the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Measurement report: Occurrence of aminiums in PM2.5 during winter in China – aminium outbreak during polluted episodes and potential constraints
Tang Liu
Yi-Jia Ma
Qi-Bin Sun
Hong-Wei Xiao
Hao Xiao
Hua-Yun Xiao
Cong-Qiang Liu
Amines and aminiums play an important role in particle formation, liquid-phase reactions, and climate change and have attracted considerable attention over the years. Here, we investigated the concentrations and compositions of aminiums in PM2.5 in 11 Chinese cities during the winter, focusing on the characteristics of aminiums during the polluted days and the key factors influencing aminium outbreak. Monomethylaminium was the dominant aminium species in most cities, except Taiyuan and Guangzhou, followed by dimethylaminium. Diethylaminium dominated the total aminiums in Taiyuan and Guangzhou. Thus, the main amine sources in Taiyuan and Guangzhou were significantly different from those in other cities. The concentrations of the total aminiums (TAs) in most cities increased significantly during the polluted days, while relatively weak aminium outbreaks during the polluted days occurred in Xi'an and Beijing. Additionally, the concentrations of TAs in Xi'an and Beijing were insignificantly correlated with those of PM2.5 and the major acidic aerosol components, while the opposite pattern was observed in nine other cities. Thus, acid–base chemistry was significantly associated with the formation of aminiums in PM2.5 in all cities, except Xi'an and Beijing. Based on the sensitivity analysis of the aminiums : ammonium ratio to ammonium changes, as well as excluding the effects of relative humidity and atmospheric oxidation, we proposed the possibility of the competitive uptake of ammonia versus amines on acidic aerosols or the displacement of aminiums by ammonia in Xi'an and Beijing (constraining aminium outbreaks). Overall, this study deepens the understanding of the spatiotemporal differences in aminium characteristic and formation in China. However, the uptake of amines on particles to form aminiums and the relevant influencing factors require further mechanistic research.
- Article
(8455 KB) - Full-text XML
-
Supplement
(1615 KB) - BibTeX
- EndNote
Low-molecular-weight amines are ubiquitous and important in the gaseous and particulate phases (Nielsen et al., 2012; Ge et al., 2011a; Berta et al., 2023). More than 150 amines have been identified in the atmosphere (Ge et al., 2011a). The most abundant and frequently reported amines in field observations are typically C1–C6 alkylamines, including dimethylamine, monomethylamine, trimethylamine, diethylamine, ethylamine, 1-propanamine, and 1-butanamine (X.-Y. Yang et al., 2023; Liu et al., 2023). Amines can participate in various chemical and physical processes in the atmosphere, promoting the formation and growth of new particles and contributing to the production of secondary organic aerosols (Yao et al., 2018; Tong et al., 2020; Møller et al., 2020). Amines are thus considered to have a direct or indirect impact on air quality (Li et al., 2019; Tao et al., 2016; Shen et al., 2023). Air pollution (e.g., haze) caused by high levels of atmospheric fine particles (PM2.5) has received considerable attention in China over the past decade due to rapid industrialization and urbanization (F. Liu et al., 2022; Z. Liu et al., 2022). Evidently, controlling the emission strength of amine sources and understanding the transformation of atmospheric amines can effectively reduce air pollution in cities.
The main sources of atmospheric amines during the air pollution period in cities in China are typically fossil fuel combustion and biomass burning rather than agricultural emissions (Feng et al., 2022; Z. Liu et al., 2022; Wang et al., 2022; Shen et al., 2017; Ho et al., 2016; Chang et al., 2022). Owing to the water solubility and alkalinity of amines, low-molecular-weight amines in PM2.5 during the air pollution period are mainly present in the form of amine salts (i.e., aminiums) via the gas-to-particle partitioning of gaseous amines and subsequent acid–base chemistry (Zhang et al., 2021; C. Liu et al., 2022; Ge et al., 2011a; Xie et al., 2018). It should be noted that organic amines (e.g., dimethylamine and trimethylamine) in nanoparticles (< 200 nm) may also be largely present in the organic phase (Xie et al., 2018). In addition, oxidative degradation of higher-molecular-weight amines and displacement reactions are also the potential formation pathways of aminiums in PM2.5 (Tao et al., 2021; Qiu and Zhang, 2013; Tong et al., 2020). Although previous observational studies have investigated the compositions, concentrations, sources, and formation processes of low-molecular-weight aminiums in the particle phase in urban areas of Shanghai (Liu et al., 2023), Guangzhou (Shu et al., 2023), Qingdao (Z. Liu et al., 2022), and Xuzhou (X.-Y. Yang et al., 2023) in China, there has been relatively little focus on the association between PM2.5 and amine outbreaks. A recent study conducted in Wangdu County, Hebei province, China, has suggested that amines exhibited outbreak characteristics during the haze episode (Feng et al., 2022). Climate and air pollution conditions can vary greatly from city to city, due to the vastness of China. However, it is poorly understood how the characteristics and formation processes of low-molecular-weight aminiums in PM2.5 vary between clean and polluted days in different cities in China, which may hinder the further assessment of the environmental impacts of amines with regional differences.
In winter in China, air pollution episodes are more frequent compared to other seasons. Thus, we present the measurements of aminiums in PM2.5 collected from 11 different Chinese cities during the winter (2017–2018). The aims of this study are (1) to investigate the spatial differences in the compositions and concentrations of aminiums in PM2.5, with a focus on the difference between them on clean days and polluted days, and (2) to understand the key factors controlling the formation of aminiums in PM2.5 in different cities.
2.1 Site description and sample collection
A total of 11 urban sites were selected for aerosol sample collection, including the Beijing (BJ; 40.04° N, 116.41° E), Taiyuan (TY; 37.80° N, 112.58° E), Xi'an (XA; 34.25° N, 108.98° E), Lanzhou (LZ; 36.11° N, 103.73° E), Haerbin (HEB; i.e., Harbin; 45.77° N, 126.64° E), Wulumuqi (WLMQ; i.e., Ürümqi; 43.86° N, 87.75° E), Chengdu (CD; 30.68° N, 104.14° E), Guiyang (GY; 26.58° N, 106.73° E), Guangzhou (GZ; 23.18° N, 113.35° E), Wuhan (WH; 30.55° N, 114.36° E), and Hangzhou (HZ; 30.30° N, 120.16° E) sites (Fig. S1). HZ and GZ are megacities situated in the Yangtze River Delta (YRD) and Pearl River Delta (PRD) regions, respectively, both of which have developed economies. WH is located in the central region of China. CD and GY are representative cities in southwestern China. LZ, XA, TY, BJ, and HEB are cities in northern China. WLMQ, located in northwestern China, is the largest inland city that is farthest away from the ocean in the world. Obviously, the varying geographical locations and economic development levels of different cities lead to different air pollution and climate conditions between them.
PM2.5 sampling in most cities was conducted on the rooftops of buildings (four to six floors in total) using a high-volume air sampler (Series 2031; Laoying, China) from 1 December 2017 to 21 January 2018 (winter). Specifically, the sampling periods in LZ, TY, HEB, BJ, XA, WLMQ, CD, WH, HZ, GZ, and GY were 2–30 December 2017, 2–30 December 2017, 18 December 2017–15 January 2018, 22 December 2017–21 January 2018, 22 December 2017–20 January 2018, 3–28 March 2018, 1–31 December 2017, 6–29 December 2017, 4–31 December 2017, 1–30 December 2017, and 10 December 2017–11 January 2018, respectively (Tables S1–S3). At each site, PM2.5 was sampled once every 1–2 d for ∼ 24 h on prebaked quartz fiber filters (500 °C for 8 h). Moreover, two random blank filters were collected. The total number of PM2.5 samples at each sampling site is shown in Tables S1–S3. All samples were stored at −30 °C. Meteorological data such as precipitation, wind speed, temperature, and relative humidity (RH), as well as concentrations of various pollutants, were recorded during the sampling campaigns from the adjacent environmental monitoring stations. Sampling periods were classified as either clean or polluted days, based on a daily average PM2.5 mass concentration of 75 µg m−3 (Zhang and Cao, 2015).
2.2 Chemical analysis
The extraction of low-molecular-weight aminiums in the filter samples was carried out using the method described in our recent publication (Liu et al., 2023) and in a previous study (Liu et al., 2017). Briefly, the sample was filtered using a 0.22 µm Teflon syringe filter (CNW Technologies GmbH) after extraction with Milli-Q water (∼ 18.2 MΩ cm). The aminiums in the extracts that underwent pH regulation were derivatized using 0.1 mL of benzenesulfonyl chloride (BSC). The tube containing the derivatives was sealed and agitated for 30 min. To remove excess derivatization reagents, the extracts were agitated again for 30 min at 80 °C after adding the NaOH solution (0.5 mL of 10 mol L−1). Once the mixed solution had cooled down, it was acidified with a solution of HCl to adjust the pH to 5.5. A further extraction of derivatives was carried out by adding dichloromethane. It is important to mention that the organic phase was sequentially treated with Na2CO3 solution and anhydrous Na2SO4. A stream of nitrogen gas was used to concentrate the organic extracts. Finally, the sample was analyzed using gas chromatography–mass spectrometry (GC–MS) after adding dichloromethane and hexamethylbenzene. Dimethylaminium (DMAH+), monomethylaminium (MMAH+), diethylaminium (DEAH+), ethylaminium (EAH+), propylaminium (PAH+), butylaminium (BAH+), and pyrrolidinium (PYRH+) were quantified. Aminium recoveries varied between 73 % for DMAH+ and 112 % for PAH+. The detection limits of the aminium measurements ranged from 0.8 ng mL−1 for DEAH+ to 2.8 ng mL−1 for MMAH+. Aminiums are undetectable in the blank. Detailed data quality controls were described in our recent publication (Liu et al., 2023). It should be noted that we did not consider the impact of the continuous aging of aminiums collected on the filter on the measurement results. This is mainly due to the following reasons.
-
The PM2.5 samples investigated in this study are all acidic (Tables S1–S3), promoting the protonation of amino groups.
-
The protonated amino group is difficult to undergo oxidation by oxidants (e.g., hydroxyl radicals and ozone) (Nielsen et al., 2012).
Another filter cut was extracted with Milli-Q water to measure the concentrations of inorganic ions (e.g., , , , K+, Na+, Ca2+, and Mg2+) and organic acids (e.g., acetic acid, formic acid, succinic acid, oxalic acid, glutaric acid, and methanesulfonic acid) (Xu et al., 2022a, 2023; Liu et al., 2023; Lin et al., 2023). These inorganic ions were quantified via an ion chromatograph system (Dionex Aquion, Thermo Fisher Scientific, USA) (Xu et al., 2020a).
2.3 Parameter calculation
The thermodynamic model (ISORROPIA-II) was used for the prediction of the mass concentration of aerosol liquid water (ALW) and the pH value, which was detailed in our previous studies (Xu et al., 2022b, 2023; Y. Xu et al., 2020). The ventilation coefficient (VC) can be used as an indicator to assess the state of atmospheric dilution of pollutant concentrations (Gani et al., 2019). It is calculated by multiplying the wind speed by the planetary boundary layer height (PBLH) (T. Yang et al., 2023).
3.1 Compositions of aminiums in PM2.5 in China during winter
Figure 1 shows the average percentage distributions of various aminiums in PM2.5 collected in different cities in China during winter, with a comparison between their mass fractions on clean and polluted days. MMAH+ was the predominant species among the aminiums investigated in PM2.5 in most cities in northern China, including LZ, XA, HEB, BJ, and WLMQ. MMAH+ and DMAH+ (as the second most abundant species) constituted over 63 % of the total aminium concentrations in those northern cities. The relatively minor species, including DEAH+, EAH+, PAH+, BAH+, and PYRH+, contributed between 1 % and 18 % of the total aminium concentrations. The predominance of MMAH+ was also found in cities in the YRD (HZ), central (WH), and southwestern (CD and GY) China, closely followed by DMAH+. Previous studies conducted in Xi'an (winter; China) (Ho et al., 2015), Beijing (winter; China) (Wang et al., 2022; Ho et al., 2016), Nanjing (winter; China) (Liu et al., 2023), Shanghai (winter; China) (Liu et al., 2023), Xiamen (winter; China) (Ho et al., 2016), Hong Kong (winter; China) (Ho et al., 2016), and the Arabian Sea (autumn and winter) (Gibb et al., 1999), as well as at mountain (autumn; Nanling, China) (Liu et al., 2018) and background (winter; Puding, China) (Liu et al., 2023) sites, have suggested that the mass concentration fraction of MMAH+ was highest in the measured aerosol amine salts. The Henry's constants of MMA (3.65 × 101 mol kg−1 atm−1), DMA (3.14 × 101 mol kg−1 atm−1), and EA (3.55 × 101 mol kg−1 atm−1) are relatively lower than those of the other amines investigated (e.g., 1.32 × 102 mol kg−1 atm−1 for DEA) (Ge et al., 2011b), implying that the potential of MMA, DMA, and EA to be partitioned into aqueous particles was weaker compared to DEA. Additionally, the gaseous forms of these determined aminiums typically have strong alkalinity (Ge et al., 2011b). The aerosol samples in this study were all acidic (Tables S1–S3). Thus, these results imply that the increased emissions of MMA and DMA may partially explain the higher abundance of MMAH+ and DMAH+ in PM2.5 in these investigated cities during winter.
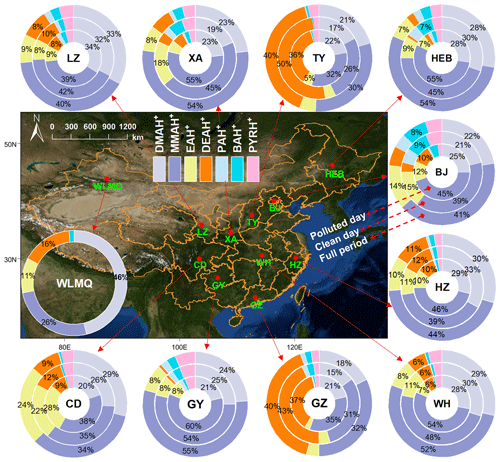
Figure 1Average percentage distributions of various aminiums in PM2.5 collected in different cities in China during winter. The map was obtained from ©MeteoInfoMap (version 3.3.0) (Chinese Academy of Meteorological Sciences, China).
In another northern city (i.e., TY), DEAH+ was the most abundant aminium species (40 % of the total aminium concentrations), followed by MMAH+ (30 %) and DMAH+(21 %). The composition characteristic of aminiums in the city of GZ (PRD area) was similar to that observed in TY (Fig. 1). Anthropogenic emissions, including vehicle exhaust and industrial production, are considered to be the main contributors to aerosol DEAH+ in urban areas (Y. Chen et al., 2022; Chen et al., 2019; X.-Y. Yang et al., 2023; Chang et al., 2022). A recent study has suggested that ethanol gasoline vehicles can emit a large amount of ethylamines, leading to the outbreak of DEAH+ during the haze episodes in Hebei Province (northern China) (Feng et al., 2022). Thus, the relative emission strength of anthropogenic DEA in the investigated amines was probably higher in TY (an inland city with application of ethanol gasoline vehicles) than in other cities. In addition, previous studies have suggested that aerosol DEAH+ can also be largely derived from marine emissions (Facchini et al., 2008; Dall'osto et al., 2019). Since GZ is a developed coastal city, local aerosol aminiums may be influenced by large gaseous DEA inputs from both local industrial production and marine sources.
The mass concentration fractions of aminiums on clean and polluted days were also compared (Fig. 1). The dominant aminium species (i.e., MMAH+, DMAH+, or DEAH+) in PM2.5 in all cities were not replaced by other aminiums from the clean days to the polluted days. This likely suggests that the main sources of atmospheric gas-phase amines in the cities did not change significantly on the polluted days. In addition, the proportions of MMAH+ (solubility of 23.76 mol kg−1 for aminium chloride form) (Ge et al., 2011b) and DMAH+ (solubility of 44.80 mol kg−1 for aminium chloride form) (Ge et al., 2011b) tended to further increase from the clean days to the polluted days, while that of DEAH+, with relatively low solubility (solubility of 20.52 mol kg−1 for aminium chloride form) (Ge et al., 2011b), showed a decreasing trend, especially in TY and GZ (where DEAH+ was dominant). The concentrations of ALW in PM2.5 were generally much higher on polluted days than on clean days, especially in the northern cities (Tables S1–S3). Clearly, liquid-phase processes likely played an important role in the formation of aminiums on polluted days.
3.2 Aminium concentrations and their linkage with PM2.5 variations
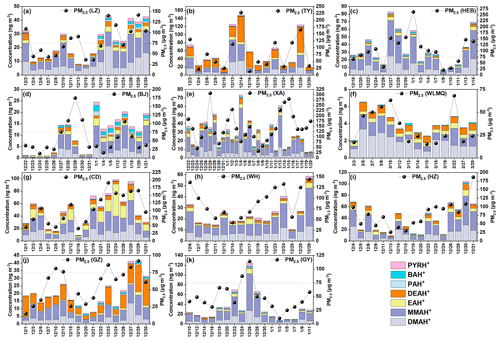
Figure 2 shows the average concentration distributions of various aminiums in PM2.5 collected in different cities in China during winter, focusing on the difference between their concentrations on clean days and polluted days. The concentrations of total aminiums (TAs) in TY ranged from 17.50 to 149.00 ng m−3, with an average of 56.90 ± 41.81 ng m−3. This average TA level was the highest among all the cities investigated. The average concentration of TAs in WLMQ was found to be the lowest (4.16 ± 1.24 ng m−3), with a range of 2.10–6.50 ng m−3. As previously mentioned, WLMQ is a vast city with a lower population density and less developed industries compared to the more developed northern and coastal cities in China. Additionally, this region is surrounded by barren mountains and sandy land (Ma et al., 2024) (Fig. 2). Apparently, the weak amine emission intensity appears to be responsible for the low levels of aminiums in the WLMQ.
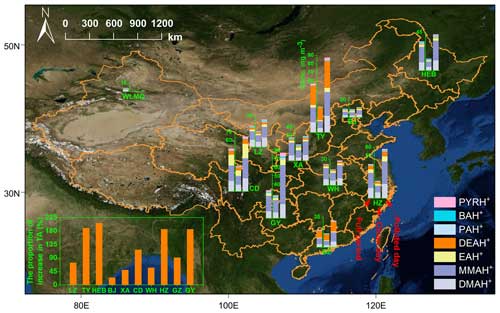
Figure 2Average concentration distributions of various aminiums in PM2.5 collected in different cities in the winter in China. The stacked bar chart (from left to right) indicates the data for the full sampling period, the clean day, and the polluted day, respectively. The column chart in the bottom-left corner shows the proportion of the increase in TA concentration from the clean days to the polluted days. The map was obtained from ©MeteoInfoMap (version 3.3.0) (Chinese Academy of Meteorological Sciences, China).
Table S4 provides an overview of the aminiums detected in atmospheric fine particles detected in different seasons and regions. The ranges of average TA concentrations in the northern cities (i.e., HEB, BJ, TY, XA, LZ, and WLMQ) generally overlapped with those measured in the coastal (GZ and HZ), central (WH), and southwestern (CD and GY) cities in this study (Tables S1–S3). Moreover, the average TA concentrations investigated here (4.16–56.90 ng m−3) were also within the observation ranges reported in previous studies (1.49–329.80 ng m−3) (Table S4) (Ho et al., 2016; Liu et al., 2023, 2018; Shen et al., 2017; Huang et al., 2016; Choi et al., 2020; Shu et al., 2023). MMAH+, as the dominant aminium species in most of the cities, showed the highest (18.33 ± 12.82 ng m−3) and lowest (1.07 ± 0.55 ng m−3) average concentrations in HEB and WLMQ, respectively. DEAH+ was the most abundant aminium species in TY and GZ, with average concentrations of 22.62 ± 17.62 ng m−3 and 8.16 ± 4.65 ng m−3, respectively (Tables S1 and S3). Two previous studies conducted in the GZ area in winter (2021 and 2015–2016) showed similar average DEAH+ (∼ 7 ng m−3) levels to this study (Liu et al., 2022b; Shu et al., 2023). However, DEAH+ was not identified as the dominant aminium component in those two previous studies. Furthermore, lower aminium concentrations (< 8 ng m−3) were generally found in most of the marine and polar regions (Dall'Osto et al., 2019; Corral et al., 2022). In general, the concentration and composition of aminiums vary spatially, which may be attributed to spatial differences in amine sources, emission intensities, and the main factors affecting aminium formation.
The average concentrations of TAs in all the investigated cities exhibited a similar variation pattern from clean to polluted days, which was characterized by higher levels on polluted days (Fig. 2). Specifically, the average aminium concentration showed an increase of up to 206 % in HEB during the polluted period. TA concentrations in LZ, TY, CD, HZ, and GZ also increased greatly by 91 % (in GZ) −190 % (in TY). It seems that PM2.5 pollution can be accompanied by an outbreak of aminiums. In contrast, a relatively small percentage increase in the TA concentration during the polluted days was found in WH (57 %), XA (50 %), and BJ (25 %). To further explore the linkage between changes in PM2.5 and fluctuations in aminiums, the temporal variations in the mass concentrations of aminiums and PM2.5 were compared across various cities (Fig. 3). The concentrations of total and major aminiums in LZ, TY, HEB, WLMQ, CD, WH, HZ, GZ, and GY showed a temporal variation that is highly similar to that of PM2.5, as indicated by a significant correlation between TAs and PM2.5 in these cities (r=0.61–0.85; P<0.05). However, high levels of PM2.5 can correspond to low levels of aminiums in XA (e.g., 29 December and 2, 14, 15, and 16 January) and BJ (e.g., 28 and 30 December). The correlations between TAs and PM2.5 in these two cities were also insignificant (P>0.05). These results suggest that the formation of aminiums in XA and BJ during the polluted period may be constrained by some special factors which will be discussed in the following discussion.
3.3 Formation of aminiums and potential ammonia suppression in aminium outbreaks
It is well documented that aminiums in PM2.5 can be formed mainly via the uptake of their gaseous form (i.e., amines) by aqueous particles, followed by acid–base neutralization reactions (Ge et al., 2011b; Xie et al., 2018; Sauerwein and Chan, 2017; Qiu and Zhang, 2013; Liu et al., 2023). Clearly, the formation of particle-phase aminiums was closely associated with the origins of the corresponding gas-phase amines (as precursors of aminiums). We found that TAs and major aminiums (e.g., MMAH+, DMAH+, and DEAH+) showed a significantly positive correlation (P<0.05) with SO2, NO2, or K+ (as indicators of fuel combustion and biomass burning) (Tian et al., 2020; Liu et al., 2023; Kunwar and Kawamura, 2014) in LZ, TY, HEB, BJ, WLMQ, CD, WH, HZ, GZ, and GY (Figs. 4 and S2). Thus, although lacking sufficient indicators (e.g., biogenic source traces) to trace the source of amines, our results can at least indicate that fossil fuel combustion or biomass burning may be important contributors to atmospheric amines in most of the investigated cities during the winter. This consideration was also supported by previous studies about the potential source analysis of aerosol aminiums in Guangzhou, Xuzhou, and Wulumuqi during the winter (X.-Y. Yang et al., 2023; Shu et al., 2023; Ma et al., 2024). In contrast, the concentrations of TAs in XA were weakly correlated (P>0.05) with those of K+, SO2, and NO2. Several studies conducted in XA have suggested that aerosol with nitrogen-containing organic compounds can be largely derived from fossil fuel combustion and biomass burning (Zhang et al., 2023a, b; He et al., 2023; Yang et al., 2024). Moreover, the traditional method of identifying amine sources through correlation analysis (Berta et al., 2023; F. Liu et al., 2022; C. Liu et al., 2022; Huang et al., 2022; Corral et al., 2022) can also have significant uncertainties, as implied by the following two cases. First, the uptake of amines by aerosol particles might be constrained by low ALW concentration, weak particle acidity, or high-ammonia levels (F. Liu et al., 2022; D. Chen et al., 2022; Ge et al., 2011b; Sauerwein and Chan, 2017; Chan and Chan, 2013; Wang et al., 2010). Second, amines might be largely decomposed by atmospheric oxidants (e.g., hydroxyl radical and ozone) (Nielsen et al., 2012; Qiu and Zhang, 2013). Thus, the abovementioned weak correlations between aminiums and indicators in XA cannot definitely indicate that the contributions of fossil fuel combustion and biomass burning to amines in XA were insignificant. Presumably, the prerequisite for amine source apportionment using the correlation between aminiums and indicators is that the gas-phase amines can be largely converted into aminiums in PM2.5 through secondary processes without the influence of constrained factors. To further explore this issue, the following discussion focuses on the main factors affecting the formation of aminiums in particles.
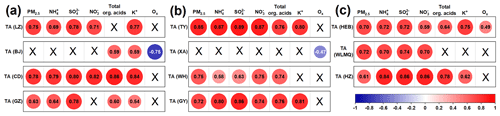
Figure 4Diagrams presenting the correlations between the concentrations of TAs and other parameters at (a–c) different sites. The colors of the different solid circles indicate different correlation coefficients (r). The size of the solid circle indicates the significance of the correlation between the two corresponding parameters; the larger circle indicates that the correlation is more significant, while the symbol “×” indicates that the P value is greater than 0.05.
The concentrations of TAs in LZ, TY, HEB, WLMQ, CD, WH, HZ, GZ, and GY showed significantly positive correlations (P<0.01) with those of the acidic components (e.g., NO, SO, organic acids, and acidity, expressed as [(NO 2SO) – NH]) (Feng et al., 2022), whereas an insignificant correlation (P > 0.05) was found between them in BJ and XA (Figs. 4 and S3). Thus, acid–base chemistry was tightly associated with the formation of aminiums in PM2.5 at all sites, except BJ and XA. A recent laboratory study has suggested that amines can be neutralized by H3O+ to form aminiums within picoseconds under conditions of high concentrations of particle sulfuric acid (Zhang et al., 2021). In addition, it has also been found that organic acids (e.g., formic acid) are able to participate in the nucleation of methanesulfonic acid–methylamine through an acid–base reaction (Zhang et al., 2022). The particles are acidic (especially on polluted days) at all study sites, with an average pH value ranging from 2.4 to 5.7 (Tables S1–S3). Amines can also partition into the particles by direct dissolution under high RH conditions (Ge et al., 2011b). Significantly increased RH values (i.e., high ALW) (Fig. 5a) and acidic components (Fig. 5b) on polluted days were also observed in XA and BJ. Nevertheless, the insignificant correlation between aminiums and acidic components and ALW concentrations in XA and BJ, together with a relatively small proportional increase in aminiums (Fig. 2) from clean to polluted days at these two sites, suggests that besides acidity and RH, there were other key factors affecting aminium formation in XA and BJ. As we know, the oxidative degradation of amines is one of the main pathways for the removal of atmospheric amines (Qiu and Zhang, 2013; Murphy et al., 2007). Furthermore, for atmospheric oxidants (e.g., hydroxyl radical) reacting with low-molecular-weight alkylamines, a negative temperature dependence of the rate coefficients has been reported (Nielsen et al., 2012). However, the winter air temperature in northern China was relatively low (<0 °C in XA and BJ) (Tables S1–S3); moreover, there was no significant change in the atmospheric oxidation (indicated by Ox levels (Ox = O3 + NO2)) of polluted and clean days in XA (higher Ox level during clean days) and BJ. In particular, the protonated amino group has been suggested to be difficult to undergo oxidation by hydroxyl radicals and ozone (Nielsen et al., 2012). Accordingly, atmospheric oxidation and temperature may not be the main factors affecting changes in aminium concentrations from clean to polluted days. Furthermore, the insignificant correlation between aminiums and acidic components in XA and BJ suggests that other factors affecting aminium formation must be considered.
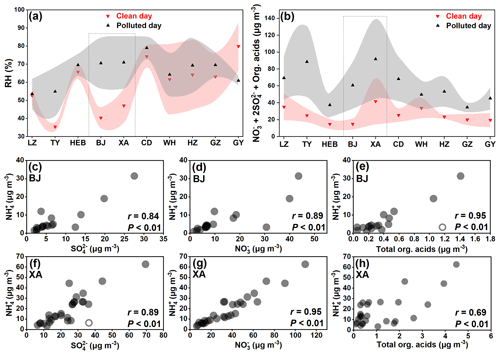
Figure 5The values of (a) RH and the concentrations of (b) major acidic components (expressed as NO + 2SO total organic acids) on clean and polluted days in different cities. The triangle and the shaded area represent the mean value and the associated standard deviation, respectively. The correlations of NH with the concentrations of NO, SO, and total organic acids at (c–e) BJ and (f–h) XA are shown. Open circles represent outliers.
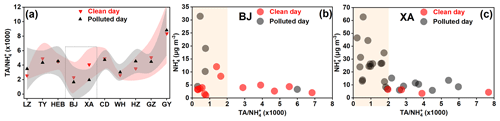
Figure 6The (a) average ratio of TA : NH on clean and polluted days in different cities. The triangle and the shaded area represent the mean value and the associated standard deviation, respectively. Scatterplots of the mass concentration of NH with the ratio of TAs to NH at the (b) BJ and (c) XA sites.
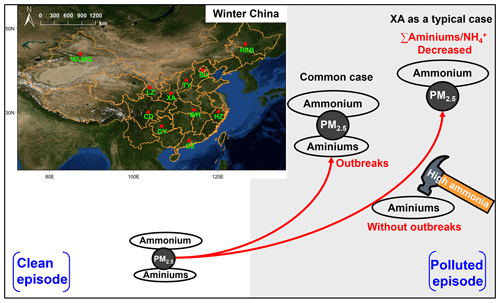
Furthermore, we found that the concentrations of NH were strongly (P<0.01) correlated with those of acidic components in XA and BJ (Fig. 5c–h). This indicates that the acidity of the particles was sufficient for the uptake of ammonia to form ammonium at these two study sites. Typically, the concentration of ammonia in the atmosphere is 1 to 3 orders of magnitude higher than that of low-molecular-weight alkylamines (Zheng et al., 2015; You et al., 2014; Yao et al., 2016; Wang et al., 2010). The uptake coefficient of alkylamines on acidic particles is lower than that of ammonia (Wang et al., 2010); moreover, Wang et al. (2010) proposed that fresh H2SO4 particles can be overwhelmingly neutralized by ammonia when both amines and ammonia are present in the air. In particular, although the strong acidic condition was conducive to the formation of aminiums, amines and ammonia may compete for uptake into acidic aerosol particles (Chen et al., 2022a). Thus, the constraint of ammonia on amine uptake at much higher ammonia levels than amine levels may be a possible explanation for the insignificant acid-dependent aminium formation in XA and BJ (Fig. 4a, b).
To further explore the role of ammonia (or ammonium) in aminium formation, the average ratios of TAs to NH on clean and polluted days in different cities were examined (Fig. 6a and Tables S1–S3). The average ratios of TAs to NH were found to be lower in XA and BJ, especially on the polluted days, which was similar to the characteristics of the TA : (NH3+ NH) ratios (Fig. S4). The sensitivity analysis of the TA : NH ratio (the lowest in XA and BJ) to NH changes (Figs. 6b, c and S5) suggests that when TA : NH > 2, the NH concentrations in XA and BJ remained at a relatively low level (less than 6 and 15 µg m−3 in BJ and XA, respectively) with the increase in the TA : NH ratio, indicating that the formation of aminiums was not limited by ammonia at low amine and ammonium levels (in this case, TA was significantly (P<0.01) correlated with NH). When TA : NH, the formation of aminiums may be constrained by higher amine and ammonium levels, which can also be supported by the insignificant (P>0.05) correlation between TA and NH in this case. In contrast, the distributions of the ratios of TA : NH in other cities were mainly in regions greater than 2 (i.e., the position where the X axis is equal to 2) (Fig. S5). The TA concentrations were thus significantly positively correlated with ammonium in these cities (excepting BJ and XA) (Fig. 4). A recent study on the uptake of marine aerosol DMA by acidic aerosols has found that the concentrations of particle DMAH+ generally decreased with increasing atmospheric ammonia concentrations (D. Chen et al., 2022); moreover, these researchers proposed the possibility that aminiums can be displaced by ammonia in a high-ammonia environment. Accordingly, high atmospheric ammonia levels can indeed constrain the conversion of amines to aminiums, even if the aerosol is acidic. In addition, due to the lower VC values (Tables S1–S3) on polluted days compared to clean days, the atmospheric amines were less able to diffuse on polluted days. This may result in an accumulation of aminiums on polluted days via acid–base chemistry. However, a large decrease in the average TA : NH and TA : (NH3 + NH) ratios from clean to polluted days occurred in XA (t test; P<0.05) (Figs. 6a, S4 and Tables S1–S3), followed by BJ. These results indicate that the uptake of amines on acidic particles relative to that of ammonia was significantly reduced from clean to polluted days in XA. It should be noted that this reduced case may also occur in BJ, while it is statistically insignificant. Presumably, the aminiums : ammonium ratio was likely an important indicator that reveals the competitive uptake of ammonia against amines on acidic aerosols or the displacement of aminiums by ammonia in a high-ammonia environment. Thus, this study provides a special field case that emphasizes the potential suppression of ammonia on aminium outbreaks during the polluted days.
The concentrations, compositions, and temporal and spatial variations in the aminiums in PM2.5 in 11 different Chinese cities during the winter were systematically investigated to reveal the key factors affecting the aminium outbreak during the polluted days. Specifically, MMAH+ was the dominant species among the aminiums investigated in PM2.5 in most cities, including LZ, XA, HEB, BJ, WLMQ, HZ, WH, CD, and GY, followed by DMAH+. In contrast, DEAH+ was found to be the most abundant aminium species in TY and GZ, followed by MMAH+ and DMAH+. This result can be attributed to the fact that the main sources of amines in TY and GZ were significantly different from those in other cities. However, due to the lack of amine emission inventories and sufficient tracers in these investigated cities, this study did not provide a detailed analysis of the specific sources of amines in these investigated cities.
We found that the concentrations of TAs and major aminiums in all cities showed a similar pattern of variation from the clean days to the polluted days, which was characterized by higher levels on the polluted days. However, the lowest percentage increase in TA concentration during the polluted days was found in XA (50 %) and BJ (25 %). Moreover, the concentrations of TAs in XA and BJ were insignificantly (P>0.05) correlated with those of PM2.5 and the main acidic components in PM2.5. However, the significant correlations of TAs with PM2.5 and the main acidic components were observed in other cities. Thus, acid–base chemistry was strongly associated with the formation of aminiums in PM2.5 in all cities, with the exception of XA and BJ. The concentrations of NH were significantly (P<0.01) correlated with those of the acidic components in XA and BJ, indicating that the acidity of the particles was sufficient for the uptake of ammonia to form ammonium at these two sites. Furthermore, based on the sensitivity analysis of the TA : NH ratio (the lowest in XA and BJ) to NH changes, as well as excluding the effects of ALW and atmospheric oxidation, we proposed a possibility about the competitive uptake of ammonia against amines on acidic aerosols in the ambient atmosphere in XA and BJ. This consideration may explain the insignificant acid-dependent aminium formation in XA and BJ. The main finding of this study has been illustrated in a diagram (Fig. 7).
In general, this study has preliminarily explored the characteristics of aminiums, ammonium, and PM2.5 from the clean days to the polluted days, according to the observational data from 11 different Chinese cities, highlighting the possibility of the competitive uptake of ammonia versus amines on acidic aerosols or the displacement of aminiums by ammonia under a high-ammonia condition. Although a recent study has also demonstrated the possibility of individual aminium being displaced by ammonia in an environment of high-ammonia level (D. Chen et al., 2022), the uptake of amines on particles to form aminiums and the mechanisms of relevant influencing factors are still not fully understood. This is because acidity, environmental ammonia and amine content, temperature, and liquid-phase reactions all affect the uptake of amines, although the acid–base neutralization of amines seems to be the most important pathway for amine uptake. Furthermore, if the uptake of amines is significantly constrained by the aforementioned factors, the traditional source-apportionment methods using correlation analysis between particle aminiums and tracers will have significant uncertainty due to the weakened partitioning of the amines into the particle phase (i.e., causing insignificant correlations between aminiums and indicators). Further laboratory validation experiments are required to substantiate this inference. In addition, it is essential to conduct prolonged observational research in settings with elevated ammonia levels and depleted amine concentrations in the near future.
The data in this study are available at https://doi.org/10.5281/zenodo.11102019 (Xu et al., 2024).
Four tables (Tables S1–S4) and five extensive figures (Figs. S1–S5) are provided in the Supplement. The supplement related to this article is available online. The supplement related to this article is available online at: https://doi.org/10.5194/acp-24-10531-2024-supplement.
YX and HYX designed the study. YX, YJM, QBS, HWX, and HX performed field measurements and sample collection. TL performed chemical analysis. YX performed data analysis. YX wrote the original draft. YX, HYX, and CQL reviewed and edited the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Regarding the maps used in this paper, please note that Figs. 1, 2, and 7 contain disputed territories.
The authors are very grateful to the editor and the anonymous referees for the kind and valuable comments that improved the paper.
This research has been supported by the National Natural Science Foundation of China (grant no. 42303081) and the Education and Scientific Research Project of Shanghai (grant no. 22YF1418700).
This paper was edited by Roya Bahreini and reviewed by three anonymous referees.
Berta, V. Z., Russell, L. M., Price, D. J., Chen, C.-L., Lee, A. K. Y., Quinn, P. K., Bates, T. S., Bell, T. G., and Behrenfeld, M. J.: Non-volatile marine and non-refractory continental sources of particle-phase amine during the North Atlantic Aerosols and Marine Ecosystems Study (NAAMES), Atmos. Chem. Phys., 23, 2765–2787, https://doi.org/10.5194/acp-23-2765-2023, 2023.
Chan, L. P. and Chan, C. K.: Role of the Aerosol Phase State in Ammonia/Amines Exchange Reactions, Environ. Sci. Technol., 47, 5755–5762, https://doi.org/10.1021/es4004685, 2013.
Chang, Y., Wang, H., Gao, Y., Jing, S. a., Lu, Y., Lou, S., Kuang, Y., Cheng, K., Ling, Q., Zhu, L., Tan, W., and Huang, R.-J.: Nonagricultural Emissions Dominate Urban Atmospheric Amines as Revealed by Mobile Measurements, Geophys. Res. Lett., 49, e2021GL097640, https://doi.org/10.1029/2021GL097640, 2022.
Chen, D., Yao, X., Chan, C. K., Tian, X., Chu, Y., Clegg, S. L., Shen, Y., Gao, Y., and Gao, H.: Competitive Uptake of Dimethylamine and Trimethylamine against Ammonia on Acidic Particles in Marine Atmospheres, Environ. Sci. Technol., 56, 5430–5439, https://doi.org/10.1021/acs.est.1c08713, 2022a.
Chen, Y., Lin, Q., Li, G., and An, T.: A new method of simultaneous determination of atmospheric amines in gaseous and particulate phases by gas chromatography-mass spectrometry, J. Environ. Sci., 114, 401–411, https://doi.org/10.1016/j.jes.2021.09.027, 2022b.
Chen, Y., Tian, M., Huang, R.-J., Shi, G., Wang, H., Peng, C., Cao, J., Wang, Q., Zhang, S., Guo, D., Zhang, L., and Yang, F.: Characterization of urban amine-containing particles in southwestern China: seasonal variation, source, and processing, Atmos. Chem. Phys., 19, 3245–3255, https://doi.org/10.5194/acp-19-3245-2019, 2019.
Choi, N. R., Lee, J. Y., Ahn, Y. G., and Kim, Y. P.: Determination of atmospheric amines at Seoul, South Korea via gas chromatography/tandem mass spectrometry, Chemosphere, 258, 127367, https://doi.org/10.1016/j.chemosphere.2020.127367, 2020.
Corral, A. F., Choi, Y., Collister, B. L., Crosbie, E., Dadashazar, H., DiGangi, J. P., Diskin, G. S., Fenn, M., Kirschler, S., Moore, R. H., Nowak, J. B., Shook, M. A., Stahl, C. T., Shingler, T., Thornhill, K. L., Voigt, C., Ziemba, L. D., and Sorooshian, A.: Dimethylamine in cloud water: a case study over the northwest Atlantic Ocean, Environ. Sci.-Atmos., 2, 1534–1550, https://doi.org/10.1039/D2EA00117A, 2022.
Dall'Osto, M., Airs, R. L., Beale, R., Cree, C., Fitzsimons, M. F., Beddows, D., Harrison, R. M., Ceburnis, D., O'Dowd, C., Rinaldi, M., Paglione, M., Nenes, A., Decesari, S., and Simó, R.: Simultaneous Detection of Alkylamines in the Surface Ocean and Atmosphere of the Antarctic Sympagic Environment, ACS Earth and Space Chem., 3, 854–862, https://doi.org/10.1021/acsearthspacechem.9b00028, 2019.
Facchini, M. C., Decesari, S., Rinaldi, M., Carbone, C., Finessi, E., Mircea, M., Fuzzi, S., Moretti, F., Tagliavini, E., Ceburnis, D., and O'Dowd, C. D.: Important Source of Marine Secondary Organic Aerosol from Biogenic Amines, Environ. Sci. Technol., 42, 9116–9121, https://doi.org/10.1021/es8018385, 2008.
Feng, X., Wang, C., Feng, Y., Cai, J., Zhang, Y., Qi, X., Li, Q., Li, J., and Chen, Y.: Outbreaks of Ethyl-Amines during Haze Episodes in North China Plain: A Potential Source of Amines from Ethanol Gasoline Vehicle Emission, Environ. Sci. Technol. Lett., 9, 306–311, 10.1021/acs.estlett.2c00145, 2022.
Gani, S., Bhandari, S., Seraj, S., Wang, D. S., Patel, K., Soni, P., Arub, Z., Habib, G., Hildebrandt Ruiz, L., and Apte, J. S.: Submicron aerosol composition in the world's most polluted megacity: the Delhi Aerosol Supersite study, Atmos. Chem. Phys., 19, 6843–6859, https://doi.org/10.5194/acp-19-6843-2019, 2019.
Ge, X., Wexler, A. S., and Clegg, S. L.: Atmospheric amines – Part I. A review, Atmos. Environ., 45, 524–546, https://doi.org/10.1016/j.atmosenv.2010.10.012, 2011a.
Ge, X., Wexler, A. S., and Clegg, S. L.: Atmospheric amines – Part II. Thermodynamic properties and gas/particle partitioning, Atmos. Environ., 45, 561–577, https://doi.org/10.1016/j.atmosenv.2010.10.013, 2011b.
Gibb, S. W., Mantoura, R. F. C., and Liss, P. S.: Ocean-atmosphere exchange and atmospheric speciation of ammonia and methylamines in the region of the NW Arabian Sea, Global Biogeochem. Cy., 13, 161–178, https://doi.org/10.1029/98GB00743, 1999.
He, K., Fu, T., Zhang, B., Xu, H., Sun, J., Zou, H., Zhang, Z., Hang Ho, S. S., Cao, J., and Shen, Z.: Examination of long-time aging process on volatile organic compounds emitted from solid fuel combustion in a rural area of China, Chemosphere, 333, 138957, https://doi.org/10.1016/j.chemosphere.2023.138957, 2023.
Ho, K.-F., Ho, S. S. H., Huang, R.-J., Chuang, H.-C., Cao, J.-J., Han, Y., Lui, K.-H., Ning, Z., Chuang, K.-J., Cheng, T.-J., Lee, S.-C., Hu, D., Wang, B., and Zhang, R.: Chemical composition and bioreactivity of PM2.5 during 2013 haze events in China, Atmos. Environ., 126, 162–170, https://doi.org/10.1016/j.atmosenv.2015.11.055, 2016.
Ho, K. F., Ho, S. S. H., Huang, R.-J., Liu, S. X., Cao, J.-J., Zhang, T., Chuang, H.-C., Chan, C. S., Hu, D., and Tian, L.: Characteristics of water-soluble organic nitrogen in fine particulate matter in the continental area of China, Atmos. Environ., 106, 252–261, https://doi.org/10.1016/j.atmosenv.2015.02.010, 2015.
Huang, S., Song, Q., Hu, W., Yuan, B., Liu, J., Jiang, B., Li, W., Wu, C., Jiang, F., Chen, W., Wang, X., and Shao, M.: Chemical composition and sources of amines in PM2.5 in an urban site of PRD, China, Environ. Res., 212, 113261, https://doi.org/10.1016/j.envres.2022.113261, 2022.
Huang, X., Deng, C., Zhuang, G., Lin, J., and Xiao, M.: Quantitative analysis of aliphatic amines in urban aerosols based on online derivatization and high performance liquid chromatography, Environ. Sci.-Process. Impact., 18, 796-801, 10.1039/C6EM00197A, 2016.
Kunwar, B. and Kawamura, K.: One-year observations of carbonaceous and nitrogenous components and major ions in the aerosols from subtropical Okinawa Island, an outflow region of Asian dusts, Atmos. Chem. Phys., 14, 1819–1836, https://doi.org/10.5194/acp-14-1819-2014, 2014.
Li, G., Liao, Y., Hu, J., Lu, L., Zhang, Y., Li, B., and An, T.: Activation of NF-κB pathways mediating the inflammation and pulmonary diseases associated with atmospheric methylamine exposure, Environ. Pollut., 252, 1216–1224, https://doi.org/10.1016/j.envpol.2019.06.059, 2019.
Lin, X., Xu, Y., Zhu, R.-G., Xiao, H.-W., and Xiao, H.-Y.: Proteinaceous Matter in PM2.5 in Suburban Guiyang, Southwestern China: Decreased Importance in Long-Range Transport and Atmospheric Degradation, J. Geophys. Res.-Atmos., 128, e2023JD038516, https://doi.org/10.1029/2023JD038516, 2023.
Liu, C., Li, H., Zheng, H., Wang, G., Qin, X., Chen, J., Zhou, S., Lu, D., Liang, G., Song, X., Duan, Y., Liu, J., Huang, K., and Deng, C.: Ocean Emission Pathway and Secondary Formation Mechanism of Aminiums Over the Chinese Marginal Sea, J. Geophys. Res.-Atmos., 127, e2022JD037805, https://doi.org/10.1029/2022JD037805, 2022.
Liu, F., Bi, X., Zhang, G., Peng, L., Lian, X., Lu, H., Fu, Y., Wang, X., Peng, P. A., and Sheng, G.: Concentration, size distribution and dry deposition of amines in atmospheric particles of urban Guangzhou, China, Atmos. Environ., 171, 279–288, https://doi.org/10.1016/j.atmosenv.2017.10.016, 2017.
Liu, F., Bi, X., Zhang, G., Lian, X., Fu, Y., Yang, Y., Lin, Q., Jiang, F., Wang, X., Peng, P. A., and Sheng, G.: Gas-to-particle partitioning of atmospheric amines observed at a mountain site in southern China, Atmos. Environ., 195, 1–11, https://doi.org/10.1016/j.atmosenv.2018.09.038, 2018.
Liu, F., Zhang, G., Lian, X., Fu, Y., Lin, Q., Yang, Y., Bi, X., Wang, X., Peng, P. A., and Sheng, G.: Influence of meteorological parameters and oxidizing capacity on characteristics of airborne particulate amines in an urban area of the Pearl River Delta, China, Environ. Res., 212, 113212, https://doi.org/10.1016/j.envres.2022.113212, 2022.
Liu, T., Xu, Y., Sun, Q.-B., Xiao, H.-W., Zhu, R.-G., Li, C.-X., Li, Z.-Y., Zhang, K.-Q., Sun, C.-X., and Xiao, H.-Y.: Characteristics, Origins, and Atmospheric Processes of Amines in Fine Aerosol Particles in Winter in China, J. Geophys. Res.-Atmos., 128, e2023JD038974, https://doi.org/10.1029/2023JD038974, 2023.
Liu, Z., Li, M., Wang, X., Liang, Y., Jiang, Y., Chen, J., Mu, J., Zhu, Y., Meng, H., Yang, L., Hou, K., Wang, Y., and Xue, L.: Large contributions of anthropogenic sources to amines in fine particles at a coastal area in northern China in winter, Sci. Total Environ., 839, 156281, https://doi.org/10.1016/j.scitotenv.2022.156281, 2022.
Ma, Y.-J., Xu, Y., Yang, T., Xiao, H.-W., and Xiao, H.-Y.: Measurement report: Characteristics of nitrogen-containing organics in PM2.5 in Ürümqi, northwestern China – differential impacts of combustion of fresh and aged biomass materials, Atmos. Chem. Phys., 24, 4331–4346, https://doi.org/10.5194/acp-24-4331-2024, 2024.
Møller, K. H., Berndt, T., and Kjaergaard, H. G.: Atmospheric Autoxidation of Amines, Environ. Sci. Technol., 54, 11087–11099, https://doi.org/10.1021/acs.est.0c03937, 2020.
Murphy, S. M., Sorooshian, A., Kroll, J. H., Ng, N. L., Chhabra, P., Tong, C., Surratt, J. D., Knipping, E., Flagan, R. C., and Seinfeld, J. H.: Secondary aerosol formation from atmospheric reactions of aliphatic amines, Atmos. Chem. Phys., 7, 2313–2337, https://doi.org/10.5194/acp-7-2313-2007, 2007.
Nielsen, C. J., Herrmann, H., and Weller, C.: Atmospheric chemistry and environmental impact of the use of amines in carbon capture and storage (CCS), Chem. Soc. Rev., 41, 6684–6704, https://doi.org/10.1039/C2CS35059A, 2012.
Qiu, C. and Zhang, R.: Multiphase chemistry of atmospheric amines, Phys. Chem. Chem. Phys., 15, 5738–5752, https://doi.org/10.1039/C3CP43446J, 2013.
Sauerwein, M. and Chan, C. K.: Heterogeneous uptake of ammonia and dimethylamine into sulfuric and oxalic acid particles, Atmos. Chem. Phys., 17, 6323–6339, https://doi.org/10.5194/acp-17-6323-2017, 2017.
Shen, W., Ren, L., Zhao, Y., Zhou, L., Dai, L., Ge, X., Kong, S., Yan, Q., Xu, H., Jiang, Y., He, J., Chen, M., and Yu, H.: C1-C2 alkyl aminiums in urban aerosols: Insights from ambient and fuel combustion emission measurements in the Yangtze River Delta region of China, Environ. Pollut., 230, 12–21, https://doi.org/10.1016/j.envpol.2017.06.034, 2017.
Shen, X., Chen, J., Li, G., and An, T.: A new advance in the pollution profile, transformation process, and contribution to aerosol formation and aging of atmospheric amines, Environ. Sci.-Atmos., 3, 444–473, https://doi.org/10.1039/D2EA00167E, 2023.
Shu, Q., Pei, C., Lin, X., Hong, D., Lai, S., and Zhang, Y.: Variations of aminiums in fine particles at a suburban site in Guangzhou, China: Importance of anthropogenic and natural emissions, Particuology, 80, 140–147, https://doi.org/10.1016/j.partic.2022.11.019, 2023.
Tao, Y., Ye, X., Jiang, S., Yang, X., Chen, J., Xie, Y., and Wang, R.: Effects of amines on particle growth observed in new particle formation events, J. Geophys. Res.-Atmos., 121, 324–335, https://doi.org/10.1002/2015JD024245, 2016.
Tao, Y., Liu, T., Yang, X., and Murphy, J. G.: Kinetics and Products of the Aqueous Phase Oxidation of Triethylamine by OH, ACS Earth Space Chem., 5, 1889–1895, https://doi.org/10.1021/acsearthspacechem.1c00162, 2021.
Tian, D., Fan, J., Jin, H., Mao, H., Geng, D., Hou, S., Zhang, P., and Zhang, Y.: Characteristic and Spatiotemporal Variation of Air Pollution in Northern China Based on Correlation Analysis and Clustering Analysis of Five Air Pollutants, J. Geophys. Res.-Atmos., 125, e2019JD031931, https://doi.org/10.1029/2019JD031931, 2020.
Tong, D., Chen, J., Qin, D., Ji, Y., Li, G., and An, T.: Mechanism of atmospheric organic amines reacted with ozone and implications for the formation of secondary organic aerosols, Sci. Total Environ., 737, 139830, https://doi.org/10.1016/j.scitotenv.2020.139830, 2020.
Wang, L., Lal, V., Khalizov, A. F., and Zhang, R.: Heterogeneous Chemistry of Alkylamines with Sulfuric Acid: Implications for Atmospheric Formation of Alkylaminium Sulfates, Environ. Sci. Technol., 44, 2461–2465, https://doi.org/10.1021/es9036868, 2010.
Wang, M., Wang, Q., Ho, S. S. H., Li, H., Zhang, R., Ran, W., Qu, L., Lee, S.-c., and Cao, J.: Chemical characteristics and sources of nitrogen-containing organic compounds at a regional site in the North China Plain during the transition period of autumn and winter, Sci. Total Environ., 812, 151451, https://doi.org/10.1016/j.scitotenv.2021.151451, 2022.
Xie, H., Feng, L., Hu, Q., Zhu, Y., Gao, H., Gao, Y., and Yao, X.: Concentration and size distribution of water-extracted dimethylaminium and trimethylaminium in atmospheric particles during nine campaigns - Implications for sources, phase states and formation pathways, Sci. Total Environ., 631–632, 130–141, https://doi.org/10.1016/j.scitotenv.2018.02.303, 2018.
Xu, Y., Xiao, H. Y., Wu, D. S., and Long, C. J.: Abiotic and biological degradation of atmospheric proteinaceous matter can contribute significantly to dissolved amino acids in wet deposition, Environ. Sci. Technol., 54, 6551–6561. https://doi.org/10.1021/acs.est.0c00421, 2020a.
Xu, Y., Miyazaki, Y., Tachibana, E., Sato, K., Ramasamy, S., Mochizuki, T., Sadanaga, Y., Nakashima, Y., Sakamoto, Y., Matsuda, K., and Kajii, Y.: Aerosol Liquid Water Promotes the Formation of Water-Soluble Organic Nitrogen in Submicrometer Aerosols in a Suburban Forest, Environ. Sci. Technol., 54, 1406–1414, https://doi.org/10.1021/acs.est.9b05849, 2020b.
Xu, Y., Dong, X.-N., Xiao, H.-Y., He, C., and Wu, D.-S.: Water-Insoluble Components in Rainwater in Suburban Guiyang, Southwestern China: A Potential Contributor to Dissolved Organic Carbon, J. Geophys. Res.-Atmos., 127, e2022JD037721, https://doi.org/10.1029/2022JD037721, 2022a.
Xu, Y., Dong, X.-N., Xiao, H.-Y., Zhou, J.-X., and Wu, D.-S.: Proteinaceous Matter and Liquid Water in Fine Aerosols in Nanchang, Eastern China: Seasonal Variations, Sources, and Potential Connections, J. Geophys. Res.-Atmos., 127, e2022JD036589, https://doi.org/10.1029/2022JD036589, 2022b.
Xu, Y., Dong, X.-N., He, C., Wu, D.-S., Xiao, H.-W., and Xiao, H.-Y.: Mist cannon trucks can exacerbate the formation of water-soluble organic aerosol and PM2.5 pollution in the road environment, Atmos. Chem. Phys., 23, 6775–6788, https://doi.org/10.5194/acp-23-6775-2023, 2023.
Xu, Y., Liu, T., Ma, Y. J., Sun, Q. B., Xiao, H. W., Xiao, H., Xiao, H. Y., and Liu, C. Q.: Measurement report: Occurrence of aminiums in PM2.5 during winter in China: aminium outbreak during polluted episodes and potential constraints, Zenodo [data set], https://doi.org/10.5281/zenodo.11102019, 2024.
Yang, T., Xu, Y., Ye, Q., Ma, Y.-J., Wang, Y.-C., Yu, J.-Z., Duan, Y.-S., Li, C.-X., Xiao, H.-W., Li, Z.-Y., Zhao, Y., and Xiao, H.-Y.: Spatial and diurnal variations of aerosol organosulfates in summertime Shanghai, China: potential influence of photochemical processes and anthropogenic sulfate pollution, Atmos. Chem. Phys., 23, 13433–13450, https://doi.org/10.5194/acp-23-13433-2023, 2023.
Yang, X.-Y., Cao, F., Fan, M.-Y., Lin, Y.-C., Xie, F., and Zhang, Y.-L.: Seasonal variations of low molecular alkyl amines in PM2.5 in a North China Plain industrial city: Importance of secondary formation and combustion emissions, Sci. Total Environ., 857, 159371, https://doi.org/10.1016/j.scitotenv.2022.159371, 2023.
Yang, X., Huang, S., Li, D., Xu, H., Zeng, Y., Yang, L., Wang, D., Zhang, N., Cao, J., and Shen, Z.: Water-soluble organic matter with various polarities in PM2.5 over Xi'an, China: Abundance, functional groups, and light absorption, Particuology, 84, 281-289, https://doi.org/10.1016/j.partic.2023.07.005, 2024.
Yao, L., Wang, M.-Y., Wang, X.-K., Liu, Y.-J., Chen, H.-F., Zheng, J., Nie, W., Ding, A.-J., Geng, F.-H., Wang, D.-F., Chen, J.-M., Worsnop, D. R., and Wang, L.: Detection of atmospheric gaseous amines and amides by a high-resolution time-of-flight chemical ionization mass spectrometer with protonated ethanol reagent ions, Atmos. Chem. Phys., 16, 14527–14543, https://doi.org/10.5194/acp-16-14527-2016, 2016.
Yao, L., Garmash, O., Bianchi, F., Zheng, J., Yan, C., Kontkanen, J., Junninen, H., Mazon, S. B., Ehn, M., Paasonen, P., Sipilä, M., Wang, M., Wang, X., Xiao, S., Chen, H., Lu, Y., Zhang, B., Wang, D., Fu, Q., Geng, F., Li, L., Wang, H., Qiao, L., Yang, X., Chen, J., Kerminen, V.-M., Petäjä, T., Worsnop, D. R., Kulmala, M., and Wang, L.: Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity, Science, 361, 278–281, https://doi.org/10.1126/science.aao4839, 2018.
You, Y., Kanawade, V. P., de Gouw, J. A., Guenther, A. B., Madronich, S., Sierra-Hernández, M. R., Lawler, M., Smith, J. N., Takahama, S., Ruggeri, G., Koss, A., Olson, K., Baumann, K., Weber, R. J., Nenes, A., Guo, H., Edgerton, E. S., Porcelli, L., Brune, W. H., Goldstein, A. H., and Lee, S.-H.: Atmospheric amines and ammonia measured with a chemical ionization mass spectrometer (CIMS), Atmos. Chem. Phys., 14, 12181–12194, https://doi.org/10.5194/acp-14-12181-2014, 2014.
Zhang, B., Shen, Z., He, K., Sun, J., Huang, S., Xu, H., Li, J., Ho, S. S. H., and Cao, J.-J.: Insight into the Primary and Secondary Particle-Bound Methoxyphenols and Nitroaromatic Compound Emissions from Solid Fuel Combustion and the Updated Source Tracers, Environ. Sci. Technol., 57, 14280–14288, https://doi.org/10.1021/acs.est.3c04370, 2023a.
Zhang, B., Shen, Z., He, K., Zhang, L., Huang, S., Sun, J., Xu, H., Li, J., Yang, L., and Cao, J.: Source Profiles of Particle-Bound Phenolic Compounds and Aromatic Acids From Fresh and Aged Solid Fuel Combustion: Implication for the Aging Mechanism and Newly Proposed Source Tracers, J. Geophys. Res.-Atmos., 128, e2023JD039758, https://doi.org/10.1029/2023JD039758, 2023b.
Zhang, R., Shen, J., Xie, H.-B., Chen, J., and Elm, J.: The role of organic acids in new particle formation from methanesulfonic acid and methylamine, Atmos. Chem. Phys., 22, 2639–2650, https://doi.org/10.5194/acp-22-2639-2022, 2022.
Zhang, W., Zhong, J., Shi, Q., Gao, L., Ji, Y., Li, G., An, T., and Francisco, J. S.: Mechanism for Rapid Conversion of Amines to Ammonium Salts at the Air–Particle Interface, J. Am. Chem. Soc., 143, 1171–1178, https://doi.org/10.1021/jacs.0c12207, 2021.
Zhang, Y.-L. and Cao, F.: Fine particulate matter (PM2.5) in China at a city level, Sci. Rep., 5, 14884, https://doi.org/10.1038/srep14884, 2015.
Zheng, J., Ma, Y., Chen, M., Zhang, Q., Wang, L., Khalizov, A. F., Yao, L., Wang, Z., Wang, X., and Chen, L.: Measurement of atmospheric amines and ammonia using the high resolution time-of-flight chemical ionization mass spectrometry, Atmos. Environ., 102, 249–259, https://doi.org/10.1016/j.atmosenv.2014.12.002, 2015.