the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
SO2 enhances aerosol formation from anthropogenic volatile organic compound ozonolysis by producing sulfur-containing compounds
Zhaomin Yang
Narcisse T. Tsona
Xin Luo
Sulfur dioxide (SO2) can affect aerosol formation in the atmosphere, but the underlying mechanisms remain unclear. Here, we investigate aerosol formation and composition from the ozonolysis of cyclooctene with and without SO2 addition in a smog chamber. Liquid chromatography equipped with high-resolution tandem mass spectrometry measurements indicates that monomer carboxylic acids and corresponding dimers with acid anhydride and aldol structures are important components in particles formed in the absence of SO2. A 9.4–12.6-times increase in particle maximum number concentration is observed in the presence of 14–192 ppb SO2. This increase is largely attributed to sulfuric acid (H2SO4) formation from the reactions of stabilized Criegee intermediates with SO2. In addition, a number of organosulfates (OSs) are detected in the presence of SO2, which are likely products formed from the heterogeneous reactions of oxygenated species with H2SO4. The molecular structures of OSs are also identified based on tandem mass spectrometry analysis. It should be noted that some of these OSs have been found in previous field studies but were classified as compounds from unknown sources or of unknown structures. The observed OSs are less volatile than their precursors and are therefore more effective contributors to particle formation and growth, partially leading to the increase in particle volume concentration under SO2-presence conditions. Our results provide an in-depth molecular-level insight into how SO2 alters particle formation and composition.
- Article
(2893 KB) - Full-text XML
-
Supplement
(2091 KB) - BibTeX
- EndNote
Secondary organic aerosol (SOA) accounts for a large fraction of the organic aerosol mass. The atmospheric oxidation of anthropogenic volatile organic compounds (AVOCs) can produce low-volatility organic products that contribute to SOA formation and growth (Kelly et al., 2018; Fan et al., 2020). The oxidation of AVOCs can dominate SOA formation under severe haze episodes (Nie et al., 2022; He et al., 2020; Huang et al., 2019; Qiu et al., 2020). Thus, AVOCs have been commonly considered as significant SOA precursors. SOA can negatively impact air quality, global climate, and public health (Nault et al., 2021; Zhu et al., 2017). To better understand air pollution and develop effective particle control strategies, it is necessary to investigate the formation mechanism and molecular composition of anthropogenic SOA.
Recently, the impacts of inorganic gases on aerosol chemistry have received significant attention (Deng et al., 2022). In particular, there is increasing evidence that sulfur dioxide (SO2) can modulate SOA formation and composition (Ye et al., 2018; Stangl et al., 2019; Liu et al., 2017). Liu et al. (2017) reported that SOA formation from cyclohexene photooxidation was inhibited by atmospherically relevant concentrations of SO2, as a result of the reaction of hydroxyl radical (•OH) with SO2 (to form sulfuric acid (H2SO4)) competing with the •OH reaction with cyclohexene. They demonstrated that H2SO4-catalyzed SOA enhancement was not sufficient to compensate for the loss of •OH reactivity towards cyclohexene, leading to the suppression in cyclohexene SOA formation. On the other hand, SO2 can enhance SOA formation and alter SOA composition by interacting with organic peroxides or stabilized Criegee intermediate (sCI) during the ozonolysis of alkenes (Stangl et al., 2019; Ye et al., 2018). For instance, under humid conditions, the reactive uptake of SO2 into organic aerosols was obvious and reactions of SO2 with organic peroxides could contribute to organosulfate (OS) formation (S. Wang et al., 2021; Ye et al., 2018). H2SO4 originating from sCI-induced oxidation of SO2 is also linked to OS production (Stangl et al., 2019). OSs have been detected in different SO2-alkene interaction areas (Hettiyadura et al., 2019; Wang et al., 2018; Bruggemann et al., 2020). Ubiquitous OSs may be used as tracers of SOA influenced by SO2 emissions (Bruggemann et al., 2020). To gain further mechanistic insights into the complex roles of SO2 in SOA formation, it is important to explore the chemical nature and formation mechanism of OSs.
Cycloalkenes emitted from diesel vehicles and industrial processes are a crucial class of AVOCs in the atmosphere. They can be used to explore key chemical processes involved in atmospheric oxidation and SOA formation (Räty et al., 2021). However, SOA formation chemistry from cycloalkenes has received less attention than that from linear or branched alkenes, leading to significant uncertainties in our understanding of SOA. Recent studies have reported that ozonolysis of cycloalkenes could form highly oxidized products and have considerable SOA yield (Räty et al., 2021; Rissanen, 2018). Among the most common cycloalkenes (with 5 to 8 carbon atoms), cyclooctene has the largest potential for SOA formation (Keywood et al., 2004). Ozonolysis is the dominant oxidation pathway of cyclooctene, with a reaction rate constant of 4.51 × 10−16 cm3 molec.−1 s−1 (298 K). Urban atmosphere is highly complex and may contain various concentrations of cycloalkenes and SO2, which complicates SOA formation and composition. While most previous studies have identified compounds containing carbon, hydrogen, and oxygen atoms (CHO compounds) as important contributors to cycloalkene SOA (Hamilton et al., 2006; Gao et al., 2004; Räty et al., 2021), the potential of OS formation from the ozonolysis of cyclooctene in the presence of SO2 and the chemical processes behind OS formation remain unclear.
Given the significance of cycloalkene and SO2 emissions in aerosol formation, we investigated the effects of SO2 on the formation and chemical composition of cyclooctene SOA. Aerosol particles were formed from the ozonolysis of cyclooctene in the absence and presence of SO2 in a smog chamber. Structural identifications of the observed products were reported and corresponding formation mechanisms were proposed. We report the mechanism, showing how SO2 impacts particle formation and growth based on the observation of sulfuring-containing compounds. Our results provide a more comprehensive mechanistic understanding of the roles of SO2 in modulating SOA formation and composition.
2.1 Particle production
Particle formation from the ozonolysis of cyclooctene (k298 K=4.51 × 10−16 cm3 molec.−1 s−1) was carried out under dark conditions in a 1.2 m3 Teflon chamber housed in a temperature-controlled room. A summary of experimental conditions and results is listed in Table 1. Detailed experimental equipment and methods have been described in our previous studies (Yang et al., 2022, 2021). Particle formation experiments were operated in a batch mode. Briefly, cyclooctene was introduced into the chamber by passing zero air through a tube containing a known volume of cyclooctene (95 %, Alfa). Then, cyclohexane (99.5 %, Aladdin) was injected in excess (∼ 130 ppm) into the chamber so that more than 98 % of •OH generated during the ozonolysis of cyclooctene was scavenged. Control experiments showed that the presence of cyclohexane could lead to a significant decrease in particle volume concentration (Fig. S1 in the Supplement). When desired, SO2 was added to the chamber from an SO2 calibration cylinder. Initial concentration ratios of SO2 to cyclooctene were in the range of ∼ 0.07–1 ppb ppb−1 to simulate different polluted atmospheric conditions. The reactor was stabilized for 20 min under dark conditions to allow for mixing of species. Finally, ozonolysis of cyclooctene was initiated by introducing ozone (O3) produced via a commercial ozone generator (WH-H-Y5Y, Wohuan, China). All experiments were performed at room temperature (∼ 295 K) and atmospheric pressure (∼ 1 atm) without seed particles. Temperature and relative humidity (RH) inside the chamber were measured with a hygrometer (Model 645, Testo AG, Germany). O3 and SO2 concentrations over the course of ozonolysis were monitored by a Thermo Scientific model 49i O3 analyzer and a Thermo Scientific model 43i-TLE SO2 analyzer, respectively. The detection limits of O3 analyzer and SO2 analyzer were 0.5 and 0.05 ppb, respectively. Size distributions and volume concentrations of particles were continuously recorded using a scanning mobility particle sizer (SMPS), which consisted of a differential mobility analyzer (Model 3082, TSI, USA) and an ultrafine condensation particle counter (Model 3776, TSI, USA). The particle volume concentration was measured continuously until we observed a decrease. The particle formation experiments proceeded for 300 min before the collection of aerosol particles.
Table 1Experimental conditions and results for particle-formation experiments.
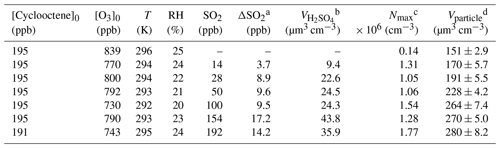
a ΔSO2 represents the consumed SO2 concentration during the ozonolysis of cyclooctene. b The volume concentration of particle-phase H2SO4 assuming a full conversion of SO2 to H2SO4 with a density of 1.58 g cm−3 under moderate humidity conditions (Wyche et al., 2009; Ye et al., 2018). c Nmax denotes the maximum number concentration of aerosol particles during the ozonolysis of cyclooctene. d Vparticle is the volume concentration of aerosol particles, which has been corrected for wall loss of particles. Errors represent standard deviation for particle-formation experiments.
2.2 Particle collection and chemical characterization
Aerosol particles were collected on aluminum foils using a 14-stage low-pressure impactor (DLPI+, Dekati Ltd, Finland). All samples were stored in a −20 ∘C freezer until analysis. Offline functional group measurements of aerosol particles were performed using an attenuated total reflectance-Fourier transform infrared spectrometer (ATR-FTIR, Vertex 70, Bruker, Germany). Before each measurement, the diamond crystal was thoroughly cleaned with ethanol and ultrapure water to eliminate the interference of ambient contaminants on functional group measurements of aerosol particles. ATR-FTIR spectra of blank aluminum foils and aerosol samples were recorded in the range of 4000–600 cm−1 at a resolution of 4 cm−1 with 64 scans. The data of ATR-FTIR spectra were recorded with the OPUS software.
Aerosol particles were also collected on polytetrafluoroethylene (PTFE) filters (0.22 µm pore size, 47 mm diameter, TJMF50, Jinteng, China). The whole sample filters were extracted twice into 5 mL of methanol (Optima® LC-MS grade, Fisher Scientific) by ice sonication (KQ5200E, Kunshan Ultrasonic Instruments, China) for 20 min. Extracts were then filtered, concentrated to near dryness, and subsequently reconstituted in 200 µL of 50:50 () methanol and ultrapure water. Blank filters were also subjected to the same extraction and preparation procedure. Obtained extracts of blank and sample filters were analyzed using a Thermo Scientific ultrahigh-performance liquid chromatograph, which was coupled with a high-resolution Q Exactive Focus Hybrid Quadrupole-Orbitrap mass spectrometer equipped with an electrospray ionization (ESI) source (UHPLC/ESI-HRMS). Samples were first separated on an Atlantis T3 C18 column (100 Å pores, 3 µm particle size, 2.1 mm × 150 mm, Waters, USA) at 35 ∘C. The used binary mobile-phase system consisted of ultrapure water with 0.1 % () formic acid (A) and methanol with 0.1 % () formic acid (B). The LC gradient employed was as follows: 0–3 min at 3 % B, 3–25 min increased linearly to 50 % B, 25–43 min ramped linearly to 90 % B, 43–48 min returned to 3 % B, and 48–60 min B held constant at 3 % to re-equilibrate the column. The injected volume of samples and flow rate were 2 µL and 200 µL min−1, respectively. The ESI source was operated in both positive (+) and negative (−) ion modes to ionize analyte components with a scan range of mass-to-charge () of 50 to 750. LC/ESI-MS parameter settings were as follows: 3.5 kV spray voltage (+), −3.0 kV spray voltage (−), 50 V S-lens radiofrequency (RF) level (+), 50 V S-lens RF level (−), 320 ∘C capillary temperature, 2.76 × 105 Pa sheath gas (nitrogen) pressure, and 3.33 L min−1 auxiliary gas (nitrogen) flow. Data-dependent tandem mass spectrometry (MS/MS) analysis was also carried out by high-energy collision-induced dissociation (CID) with stepped collision energies of 20, 40, and 60 eV. For MS/MS experiments, an isolation width of 2 units was applied. Other parameters were also selected in MS/MS experiments as follows: 2 × 105 automatic gain control (AGC) target, 50 ms maximum IT, 3 loop count, 1 × 105 minimum AGC target, 2–6 s apex trigger, and 6 s dynamic exclusion. The mass resolution of MS and MS/MS were 70 000 (full width at half maximum, FWHM, at 200) and 17 500, respectively. Detailed data processes are reported elsewhere (Yang et al., 2021, 2022).
The double bond equivalent (DBE) value is the combined number of rings and double bonds in the product CcHhOoNnSs (Here, subscripts c, h, o, n, and s represent the number of carbon, hydrogen, oxygen, nitrogen, and sulfur atom in the product CcHhOoNnSs.) and could be calculated according to Eq. (1). For organosulfate, the two S = O bonds in the sulfate group were not considered based on calculations in previous studies (Wang et al., 2016; Riva et al., 2016b; Kuang et al., 2016). The DBE value of organosulfate reflects the unsaturation degree of its side carbon chain.
Kendrick mass defect (KMD) analysis could provide chemical insights into the chemical compositions of complex organic mixtures (Kundu et al., 2017; Kenseth et al., 2020). The KMD value is the same for homologous species that differ from each other only by their base units. CH2 and the oxygen atom (O) are usually chosen as base units for Kendrick analysis of complex organic mass spectra. Kendrick mass (KM) could be converted into a new mass scale from the IUPAC mass (Eqs. 2 and 4). KMD is determined as the difference between the nominal mass of a compound (the rounded integer mass) and KM (Eqs. 3 and 5).
The saturation mass concentration (Co, µg m−3) of product i was also calculated based on its elemental composition using the following expression (Li et al., 2016):
where is the reference carbon number; and , represent the numbers of carbon, oxygen, nitrogen, and sulfur atoms, respectively; bC,bO, bN, and bS denote the contribution of each carbon, oxygen, nitrogen, and sulfur atom to ; and bCO is the carbon–oxygen nonideality.
2.3 Wall loss corrections
The wall loss rates of O3 and SO2 inside the chamber were determined to be 2.05 × 10−4 and 2.02 × 10−4 min−1 (Fig. S2), respectively, indicating that the losses of these two gas-phase species to the chamber walls were negligible over the course of experiments. The wall loss of cyclooctene (5.23 × 10−6 min−1) was also negligible, while its oxidation products may deposit to the inner walls. However, wall losses of gas-phase products could be mitigated due to excess O3 concentration (Sect. S1 in the Supplement). The quick oxidation and nucleation could provide attractive condensation surfaces for oxidation products, thereby reducing the product wall losses to some extent (Stirnweis et al., 2017). Although wall losses of organic vapors may underestimate the particle mass, this work mainly focuses on the characterization of particle composition rather than the absolute SOA yield.
Independent wall-loss experiments of ammonium sulfate ((NH4)2SO4) particles were also performed to determine the size-dependent wall-loss rate constants of particles inside the chamber. An aqueous (NH4)2SO4 solution was added to a TSI Model 3076 atomizer to produce droplets. The droplets were passed through a silica gel diffusion dryer to get dry (NH4)2SO4 particles and then these were injected into the chamber. The size distributions of (NH4)2SO4 particles were characterized using the SMPS for 6 h. The relationship between the wall-loss rate (k, h−1) of particles and their size (dp, nm) can be expressed as k(dp)=1.20 × 10−7 × × based on a size-dependent particle wall-loss correction method.
3.1 SO2 effects on aerosol formation
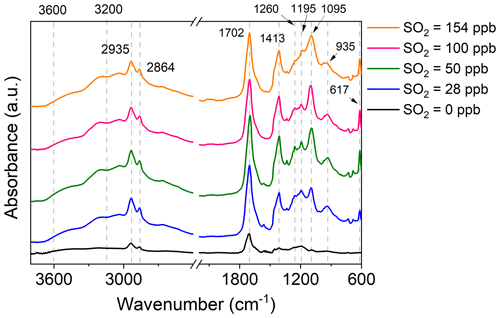
Insights into SO2 effects on particle formation could be gained through investigating the number and volume concentration as well as the size distribution of particles under various SO2 level conditions. In the absence of SO2, the particle number concentration increased in a burst within the first 20 min of cyclooctene ozonolysis and then decreased because of their coagulation and wall deposition, while the particle volume concentration increased gradually and reached its maximum within 240 min (Fig. S3). An elevating SO2 level can result in significant increases in the number and volume concentration of particles (Fig. 1a), which is consistent with observations from previous studies (Ye et al., 2018; Yang et al., 2021). We observed a 9.4–12.6-times increase in particle maximum number concentration in the presence of 14–192 ppb SO2 (Table 1). The promoted effect of SO2 is shown more clearly in Fig. 1b, where SO2 was seen to be consumed on a similar timescale to particle formation. Specifically, upon initiation of cyclooctene ozonolysis, SO2 concentration decreased and the particle volume concentration increased simultaneously. After cyclooctene was completely consumed, both SO2 consumption and particle production slowed down. SO2 consumption and particle formation resumed when more cyclooctene was introduced into the reactor. This result indicates that SO2 may react with certain highly reactive species produced from cyclooctene ozonolysis. For instance, reactions of SO2 with sCI could form H2SO4 (Boy et al., 2013), which is a key species for new particle formation (Lehtipalo et al., 2018; Yao et al., 2018). Inorganic sulfate absorption at 617 cm−1 was observed in the ATR-FTIR spectra of particles formed in cyclooctene–O3–SO2 systems (Fig. 2) (Hawkins et al., 2010; Coury and Dillner, 2008), indicating the formation of H2SO4. We assumed that all consumed SO2 was converted to particle-phase H2SO4, which represents an upper limit of H2SO4 formation (Wyche et al., 2009; Ye et al., 2018). The amount of H2SO4 produced could not fully account for the enhancement of particle volume concentration (Table 1). H2SO4 has been considered as an important driver of particle acidity (Tilgner et al., 2021). Acid catalysis induced by H2SO4 may also promote the formation of additional organic products, leading to the increase in particle volume concentration (Deng et al., 2021).
SO2 can also affect the growth of new aerosol particles (Fig. 1c). Once O3 was introduced into the reactor, aerosol particles were produced rapidly. After cyclooctene was depleted, the aerosol particle mass increased slowly. The initial stage of particle formation was then defined as the time from reaction initiation to the complete consumption of cyclooctene (∼ 10 min). From Fig. 1c, in the initial stage of ozonolysis (10 min), particles formed in cyclooctene–O3–SO2 systems had a smaller size mode than those formed in the cyclooctene–O3 system, which may be attributed to the following two factors: first, oligomers formed from sCI reactions with organic species could partition into the condensed phase to contribute to particle growth (Riva et al., 2017). SO2 presence may lead to a decrease in these oligomers because SO2 can compete with organic species to react with sCI. Second, counterbalancing the reduction of oligomers via sCI + SO2 reactions is the production of H2SO4. The production of more new particles in cyclooctene–O3–SO2 systems could provide more condensation sinks. Organic vapors that can condense onto particles are dispersed via new particles, resulting in small particle size at the initial phase of cyclooctene–O3–SO2 systems (Stangl et al., 2019). Interestingly, particles could grow quickly in the presence of SO2. At 300 min reaction time, particles formed in the presence of SO2 even had slightly larger sizes than those formed in the absence of SO2. H2SO4-catalyzed heterogenous reactions could produce lower-volatility organic species from higher-volatility reactants in the aerosol phase (Yang et al., 2020; Han et al., 2016). Semi-volatile species could undergo evaporation after partitioning to the aerosol phase while low-volatility products generally have a negligible evaporation rate from the aerosol phase. Low-volatility products formed via H2SO4-catalyzed heterogenous reactions could build particle mass at a rate almost equal to the condensation rate and, thus, effectively facilitate particle growth in cyclooctene–O3–SO2 systems (Apsokardu and Johnston, 2018).
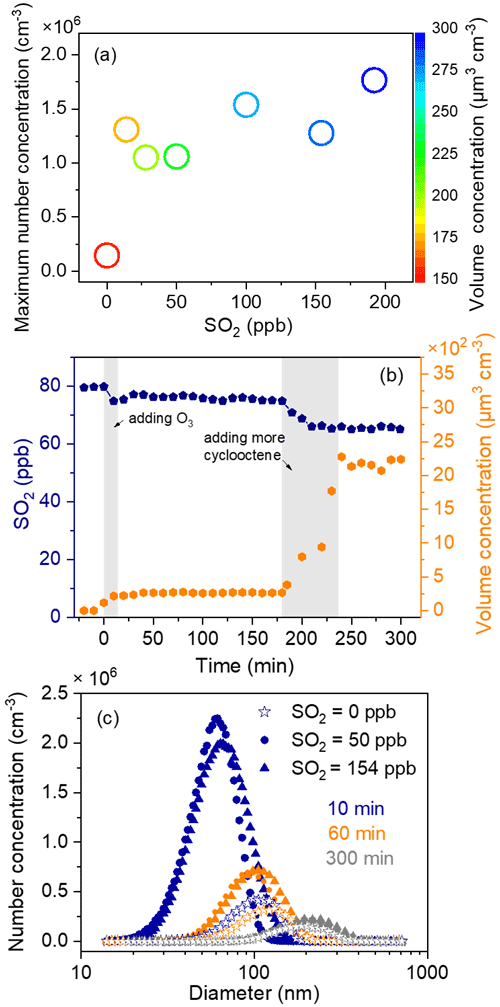
Figure 1Particle formation from the ozonolysis of cyclooctene under various SO2 conditions. (a) Maximum particle number concentration as a function of initial SO2 level. Circle color represents particle volume concentration. (b) Temporal profiles of SO2 concentration and particle volume concentration. (c) Size distributions of aerosol particles formed with various SO2 concentrations at 10, 60, and 300 min after the initiation of cyclooctene ozonolysis.
3.2 Aerosol chemical composition under SO2-free conditions
Figure 3 shows the base peak chromatograms (BPCs) of cyclooctene-derived particles in the absence of SO2. The chromatograms of blank filter clearly showed no peaks eluted at retention times (RTs) between 0 and 30 min, while there were several significant peaks for cyclooctene SOA chromatograms in both positive and negative ion modes. Each chromatogram peak of cyclooctene SOA represents at least one ion, and major peaks are only labeled with the mass of the most abundant single ion. Compared to the negative chromatogram of cyclooctene SOA, the corresponding label ions in the positive chromatogram were 24 Da higher in mass. This is consistent with the fact that many ions produce adducts with sodium ion ([M + Na]+) in positive ion mode, while negative ion mode leads to the production of deprotonated ions ([M – H]−) (Mackenzie-Rae et al., 2018). From Fig. 3, products with molecular weight (MW) <200 Da eluted from the column at shorter RTs than those with MW >200 Da. Low-molecular-weight products (MW <200 Da) likely correspond to small monomer type compounds (hereafter termed monomeric products), which directly originate from the ozonolysis of cyclooctene. Compounds with MW >200 Da mainly dominate the later part of the chromatogram, and they may be homo- or heterodimeric species (hereafter denoted dimeric products) formed using two monomeric products as building blocks.
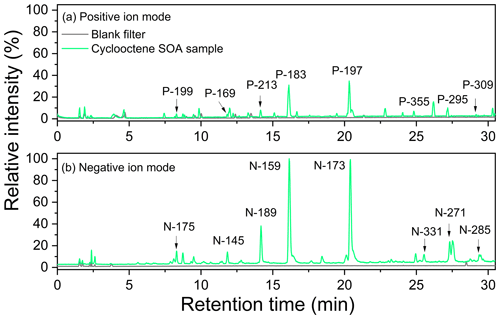
Figure 3Base peak chromatograms of both blank filter and particles generated from the ozonolysis of cyclooctene in the absence of SO2. Labels represent the most abundant single ion of each peak. (a) Positive ion mode. (b) Negative ion mode.
Possible structures of major monomeric products were proposed based on their accurate , fragmentation mass spectra, and previous mechanistic insights. Note that the fragmentation of [M + Na]+ is relatively difficult (Zhao et al., 2016) and, thus, the positive ion mode was not further analyzed in providing structural insights in the current study. The negative chromatogram peaks with RT at 11.85 min (N-145), 16.13 min (N-159), and 20.41 min (N-173) were significant peaks for cyclooctene SOA (Fig. 3b), and they were assigned neutral formulas of C6H10O4, C7H12O4, and C8H14O4, respectively. As shown in Fig. 4, MS/MS spectra of monomer C6H10O4, C7H12O4, and C8H14O4 were similar. Taking C8H14O4 as an example (Fig. 4c), its fragmentation mass spectrum was characterized by a loss of 44 Da (CO2), suggesting the presence of a carboxyl group. The neutral loss of 18 Da (H2O) upon fragmentation of the parent ion (C8H13O, ) led to the production of an ion with 155.07143. The loss of H2O is an unspecific fragmentation mechanism, which likely originates from a carboxyl or hydroxyl group (Noziere et al., 2015). The fragment ion () representing the simultaneous neutral losses of CO2 and H2O was also formed. MS/MS spectra can result from multiple isomeric structures in many cases (Wang et al., 2019). Yasmeen et al. (2011) showed the detailed fragmentation spectrum for the dicarboxylic acid standard (azelaic acid) and indicated that deprotonated azelaic acid also showed losses of H2O, CO2, and CO2+ H2O. In addition, Noziere et al. (2015) showed that the neutral losses of CO2 and H2O indicates two carboxyl groups. Thus, monomer C8H14O4 was tentatively assigned to suberic acid and the corresponding fragmentation pathway for C8H13O is proposed in Fig. S4. The fragment ions originating from losses of H2O, CO2, and CO2+ H2O were also observed in MS/MS spectra of C6H10O4 and C7H12O4, indicative of adipic acid and pimelic acid, respectively. Carboxylic acids have also been observed in SOA produced from previous alkene ozonolysis (Hamilton et al., 2006; Kenseth et al., 2020; Mackenzie-Rae et al., 2018; Zhang et al., 2015). Carboxylic acids represent a significant class of aerosol components, and they play a significant role in particle chemistry via their influences on particle acidity and through direct involvement in certain heterogeneous reactions to produce low-volatility species (Millet et al., 2015). More experiments using available authentic standards are necessary to better understand their structures, sources, and formation mechanism. Other prominent monomer peaks at RTs of 8.30 min (N-175) and 14.18 min (N-189) corresponded to compounds with neutral formula, namely C7H12O5 and C8H14O5. The losses of H2O, CO, and CO2 in the MS/MS spectrum of C7H12O5 indicated hydroxyl, terminal carbonyl, and carboxyl groups, respectively (Mackenzie-Rae et al., 2018; Riva et al., 2016a), and C7H12O5 was identified as hydroxy-containing oxoheptanoic acid (Fig. S5a and c). Monomer C8H14O5 only showed losses of H2O and CO (Fig. S5b), and it is difficult to determine the specific type and positioning of oxygen-containing functionalities within C8H14O5 with five oxygen atoms based on its MS/MS spectrum.
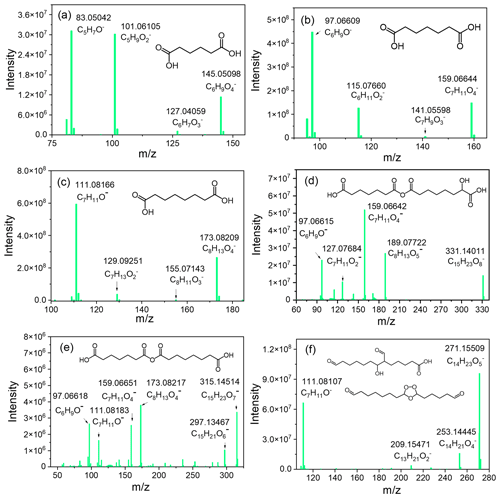
Figure 4MS/MS spectra of major monomers and dimers. Monomers: (a) C6H10O4, (b) C7H12O4, and (c) C8H14O4. Dimers: (d) C15H24O8, (e) C15H24O7, and (f) C14H24O5.
The labeled dimer peaks in negative ion mode corresponded to [M − H]− ion masses of 271, 285, and 331 (Fig. 3b), which were assigned neutral formulas of C14H24O5, C15H26O5, and C15H24O8, respectively. The number of fragment ions of dimers are generally limited, and determining the exact structure of dimers is less certain compared to monomers (Witkowski and Gierczak, 2017). Therefore, only a decrease in molecular structure possibilities is provided. For dimer C15H24O8, fragment ions 159.06642 (C7H11O) and 189.07722 (C8H13O) were detected in the MS/MS spectrum (Fig. 4d). When dimers are subjected to CID, fragment ions corresponding to their building blocks are commonly observed (Witkowski and Gierczak, 2017; Hall and Johnston, 2012). Based on this rule, it could be concluded that dimer C15H24O8 was an association product of C7H12O4 and C8H14O5. Similarly, for dimer C15H24O7, there were two significant product ions of C15H23O, with accurate masses of 159.06651 (C7H11O) and 173.08217 (C8H13O) (Fig. 4e). Furthermore, fragment ions corresponding to secondary loss of CO2+ H2O from product ions C7H11O and C8H13O were also observed. The fragmentation spectrum of C15H24O7 was similar to the MS/MS spectra of C7H12O4 and C8H14O4 (Fig. 4b and c), suggesting again that C7H12O4 and C8H14O4 may be the building blocks of C15H24O7. Acid-catalyzed heterogeneous processes can result in the formation of high-molecular-weight dimers in both biogenic and anthropogenic systems (Barsanti et al., 2017). Carboxylic acid monomers formed could be important sources of particle acidity in the absence of SO2. Dimers C15H24O7 and C15H24O8 may be produced by heterogeneous reactions involving the loss of a water molecule, and the linkage between building blocks is an acid anhydride (Fig. S6) (Hamilton et al., 2006). Another abundant dimer peak (N-271) in the negative chromatogram was identified as C14H23O with mass accuracy of −0.02492 ppm. C14H23O could dissociate to the product ions of C14H21O, C13H21O, and C7H11O− (Fig. 4f). Both secondary ozonide and aldol structures shown in Fig. 4f could match the assigned elemental formula of C14H24O5. However, the neutral losses of H2O and CO2 were not easily produced by secondary ozonide, but were more likely for the aldol structure (Hall and Johnston, 2012). Aldol condensation products were also among the most commonly observed species in previous ozonolysis of alkenes (Zhao et al., 2016; Kenseth et al., 2018; Kristensen et al., 2016). Therefore, C14H24O5 as shown in Fig. 4f is likely an aldol condensation product.
To examine the overall composition of particles, average mass spectra (Fig. S7) corresponding to the chromatogram where particle components eluted were also analyzed. Figure 5 summarizes the oxidation products observed in particles mapped in O-KMD and van Krevelen plot. The molecular formulas of identified oxidation products could be largely classified into homologous series of monomers and dimers (Fig. 5a and b). The elemental composition distribution of products measured in positive and negative ion modes was similar, with most monomers and dimers having ratios ranging from 0.2 to 0.8, and ratios ranging from 1.2 to 1.8 (Fig. 5c). Lines with slopes of 0, −0.5, −1, and −2 in Fig. 5c can be used to illustrate the addition of hydroxyl/peroxide, carboxylic acid (with fragmentation), carboxylic acid (without fragmentation), and carbonyl groups to a saturated carbon chain, respectively (Heald et al., 2010). As shown in Fig. 5c, cyclooctene SOA occupied a relatively wide range in the van Krevelen diagram, and there are a large number of points scattered in the space between lines with slopes of −0.5 and −2. This behavior is consistent with the importance of high-abundance carboxylic acids in the above analysis.
3.3 SO2 effects on aerosol chemical composition
To obtain further detailed mechanisms regarding SO2 effects and determine whether heterogeneous processes occur, aerosol samples were analyzed using ATR-FTIR and LC/ESI-MS. Both IR and MS analysis of particles revealed changes in aerosol chemical composition induced by SO2 addition.
3.3.1 Characteristics of functional group in aerosol-phase products
Figure 2 shows ATR-FTIR spectra of aerosol particles. Hydroxy (3600–3200 cm−1), alkyl (2935 and 2864 cm−1), and carbonyl (1702 cm−1) groups were identified in particles collected from the cyclooctene–O3 system (Table S1 in the Supplement). These particles also had a broad absorption across the 1500–800 cm−1 region, which may arise from C–H deformation in 1480–1350 cm−1, C–C stretching in 1250–1120 cm−1, and C–O stretching in different regions for various oxygenated species (Hung et al., 2013). Three additional absorption bands at 1413, 1095, and 617 cm−1 were observed in ATR-FTIR spectra of particles formed with the introduction of SO2 (Tammer, 2004; Lal et al., 2012). Absorption bands at 1413 and 1095 cm−1 may be associated with asymmetric and symmetric stretching of −SO2− while inorganic sulfates could give rise to strong absorption at 617 cm−1. The presence of absorption bands of sulfur-containing groups suggests that SO2 addition can result in the production of sulfur-containing compounds.
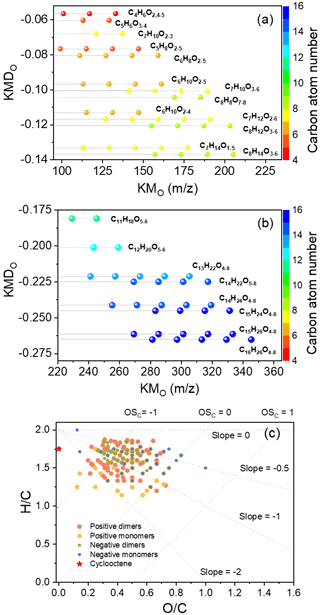
Figure 5Oxidation products observed in particles produced from the ozonolysis of cyclooctene in the absence of SO2. Oxygen (O) Kendrick mass defect plots of (a) monomers and (b) dimers. (c) Van Krevelen diagram.
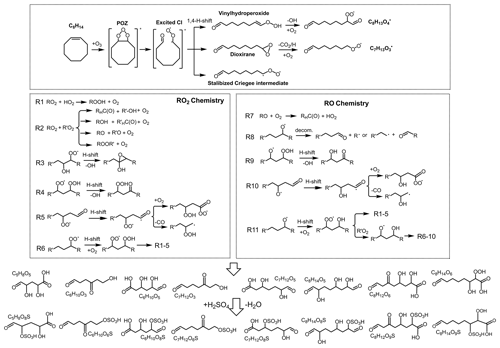
Figure 6Simplified formation schemes for the selected organosulfates formed from the ozonolysis of cyclooctene.
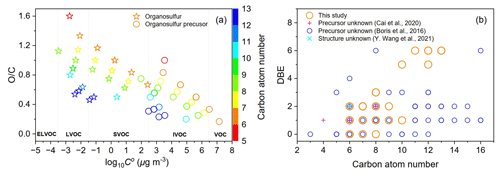
Figure 7(a) Two-dimensional volatility–oxidation space of the identified organosulfurs and their precursors. (b) Carbon atom number distribution of organosulfurs observed in the current work and in the studies of Cai et al. (2020), Boris et al. (2016), and Y. Wang et al. (2021). Detailed formulae of these OSs can be found in Table S3. Organosulfurs from previous studies are of unknown origin or unknown structure.
3.3.2 Organosulfate formation in the presence of SO2
In addition to CHO compounds, products with CcHhOoSs elemental formulas were identified in the presence of SO2 (Fig. S8). OS could undergo highly efficient ionization to give deprotonated molecular ions in negative ion mode. Based on MS/MS analysis, unambiguous identification of OS can be achieved, since OSs could give characteristic fragment ions at 80 (SO), 81 (HSO), and/or 97 (HSO) in their MS/MS spectra (Figs. S9–S17). Accurate mass measurements of OSs as well as their retention times and DBE values are provided in Table S2. The proposed structure and fragmentation scheme of each OS and the corresponding precursor are presented in Figs. S9–S17. For instance, OS-209 and OS-223 showed prominent product ions for losses of HSO and SO (Figs. S11–S12), confirming the organosulfate moiety. Neither a hydroxyl nor a carboxyl group fragment ion (i.e., −H2O or −CO2) was observed in their MS/MS spectra. C6H10O3 and C7H12O3 were proposed as the precursors of OS-209 and OS-223, respectively. MS/MS spectra of C6H10O3 and C7H12O3 were characterized by loss of CO, indicating a terminal carbonyl group (Figs. S11–S12). Considering structural features of OS precursor measurements as well as OS-209 and OS-223 all corresponding to DBE = 2, two carbonyl groups could well explain the observed MS/MS spectra of OS-209 and OS-223. The organosulfate substituent was expected to attach to a terminal carbon atom. Although the carbonyl group is more readily observed in positive ion mode, ESI-MS is also highly sensitive to carbonyl compounds containing sulfate substituents and thereby gives intense [M − H]− ions in negative ion mode (Riva et al., 2016b).
A relatively high abundance of OS is helpful for the acquisition of MS/MS data, and therefore high-abundance [M − H]− ions were chosen as representative candidates to clarify the precursors and formation pathways of OSs. A simplified chemical mechanism describing OS production from the ozonolysis of cyclooctene (C8H14) is proposed in Fig. 6. The ozonolysis of cyclooctene (C8H14) can be initiated by O3 addition to the endocyclic double bond, forming an energy-rich primary ozonide (POZ). POZ can decompose rapidly to an excited CI containing both a terminal carbonyl and carbonyl oxide group. The excited CI could lead to the formation of sCI, vinylhydroperoxide, and dioxirane, illustrating the multiplicity and the complexity of cyclooctene ozonolysis. SCI is mainly capable of involvement in bimolecular reactions to form carboxylic acids and acid esters. Vinylhydroperoxide rapidly decomposes into an alkyl radical (C8H13O2•) and an •OH. Molecular oxygen could be subsequently added to C8H13O2• to produce an alkyl peroxy radical (RO2, C8H13O4•). Dioxirane intermediate may also undergo decomposition and produce C7H13O3•. C8H13O4• and C7H13O3• are considered as the starting point of the RO2• and alkoxy radical (RO•) chemistry, resulting in termination CHO compounds with hydroperoxy, carbonyl, or hydroxy groups (Fig. 6). Acid-catalyzed heterogenous reactions of CHO products have been evidenced to play a major role in OS formation in the atmosphere (Riva et al., 2016c, b). Although acidic seed particles were not directly injected into the reactor during cyclooctene ozonolysis, SO2-induced H2SO4 may create acidic conditions for the occurrence of heterogeneous reactions. In the case of CHO products with a hydroxyl group, H2SO4 could protonate the hydroxyl group, leading to the formation of OS and water. The low RH (∼ 20 %) of ozonolysis was helpful for shifting the reaction equilibrium in favor of OS production.
Detailed information about the volatility of oxidation products is necessary to evaluate their potential to contribute to aerosol formation. As shown in Fig. 7a, the products could be categorized into intermediate-volatility OCs (IVOCs), semi-volatile OCs (SVOCs), low-volatility OCs (LVOCs), and extremely low-volatility OCs (ELVOC), with Co in the range of 300–3 × 106, 0.3–300, 3 × 10−4–0.3, and <3 × 10−4 µg m−3, respectively (Donahue et al., 2011). The saturation mass concentration of OSs spanned more than six orders of magnitude (Fig. 7a), suggesting their inherent chemical complexity. A large number of OSs are SVOCs and LVOCs, while their precursors are classified as IVOCs and SVOCs, indicating that the SO2 presence facilitates the reduction of product volatility (Yang et al., 2020; Han et al., 2016). Lower-volatility OSs generated from acid-catalyzed heterogenous reactions may build particle mass at a faster rate compared to their higher-volatility precursors, and thereby benefit the formation and growth of particles in the presence of SO2.
Figure 7b displays the DBE–carbon atom number space for organosulfur compounds. There are some overlaps of organosulfur compounds detected in this work with previous data from field observations (Y. Wang et al., 2021; Boris et al., 2016; Cai et al., 2020). For example, Y. Wang et al. (2021) comprehensively analyzed OS in PM2.5 filter samples collected at an urban site in Shanghai, China, and observed the presence of C6H10O6S (Fig. 7b, cyan cross). In the absence of chromatographic data such as retention times, C6H10O6S was tentatively assigned to diesel vapor-derived OS. Alkenes are important components of diesel and cyclooctene may be also responsible for C6H10O6S formation in the atmosphere. The overlaps of organosulfur compounds indicate that the ozonolysis of cycloalkenes in the presence of SO2 is likely an important source of organosulfur compounds in the ambient atmosphere. In addition, our work further suggests that the sources of OS cannot be determined only based on their elemental formula, and techniques that enable the identification of molecular structures (e.g., MS/MS) are greatly beneficial in field studies. The identified molecular structures of OSs in this study are also helpful in source apportionment in field studies.
We have explored O3-initiated oxidation of cyclooctene in the absence and presence of SO2, with a focus on the mechanism by which SO2 impacts particle formation and composition. Cyclooctene can produce a large number of particles upon reacting with O3. Higher SO2 concentration led to higher particle number concentration as a result of H2SO4 formation from the reactions of sCI with SO2.
Cyclooctene SOA mainly consisted of carboxylic acids and corresponding dimers with acid anhydride and aldol structures when SO2 was not added. SO2 addition can induce changes in particle chemical composition through the formation of OSs. Some OSs, classified as compounds of unknown origin or unknown structure in previous field studies, were also observed in this work. The OSs found here are less volatile than their precursors, indicating the stronger ability of OS for particle formation. The formation of OSs can in part lead to the increase in particle volume concentrations in the presence of SO2.
The results here suggest that SO2 can influence aerosol particle formation and composition by producing sulfur-containing compounds (i.e., H2SO4 and OSs). Nevertheless, the observed number of OSs may be amplified by the high SO2 concentration used in the present work. In order to determine the actual mass yields of OSs and better quantify roles of SO2 in particle formation, further experiments using ambient SO2 levels and authentic standards are warranted.
Experimental data are available upon request to the corresponding author.
The supplement related to this article is available online at: https://doi.org/10.5194/acp-23-417-2023-supplement.
ZY designed the experiments and carried them out. ZY performed data analysis with assistance from XL, NTT, KL, and LD. ZY prepared the paper with contributions from all co-authors. NTT, KL, and LD commented on the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research has been supported by the National Natural Science Foundation of China (grant no. 22076099), the Department of Education of Shandong Province (grant no. 2019KJD007), and the Fundamental Research Fund of Shandong University (grant no. 2020QNQT012).
This paper was edited by Dantong Liu and reviewed by two anonymous referees.
Apsokardu, M. J. and Johnston, M. V.: Nanoparticle growth by particle-phase chemistry, Atmos. Chem. Phys., 18, 1895–1907, https://doi.org/10.5194/acp-18-1895-2018, 2018.
Barsanti, K. C., Kroll, J. H., and Thornton, J. A.: Formation of Low-Volatility Organic Compounds in the Atmosphere: Recent Advancements and Insights, J. Phys. Chem. Lett., 8, 1503–1511, https://doi.org/10.1021/acs.jpclett.6b02969, 2017.
Boris, A. J., Lee, T., Park, T., Choi, J., Seo, S. J., and Collett Jr., J. L.: Fog composition at Baengnyeong Island in the eastern Yellow Sea: detecting markers of aqueous atmospheric oxidations, Atmos. Chem. Phys., 16, 437–453, https://doi.org/10.5194/acp-16-437-2016, 2016.
Boy, M., Mogensen, D., Smolander, S., Zhou, L., Nieminen, T., Paasonen, P., Plass-Dülmer, C., Sipilä, M., Petäjä, T., Mauldin, L., Berresheim, H., and Kulmala, M.: Oxidation of SO2 by stabilized Criegee intermediate (sCI) radicals as a crucial source for atmospheric sulfuric acid concentrations, Atmos. Chem. Phys., 13, 3865–3879, https://doi.org/10.5194/acp-13-3865-2013, 2013.
Bruggemann, M., Xu, R., Tilgner, A., Kwong, K. C., Mutzel, A., Poon, H. Y., Otto, T., Schaefer, T., Poulain, L., Chan, M. N., and Herrmann, H.: Organosulfates in Ambient Aerosol: State of Knowledge and Future Research Directions on Formation, Abundance, Fate, and Importance, Environ. Sci. Technol., 54, 3767–3782, https://doi.org/10.1021/acs.est.9b06751, 2020.
Cai, D., Wang, X., Chen, J., and Li, X.: Molecular characterization of organosulfates in highly polluted atmosphere using ultra-high-resolution mass spectrometry, J. Geophys. Res.-Atmos., 125, e2019JD032253, https://doi.org/10.1029/2019jd032253, 2020.
Coury, C. and Dillner, A. M.: A method to quantify organic functional groups and inorganic compounds in ambient aerosols using attenuated total reflectance FTIR spectroscopy and multivariate chemometric techniques, Atmos. Environ., 42, 5923–5932, https://doi.org/10.1016/j.atmosenv.2008.03.026, 2008.
Deng, H., Lakey, P. S. J., Wang, Y., Li, P., Xu, J., Pang, H., Liu, J., Xu, X., Li, X., Wang, X., Zhang, Y., Shiraiwa, M., and Gligorovski, S.: Daytime SO2 chemistry on ubiquitous urban surfaces as a source of organic sulfur compounds in ambient air, Sci. Adv., 8, eabq6830, https://doi.org/10.1126/sciadv.abq6830, 2022.
Deng, Y., Inomata, S., Sato, K., Ramasamy, S., Morino, Y., Enami, S., and Tanimoto, H.: Temperature and acidity dependence of secondary organic aerosol formation from α-pinene ozonolysis with a compact chamber system, Atmos. Chem. Phys., 21, 5983–6003, https://doi.org/10.5194/acp-21-5983-2021, 2021.
Donahue, N. M., Epstein, S. A., Pandis, S. N., and Robinson, A. L.: A two-dimensional volatility basis set: 1. organic-aerosol mixing thermodynamics, Atmos. Chem. Phys., 11, 3303–3318, https://doi.org/10.5194/acp-11-3303-2011, 2011.
Fan, Y., Liu, C.-Q., Li, L., Ren, L., Ren, H., Zhang, Z., Li, Q., Wang, S., Hu, W., Deng, J., Wu, L., Zhong, S., Zhao, Y., Pavuluri, C. M., Li, X., Pan, X., Sun, Y., Wang, Z., Kawamura, K., Shi, Z., and Fu, P.: Large contributions of biogenic and anthropogenic sources to fine organic aerosols in Tianjin, North China, Atmos. Chem. Phys., 20, 117–137, https://doi.org/10.5194/acp-20-117-2020, 2020.
Gao, S., Keywood, M., Ng, N. L., Surratt, J., Varutbangkul, V., Bahreini, R., Flagan, R. C., and Seinfeld, J. H.: Low-molecular-weight and oligomeric components in secondary organic aerosol from the ozonolysis of cycloalkenes and alpha-pinene, J. Phys. Chem. A, 108, 10147–10164, https://doi.org/10.1021/jp047466e, 2004.
Hall, W. A. and Johnston, M. V.: Oligomer Formation Pathways in Secondary Organic Aerosol from MS and MS/MS Measurements with High Mass Accuracy and Resolving Power, J. Am. Soc. Mass Spectr., 23, 1097–1108, https://doi.org/10.1007/s13361-012-0362-6, 2012.
Hamilton, J. F., Lewis, A. C., Reynolds, J. C., Carpenter, L. J., and Lubben, A.: Investigating the composition of organic aerosol resulting from cyclohexene ozonolysis: low molecular weight and heterogeneous reaction products, Atmos. Chem. Phys., 6, 4973–4984, https://doi.org/10.5194/acp-6-4973-2006, 2006.
Han, Y., Stroud, C. A., Liggio, J., and Li, S.-M.: The effect of particle acidity on secondary organic aerosol formation from α-pinene photooxidation under atmospherically relevant conditions, Atmos. Chem. Phys., 16, 13929–13944, https://doi.org/10.5194/acp-16-13929-2016, 2016.
Hawkins, L. N., Russell, L. M., Covert, D. S., Quinn, P. K., and Bates, T. S.: Carboxylic acids, sulfates, and organosulfates in processed continental organic aerosol over the southeast Pacific Ocean during VOCALS-REx 2008, J. Geophys. Res., 115, D13201, https://doi.org/10.1029/2009jd013276, 2010.
He, X., Wang, Q., Huang, X. H. H., Huang, D. D., Zhou, M., Qiao, L., Zhu, S., Ma, Y.-g., Wang, H.-l., Li, L., Huang, C., Xu, W., Worsnop, D. R., Goldstein, A. H., and Yu, J. Z.: Hourly measurements of organic molecular markers in urban Shanghai, China: Observation of enhanced formation of secondary organic aerosol during particulate matter episodic periods, Atmos. Environ., 240, 117807, https://doi.org/10.1016/j.atmosenv.2020.117807, 2020.
Heald, C. L., Kroll, J. H., Jimenez, J. L., Docherty, K. S., DeCarlo, P. F., Aiken, A. C., Chen, Q., Martin, S. T., Farmer, D. K., and Artaxo, P.: A simplified description of the evolution of organic aerosol composition in the atmosphere, Geophys. Res. Lett., 37, L08803, https://doi.org/10.1029/2010gl042737, 2010.
Hettiyadura, A. P. S., Al-Naiema, I. M., Hughes, D. D., Fang, T., and Stone, E. A.: Organosulfates in Atlanta, Georgia: anthropogenic influences on biogenic secondary organic aerosol formation, Atmos. Chem. Phys., 19, 3191–3206, https://doi.org/10.5194/acp-19-3191-2019, 2019.
Huang, G., Liu, Y., Shao, M., Li, Y., Chen, Q., Zheng, Y., Wu, Z., Liu, Y., Wu, Y., Hu, M., Li, X., Lu, S., Wang, C., Liu, J., Zheng, M., and Zhu, T.: Potentially Important Contribution of Gas-Phase Oxidation of Naphthalene and Methylnaphthalene to Secondary Organic Aerosol during Haze Events in Beijing, Environ. Sci. Technol., 53, 1235–1244, https://doi.org/10.1021/acs.est.8b04523, 2019.
Hung, H. M., Chen, Y. Q., and Martin, S. T.: Reactive aging of films of secondary organic material studied by infrared spectroscopy, J. Phys. Chem. A, 117, 108–116, https://doi.org/10.1021/jp309470z, 2013.
Kelly, J. M., Doherty, R. M., O'Connor, F. M., and Mann, G. W.: The impact of biogenic, anthropogenic, and biomass burning volatile organic compound emissions on regional and seasonal variations in secondary organic aerosol, Atmos. Chem. Phys., 18, 7393–7422, https://doi.org/10.5194/acp-18-7393-2018, 2018.
Kenseth, C. M., Huang, Y., Zhao, R., Dalleska, N. F., Hethcox, J. C., Stoltz, B. M., and Seinfeld, J. H.: Synergistic O3 + OH oxidation pathway to extremely low-volatility dimers revealed in beta-pinene secondary organic aerosol, P. Natl. Acad. Sci. USA, 115, 8301–8306, https://doi.org/10.1073/pnas.1804671115, 2018.
Kenseth, C. M., Hafeman, N. J., Huang, Y., Dalleska, N. F., Stoltz, B. M., and Seinfeld, J. H.: Synthesis of Carboxylic Acid and Dimer Ester Surrogates to Constrain the Abundance and Distribution of Molecular Products in alpha-Pinene and beta-Pinene Secondary Organic Aerosol, Environ. Sci. Technol., 54, 12829–12839, https://doi.org/10.1021/acs.est.0c01566, 2020.
Keywood, M. D., Varutbangkul, V., Bahreini, R., Flagan, R. C., and Seinfeld, J. H.: Secondary organic aerosol formation from the ozonolysis of cycloalkenes and related compounds, Environ. Sci. Technol., 38, 4157–4164, https://doi.org/10.1021/es035363o, 2004.
Kristensen, K., Watne, Å. K., Hammes, J., Lutz, A., Petäjä, T., Hallquist, M., Bilde, M., and Glasius, M.: High-Molecular Weight Dimer Esters Are Major Products in Aerosols from α-Pinene Ozonolysis and the Boreal Forest, Environ. Sci. Technol. Lett., 3, 280–285, https://doi.org/10.1021/acs.estlett.6b00152, 2016.
Kuang, B. Y., Lin, P., Hu, M., and Yu, J. Z.: Aerosol size distribution characteristics of organosulfates in the Pearl River Delta region, China, Atmos. Environ., 130, 23–35, https://doi.org/10.1016/j.atmosenv.2015.09.024, 2016.
Kundu, S., Fisseha, R., Putman, A. L., Rahn, T. A., and Mazzoleni, L. R.: Molecular formula composition of β-caryophyllene ozonolysis SOA formed in humid and dry conditions, Atmos. Environ., 154, 70–81, https://doi.org/10.1016/j.atmosenv.2016.12.031, 2017.
Lal, V., Khalizov, A. F., Lin, Y., Galvan, M. D., Connell, B. T., and Zhang, R.: Heterogeneous reactions of epoxides in acidic media, J. Phys. Chem. A, 116, 6078–6090, https://doi.org/10.1021/jp2112704, 2012.
Lehtipalo, K., Yan, C., Dada, L., Bianchi, F., Xiao, M., Wagner, R., Stolzenburg, D., Ahonen, L. R., Amorim, A., Baccarini, A., Bauer, P. S., Baumgartner, B., Bergen, A., Bernhammer, A. K., Breitenlechner, M., Brilke, S., Buchholz, A., Mazon, S. B., Chen, D. X., Chen, X. M., Dias, A., Dommen, J., Draper, D. C., Duplissy, J., Ehn, M., Finkenzeller, H., Fischer, L., Frege, C., Fuchs, C., Garmash, O., Gordon, H., Hakala, J., He, X. C., Heikkinen, L., Heinritzi, M., Helm, J. C., Hofbauer, V., Hoyle, C. R., Jokinen, T., Kangasluoma, J., Kerminen, V. M., Kim, C., Kirkby, J., Kontkanen, J., Kurten, A., Lawler, M. J., Mai, H. J., Mathot, S., Mauldin, R. L., Molteni, U., Nichman, L., Nie, W., Nieminen, T., Ojdanic, A., Onnela, A., Passananti, M., Petaja, T., Piel, F., Pospisilova, V., Quelever, L. L. J., Rissanen, M. P., Rose, C., Sarnela, N., Schallhart, S., Schuchmann, S., Sengupta, K., Simon, M., Sipila, M., Tauber, C., Tome, A., Trostl, J., Vaisanen, O., Vogel, A. L., Volkamer, R., Wagner, A. C., Wang, M. Y., Weitz, L., Wimmer, D., Ye, P. L., Ylisirnio, A., Zha, Q. Z., Carslaw, K. S., Curtius, J., Donahue, N. M., Flagan, R. C., Hansel, A., Riipinen, I., Virtanen, A., Winkler, P. M., Baltensperger, U., Kulmala, M., and Worsnop, D. R.: Multicomponent new particle formation from sulfuric acid, ammonia, and biogenic vapors, Sci. Adv., 4, eaau5363, https://doi.org/10.1126/sciadv.aau5363, 2018.
Li, Y., Pöschl, U., and Shiraiwa, M.: Molecular corridors and parameterizations of volatility in the chemical evolution of organic aerosols, Atmos. Chem. Phys., 16, 3327–3344, https://doi.org/10.5194/acp-16-3327-2016, 2016.
Liu, S., Jia, L., Xu, Y., Tsona, N. T., Ge, S., and Du, L.: Photooxidation of cyclohexene in the presence of SO2: SOA yield and chemical composition, Atmos. Chem. Phys., 17, 13329–13343, https://doi.org/10.5194/acp-17-13329-2017, 2017.
Mackenzie-Rae, F. A., Wallis, H. J., Rickard, A. R., Pereira, K. L., Saunders, S. M., Wang, X., and Hamilton, J. F.: Ozonolysis of α-phellandrene – Part 2: Compositional analysis of secondary organic aerosol highlights the role of stabilised Criegee intermediates, Atmos. Chem. Phys., 18, 4673–4693, https://doi.org/10.5194/acp-18-4673-2018, 2018.
Millet, D. B., Baasandorj, M., Farmer, D. K., Thornton, J. A., Baumann, K., Brophy, P., Chaliyakunnel, S., de Gouw, J. A., Graus, M., Hu, L., Koss, A., Lee, B. H., Lopez-Hilfiker, F. D., Neuman, J. A., Paulot, F., Peischl, J., Pollack, I. B., Ryerson, T. B., Warneke, C., Williams, B. J., and Xu, J.: A large and ubiquitous source of atmospheric formic acid, Atmos. Chem. Phys., 15, 6283–6304, https://doi.org/10.5194/acp-15-6283-2015, 2015.
Nault, B. A., Jo, D. S., McDonald, B. C., Campuzano-Jost, P., Day, D. A., Hu, W., Schroder, J. C., Allan, J., Blake, D. R., Canagaratna, M. R., Coe, H., Coggon, M. M., DeCarlo, P. F., Diskin, G. S., Dunmore, R., Flocke, F., Fried, A., Gilman, J. B., Gkatzelis, G., Hamilton, J. F., Hanisco, T. F., Hayes, P. L., Henze, D. K., Hodzic, A., Hopkins, J., Hu, M., Huey, L. G., Jobson, B. T., Kuster, W. C., Lewis, A., Li, M., Liao, J., Nawaz, M. O., Pollack, I. B., Peischl, J., Rappenglück, B., Reeves, C. E., Richter, D., Roberts, J. M., Ryerson, T. B., Shao, M., Sommers, J. M., Walega, J., Warneke, C., Weibring, P., Wolfe, G. M., Young, D. E., Yuan, B., Zhang, Q., de Gouw, J. A., and Jimenez, J. L.: Secondary organic aerosols from anthropogenic volatile organic compounds contribute substantially to air pollution mortality, Atmos. Chem. Phys., 21, 11201–11224, https://doi.org/10.5194/acp-21-11201-2021, 2021.
Nie, W., Yan, C., Huang, D. D., Wang, Z., Liu, Y., Qiao, X., Guo, Y., Tian, L., Zheng, P., Xu, Z., Li, Y., Xu, Z., Qi, X., Sun, P., Wang, J., Zheng, F., Li, X., Yin, R., Dallenbach, K. R., Bianchi, F., Petäjä, T., Zhang, Y., Wang, M., Schervish, M., Wang, S., Qiao, L., Wang, Q., Zhou, M., Wang, H., Yu, C., Yao, D., Guo, H., Ye, P., Lee, S., Li, Y. J., Liu, Y., Chi, X., Kerminen, V.-M., Ehn, M., Donahue, N. M., Wang, T., Huang, C., Kulmala, M., Worsnop, D., Jiang, J., and Ding, A.: Secondary organic aerosol formed by condensing anthropogenic vapours over China's megacities, Nat. Geosci., 15, 255–261, https://doi.org/10.1038/s41561-022-00922-5, 2022.
Noziere, B., Kalberer, M., Claeys, M., Allan, J., D'Anna, B., Decesari, S., Finessi, E., Glasius, M., Grgic, I., Hamilton, J. F., Hoffmann, T., Iinuma, Y., Jaoui, M., Kahnt, A., Kampf, C. J., Kourtchev, I., Maenhaut, W., Marsden, N., Saarikoski, S., Schnelle-Kreis, J., Surratt, J. D., Szidat, S., Szmigielski, R., and Wisthaler, A.: The molecular identification of organic compounds in the atmosphere: state of the art and challenges, Chem. Rev., 115, 3919–3983, https://doi.org/10.1021/cr5003485, 2015.
Qiu, X., Wang, S., Ying, Q., Duan, L., Xing, J., Cao, J., Wu, D., Li, X., Chengzhi, X., Yan, X., Liu, C., and Hao, J.: Importance of Wintertime Anthropogenic Glyoxal and Methylglyoxal Emissions in Beijing and Implications for Secondary Organic Aerosol Formation in Megacities, Environ. Sci. Technol., 54, 11809–11817, https://doi.org/10.1021/acs.est.0c02822, 2020.
Räty, M., Peräkylä, O., Riva, M., Quéléver, L., Garmash, O., Rissanen, M., and Ehn, M.: Measurement report: Effects of NOx and seed aerosol on highly oxygenated organic molecules (HOMs) from cyclohexene ozonolysis, Atmos. Chem. Phys., 21, 7357–7372, https://doi.org/10.5194/acp-21-7357-2021, 2021.
Rissanen, M. P.: NO2 Suppression of Autoxidation-Inhibition of Gas-Phase Highly Oxidized Dimer Product Formation, ACS Earth Space Chem., 2, 1211–1219, https://doi.org/10.1021/acsearthspacechem.8b00123, 2018.
Riva, M., Budisulistiorini, S. H., Zhang, Z., Gold, A., and Surratt, J. D.: Chemical characterization of secondary organic aerosol constituents from isoprene ozonolysis in the presence of acidic aerosol, Atmos. Environ., 130, 5–13, https://doi.org/10.1016/j.atmosenv.2015.06.027, 2016a.
Riva, M., Da Silva Barbosa, T., Lin, Y.-H., Stone, E. A., Gold, A., and Surratt, J. D.: Chemical characterization of organosulfates in secondary organic aerosol derived from the photooxidation of alkanes, Atmos. Chem. Phys., 16, 11001–11018, https://doi.org/10.5194/acp-16-11001-2016, 2016b.
Riva, M., Budisulistiorini, S. H., Chen, Y., Zhang, Z., D'Ambro, E. L., Zhang, X., Gold, A., Turpin, B. J., Thornton, J. A., Canagaratna, M. R., and Surratt, J. D.: Chemical Characterization of Secondary Organic Aerosol from Oxidation of Isoprene Hydroxyhydroperoxides, Environ. Sci. Technol., 50, 9889–9899, https://doi.org/10.1021/acs.est.6b02511, 2016c.
Riva, M., Budisulistiorini, S. H., Zhang, Z., Gold, A., Thornton, J. A., Turpin, B. J., and Surratt, J. D.: Multiphase reactivity of gaseous hydroperoxide oligomers produced from isoprene ozonolysis in the presence of acidified aerosols, Atmos. Environ., 152, 314–322, https://doi.org/10.1016/j.atmosenv.2016.12.040, 2017.
Stangl, C. M., Krasnomowitz, J. M., Apsokardu, M. J., Tiszenkel, L., Ouyang, Q., Lee, S., and Johnston, M. V.: Sulfur dioxide modifies aerosol particle formation and growth by ozonolysis of monoterpenes and isoprene, J. Geophys. Res.-Atmos., 124, 4800–4811, 2019.
Stirnweis, L., Marcolli, C., Dommen, J., Barmet, P., Frege, C., Platt, S. M., Bruns, E. A., Krapf, M., Slowik, J. G., Wolf, R., Prévôt, A. S. H., Baltensperger, U., and El-Haddad, I.: Assessing the influence of NOx concentrations and relative humidity on secondary organic aerosol yields from α-pinene photo-oxidation through smog chamber experiments and modelling calculations, Atmos. Chem. Phys., 17, 5035–5061, https://doi.org/10.5194/acp-17-5035-2017, 2017.
Tammer, M.: G. Sokrates: Infrared and Raman characteristic group frequencies: tables and charts, Colloid Polym. Sci., 283, 235–235, https://doi.org/10.1007/s00396-004-1164-6, 2004.
Tilgner, A., Schaefer, T., Alexander, B., Barth, M., Collett Jr., J. L., Fahey, K. M., Nenes, A., Pye, H. O. T., Herrmann, H., and McNeill, V. F.: Acidity and the multiphase chemistry of atmospheric aqueous particles and clouds, Atmos. Chem. Phys., 21, 13483–13536, https://doi.org/10.5194/acp-21-13483-2021, 2021.
Wang, S., Liu, T., Jang, J., Abbatt, J. P. D., and Chan, A. W. H.: Heterogeneous interactions between SO2 and organic peroxides in submicron aerosol, Atmos. Chem. Phys., 21, 6647–6661, https://doi.org/10.5194/acp-21-6647-2021, 2021.
Wang, X. K., Rossignol, S., Ma, Y., Yao, L., Wang, M. Y., Chen, J. M., George, C., and Wang, L.: Molecular characterization of atmospheric particulate organosulfates in three megacities at the middle and lower reaches of the Yangtze River, Atmos. Chem. Phys., 16, 2285–2298, https://doi.org/10.5194/acp-16-2285-2016, 2016.
Wang, Y., Ma, Y., Li, X., Kuang, B. Y., Huang, C., Tong, R., and Yu, J. Z.: Monoterpene and Sesquiterpene alpha-Hydroxy Organosulfates: Synthesis, MS/MS Characteristics, and Ambient Presence, Environ. Sci. Technol., 53, 12278–12290, https://doi.org/10.1021/acs.est.9b04703, 2019.
Wang, Y., Zhao, Y., Wang, Y., Yu, J.-Z., Shao, J., Liu, P., Zhu, W., Cheng, Z., Li, Z., Yan, N., and Xiao, H.: Organosulfates in atmospheric aerosols in Shanghai, China: seasonal and interannual variability, origin, and formation mechanisms, Atmos. Chem. Phys., 21, 2959–2980, https://doi.org/10.5194/acp-21-2959-2021, 2021.
Wang, Y., Hu, M., Guo, S., Wang, Y., Zheng, J., Yang, Y., Zhu, W., Tang, R., Li, X., Liu, Y., Le Breton, M., Du, Z., Shang, D., Wu, Y., Wu, Z., Song, Y., Lou, S., Hallquist, M., and Yu, J.: The secondary formation of organosulfates under interactions between biogenic emissions and anthropogenic pollutants in summer in Beijing, Atmos. Chem. Phys., 18, 10693–10713, https://doi.org/10.5194/acp-18-10693-2018, 2018.
Witkowski, B. and Gierczak, T.: Characterization of the limonene oxidation products with liquid chromatography coupled to the tandem mass spectrometry, Atmos. Environ., 154, 297–307, https://doi.org/10.1016/j.atmosenv.2017.02.005, 2017.
Wyche, K. P., Monks, P. S., Ellis, A. M., Cordell, R. L., Parker, A. E., Whyte, C., Metzger, A., Dommen, J., Duplissy, J., Prevot, A. S. H., Baltensperger, U., Rickard, A. R., and Wulfert, F.: Gas phase precursors to anthropogenic secondary organic aerosol: detailed observations of 1,3,5-trimethylbenzene photooxidation, Atmos. Chem. Phys., 9, 635–665, https://doi.org/10.5194/acp-9-635-2009, 2009.
Yang, Z., Tsona, N. T., Li, J., Wang, S., Xu, L., You, B., and Du, L.: Effects of NOx and SO2 on the secondary organic aerosol formation from the photooxidation of 1,3,5-trimethylbenzene: A new source of organosulfates, Environ. Pollut., 264, 114742, https://doi.org/10.1016/j.envpol.2020.114742, 2020.
Yang, Z., Xu, L., Tsona, N. T., Li, J., Luo, X., and Du, L.: SO2 and NH3 emissions enhance organosulfur compounds and fine particle formation from the photooxidation of a typical aromatic hydrocarbon, Atmos. Chem. Phys., 21, 7963–7981, https://doi.org/10.5194/acp-21-7963-2021, 2021.
Yang, Z., Tsona, N. T., George, C., and Du, L.: Nitrogen-Containing Compounds Enhance Light Absorption of Aromatic-Derived Brown Carbon, Environ. Sci. Technol., 56, 4005–4016, https://doi.org/10.1021/acs.est.1c08794, 2022.
Yao, L., Garmash, O., Bianchi, F., Zheng, J., Yan, C., Kontkanen, J., Junninen, H., Mazon, S. B., Ehn, M., Paasonen, P., Sipila, M., Wang, M., Wang, X., Xiao, S., Chen, H., Lu, Y., Zhang, B., Wang, D., Fu, Q., Geng, F., Li, L., Wang, H., Qiao, L., Yang, X., Chen, J., Kerminen, V.-M., Petaja, T., Worsnop, D. R., Kulmala, M., and Wang, L.: Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity, Science, 361, 278–281, https://doi.org/10.1126/science.aao4839, 2018.
Yasmeen, F., Szmigielski, R., Vermeylen, R., Gomez-Gonzalez, Y., Surratt, J. D., Chan, A. W., Seinfeld, J. H., Maenhaut, W., and Claeys, M.: Mass spectrometric characterization of isomeric terpenoic acids from the oxidation of α-pinene, β-pinene, d-limonene, and Δ3-carene in fine forest aerosol, J. Mass Spectrom., 46, 425–442, https://doi.org/10.1002/jms.1911, 2011.
Ye, J., Abbatt, J. P. D., and Chan, A. W. H.: Novel pathway of SO2 oxidation in the atmosphere: reactions with monoterpene ozonolysis intermediates and secondary organic aerosol, Atmos. Chem. Phys., 18, 5549–5565, https://doi.org/10.5194/acp-18-5549-2018, 2018.
Zhang, X., McVay, R. C., Huang, D. D., Dalleska, N. F., Aumont, B., Flagan, R. C., and Seinfeld, J. H.: Formation and evolution of molecular products in alpha-pinene secondary organic aerosol, P. Natl. Acad. Sci. USA, 112, 14168–14173, https://doi.org/10.1073/pnas.1517742112, 2015.
Zhao, Y., Wingen, L. M., Perraud, V., and Finlayson-Pitts, B. J.: Phase, composition, and growth mechanism for secondary organic aerosol from the ozonolysis of α-cedrene, Atmos. Chem. Phys., 16, 3245–3264, https://doi.org/10.5194/acp-16-3245-2016, 2016.
Zhu, J., Penner, J. E., Lin, G., Zhou, C., Xu, L., and Zhuang, B.: Mechanism of SOA formation determines magnitude of radiative effects, P. Natl. Acad. Sci. USA, 114, 12685–12690, https://doi.org/10.1073/pnas.1712273114, 2017.