the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Quantification and characterization of primary biological aerosol particles and microbes aerosolized from Baltic seawater
Gabriel Pereira Freitas
Rachel Ann Foster
Paul Zieger
Ernst Douglas Nilsson
Piotr Markuszewski
Matthew Edward Salter
Primary biological aerosol particles (PBAPs) can influence the climate and affect human health. To investigate the aerosolization of PBAPs by sea spray aerosol (SSA), we conducted ship-based campaigns in the central Baltic Sea near Östergarnsholm in May and August 2021. Using a plunging-jet sea spray simulation chamber filled with local seawater, we performed controlled chamber experiments to collect filters and measure aerosols. We determined the abundance of microbial cells in the chamber air and seawater using staining and fluorescence microscopy, normalizing these values to sodium concentrations to calculate enrichment factors. Our results showed that microbes were enriched in the aerosol by 13 to 488 times compared to the underlying seawater, with no significant enrichment observed in the sea surface microlayer. Microbial abundances obtained through microscopy were compared with estimates of fluorescent PBAPs (fPBAPs) using a single-particle fluorescence spectrometer. We estimated microbial emission fluxes using two independent approaches: (1) applying the enrichment factors derived from this study with mass flux estimates from previous SSA parameterizations and (2) using a scaling approach from a companion study. Both methods produced microbial emission flux estimates that were in good agreement and of the same order of magnitude as previous studies, while fPBAP emission flux estimates were significantly lower. Furthermore, 16S rRNA sequencing identified the diversity of bacteria enriched in the nascent SSA compared to the underlying seawater.
- Article
(3067 KB) - Full-text XML
-
Supplement
(2856 KB) - BibTeX
- EndNote
Primary biological aerosol particles (PBAPs) are airborne particles that include microbes (bacteria, archaea, and picoeukaryotes), viruses, pollen, and spores, which can exist as agglomerates, single particles, or cell fractions. Before aerosolization, these particles are referred to as microorganisms. Although PBAPs constitute less than 0.1 % of aerosol particles by number (Mayol et al., 2017), they play a significant role in the chemical and biological composition of aerosols. For instance, PBAPs are efficient cloud condensation nuclei and ice nuclei (Wilson et al., 2015; DeMott et al., 2016; Šantl-Temkiv et al., 2019), potentially affecting cloud properties such as phase, albedo, and lifetime and thus influencing Earth's climate and biogeochemical cycles (Fröhlich-Nowoisky et al., 2016). Additionally, PBAPs can impact human health and well-being (Genitsaris et al., 2011; Smets et al., 2016; May et al., 2018), though their specific effects remain inadequately understood (Alsante et al., 2021).
Once aerosolized, PBAPs can be transported over hundreds to thousands of kilometres, with residence times ranging from days to weeks (Mayol et al., 2017). The transport and residence times of PBAPs depend on their size, density, and shape (Tesson and Šantl-Temkiv, 2018) as well as atmospheric conditions such as wind speed, direction, and precipitation. The impact of marine PBAPs on ecosystems is influenced by their fluxes between the ocean and atmosphere, their atmospheric transport distance and altitude, and their viability during transport (Alsante et al., 2021). Therefore, it is crucial to better characterize the air–sea exchange of marine microorganisms, their dispersal in the atmosphere, and their ability to adapt to atmospheric conditions.
PBAPs from the oceans are released into the atmosphere along with sea spray aerosol (SSA) (Després et al., 2012), a significant natural aerosol source. SSA forms when waves break and trap air as bubbles in seawater. These bubbles rise to the surface, scavenge biogenic material, and burst at the surface, generating film drops from the disintegration of the bubble film cap and jet drops from the collapse of the bubble cavity (Blanchard, 1963, 1983; Lewis and Schwartz, 2004). Film drops are numerous, are typically smaller than <1 µm, and are enriched in organic materials from the surface microlayer (SML) such as cell fragments and small microorganisms, e.g. bacteria and viral particles (Blanchard and Syzdek, 1982; Rastelli et al., 2017; Michaud et al., 2018). The SML has a distinct microbial community composition and hydrographic conditions compared to the underlying water (Franklin et al., 2005; Joux et al., 2006; Cunliffe et al., 2009; Stolle et al., 2011). In contrast, jet drops have a radius that is typically larger than 1 µm and consist mainly of sea salt, water-soluble organics, and larger microorganisms from underlying waters (Wang et al., 2017).
Our understanding of the atmospheric abundance and diversity of PBAPs over open oceans is limited due to spatial and temporal sampling constraints. Additionally, the low concentration of PBAPs necessitates either high sample flows or long sampling times to obtain sufficient biomass for downstream analyses such as microscopy, flow cytometry, or DNA sequencing. Consequently, the role of oceans as a source or sink of PBAPs is not fully resolved (Burrows et al., 2009b, a; Amato et al., 2023), particularly regarding microbial emission fluxes in remote open-ocean and coastal regions (Amato et al., 2023).
Estimates of microbial emission fluxes from the oceans vary, with a global average of around 60 cells m−2s−1 (Burrows et al., 2009b) and regional studies showing fluxes between 10 and 100 cells m−2s−1 (Mayol et al., 2014, 2017; Hu et al., 2017). However, direct measurements of microbial emission fluxes are rare due to the lack of suitable bioaerosol measurement techniques for traditional eddy covariance flux measurements. Recent advancements in real-time single-particle analysis instruments using ultraviolet-light-induced fluorescence allow continuous monitoring of fluorescent PBAPs (fPBAPs) (e.g. Huffman et al., 2020; Santander et al., 2021; Freitas et al., 2022). Examples of real-time single-particle analysis instruments are the Wideband Integrated Bioaerosol Sensor (WIBS), the Multiparameter Bioaerosol Sensor (MBS; Ruske et al., 2017), the Spectral Intensity Bioaerosol Spectrometer (SIBS; Könemann et al., 2019), the PA-300 (Kiselev et al., 2013), and the Rapid-E (Šaulienė et al., 2019). Despite this progress, these instruments are limited to detecting particles larger than some microorganisms (e.g. bacteria as small as 0.2 µm in diameter; Schulz and Jørgensen, 2001) and may underestimate the total PBAP abundance. Additionally, these instruments cannot provide information on the diversity of microbial community composition, which is important because studies have shown significant variations in airborne microbial communities across different oceanic regions (Seifried et al., 2015; Michaud et al., 2018; Mayol et al., 2017; Lang-Yona et al., 2022). Comprehensive global studies of the diversity of airborne microbes, especially at pristine marine sites, are still lacking. Currently, only one study of global airborne microbial communities exists, and it does not include pristine marine sampling sites (Tignat-Perrier et al., 2019).
Previous mesocosm studies have shown that microbes are significantly enriched in SSA compared to underlying seawater (Carlucci and Williams, 1965; Cipriano, 1979; Blanchard and Syzdek, 1972, 1982; Marks et al., 2001; Aller et al., 2005; Rastelli et al., 2017; Zinke et al., 2024b), with enrichment factors ranging between 10 and 2500. Advancements in both culture-based and culture-independent approaches have provided new insights into airborne microbial communities. Certain microbial taxa, particularly those with hydrophobic surface properties, have been found to be selectively aerosolized (Fahlgren et al., 2015; Rastelli et al., 2017; Perrott et al., 2017; Michaud et al., 2018; Freitas et al., 2022; Zinke et al., 2024b). These hydrophobic properties may enhance their transport to the sea surface and their inclusion in SSA. Fahlgren et al. (2015) suggested that pigmentation might also influence selective aerosolization by affecting surface properties. Marks et al. (2019) suggested that the enrichment of bacteria and diatoms in SSA could result from anionic bubble surfaces attracting cells with typically negative charges on their outer membranes. When these bubbles reach the water surface and burst, the collected microorganisms are ejected into the air, with the initial or secondary jet droplets projected upward from the sub-bubble cationic vortex.
In this study, we conducted two ship-based campaigns in the Baltic Sea to investigate the air–sea exchange of fPBAPs in SSA using chamber experiments. We aimed to quantify the contribution of marine fPBAPs to the atmospheric aerosol load and identify bacterial taxa preferentially aerosolized by SSA. We employed filter-based sampling for airborne microbes and continuous fPBAP measurements, calculated microbial enrichment factors in SSA compared to subsurface seawater, and used two approaches to estimate microbial emission fluxes from coastal Baltic Sea areas. Additionally, 16S rRNA gene sequencing of subsurface seawater, SML samples, and chamber-generated SSA samples allowed us to investigate the enrichment of specific bacterial taxa in the SML and aerosol relative to underlying seawater.
2.1 Experimental set-up and sample collection
Aerosol and seawater samples were collected during two cruises in the Baltic Sea on board the Research Vessel (R/V) Oceania (18 to 29 May 2021) and R/V Electra (9 to 22 August 2021). Both ships were stationed near the Integrated Carbon Observation System (ICOS) eddy covariance flux station on Östergarnsholm, east of Gotland (57°25′48.4" N, 18°59′02.9" E). In addition to the station at Östergarnsholm (between 19 and 24 May), R/V Oceania also conducted a transect through the Baltic Proper (Fig. S1 in the Supplement).
Nascent SSA was generated using a plunging-jet sea spray simulation chamber (total volume of 200 L) filled with local seawater (volume of 90 L) from the ship's flow-through system at a depth of 1.5 m. This chamber, described in detail in Salter et al. (2014), allowed us to collect nascent SSA on filters and conduct online aerosol measurements under controlled conditions, excluding terrestrial sources, while also monitoring seawater properties. To prevent contamination from ambient air, the chamber headspace was continuously flushed with particle-free air generated by a dry-air generator equipped with a HEPA filter (Kaeser, model Dental T, Germany, during the R/V Oceania campaign and Dürr Dental SE, model Silver Airline Trio 160 lpm, Germany, during the R/V Electra campaign). The chamber can operate in flow-through mode, continuously replacing seawater from the ship's seawater inlet. However, during the second campaign, whenever R/V Electra had to leave its anchored position and return to harbour, the chamber operated in closed mode, meaning that the seawater was recirculated in the chamber until the ship was back at its station.
During the R/V Oceania campaign, surface seawater salinity and temperature were measured with a thermosalinograph (SBE21, Sea-Bird Scientific, USA), and dissolved oxygen in the water was measured with an oxygen meter (Fibox 4 trace, PreSens Precision Sensing GmbH, Germany), in line with the flow-through system. During the R/V Electra campaign, seawater salinity and temperature were measured with a conductivity sensor (Aanderaa 4120, Norway), and dissolved oxygen was measured with an optode (Aanderaa 4175, Norway) inside the chamber. The daily average concentrations of chlorophyll a in the seawater at the locations where the ships were anchored close to Östergarnsholm were determined using re-analysis data (Woźniak et al., 2011a, b; Konik et al., 2019).
Bulk aerosol samples were collected using filters attached to the sea spray simulation chamber, positioned approximately 45 cm above the water surface. Samples for cell enumeration and ion chromatography were collected on black polycarbonate filters (pore size 0.2 µm and diameter 25 mm; Merck Millipore Ltd., Ireland), while samples for DNA analysis were collected on Supor filters (0.2 µm pore size and diameter 25 mm; Pall Corporation, USA). Aerosol filter samples were collected for 24 h at a flow rate of 5 L min−1 controlled by mass flow controllers (hereafter referred to as MFCs, model 5850E, Brooks Instrument, USA).
Water samples for cell enumeration (100 mL), ion chromatography (10 mL), and DNA analysis (500 mL) were collected each time the aerosol filters were exchanged. Additionally, SML samples (500 mL total volume) for cell enumeration, ion chromatography, and DNA analysis were collected once a day during the R/V Oceania campaign by lowering a Garett screen (Garrett, 1965) over the side of the ship. During the R/V Electra campaign, SML samples (500 mL) were collected from a smaller boat using a glass plate (20×35 cm) and a squeegee (Harvey and Burzell, 1972). Glass-plate samplers typically have sampling depths of 20–150 µm, while mesh screen samplers have sampling depths of 150–400 µm (Cunliffe and Wurl, 2017).
Low biomass in aerosol samples, as collected in this study, poses a risk of contamination, making it crucial to ensure sterilized conditions, use sterile techniques, and take operational blanks to verify that measurements are not biased by contamination (Šantl-Temkiv et al., 2020; Dommergue et al., 2019). To ensure sterility, the sampling equipment (sampling bottles, filter holders, and tubes) was autoclaved prior to each campaign. Additionally, equipment used repeatedly was cleaned in a 10 % bleach bath after each sampling time. Handling blanks were collected throughout the campaigns for each analysis method. This involved placing the filters in their respective filter holders without drawing any air through them and immediately removing them again. All the samples were stored at −20 °C until further analysis.
A total of 11 filter samples were collected each during the R/V Oceania and Electra campaigns. However, due to poor staining, cells could not confidently be enumerated during the R/V Oceania campaign, so these samples will only be discussed in terms of microbial community composition. Conversely, samples collected during the R/V Electra campaign will be discussed in terms of cell abundance, enrichment, and community composition. For more details, an overview of all the samples and methods applied to each sample can be found in Tables S1 and S2 in the Supplement.
2.2 Cell enumeration
During the R/V Electra campaign, the filter samples designated for cell enumeration underwent immediate sonication in a sonicator bath (model 1510, Branson Ultrasonics, USA) at 40 Hz for 1 min in 5 mL of ultrapure water to extract the cells from the filters. An aliquot of 0.5 mL was reserved for ion chromatography analysis. The remaining suspension was chemically fixed with 4 % paraformaldehyde solution (w:v) for 45 min and stained with 4',6-diamidino-2-phenylindole (DAPI) at a concentration of 10 µg mL−1 for 15 min. The stained suspension was then filtered through another black polycarbonate filter (0.2 µm pore size, 25 mm diameter; Merck Millipore Ltd., Ireland) rinsed with phosphate-buffered saline (1X PBS, pH 7.4). Subsequently, the filters were mounted on slides using an anti-fade solution (Epredia Lab Vision PermaFluor Aqueous Mounting Medium, Fisher Scientific, Sweden) along with cover slips and stored at −20 °C until enumeration was conducted on land. To assess the extraction efficiency and account for cells that may have remained on the extracted filter, these were also fixed, stained, and analysed alongside the extracts. Similarly, handling blank filters were processed, following the same steps as for the aerosol filters. Additionally, up to 100 mL of bulk seawater samples and SML samples were fixed, stained, and filtered and the resulting filters mounted on slides and stored at −20 °C. Details regarding the exact seawater volume that passed through each filter are available in Tables S1 and S2.
For enumeration, a fluorescence microscope (BX60, Olympus Corporation, Japan) equipped with a UV filter set (excitation wavelength approximately 365 nm) was used. Counting of positively stained cells was performed at 1000 times magnification and used the FIJI software (Schindelin et al., 2012). Positively stained cells (including bacteria, fragments, spores, and large viruses) were counted in 20 random fields per sample, corresponding to an area of 0.3 mm2 of the filter, to ensure reliable counting statistics.
2.3 Inorganic ion analysis
The extracted aerosol and seawater samples were analysed for their ionic composition using a Dionex Aquion IC system (Thermo Fisher Scientific, USA). An AS22 column (eluent 20 mM methanesulfonic acid pumped at 1 mL min−1) and a CS12A column (eluent 1.5 mM NaHCO3 and 4.5 mM Na2CO3 pumped at 1.2 mL min−1) were used to determine the concentrations of major anions (chloride, Cl−; sulfate, SO) and cations (sodium, Na+; potassium, K+; magnesium, Mg2+; calcium, Ca2+), respectively. The injected volume was 25 µL. Seawater samples were diluted 1:20 with ultrapure water to match the analytical range of the aerosol extracts.
To ensure analytical quality, certified reference samples (QC DWB, Eurofins Miljø Luft A/S, Denmark) were used for checks. Systematic errors were less than 2 % for all ionic components, except for Ca2+, which had an error of less than 3 %. Random errors were approximately 0.1 % for samples analysed after the R/V Oceania campaign and slightly higher after the R/V Electra campaign (0.2 %, 1.3 %, 0.5 %, 0.2 %, 3.4 %, and 3.2 % for Na+, K+, Mg2+, Ca2+, Cl−, and SO, respectively).
The limits of detection (LODs), defined as 3 standard deviations of the ultrapure water blanks, were 0.006, 0, 0.001, 0.057, and 0.052 mg L−1 for Na+, K+, Mg2+, Ca2+, and Cl−, respectively, for samples collected during the R/V Oceania campaign. For samples collected during the R/V Electra campaign, the LODs were 0.004, 0.003, 0.002, 0.008, 0.012, and 0.001 mg L−1 for Na+, K+, Mg2+, Ca2+, Cl−, and SO, respectively. The limits of quantification (LOQs), defined as 10 standard deviations of the ultrapure water blanks, were 0.021, 0, 0.002, 0.192, and 0.174 mg L−1 for Na+, K+, Mg2+, Ca2+, and Cl−, respectively, for samples collected during the R/V Oceania campaign. For samples collected during the R/V Electra campaign, the LOQs were 0.015, 0.008, 0.007, 0.027, 0.040, and 0.005 mg L−1 for Na+, K+, Mg2+, Ca2+, Cl−, and SO, respectively.
2.4 Calculation of enrichment factors
We normalized the cell concentration per millilitre of extract from the filter samples and the corresponding seawater samples (referred to as X) using their respective Na+ concentrations to determine the enrichment factors (EFs) of aerosolized cells relative to seawater. The formula for calculating the EFs is as follows:
In this equation, is the ratio of cell concentration (X) to Na+ concentration in the aerosol samples and is the same ratio in the corresponding seawater samples.
2.5 DNA analysis
The DNA extraction and sequencing procedure used in this study is detailed in Zinke et al. (2024b). Briefly, nucleic acids were extracted from seawater and aerosol filter samples using the Plant Easy Mini Kit (Qiagen, Germany) with a final elution volume of 25 µL and amplified the V3–V4 region of the 16S rRNA gene through a triple polymerase chain reaction (PCR) using the Bac341F primer 5'-CCT ACG GGN GGC WGC AG-3' and the Bac805R primer 5'-GAC TAC HVG GGT ATC TAA TCC-3' (Herlemann et al., 2011; Hugerth et al., 2014). These primers were optimized for bacteria and might not amplify archaea as effectively (Hugerth et al., 2014). The 16S rRNA gene amplification was performed at the Department of Biology, Aarhus University, Denmark, and followed a modified Illumina protocol (16S Metagenomic Sequencing Library Preparation, Part 15044223 Rev. B). A detailed description of the triple PCR amplification can be found in Sect. S2 in the Supplement. It should be noted that the triple PCR may have introduced biases due to over-amplification of certain taxa due to differences in template abundances, differences in amplification efficiency (Polz and Cavanaugh, 1998), or inhibition of amplification by self-annealing of the most abundant templates (Suzuki and Giovannoni, 1996). The nf-core/ampliseq workflow version 2.3.2 (Straub et al., 2020) was used to analyse the sequencing data (see Sect. S3 for a more detailed description of the workflow). The R Phyloseq package (McMurdie and Holmes, 2013) and the R Vegan package (Oksanen, 2010) were used to analyse and visualize the processed data; 175 amplicon sequence variants (ASVs) were identified as contaminants and were removed from further analysis using the decontam package (Davis et al., 2017). A list of those ASVs is provided in Table S3. The R breakaway package (Willis and Bunge, 2015) was used to estimate the alpha diversity and similarities in the seawater, and chamber aerosol samples were assessed using non-metric multi-dimensional scaling (NMDS) analysis based on the Bray–Curtis similarity index. An analysis of similarity (ANOSIM) test was conducted (Clarke, 1993) to test for significant differences between the different sample types. Taxonomic trees were generated using PhyloT and iTOL (Letunic and Bork, 2021). To investigate the bacteria that were more likely to be aerosolized or remain in the seawater, aerosolization factors (AFs) were calculated as the mean relative abundance in the aerosols in the headspace of the sea spray simulation chamber (CSSC) divided by the mean relative abundance in the seawater (Cseawater):
2.6 Aerosol particle measurements
During both campaigns, the size distribution of the aerosols produced in the chamber was measured using a custom-built differential mobility particle sizer (DMPS), consisting of a Vienna-type differential mobility analyser (DMA) and a condensation particle counter (CPC, model 3772, TSI, USA), and a white-light optical particle size spectrometer (WELAS 2300 HP sensor and Promo 2000 H, Palas GmbH, Germany, hereafter called OPSS). The DMPS measured particles with electrical mobility diameters between 0.015 and 0.906 µm distributed at a flow rate of 1 L min−1, while the OPSS measured particles with optical diameters between 0.150 and 10 µm at a flow rate of 5 L min−1. The size distributions were combined at 350 nm. A more detailed description of the aerosol sizing instrumentation is given in Zinke et al. (2024c). During the R/V Electra campaign, we conducted additional concentration and fluorescence measurements of coarse particles (CPs), fluorescent particles (FPs), and fPBAPs with optical diameters larger than 0.8 µm using the single-particle MBS (University of Hertfordshire, UK). The MBS had a sample flow of 0.3 L min−1 and a sheath flow of 1.55 L min−1. It uses a low-power laser with a wavelength of 635 nm to detect incoming particles and a scattering signal to determine the size of each individual particle. A xenon flash lamp with a wavelength of 280 nm excites fluorescence, which is recorded in eight equidistant channels within the spectral ranges of 305 and 655 nm. Further details about the MBS can be found in Ruske et al. (2017). The particular MBS data processing used here is described in Freitas et al. (2022), which is based on a similar set-up of the MBS connected to the sea spray chamber. Briefly, if a particle's fluorescence signal exceeds 3 times the standard deviation (3γ) of the background signal, it is classified as a fluorescent particle (FP), and if it exceeds 9γ in any of the fluorescence channels, it is classified as a highly fluorescent particle (HFP). This threshold has been used in previous studies by Savage et al. (2017) and Freitas et al. (2022). If the maximum fluorescent signal is observed at 364 nm, particles are classified as marine fPBAPs (Freitas et al., 2022). Freitas et al. (2022) showed that particles with a maximum at 364 nm were only present in real seawater but were absent after filtration of the seawater.
To ensure dry air for aerosol sampling, the aerosol-particle-laden air from the sea spray simulation chamber was passed through a Nafion dryer (MD-700-36F, Perma Pure, USA) located in front of the MBS. The dryer was supplied with a sheath flow of dry, particle-free air at a flow rate of 10 L min−1. The relative humidity and temperature of the sampled air were monitored with sensors (HYTELOG-USB, B+B Thermo-Technik GmbH) mounted in front of the MBS sampling inlets. The average RH was 19.1±3.0 % and the average temperature of the dried chamber air was 26.4±1.6 °C.
A schematic of the experimental set-up is depicted in Fig. 1.
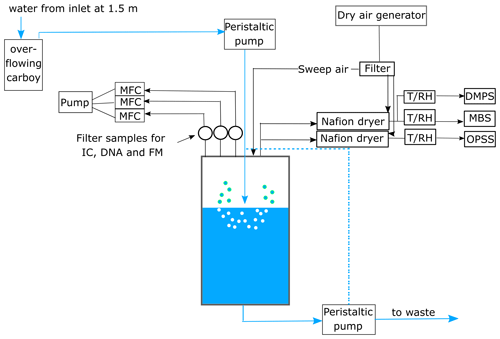
Figure 1Schematic of the experimental set-up. The seawater in the chamber was constantly plunged and refilled from the ship's seawater intake. The sea spray aerosol (SSA) produced in the chamber was sampled onto three filters for cell enumeration using fluorescence microscopy (FM), ion chromatography (IC), and DNA analysis. The flow through each filter was controlled using mass flow controllers (MFCs). Aerosol number and size were measured with a differential mobility particle sizer (DMPS) and an optical particle size spectrometer (OPSS). Additional online measurements of fluorescent particles were conducted using the Multiparameter Bioaerosol Sensor (MBS). Prior to sampling, the aerosol-laden air was dried using a Nafion dryer and the temperature (T) and relative humidity (RH) in the sampling line were monitored using a T/RH sensor. The dashed blue line indicates the water flow when the chamber was operated in closed mode.
2.7 Aerosol eddy covariance flux measurements
Additional data were obtained from flux measurements made on Östergarnsholm, as described in detail in Zinke et al. (2024c). Briefly, aerosol eddy covariance fluxes were measured at 12 m above sea level using an ultrasonic anemometer (Gill HS, Gill Instruments Ltd, UK) that recorded the three-dimensional wind speed and atmospheric temperature at a frequency of 20 Hz. Fluctuations in H2O and CO2 were recorded at the same frequency using a LI-7500A (LI-COR Environmental Ltd, UK). The concentration of ambient aerosols with diameters µm was measured at ambient relative humidity with a time resolution of 1 s using an optical particle counter (OPC, model 1.109, Grimm Aerosol Technik GmbH, Germany). For detailed information about the flux calculations, we refer the reader to Zinke et al. (2024c).
3.1 Sea spray chamber experiments on board R/V Electra
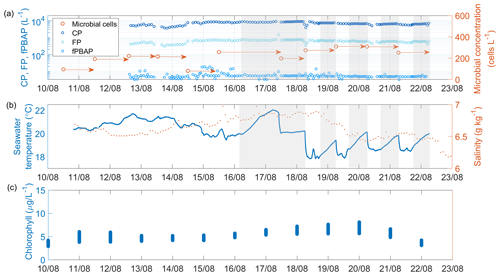
Figure 2Time series of (a) coarse particles (CPs), fluorescent particles (FPs), fluorescent primary biological aerosol particles (fPBAPs), and airborne microbial concentration measured in the headspace of the chamber; (b) seawater temperature and salinity; and (c) concentration of chlorophyll a (from re-analysis data) in the seawater measured during the R/V Electra campaign. The arrows in panel (a) mark the duration of the filter collection. Times when the chamber was operated in closed mode are shaded in grey.
Time series of seawater properties such as temperature, salinity, and chlorophyll a as well as the concentrations of MBS-derived fPBAPs and airborne microbial cells determined from filters measured in the sea spray chamber experiments during the R/V Electra campaign are shown in Fig. 2. The average seawater temperature inside the chamber remained stable, initially around 20 ± 1.1 °C and later around 18±1.1 °C towards the end of the campaign. However, during the closed-mode operations, the temperature rose by an average of 1.5±0.4 °C due to the lack of fresh seawater input. Throughout the campaign, the seawater salinity remained constant at 6.7 ± 0.1 g kg−1.
The average chlorophyll-a concentration, derived from re-analysis data, remained stable at 5.2±1.1 mg m−3 throughout the R/V Electra campaign. Chlorophyll a serves as an indirect indicator of biomass in surface seawater and has been utilized here to gauge emissions of organic matter and microbes from ocean surfaces. However, recent studies by Quinn et al. (2014) and Freitas et al. (2022) suggested that correlating chlorophyll-a concentrations directly with organic matter and microbial emissions may not be straightforward. Despite the consistent daily averages during the R/V Electra campaign, significant correlations between chlorophyll-a concentrations and fPBAP emissions were not observed. The growth of plankton biomass in the Baltic Sea follows a distinct seasonal cycle characterized by three prominent peaks (Wasmund et al., 1996; Stoń-Egiert and Ostrowska, 2022; Skjevik et al., 2022). These peaks typically occur during spring (March/April), when diatoms and dinoflagellates bloom; summer (July/August), marked by the proliferation of large filamentous cyanobacteria; and autumn (September/October), when diatoms bloom again (the timings of the blooms noted here are general averages, and on occasion there are anomalies). The R/V Oceania campaign, conducted in May, fell between the spring and summer blooms, whereas the R/V Electra campaign, carried out in August, coincided with the late-summer bloom. Figure S2 displays a yearly time series of the re-analysed chlorophyll-a concentration in the vicinity of Östergarnsholm.
The average concentration of cells as determined by fluorescence microscopy was mL−1 in the bulk seawater (SW) and mL−1 in the SML (Fig S3). Surprisingly, the number of cells in the SML samples did not differ significantly from those in the bulk seawater (Pearson's correlation coefficient r=0.94, p=0.002, and n=7). This contrasts with previous studies that reported higher cell concentrations in the SML (Sieburth et al., 1976; Aller et al., 2005; Rastelli et al., 2017).
The average concentration of the cells in the chamber's headspace increased from 178.4±45.9 cells L−1 to 241.0±77.5 cells L−1 when the plunging-jet flow rate increased from 1.3 to 2.6 L min−1. In contrast, the concentration of the blank filter samples was 4 orders of magnitude lower, with only cells per blank filter compared to cells per aerosol filter. Furthermore, increasing the plunging-jet flow rate resulted in higher concentrations of CPs and FPs (4865±1291 CPs L−1 at 1.3 L min−1 compared to 7316±1698 CPs L−1 at 2.6 L min−1 and 422±122 FPs L−1 at 1.3 L min−1 compared to 646±153 FPs L−1 at 2.6 L min−1, respectively). Surprisingly, the concentration of fPBAPs did not show a significant increase (5.4±2.4 L−1 at 1.3 L min−1 compared to 5.7±1.9 L−1 at 2.6 L min−1). Notably, fPBAPs represented only a minor fraction of all the FPs (median 1.1 %) and an even smaller proportion of CPs, which decreased from 0.09 % to 0.07 % when the jet flow rate increased but remained fairly constant even when the chamber was operated in closed mode (Fig. S4). This suggests that the chamber's large volume provided a sufficient reservoir of fPBAPs to balance cell multiplication with the removal by aerosolization.
These ratios are higher than those reported in a previous study by Freitas et al. (2022), who conducted similar experiments in the Baltic Sea near Gotland in June 2018 using the same experimental set-up and reported fPBAP / CP ratios of 0.05 %. One potential factor influencing the airborne fPBAP concentration could be seasonal differences in the abundance of biological and fluorescent matter in surface water between August 2021 and June 2018 (i.e. chlorophyll a was <2 mg m−3 in June 2018 compared to 5.2±1.1 mg m−3 in August 2021).
The fPBAP estimates obtained in this study were nearly 2 orders of magnitude lower than the cell abundance estimates obtained from fluorescence microscopy. This discrepancy may be attributed to two main factors. Firstly, the limitations of the MBS, which only detects particles with diameters larger than 0.8 µm, could lead to undersampling of smaller microbial cells, viruses, and cell fragments. Secondly, while the MBS provides an estimate of particle-attached microbes and cells emitted as agglomerates, as they are present in the ambient atmosphere, fluorescence microscopy of the extracted filters only provides estimates of single cell numbers. This distinction is significant because cell clusters are likely to break up during the sonication step (Zinke et al., 2024b).
3.1.1 Enrichment factors of microbes in chamber air and the SML compared to the underlying bulk seawater
To assess the EFs of microbial cells in the sea spray simulation chamber aerosol relative to the underlying seawater, we normalized the estimated cell abundances by the respective sodium concentrations. This normalization adjusts for changes in the SSA emission flux within the chamber, which is influenced by experimental conditions such as the plunging-jet speed. A time series of the microbial cell and sodium concentrations in both seawater and chamber aerosol samples is provided in Fig. S3. The mass fractions of sodium in the aerosol and seawater samples remained relatively constant throughout both campaigns, with average values of approximately 30.5±2.5 % and 34±1% in the aerosol and 32.6±0.3 % and 32±0.1 % in the seawater during the R/V Oceania and R/V Electra campaigns, respectively (Fig. S5).
The derived EFs ranged from 13 to 488 (mean 125.4±143.3) and decreased from 262.6±162.4 to 47.0±36.2 as the plunging-jet flow rate increased from 1.3 to 2.6 L min−1. This decrease could be attributed to the higher jet flow rate potentially preventing the microbial enrichment in the SML.
In contrast to previous studies (Sieburth et al., 1976; Aller et al., 2005; Rastelli et al., 2017), we observed no significant enrichment of microbes in the SML compared to the underlying SW. The EF between the SML and SW ranged from 0.34 to 1.74, with a mean of 0.92±0.51. One possible explanation for this lack of enrichment is the average wind speed of 6.7±2.5 m s−1 encountered during the R/V Electra campaign, which may have been high enough to prevent microbial enrichment in the SML due to wind-induced mixing. We observed a negative correlation (, p=0.01) between wind speed and the SML EF, with the highest SML EF occurring on the day with the lowest wind speed (Fig. S6). This result is consistent with the findings of Rahlff et al. (2017), who found that wind speeds above 4.1 m s−1 prevent microbial enrichment in the SML. Notably, several studies have reported that the SML can persist at wind speeds above 6.6 m s−1, and enrichment of organic matter has been observed at wind speeds of up to 10 m s−1 (e.g. Carlson, 1983; Wurl et al., 2011). It is important to recognize that the SML is operationally defined and likely persists even at moderate to high wind speeds. Despite this persistence, there are clearly periods, especially at higher wind speeds, when microbes are not enriched in the SML. Another possible explanation for the lack of enrichment in our study could be the dilution of the sample with subsurface water during glass-plate sampling.
3.1.2 Comparison of microbial abundance estimates and enrichment factors with previous mesocosm studies
In early investigations, Blanchard and Syzdek (1972) conducted mesocosm experiments using a suspension of Serratia marcescens in distilled water in order to observe and quantify dynamics in EF production. They observed significantly higher EFs in the initial jet drops, with values of up to 1200 compared to the final jet drops with an EF value of 8. Additionally, Blanchard and Syzdek (1982) reported EFs ranging between 10 and 20 for film drops, while Cipriano (1979) conducted a separate mesocosm study using a suspension of S. marinorubra in seawater from Long Island and reported EFs ranging from 50 to 100 for film drops. While we did not distinguish between film and jet drops in the current study, we can expect the lower salinity of the Baltic seawater to lead to the presence of larger bubbles that tend to break up into film drops (Zinke et al., 2022), which might explain why the EFs observed in the current study fall closer to the estimate of Cipriano (1979).
Other mesocosm studies have used culture-based approaches to enumerate the abundance of airborne microbes in the Baltic Sea and Arctic Ocean, observing lower concentrations than our study (Marks et al., 2001; Hultin et al., 2011; Fahlgren et al., 2015). However, it should be considered that not all microbes form visible colonies (less than 1 % of bacteria are culturable), which could explain the lower concentrations reported in these studies. Marks et al. (2001), who conducted mesocosm experiments with Baltic seawater from the Gulf of Gdańsk in July 1997 and March 1998, estimated EFs of mesophilic and psychrophilic bacteria ranging between 37–2545 and 14–585, respectively. Hultin et al. (2011), who conducted mesocosm experiments in the Baltic Sea in May–June and September 2005, provided an upper estimate of the total airborne bacteria concentration of 104–106 cells m−3 (or 101–103 cells L−1) by assuming that the transport efficiency of all bacteria is equal to that of colony-forming units (CFUs). This estimate falls approximately within the same range as the concentrations obtained from the current study.
Other mesocosm studies that reported airborne microbial concentrations comparable to the estimates obtained from the current study were conducted by Aller et al. (2005), Rastelli et al. (2017), and Zinke et al. (2024b). Aller et al. (2005) used inverted funnels and frits to generate SSA directly from seawater on a floating catamaran in June–September 2003 in the Long Island Sound, New York. They enumerated DAPI-stained bacteria with fluorescence microscopy and reported an EF of approximately 10. However, frits have been shown to produce a narrower bubble size spectrum than the spectrum observed in breaking waves, with a bias towards smaller bubbles (Stokes et al., 2013). These smaller bubbles tend to produce jet drops, possibly explaining the lower EF compared to our study.
Rastelli et al. (2017) conducted mesocosm experiments in June–July 2006 in the north-eastern Atlantic (close to Ireland) using a plunging-jet sea spray simulation chamber. Samples were collected with a five-stage Berner impactor and analysed with fluorescence microscopy to estimate the prokaryote abundance in the aerosol. They reported size-dependent enrichment of bacteria in SSA with an EF value of approximately 45 for particles with diameters less than 1.2 µm and significantly lower enrichment for particles with diameters larger than 1.2 µm. Similarly, Michaud et al. (2018) determined the abundance of airborne bacteria from wave channel experiments at Scripps Pier in San Diego, USA, using flow cytometry and reported EFs of approximately 11 in SSA compared to the bulk seawater.
Recently, in a mesocosm study conducted in June–July 2022 in the north-eastern Atlantic, Zinke et al. (2024b) derived an average EF of 48.6±35.6 (ranging between 9 and 158). While this study used a similar approach to that employed in the current study, it used a smaller sea spray simulation chamber that was operated in closed mode, meaning that the water was not replaced over the duration of each 48 h experiment. As a result of this study, selective growth in the seawater was observed, which might have impacted the enrichment of certain bacteria taxa in the chamber air. In the current study, we have employed an approach where the seawater is continuously replaced to prevent such selective growth, except for periods when the ship had to leave its anchored position close to Östergarnsholm.
It is important to note that, of the studies discussed above, Rastelli et al. (2017) and Zinke et al. (2024b) are the only ones that normalized their EFs using the sodium concentration. This is important because it is essential to consider that the emission fluxes of microbes or fPBAPs obtained from mesocosm experiments can be influenced by various factors, such as the biogeochemical properties of seawater (e.g. salinity, seawater temperature, and the presence of surfactants) and the experimental set-up (e.g. the bubble-production method, bubbling rate, proximity of the sampling ports to the water surface, chamber size, and closed or flow-through operation mode). Therefore, caution should be exercised when comparing results from experiments with different set-ups. By normalizing the EFs using the sodium concentration, it becomes possible to account for changes in SSA emission fluxes. To facilitate meaningful comparisons across different studies, we strongly advocate normalizing the EFs using relevant ionic concentrations, such as sodium. This approach will help ensure a more robust and accurate assessment of the enrichment and emission of microbial cells and fPBAPs from seawater into the atmosphere. A summary table of the above-mentioned studies can be found in Zinke et al. (2024b).
3.1.3 Estimation of marine microbial and fPBAP fluxes from the chamber experiments
Emission fluxes of microbial cells and fPBAPs (per m−2 s−1) were derived using two independent approaches. In the first approach, microbial emission fluxes were derived by multiplying the EFs derived in this study by mass emission estimates from the existing SSA parameterizations of Mårtensson et al. (2003), Salter et al. (2015), and Zinke et al. (2024c):
where is the fraction of the sodium mass to the total sea salt mass in seawater (here we used the mass fraction of sodium in the aerosol, 0.32, which was measured during the R/V Electra campaign), is the ratio of cell abundance to sodium mass measured per millilitre of seawater, ρ is the sea salt aerosol density (here we used 2.017 g cm−3; Zieger et al., 2017), and is the size-resolved number emission from one of the sea salt source parameterizations (so X is either Mårtensson, Salter, or Zinke). The mass fluxes were integrated over a size range of µm, which corresponds to the range in which the Mårtensson et al. (2003) parameterization is valid. The parameterizations of Mårtensson et al. (2003) and Salter et al. (2015) were derived for salinities of 33 and 35 g kg−1, respectively. Since the current study was conducted under brackish conditions (S∼6.7 g kg−1), a correction factor had to be applied for these two parameterizations. This correction factor is based on the assumption of a linear relationship between salinity and SSA volume, which is supported by the findings of Zinke et al. (2022). On the other hand, the parameterization by Zinke et al. (2024c) was derived for the exact conditions of the current study. While the parameterizations by Mårtensson et al. (2003) and Salter et al. (2015) are based on purely inorganic sea salt, the parameterization by Zinke et al. (2024c) was derived for SSA containing organics. Unfortunately, the amount of organics in the SSA is unknown.
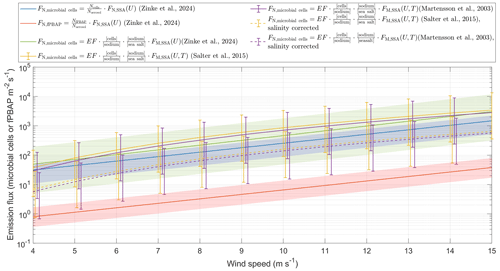
Figure 3Estimation of single-cell and fPBAP emission fluxes at different wind speeds using two different approaches. The red and blue lines show fPBAP and microbial fluxes that were estimated by multiplying the ratio of microbial cells or fPBAPs to all aerosols by the wind-speed-dependent parameterization of Zinke et al. (2024c). The green, yellow, and purple lines were estimated by multiplying the enrichment factors by the mass flux estimated from the parameterizations of Zinke et al. (2024c), Salter et al. (2015), and Mårtensson et al. (2003), respectively. The dashed yellow and purple lines show the estimates based on Salter et al. (2015) and Mårtensson et al. (2003) and account for the difference in salinities between those parameterizations and the current study. The lines are the mean flux estimates, while the shaded areas and error bars indicate the range of the minimum to maximum flux estimates. All the number and mass fluxes were integrated over a size range of µm, which corresponds to the range in which the Mårtensson et al. (2003) parameterization is valid.
The second approach is based on a comparison of the particle concentration measured from the chamber experiments with in situ eddy covariance fluxes measured on Östergarnsholm (Zinke et al., 2024c). From this comparison we derived a chamber-specific scaling factor which allows us to scale the airborne cell counts to microbial fluxes (cells m−2s−1). Using this scaling factor, we estimated an average microbial flux of 45.9±13.4 cells m−2s−1 (range 16–63 cells m−2 s−1) and an average fPBAP flux of 1.12±0.47 fPBAPs m−2 s−1 (range 0.6–6.1 fPBAPs m−2 s−1) under the given experimental conditions. Furthermore, by multiplying the wind-speed-dependent parameterization derived in Zinke et al. (2024c) by a factor of 0.00086 (mean, range 0.0003–0.0013), which corresponds to the ratio of cells determined from fluorescence microscopy to the total aerosol concentration (integrated over the size range between 0.02 and 2.8 µm), we were able to derive microbial emission fluxes for different wind speeds (Fig. 3). A two-sample Kolmogorov–Smirnov test (Massey Jr, 1951) revealed no significant differences between the estimates from the two different approaches used in this study (p=0.99) at a significance level of 5 %.
While the majority of the microbial cells (99 %) that were measured with fluorescence microscopy (FM) were smaller than 2.8 µm (Fig. S7), 39 % of the fPBAPs were larger than 2.8 µm. As such, using the same range to estimate fPBAP fluxes would result in a grave underestimation of the fPBAP fluxes. Instead, we calculated the ratio of fPBAPs to total aerosols for the entire size range measured by the DMPS and OPSS ( µm), which was (mean, range –). The fPBAP emission flux was then estimated by multiplying this factor by the total aerosol number flux integrated over this size range. The estimated fPBAP emissions were more than 1 order of magnitude lower than the microbial emission fluxes, likely due to the different measurement approaches (single cells from fluorescence microscopy versus particle-attached cells or cell agglomerates from the MBS measurements) discussed in Sect. 3.1.
Only a few other studies have attempted to estimate the emission fluxes of microbes or PBAPs. Mayol et al. (2014), who conducted ambient measurements in the North Atlantic, derived a flux of prokaryotes (defined as cells with diameters < 1 µm) of 42 cells m−2 s−1 by multiplying the abundance in seawater by the SSA source function of Andreas (1998). They further estimated a deposition flux of 49 prokaryotic cells m−2 s−1 by multiplying the atmospheric concentration of prokaryotes by the settling velocity, which depends on the particle size and density. By subtracting the deposition flux from the emission flux, they derived a net flux of −6.49 prokaryotic cells m−2 s−1. Similarly, Mayol et al. (2017) estimated the total emission flux of prokaryotes over the subtropical and tropical oceans (Atlantic, Pacific, and Indian) to be cells m−2 d−1 (or 0.01–23 cells m−2 s−1) and the deposition flux to be up to 6×106 cells m−2 d−1 (or 69.4 cells m−2 s−1). Hu et al. (2017) used the same approach to estimate bacteria fluxes from ambient measurements in the Kuroshio extension and reported values of the order of 1×102 cells m−2 s−1. Hu et al. (2017) further estimated the wind-speed-dependent emission fluxes of bacteria using enrichment factors and seawater concentrations of bacteria (see their Fig. 3a and Table S1). It should be noted that the studies by Mayol et al. (2014), Mayol et al. (2017), and Hu et al. (2017) were conducted under different conditions to the current studies (i.e. in high-salinity oceans with different wave states and varying distances to land). Even so, the emission flux estimate obtained from the current study falls within the same range as the estimates from those studies. These previous studies suggest that the deposition flux of marine prokaryotes outweighs their emission flux (Mayol et al., 2017; Hu et al., 2017), implying that the ocean acts as a sink rather than a source of microbes (except at high wind speeds >8 m s−1; Hu et al., 2017). However, these studies only provide a posteriori estimates of the fluxes that might also be affected by terrestrial sources due to their proximity to land. Combining eddy covariance systems with high-frequency online fPBAP measurements would allow direct measurements of fPBAP fluxes and could help determine whether the oceans are actually a sink or a small source of fPBAPs.
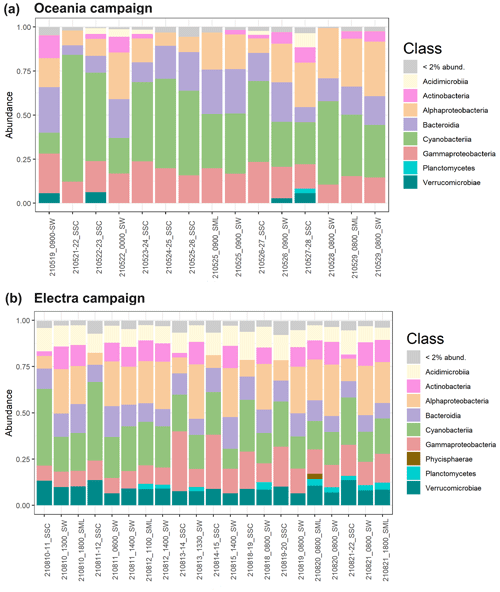
3.1.4 Bacterial community composition in the seawater and chamber aerosols
Using 16S rRNA sequencing, we investigated the bacterial community compositions of three different sample types: bulk SW, the SML, and nascent SSA generated in a sea spray simulation chamber (SSC) during both campaigns (Fig. 4). We compared the ASVs present in SW and SSC samples from both research campaigns. Our analysis revealed that 3033 ASVs were common to all samples, regardless of type or campaign. In the SW samples, 773 ASVs were solely detected during the R/V Electra campaign and 366 ASVs were solely detected during the R/V Oceania campaign. In the SSC samples, 357 ASVs were solely detected during the R/V Oceania campaign and 102 ASVs were solely detected during the R/V Electra campaign (Fig. S8). A comparison of the communities in the SW, SML, and SSC using the ANOSIM statistical test revealed distinct differences between the communities in SSC and SW (with dissimilarity values of r=0.76 and p=0.002 for the R/V Oceania campaign and r=1 and p=0.002 for the R/V Electra campaign) and SSC and SML (r=0.67 and p=0.05 for the R/V Oceania campaign and r=1 and p=0.004 for the R/V Electra campaign). This is surprising given that the bacteria sampled from the headspace of the chamber were expected to originate from the seawater. One possible explanation for this could be selective aerosolization of certain taxa. Fahlgren et al. (2015) also reported that bacterial communities in aerosols differed greatly from their corresponding seawater communities. Their conclusion was that some taxa were selectively enriched in aerosols and sometimes even removed entirely from the seawater samples, while others were barely aerosolized at all. Another possible explanation could be the presence of free DNA fragments in the headspace of the chamber that can pass through the HEPA filters used to flush the headspace with particle-free air (Zinke et al., 2024b). Subsequently, these DNA fragments might have been over-amplified by triple PCR. Furthermore, we cannot fully exclude the possibility that misclassifications were introduced by the pipeline due to limitations of the reference database, sequencing artifacts, or chimeras that might have slipped through the correction steps in the pipeline.
No significant difference was observed between the SML and SW for both campaigns ( and p=0.88 for the R/V Oceania campaign; r=0.14 and p=0.18 for the R/V Electra campaign), suggesting that the bacterial community in the SML was mixed well with the underlying bulk water.
Regarding the alpha diversity richness index, the samples from the R/V Oceania campaign showed slightly less diversity (270±54 in SSC, 315±69 in SW, and 313±32 in the SML) than those from the R/V Electra campaign (427±58 in SSC, 472±38 in SW, and 379±61 in the SML; see also Fig. S9a). In line with this observation, previous studies at different study sites reported seasonal cycles in the bacterial diversity with a minimum during spring and a maximum during late summer and autumn that were governed by environmental parameters such as seawater temperature, day length, and nutrient availability (e.g. Fuhrman et al., 2006; Andersson et al., 2010). In terms of beta diversity, the samples from the two campaigns formed distinct clusters (Fig. 7b). The SSC samples clustered separately from the SW samples during both campaigns. During the R/V Electra campaign, the SML samples showed slightly less diversity than SW samples in terms of alpha diversity but were closely clustered in terms of beta diversity. In the R/V Oceania data, no distinct difference between the SW and SML samples was observed in terms of alpha or beta diversity.
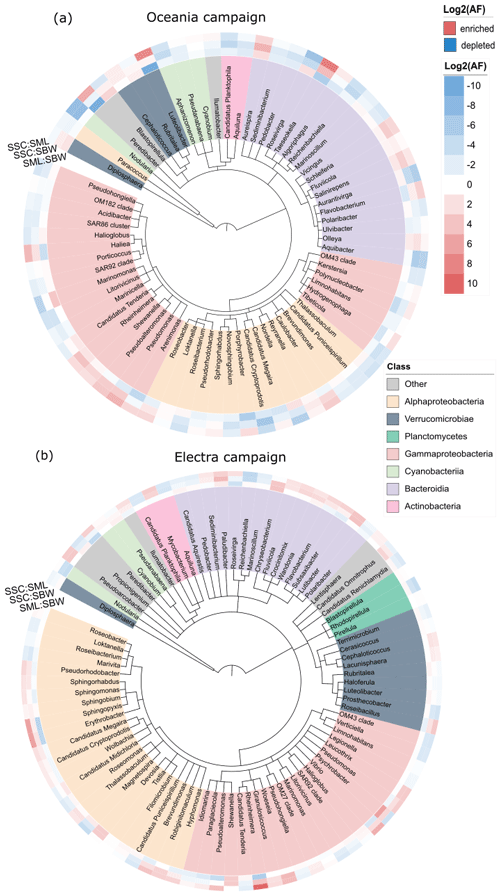
Figure 4Taxonomic trees at the genus level for (a) the R/V Oceania campaign and (b) the R/V Electra campaign for taxa abundant in the bulk seawater (SBW), surface microlayer (SML), and sea spray simulation chamber (SSC) samples, colour-coded according to class. The heat maps indicate enrichment in the SML compared to SBW (inner circle), SSC compared to SBW (middle circle), and SSC compared to the SML (outer circle). The tree was generated using PhyloT and iTOL (Letunic and Bork, 2021).
We did not observe a significant enrichment of certain taxa in the SML when compared to the SW (Fig. S10), which is in line with the finding that there was no enrichment in terms of the total cell abundance. It is possible that high-wind conditions prevented the formation of a distinct bacterioneuston community in the SML due to constant mixing of the SML with the underlying bulk seawater as discussed in Sect. 3.1.1.
In terms of enrichment in the aerosol, we observed an increased abundance of Gammaproteobacteria in the chamber aerosol, with a relative abundance of 23 % compared to 12 % in the seawater during the R/V Electra campaign (Fig. 5b). In contrast to the R/V Electra campaign, Gammaproteobacteria showed no significant enrichment during the R/V Oceania campaign, with relative abundances between 17 % and 19 % in both chamber air and seawater (Fig. 5a). An enrichment of Gammaproteobacteria in aerosol has been reported in previous studies in the Baltic Sea (Fahlgren et al., 2010; Seifried et al., 2015; Freitas et al., 2022). However, in contrast to these studies, we did not find a significant enrichment in Acidimicrobiia, Bacteroidia, and Verrucomicrobia during the R/V Electra campaign (with relative abundances of 10 %–11 % in all the sample types) and a depletion of Bacteroidia during the R/V Oceania campaign (14 % in chamber air compared to 21 % in seawater). Furthermore, we observed decreased abundances of Actinobacteria and Alphaproteobacteria in the chamber air compared to the seawater during both campaigns (2 % Actinobacteria and 9 %–10 % Alphaproteobacteria in chamber air compared to 11 % Actinobacteria and 24 %–25 % Alphaproteobacteria in seawater during both campaigns). The differences between the previous studies and the current study could be explained by spatial and seasonal differences. Furthermore, both Fahlgren et al. (2010), who conducted measurements at a coastal site in Kalmar, and Seifried et al. (2015), who conducted measurements in the Kattegat, measured in ambient air, and as such the community compositions from their studies likely also contained bacteria from terrestrial sources, depending on the wind direction. During the R/V Electra and R/V Oceania campaigns, we observed an enrichment of Cyanobacteria in the SSC (with relative abundances in the SSC of 27 % and 52 % compared to 12 % and 28 % in SW). Similarly, Lewandowska et al. (2017), who conducted ambient measurements of bioaerosols over land (Gdynia) and at sea (Gulf of Gdańsk, south-western Baltic), identified picoplanktonic Cyanobacteria in the air.
We conducted two ship-based campaigns in the Baltic Sea in May and August 2021, where we performed chamber experiments to investigate emissions of fPBAPs with SSA. By normalizing the microbial cell abundances in the aerosol and seawater obtained from fluorescence microscopy with the sodium concentrations in each medium, we found that microbes were 13–488 times more enriched in the aerosol compared to the bulk seawater. Emission fluxes of microbes were estimated using two independent approaches: in the first approach, the EFs derived from this study were multiplied by mass emission estimates from existing sea salt parameterizations (Mårtensson et al., 2003; Salter et al., 2015; Zinke et al., 2024c). The second scaling approach is based on a companion study (Zinke et al., 2024c), where we scaled the chamber experiments to EC flux measurements on a nearby island. Using the wind-speed-dependent parameterization that was derived from Zinke et al. (2024c), we derived microbial and fPBAP emission estimates for a wind speed range between 4 and 15 m s−1. The microbial emission flux estimates from both approaches agreed fairly well and were of the same order of magnitude as reported values from previous studies. The estimated fPBAP fluxes were however significantly lower. This study is the first to derive microbial and fPBAP flux estimates from low-salinity (eutrophic) waters such as the Baltic Sea, addressing a research gap outlined by Šantl-Temkiv et al. (2022) and Amato et al. (2023), who emphasized the need for microbial emission flux estimates over marine regions.
The 16S rRNA sequencing revealed no significant differences between the bulk seawater and the SML. Similarly, no significant enrichment in terms of cell abundance could be observed in the surface microlayer. One possible explanation for this finding could be that the average wind speed of 6 m s−1 encountered during this study generated mixing in the seawater, preventing the enrichment of certain taxa in the SML. Another possible explanation could be dilution of the SML with underlying bulk water during the glass-plate sampling.
Future studies should conduct long-term ambient measurements to investigate seasonal cycles of PBAPs and microbial emissions covering all seasons and different oceanic waters. These measurements could be incorporated into existing networks, combining meteorological and aerosol measurements to gain a comprehensive understanding of surface–atmosphere exchange processes. Furthermore, future studies should focus on the viability and metabolic activity of airborne microbes and particularly on the role of phenotypic traits (e.g. pigmentation or hydrophobic surface properties) and atmospheric conditions in their survival and dispersal in the atmosphere. With a potential increase in harmful algae blooms due to climate warming, it is crucial to improve our understanding of whether harmful algae and the associated pathogenic microbes can become airborne from seawater and remain viable during aerosolization and atmospheric dispersal. Moreover, determining the fraction of airborne PBAPs that can act as ice-nucleating particles is essential for understanding their potential impacts on the climate.
The data from this study are available from the Bolin Centre for Climate Research database (https://doi.org/10.17043/zinke-2024-baltic-bioaerosols-1; Zinke et al., 2024a). Additionally, the sequencing data are publicly available from the NCBI database (accession no. PRJNA1110170; http://www.ncbi.nlm.nih.gov/bioproject/1110170, National Library of Medicine, 2024).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-24-13413-2024-supplement.
JZ, RAF, MES, GPF, PZ, and EDN designed the experiments. JZ carried out the chamber experiments with the help of GPF and PZ. The data analysis and visualization were conducted by JZ. GPF processed and provided the MBS data. JZ prepared the manuscript with contributions from all the co-authors.
At least one of the (co-)authors is a member of the editorial board of Atmospheric Chemistry and Physics. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We gratefully acknowledge the financial support provided by the Bolin Centre for Climate Research at Stockholm University and the Swedish Research Council – Vetenskapsrådet (grant nos. 2018-05045_VR and 2018-04255_VR). Rachel Ann Foster is funded by the Knut and Alice Wallenberg Foundation. The aerosol flux tower and system were financed initially by the Svenska Forskningsrådet (FORMAS) (grant no. 2007-1362_FORMAS) and have since received contributions from several FORMAS and VR projects. We are obligated to the Institute of Oceanology of the Polish Academy of Science, which allowed us to use their R/V Oceania free of charge. We thank the crews and captains of R/V Oceania and R/V Electra and the technical staff at ACES, Stockholm University, for their support. Thanks go to Daniel Lundin (Centre for Ecology and Evolution in Microbial model Systems, Linnaeus University, Kalmar, Sweden) for support with the bio-informatic analyses. Additionally, we extend our thanks to Tina Šantl-Temkiv, Marie Braad Lund, and Britta Poulsen from the Department of Biology at Aarhus University, Denmark, for their assistance with sequencing the samples. We are grateful to Christian D. F. Castenschiold (Department of Biology, Aarhus University) for sharing his R script for generating the scatterplots. Furthermore, we thank Pär Helmquist (ACES) for his assistance with the ion chromatography analysis. We acknowledge the helpful suggestions of two anonymous reviewers that helped to improve our manuscript.
This research has been supported by VR (grant nos. 2018-05045 and 2018-04255) and FORMAS (grant no. 2007-1362).
The publication of this article was funded by the Swedish Research Council, Forte, FORMAS, and Vinnova.
This paper was edited by Eija Asmi and reviewed by two anonymous referees.
Aller, J. Y., Kuznetsova, M. R., Jahns, C. J., and Kemp, P. F.: The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols, J. Aerosol Sci., 36, 801–812, https://doi.org/10.1016/j.jaerosci.2004.10.012, 2005. a, b, c, d, e
Alsante, A. N., Thornton, D. C. O., and Brooks, S. D.: Ocean Aerobiology, Front. Microbiol., 12, 764178, https://doi.org/10.3389/fmicb.2021.764178, 2021. a, b
Amato, P., Mathonat, F., Nuñez Lopez, L., Péguilhan, R., Bourhane, Z., Rossi, F., Vyskocil, J., Joly, M., and Ervens, B.: The aeromicrobiome: the selective and dynamic outer-layer of the Earth’s microbiome, Frontiers in Microbiology, 14, 1186847, https://doi.org/10.3389/fmicb.2023.1186847, 2023. a, b, c
Andersson, A. F., Riemann, L., and Bertilsson, S.: Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities, The ISME J., 4, 171–181, 2010. a
Andreas, E. L.: A new sea spray generation function for wind speeds up to 32 m s−1, Phys. Oceanogr., 28, 2175–2184, 1998. a
Blanchard, D. C.: The electrification of the atmosphere by particles from bubbles in the sea, Prog. Oceanogr., 1, 73–112, https://doi.org/10.1016/0079-6611(63)90004-1, 1963. a
Blanchard, D. C.: Air-Sea Exchange of Gases and Particles, chap. The Production, Distribution, and Bacterial Enrichment of the Sea-Salt Aerosol, 407–454 pp., D. Reidel Publishing Company, https://doi.org/10.1007/978-94-009-7169-1_7, 1983. a
Blanchard, D. C. and Syzdek, L. D.: Concentration of bacteria in jet drops from bursting bubbles, J. Geophys. Res., 77, 5087–5099, 1972. a, b
Blanchard, D. C. and Syzdek, L. D.: Water-to-air transfer and enrichment of bacteria in drops from bursting bubbles, Appl. Environ. Microbiol., 43, 1001–1005, 1982. a, b, c
Burrows, S. M., Butler, T., Jöckel, P., Tost, H., Kerkweg, A., Pöschl, U., and Lawrence, M. G.: Bacteria in the global atmosphere – Part 2: Modeling of emissions and transport between different ecosystems, Atmos. Chem. Phys., 9, 9281–9297, https://doi.org/10.5194/acp-9-9281-2009, 2009a. a
Burrows, S. M., Elbert, W., Lawrence, M. G., and Pöschl, U.: Bacteria in the global atmosphere – Part 1: Review and synthesis of literature data for different ecosystems, Atmos. Chem. Phys., 9, 9263–9280, https://doi.org/10.5194/acp-9-9263-2009, 2009b. a, b
Carlson, D.: Dissolved organic materials in surface microlayers: Temporal and spatial variability and relation to sea state, Limnol. Oceanogr., 28, 415–431, 1983. a
Carlucci, A. and Williams, P.: Concentration of bacteria from sea water by bubble scavenging, ICES J. Mar. Sci., 30, 28–33, 1965. a
Cipriano, R. J.: Bubble and Aerosol Spectra Produced by a Laboratory Simulation of a Breaking Wave, State University of New York at Albany, ISBN 9798661621832, 1979. a, b, c
Clarke, K. R.: Non-parametric multivariate analyses of changes in community structure, Aust. J. Ecol., 18, 117–143, 1993. a
Cunliffe, M. and Wurl, O.: Sampling the sea surface microlayer, Hydrocarbon and lipid microbiology protocols: field studies, 255–261 pp., https://doi.org/10.1007/8623_2015_83, 2017. a
Cunliffe, M., Salter, M., Mann, P. J., Whiteley, A. S., Upstill-Goddard, R. C., and Murrell, J. C.: Dissolved organic carbon and bacterial populations in the gelatinous surface microlayer of a Norwegian fjord mesocosm, FEMS Microbiol. Lett., 299, 248–254, 2009. a
Davis, N., Proctor, D., Holmes, S., Relman, D., and Callahan, B.: Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data, bioRxiv: 221499, https://doi.org/10.1186/s40168-018-0605-2, 2017. a
DeMott, P. J., Hill, T. C., McCluskey, C. S., Prather, K. A., Collins, D. B., Sullivan, R. C., Ruppel, M. J., Mason, R. H., Irish, V. E., Lee, T., et al.: Sea spray aerosol as a unique source of ice nucleating particles, P. Natl. Acad. Sci. USA, 113, 5797–5803, 2016. a
Després, V., Huffman, J. A., Burrows, S. M., Hoose, C., Safatov, A., Buryak, G., Fröhlich-Nowoisky, J., Elbert, W., Andreae, M., Pöschl, U., and Jaenicke, R.: Primary biological aerosol particles in the atmosphere: a review, Tellus B, 64, 15598, 2012. a
Dommergue, A., Amato, P., Tignat-Perrier, R., Magand, O., Thollot, A., Joly, M., Bouvier, L., Sellegri, K., Vogel, T., Sonke, J. E., Jaffrezo, J.-L., Andrade, M., Moreno, I., Labuschagne, C., Martin, L., Zhang, Q., and Larose, C.: Methods to investigate the global atmospheric microbiome, Front. Microbiol., 10, 243, 2019. a
Fahlgren, C., Hagström, A., Nilsson, D., and Zweifel, U. L.: Annual variations in the diversity, viability, and origin of airborne bacteria, Appl. Environ. Microbiol., 76, 3015–3025, 2010. a, b
Fahlgren, C., Gómez-Consarnau, L., Zábori, J., Lindh, M. V., Krejci, R., Mårtensson, E. M., Nilsson, D., and Pinhassi, J.: Seawater mesocosm experiments in the A rctic uncover differential transfer of marine bacteria to aerosols, Environ. Microbiol. Rep., 7, 460–470, https://doi.org/10.1111/1758-2229.12273, 2015. a, b, c, d
Franklin, M. P., McDonald, I. R., Bourne, D. G., Owens, N. J. P., Upstill-Goddard, R. C., and Murrell, J. C.: Bacterial diversity in the bacterioneuston (sea surface microlayer): The bacterioneuston through the looking glass, Environ. Microbiol., 7, 723–736, 2005. a
Freitas, G. P., Stolle, C., Kaye, P. H., Stanley, W., Herlemann, D. P., Salter, M. E., and Zieger, P.: Emission of primary bioaerosol particles from Baltic seawater, Environ. Sci.-Atmos., 2, 1170–1182, 2022. a, b, c, d, e, f, g, h, i
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., Lang-Yona, N., Burrows, S. M., Gunthe, S. S., Elbert, W., Su, H., Hoor, P., Thines, E., Hoffmann, T., Després, V. R., and Pöschl, U.: Bioaerosols in the Earth system: Climate, health, and ecosystem interactions, Atmos. Res., 182, 346–376, 2016. a
Fuhrman, J. A., Hewson, I., Schwalbach, M. S., Steele, J. A., Brown, M. V., and Naeem, S.: Annually reoccurring bacterial communities are predictable from ocean conditions, P. Natl. Acad. Sci. USA, 103, 13104–13109, 2006. a
Garrett, W. D.: Collection of slick-forming materials from the sea surface, Limnol. Oceanogr., 10, 602–605, 1965. a
Genitsaris, S., Kormas, K., and Moustaka-Gouni, M.: Airborne algae and cyanobacteria: occurrence and related health effects, Front. Biosci., 3, 772–787, 2011. a
Harvey, G. W. and Burzell, L. A.: A simple microlayer method for small samples, Limnol. Oceanogr., 17, 156–157, 1972. a
Herlemann, D. P., Labrenz, M., Jürgens, K., Bertilsson, S., Waniek, J. J., and Andersson, A. F.: Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea, The ISME J., 5, 1571–1579, 2011. a
Hu, W., Murata, K., Fukuyama, S., Kawai, Y., Oka, E., Uematsu, M., and Zhang, D.: Concentration and viability of airborne bacteria over the Kuroshio extension region in the Northwestern Pacific Ocean: Data from three cruises, J. Geophys. Res.-Atmos., 122, 12–892, 2017. a, b, c, d, e, f
Huffman, J. A., Perring, A. E., Savage, N. J., Clot, B., Crouzy, B., Tummon, F., Shoshanim, O., Damit, B., Schneider, J., Sivaprakasam, V., Zawadowicz, M. A., Crawford, I., Gallagher, M., Topping, D., Doughty, D. C., Hill, S. C., and Pan, Y.: Real-time sensing of bioaerosols: Review and current perspectives, Aerosol Sci. Technol., 54, 465–495, 2020. a
Hugerth, L. W., Wefer, H. A., Lundin, S., Jakobsson, H. E., Lindberg, M., Rodin, S., Engstrand, L., and Andersson, A. F.: DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies, Appl. Environ. Microbiol., 80, 5116–5123, 2014. a, b
Hultin, K. A. H., Krejci, R., Pinhassi, J., Gomez-Consarnau, L., Mårtensson, E. M., Hagström, A., and Nilsson, E. D.: Aerosol and bacterial emissions from Baltic seawater, Atmos. Res., 99, 1–14, https://doi.org/10.1016/j.atmosres.2010.08.018, 2011. a, b
Joux, F., Agogué, H., Obernosterer, I., Dupuy, C., Reinthaler, T., Herndl, G. J., and Lebaron, P.: Microbial community structure in the sea surface microlayer at two contrasting coastal sites in the northwestern Mediterranean Sea, Aqua. Microbial Ecol., 42, 91–104, 2006. a
Kiselev, D., Bonacina, L., and Wolf, J.-P.: A flash-lamp based device for fluorescence detection and identification of individual pollen grains, Rev. Sci. Instrum., 84, 033302, https://doi.org/10.1063/1.4793792, 2013. a
Könemann, T., Savage, N., Klimach, T., Walter, D., Fröhlich-Nowoisky, J., Su, H., Pöschl, U., Huffman, J. A., and Pöhlker, C.: Spectral Intensity Bioaerosol Sensor (SIBS): an instrument for spectrally resolved fluorescence detection of single particles in real time, Atmos. Meas. Tech., 12, 1337–1363, https://doi.org/10.5194/amt-12-1337-2019, 2019. a
Konik, M., Kowalewski, M., Bradtke, K., and Darecki, M.: The operational method of filling information gaps in satellite imagery using numerical models, Int. J. Appl. Earth Observ. Geoinfo., 75, 68–82, 2019. a
Lang-Yona, N., Flores, J. M., Haviv, R., Alberti, A., Poulain, J., Belser, C., Trainic, M., Gat, D., Ruscheweyh, H.-J., Wincker, P., et al.: Terrestrial and marine influence on atmospheric bacterial diversity over the north Atlantic and Pacific Oceans, Commun. Earth Environ., 3, 121, https://doi.org/10.1038/s43247-022-00441-6 , 2022. a
Letunic, I. and Bork, P.: Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation, Nucleic Acids Res., 49, W293–W296, 2021. a, b
Lewandowska, A. U., Śliwińska-Wilczewska, S., and Woźniczka, D.: Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea, Mar. Pollut. Bull., 125, 30–38, 2017. a
Lewis, E. R. and Schwartz, S. E.: Sea Salt Aerosol Production: Mechanisms, Methods, Measurements and Models – A Critical Review, Geophysical Monograph Series, Vol. 152, American Geophysical Union, ISBN 978-0-875-90417-7, 2004. a
Mårtensson, E. M., Nilsson, E. D., de Leeuw, G., Cohen, L. H., and Hansson, H. C.: Laboratory simulations and parameterization of the primary marine aerosol production, J. Geophys. Res.-Atmos., 108, 4297, https://doi.org/10.1029/2002JD002263, 2003. a, b, c, d, e, f, g, h
Marks, R., Kruczalak, K., Jankowska, K., and Michalska, M.: Bacteria and fungi in air over the Gulf of Gdańsk and Baltic sea, J. Aerosol Sci., 32, 237–250, 2001. a, b, c
Marks, R., Górecka, E., McCartney, K., and Borkowski, W.: Rising bubbles as mechanism for scavenging and aerosolization of diatoms, J. Aerosol Sci., 128, 79–88, 2019. a
Massey Jr, F. J.: The Kolmogorov-Smirnov test for goodness of fit, J. Am. Stat. Assoc., 46, 68–78, https://doi.org/10.1080/01621459.1951.10500769, 1951. a
May, N. W., Olson, N. E., Panas, M., Axson, J. L., Tirella, P. S., Kirpes, R. M., Craig, R. L., Gunsch, M. J., China, S., Laskin, A., Ault, A. P., and Pratt, K. A.: Aerosol emissions from great lakes harmful algal blooms, Environ. Sci. Technol., 52, 397–405, https://doi.org/10.1021/acs.est.7b03609, 2018. a
Mayol, E., Jiménez, M. A., Herndl, G. J., Duarte, C. M., and Arrieta, J. M.: Resolving the abundance and air-sea fluxes of airborne microorganisms in the North Atlantic Ocean, Front. Microbiol., 5, 557, 2014. a, b, c
Mayol, E., Arrieta, J. M., Jiménez, M. A., Martínez-Asensio, A., Garcias-Bonet, N., Dachs, J., González-Gaya, B., Royer, S.-J., Benítez-Barrios, V. M., Fraile-Nuez, E., and Duarte, C. M.: Long-range transport of airborne microbes over the global tropical and subtropical ocean, Nat. Commun., 8, 1–9, 2017. a, b, c, d, e, f, g
McMurdie, P. J. and Holmes, S.: phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data, PloS one, 8, e61217, https://doi.org/10.1371/journal.pone.0061217, 2013. a
Michaud, J. M., Thompson, L. R., Kaul, D., Espinoza, J. L., Richter, R. A., Xu, Z. Z., Lee, C., Pham, K. M., Beall, C. M., Malfatti, F., Azam, F., Knight, R., Burkart, M. D., Dupont, C. L., and Prather, K. A.: Taxon-specific aerosolization of bacteria and viruses in an experimental ocean-atmosphere mesocosm, Nat. Commun., 9, 1–10, https://doi.org/10.1038/s41467-018-04409-z, 2018. a, b, c, d
National Library of Medicine: Characterization of bacteria emissions from the Baltic Sea, Raw sequence reads, Stockholm University, Accession number PRJNA1110170 http://www.ncbi.nlm.nih.gov/bioproject/1110170 (last access: 4 December 2024), 2024. a
Oksanen, J.: Vegan: community ecology package, 2.6-8 [code], http://CRAN.R-project.org/package=vegan (last access: 3 December), 2010. a
Perrott, P., Turgeon, N., Gauthier-Levesque, L., and Duchaine, C.: Preferential aerosolization of bacteria in bioaerosols generated in vitro, J. Appl. Microbiol., 123, 688–697, 2017. a
Polz, M. F. and Cavanaugh, C. M.: Bias in template-to-product ratios in multitemplate PCR, Appl. Environ. Microbiol., 64, 3724–3730, 1998. a
Quinn, P. K., Bates, T. S., Schulz, K. S., Coffman, D. J., Frossard, A. A., Russell, L. M., Keene, W. C., and Kieber, D. J.: Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol, Nat. Geosci., 7, 228–232, https://doi.org/10.1038/NGEO2092, 2014. a
Rahlff, J., Stolle, C., Giebel, H.-A., Brinkhoff, T., Ribas-Ribas, M., Hodapp, D., and Wurl, O.: High wind speeds prevent formation of a distinct bacterioneuston community in the sea-surface microlayer, FEMS Microbiol. Ecol., 93, fix041, https://doi.org/10.1093/femsec/fix041, 2017. a
Rastelli, E., Corinaldesi, C., Dell’Anno, A., Lo Martire, M., Greco, S., Cristina Facchini, M., Rinaldi, M., O’Dowd, C., Ceburnis, D., and Danovaro, R.: Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: an experimental approach, Sci. Rep., 7, 1–10, https://doi.org/10.1038/s41598-017-10563-z, 2017. a, b, c, d, e, f, g, h
Ruske, S., Topping, D. O., Foot, V. E., Kaye, P. H., Stanley, W. R., Crawford, I., Morse, A. P., and Gallagher, M. W.: Evaluation of machine learning algorithms for classification of primary biological aerosol using a new UV-LIF spectrometer, Atmos. Meas. Tech., 10, 695–708, https://doi.org/10.5194/amt-10-695-2017, 2017. a, b
Salter, M. E., Nilsson, E. D., Butcher, A., and Merete, B.: On the seawater temperature dependence of continuous plunging jet derived sea spray aerosol, J. Geophys. Res.-Atmos., 119, 9052–9072, https://doi.org/10.1002/2013JD021376, 2014. a
Salter, M. E., Zieger, P., Acosta Navarro, J. C., Grythe, H., Kirkevåg, A., Rosati, B., Riipinen, I., and Nilsson, E. D.: An empirically derived inorganic sea spray source function incorporating sea surface temperature, Atmos. Chem. Phys., 15, 11047–11066, https://doi.org/10.5194/acp-15-11047-2015, 2015. a, b, c, d, e, f
Santander, M. V., Mitts, B. A., Pendergraft, M. A., Dinasquet, J., Lee, C., Moore, A. N., Cancelada, L. B., Kimble, K. A., Malfatti, F., and Prather, K. A.: Tandem fluorescence measurements of organic matter and bacteria released in sea spray aerosols, Environ. Sci. Technol., 55, 5171–5179, 2021. a
Šantl-Temkiv, T., Lange, R., Beddows, D., Rauter, U., Pilgaard, S., Dall’Osto, M., Gunde-Cimerman, N., Massling, A., and Wex, H.: Biogenic sources of ice nucleating particles at the high Arctic site villum research station, Environ. Sci. Technol., 53, 10580–10590, 2019. a
Šantl-Temkiv, T., Sikoparija, B., Maki, T., Carotenuto, F., Amato, P., Yao, M., Morris, C. E., Schnell, R., Jaenicke, R., Pöhlker, C., DeMott, P. J., Hill, T. C. J., and Huffman, J. A.: Bioaerosol field measurements: Challenges and perspectives in outdoor studies, Aerosol Sci. Technol., 54, 520–546, 2020. a
Šantl-Temkiv, T., Amato, P., Casamayor, E. O., Lee, P. K., and Pointing, S. B.: Microbial ecology of the atmosphere, FEMS Microbiol. Rev., 46, fuac009, https://doi.org/10.1093/femsre/fuac009, 2022. a
Šaulienė, I., Šukienė, L., Daunys, G., Valiulis, G., Vaitkevičius, L., Matavulj, P., Brdar, S., Panic, M., Sikoparija, B., Clot, B., Crouzy, B., and Sofiev, M.: Automatic pollen recognition with the Rapid-E particle counter: the first-level procedure, experience and next steps, Atmos. Meas. Tech., 12, 3435–3452, https://doi.org/10.5194/amt-12-3435-2019, 2019. a
Savage, N. J., Krentz, C. E., Könemann, T., Han, T. T., Mainelis, G., Pöhlker, C., and Huffman, J. A.: Systematic characterization and fluorescence threshold strategies for the wideband integrated bioaerosol sensor (WIBS) using size-resolved biological and interfering particles, Atmos. Meas. Tech., 10, 4279–4302, https://doi.org/10.5194/amt-10-4279-2017, 2017. a
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., and Cardona, A.: Fiji: an open-source platform for biological-image analysis, Nat. Methods, 9, 676–682, 2012. a
Schulz, H. N. and Jørgensen, B. B.: Big bacteria, Annu. Rev. Microbiol., 55, 105–37, https://doi.org/10.1146/annurev.micro.55.1.105, 2001. a
Seifried, J. S., Wichels, A., and Gerdts, G.: Spatial distribution of marine airborne bacterial communities, MicrobiologyOpen, 4, 475–490, 2015. a, b, c
Sieburth, J. M., Willis, P., Johnson, K. M., Burney, C. M., Lavoie, D. M., Hinga, K. R., Caron, D. A., French, III, F. W., Johnson, P. W., and Davis, P. G.: Dissolved Organic Matter and Heterotrophic Microneuston in the Surface Microlayers of the North Atlantic, Science, 194, 1415–1418, 1976. a, b
Skjevik, A.-T., Wesslander, K., Viktorsson, L., and Nilsson, M.: The Swedish National Marine Monitoring Programme 2021, 49 pp., https://www.smhi.se/publikationer/publikationer/the-swedish-national-marine-monitoring-programme-2023-1.210240 (last access: 3 December 2024), 2022. a
Smets, W., Moretti, S., Denys, S., and Lebeer, S.: Airborne bacteria in the atmosphere: Presence, purpose, and potential, Atmos. Environ., 139, 214–221, 2016. a
Stokes, M. D., Deane, G. B., Prather, K., Bertram, T. H., Ruppel, M. J., Ryder, O. S., Brady, J. M., and Zhao, D.: A Marine Aerosol Reference Tank system as a breaking wave analogue for the production of foam and sea-spray aerosols, Atmos. Meas. Tech., 6, 1085–1094, https://doi.org/10.5194/amt-6-1085-2013, 2013. a
Stolle, C., Labrenz, M., Meeske, C., and Jürgens, K.: Bacterioneuston community structure in the southern Baltic sea and its dependence on meteorological conditions, Appl. Environ. Microbiol., 77, 3726–3733, 2011. a
Stoń-Egiert, J. and Ostrowska, M.: Long-term changes in phytoplankton pigment contents in the Baltic Sea: Trends and spatial variability during 20 years of investigations, Cont. Shelf Res., 236, 104666, https://doi.org/10.1016/j.csr.2022.104666, 2022. a
Straub, D., Blackwell, N., Langarica-Fuentes, A., Peltzer, A., Nahnsen, S., and Kleindienst, S.: Interpretations of environmental microbial community studies are biased by the selected 16S rRNA (gene) amplicon sequencing pipeline, Front. Microbiol., 11, 550420, 2020. a
Suzuki, M. T. and Giovannoni, S. J.: Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR, Appl. Environ. Microbiol., 62, 625–630, 1996. a
Tesson, S. V. and Šantl-Temkiv, T.: Ice nucleation activity and aeolian dispersal success in airborne and aquatic microalgae, Front. Microbiol., 9, 2681, https://doi.org/10.3389/fmicb.2018.02681, 2018. a
Tignat-Perrier, R., Dommergue, A., Thollot, A., Keuschnig, C., Magand, O., Vogel, T. M., and Larose, C.: Global airborne microbial communities controlled by surrounding landscapes and wind conditions, Sci. Rep., 9, 14441, 2019. a
Wang, X., Deane, G. B., Moore, K. A., Ryder, O. S., Stokes, M. D., Beall, C. M., Collins, D. B., Santander, M. V., Burrows, S. M., Sultana, C. M., et al.: The role of jet and film drops in controlling the mixing state of submicron sea spray aerosol particles, P. Natl. Acad. Sci. USA, 114, 6978–6983, https://doi.org/10.1073/pnas.1702420114, 2017. a
Wasmund, N., Breuel, G., Edler, L., Kuosa, H., Olsonen, R., Schultz, H., Pys-Wolska, M., and Wrzolek, L.: Pelagic biology, Baltic Sea Env. Proc., 64, 89–100, 1996. a
Willis, A. and Bunge, J.: Estimating diversity via frequency ratios, Biometrics, 71, 1042–1049, 2015. a
Wilson, T. W., Ladino, L. A., Alpert, P. A., Breckels, M. N., Brooks, I. M., Browse, J., Burrows, S. M., Carslaw, K. S., Huffman, J. A., Judd, C., et al.: A marine biogenic source of atmospheric ice-nucleating particles, Nature, 525, 234–238, https://doi.org/10.1038/nature14986, 2015. a
Woźniak, B., Bradtke, K., Darecki, M., Dera, J., Dudzińska-Nowak, J., Dzierzbicka-Głowacka, L., Ficek, D., Furmańczyk, K., Kowalewski, M., Kreżel, A., Majchrowski, R., Ostrowska, M., Paszkuta, M., Stoń-Egiert, J., Stramska, M., and Zapadka, T.: SatBałtyk–A Baltic environmental satellite remote sensing system–an ongoing project in Poland. Part 1: Assumptions, scope and operating range, Oceanologia, 53, 897–924, 2011a. a
Woźniak, B., Bradtke, K., Darecki, M., Dera, J., Dudzińska-Nowak, J., Dzierzbicka-Głowacka, L., Ficek, D., Furmańczyk, K., Kowalewski, M., Kreżel, A., Majchrowski, R., Ostrowska, M., Paszkuta, M., Stoń-Egiert, J., Stramska, M., and Zapadka, T.: SatBałtyk–A Baltic environmental satellite remote sensing system–an ongoing project in Poland. Part 2: Practical applicability and preliminary results, Oceanologia, 53, 925–958, 2011b. a
Wurl, O., Wurl, E., Miller, L., Johnson, K., and Vagle, S.: Formation and global distribution of sea-surface microlayers, Biogeosciences, 8, 121–135, https://doi.org/10.5194/bg-8-121-2011, 2011. a
Zieger, P., Väisänen, O., Corbin, J. C., Partridge, D. G., Bastelberger, S., Mousavi-Fard, M., Rosati, B., Gysel, M., Krieger, U. K., Leck, C., Nenes, A., Riipinen, I., Virtanen, A., and Salter, M. E.: Revising the hygroscopicity of inorganic sea salt particles, Nat. Commun., 8, 1–10, https://doi.org/10.1038/ncomms15883, 2017. a
Zinke, J., Nilsson, E. D., Zieger, P., and Salter, M. E.: The Effect of Seawater Salinity and Seawater Temperature on Sea Salt Aerosol Production, J. Geophys. Res.-Atmos., 127, e2021JD036005, https://doi.org/10.1029/2021JD036005, 2022. a, b
Zinke, J., Freitas, G., Foster, R., Zieger, P., Nilsson, E., Markuszewski, P., and Salter, M.: Measurements of bioaerosols, bacteria and ionic composition in sea spray aerosols from experiments in the Baltic Sea, Bolin Centre for Climate Research [data set], https://doi.org/10.17043/zinke-2024-baltic-bioaerosols-1, 2024a. a
Zinke, J., Freitas, G., Salter, M. E., Lundin, D., Aggarwal, S., Zieger, P., Mohr, C., and Foster, R. A.: Quantification and characterization of bacteria emission over the North-Eastern Atlantic using mesocosm experiments, Environ. Sci. Technol. Air, 1, 162–174, https://doi.org/10.1021/acsestair.3c00017, 2024b. a, b, c, d, e, f, g, h, i
Zinke, J., Nilsson, E. D., Markuszewski, P., Zieger, P., Mårtensson, E. M., Rutgersson, A., Nilsson, E., and Salter, M. E.: Sea spray emissions from the Baltic Sea: comparison of aerosol eddy covariance fluxes and chamber-simulated sea spray emissions, Atmos. Chem. Phys., 24, 1895–1918, https://doi.org/10.5194/acp-24-1895-2024, 2024a. a, b, c, d, e, f, g, h, i, j, k, l, m