the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Measurement report: High contribution of N2O5 uptake to particulate nitrate formation in NO2-limited urban areas
Ziyi Lin
Chuanyou Ying
Lingling Xu
Xiaoting Ji
Keran Zhang
Feng Zhang
Gaojie Chen
Lingjun Li
Chen Yang
Yuping Chen
Ziying Chen
Particulate nitrate (p) is a major component of fine particles in Chinese urban areas. However, the relative contributions of p formation pathways in urban areas remain poorly quantified, particularly under the NO2-limited regime that governs its formation (as defined by the ratio), which hinders effective particulate pollution control. In this study, comprehensive winter field observations were conducted in urban Xiamen, Southeast China. We observed significantly elevated nighttime p levels concurrent with increased N2O5 concentrations. Quantification using an observation-constrained model revealed that N2O5 uptake contributed 51.2 % to total p formation, which was comparable to that of the OH+NO2 reaction. The N2O5 uptake was found to be mainly driven by nocturnal NO3 oxidation capacity (modulated by NO2 and O3 levels) rather than by heterogeneous reaction conditions. Sensitivity simulations further demonstrated that p formation rate was more sensitive to NOx variations than to VOCs variations. Implementing NOx control measures at nighttime was shown to effectively reduce p by abating N2O5 uptake while simultaneously preventing daytime O3 increase. Our findings enhance the understanding of p formation in NO2-limited urban areas and provide valuable insights for developing joint PM2.5 and O3 mitigation strategies.
- Article
(4940 KB) - Full-text XML
-
Supplement
(1688 KB) - BibTeX
- EndNote
Fine particulate matter (PM2.5) contributes to various atmospheric environmental issues, including visibility deterioration, radiative forcing change, and adverse impacts on human health (Seinfeld, 1989; Lelieveld et al., 2015). Among its chemical components, particulate nitrate (p) has attracted increasing attention due to its rising mass fraction in PM2.5 and its nonlinear responses to emission mitigation strategies (Xie et al., 2022; Zhai et al., 2021; Li et al., 2021; Zhang et al., 2021; Zhou et al., 2022; Zong et al., 2022; Wang et al., 2020). The primary formation pathways of p include gas-phase oxidation through the reaction of hydroxyl radicals (OH) and nitrogen dioxides (NO2) (R1 and R2), and heterogeneous uptake of dinitrogen pentoxide (N2O5) which is produced via NO2 oxidation by nitrate radicals (NO3) (R3–R5) (Brown and Stutz, 2012). It is well recognized that the OH+NO2 reaction dominates in daytime, while N2O5 uptake dominates in nighttime. During nocturnal p formation, particulate chlorides can induce N2O5 heterogeneous uptake to produce ClNO2, thereby competing with p formation.
Many studies have focused on quantifying the potential formation pathways of p in urban areas of China. In major urban agglomerations such as the Beijing-Tianjin-Hebei (BTH) region (Chen et al., 2020; Ma et al., 2023; Zhao et al., 2023), Yangtze River Delta (YRD) (Sun et al., 2022; Zhai et al., 2023; Zhang et al., 2023b), and Pearl River Delta (PRD) (Yang et al., 2022; Niu et al., 2022; Cheng et al., 2024), p formation was typically dominated by the gas-phase oxidation of OH+NO2. In contrast, under special conditions such as the COVID-19 pandemic and PM2.5 pollution events (Yan et al., 2023; Zhai et al., 2023), N2O5 uptake became the main pathway. Previous research has demonstrated that the formation rate of p via N2O5 uptake is closely related to its precursor NO2 and O3, and the N2O5 formation can be classified into NO2-limited and O3-limited regimes based on the ratio (Ma et al., 2023). The winter ratios in the BTH, YRD, and PRD regions were generally above 1, placing N2O5 formation in the O3-limited or transition regime (Ma et al., 2023; Wen et al., 2018; Li et al., 2021; Zhang et al., 2023b). However, the N2O5 uptake served as the dominant pathway for p formation, typically under NO2-limited conditions (e.g., reduced emissions during the pandemic) or under large aerosol surface areas (e.g., severe particulate pollution episodes). Collectively, these findings indicate that spatial variations in NO2 and O3 levels are likely a key driver of regional differences in the dominant formation pathways of p. The formation of p primarily depends on precursors OH, NO2, and O3, with OH and O3 concentrations being influenced by VOCs and NOx emissions. Thus, the different formation pathways of p result in complex responses to NOx/VOCs emissions. The response of p formation via OH+NO2 to precursors variation is relatively well-understood, as most Chinese urban areas are located in VOC-limited regimes for O3 (Wang et al., 2023a, 2022c; Zhang et al., 2023a; Mao et al., 2022), and ammonia-rich regimes for p (Xing et al., 2018; Sun et al., 2022; Fu et al., 2024; Liu et al., 2019). Under these conditions, VOCs reduction suppresses p formation by decreasing OH concentrations, whereas NOx reduction enhances p formation by weakening the NOx titration effect. Given the regional variations in the ratio across urban areas of China (Ma et al., 2023), the response of p formation via N2O5 uptake to precursor changes (VOCs, O3) likely exhibits spatial heterogeneity. A recent study has revealed that under O3-limited conditions for N2O5 formation (Zhang et al., 2023b), reducing NOx emissions had negligible effects, while reducing VOCs decreased the consumption of NO3 by VOCs, thereby enhancing p formation from N2O5 uptake. However, the response of p formation to precursors under NO2-limited conditions remains unclear. Aside from precursor availability, N2O5 uptake is also greatly influenced by heterogeneous reaction conditions like aerosol composition and aerosol surface area (Mcduffie et al., 2018b, a; Tham et al., 2018; Yu et al., 2020), which introduces additional uncertainty in determining the contribution of p formation pathways and the effectiveness of precursor control strategies.
The ratios in southeastern China predominantly fell within the NO2-limited regime for N2O5 formation (Ma et al., 2023). Xiamen, as one of the most developed cities in southeastern China, exhibits relatively better air quality with low levels of VOCs and NOx compared to China's megacities (Table S1 in the Supplement). This pattern well represents the future urban atmospheric conditions following the implementation of air pollution control measures in China. From December 2022–February 2023, we conducted comprehensive multi-parameter observations in urban Xiamen, including N2O5 and related chemical constituents. An observation-constrained box model incorporating the heterogeneous reaction parameters was utilized to quantify the rates of different p formation pathways. An explainable machine learning (ML) method was applied to identify the driving factors for high p formation rate via N2O5 uptake. Additionally, multi-scenario simulations were performed to examine the joint responses of p and O3 formation to various NOx and VOCs emissions. These findings enhance our understanding of p formation pathways and their environmental implications in NO2-limited regions, providing valuable insights for developing joint PM2.5 and O3 mitigation strategies.
2.1 Field observation
Field observations were conducted during the winter period from 1 December 2022–3 February 2023, at an urban site (marked by the red star in Fig. S1 in the Supplement) in Xiamen, which is located in the southeastern coastal region of China. Detailed site information has been described in our previous studies (Yang et al., 2023; Liu et al., 2022). Trace gases (including PAN, HCHO, HONO, VOCs, O3, NOx, CO, and SO2), chemical components in PM2.5 (including organic carbon and elemental carbon, , , , Cl−), PM2.5 mass concentration, and meteorological parameters (including ambient temperature (T), relative humidity (RH), atmospheric pressure (P), wind speed (WS), wind direction (WD), and photolysis rates) were continuously measured during the campaign. Detailed information about measurement methods and instruments is summarized in Sect. S1 in the Supplement. In addition, boundary layer height (BLH) data were obtained from the ERA5 dataset (Hersbach et al., 2020).
A chemical ionization time-of-flight mass spectrometer equipped with an iodide source (iodide-TOF-CIMS, Aerodyne Research Inc., USA) was deployed to measure N2O5 and ClNO2. A nearly 2 m long perfluoroalkoxy (PFA) tube with a 14-inch inner diameter was used for sampling. The total sampling flow rate was set as 10 standard liters per minute (SLPM), of which only 2SLPM was diverted to the CIMS. A nitrogen (N2) flow (99.999 %, 2.7 SLPM), carrying methyl iodide (CH3I) vapor released from a heated permeation tube, passed through a soft X-ray source (Tofwerk AG, P-type) to generate reagent ions I−. The I− was combined with the target gas in an ion molecule reaction (IMR) chamber and then detected by the ToF-CIMS. Ambient N2O5 and ClNO2 were detected as the I(N2O5)− and I(ClNO2)− clusters at 235 and 208 . The detailed calibration procedures of N2O5 and ClNO2 are described in Sect. S2, following established methods (Wang et al., 2022b, a; Thaler et al., 2011). Briefly, N2O5 was generated from the reaction between O3 and excessive NO2, while ClNO2 was synthesized via the reaction of Cl2 (6 ppm in N2) with a moist mixture of NaNO2 and NaCl. The calibration curves for N2O5 and ClNO2 at different RH are shown in Fig. S2, with mean sensitivities of 0.110±0.063 and , respectively. The instrument background was determined by introducing dry N2 into the inlet for 20 min. Based on three times the standard deviation (3σ) of the background signal, the typical 1 min detection limits for N2O5 and ClNO2 were estimated to be 0.61 and 1.3 ppt, respectively.
2.2 Determination of p formation rate
The iterative box model developed by Wagner et al. with a simplified mechanism was employed to obtain key parameters of the N2O5 uptake process (Wagner et al., 2013), including the loss rate of N2O5 (kN2O5) and the production yield of ClNO2 (φClNO2, see in Sect. S3). To validate the iterative box model results, these parameters were calculated concurrently based on the classical steady-state approximation method (Sect. S4) (Brown et al., 2003; Chen et al., 2022). The derived parameters of N2O5 uptake were adopted for subsequent multiphase box model.
A Framework for 0-D Atmospheric Modeling (F0AM), incorporating the Master Chemical Mechanism (MCM v3.3.1) and heterogeneous mechanisms (Table S2), was employed to simulate nitrate formation rates for each day during the study period (Wolfe et al., 2016; Atkinson and Arey, 2003; Jenkin et al., 2015). The heterogeneous parameters derived from the iterative box model were implemented in F0AM. In addition, hourly interval data of trace gases, photochemically active species, meteorological variables, and reanalysis data were also applied to constrain the multiphase chemical box model. Detailed model configurations are provided in Sect. S5. As shown in Fig. S3, the model performed well in simulating the trends of N2O5 and ClNO2 with R2 of 0.88 and 0.49, respectively. However, a systematic underestimation existed in the simulated N2O5 and ClNO2 concentrations, which likely resulted from the model configuration including overestimated physical removal rates, elevated concentration of intermediate VOC species, or uncertainties in transport processes. Consequently, the simulated p formation from N2O5 uptake in this study could be regarded as a lower limit. The simulated OH concentrations agreed well with parameterized method suggested by Ehhalt and Rohrer (Fig. S4, R2=0.86) (Ehhalt and Rohrer, 2000). Based on model simulation and precursor observations, we quantified p formation rates through both OH+NO2 and N2O5 uptake pathways by model integral.
2.3 Identification of influencing factors for p formation via N2O5 uptake
Extreme gradient boosting (XGBoost), a machine learning technique, has been widely applied in atmospheric chemistry research (Gui et al., 2020; Wang et al., 2023b; Requia et al., 2020). Here, we built a XGBoost model to reproduce the p formation rate via N2O5 uptake with selected variables. The model was built using the “xgboost” library (https://github.com/dmlc/xgboost/tree/master, last access: 8 November 2025) in a python environment. Explanatory variables included meteorological parameters (BLH, T, and RH), nocturnal atmospheric oxidation capacity P(NO3) calculated by , TVOCs, the logarithm of the ratio of NO2 to O3 (), NO, and heterogeneous uptake parameters (φClNO2 and kN2O5). Only nighttime (18:00–06:00 LT the next day) data were considered to identify key drivers of p formation via N2O5 uptake. The hyperparameters of the XGBoost model were tuned by grid searching method and the established model was evaluated using R2, Mean Absolute Error (MAE) and Root Mean Square Error (RMSE). By incorporating SHAP interpretation, the XGBoost-SHAP method could quantify factor contributions through SHAP values, where absolute SHAP values denote the relative importance. Detailed description and setup of the XGBoost-SHAP method can be found in Sect. S6 and our previous study (Lin et al., 2024).
2.4 Emission scenario modelling
Using the aforementioned multiphase chemical box model, we investigated changes in formation rates of p () and O3 (PO3) under different VOCs and NOx emission scenarios. The base model simulation was performed using mean diurnal values from the winter 2022 observations. A series of emission scenarios were tested by scaling normalized VOCs and NOx concentrations from 0–2 times baseline levels to examine their impacts on and PO3. Prior to each scenario simulation, 3d spin-up was set to stabilize intermediate species concentrations. Isopleth diagrams of simulated and PO3 were obtained from the base scenario and 120 emission change scenarios. In addition, response strength (RS) was calculated using Eq. (2) as an indicator of emission sensitivity.
where, ki is the corresponding chemical reaction rate constants.
where, Xi and Xbase are the mean formation rates of dependent variables e.g. , PO3 in scenario i and base simulations, respectively. Vi and Vbase are the emission rates for the scenario i and base simulations, respectively. Notably, the emission rates ranged from 0–2 times baseline levels, with the base simulation emission rate normalized to 1.
3.1 Overview of observations
The mean diurnal patterns of p, gaseous pollutants and relevant meteorological parameters are shown in Fig. 1. During the entire observation period, mean concentrations of NO2, O3, total VOCs, and PM2.5 were 10.9 ppb, 27.3 ppb, 18.2 ppb, and 14.3 µg m−3, respectively, lower than those observed in most of China's key cities (refer to Table S1). Despite the low NOx levels, p contributed 29.5 % to PM2.5 mass concentration, which was higher than proportions reported in Beijing urban area (24.7 %) (Ma et al., 2023), Guangdong (24.0 %) (Yun et al., 2018), and Nanjing (24 %–27 %) (Huang et al., 2020). This discrepancy suggests efficient conversion from NO2 to p in the study area. In addition, the proportion of p increased with rising PM2.5 concentration (Fig. S6), indicating its importance to particulate pollution. This is consistent with the phenomenon widespread in urban areas of China where p became dominant in inorganic aerosols despite NOx reduction, underscoring the need for efficient p control strategies (Zhai et al., 2021; Zhao et al., 2020; Zhang et al., 2022).
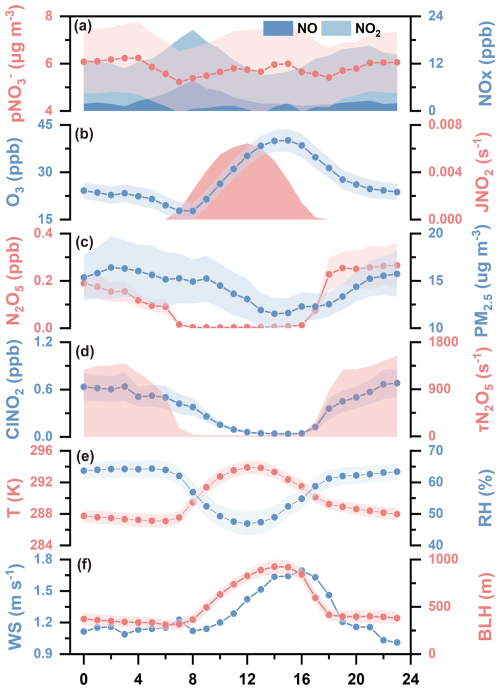
Figure 1Diurnal variations of key parameters during the winter of 2022. The concentrations of p, NOx, O3, N2O5, PM2.5 and ClNO2. The levels of the photolysis frequencies of NO2 (JNO2), ambient temperature (T), relative humidity (RH), the lifetime of N2O5 (τN2O5), wind speed (WS) and the boundary layer height (BLH). Shaded areas of p, O3, N2O5, PM2.5, ClNO2, T, RH and BLH represent 95 % confidence intervals.
The diurnal pattern of p exhibited a bimodal characteristic, with peaks occurring at 04:00 and 15:00 LT, respectively. The daytime peak (07:00–17:00 LT was accompanied by low concentrations of NOx and high levels of O3 and JNO2, indicating that active photochemical conditions promoted daytime p formation. During the nighttime (18:00–06:00 LT the next day), p concentrations increased together with NO2, N2O5 and ClNO2 from 18:00 LT onward and remained elevated until early morning. This nighttime accumulation can be attributed to two factors. First, lower temperature, shallower boundary layer height, and reduced wind speed at night favored the accumulation of p and related nitrogen-containing species. Second, higher RH and PM2.5 concentrations at night enhanced aerosol water content and surface area, providing favorable conditions for heterogeneous hydrolysis of N2O5 to form p. The mean concentration of N2O5 was 0.19±0.26 ppb (peaking at 2.52 ppb), which is relatively higher than values reported for China's megacities (Chen et al., 2020; Wang et al., 2017; Tham et al., 2018; Wang et al., 2022a; Liu et al., 2025; Li et al., 2023). Moreover, the observed elevation in nighttime ClNO2, primarily produce via the reaction of N2O5 with Cl-containing particles, strongly supports the presence of active heterogeneous processes of N2O5. Collectively, these findings imply a likely significant contribution of N2O5 uptake to p formation during the nighttime.
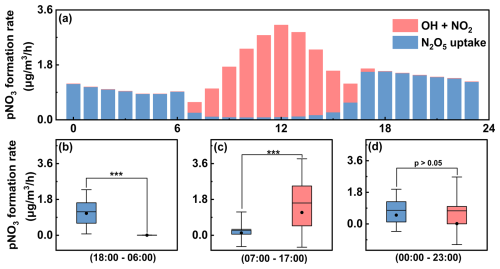
Figure 2Simulated rates of key p formation pathways obtained from the chemical box model incorporating heterogeneous parameters. Diurnal formation rates of p via the OH+NO2 and N2O5 uptake pathways (a) and comparison of the two pathways during the nighttime (b), daytime (c), and the whole day (d). Note that the results in panel (a) represent the mean simulated formation rates over the entire observation period. The box shows the 25th–75th percentiles with whiskers representing the 5th–95th percentiles. The black line and dot inside the box represent the mean and median values, respectively. Statistical significance was determined using pair-sample t-tests with *** indicating p<0.001.
3.2 High contribution of N2O5 uptake to p formation in NO2-limited conditions
In view of the observed importance of daytime and nighttime p formation, we further employed an observation-constrained model to quantify the potential formation pathways, including the gas-phase reaction of OH+NO2 and heterogeneous N2O5 uptake. This model incorporated heterogeneous chemical mechanisms, with key heterogeneous parameters (e.g. kN2O5 and φClNO2) obtained through simulation (See Methods for details). As shown in Fig. S7, the simulated kN2O5 and φClNO2 exhibited good agreement with the classical steady-state method (R2=0.76 and 0.73, respectively), demonstrating the model's capability to characterize heterogeneous uptake processes and thereby effectively evaluate p formation processes.
As illustrated in Fig. 2a, the diurnal pattern of p formation rates exhibited a classical characteristic, with daytime dominated by gas-phase oxidation and nighttime dominated by N2O5 uptake. Specifically, the daytime OH+NO2 reaction had a mean p formation rate of 1.62 , while the nighttime N2O5 uptake pathway showed a formation rate of 1.18 (Fig. 2b and c). For the whole day, N2O5 uptake contributed an average of 51.2 % to p formation, which was comparable to the contribution of the OH+NO2 pathway (Fig. 2d). To exclude year-specific effects, we further analyzed p formation during the winters from 2019–2023. The results revealed that the p formation rates via N2O5 uptake (0.75–1.40 ) were comparable to those from the OH+NO2 reaction (0.88–1.66 ; Fig. 3a), with the N2O5 uptake pathway consistently accounting for approximately half of the total p formation in the study area (Fig. 3b). Such a high contribution of N2O5 uptake to p is generally uncommon in urban areas. A study in urban Beijing showed that during non-polluted periods, N2O5 uptake contributed only 18.9 % to nitrate formation rates (Ma et al., 2023). Similarly, the contributions of N2O5 uptake were 10 %–38 % and 4 % in urban areas of the YRD (Sun et al., 2022; Zhai et al., 2023; Zhang et al., 2023b) and PRD regions(Yang et al., 2022), respectively.
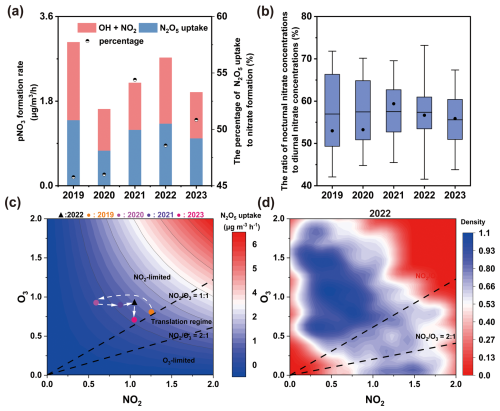
Figure 3Inter-annual patterns of key p formation pathways in urban Xiamen. The average p formation rate from OH+NO2 and N2O5 uptake (a), and the average ratio of the sum of nocturnal p concentrations to the sum of all-day p concentration (b) in different winters from 2019–2023 based on the measured p in PM2.5. The sensitivity of nocturnal p formation via N2O5 uptake to NO2 and O3 from 2019–2023 (c). And probability distribution of observed at nighttime in winter 2022 (d). The observed periods of different winters from 2019–2023 are summarized in Table S3. In panel (b), the black line and the solid circle in the boxplot represent the mean and median value, respectively. In panel (c), the black triangle indicates the base case of winter 2022, solid circles in different colors represent the average NO2 to O3 ratios in different years, and the predicted average formation rate of N2O5 uptake as the normalized emissions (average concentrations of O3 and NO2) varied between 0–2.
Previous studies have found that nocturnal p formation via N2O5 uptake strongly depends on the ratio of NO2 to O3 (Ma et al., 2023). This process is suppressed in the O3-limited regime () but enhanced in the NO2-limited regime (). The COVID-19 lockdown period was a typical example of this ratio dependence(Yan et al., 2023). In regions like Beijing, substantial reductions in NOx emissions caused a shift in nocturnal p formation from the O3-limited to the NO2-limited regime. This shift resulted in elevated nighttime O3 levels and a weakened NO titration effect, collectively promoting N2O5 formation and subsequent p formation. The sensitivity of p formation via N2O5 uptake to NO2 and O3 during the campaign is presented in Fig. 3c and d. The observed mean values of (0.40) and the probability distributions of ratios both indicate that N2O5 uptake was in the NO2-limited regime. Based on NO2 and O3 observational data during 2015–2021 from the China National Environmental Monitoring Centre(Ma et al., 2023), most key urban regions in China (e.g., the NCP, YRD, and Beijing) were found to lie in the O3-limited or transition regimes (), whereas nocturnal p formation in southeastern China was distinctly in NO2-limited regime. These results confirm that the dominant p formation mechanisms in our study area significantly differs from those in most urban areas of China, which might be attributed to the dependence of N2O5 uptake on precursor NO2 and O3. In addition, the dominance of N2O5 uptake in p formation also occurred during haze pollution periods (Zhai et al., 2023; Wang et al., 2017), where increased aerosol surface area under high particulate loadings created favorable conditions for N2O5 heterogeneous reactions. Therefore, to evaluate the role of precursors, we conducted a comprehensive analysis of the factors driving p formation via N2O5 uptake.
3.3 Driving factors of p formation via nocturnal N2O5 uptake
The N2O5 uptake rate is influenced by multiple factors including precursor levels, meteorological parameters, and heterogeneous reaction conditions (Ma et al., 2023; Chen et al., 2020, 2024). A machine learning method integrating these factors was employed to identify the key drivers of p formation via N2O5 uptake. The relative importance of each factor was evaluated by absolute SHAP values (Fig. 4a), and their impacts were elucidated by examining the relationships between individual factors and their corresponding SHAP values (Fig. 4b–e and S8). Results showed that the nocturnal NO3 formation rate (P(NO3)), an integrated indicator of nocturnal atmospheric oxidation capacity (Wang et al., 2021), was the most important factor. The steep slope of the positive correlation between P(NO3) and SHAP values indicated that P(NO3) strongly enhances p formation via N2O5 uptake. P(NO3) is primarily formed through the reaction between NO2 and O3 (), suggesting that NO2 and O3 mainly influenced p formation via N2O5 by modulating NO3 radical formation. Notably, the factor had relatively low importance, indicating concentrations of precursors were more important than ratio in determining p formation via N2O5 uptake under extremely NO2-limited condition (mean was 0.40). Furthermore, as shown in Fig. S8b, and its SHAP value show a positive correlation when is less than 0. This indicates that under NO2-limited conditions (, i.e., ), p formation via N2O5 uptake was driven by the elevated NO2.
Compared with P(NO3), other factors exhibited weaker effects on p formation rate via N2O5 uptake. φClNO2 emerged as the second most important factor and showed a negative correlation with SHAP values (Fig. 4c), illustrating that ClNO2 formation inhibited p formation. This inhibitory effect could be attributed to high concentrations of Cl-containing particles () in the study area. Chloride-containing aerosols promote N2O5 uptake to produce more ClNO2 (as evidenced by the positive correlation between φClNO2 and chloride ions, Fig. S9), while simultaneously reducing p formation (R5). Additionally, the nighttime produced ClNO2 can undergo photolysis in following day to release Cl radicals, which further promote O3 formation. This indirect effect must be considered when formulating control measures for particulate matter pollution. Interestingly, as shown in Table S4 (Tham et al., 2016; Wang et al., 2018; Yun et al., 2018; Morgan et al., 2015), although the simulated kN2O5 () was higher than values reported in Beijing (), Guangdong (), and UK (), kN2O5 exerted only a weak positive effect on N2O5 uptake (Fig. 4d). The large difference existing in the importance of P(NO3) and kN2O5 indicated that the p formation rate via N2O5 uptake process was more limited by precursor levels rather than heterogeneous uptake conditions. Similar phenomenon was also found in winter in urban Beijing and Northern Utah mountain basins (Mcduffie et al., 2019; Chen et al., 2020). The total concentrations of the observed VOCs (TVOCs) showed a weak negative correlation with N2O5 uptake (Fig. 4e). Similar to existing research (Hu et al., 2023), specific VOC species, such as styrene, 2-butene, and isoprene, can readily consume NO3 radicals (Fig. S10), thereby inhibiting N2O5 formation. However, the loss of N2O5 through the reaction between VOCs and NO3 was relatively limited compared to its direct uptake, as determined by our calculations (Text S4), which supported the SHAP analysis.
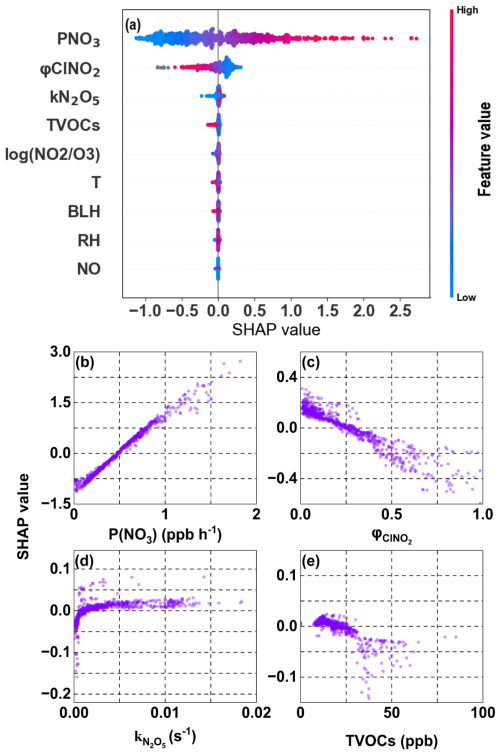
Figure 4Feature importance (a) and the effects of key factors on p formation via N2O5 uptake (b–e) obtained by the XGBoost-SHAP method. The relationships between SHAP values and major features: P(NO3) (b), φClNO2(c), kN2O5 (d), and TVOCs (e). Feature importance ranking (a) is determined by mean absolute SHAP values (descending order, top to bottom). Relationships between SHAP values and other factors are shown in Fig. S8.
Moreover, we found that the effects of φClNO2, kN2O5, and TVOCs on p formation via N2O5 uptake were subject to P(NO3) levels (Fig. 5a–c). Specifically, the negative effect of φClNO2 and the positive effect of kN2O5 on p formation via N2O5 uptake became statistically significant when P(NO3) exceeded approximately 1.0 and 0.5 ppb h−1, respectively. The negative correlation slope of TVOCs versus p formation via N2O5 uptake intensified with increasing P(NO3) levels, indicating that the N2O5 removal effect was enhanced through VOC-induced NO3 depletion. These findings highlight the critical role of precursor NO2 and O3 in nocturnal p formation, demonstrating that these precursors mainly affect this pathway by modulating NO3 radical formation.
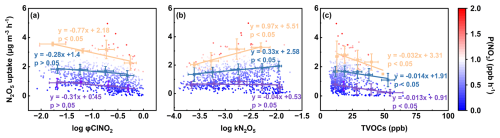
Figure 5Relationships between p formation via N2O5 uptake and φClNO2 (a), kN2O5 (b), and TVOCs (c) colored by P(NO3). Linear fit curves in purple, blue and orange represent the fitting results for P(NO3) in the ranges of 0–0.5, 0.5–1.0 and , respectively.
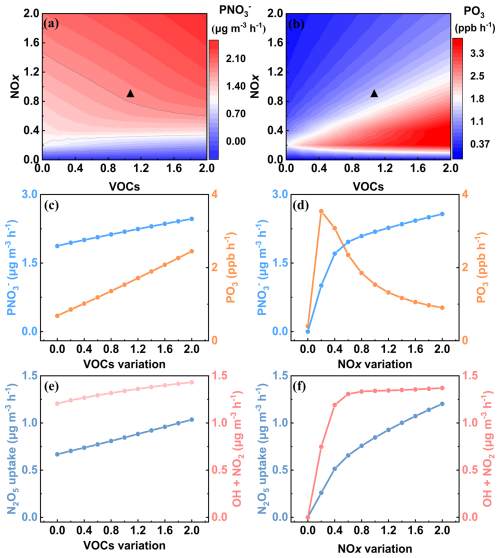
Figure 6Results of multi-scenario simulations obtained from an observation-constrained box model. Isopleths of simulated (a) and PO3 (b) with normalized VOCs and NOx. Simulated mean formation rates of p and O3 (c, d), as well as p formation rates via N2O5 uptake and OH+NO2 (e, f) with normalized VOCs and NOx. The and PO3 denote the formation rates of p and O3, respectively. The simulated results are daily mean values, and the black triangle indicates the base case for winter 2022. In addition, the results in panel (c–f) were obtained by maintaining either NOx or VOCs at the base emission rate while varying the other.
3.4 Optimal mitigation strategies of p under NO2-limited conditions
The above results revealed that p formation through both the daytime OH+NO2 reaction and nocturnal heterogeneous N2O5 uptake was closely linked to VOCs–NOx–O3 chemistry (Yang et al., 2022). Using a multiphase box model, we systematically examined the responses of both p and O3 to varying NOx and VOC emission scenarios. Figure 6a shows p formation located in the transition regime of VOCs and NOx. The formation rate of p () decreased with the reductions of VOCs and NOx, and this trend became more pronounced under aggressive NOx reduction scenarios (Fig. 6c and d). Figure S11a and b reveal that the mean response strength (RS, as defined in Methods) of to NOx was 0.75, higher than that for VOCs (RS=0.29), suggesting that NOx reduction had a greater potential for p mitigation compared to VOCs control. However, NOx and VOCs reductions exerted different impacts on O3 formation rate (PO3). In our study area, PO3 located in the VOC-limited regime (Fig. 6b). We found that PO3 declined with VOCs reduction but increased with NOx reduction until NOx dropped below 20 % of the base (Fig. 5c and d). Moreover, detailed results distinguishing daytime and nighttime major formation pathways of p are presented in Fig. 6e and f and Fig. S11c and d. For VOC reduction scenarios, both the OH+NO2 reaction and N2O5 uptake pathways showed declining nitrate formation rates, with comparable RS of 0.11 and 0.18, respectively. This occurs because reduced VOCs concentrations decrease OH radical and O3 concentrations, thereby suppressing p formation via both pathways. In contrast, NOx reduction yielded more complex behavior. The OH+NO2 reaction rates remained nearly constant until NOx dropped to 60 % of the base. This stability arises because NOx reduction diminishes the NO titration effect on O3, thereby increasing OH radicals through O3 photolysis. The competing effects of NOx reduction and OH enhancement led to an initial plateau in the OH+NO2 reaction rate before its eventual decline. Differently, the N2O5 uptake rate decreased consistently and significantly with NOx abatement, exhibiting a high mean RS value of 0.61. This phenomenon was closely associated with the NO2-limited regime of N2O5 uptake in the study area. As shown in Fig. S12, the variation trends of , P(O3), OH+NO2, and N2O5 uptake were consistent across all VOCs/NOx combinations, indicating that the results robustly reflect the response mechanisms to precursor emission changes.
As mentioned above, while VOCs reduction proved effective in mitigating both p and O3, its effectiveness in reducing p remained limited compared to NOx reduction. However, the effectiveness of NOx reduction exhibited significant regional and temporal variations. In China's megacities, including PRD, YRD, and BTH regions, p initially increased and then decreased in response to the reduction of NOx emissions (Li et al., 2021; Zhang et al., 2023b; Yang et al., 2022). Under high-NOx conditions, mild NOx reduction would raise daytime OH and O3 concentrations (Zhang et al., 2023b), rendering OH (rather than NOx) the limiting factor for the OH+NO2 reaction, which consequently enhanced daytime p formation. Additionally, as the season most susceptible to PM pollution, wintertime N2O5 formation in these regions was in an O3-limited or transition regime (Ma et al., 2023), wherein the elevated daytime O3 significantly enhanced NO3 radical generation, thereby promoting nocturnal N2O5 uptake and subsequent p formation. Conversely, in NO2-limited regions (e.g., southeastern China), NOx reduction showed limited impact on daytime p formation via the OH+NO2 pathway but effectively suppressed nighttime p formation via N2O5 uptake. This approach concurrently reduced ClNO2 formation from N2O5 heterogeneous processes, consequently diminishing next-day Cl radical generation and its positive feedback on O3 formation. Considering NOx reduction during the daytime would cause O3 formation and only a slight reduction in p, it is preferable to regulate NOx at night (18:00–06:00 LT the next day). Our findings demonstrate that in regions with a NO2-limited for p formation, targeted NOx reduction can synergistically decrease both p and O3 concentrations, highlighting the critical need to tailor mitigation strategies for different regions.
Our observations revealed a bimodal diurnal pattern of p in winter in urban Xiamen. The co-occurrence of elevated nighttime p levels with increased N2O5 implied a significant contribution of N2O5 uptake to p formation. Quantitative model analysis showed that N2O5 uptake contributed 51.2 % of the total p, which was comparable to the OH+NO2 reaction. This high contribution of N2O5 uptake to p is not commonly observed across Chinese cities. Comparative analysis among different cities suggests that this phenomenon is likely associated with NO2-limited conditions for N2O5 uptake in our study area. Machine learning results further demonstrated that p formation via N2O5 uptake was driven by nocturnal atmospheric oxidation capacity (PNO3) rather than heterogeneous uptake conditions. The underlying mechanism is that the weakened NOx titration effects lead to nighttime O3 accumulation, which promotes NO3 radical generation and consequently enhances N2O5 and p formation. The joint response of p and O3 to various NOx and VOCs emission scenarios indicated that p was more sensitive to NOx reduction than to VOCs reduction. However, mild NOx reduction showed limited effectiveness in reducing daytime p while simultaneously increasing O3 concentrations. Our findings suggest that NOx reduction is more effective when implemented during nighttime, particularly in regions where N2O5 formation is NO2-limited. This approach can effectively control p formation by suppressing nocturnal NO3 radical generation and consequently inhibiting N2O5 uptake, while simultaneously alleviate O3 pollution by reducing ClNO2 formation. With continuous NOx and VOCs emission reductions and renewable energy adoption in China, urban areas are transitioning from NOx-saturated to NOx-limited conditions, potentially increasing the importance of the N2O5 uptake pathway. In this context, comprehensive assessment of NOx reduction impacts on urban p and O3 pollution, along with the development of region-specific mitigation strategies, becomes critically important.
Data analysis methods are available from the authors upon request.
The dataset for this paper can be accessed at https://doi.org/10.6084/m9.figshare.29670629 (Lin et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-17747-2025-supplement.
ZL contributed to the methodology, data curation, software, analysis and writing of the original draft. LX and JC contributed to the conceptualization, investigation, data curation, reviewing and editing the text, supervision, and funding acquisition. Ch. Y, XJ, KZ, FZ, GC, LL, CY, YC, and ZC provided useful advice and revised the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We acknowledge the data contributed by the field campaign team.
This research has been supported by the National Natural Science Foundation of China (U22A20578), the guiding project of seizing the commanding heights of a “self-purifying city” (IUE-CERAE-202402), the National Key Research and Development Program (2022YFC3700304), the STS Plan Supporting Project of the Chinese Academy of Sciences in Fujian Province (2023T3013), and the Xiamen Atmospheric Environment Observation and Research Station of Fujian Province.
This paper was edited by Eleanor Browne and reviewed by two anonymous referees.
Atkinson, R. and Arey, J.: Atmospheric degradation of volatile organic compounds, Chem. Rev., 103, 4605–4638, https://doi.org/10.1021/cr0206420, 2003.
Brown, S. S. and Stutz, J.: Nighttime radical observations and chemistry, Chem. Soc. Rev., 41, 6405–6447, https://doi.org/10.1039/c2cs35181a, 2012.
Brown, S. S., Stark, H., and Ravishankara, A. R.: Applicability of the steady state approximation to the interpretation of atmospheric observations of NO3 and N2O5, J. Geophys. Res.-Atmos., 108, 4539, https://doi.org/10.1029/2003jd003407, 2003.
Chen, X., Wang, H., and Lu, K.: Interpretation of NO3−N2O5 observation via steady state in high-aerosol air mass: the impact of equilibrium coefficient in ambient conditions, Atmos. Chem. Phys., 22, 3525–3533, https://doi.org/10.5194/acp-22-3525-2022, 2022.
Chen, X., Ma, W., Zheng, F. X., Wang, Z. C., Hua, C. J., Li, Y. R., Wu, J., Li, B. D., Jiang, J. K., Yan, C., Petäjä, T., Bianchi, F., Kerminen, V. M., Worsnop, D. R., Liu, Y. C., Xia, M., and Kulmala, M.: Identifying Driving Factors of Atmospheric N2O5 with Machine Learning, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.4c00651, 2024.
Chen, X. R., Wang, H. C., Lu, K. D., Li, C. M., Zhai, T. Y., Tan, Z. F., Ma, X. F., Yang, X. P., Liu, Y. H., Chen, S. Y., Dong, H. B., Li, X., Wu, Z. J., Hu, M., Zeng, L. M., and Zhang, Y. H.: Field Determination of Nitrate Formation Pathway in Winter Beijing, Environ. Sci. Technol., 54, 9243–9253, https://doi.org/10.1021/acs.est.0c00972, 2020.
Cheng, C. L., Yang, S. X., Yuan, B., Pei, C. L., Zhou, Z. H., Mao, L. Y., Liu, S. L., Chen, D. Y., Cheng, X. Y., Li, M., Shao, M., and Zhou, Z.: The significant contribution of nitrate to a severe haze event in the winter of Guangzhou, China, Sci. Total Environ., 909, https://doi.org/10.1016/j.scitotenv.2023.168582, 2024.
Ehhalt, D. H. and Rohrer, F.: Dependence of the OH concentration on solar UV, J. Geophys. Res.-Atmos., 105, 3565–3571, https://doi.org/10.1029/1999jd901070, 2000.
Fu, X. X., Wang, X. M., Liu, T. Y., He, Q. F., Zhang, Z., Zhang, Y. L., Song, W., Dai, Q. W., Chen, S., and Dong, F. Q.: Secondary inorganic aerosols and aerosol acidity at different PM2.5 pollution levels during winter haze episodes in the Sichuan Basin, China, Sci. Total Environ., 918, https://doi.org/10.1016/j.scitotenv.2024.170512, 2024.
Gui, K., Che, H. Z., Zeng, Z. L., Wang, Y. Q., Zhai, S. X., Wang, Z. M., Luo, M., Zhang, L., Liao, T. T., Zhao, H. J., Li, L., Zheng, Y., and Zhang, X. Y.: Construction of a virtual PM2.5 observation network in China based on high-density surface meteorological observations using the Extreme Gradient Boosting model, Environ. Int., 141, https://doi.org/10.1016/j.envint.2020.105801, 2020.
Hersbach, H., Bell, B., Berrisford, P., Hirahara, S., Horányi, A., Muñoz-Sabater, J., Nicolas, J., Peubey, C., Radu, R., Schepers, D., Simmons, A., Soci, C., Abdalla, S., Abellan, X., Balsamo, G., Bechtold, P., Biavati, G., Bidlot, J., Bonavita, M., De Chiara, G., Dahlgren, P., Dee, D., Diamantakis, M., Dragani, R., Flemming, J., Forbes, R., Fuentes, M., Geer, A., Haimberger, L., Healy, S., Hogan, R. J., Hólm, E., Janisková, M., Keeley, S., Laloyaux, P., Lopez, P., Lupu, C., Radnoti, G., de Rosnay, P., Rozum, I., Vamborg, F., Villaume, S., and Thépaut, J. N.: The ERA5 global reanalysis, Q. J. Roy. Meteor. Soc., 146, 1999–2049, https://doi.org/10.1002/qj.3803, 2020.
Hu, H., Wang, H., Lu, K., Wang, J., Zheng, Z., Xu, X., Zhai, T., Chen, X., Lu, X., Fu, W., Li, X., Zeng, L., Hu, M., Zhang, Y., and Fan, S.: Variation and trend of nitrate radical reactivity towards volatile organic compounds in Beijing, China, Atmos. Chem. Phys., 23, 8211–8223, https://doi.org/10.5194/acp-23-8211-2023, 2023.
Huang, X., Ding, A. J., Wang, Z. L., Ding, K., Gao, J., Chai, F. H., and Fu, C. B.: Amplified transboundary transport of haze by aerosol-boundary layer interaction in China, Nat. Geosci., 13, 428–434, https://doi.org/10.1038/s41561-020-0583-4, 2020.
Jenkin, M. E., Young, J. C., and Rickard, A. R.: The MCM v3.3.1 degradation scheme for isoprene, Atmos. Chem. Phys., 15, 11433–11459, https://doi.org/10.5194/acp-15-11433-2015, 2015.
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., and Pozzer, A.: The contribution of outdoor air pollution sources to premature mortality on a global scale, Nature. 525, 367–371, https://doi.org/10.1038/nature15371, 2015.
Li, F. B., Huang, D. D., Nie, W., Tham, Y. J., Lou, S. R., Li, Y. Y., Tian, L. H., Liu, Y. L., Zhou, M., Wang, H. C., Qiao, L. P., Wang, H. L., Wang, Z., Huang, C., and Li, Y. J.: Observation of nitrogen oxide-influenced chlorine chemistry and source analysis of Cl2 in the Yangtze River Delta, China, Atmos. Environ., 306, https://doi.org/10.1016/j.atmosenv.2023.119829, 2023.
Li, M., Zhang, Z., Yao, Q., Wang, T., Xie, M., Li, S., Zhuang, B., and Han, Y.: Nonlinear responses of particulate nitrate to NOx emission controls in the megalopolises of China, Atmos. Chem. Phys., 21, 15135–15152, https://doi.org/10.5194/acp-21-15135-2021, 2021.
Lin, Z., Xu, L., Yang, C., Chen, G., Ji, X., Li, L., Zhang, K., Hong, Y., Li, M., Fan, X., Hu, B., Zhang, F., and Chen, J.: Trends of peroxyacetyl nitrate and its impact on ozone over 2018–2022 in urban atmosphere, npj Clim. Atmos. Sci., 7, 192, https://doi.org/10.1038/s41612-024-00746-7, 2024.
Lin, Z., Ying, C., Xu, L., Ji, X., Zhang, K., Zhang, F., Chen, G., Li, L., Yang, C., Chen, Y., Chen, Z., Chen, J. Data availability about the measurement report titled “Measurement report: High contribution of N2O5 uptake to particulate nitrate formation in NO2-limited urban areas”, figshare [data set], https://doi.org/10.6084/m9.figshare.29670629.v2, 2025.
Liu, M. X., Huang, X., Song, Y., Tang, J., Cao, J. J., Zhang, X. Y., Zhang, Q., Wang, S. X., Xu, T. T., Kang, L., Cai, X. H., Zhang, H. S., Yang, F. M., Wang, H. B., Yu, J. Z., Lau, A. K. H., He, L. Y., Huang, X. F., Duan, L., Ding, A. J., Xue, L. K., Gao, J., Liu, B., and Zhu, T.: Ammonia emission control in China would mitigate haze pollution and nitrogen deposition, but worsen acid rain, P. Natl. Acad. Sci. USA, 116, 7760–7765, https://doi.org/10.1073/pnas.1814880116, 2019.
Liu, T., Hong, Y., Li, M., Xu, L., Chen, J., Bian, Y., Yang, C., Dan, Y., Zhang, Y., Xue, L., Zhao, M., Huang, Z., and Wang, H.: Atmospheric oxidation capacity and ozone pollution mechanism in a coastal city of southeastern China: analysis of a typical photochemical episode by an observation-based model, Atmos. Chem. Phys., 22, 2173–2190, https://doi.org/10.5194/acp-22-2173-2022, 2022.
Liu, Y., Wang, Y., Ma, P., Ma, Y., Pan, Y., Ma, W., Li, S., Liu, P., Liao, Z., Liu, Z., Chu, B., Ma, Q., Quan, J., and He, H.: Formation of Nitrate in the Residual Layer of Beijing: Pathways Evaluation and Contributions to the Ground Level, Environ. Sci. Technol., 59, 9699–9708, https://doi.org/10.1021/acs.est.5c02981, 2025.
Ma, P. K., Quan, J. N., Dou, Y. J., Pan, Y. B., Liao, Z. H., Cheng, Z. G., Jia, X. C., Wang, Q. Q., Zhan, J. L., Ma, W., Zheng, F. X., Wang, Y. Z., Zhang, Y. S., Hua, C. J., Yan, C., Kulmala, M., Liu, Y. A., Huang, X., Yuan, B., Brown, S. S., and Liu, Y. C.: Regime-Dependence of Nocturnal Nitrate Formation via N2O5 Hydrolysis and Its Implication for Mitigating Nitrate Pollution, Geophys. Res. Lett., 50, https://doi.org/10.1029/2023gl106183, 2023.
Mao, J. Y., Yan, F. H., Zheng, L. M., You, Y. C., Wang, W. W., Jia, S. G., Liao, W. H., Wang, X. M., and Chen, W. H.: Ozone control strategies for local formation- and regional transport-dominant scenarios in a manufacturing city in southern China, Sci. Total Environ., 813, https://doi.org/10.1016/j.scitotenv.2021.151883, 2022.
McDuffie, E. E., Fibiger, D. L., Dubé, W. P., Hilfiker, F. L., Lee, B. H., Jaeglé, L., Guo, H. Y., Weber, R. J., Reeves, J. M., Weinheimer, A. J., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Dibb, J. E., Veres, P., Ebben, C., Sparks, T. L., Wooldridge, P. J., Cohen, R. C., Campos, T., Hall, S. R., Ullmann, K., Roberts, J. M., Thornton, J. A., and Brown, S. S.: ClNO2 Yields From Aircraft Measurements During the 2015 WINTER Campaign and Critical Evaluation of the Current Parameterization, J. Geophys. Res.-Atmos., 123, 12994–13015, https://doi.org/10.1029/2018jd029358, 2018a.
McDuffie, E. E., Fibiger, D. L., Dubé, W. P., Lopez-Hilfiker, F., Lee, B. H., Thornton, J. A., Shah, V., Jaeglé, L., Guo, H. Y., Weber, R. J., Reeves, J. M., Weinheimer, A. J., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Dibb, J. E., Veres, P., Ebben, C., Sparks, T. L., Wooldridge, P. J., Cohen, R. C., Hornbrook, R. S., Apel, E. C., Campos, T., Hall, S. R., Ullmann, K., and Brown, S. S.: Heterogeneous N2O5 Uptake During Winter: Aircraft Measurements During the 2015 WINTER Campaign and Critical Evaluation of Current Parameterizations, J. Geophys. Res.-Atmos., 123, 4345–4372, https://doi.org/10.1002/2018jd028336, 2018b.
McDuffie, E. E., Womack, C. C., Fibiger, D. L., Dube, W. P., Franchin, A., Middlebrook, A. M., Goldberger, L., Lee, B. H., Thornton, J. A., Moravek, A., Murphy, J. G., Baasandorj, M., and Brown, S. S.: On the contribution of nocturnal heterogeneous reactive nitrogen chemistry to particulate matter formation during wintertime pollution events in Northern Utah, Atmos. Chem. Phys., 19, 9287–9308, https://doi.org/10.5194/acp-19-9287-2019, 2019.
Morgan, W. T., Ouyang, B., Allan, J. D., Aruffo, E., Di Carlo, P., Kennedy, O. J., Lowe, D., Flynn, M. J., Rosenberg, P. D., Williams, P. I., Jones, R., McFiggans, G. B., and Coe, H.: Influence of aerosol chemical composition on N2O5 uptake: airborne regional measurements in northwestern Europe, Atmos. Chem. Phys., 15, 973–990, https://doi.org/10.5194/acp-15-973-2015, 2015.
Niu, Y. B., Zhu, B., He, L. Y., Wang, Z., Lin, X. Y., Tang, M. X., and Huang, X. F.: Fast Nocturnal Heterogeneous Chemistry in a Coastal Background Atmosphere and Its Implications for Daytime Photochemistry, J. Geophys. Res.-Atmos., 127, https://doi.org/10.1029/2022jd036716, 2022.
Requia, W. J., Di, Q., Silvern, R., Kelly, J. T., Koutrakis, P., Mickley, L. J., Sulprizio, M. P., Amini, H., Shi, L. H., and Schwartz, J.: An Ensemble Learning Approach for Estimating High Spatiotemporal Resolution of Ground-Level Ozone in the Contiguous United States, Environ. Sci. Technol., 54, 11037–11047, https://doi.org/10.1021/acs.est.0c01791, 2020.
Seinfeld, J. H.: Urban air-pollution – state of the science, Science, 243, 745–752, https://doi.org/10.1126/science.243.4892.745, 1989.
Sun, J., Qin, M., Xie, X., Fu, W., Qin, Y., Sheng, L., Li, L., Li, J., Sulaymon, I. D., Jiang, L., Huang, L., Yu, X., and Hu, J.: Seasonal modeling analysis of nitrate formation pathways in Yangtze River Delta region, China, Atmos. Chem. Phys., 22, 12629–12646, https://doi.org/10.5194/acp-22-12629-2022, 2022.
Thaler, R. D., Mielke, L. H., and Osthoff, H. D.: Quantification of Nitryl Chloride at Part Per Trillion Mixing Ratios by Thermal Dissociation Cavity Ring-Down Spectroscopy, Anal. Chem., 83, 2761–2766, https://doi.org/10.1021/ac200055z, 2011.
Tham, Y. J., Wang, Z., Li, Q., Yun, H., Wang, W., Wang, X., Xue, L., Lu, K., Ma, N., Bohn, B., Li, X., Kecorius, S., Größ, J., Shao, M., Wiedensohler, A., Zhang, Y., and Wang, T.: Significant concentrations of nitryl chloride sustained in the morning: investigations of the causes and impacts on ozone production in a polluted region of northern China, Atmos. Chem. Phys., 16, 14959–14977, https://doi.org/10.5194/acp-16-14959-2016, 2016.
Tham, Y. J., Wang, Z., Li, Q., Wang, W., Wang, X., Lu, K., Ma, N., Yan, C., Kecorius, S., Wiedensohler, A., Zhang, Y., and Wang, T.: Heterogeneous N2O5 uptake coefficient and production yield of ClNO2 in polluted northern China: roles of aerosol water content and chemical composition, Atmos. Chem. Phys., 18, 13155–13171, https://doi.org/10.5194/acp-18-13155-2018, 2018.
Wagner, N. L., Riedel, T. P., Young, C. J., Bahreini, R., Brock, C. A., Dubé, W. P., Kim, S., Middlebrook, A. M., Öztürk, F., Roberts, J. M., Russo, R., Sive, B., Swarthout, R., Thornton, J. A., VandenBoer, T. C., Zhou, Y., and Brown, S. S.: N2O5 uptake coefficients and nocturnal NO2 removal rates determined from ambient wintertime measurements, J. Geophys. Res.-Atmos., 118, 9331–9350, https://doi.org/10.1002/jgrd.50653, 2013.
Wang, H., Lu, K., Guo, S., Wu, Z., Shang, D., Tan, Z., Wang, Y., Le Breton, M., Lou, S., Tang, M., Wu, Y., Zhu, W., Zheng, J., Zeng, L., Hallquist, M., Hu, M., and Zhang, Y.: Efficient N2O5 uptake and NO3 oxidation in the outflow of urban Beijing, Atmos. Chem. Phys., 18, 9705–9721, https://doi.org/10.5194/acp-18-9705-2018, 2018.
Wang, H., Peng, C., Wang, X., Lou, S., Lu, K., Gan, G., Jia, X., Chen, X., Chen, J., Wang, H., Fan, S., Wang, X., and Tang, M.: N2O5 uptake onto saline mineral dust: a potential missing source of tropospheric ClNO2 in inland China, Atmos. Chem. Phys., 22, 1845–1859, https://doi.org/10.5194/acp-22-1845-2022, 2022a.
Wang, H., Yuan, B., Zheng, E., Zhang, X., Wang, J., Lu, K., Ye, C., Yang, L., Huang, S., Hu, W., Yang, S., Peng, Y., Qi, J., Wang, S., He, X., Chen, Y., Li, T., Wang, W., Huangfu, Y., Li, X., Cai, M., Wang, X., and Shao, M.: Formation and impacts of nitryl chloride in Pearl River Delta, Atmos. Chem. Phys., 22, 14837–14858, https://doi.org/10.5194/acp-22-14837-2022, 2022b.
Wang, H. C., Lu, K. D., Chen, X. R., Zhu, Q. D., Chen, Q., Guo, S., Jiang, M. Q., Li, X., Shang, D. J., Tan, Z. F., Wu, Y. S., Wu, Z. J., Zou, Q., Zheng, Y., Zeng, L. M., Zhu, T., Hu, M., and Zhang, Y. H.: High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway, Environ. Sci. Technol. Letters. 4, 416–420, https://doi.org/10.1021/acs.estlett.7b00341, 2017.
Wang, H. C., Lu, K. D., Chen, S. Y., Li, X., Zeng, L. M., Hu, M., and Zhang, Y. H.: Characterizing nitrate radical budget trends in Beijing during 2013–2019, Sci. Total Environ., 795, https://doi.org/10.1016/j.scitotenv.2021.148869, 2021.
Wang, W. J., Li, X., Cheng, Y. F., Parrish, D. D., Ni, R. J., Tan, Z. F., Liu, Y., Lu, S. H., Wu, Y. S., Chen, S. Y., Lu, K. D., Hu, M., Zeng, L. M., Shao, M., Huang, C., Tian, X. D., Leung, K. M., Chen, L. F., Fan, M., Zhang, Q., Rohrer, F., Wahner, A., Pöschl, U., Su, H., and Zhang, Y. H.: Ozone pollution mitigation strategy informed by long-term trends of atmospheric oxidation capacity, Nat. Geosci., 16, 1080–1081, https://doi.org/10.1038/s41561-023-01334-9, 2023a.
Wang, Y. H., Gao, W. K., Wang, S., Song, T., Gong, Z. Y., Ji, D. S., Wang, L. L., Liu, Z. R., Tang, G. Q., Huo, Y. F., Tian, S. L., Li, J. Y., Li, M. G., Yang, Y., Chu, B. W., Petäjä, T., Kerminen, V. M., He, H., Hao, J. M., Kulmala, M., Wang, Y. S., and Zhang, Y. H.: Contrasting trends of PM2.5 and surface-ozone concentrations in China from 2013 to 2017, Natl. Sci. Rev., 7, 1331–1339, https://doi.org/10.1093/nsr/nwaa032, 2020.
Wang, Y. R., Yang, X. Y., Wu, K., Mei, H., De Smedt, I., Wang, S. G., Fan, J., Lyu, S., and He, C.: Long-term trends of ozone and precursors from 2013 to 2020 in a megacity (Chengdu), China: Evidence of changing emissions and chemistry, Atmos. Res., 278, https://doi.org/10.1016/j.atmosres.2022.106309, 2022c.
Wang, Y. T., Zhao, Y., Liu, Y. M., Jiang, Y. Q., Zheng, B., Xing, J., Liu, Y., Wang, S., and Nielsen, C. P.: Sustained emission reductions have restrained the ozone pollution over China, Nat. Geosci., 16, 967–974, https://doi.org/10.1038/s41561-023-01284-2, 2023b.
Wen, L., Xue, L., Wang, X., Xu, C., Chen, T., Yang, L., Wang, T., Zhang, Q., and Wang, W.: Summertime fine particulate nitrate pollution in the North China Plain: increasing trends, formation mechanisms and implications for control policy, Atmos. Chem. Phys., 18, 11261–11275, https://doi.org/10.5194/acp-18-11261-2018, 2018.
Wolfe, G. M., Marvin, M. R., Roberts, S. J., Travis, K. R., and Liao, J.: The Framework for 0-D Atmospheric Modeling (F0AM) v3.1, Geosci. Model Dev., 9, 3309–3319, https://doi.org/10.5194/gmd-9-3309-2016, 2016.
Xie, X. D., Hu, J. L., Qin, M. M., Guo, S., Hu, M., Wang, H. L., Lou, S. R., Li, J. Y., Sun, J. J., Li, X., Sheng, L., Zhu, J. L., Chen, G. Y., Yin, J. J., Fu, W. X., Huang, C., and Zhang, Y. H.: Modeling particulate nitrate in China: Current findings and future directions, Environ. Int., 166, https://doi.org/10.1016/j.envint.2022.107369, 2022.
Xing, J., Ding, D., Wang, S., Zhao, B., Jang, C., Wu, W., Zhang, F., Zhu, Y., and Hao, J.: Quantification of the enhanced effectiveness of NOx control from simultaneous reductions of VOC and NH3 for reducing air pollution in the Beijing–Tianjin–Hebei region, China, Atmos. Chem. Phys., 18, 7799–7814, https://doi.org/10.5194/acp-18-7799-2018, 2018.
Yan, C., Tham, Y. J., Nie, W., Xia, M., Wang, H. C., Guo, Y. S., Ma, W., Zhan, J. L., Hua, C. J., Li, Y. Y., Deng, C. J., Li, Y. R., Zheng, F. X., Chen, X., Li, Q. Y., Zhang, G., Mahajan, A. S., Cuevas, C. A., Huang, D. D., Wang, Z., Sun, Y. L., Saiz-Lopez, A., Bianchi, F., Kerminen, V. M., Worsnop, D. R., Donahue, N. M., Jiang, J. K., Liu, Y. C., Ding, A. J., and Kulmala, M.: Increasing contribution of nighttime nitrogen chemistry to wintertime haze formation in Beijing observed during COVID-19 lockdowns, Nat. Geosci., 16, 975–981, https://doi.org/10.1038/s41561-023-01285-1, 2023.
Yang, C., Dong, H. S., Chen, Y. P., Xu, L. L., Chen, G. J., Fan, X. L., Wang, Y. H., Tham, Y. J., Lin, Z. Y., Li, M. R., Hong, Y. W., and Chen, J. S.: New Insights on the Formation of Nucleation Mode Particles in a Coastal City Based on a Machine Learning Approach, Environ. Sci. Technol., 58, 1187–1198, https://doi.org/10.1021/acs.est.3c07042, 2023.
Yang, S., Yuan, B., Peng, Y., Huang, S., Chen, W., Hu, W., Pei, C., Zhou, J., Parrish, D. D., Wang, W., He, X., Cheng, C., Li, X.-B., Yang, X., Song, Y., Wang, H., Qi, J., Wang, B., Wang, C., Wang, C., Wang, Z., Li, T., Zheng, E., Wang, S., Wu, C., Cai, M., Ye, C., Song, W., Cheng, P., Chen, D., Wang, X., Zhang, Z., Wang, X., Zheng, J., and Shao, M.: The formation and mitigation of nitrate pollution: comparison between urban and suburban environments, Atmos. Chem. Phys., 22, 4539–4556, https://doi.org/10.5194/acp-22-4539-2022, 2022.
Yu, C., Wang, Z., Xia, M., Fu, X., Wang, W., Tham, Y. J., Chen, T., Zheng, P., Li, H., Shan, Y., Wang, X., Xue, L., Zhou, Y., Yue, D., Ou, Y., Gao, J., Lu, K., Brown, S. S., Zhang, Y., and Wang, T.: Heterogeneous N2O5 reactions on atmospheric aerosols at four Chinese sites: improving model representation of uptake parameters, Atmos. Chem. Phys., 20, 4367–4378, https://doi.org/10.5194/acp-20-4367-2020, 2020.
Yun, H., Wang, W., Wang, T., Xia, M., Yu, C., Wang, Z., Poon, S. C. N., Yue, D., and Zhou, Y.: Nitrate formation from heterogeneous uptake of dinitrogen pentoxide during a severe winter haze in southern China, Atmos. Chem. Phys., 18, 17515–17527, https://doi.org/10.5194/acp-18-17515-2018, 2018.
Zhai, S. X., Jacob, D. J., Wang, X., Liu, Z. R., Wen, T. X., Shah, V., Li, K., Moch, J. M., Bates, K. H., Song, S. J., Shen, L., Zhang, Y. Z., Luo, G., Yu, F. Q., Sun, Y. L., Wang, L. T., Qi, M. Y., Tao, J., Gui, K., Xu, H. H., Zhang, Q., Zhao, T. L., Wang, Y. S., Lee, H. C., Choi, H., and Liao, H.: Control of particulate nitrate air pollution in China, Nat. Geosci., 14, 389–395, https://doi.org/10.1038/s41561-021-00726-z, 2021.
Zhai, T., Lu, K., Wang, H., Lou, S., Chen, X., Hu, R., and Zhang, Y.: Elucidate the formation mechanism of particulate nitrate based on direct radical observations in the Yangtze River Delta summer 2019, Atmos. Chem. Phys., 23, 2379–2391, https://doi.org/10.5194/acp-23-2379-2023, 2023.
Zhang, R., Han, Y. H., Shi, A. J., Sun, X. S., Yan, X., Huang, Y. H., and Wang, Y.: Characteristics of ambient ammonia and its effects on particulate ammonium in winter of urban Beijing, China, Environ. Sci. Pollut. R., 28, 62828–62838, https://doi.org/10.1007/s11356-021-14108-w, 2021.
Zhang, X., Ma, Q., Chu, W. H., Ning, M., Liu, X. Q., Xiao, F. J., Cai, N. N., Wu, Z. J., and Yan, G.: Identify the key emission sources for mitigating ozone pollution: A case study of urban area in the Yangtze River Delta region, China, Sci. Total Environ., 892, https://doi.org/10.1016/j.scitotenv.2023.164703, 2023a.
Zhang, Y., Lei, R., Cui, S., Wang, H., Chen, M., and Ge, X.: Spatiotemporal trends and impact factors of PM2.5 and O3 pollution in major cities in China during 2015–2020, Chinese Sci. Bull., 67, 2029–2042, 2022.
Zhang, Y. N., Wang, H. L., Huang, L. B., Qiao, L. P., Zhou, M., Mu, J. S., Wu, C., Zhu, Y. J., Shen, H. Q., Huang, C., Wang, G. H., Wang, T., Wang, W. X., and Xue, L. K.: Double-Edged Role of VOCs Reduction in Nitrate Formation: Insights from Observations during the China International Import Expo 2018, Environ. Sci. Technol., 57, 15979–15989, https://doi.org/10.1021/acs.est.3c04629, 2023b.
Zhao, S. P., Yin, D. Y., Yu, Y., Kang, S. C., Qin, D. H., and Dong, L. X.: PM2.5 and O3 pollution during 2015–2019 over 367 Chinese cities: Spatiotemporal variations, meteorological and topographical impacts, Environ. Pollut., 264, https://doi.org/10.1016/j.envpol.2020.114694, 2020.
Zhao, X. X., Zhao, X. J., Liu, P. F., Chen, D., Zhang, C. L., Xue, C. Y., Liu, J. F., Xu, J., and Mu, Y. J.: Transport Pathways of Nitrate Formed from Nocturnal N2O5 Hydrolysis Aloft to the Ground Level in Winter North China Plain, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.3c00086, 2023.
Zhou, M., Nie, W., Qiao, L. P., Huang, D. D., Zhu, S. H., Lou, S. R., Wang, H. L., Wang, Q., Tao, S. K., Sun, P., Liu, Y. W., Xu, Z., An, J. Y., Yan, R. S., Su, H., Huang, C., Ding, A. J., and Chen, C. H.: Elevated Formation of Particulate Nitrate From N2O5 Hydrolysis in the Yangtze River Delta Region From 2011 to 2019, Geophys. Res. Lett., 49, https://doi.org/10.1029/2021gl097393, 2022.
Zong, Z., Tian, C. G., Sun, Z. Y., Tan, Y., Shi, Y. J., Liu, X. H., Li, J., Fang, Y. T., Chen, Y. J., Ma, Y. H., Gao, H. W., Zhang, G., and Wang, T.: Long-Term Evolution of Particulate Nitrate Pollution in North China: Isotopic Evidence From 10 Offshore Cruises in the Bohai Sea From 2014 to 2019, J. Geophys. Res.-Atmos., 127, https://doi.org/10.1029/2022jd036567, 2022.
