAS, albeit at concentrations lower than those typically found in industrial effluents. Despite this, studies on PFAS atmospheric emissions from sewage have primarily focused on WWTPs processing industrial effluents or a mix of industrial and domestic sources. Consequently, a knowledge gap exists regarding the release of PFAS to the atmosphere, particularly their association with PM10 aerosols, during the treatment of domestic wastewater, especially under conditions of vigorous aeration.
The aim of the current study is to assess the aerosolisation potential of PFAS during a WWTP process that involves vigorous aeration steps. Special emphasis is given to (a) legacy PFAS, such as PFOS and PFOA, as they are still observed in sewage after 15 and 5 years of restrictions, respectively, and (b) new-generation and replacement PFAS such as FTS. To achieve this, PM10 samples collected from a scaled-down AS tank processing domestic wastewater (from a population of >10 000 people) in the United Kingdom (UK) were screened for 15 PFAS (C4–C11) including legacy and new-generation replacement compounds such as FTS.
2.1 Materials and chemicals
The materials and chemicals (the full names of the listed chemicals are given in Tables S1 and S2 of the Supplement) include 10 mL headspace glass vials (Chromacol 10-HSV, Thermo Scientific); metal screw caps (Chromacol 18-MSC, Thermo Scientific); polytetrafluoroethylene (PTFE) septa (Chromacol 18-ST101 Thermo Scientific); PTFE membrane filters (Iso-Disc PTFE-13−4, 13 mm × 0.45 µm); glass fibre filters (GFFs) (47 mm, Advantec®, model no. GB-100R); the EPA 533 PAR mix containing 25 PFAS, i.e. PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUdA, PFDoA, HFPO-DA, PFMPA, PFMBA, 3,6-OPFHpA, L-PFBS, L-PFPeS, PFHxS, L-PFHpS, PFOS, 4:2 FTS, 6:2 FTS, 8:2 FTS, NaDONA, 9Cl-PF3ONS, 11Cl-PF3OudS, and PFEESA, each having a concentration of 0.5 ng µL−1 (Wellington laboratories Inc, Canada); EPA533ES isotope dilution standard mixture containing 16 mass-labelled (13C) PFAS, i.e. M3PFBS, M5PFHxA, M6PFDA, M3PFHxS, M8PFOS, MPFBA, M5PFPeA, M4PFHpA, M8PFOA, M9PFNA, M7PFUdA, MPFDoA, M2-4:2 FTS, M2-6:2 FTS, M2-8:2 FTS, and M3HFPO, with concentrations of 0.5–2.0 ng µL−1; liquid chromatography (LC)–mass spectrometry (MS)-grade water (Optima™, Fisher Scientific); methanol, LC–MS grade (Optima™, Fisher Scientific); formic acid, LC–MS grade (Optima™, Fisher Chemicals); and ammonium acetate, LC–MS grade (Optima™, Fisher chemicals).
2.2 Sampling site
The PM10 samples were collected above a scaled-down AS tank processing municipal wastewater equivalent to that of a population of >10 000 people (the location of the facility is anonymised due to a non-disclosure agreement). The AS tank, constructed from high-density polyethylene (HDPE), contains an aeration basin of volume 3.06 m3. The aeration basin of the AS tank is connected to a secondary clarifier (of volume 0.86 m3) where sewage, after aeration, is allowed to settle. The AS tank continuously receives and processes primary treated sewage (with a solid retention time (SRT) of 10 d) from the parent large-scale WWTP using pumps.
2.3 PM10 sample collection
The MiniVol™ tactical air sampler (Air Metrics, United States of America) used for PM10 sampling was installed near the aeration tank (<0.2 m from the aeration tank) with the sampling head slightly above the rim of the tank (10 cm above). The PM10 samples were collected on GFFs at 10 L min−1. Prior to sampling, GFFs were baked at 450 °C for 24 h to eliminate potential organic contaminants. The samples were collected during two sampling periods: between (1) 2–6 October 2023 and (2) 4–8 March 2024. PM10 samples were collected separately during the day (between 10:00 and 15:00 GMT) and night (between 15:00 and 10:00 GMT the next day). The sampling dates and durations are given in Table 1.
GFFs with PM10 were rolled using prewashed stainless-steel tweezers, keeping aerosol content inside, and placed into a prewashed 10 mL headspace glass vial. A total of 5 mL methanol (LC–MS grade) was added to the vial to disinfect the filters from potential pathogenic microorganisms and extract the organic compounds including PFAS. The samples were then stored at 5 °C until the day of analysis. The vials, PTFE septa, and metal screw caps were prewashed with LC–MS-grade methanol and dried before use to remove potential PFAS contamination. PFAS leaching from the vials, PTFE septa, and metal screw caps was assessed in another study, which reported minimal PFAS leachables from these consumables (Kourtchev et al., 2022). Two types of blanks were used to evaluate possible PFAS contamination from handling the filters. These include (1) baked filters (BFs) and (2) baked filters placed in a MiniVol® air sampler and collecting air above the AS tank at 10 L min−1 for 2 min (field blanks, FLDBs).
It is important to note that the use of GFFs and quartz fibre filters (QFFs) during PM sampling has been reported to cause positive sampling artefacts, such as the adsorption of gas-phase organic compounds (Chang et al., 2025; Turpin et al., 1994). Previous studies have shown that certain PFAS, such as PFOS and PFOA, can partition from aqueous aerosols to the gas phase (Ahrens et al., 2012; McMurdo et al., 2008). As a result, the GFFs used in our study may also include a small fraction of gas-phase PFAS. Consequently, the reported PM10 concentrations of PFAS in our study might be overestimated.
2.4 Extraction and analysis of PM10 GFF samples
The GFF samples stored in methanol were spiked with internal standards (IS), a mixture of 16 13C PFAS-labelled compounds (EPA533 ES, Wellington laboratories Inc, Canada) at concentrations of 20 ng L−1 for M2-4:2 FTS, M2-6:2 FTS, and M2-8:2 FTS and 5 ng L−1 for the remaining compounds, and extracted using the procedure published in Kourtchev et al. (2022).
Briefly, the vial content was subjected to ultrasonic agitation for 40 min. The methanol extracts were then filtered through a 0.45 µm PTFE syringe filter prewashed with methanol (3 times with 5 mL). The PTFE filters used in our study were assessed for PFAS leaching potential in Kourtchev et al. (2022). Minimal leaching of PFAS was observed from the PTFE filters after purging them with 5 mL LC–MS-grade methanol three times (total volume of 15 mL) (Kourtchev et al., 2022). The extracts were then reduced by volume to 1 mL under gentle nitrogen flow.
The methanolic extract was then topped up with 4 mL of LC–MS-grade water providing the 80:20 () water : methanol ratio required for the online solid-phase extraction (SPE) (Kourtchev et al., 2022). The vial content was homogenised by vortex mixing and analysed using online SPE LC–high-resolution mass spectrometry (HRMS) with the method published elsewhere (Kourtchev et al., 2022). The analytical method is validated for screening and quantifying 15 PFAS comprising PFBA, PFPeA, PFBS, 4:2 FTS, PFHxA, PFPeS, PFHxS, PFHpA, PFOA, PFHpS, PFNA, PFOS, 8:2 FTS, PFDA, and PFUdA. Therefore, to ensure the accuracy, reliability, and reproducibility of analytical results, the current study focused only on those fully validated analytes.
The online SPE and chromatographic separation were carried out using an EQuan MAX Plus Thermo Scientific™ Vanquish™ UHPLC system and a Thermo Scientific™ TriPlus™ RSH autosampler. Online SPE was performed using a Thermo Scientific™ Hypersil GOLD aQ Column (20 × 2.1 mm, 12 µm column). A total of 0.1 % formic acid in water was used as the loading phase for the online SPE. Following online SPE, the chromatographic separation was achieved using a Waters® CORTECS C18 column (90 Å, 100 × 2.1 mm, 2.7 µm analytical column). The eluents used for chromatographic separation were (a) 2 mM ammonium acetate in 10 % methanol and (b) 100 % methanol. A Q Exactive™ Focus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher, Bremen, Germany) fitted with an electrospray ionisation (ESI) (Ion Max™) source was employed for the mass spectrometric analysis. The mass spectrometric analysis was performed in single ion monitoring (SIM) negative ionisation mode. The mass spectrometer was calibrated prior to analysis to have a mass accuracy of ≤5 ppm. The limit of detection (LOD) values for the analytes in this study were similar to those reported by Kourtchev et al. (2022), with the exception for the LOD for PFBA. This was 1.47 ng L−1, which is higher than the value reported by Kourtchev et al. (2022) and could potentially be due to higher background levels of the analyte in the system blanks. The maximum concentration values of the PFAS detected in the field blanks and baked filter blanks were subtracted from the PFAS concentrations detected in the samples.
2.5 Quality assurance (QA) and quality control (QC)
Several steps were taken to ensure the QA and QC during the sampling and analysis. PFAS-containing consumables were avoided as much as possible during the sampling, extraction, and LC–HRMS analysis. To prevent accumulation of PFAS in the LC–HRMS system, prior to the analysis, the system was flushed with the mobile at composition of 60:40 A:B (A denotes 2 mM ammonium acetate in 10 % methanol, and B denotes 100 % methanol) and 0.3 mL min−1 flow rate, overnight (Kourtchev et al., 2022). System suitability tests (SSTs) were performed before the analysis of each batch to ensure the adequate performance of the LC–HRMS system. Pass criteria were evaluated based on the chromatographic peak area and height, retention times, mass accuracy, and peak-tailing factors. System blanks (“zero volume”) and 80:20 water:methanol () blanks were injected at the start of the batch, in between the samples, and at the end of the batch to monitor a potential PFAS carryover. The zero-volume blanks and 80:20 water : methanol blanks reported PFAS concentrations lower than the method limit of quantification (LOQ) values.
3.1 PFAS composition of PM10 above the AS tank
Figure 1 shows the concentrations of PFAS detected in PM10 samples collected above the AS tank during the two sampling periods. Out of the 15 target PFAS, 8 compounds were detected across the collected samples.
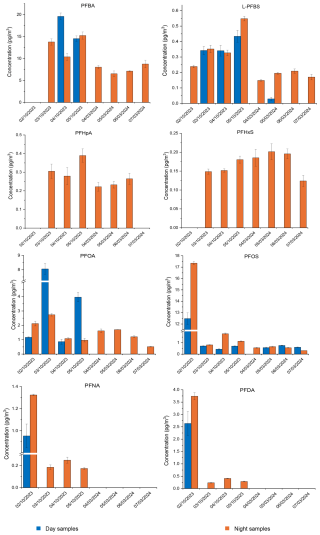
Figure 1Concentrations of PFBA, L-PFBS, PFHpA, PFHxS, PFOA, PFOS, PFNA, and PFDA in the PM10 samples collected from the AS tank in October 2023 and March 2024. The absence of data points on certain sampling days indicates that the compound was either not detected or below the method LOD. The error bars represent the standard deviation of the value from three replicate injections. The data of Fig. 1 are shown in Tables S3 and S4.
The detected PFAS include short-chain PFBA and PFBS; medium-chain PFHpA, PFHxS, PFOA, PFNA, and PFOS; and long-chain PFDA. The most abundant of the PFAS detected in the PM10 from both sampling campaigns was a short-chain PFBA with a maximum concentration of 19.6 ± 0.8 pg m−3 in October 2023 and 8.8 ± 0.9 pg m−3 in March 2024. PFBA is one of the most volatile PFAS observed in our study. Further, short-chain PFAS such as PFBA and PFBS are reported to have lower aerosolisation tendencies compared to long-chain compounds (e.g. PFOS and PFOA) (Johansson et al., 2019; Pandamkulangara Kizhakkethil et al., 2024). Despite being volatile and having a low aerosolisation tendency, the presence of PFBA in the PM10 aerosols at considerable concentrations in our study could potentially be due to the presence of high levels of PFBA in the sewage during the sampling period.
The concentration of PFAS detected in the samples from October 2023 followed the order PFBA > PFOS > PFOA > PFDA, with maximum concentrations recorded at 17.4 ± 0.2 pg m−3 for PFOS, 8.1 ± 0.4 pg m−3 for PFOA, and 3.7 ± 0.1 pg m−3 for PFDA. The samples collected during March 2024 showed a different pattern, with PFOA (1.70 ± 0.01 pg m−3) having the highest concentration after PFBA, followed by PFOS at 0.76 ± 0.02 pg m−3. It has been reported that the aerosolisation of PFAS from contaminated water depends on the carbon chain length, functional groups, and organic content, with higher aerosol enrichment for long-chain PFAS and perfluorosulfonic acids (PFSA) compared to PFCA (Johansson et al., 2019; Pandamkulangara Kizhakkethil et al., 2024; Reth et al., 2011; Sha et al., 2024). However, it is interesting to note that the PFAS levels in the PM10 in our study followed a reverse order with short-chain PFBA detected at higher values. It should be noted that the concentrations of PFAS in the wastewater were not measured in our study.
The detected PFAS have been associated with different sources. For example, PFOS, PFNA, and PFOA have historically been produced and used in the manufacturing of numerous products, such as firefighting foam, fluoropolymers, textiles, leather, paper, and lubricants (ATSDR, 2015; Buck et al., 2011; de Alba-Gonzalez et al., 2024; Wang et al., 2014). PFHxS and its salts and related compounds have been used in applications such as firefighting foam, coatings, electronics and semiconductors, and polishing agents (in many of these applications, PFHxS has been introduced as a replacement for PFOS) (UNEP/POPS/POPRC.15/7/Add.1, 2019). PFBA and PFBS have been used as replacements for legacy and longer-chain PFAS (Ateia et al., 2019; Christian, 2024; Wang et al., 2013). PFBA is used in the manufacturing of food packaging materials, carpets, and fluorosurfactants (Christian, 2024; US EPA, 2022). PFBS and PFBS-based compounds are used in applications such as metal plating and as a flame retardant and surfactant (Wang et al., 2013). PFDA, a long-chain PFAS identified in the PM in this study, has been reported as a breakdown product of stain- and greaseproof coatings on food packaging, furniture, and carpets (Christian, 2024). Laundry water could potentially be one of the sources of PFAS in the sewage since the WWTP receives a major portion of the sewage from households (Clara et al., 2008).
Clear differences were observed in the concentrations of PFAS in PM10 samples from the two sampling campaigns. In general, concentrations of all detected PFAS except PFHxS were higher in the samples collected in October 2023 compared to the March 2024 samples. For example, the highest concentration of PFBA reported during the March 2024 period was less than half of that reported in the October 2023 period. PFNA and PFDA were absent in the samples from March 2024, but they were detected in the October 2023 samples. The concentrations of PFHxS and PFHpA reported during both sampling periods were higher than the method LOD but slightly lower than the method LOQ values. There are several potential reasons for the observed seasonal differences in the concentrations of PFAS, which include the pH value, density, and composition of the wastewater. The pH of the contaminated water has been reported to influence the atmospheric transfer of PFAS (Ahrens et al., 2012; Barton et al., 2007; Pandamkulangara Kizhakkethil et al., 2024; Vierke et al., 2013). For example, the average pH of the wastewater in October 2023 was 7.5, whereas the average pH was 9.3 during the March 2024 sampling campaign. Additionally, the sewage density and potentially the composition were different during the two sampling periods (the pH and density data are not shown in the paper due to the non-disclosure agreement). PFAS are well known for their sorption to biosolids in sewage (Ebrahimi et al., 2021; Link et al., 2024). During the March 2024 sampling period, the sewage was thicker compared to October 2023, potentially leading to higher sorption of PFAS in the biosolids and consequently lower PM10-associated emissions. It should be noted that the sewage composition was not static during the sampling periods. The SRT of the AS tank was 10 d, and the chamber received and processed primary treated wastewater from the parent WWTP continuously. Therefore, the variation in the sewage composition could potentially explain the differences in the airborne PFAS concentration. Moreover, the surface runoff, linked to rainfall, could also be a factor influencing the overall PFAS levels, as it may introduce additional contaminants into the wastewater system. Additionally, dilution of PFAS levels in the sewage due to rainfall could also affect the airborne PFAS concentration. Seasonal variations in PFAS PM10 levels could also be due to changes in household activities throughout the year and thus changing concentrations in domestic wastewater entering the WWTP.
Since the sampling campaigns were conducted in two different seasons, the atmospheric conditions, e.g. temperature or relative humidity (see Figs. S1–S4 of the Supplement for the average temperature and relative humidity at the sampling periods), could also influence the PFAS partitioning into aerosols from the contaminated water (Ahrens et al., 2012). It should be noted that the absence of PFNA and PFDA in the March 2024 samples could be attributed to lower concentration of these analytes in the sewage resulting in PFAS PM10-bound concentrations below the method LOD.
Several PFAS exhibited day and night variations in PM10 samples. For example, the PFBA concentration was higher during the day compared to the night on specific sampling days of October 2023. On the other hand, PFBA concentration during the day was close to the background levels during the March 2024 campaign. PFHpA and PFHxS were not detected in the day samples during both sampling campaigns. Legacy PFOS and PFOA showed higher concentrations during the day on specific sampling days. The difference in the diurnal concentrations could potentially be due to variability in the composition of the wastewater at the respective sampling time. The diurnal variations in the environmental conditions such as temperature and relative humidity could have also contributed to the observed higher PFAS concentrations observed in the specific day samples compared to the night samples. The shorter sampling duration of day samples compared to the night samples likely led to a lower aerosol mass load on the filters, resulting in several PFAS mass loads below the LODs, which could explain the observed diurnal differences in PFAS concentrations.
3.2 Comparison to previous studies
The observation of high levels of PFBA in our study is consistent with the results of Weinberg et al. (2011), who identified PFBA (up to 8.4 pg m−3) as the most abundant PFAS in the PM samples (TSP) collected above the aeration tanks of two WWTPs processing a mixture of domestic and industrial wastewater in northern Germany. PFBA was also identified as the dominant ionic PFAS in the atmosphere of WWTPs in other studies (Shoeib et al., 2016; Lin et al., 2022). For example, air samples collected using sorbent-impregnated polyurethane foam (SIP) passive air samplers at WWTPs employing AS (processing mixed wastewater), secondary extended aeration (one processing domestic and the other two processing mixed wastewater), and facultative seasonal-discharge lagoons (processing domestic wastewater) in Canada detected PFBA in up to 60 ± 21 % of the total PFCA detected (Shoeib et al., 2016). Similarly, Lin et al. (2022) reported PFBA at considerable levels in the atmosphere near the aeration tanks of two WWTPs and above a WWTP using CEPT (processing wastewater from urban areas) in Hong Kong SAR, China. The concentrations of PFBA in TSP reported by Lin et al. (2022), with maximum values of 9.17 and 15.6 pg m−3 near the aeration tanks, are comparable to the values reported in our study.
The high PM10-associated concentration of PFBA in our study could potentially be explained by the recent increase in the use of short-chain PFAS as a replacement for legacy PFOS and PFOA (Ateia et al., 2019; Wang et al., 2013).
The concentrations of PFAS in PM10 reported in our study, except for PFHxS, were higher than those measured by Weinberg et al. (2011) in the particulate phase (TSP) above the aeration tanks of a WWTP that processed a mix of domestic and industrial waste in northern Germany. For example, during the October 2023 sampling period, legacy PFAS such as PFOS and PFOA were detected in our study at levels up to 17.4 ± 0.2 pg m−3 and 8.1 ± 0.4 pg m−3, respectively. In contrast, the maximum concentrations of PFOS and PFOA during March 2024 were 0.76 ± 0.02 and 1.70 ± 0.01 pg m−3, respectively. Weinberg et al. (2011) measured PFOS and PFOA concentrations in the TSP to be up to 0.9 and 1.3 pg m−3, respectively. The difference in the PFAS emission levels could potentially be due to the difference in PFAS composition in the wastewater. The PFAS composition in wastewater across the European Union (EU) has been reported to differ depending on the region (Lenka et al., 2021).
The PFDA concentrations, up to 1.31 pg m−3, in the TSP samples reported by Lin et al. (2022) above the aeration tanks of WWTPs in Hong Kong SAR, China, were lower than the PFDA levels observed in our study during the October 2023 period (3.7 ± 0.1 pg m−3). However, for other PFAS compounds such as PFBS, PFHxS, PFHpA, PFOA, PFOS, and PFNA, Lin et al. (2022) reported considerably higher values in the TSP samples compared to those observed in our study. Lin et al. (2022) investigated the distribution of PFAS across 11 PM size fractions (ranging from 0.1 to >18 µm) collected from three WWTPs (two using aeration and one using CEPT), as well as a landfill and two reference sites. PFOS in PM from all studied WWTPs (treating urban wastewater) showed a major distribution around the PM fractions with aerodynamic diameters between 0.1 and 10 µm. Similarly, PFBA and PFBS were also found to be primarily associated with particles of aerodynamic diameters less than 10 µm, indicating that the PM10 collected in our study could have potentially captured a majority of the PFAS-bound particles. The reported values in our study therefore provide insights into the total aerosol-bound emissions of studied PFAS during the WWT process.
The PFAS reported in our study were significantly lower than the PM (TSP) values reported by Vierke et al. (2011) (processing wastewater from Ontario, an urban area in Canada). For example, the average PM concentrations of PFOS and PFOA above the aeration tank of a WWTP in Canada study were 3900 and 71 pg m−3, respectively (Vierke et al., 2011). Similarly, Qiao et al. (2024) also reported considerably higher values for legacy PFOS (1.7–65.1 pg m−3) and PFOA (3.1–101 pg m−3) in the TSP samples above the influent and aeration tanks of two WWTPs (one processing domestic wastewater and the other one processing industrial wastewater) in China.
It is interesting to note that the PFAS levels in PM10 reported in our study are comparable to those reported by Weinberg et al. (2011) in the TSP samples, which is the only study that has investigated atmospheric PFAS levels in European WWTPs. The similarity in the TSP and PM10 concentrations could be due to PFAS being mainly associated with aerosols having aerodynamic diameters less than 10 µm, as shown for several types of sewage in Lin et al. (2022). In contrast, higher PFAS levels in TSP samples were reported in all other studies conducted at WWTPs in Canada and China (Lin et al., 2022; Vierke et al., 2011; Qiao et al., 2024). The differences in PFAS levels in PM could potentially be due to variations in wastewater composition in these regions. For example, the WWTP studied by Vierke et al. (2011) is situated in Ontario, a heavily industrialised city in Canada. Similarly, the WWTPs investigated by Lin et al. (2022) and Qiao et al. (2024) are located in China (Hong Kong SAR and Tianjin, respectively), one of the most heavily industrialised countries in the world. The facility in our study processes sewage mainly from households (for approximately 30 000 people) rather than industries, which may contain lower PFAS levels in the sewage and thus in aerosol. The total PFAS concentrations associated with PM10 fractions in our study were 15.49 pg m−3 in October 2023 and 4.25 pg m−3 in March 2024 (see Tables S3 and S4 of the Supplement), which is comparable (2–13 pg m−3) to concentrations in the TSP from mixed wastewater in northern Germany reported by Weinberg et al. (2011). It is important to note that the latter study considered the same set of ionic PFAS as our study but included two additional analytes, i.e. PFDoA and perfluorooctanesulfonamide (PFOSA), which were not targeted by our method.
In this study, we investigated, for the first time, the PFAS concentrations associated with the health-relevant PM10 fraction of airborne aerosols emitted during the AS aeration process at a WWTP processing domestic wastewater. PM10 samples were collected over two sampling campaigns in two different seasons (i.e. October 2023 and March 2024) above a scaled-down AS tank consisting of an aeration basin of volume ∼ 3 m3, treating wastewater equivalent to that produced by >10 000 people. Eight PFAS were observed across the collected PM10 samples. These include legacy PFOS and PFOA, which were detected in concentrations of up to 17.4 ± 0.2 and 8.1 ± 0.4 pg m−3, respectively, in the samples from October 2023.
The presence of legacy PFOS and PFOA in the PM even after a decade-long restriction raises concern and suggests that PFOS- and PFOA-containing products are still in use or in the recirculation cycle. More studies are needed to understand if these legacy compounds could have been formed in the wastewater during the treatment process from the degradation of precursor compounds such as fluorotelomer alcohols (FTOHs), PFOSA, perfluorooctane sulfonamidoethanols (FOSEs), and polyfluoroalkyl phosphate esters (PAPs) as suggested by Dauchy et al. (2017), Xiao (2022), and Ao et al. (2024).
The presence of PFBA at high concentrations in the collected samples potentially suggests an increased shift towards the use of short-chain PFAS as a replacement for legacy PFAS.
Our results indicate that WWT processes involving aeration could aerosolise and transfer PFAS into the atmosphere. Considering the sheer number of different PFAS that are in production and used today, the estimated total PFAS concentrations likely represent only a fraction of the actual emissions during the aeration process.
To the best of our knowledge, this is the first study to investigate the presence of PFAS in the PM10 fraction of the airborne aerosols from the AS aeration process in a WWTP in the UK and Europe.
Future research should consider the simultaneous characterisation of wastewater PFAS levels alongside PM measurements to improve understanding of the relationships among airborne PFAS emissions. Expanding the range of monitored PFAS beyond the 15 fully validated targets in our study, particularly including neutral PFAS such as FTOHs and FOSEs, would enhance our understanding of their role in WWTP aerosolisation. Additionally, incorporating gas-phase sampling would be valuable in assessing the potential partitioning of PFAS into the gaseous phase, further refining our understanding of their atmospheric behaviour.
The data are not publicly accessible due to a non-disclosure agreement with a wastewater treatment company, which is also anonymised. The data of Fig. 1 are shown in Tables S3 and S4.
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-5947-2025-supplement.
JPK conceived the study and performed field measurements, sampling, sample analysis, data processing, and interpretation. AB co-supervised JPK. ZS co-supervised JPK and provided resources. IK conceived and led the study; supervised the project; obtained funding; provided the resources; and performed field measurements, sampling, and data interpretation. JPK and IK prepared the original draft of the paper. All authors contributed to reviewing and editing the manuscript.
At least one of the (co-)authors is a member of the editorial board of Atmospheric Chemistry and Physics. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors wish to acknowledge Coventry University QR funding for providing a Trailblazers PhD studentship (acquired by Ivan Kourtchev) to Jishnu Pandamkulangara Kizhakkethil and the Centre for Agroecology, Water and Resilience (CAWR), Coventry University, for providing financial support.
This research has been supported by the Coventry University Research Excellence Development Fund (Emerging Contaminants and Bioaerosols from Wastewater Treatment Plants: An Underrecognised Threat to Air Quality and Human Health).
This paper was edited by Jason Surratt and reviewed by two anonymous referees.
Abbey, D. E., Hwang, B. L., Burchette, R. J., Vancuren, T., and Mills, P. K.: Estimated long-term ambient concentrations of PM10 and development of respiratory symptoms in a nonsmoking population, Arch. Environ. Health Int. J., 50, 139–152, https://doi.org/10.1080/00039896.1995.9940891, 1995.
Ahrens, L., Shoeib, M., Harner, T., Lee, S. C., Guo, R., and Reiner, E. J.: Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere, Environ. Sci. Technol., 45, 8098–8105, https://doi.org/10.1021/es1036173, 2011.
Ahrens, L., Harner, T., Shoeib, M., Lane, D. A., and Murphy, J. G.: Improved characterization of gas–particle partitioning for per- and polyfluoroalkyl substances in the atmosphere using annular diffusion denuder samplers, Environ. Sci. Technol., 46, 7199–7206, https://doi.org/10.1021/es300898s, 2012.
Ao, J., Tang, W., Liu, X., Ao, Y., Zhang, Q., and Zhang, J.: Polyfluoroalkyl phosphate esters (PAPs) as PFAS substitutes and precursors: An overview, J. Hazard. Mater., 464, 133018, https://doi.org/10.1016/j.jhazmat.2023.133018, 2024.
Ateia, M., Maroli, A., Tharayil, N., and Karanfil, T.: The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review, Chemosphere, 220, 866–882, https://doi.org/10.1016/j.chemosphere.2018.12.186, 2019.
ATSDR: Draft toxicological profile for perfluoroalkyls, https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (last access: 6 September 2024), 2015.
Barton, C. A., Kaiser, M. A., and Russell, M. H.: Partitioning and removal of perfluorooctanoate during rain events: The importance of physical-chemical properties, J. Environ. Monit., 9, 839–846, https://doi.org/10.1039/B703510A, 2007.
Beach, S. A., Newsted, J. L., Coady, K., and Giesy, J. P.: Ecotoxicological evaluation of perfluorooctanesulfonate (PFOS), in: Reviews of environmental contamination and toxicology: Continuation of residue reviews, edited by: Albert, L. A., de Voogt, P., Gerba, C. P., Hutzinger, O., Knaak, J. B., Mayer, F. L., Morgan, D. P., Park, D. L., Tjeerdema, R. S., Whitacre, D. M., Yang, R. S. H., Ware, G. W., Nigg, H. N., Doerge, D. R., and Gunther, F. A., Springer New York, New York, NY, 133–174, https://doi.org/10.1007/0-387-32883-1_5, 2006.
Boucher, O., Randall, D., Artaxo, P., Bretherton, C., Feingold, G., Forster, P., Kerminen, V.-M, Kondo, Y., Liao, H., Lohmann, U., Rasch, P., Satheesh, S. K., Sherwood, S., Stevens, B., and Zhang, X. Y.: Clouds and aerosols. in: Climate change 2013: The physical science basis. contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, edited by: Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V., and Midgley, P. M., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter07_FINAL-1.pdf (last access: 8 June 2025), 2013.
Brendel, S., Fetter, É., Staude, C., Vierke, L., and Biegel-Engler, A.: Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH, Environ. Sci. Eur., 30, 9, https://doi.org/10.1186/s12302-018-0134-4, 2018.
Brusseau, M. L., Anderson, R. H., and Guo, B.: PFAS concentrations in soils: Background levels versus contaminated sites, Sci. Total Environ., 740, 140017, https://doi.org/10.1016/j.scitotenv.2020.140017, 2020.
Buck, R. C., Franklin, J., Berger, U., Conder, J. M., Cousins, I. T., de Voogt, P., Jensen, A. A., Kannan, K., Mabury, S. A., and van Leeuwen, S. P. J.: Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins, Integr. Environ. Assess. Manag., 7, 513–541, https://doi.org/10.1002/ieam.258, 2011.
Chang, N. Y., Eichler, C. M. A., Amparo, D. E., Zhou, J., Baumann, K., Cohen Hubal, E. A., Surratt, J. D., Morrison, G. C., and Turpin, B. J.: Indoor air concentrations of PM2.5 quartz fiber filter-collected ionic PFAS and emissions to outdoor air: findings from the IPA campaign, Environ. Sci.-Proc. Imp., https://doi.org/10.1039/D4EM00359D, 2025.
Chen, S.-L., Chang, S.-W., Chen, Y.-J., and Chen, H.-L.: Possible warming effect of fine particulate matter in the atmosphere, Commun. Earth Environ., 2, 208, https://doi.org/10.1038/s43247-021-00278-5, 2021.
Christian, N. P.: Chemical toxicity of per- and poly-fluorinated alkyl substances (PFAS), in: Encyclopedia of Toxicology (Fourth Edition), edited by: Wexler, P., Academic Press, Oxford, 747–756, https://doi.org/10.1016/B978-0-12-824315-2.01052-6, 2024.
Clara, M., Scharf, S., Weiss, S., Gans, O., and Scheffknecht, C.: Emissions of perfluorinated alkylated substances (PFAS) from point sources – identification of relevant branches, Water Sci. Technol., 58, 59–66, https://doi.org/10.2166/wst.2008.641, 2008.
Dauchy, X., Boiteux, V., Bach, C., Colin, A., Hemard, J., Rosin, C., and Munoz, J.-F.: Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility, Sci. Total Environ., 576, 549–558, https://doi.org/10.1016/j.scitotenv.2016.10.130, 2017.
de Alba-Gonzalez, M., del Carmen González-Caballero, M., and Tarazona, J. V.: Perfluorooctanoic acid, in: Encyclopedia of Toxicology (Fourth Edition), edited by: Wexler, P., Academic Press, Oxford, 367–376, https://doi.org/10.1016/B978-0-12-824315-2.00760-0, 2024.
European Union: Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance), http://data.europa.eu/eli/dir/2020/2184/oj (last access: 4 September 2024), 2020.
Domingo, J. L. and Nadal, M.: Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature, Environ. Res., 177, 108648, https://doi.org/10.1016/j.envres.2019.108648, 2019.
Ebrahimi, F., Lewis, A. J., Sales, C. M., Suri, R., and McKenzie, E. R.: Linking PFAS partitioning behavior in sewage solids to the solid characteristics, solution chemistry, and treatment processes, Chemosphere, 271, 129530, https://doi.org/10.1016/j.chemosphere.2020.129530, 2021.
ECHA: Per- and polyfluoroalkyl substances (PFAS), https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (last access: 4 September 2024), 2022a.
ECHA: Proposal to ban “forever chemicals” in firefighting foams throughout the EU, European Chemicals Agency, https://echa.europa.eu/de/ (last access: 4 September 2024), 2022b.
Eriksson, U., Haglund, P., and Kärrman, A.: Contribution of precursor compounds to the release of per- and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs), J. Environ. Sci., 61, 80–90, https://doi.org/10.1016/j.jes.2017.05.004, 2017.
Fenton, S. E., Ducatman, A., Boobis, A., DeWitt, J. C., Lau, C., Ng, C., Smith, J. S., and Roberts, S. M.: Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research, Environ. Toxicol. Chem., 40, 606–630, https://doi.org/10.1002/etc.4890, 2021.
Glüge, J., Scheringer, M., Cousins, I. T., DeWitt, J. C., Goldenman, G., Herzke, D., Lohmann, R., Ng, C. A., Trier, X., and Wang, Z.: An overview of the uses of per- and polyfluoroalkyl substances (PFAS), Environ. Sci.-Proc. Imp., 22, 2345–2373, https://doi.org/10.1039/D0EM00291G, 2020.
Gobelius, L., Glimstedt, L., Olsson, J., Wiberg, K., and Ahrens, L.: Mass flow of per- and polyfluoroalkyl substances (PFAS) in a Swedish municipal wastewater network and wastewater treatment plant, Chemosphere, 336, 139182, https://doi.org/10.1016/j.chemosphere.2023.139182, 2023.
Goldstein, A. H. and Galbally, I. E.: Known and unexplored organic constituents in the Earth's atmosphere, Environ. Sci. Technol., 41, 1514–1521, https://doi.org/10.1021/es072476p, 2007.
Gomis, M. I., Vestergren, R., Borg, D., and Cousins, I. T.: Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives, Environ. Int., 113, 1–9, https://doi.org/10.1016/j.envint.2018.01.011, 2018.
Johansson, J. H., Salter, M. E., Acosta Navarro, J. C., Leck, C., Nilsson, E. D., and Cousins, I. T.: Global transport of perfluoroalkyl acids via sea spray aerosol, Environ. Sci.-Proc. Imp., 21, 635–649, https://doi.org/10.1039/C8EM00525G, 2019.
Kourtchev, I., Hellebust, S., Heffernan, E., Wenger, J., Towers, S., Diapouli, E., and Eleftheriadis, K.: A new on-line SPE LC-HRMS method for the analysis of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in PM2.5 and its application for screening atmospheric particulates from Dublin and Enniscorthy, Ireland, Sci. Total Environ., 835, 155496, https://doi.org/10.1016/j.scitotenv.2022.155496, 2022.
Lenka, S. P., Kah, M., and Padhye, L. P.: A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants, Water Res., 199, 117187, https://doi.org/10.1016/j.watres.2021.117187, 2021.
Lesmeister, L., Lange, F. T., Breuer, J., Biegel-Engler, A., Giese, E., and Scheurer, M.: Extending the knowledge about PFAS bioaccumulation factors for agricultural plants – A review, Sci. Total Environ., 766, 142640, https://doi.org/10.1016/j.scitotenv.2020.142640, 2021.
Li, Y., Liu, Y., Shi, G., Liu, C., Hao, Q., and Wu, L.: Occurrence and risk assessment of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in surface water, groundwater and sediments of the Jin River Basin, Southeastern China, Bull. Environ. Contam. Toxicol., 108, 1026–1032, https://doi.org/10.1007/s00128-021-03435-w, 2022.
Lin, H., Lao, J.-Y., Wang, Q., Ruan, Y., He, Y., Lee, P. K. H., Leung, K. M. Y., and Lam, P. K. S.: Per- and polyfluoroalkyl substances in the atmosphere of waste management infrastructures: Uncovering secondary fluorotelomer alcohols, particle size distribution, and human inhalation exposure, Environ. Int., 167, 107434, https://doi.org/10.1016/j.envint.2022.107434, 2022.
Link, G. W., Reeves, D. M., Cassidy, D. P., and Coffin, E. S.: Per- and polyfluoroalkyl substances (PFAS) in final treated solids (Biosolids) from 190 Michigan wastewater treatment plants, J. Hazard. Mater., 463, 132734, https://doi.org/10.1016/j.jhazmat.2023.132734, 2024.
Liu, S., Yang, R., Yin, N., and Faiola, F.: The short-chain perfluorinated compounds PFBS, PFHxS, PFBA and PFHxA, disrupt human mesenchymal stem cell self-renewal and adipogenic differentiation, J. Environ. Sci., 88, 187–199, https://doi.org/10.1016/j.jes.2019.08.016, 2020.
McMurdo, C. J., Ellis, D. A., Webster, E., Butler, J., Christensen, R. D., and Reid, L. K.: Aerosol enrichment of the surfactant PFO and mediation of the water–air transport of gaseous PFOA, Environ. Sci. Technol., 42, 3969–3974, https://doi.org/10.1021/es7032026, 2008.
Moneta, B. G., Feo, M. L., Torre, M., Tratzi, P., Aita, S. E., Montone, C. M., Taglioni, E., Mosca, S., Balducci, C., Cerasa, M., Guerriero, E., Petracchini, F., Cavaliere, C., Laganà, A., and Paolini, V.: Occurrence of per- and polyfluorinated alkyl substances in wastewater treatment plants in Northern Italy, Sci. Total Environ., 894, 165089, https://doi.org/10.1016/j.scitotenv.2023.165089, 2023.
Müller, V., Kindness, A., and Feldmann, J.: Fluorine mass balance analysis of PFAS in communal waters at a wastewater plant from Austria, Water Res., 244, 120501, https://doi.org/10.1016/j.watres.2023.120501, 2023.
Nguyen, D., Stults, J., Devon, J., Novak, E., Lanza, H., Choi, Y., Lee, L., and Schaefer, C. E.: Removal of per- and polyfluoroalkyl substances from wastewater via aerosol capture, J. Hazard. Mater., 465, 133460, https://doi.org/10.1016/j.jhazmat.2024.133460, 2024.
Nguyen, M. A., Wiberg, K., Ribeli, E., Josefsson, S., Futter, M., Gustavsson, J., and Ahrens, L.: Spatial distribution and source tracing of per- and polyfluoroalkyl substances (PFASs) in surface water in Northern Europe, Environ. Pollut., 220, 1438–1446, https://doi.org/10.1016/j.envpol.2016.10.089, 2017.
O'Connor, J., Bolan, N. S., Kumar, M., Nitai, A. S., Ahmed, M. B., Bolan, S. S., Vithanage, M., Rinklebe, J., Mukhopadhyay, R., Srivastava, P., Sarkar, B., Bhatnagar, A., Wang, H., Siddique, K. H. M., and Kirkham, M. B.: Distribution, transformation and remediation of poly- and per-fluoroalkyl substances (PFAS) in wastewater sources, Process Saf. Environ. Prot., 164, 91–108, https://doi.org/10.1016/j.psep.2022.06.002, 2022.
Pandamkulangara Kizhakkethil, J., Shi, Z., Bogush, A., and Kourtchev, I.: Aerosolisation of per- and polyfluoroalkyl substances (PFAS) during aeration of contaminated aqueous solutions, Atmos. Environ., 334, 120716, https://doi.org/10.1016/j.atmosenv.2024.120716, 2024.
Phong Vo, H. N., Ngo, H. H., Guo, W., Hong Nguyen, T. M., Li, J., Liang, H., Deng, L., Chen, Z., and Hang Nguyen, T. A.: Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation, J. Water Process Eng., 36, 101393, https://doi.org/10.1016/j.jwpe.2020.101393, 2020.
Podder, A., Sadmani, A. H. M. A., Reinhart, D., Chang, N.-B., and Goel, R.: Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects, J. Hazard. Mater., 419, 126361, https://doi.org/10.1016/j.jhazmat.2021.126361, 2021.
Pope III, C. A., Schwartz, J., and Ransom, M. R.: Daily mortality and PM10 pollution in Utah Valley, Arch. Environ. Health., 47, 211–217, https://doi.org/10.1080/00039896.1992.9938351, 1992.
Pope III, C. A., Coleman, N., Pond, Z. A., and Burnett, R. T.: Fine particulate air pollution and human mortality: 25+ years of cohort studies, Environ. Res., 183, 108924, https://doi.org/10.1016/j.envres.2019.108924, 2020.
Qiao, B., Chen, H., Song, D., Yu, H., Baqar, M., Li, X., Zhao, L., Yao, Y., and Sun, H.: Multimedia distribution and release characteristics of emerging PFAS in wastewater treatment plants in Tianjin, China, J. Hazard. Mater., 475, 134879, https://doi.org/10.1016/j.jhazmat.2024.134879, 2024.
Reth, M., Berger, U., Broman, D., Cousins, I. T., Nilsson, E. D., and McLachlan, M. S.: Water-to-air transfer of perfluorinated carboxylates and sulfonates in a sea spray simulator, Environ. Chem., 8, 381–388, https://doi.org/10.1071/EN11007, 2011.
Saikat, S., Kreis, I., Davies, B., Bridgman, S., and Kamanyire, R.: The impact of PFOS on health in the general population: a review, Environ. Sci.-Proc. Imp., 15, 329–335, https://doi.org/10.1039/C2EM30698K, 2013.
Semerád, J., Hatasová, N., Grasserová, A., Černá, T., Filipová, A., Hanč, A., Innemanová, P., Pivokonský, M., and Cajthaml, T.: Screening for 32 per- and polyfluoroalkyl substances (PFAS) including GenX in sludges from 43 WWTPs located in the Czech Republic – Evaluation of potential accumulation in vegetables after application of biosolids, Chemosphere, 261, 128018, https://doi.org/10.1016/j.chemosphere.2020.128018, 2020.
Sha, B., Ungerovich, E., Salter, M. E., Cousins, I. T., and Johansson, J. H.: Enrichment of Perfluoroalkyl Acids on Sea Spray Aerosol in Laboratory Experiments: The Role of Dissolved Organic Matter, Air Entrainment Rate and Inorganic Ion Composition, Environ. Sci. Tech. Let., 11, 746–751, https://doi.org/10.1021/acs.estlett.4c00287, 2024.
Shoeib, M., Schuster, J., Rauert, C., Su, K., Smyth, S.-A., and Harner, T.: Emission of poly and perfluoroalkyl substances, UV-filters and siloxanes to air from wastewater treatment plants, Environ. Pollut., 218, 595–604, https://doi.org/10.1016/j.envpol.2016.07.043, 2016.
Solan, M. E. and Lavado, R.: The use of in vitro methods in assessing human health risks associated with short-chain perfluoroalkyl and polyfluoroalkyl substances (PFAS), J. Appl. Toxicol., 42, 1298–1309, https://doi.org/10.1002/jat.4270, 2022.
Sunderland, E. M., Hu, X. C., Dassuncao, C., Tokranov, A. K., Wagner, C. C., and Allen, J. G.: A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects, J. Expo. Sci. Environ. Epidemiol., 29, 131–147, https://doi.org/10.1038/s41370-018-0094-1, 2019.
Taylor, K. E. and Penner, J. E.: Response of the climate system to atmospheric aerosols and greenhouse gases, Nature, 369, 734–737, https://doi.org/10.1038/369734a0, 1994.
Turpin, B. J., Huntzicker, J. J., and Hering, S. V.: Investigation of organic aerosol sampling artifacts in the Los Angeles basin, Atmos. Environ., 28, 3061–3071, https://doi.org/10.1016/1352-2310(94)00133-6, 1994.
UNEP/POPS/POPRC.15/7/Add.1: Report of the persistent organic pollutants review committee on the work of its fifteenth meeting, addendum; Risk management evaluation on perfluorohexane sulfonic acid (PFHxS), its salts and PFHxS-related compounds, https://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.15-7-Add.1.English.PDF (last access: 6 September 2024), 2019.
US EPA: IRIS toxicological review of perfluorobutanoic acid (PFBA, CASRN 375-22-4) and related salts, https://iris.epa.gov/static/pdfs/0701_summary.pdf (last access: 11 September 2024), 2022.
US EPA: Final PFAS national primary drinking water regulation, https://www.epa.gov/sdwa/, last access: 4 September 2024a.
US EPA: Our current understanding of the human health and environmental risks of PFAS, https://www.epa.gov/pfas/, last access: 4 September 2024b.
Vierke, L., Ahrens, L., Shoeib, M., Reiner, E. J., Guo, R., Palm, W., Ebinghaus, R., and Harner, T.: Air concentrations and particle–gas partitioning of polyfluoroalkyl compounds at a wastewater treatment plant, Environ. Chem., 8, 363–371, https://doi.org/10.1071/EN10133, 2011.
Vierke, L., Berger, U., and Cousins, I. T.: Estimation of the acid dissociation constant of perfluoroalkyl carboxylic acids through an experimental investigation of their water-to-air transport, Environ. Sci. Technol., 47, 11032–11039, https://doi.org/10.1021/es402691z, 2013.
Vohra, K., Vodonos, A., Schwartz, J., Marais, E. A., Sulprizio, M. P., and Mickley, L. J.: Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem, Environ. Res., 195, 110754, https://doi.org/10.1016/j.envres.2021.110754, 2021.
Wang, Z., Cousins, I. T., Scheringer, M., and Hungerbühler, K.: Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors, Environ. Int., 60, 242–248, https://doi.org/10.1016/j.envint.2013.08.021, 2013.
Wang, Z., Cousins, I. T., Scheringer, M., Buck, R. C., and Hungerbühler, K.: Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: The remaining pieces of the puzzle, Environ. Int., 69, 166–176, https://doi.org/10.1016/j.envint.2014.04.006, 2014.
Wang, Z., Cousins, I. T., Scheringer, M., and Hungerbuehler, K.: Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions, Environ. Int., 75, 172–179, https://doi.org/10.1016/j.envint.2014.11.013, 2015.
Weinberg, I., Dreyer, A., and Ebinghaus, R.: Waste water treatment plants as sources of polyfluorinated compounds, polybrominated diphenyl ethers and musk fragrances to ambient air, Environ. Pollut., 159, 125–132, https://doi.org/10.1016/j.envpol.2010.09.023, 2011.
Xiao, F.: An overview of the formation of PFOA and PFOS in drinking-water and wastewater treatment processes, J. Environ. Eng., 148, 1822001, https://doi.org/10.1061/(ASCE)EE.1943-7870.0001986, 2022.
Xiao, F., Simcik, M. F., Halbach, T. R., and Gulliver, J. S.: Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: Migration and implications for human exposure, Water Res., 72, 64–74, https://doi.org/10.1016/j.watres.2014.09.052, 2015.
Zareitalabad, P., Siemens, J., Hamer, M., and Amelung, W.: Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater – a review on concentrations and distribution coefficients, Chemosphere, 91, 725–732, https://doi.org/10.1016/j.chemosphere.2013.02.024, 2013.
Zhang, H., Li, L., Song, J., Akhter, Z. H., and Zhang, J.: Understanding aerosol–climate–ecosystem interactions and the implications for terrestrial carbon sink using the Community Earth System Model, Agric. For. Meteorol., 340, 109625, https://doi.org/10.1016/j.agrformet.2023.109625, 2023.
Zhou, J., Baumann, K., Mead, R. N., Skrabal, S. A., Kieber, R. J., Avery, G. B., Shimizu, M., DeWitt, J. C., Sun, M., Vance, S. A., Bodnar, W., Zhang, Z., Collins, L. B., Surratt, J. D., and Turpin, B. J.: PFOS dominates PFAS composition in ambient fine particulate matter (PM2.5) collected across North Carolina nearly 20 years after the end of its US production, Environ. Sci.-Proc. Imp., 23, 580–587, https://doi.org/10.1039/D0EM00497A, 2021.
Zhou, J., Baumann, K., Surratt, J. D., and Turpin, B. J.: Legacy and emerging airborne per- and polyfluoroalkyl substances (PFAS) collected on PM2.5 filters in close proximity to a fluoropolymer manufacturing facility, Environ. Sci.-Proc. Imp., 24, 2272–2283, https://doi.org/10.1039/D2EM00358A, 2022.
Zhou, R.-X., Liao, H.-J., Hu, J.-J., Xiong, H., Cai, X.-Y., and Ye, D.-W.: Global burden of lung cancer attributable to household fine particulate matter pollution in 204 countries and territories, 1990 to 2019, J. Thorac. Oncol., 19, 883–897, https://doi.org/10.1016/j.jtho.2024.01.014, 2024.