the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Ammonium nitrate promotes sulfate formation through uptake kinetic regime
Zemin Feng
Feixue Zheng
Xiaolei Bao
Pengfei Liu
Yanli Ge
Yan Zhao
Tao Jiang
Yunwen Liao
Yusheng Zhang
Xiaolong Fan
Yonghong Wang
Jing Cai
Federico Bianchi
Tuukka Petäjä
Yujing Mu
Markku Kulmala
Although the anthropogenic emissions of SO2 have decreased significantly in China, the decrease in SO in PM2.5 is much smaller than that of SO2. This implies an enhanced formation rate of SO in the ambient air, and the mechanism is still under debate. This work investigated the formation mechanism of particulate sulfate based on statistical analysis of long-term observations in Shijiazhuang and Beijing supported with flow tube experiments. Our main finding was that the sulfur oxidation ratio (SOR) was exponentially correlated with ambient RH in Shijiazhuang (SOR = ) and Beijing (SOR = ). In Shijiazhuang, the SOR is linearly correlated with the ratio of aerosol water content (AWC) in PM2.5 (SOR = ). Our results suggest that uptake of SO2 instead of oxidation of S(IV) in the particle phase is the rate-determining step for sulfate formation. NH4NO3 plays an important role in the AWC and the change of particle state, which is a crucial factor determining the uptake kinetics of SO2 and the enhanced SOR during haze days. Our results show that NH3 significantly promoted the uptake of SO2 and subsequently the SOR, while NO2 had little influence on SO2 uptake and SOR in the presence of NH3.
- Article
(2039 KB) - Full-text XML
-
Supplement
(1479 KB) - BibTeX
- EndNote
Atmospheric particulate matter (PM) is a worldwide concern due to its adverse effect on human health, such as its association with respiratory and cardiovascular diseases, lung cancer, and premature death (WHO, 2013; Lelieveld et al., 2015). The Chinese government has made great efforts to improve the air quality (Cheng et al., 2019). For example, the annual PM2.5 concentration in Beijing decreased from 89.5 µg m−3 in 2013 to 58 µg m−3 in 2017 due to the stringent reduction of local and regional emissions (Cheng et al., 2019; Ji et al., 2019). However, the PM2.5 concentrations in most regions of China (Cheng et al., 2019; Q. Chen et al., 2019; Huang et al., 2019; Tian et al., 2019) are still significantly higher than the PM2.5 standard recommended by the World Health Organization (WHO) (WHO, 2006). Haze events also occur with high frequency, especially, in autumn and winter.
Secondary inorganic aerosol (SIA) including sulfate (SO), nitrate (NO), ammonium (NH) and secondary organic aerosol (SOA) usually contribute to ∼70 % of PM2.5 mass concentration in different regions (Huang et al., 2014; An et al., 2019). SIA often accounts for more than a half of PM2.5 mass in severe pollution events (Zheng et al., 2015; Wang et al., 2016). Even SO exceeds more than 20 % of PM2.5 mass (Guo et al., 2014; Wang et al., 2016; Xie et al., 2015; He et al., 2018). Interestingly, the anthropogenic emissions of SO2 in 2017 reduced by ∼90 % when compared with 2000 in Beijing (Cheng et al., 2019; Lang et al., 2017). However, the decrease rate of particulate SO concentration (Lang et al., 2017; C. Li et al., 2017) is much smaller than SO2 (Lang et al., 2017; Zhang et al., 2020; Liu et al., 2021). For example, the annual mean concentration of SO decreased by 0.1 µg m−3 yr−1 from 2000 to 2013, followed by 1.9 µg m−3 yr−1 from 2013 to 2015 in Beijing, while it decreased by 3.8 µg m−3 yr−1 for SO2 (Lang et al., 2017). This implies an enhanced oxidation rate of SO2 in the atmosphere (Lang et al., 2017). However, the mechanisms and kinetics of particulate SO formation in the real atmosphere are still open questions in many regions of China although they have been extensively discussed (Ervens, 2015; Warneck, 2018).
Particulate SO can be formed through homogeneous oxidation of SO2 by hydroxyl radicals (OH) and stabilized Criegee intermediates (SCIs) in the gas phase and subsequent uptake onto particles, while the OH pathway is the dominant gas-phase oxidation pathway (Seinfeld and Pandis, 2006; L. Liu et al., 2019). Modeling studies greatly underestimated (∼54 %) SO concentration in serve pollution events in Beijing if only considering gas-phase oxidation of SO2, while the normalized mean bias (NMB) decreased significantly after heterogeneous oxidation of SO2 being considered (Zheng et al., 2015). Several heterogeneous and/or multiphase oxidation pathways, such as oxidation of SO2 or sulfite by H2O2 (Huang et al., 2015; Maaß et al., 1999; P. Liu et al., 2020; Ye et al., 2021; Liu et al., 2021), HONO (J. F. Wang et al., 2020), and O3 (Maahs, 1983) or photochemical oxidation of SO2 (Yu et al., 2017; Xie et al., 2015), catalytic oxidation of SO2 by transition metal ions (TMI) (Warneck, 2018; Martin and Good, 1991; Wang et al., 2021) and oxidation of SO2 by NO2 (He et al., 2014; Clifton et al., 1988; Wang et al., 2016; Cheng et al., 2016; Wu et al., 2019; Spindler et al., 2003) in aqueous phase and heterogeneous oxidation of SO2 on black carbon (Zhao et al., 2017; Zhang et al., 2020; Yao et al., 2020), have been proposed based on field measurements and laboratory and modeling studies. However, the relative contribution of these pathways to the SO production is still controversial. For example, the contribution of heterogeneous oxidation to SO production had been evaluated to be (48 ± 5) % based on oxygen isotopic measurements (He et al., 2018), while it was 31 % even in the nighttime calculated by an observation-based modeling (OBM) (Xue et al., 2016). Gas-phase oxidation by OH could explain 33 %–36 % of SO production in the Beijing–Tianjin–Hebei region (L. Liu et al., 2019), while it was negligible based on isotopic measurements (He et al., 2018) and OBM simulations (Xue et al., 2016). As for the oxidation of S(IV) species, which includes SO2, HSO and SO, in aqueous phase, oxidation by H2O2 (T. Liu et al., 2020; P. Liu et al., 2020; Ye et al., 2021), NO2 (J. F. Wang et al., 2020; Wang et al., 2016; Cheng et al., 2016), O3 (Fang et al., 2019), or TMI (Mn2+) (Wang et al., 2021) was proposed as the most important pathway by different researchers. However, the relative importance of these oxidation paths varied greatly among different researches. For instance, TMI-catalyzed oxidation could explain ∼69 % of aqueous sulfate formation in North China Plain based on isotopic measurements and modeling (Shao et al., 2019), while oxidation by NO2 or O2 was the dominant oxidation path (66 %–73 %) based on isotopic measurements in another study (He et al., 2018). It should be noted that some reaction mechanisms mentioned above were proposed based on case studies in short-term observations. Thus, long-term observations at different environments are required to verify whether these mechanisms are statistically important. In addition, the previous studies mainly focused on oxidation process of SO2 in particle phase, while it is unclear what are the controlling factors of the S(IV)-to-S(VI) conversion from the gas phase to the particle phase. In particular, it has been found that the mass fraction of NO and NH is increasing gradually (Lang et al., 2017; Li et al., 2018). This will modify its physical properties, such as morphology, phase-state and so on. The feedback between aerosol physics and aerosol chemistry is still poorly understood.
In this work, 1-year field observations have been performed in Shijiazhuang and Beijing, synchronously. The formation mechanism of particulate sulfate has been statistically investigated to identify the controlling factors. The role of mass transfer of SO2 and the oxidation of S(IV) in particle phase have been discussed based on flow tube experiments and field measurements. The conversion ratio of SO2 to sulfate is statistically and linearly correlated to the aerosol water content (AWC), which is strongly modulated by particulate ammonium nitrate. The reaction kinetics and other factors affecting sulfate production have also been discussed.
2.1 Field measurements
Field measurements were performed at Shijiazhuang University (SJZ; 38.0281∘ N, 114.6070∘ E) and the west campus of Beijing University of Chemical Technology (BUCT; 39.9428∘ N, 119.2966∘ E) from 15 March 2018 to 15 April 2019. The SJZ station is on a rooftop of the main teaching building (5 floors, ∼23 m above the surface), which is around 250 m from the Zhujiang Road of Shijiazhuang. The BUCT station is on a rooftop of the main building (5 floors, ∼18 m above the surface), which is around 550 m from the 3rd Ring Road of Beijing. The distance between the two stations, which are the representative cities of Beijing-Tianjin-Hebei (BTH), is 260 km (Fig. S1). Both stations are surrounded by traffic and residential emissions, thus, are typical urban observation sites. The details about the observation stations have been described in our previous work (Y. Liu et al., 2020c, b, a).
Ambient air was drawn from the roof of the corresponding building. At the SJZ station, the mass concentration of PM2.5 was measured by a beta attenuation mass monitor (BAM-1020, Met One Instruments, USA) with a smart heater (model BX-830, Met One Instruments Inc., USA) to control the RH of the incoming air to 35 % and a PM2.5 inlet (URG) to cut off the particles with diameter larger than 2.5 µm. Particle-phase total concentrations of iron and manganese were measured using a heavy-metal analyzer (EHM-X100, Skyray Instrument). Water-soluble ions (Na+, K+, Mg2+, Ca2+, NH, SO, Cl− and NO) in PM2.5 and gas pollutants (HCl, HONO, HNO3, SO2 and NH3) were measured using an analyzer for monitoring aerosols and gases (MARGA, ADI 2080, Applikon Analytical B.V., the Netherlands) with 1 h of time resolution. At the BUCT station, the mass concentration of PM2.5 was the mean concentration obtained from four surrounding monitoring stations (including Wanliu, Gucheng, Wanshouxigong and Guanyuan) of China Environmental Monitoring Centre (http://www.cnemc.cn, last access: 31 December 2020). The chemical composition of PM2.5 was measured using a time-of-flight aerosol chemical speciation monitor (ToF-ACSM, Aerodyne) after the ambient air went through a PM2.5 inlet (URG) and a Nafion dryer (MD-700-24, Perma Pure). The configuration and the operation protocol of ToF-ACSM have been described in previous work (Fröhlich et al., 2013). The ionization efficiency (IE) calibration for ACSM was performed using 300 nm dry NH4NO3 every month. Ambient air was drawn from the roof using a Teflon sampling tube (BMET-S, Beijing Saak-Mar Environmental Instrument Ltd.) with the residence time <10 s for gas-phase pollutant measurements. Trace gases including NOx, SO2, CO and O3 were measured with the corresponding analyzer (Thermo Scientific, 42i, 43i, 48i and 49i) at both the SJZ and BUCT stations. Meteorological parameters including temperature, pressure, relative humidity (RH), wind speed and wind direction were measured using weather stations (WXT 520 at HAS/SJZ station and AWS 310 at AHL/BUCT station, Vaisala).
2.2 Uptake kinetics of SO2 on dust internally mixed with NH4NO3
To understand the influence of RH on uptake kinetics (), the on dust internally mixed with NH4NO3 was measured using a coated-wall flow tube reactor. The configuration of the reactor and data process protocol have been described in detail previously (Han et al., 2013; Liu et al., 2015). The γ parameter, presenting the mass transfer kinetic of gas-phase SO2 to particle phase, is defined by the net loss rate of SO2 per collision onto the surface (Ravishankara, 1997; Usher et al., 2003), namely,
where is the net loss rate of SO2 when the surface is exposed to SO2 (molecules s−1); ω is the collision frequency (s−1); kobs, rtube, and are the first-order rate constant of SO2, the flow tube radius, and the average molecular velocity of SO2, respectively. A correction for gas-phase diffusion limitations was considered for γobs calculations using the Cooney–Kim–Davis (CKD) method (Cooney et al., 1974; Murphy and Fahey, 1987). The Brunauer–Emmett–Teller (BET) uptake coefficient () was obtained from the mass dependence of γobs as follows (Han et al., 2013; Liu et al., 2015):
where [slope] is the slope of the plot of γobs versus the sample mass in the linear regime (mg−1), Ag is the inner surface area of the sample tube (cm2) and SBET is the specific surface area of the particle sample (cm2 mg−1).
Similar to a previous work (Zhang et al., 2019), dust internally mixed with NH4NO3 was used in the kinetics study, because it was difficult to deposit enough real ambient particles onto the inner surface of the sample holder. Although the composition of the model particles is much simpler than that of ambient particles, it is still meaningful, because we mainly focused on the influence of RH or aerosol water content (AWC) on uptake kinetics of SO2. The mixture (mass ratio = 2:1) of A1 ultrafine test dust (Powder Technology Inc.) and NH4NO3 (AR, Sinopharm Chemical Reagent Co. Ltd, China) were suspended in the mixture of ethanol and water (v:v = 1:3). The inner surface of the Pyrex quartz tube (sample holder) was uniformly coated by the above mixture and dried overnight in an oven at 393 K. The sample mass was calculated according to the weighted mass of the dry tube before and after coating. NH4NO3 in the mixture was further confirmed using an ion chromatograph (Ω Metrohm 940, Applikon Analytical B.V., the Netherlands). Around 50 % of the NH4NO3 remained in the mixture even after heating and potential evaporation. To avoid the wall loss of SO2 on the sample holder, all the inner surface of the sample holder was covered with particles. The wall loss of SO2 on the remained surface (the inner surface of the outside tube and the outside surface of the sample holder) was subtracted at a steady-state condition at the corresponding RH before the uptake experiment as done in our previous work (Liu et al., 2015). The mean concentrations of SO2, NO2 and NH3 were 8.3 ± 5.2 (0.4–49.1), 31.5 ± 13.2 (2.5–85.1) and 41.0 ± 18.4 (0.3–126.4) ppb, respectively, in polluted events (with the PM2.5 concentration higher than 75 µg m−3 and the RH less than 90 %) in Shijiazhuang. The initial concentrations of SO2, NO2 and NH3 in the reactor were 190 ± 2.5, 100 ± 2.5 and 50 ± 2.5 ppb, respectively. The initial concentrations of NO2 and NH3 were close to their ambient concentrations, while a high initial SO2 concentration was used here to obtain a good signal to noise ratio for measurements. In this work, we aimed to understand the influence of AWC on the uptake kinetics of SO2. Therefore, we fixed the initial concentrations of pollutants and the temperature at 300 K. SO2 and NO2 were measured using the corresponding analyzer (Thermo 43i and 42i), and NH3 was measured by an ammonia analyzer (EAA-22, LGR, USA). The specific surface area of the mixture of A1 dust and NH4NO3 was 0.813 m2 g−1, measured by a nitrogen BET physisorption analyzer (Quantachrome Autosorb-1-C). RH from 0 to 80 % was adjusted by varying the ratio of dry to wet zero air (water bubbler) and measured by a RH sensor (HMP110, Humicap). Control experiments demonstrate that adsorption of SO2 on the quartz tube is negligible. It should be noted that the wall loss of SO2 in the presence of NH3 and/or NO2 would be larger in the absence of seed aerosols. Additional control experiments in the presence of NO2 and NH3 demonstrate that the contribution of wall loss of SO2 should be less than 3 % to the measured γ.
2.3 Calculations of AWC, aerosol pH and production rates of sulfate in aerosol liquid water
The AWC and aerosol pH in Shijiazhuang were calculated using the ISORROPIA II model using the measured concentrations of SO, NH, NH3, NO, HNO3, Cl−, HCl, Na+, Ca2+, K+ and Mg2+, as well as RH and temperature as input. The particles were assumed in metastable phase using a forward method (Song and Osada, 2020; Shi et al., 2019). The dataset with RH lower than 35 % was excluded (Pye et al., 2020) due to large uncertainties of aerosol pH (Ding et al., 2019; Guo et al., 2016; Pye et al., 2020). The pH was then calculated according to Pye et al. (2020) and Ding et al. (2019):
where is the activity coefficient of H+, and is the molality of H+. The deliquescence curves of inorganic salts were calculated at 298.5 K using the E-AIM model (Clegg et al., 1998). Then, the AWC attributed to individual salt was calculated with the mass of the salt and the mass-based growth factor at the corresponding RH. The AWC of model particles for laboratory studies was also calculated with the known composition, while the aerosol pH values in Beijing were not calculated, because the concentrations of Na+, Ca2+, K+ and Mg2+ were unavailable.
Similar to previous studies (P. Liu et al., 2020; Cheng et al., 2016), four oxidation pathways of S(IV) in aqueous-phase were accounted for, i.e., oxidation by O3, H2O2, NO2 and TMI (Fe3+ and Mn2+), according to the following equations (Seinfeld and Pandis, 2006; Cheng et al., 2016; P. Liu et al., 2020):
where M−1 s−1, M−1 s−1, M−1 s−1, M−1 s−1, K=13 M−1, M−1 s−1, and (for pH ≤ 4.2) or M−1 s−1, α=0.67 (for pH > 4.2), and k5= (1.24–1.67) ×107 M−1 s−1 (for 5.3 ≤ pH ≤ 8.7; the linear interpolated values were used for pH between 5.3 and 8.7) at 298 K (Clifton et al., 1988; P. Liu et al., 2020; Tilgner et al., 2021; Liu et al., 2021). [O3, aq], [H2O2, aq] and [NO2, aq] were calculated according to the Henry's constants, which are , 1.0×105 and M atm−1 at 298 K for O3, H2O2 and NO2, respectively (Seinfeld and Pandis, 2006). H2O2 concentrations were unavailable during our observations. It was fitted based on temperature like in previous work (Fang et al., 2019). Figure S2 shows the derived H2O2 concentrations and the diurnal curves of H2O2 in winter in Shijiazhuang. The H2O2 concentrations varied from 0.05 to 3.7 ppbv, with a mean value of 0.62 ± 0.52 ppbv. Overall, the wintertime H2O2 concentrations derived in this work are comparable with those reported in the literature (Ye et al., 2018). The concentrations of Fe3+ and Mn2+ were calculated according to the measured total Fe and Mn concentrations assuming 18 % of total Fe and 30 % of total Mn were soluble (Wang et al., 2014; Cui et al., 2008) and the precipitation equilibriums of Fe(OH)3 and Mn(OH)2 depending on pH. The concentrations of Fe and Mn before December 2018 were estimated according to their mean ratios to PM2.5 mass concentration (Wang et al., 2014), because the instrument was unavailable.
3.1 Variation of sulfate in PM2.5
Figure 1a shows the hourly mean mass concentration of PM2.5 measured at SJZ and BUCT stations from 15 March 2018 to 15 April 2019. The mass concentration of PM2.5 in Shijiazhuang generally coincided with that in Beijing. This highlights the regional characteristic of air pollution in BJH. However, Shijiazhuang usually showed significantly higher PM2.5 concentration than that in Beijing. The hourly mean PM2.5 concentration varied in the range of 0–650 µg m−3 with an annual mean concentration of 86.4 ± 77.8 µg m−3. The corresponding values in Beijing were 1.5–556 and 55.0 ± 51.0 µg m−3. Particularly, the wintertime mass concentration of PM2.5 in Shijiazhuang was around 2.4 times as that in Beijing. This is consistent with previous results that Shijiazhuang is suffering from more serious air pollution (D. Chen et al., 2019b) because of its larger density of heavy industries and more intensive emissions than in Beijing (D. Chen et al., 2019a).
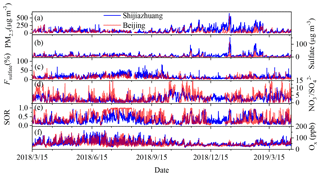
Figure 1The hourly mean (a) mass concentration of PM2.5, (b) sulfate concentration, (c) sulfate fraction in PM2.5, (d) molar ratio of nitrate to sulfate, (e) sulfur oxidation ratio (SOR) and (e) Ox (= NO2+O3) concentration in Shijiazhuang and Beijing from 15 March 2018 to 15 April 2019.
Like the mass concentration of PM2.5, both the mass concentration (Fig. 1b) and the fraction of sulfate in PM2.5 (Fig. 1c) in Shijiazhuang were usually higher than those in Beijing. The annual mean sulfate concentrations in Shijiazhuang and Beijing were 11.7 ± 12.7 and 5.4 ± 6.9 µg m−3, which annually contributed 15.3 ± 8.7 % and 10.7 ± 7.3 % to the PM2.5 mass concentrations, respectively. However, the molar ratio of NO to SO (3.37 ± 3.05), corresponding to the mass ratio 2.17 ± 1.97 in Beijing was significantly higher than that in Shijiazhuang (2.69 ± 1.80, corresponding to mass ratio of 1.77 ± 1.72) at the 0.05 level. This is consistent with the emission inventories of air pollutants, in which Shijiazhuang had larger SO2 emissions than Beijing, and vice versa for NOx emissions (Yang et al., 2019; F. Liu et al., 2017; D. Chen et al., 2019a). A decrease of sulfate concentration (5.4 ± 6.9 µg m−3) in Beijing was significant even when compared with that in PM1.0 (8.1 ± 8.3 µg m−3) measured from July 2011 to June 2012 (Sun et al., 2015), while the mass ratio of (2.17 ± 1.97) in Beijing showed an obvious increase compared with those in 2011–2012 (1.3–1.8) (Sun et al., 2015) and 2008 (0.8–1.5) (Zhang et al., 2013). This can be ascribed to the effective reduction of SO2 emissions but less effective reduction of traffic emissions in Beijing.
The ground surface concentrations of pollutants are prone to be affected by variation of mixing layer height (MLH) (Zhong et al., 2018; Tang et al., 2016). Sulfur oxidation ratio (SOR), which is defined as the molar ratio of sulfate to total sulfur (Wang et al., 2005; Fang et al., 2019),
was calculated and should be less affected by the MLH variation. As shown in Fig. 1e, the SOR in Beijing was overall higher than that in Shijiazhuang. Thus, the annual mean SOR in Beijing (0.42 ± 0.29) was comparable with that reported in literature (Fang et al., 2019), while it was significantly higher than that in Shijiazhuang (0.31 ± 0.19) at the 0.05 level. The high primary emissions of SO2 in Shijiazhuang should lead to a lower SOR than that in Beijing. On the other hand, secondary transformation of SO2 to sulfate should also have an influence on the SOR. The Ox (Ox = NO2+O3) concentration in Shijiazhuang was usually higher than that in Beijing (Fig. 1f). The annual mean Ox concentration in Shijiazhuang was 55.2 ± 22.3 ppb, which was significantly higher than that in Beijing (50.7 ± 21.5 ppb) at the 0.05 level. This is inconsistent with the observed higher SOR in Beijing if gas-phase oxidation mainly contributed to sulfate formation. These results suggest that heterogeneous and/or multiphase reactions may also play important roles in particulate sulfate formation during transport (Zheng et al., 2015; Martin and Good, 1991; Wu et al., 2019).
Figure 2a–c show the mass concentration of PM2.5 colored according to the mass concentration of sulfate, the fraction of sulfate in the soluble PM and the SOR in Shijiazhuang. In most serve pollution events, high PM2.5 mass concentration coincided with the high sulfate concentration, the fraction of sulfate and the SOR (colored in grey color). For example, the mean PM2.5 concentration was 411.7 ± 98.1 µg m−3 during the pollution event that occurred from 08:00 on 12 January 2019 to 00:00 on 15 January 2019 (All times in this paper are given in local time unless stated otherwise.). The corresponding sulfate concentration, fraction of sulfate in soluble PM and SOR were 80.6 ± 24.0 µg m−3, 39.4 ± 3.6 % and 0.79 ± 0.09, respectively. Other pollution episodes, which were highlighted in grey color in Fig. 2, showed a similar trend. The variations of the sulfate concentration, the fraction of sulfate in nonrefractory PM2.5 and the SOR with PM2.5 mass concentration in Beijing were similar to Shijiazhuang and shown in Fig. S3. These results confirm that the conversion rate of SO2 to sulfate is promoted in pollution days when compared with that in clean days.
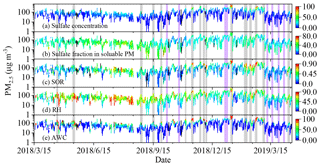
Figure 2Mass concentration of PM2.5 colored according to (a) sulfate concentration, (b) sulfate fraction in soluble PM, (c) SOR, (d) RH and (e) AWC in Shijiazhuang. The shade areas in grey indicate the pollution events with high concentration of sulfate at high RH, while the purple ones the mean pollution events with low sulfate fraction at high RH.
3.2 Role of aerosol water content in sulfate formation
Previous studies have found that severe pollution events are frequently accompanied with high RH (Zhang et al., 2018; Tang et al., 2016; Wu et al., 2018; Y. Liu et al., 2019; Clifton et al., 1988; Maahs, 1983; Martin and Good, 1991). As shown in Fig. 2d, the high concentration of sulfate positively correlated with high RH in most cases, which are shaded in grey columns. However, some pollution events (shaded in purple columns) also occurred under high RH, but the sulfate concentration or sulfate fraction in soluble PM was not so high. This means that high RH is a necessary but not a sufficient condition for sulfate conversion in severe haze pollution events. Thus, it is difficult to fully understand the general regularity behind the dataset or overemphasize the importance of a specific process in the atmosphere based on case studies. This might be the reason why contrary conclusions about the formation path of sulfate were drawn by different researchers. We statistically analyzed the relationship between the SOR and the RH. All the hourly mean data of the SOR and RH have been binned into 100 × 100 boxes. Then, the density of data points, which statistically indicates the occurrence of the events at given values of RH and SOR, was calculated using a bivariate kernel density estimator (Wand and Jones, 1993).
Figure 3a and b show the 2D kernel density graphs between the SOR and the RH in Shijiazhuang and Beijing. The color bar shows the density of data points. Although the SOR varied obviously at a certain RH, the most probable distribution of SOR could be exponentially fitted as a function of RH in Shijiazhuang (Fig. 3a); i.e., SOR = (R=0.79). This is consistent with the dependence of SOR on RH based on previous studies (Tian et al., 2019; Wu et al., 2019). It should be noted that both SOR and RH showed obvious diurnal variation (Fig. S4). Their diurnal variations were somewhat similar, but a 4 h time lag was observed between their minimum values. This means that the diurnal variations of SOR and RH might also contribute to the strong dependency of SOR on RH (Fig. 3a and b). However, the exponential dependency of SOR on RH was still observable in the night or in the day (Fig. S5a and b). It did so in winter or summer (Fig. S5c and d). This means that aqueous reactions are important for sulfate formation even if the influence of diurnal and seasonal variations are ruled out (Wang et al., 2016; Cheng et al., 2016).
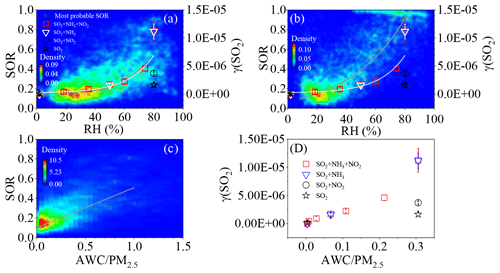
Figure 3Relationship between SOR and on dust internally mixed with NH4NO3 (2:1) and RH in (a) Shijiazhuang and (b) Beijing and the correlation of (c) SOR in Shijiazhuang and (d) with AWCPM2.5. The initial concentrations of SO2, NO2 and/or NH3 in the flow tube reactor were 190 ± 2.5, 100 ± 2.5 and/or 50 ± 2.5 ppb, respectively. The grey lines are the fitting curves for the most probable SOR, and the white lines are the fitting curves for the .
In Fig. 3a, 72.5 % of the data points of Shijiazhuang (6509 over 8980 effective points) were in the domain with the RH range of 10 %–70 % and the SOR range of 0.05–0.42, while 10.1 % of data points were in the region with the RH greater than 70 % and the SOR greater than 0.42. The first region corresponded to a lower mean PM2.5 concentration, sulfate concentration and SOR (76.1 ± 62.78 µg m−3, of 8.1 ± 6.3 µg m−3, and 0.21 ± 0.09, respectively) compared with the second one (115.7 ± 96.7 µg m−3, 22.4 ± 20.4 µg m−3 and 0.62 ± 0.14, respectively). As shown in Fig. 3b, the SOR also exponentially increased as a function of RH in Beijing. A percentage of 74.6 % of 8169 data points were in the first region. The mean PM2.5 concentration, sulfate concentration and SOR were 48.2 ± 44.8 µg m−3, 2.9 ± 3.0 µg m−3 and 0.21 ± 0.10, respectively, in the low RH region, while they were 69.9 ± 50.9 µg m−3, 9.4 ± 8.5 µg m−3 and 0.83 ± 0.15 in the high RH region. The most probable distribution of SOR in Beijing could also be exponentially fitted as a function of RH (SOR = , R=0.92). However, the SOR was more sensitive to RH in Beijing than that in Shijiazhuang. This might be explained by the increased importance of sulfate formation via gas-phase reactions in Beijing (Fang et al., 2019; Hollaway et al., 2019), because the PM2.5 mass concentrations in Beijing were significantly lower than that in Shijiazhuang (Fig. 1).
Formation of particle-phase sulfate through heterogeneous or multiphase oxidations includes the uptake of SO2 and the following oxidation in particle phase. Thus, it is meaningful to identify the rate-determining step (RDS) for understanding the evolution of the SOR. As shown in Fig. 3, the initial increased exponentially from 0 to (1.13 ± 0.21) × 10−5 when the RH increases from 2 % to 80 % in the presence of 50 ± 2.5 ppb NH3 with or without 100 ± 2.5 ppb NO2. The dependence of on RH was with a correlation coefficient of 0.96. A transition region of the versus the RH was observable when the RH ranged from 60 % to 80 %. When the RH was higher than 70 %, the increased quickly as a function of the RH. The similar dependency on RH for the and the SOR suggests that the uptake kinetic of SO2 might determine sulfate formation.
In a previous work (Zhang et al., 2019), it has been found that all the uptake of SO2 on dust or nitrate-coated dust can be transformed into sulfate over the timescale of the uptake experiment using the similar coated-wall flow tube reactor. Another study also observed a quick formation of sulfate on the surface of aqueous microdroplets under acidic conditions (pH < 3.5) without the addition of other oxidants, which was explained by the direct interfacial electron transfer from SO2 to O2 on the aqueous microdroplets (Hung et al., 2018). The pH of deliquesced NH4NO3 is 4.2 as calculated using the ISORROPIA II model. This means that oxidation of S(IV) might not be a RDS of sulfate formation. The oxidation processes can be ascribed to catalytic oxidation by O2 in the presence of transition metals, oxidation by O2 and nitric acid promoted by protons in the presence of nitrate (Zhang et al., 2019) and the oxidation by other dissolved oxidants in liquid phase (T. Chen et al., 2019; Cheng et al., 2016; Wang et al., 2016). To further validate this assumption, the formation rates of SO () in aerosol liquid phase were calculated according to the method used in previous work (P. Liu et al., 2020; Cheng et al., 2016). If oxidation of S(IV) is the rate-determining step, the formation rate should show a similar dependence on RH like the SOR.
As shown in Fig. 4a, the relative contributions of different oxidation paths of S(IV) varied obviously case by case. In summer and autumn, oxidation by H2O2 was the most important path followed by TMI. In winter, however, NO2, O3 or H2O2 could contribute to the major oxidation path. This might be the reason why these oxidation paths showed inconsistent relative importance of among different studies even using the same method, such as isotopic measurements (Shao et al., 2019; He et al., 2018). Figure 4b and c show the dependence of the formation rates of sulfate on RH in the range of 35 %–100 % in Shijiazhuang. The dataset for RH below 35 % was omitted due to the large uncertainty in aerosol pH calculations (Ding et al., 2019; Guo et al., 2016; Pye et al., 2020). The relative contributions of different oxidation paths of S(IV) also varied obviously as a function of RH. NO2 and O3 played important role in aqueous S(IV) oxidation when RH was from 35 % to 45 %, while TMI became the dominator when RH ranged from 45 % to 70 %. Above 70 % RH, the contribution of H2O2 was dominant, which is consistent with several recent studies (P. Liu et al., 2020; T. Liu et al., 2020). However, the total formation rate of sulfate in aerosol liquid phase slightly decreased as RH increased. A weak downward trend of the rate with RH was also observable in the 2D kernel density graphs as shown in Fig. 4c. This is opposite to the dependencies of the SOR and the on RH as discussed above, which means the RDS for sulfate formation should be the uptake of SO2 instead of oxidation of S(IV) in aqueous phase. We further calculated the production rate of sulfate through uptake of SO2 (mass transfer to aerosol particles) according to
where As is the surface area concentration of PM2.5, ω is the mean molecular velocity of SO2 and is the mass concentration of SO2. As shown in Fig. 4c, the probability-weighted production rate of sulfate through uptake of SO2 (the grey line) is lower than that through aqueous oxidation of S(IV), in particular, when RH is lower than 70 %. It should be noted that the mass transfer of SO2 was not assumed to be the RDS using a large mass accommodation coefficient of SO2 (α=0.11) (Cheng et al., 2016). According to the relationship between the mass accommodation coefficient (α) and the uptake coefficient (γ) of SO2 (Kulmala and Wagner, 2001), the on particles is on the same order of the . This means that mass transfer rate might have been overestimated by Cheng et al. (2016).
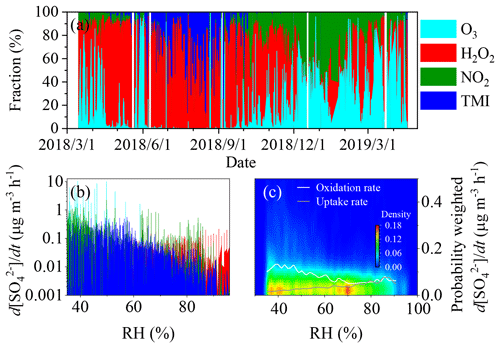
Figure 4(a) The relative importance of oxidation paths of S(IV) in aqueous phase, the dependence of (b) sulfate formation rates and (c) the probability-weighted sulfate formation rates on RH in Shijiazhuang.
Phase state is a crucial factor determining the mass transfer of pollutants from gas phase to particle phase (Davis et al., 2015; Marshall et al., 2018; Shiraiwa et al., 2011; Liu et al., 2014), while the AWC or RH greatly affects the phase state of aerosol particles (Duan et al., 2019; Y. Liu et al., 2019; Shiraiwa et al., 2017). For example, ambient particles were found to change from semisolid to liquid state when the RH was above ∼60 % (Y. Liu et al., 2019, 2017), corresponding to the AWC higher than ∼15 µg m−3 (Y. Liu et al., 2017) under the typical urban environment in Beijing based on rebound fractions measurements. It was also confirmed that haze particles displayed a solid–aqueous equilibrium state when the RH was around 60 %–80 % using an individual particle hygroscopicity system (Sun et al., 2018). As shown in Fig. S6, the most probable distribution of the AWC exponentially increased with the RH (AWC = , R=0.98) in Shijiazhuang. An obvious transition region of the RH between 60 % and 80 % was also observed. These results indicate that the liquid-phase aerosol should appear when the RH is higher than ∼60 % (Y. Liu et al., 2019, 2017) and subsequently promote the conversion of SO2 to sulfate. The SOR increased as a power function of AWC (SOR = , R=0.78), while it was linearly correlated with the ratio of AWCPM2.5 (SOR = , R=0.78) as shown in Fig. 3c. Similarly, the AWC of dust internally mixed with NH4NO3 was also calculated using the ISORROPIA II model. The also showed a similar trend as a function of AWCPM2.5 (, R=0.95) (Fig. 3d), although the ranges of AWCPM2.5 were different due to the difference in aerosol composition. This means that the fraction of aerosol liquid water governs both the conversion of SO2 to sulfate and uptake kinetics of SO2.
It should be noted that although the SOR showed a similar RH dependence as the SO2, a deviation was observed in both Shijiazhuang and Beijing (Fig. 3). The was measured at a fixed temperature and initial SO2 concentration. In the atmosphere, both of them varied obviously. This might lead to the observed deviation. On the other hand, the coexisted components such as organic aerosol and black carbon in atmospheric particles should have complicated the influence on the hygroscopicity and the phase change of particles. The difference between the model particles and the real ambient aerosol particles might also partially lead to the deviations of the RH dependence between the SOR and the . In addition, it also implies that besides the reaction in aerosol liquid phase, other reaction paths such as oxidation of SO2 by gas-phase oxidants should also play an important role in sulfate formation (Duan et al., 2019).
3.3 Influence of particle composition on AWC and sulfate formation
Besides RH, particle composition is another important factor to affect the AWC. According to the ions balance (Fig. S7a), ammonia was adequate to neutralize the anions in PM2.5, which is consistent with the results in the literature (Y. Wang et al., 2020b). In addition, (81.5 ± 15.9) % (with the median of 87.1 %) of ionic anions (nitrate, chloride and sulfate) were neutralized by ammonium (Fig. S7b). This means NH4NO3, (NH4)2SO4 and NH4Cl should be the dominant form of nitrate, sulfate and chloride in PM2.5, respectively. We further reconstructed the molecular composition from the ions based on the principles of aerosol neutralization and molecular thermodynamics (Kortelainen et al., 2017). The molecular concentrations were estimated according to the molar ratio of NH to SO () according to the following rules: (i) if , NH existed as the chemical forms of H2SO4 and NH4HSO4; (ii) , NH existed as (NH4)2SO4 and NH4HSO4; (iii) if , then the fraction NH corresponding to twice the amount of SO existed as (NH4)2SO4, and the remaining fraction of NH was associated with NO and Cl−; and (iv) the rest of NO, which was not neutralized by NH, was from NaNO3. Figure 5a and b show the variation of the molecular composition as a function of RH in Shijiazhuang. Obviously, NH4NO3 and (NH4)2SO4 were the major molecular components. Both of them showed upward trend as the RH increased. In particular, the fraction of NH4NO3 increased gradually from ∼10 % to ∼50 % when the RH increased from ∼30 % to 90 %. Correspondingly, the fraction of (NH4)2SO4 decreased as the RH increased.
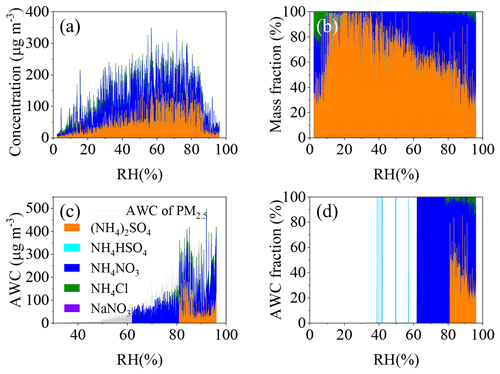
Figure 5Variations of (a) the mass concentrations and (b) the mass fractions of molecular composition in PM2.5, (c) the estimated AWC attributed to different composition and (d) the corresponding AWC fraction as a function of RH in Shijiazhuang.
It should be noted that the deliquescence RH (DRH) of NH4NO3 (61.8 %) (Onasch et al., 1999) is lower than those values of NH4Cl (78 %) (Hu et al., 2011) and (NH4)2SO4 (80 %) (Lightstone et al., 2000). We further calculated the AWC attributed to the individual molecular component based on the growth factors and mass concentrations. As shown in Fig. 5c, the sum of the AWC of individual salts is underestimated by around 13 % compared to that calculated using the ISORROPIA II model (the grey line), because the mixing state was not considered in the former method. However, we can still draw a conclusion that NH4NO3 and (NH4)2SO4 are the major contributors to the AWC. Especially NH4NO3 dominated the AWC when the RH ranged from 60 % to 80 %, in which the SOR and the were very sensitive to RH. These results suggest that NH4NO3 should be the most important mediator to AWC and subsequently the uptake of SO2 in the transition regime of RH in Fig. 3a. It should be noted that (NH4)HSO4 has a lower DRH than NH4NO3 (Y. J. Li et al., 2017). However, 98.4 % of the data points showed an higher than 2.0 in Shijiazhuang. This means that the contribution of (NH4)HSO4 to PM2.5 should be negligible because of the abundance of atmospheric NH3 in northern China. In previous work, it has been found that SO2 oxidation can be promoted by particulate nitrate through the accumulation of proton (Zhang et al., 2019) and the formation of NO+NO (Kong et al., 2014). Our results further showed the importance of NH4NO3 in the AWC, which possibly determines the phase state of particles and subsequently the uptake kinetics of SO2 and the SOR as discussed above. To further confirm the role of NH4NO3 in the uptake of SO2, uptake experiment of SO2 on pure dust has been carried out at 2 % and 80 % RH. The corresponding was 1.10 ± 1.05 × 10−7 and 1.66 ± 0.28 × 10−7 on pure dust sample in the presence of NH3 and NO2. However, as discussed above, it was 0 and 1.12 ± 0.15 × 10−5 on dust internally mixed with 33 % NH4NO3. This directly confirmed the role of NH4NO3 in SO2 uptake via aerosol liquid water.
Figure S8 shows the dependencies of the AWCPM2.5 and SOR on the fraction of the individual molecular component. Both the AWCPM2.5 and SOR statistically increased as the fraction of NH4NO3 increased (Fig. S8a and d). A weak increase followed by a decrease was observed for the AWCPM2.5 as the fraction of (NH4)2SO4 increased, while a negative correlation between the AWCPM2.5 and the fraction of NH4Cl was observed. It did so for the SOR and the fraction of NH4Cl. These phenomena were overall consistent with the sequence of their hygroscopicity. In addition, chloride is a primary pollutant mainly from coal combustion and biomass burning (Bi et al., 2019). Besides chloride, other primary particles from combustion such as soot, which were not accounted for in this work, might also decrease the uptake capability of water and subsequently be unfavorable for SO2 uptake.
To assess the relative importance of sulfate and nitrate (the major SIA component) to AWC, the sensitivity of their fraction to AWC in Shijiazhuang was tested using the ISORROPIA II model and shown in Fig. S9. The base case means the AWC was calculated using the measured concentration of the ions. Then, we reduced the fraction of NH4NO3 or (NH4)2SO4 from 0 to 80 % individually compared with the base case. Figure S9a shows the time series of the calculated AWC after reducing 50 % of NH4NO3 or (NH4)2SO4. Reduction of either NH4NO3 or (NH4)2SO4 resulted into obvious decrease of AWC during pollution events. In most cases, the reduction amplitude of AWC was larger when reducing 50 % of NH4NO3 than (NH4)2SO4. Figure S9b shows the mean ratio of AWC at a certain reduction fraction of NH4NO3 or (NH4)2SO4 to that under the base case. When NH4NO3 was reduced from 0 % to 80 %, the AWC linearly reduced from 0 % to 61.1 ± 0.1 % with a slope of 0.48 %. It varied from 0 % to 66.0 ± 0.2 % for (NH4)2SO4 (with a slope of 0.41 %). This means that the AWC is more sensitive to the fraction of NH4NO3 than (NH4)2SO4 in Shijiazhuang. This also implies the importance of NH4NO3 in the observed high AWC in haze days. On the other hand, reducing 10 % of NH4NO3 can lead to a reduction of 5.2 ± 1.0 % AWC during haze days. Subsequently, we can roughly estimate that the SOR might be reduced by ∼4 % through a linear interpolation according to the equation of the SOR and the AWCPM2.5 (SOR = ) fitted in Fig. 3c. This means reduction of NOx and NH3 should lead to additional reduction of particulate sulfate.
3.4 Influence of other factors on sulfate formation
Several studies have proposed out that NO2 can promote the oxidation of SO2 on particle surfaces and in aqueous phase. For example, laboratory studies have found that parts per million (ppm) levels of NO2 can promote sulfate formation on the surface of dust through NO+NO which is disproportionated from N2O4 intermediate (He et al., 2014; Liu et al., 2012; Ma et al., 2008) or parts per million levels of NO2 can promote the oxidation of SO2 in the deliquesced oxalic acid (Wang et al., 2016). This is supported by the evidence that a high fraction of sulfate in PM2.5 is positively correlated with NO2 concentration (Xie et al., 2015) and that a high PM2.5 concentration is accompanied with high ratio of in several case studies (He et al., 2014). The importance of the SO2 oxidation by NO2 in aqueous phase has also been confirmed in modeling studies (Cheng et al., 2016; Xue et al., 2016). However, this reaction path is still under debate because of the following reasons. (1) The concentration of NO2 in laboratory studies was about 2 orders of magnitude higher than that in ambient air. This will affect the surface concentration of the intermediate (N2O4) and the concentration of solved NO2 in aqueous phase. (2) The dissolved NO2 concentration is highly sensitive to pH. The pH value in aerosol was 5.6–6.2 estimated in modeling study (Cheng et al., 2016). However, a recent work found that it varied from 3.8 to 4.5 at RH > 30 % and showed a moderate acidity because of the thermodynamic equilibrium between NH and NH3 (Ding et al., 2019). (3) The previous calculations were conducted using a high reaction rate constant of the NO2 reaction with dissolved S(IV) (Clifton et al., 1988; Cheng et al., 2016), while a smaller value was reported in the more recent study (Spindler et al., 2003; Tilgner et al., 2021). (4) The relative importance of each path depends on the concentration of the relevant pollutants including H2O2 and TMI (P. Liu et al., 2020). Therefore, it is necessary to verify the importance of this process by long-term observation at different environments.
Figure 6 shows the 2D kernel density graph of the sulfate fraction in soluble PM and the SOR in Shijiazhuang as a function of the concentration of different gas-phase pollutants. It should be pointed out that the SOR or the should be positively correlated to NO2 concentration if it can promote the conversion of SO2 to sulfate or the uptake of SO2. However, both sulfate fraction and SOR were negatively correlated with the concentration of NO2 from the point of view of statistics. A same trend was observed in Beijing (Fig. S10). This is similar to recent studies that observed the opposite correlation between SOR and NOx concentration in the Sichuan Basin (Tian et al., 2019) and in Beijing (Fang et al., 2019). This means that NO2 concentration is statistically not a determining factor for sulfate formation in the atmosphere. This is well supported by the uptake kinetics of SO2 measured using a flow tube reactor. As shown in Fig. 3a and b, when 50 ± 2.5 ppb of NH3 presenting in the reactant gases, no difference was observable for the between the presence (red squares) and absence of 100 ± 2.5 ppb of NO2 (white triangles). This is consistent with these previous studies that found NO2 to be having no influence on SO2 uptake when NH3 was abundant in the atmosphere (Wu et al., 2019; Wang et al., 2021). In addition, it is consistent with the fact that H2O2 dominated the oxidation of S(IV) in aerosol liquid water when RH was higher than 60 % (Fig. 4a). It should be pointed out that the at 80 % RH was 1.7 ± 0.3 × 10−6 on the mixture of dust and NH4NO3 in the absence of NH3 and NO2 (Fig. 3). It increased to 3.7 ± 0.2 × 10−6 in the presence of NO2. This is consistent with the promotion effect of NO2 for converting SO2 to sulfate in the absence of NH3 as observed in both a smog chamber (Wang et al., 2016) and a bubbling reactor (T. Chen et al., 2019). However, the enhanced uptake of SO2 induced by NO2 might be too low to be measured in the presence of NH3. Therefore, the weak promotion effect by NO2 alone cannot explain the negative correlation between the SOR and the concentration of NO2 in Fig. 6f.
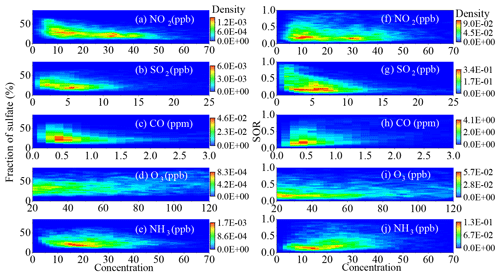
Figure 6Dependence of the sulfate fraction in soluble PM and the SOR on gaseous pollutant concentration in Shijiazhuang.
Both the fraction of sulfate and the SOR in Shijiazhuang statistically decreased as a function of SO2 and CO concentration, respectively (Fig. 6b, c, g and h). This might be explained by the high concentration of primary aerosol components when pollution events occurred with high concentrations of primary gas-phase pollutants. However, the fraction of sulfate increased as a function of O3 (Fig. 6d). When the O3 concentration was greater than 50 ppb, the SOR slightly increased with the O3 concentration (Fig. 6i). A more obvious positive dependence of sulfate fraction on O3 concentration was observed in Beijing (Fig. S10e). This means oxidation capacity also plays an important role in sulfate formation, especially in Beijing. This is consistent with the recent finding that O3 plays an important role in SO2 oxidation at different locations (Fang et al., 2019; Tian et al., 2019; Duan et al., 2019). As shown in Fig. 6j, the SOR positively correlated with the concentration of NH3 in Shijiazhuang. This means that NH3 can promote the conversion of SO2 to sulfate. This is well in agreement with laboratory studies that observed the promotion effect by NH3 to the heterogeneous reaction of SO2 on different mineral oxides (Yang et al., 2016). In addition, flow tube experiments were also carried out by exposing the internal mixing sample (2:1 dust and NH4NO3) to 200 ± 2.5 ppb SO2 in the absence of NH3 and NO2 at 2 % and 80 % RH, respectively. As shown in Fig. 3a and b, the was zero regardless of the reactants under dry condition (2 % RH), while it increased to (1.66 ± 0.28) × 10−6 at 80 % RH. However, it was significantly smaller than the ((1.13 ± 0.21) × 10−5) in the presence of 50 ± 2.5 ppb NH3 with or without 100 ± 2.5 ppb NO2. These results further confirm that NH3 can promote the uptake of SO2 at high RH, possible through enhancing the solubility of SO2 in water (T. Chen et al., 2019; Cheng et al., 2016; Wang et al., 2016), because the effective solubility of SO2 can be enhanced due to the increase of the aerosol pH.
Aerosol acidity is one of the important factors affecting the sulfate formation and the partitioning of semivolatile gases in the atmosphere (Liu et al., 2021). As shown in Fig. S11, when aerosol pH is lower than 4.5, the oxidation rate of S(IV) in aerosol liquid phase decreases with decreasing pH, because the oxidation of S(IV) by transition metals is the dominant path and is decreasing with aerosol pH. However, the oxidation rate of S(IV) increases when the aerosol pH is higher than 4.5. This can be explained by the fact that the solubility and effective Henry's law constant of SO2 are positively dependent on pH (Cheng et al., 2016; Liu et al., 2021; P. Liu et al., 2020), which is consistent with the promotion effect of sulfate formation by NH3.
Based on 1 year of observations, we confirmed that high PM2.5 mass concentration in pollution events usually coincided with the high sulfate concentration, the fraction of sulfate, and the SOR in both Beijing and Shijiazhuang. In Shijiazhuang, the SOR exponentially increased as a function of RH from the point of view of statistics, which was similar to the RH dependence of the on the model particles containing 33 % NH4NO3 in the presence of NH3. The SOR and linearly increased as a function of the fraction of aerosol water content in PM2.5. The enhanced uptake coefficient of SO2 at high RH after the liquid-phase aerosol appeared might explain the increased SOR, because uptake of SO2 was the rate-determining step for the conversion of SO2 to particulate sulfate. NH4NO3 played an important role in the AWC, the phase state of aerosol particles and subsequently the uptake kinetics of SO2 in haze days under high RH conditions.
The contribution of nitrate to PM2.5 is increasing in China (Li et al., 2018; Tian et al., 2019) due to the intensive emissions of NOx from steel production and cement manufacturing (Wu et al., 2018; Qi et al., 2017) as well as the increasing NOx emissions from traffic (Liu et al., 2007; Wang et al., 2011). The mean fraction of nitrate in PM2.5 was 21.4 ± 12.4 % in Shijiazhuang and 15.8 ± 13.4 % in Beijing. They were close to the reported values in PM1.0 during the summer of Beijing (24 %) (Li et al., 2018) and in PM2.5 during the winter of Chengdu (23.3 %) and Chongqing (17.5 %) (Tian et al., 2019). It has been found that the fraction of nitrate and ammonium usually increases as a function of PM2.5 mass concentration (Li et al., 2018). Therefore, NOx should be an urgent air pollutant in the future in China even from the point view of its contribution to PM2.5 mass.
As observed in this work, NH4NO3 has an important contribution to PM2.5 mass concentration, the aerosol water content and subsequently the phase state of particles in the RH range of 60 %–80 %. Reduction of NOx emissions should lead to a decrease in NH4NO3 concentration and subsequently the AWC during serve pollution events. This will lead to an additional reduction of SO2 uptake and the formation of particulate sulfate through aqueous reactions. Based on our rough estimation, 4 % of sulfate might be reduced due to aqueous reaction in Shijiazhuang if the mass concentration of NH4NO3 was reduced by 10 %. More work is required to quantitatively assess the contribution of nitrate to sulfate formation from aqueous reactions in the future. It should be noted that ozone pollution becomes more and more important in China (Z. Chen et al., 2019; Ziemke et al., 2019). This requires us to harmoniously reduce NOx and volatile organic compounds in the near future. It is also important to take actions on NH3 emission control in the future as NH3 can significantly promote the uptake of SO2 in liquid-phase aerosol.
The experimental data are available upon request to the corresponding authors.
The supplement related to this article is available online at: https://doi.org/10.5194/acp-21-13269-2021-supplement.
YoL and XB designed the experiments. YoL and YuL wrote the paper. ZF, FZ, YZ, XF, CY, BC, YW, WD and JC carried out measurements at BUCT. XB and TJ carried out measurements at SJZ. YG, YZ and YoL carried out flow tube experiments. PL, YM and YoL performed sulfate formation calculations. YuL, FB, TP, YM, HH and MK revised the paper.
The authors declare that they have no conflict of interest.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The research was financially supported by the National Natural Science Foundation of China (92044301), the Ministry of Science and Technology of the People's Republic of China (2019YFC0214701), Academy of Finland via the Center of Excellence in Atmospheric Sciences (272041, 316114, 315203 and 1307537), and European Research Council via ATM-GTP 266 (742206) and via ERA-NET-Cofund through SMart URBan Solutions for air quality, disasters and city growth (SMURBS/ERA-PLANET), the Strategic Priority Research Program of Chinese Academy of Sciences and Beijing University of Chemical Technology.
This research has been supported by the National Natural Science Foundation of China (grant no. 92044301) and the Ministry of Science and Technology of the People's Republic of China (grant no. 2019YFC0214701), Academy of Finland via the Center of Excellence in Atmospheric Sciences (272041, 316114, 315203 and 1307537), and European Research Council via ATM-GTP 266 (742206) and via ERA-NET-Cofund through SMart URBan Solutions for air quality, disasters and city growth (SMURBS/ERA PLANET), the Strategic Priority Research Program of Chinese Academy of Sciences and Beijing University of Chemical Technology.
This paper was edited by Harald Saathoff and reviewed by two anonymous referees.
An, Z., Huang, R.-J., Zhang, R., Tie, X., Li, G., Cao, J., Zhou, W., Shi, Z., Han, Y., Gu, Z., and Ji, Y.: Severe haze in northern China: A synergy of anthropogenic emissions and atmospheric processes, P. Natl. Acad. Sci. USA, 116, 8657–8666, https://doi.org/10.1073/pnas.1900125116, 2019.
Bi, X., Dai, Q., Wu, J., Zhang, Q., Zhang, W., Luo, R., Cheng, Y., Zhang, J., Wang, L., Yu, Z., Zhang, Y., Tian, Y., and Feng, Y.: Characteristics of the main primary source profiles of particulate matter across China from 1987 to 2017, Atmos. Chem. Phys., 19, 3223–3243, https://doi.org/10.5194/acp-19-3223-2019, 2019.
Chen, D., Liu, Z., Ban, J., and Chen, M.: The 2015 and 2016 wintertime air pollution in China: SO2 emission changes derived from a WRF-Chem/EnKF coupled data assimilation system , Atmos. Chem. Phys., 19, 8619–8650, https://doi.org/10.5194/acp-19-8619-2019, 2019a.
Chen, D., Liu, Z., Ban, J., Zhao, P., and Chen, M.: Retrospective analysis of 2015–2017 wintertime PM2.5 in China: response to emission regulations and the role of meteorology, Atmos. Chem. Phys., 19, 7409–7427, https://doi.org/10.5194/acp-19-7409-2019, 2019b.
Chen, Q., Sheng, L. F., Gao, Y., Miao, Y. C., Hai, S. F., Gao, S. H., and Gao, Y.: The Effects of the Trans-Regional Transport of PM2.5 on a Heavy Haze Event in the Pearl River Delta in January 2015, Atmosphere, 10, 237, https://doi.org/10.3390/atmos10050237, 2019.
Chen, T., Chu, B., Ge, Y., Zhang, S., Ma, Q., He, H., and Li, S.-M.: Enhancement of aqueous sulfate formation by the coexistence of under high ionic strengths in aerosol water, Environ. Pollut., 252, 236–244, https://doi.org/10.1016/j.envpol.2019.05.119, 2019.
Chen, Z., Zhuang, Y., Xie, X., Chen, D., Cheng, N., Yang, L., and Li, R.: Understanding long-term variations of meteorological influences on ground ozone concentrations in Beijing During 2006–2016, Environ. Pollut., 245, 29–37, https://doi.org/10.1016/j.envpol.2018.10.117, 2019.
Cheng, J., Su, J., Cui, T., Li, X., Dong, X., Sun, F., Yang, Y., Tong, D., Zheng, Y., Li, Y., Li, J., Zhang, Q., and He, K.: Dominant role of emission reduction in PM2.5 air quality improvement in Beijing during 2013–2017: a model-based decomposition analysis, Atmos. Chem. Phys., 19, 6125–6146, https://doi.org/10.5194/acp-19-6125-2019, 2019.
Cheng, Y., Zheng, G., Wei, C., Mu, Q., Zheng, B., Wang, Z., Gao, M., Zhang, Q., He, K., Carmichael, G., Pöschl, U., and Su, H.: Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China, Sci. Adv., 2, e1601530, https://doi.org/10.1126/sciadv.1601530, 2016.
Clegg, S. L., Brimblecombe, P., and Wexler, A. S.: Thermodynamic Model of the System HNHNaSONOClH2O at 298.15 K, J. Phys. Chem. A, 102, 2155–2171, https://doi.org/10.1021/jp973043j, 1998.
Clifton, C. L., Altstein, N., and Huie, R. E.: Rate constant for the reaction of nitrogen dioxide with sulfur(IV) over the pH range 5.3–13, Environ. Sci. Technol., 22, 586–589, https://doi.org/10.1021/es00170a018, 1988.
Cooney, D. O., Kim, S.-S., and Davis, E. J.: Analyses of mass transfer in hemodialyzers for laminar blood flow and homogeneous dialysate, Chem. Eng. Sci., 29, 1731–1738, https://doi.org/10.1016/0009-2509(74)87031-4, 1974.
Cui, R., Guo, X., Deng, F., and Liu, H.: Analysis of Water-soluble Ions and Elements in PM10 and PM2.5, J. Environ. Health, 25, 291–294, https://doi.org/10.16241/j.cnki.1001-5914.2008.04.001, 2008 (in Chinese).
Davis, R. D., Lance, S., Gordon, J. A., Ushijima, S. B., and Tolbert, M. A.: Contact efflorescence as a pathway for crystallization of atmospherically relevant particles, P. Natl. Acad. Sci. USA, 112, 15815–15820, https://doi.org/10.1073/pnas.1522860113, 2015.
Ding, J., Zhao, P., Su, J., Dong, Q., Du, X., and Zhang, Y.: Aerosol pH and its driving factors in Beijing, Atmos. Chem. Phys., 19, 7939–7954, https://doi.org/10.5194/acp-19-7939-2019, 2019.
Duan, J., Huang, R.-J., Lin, C., Dai, W., Wang, M., Gu, Y., Wang, Y., Zhong, H., Zheng, Y., Ni, H., Dusek, U., Chen, Y., Li, Y., Chen, Q., Worsnop, D. R., O'Dowd, C. D., and Cao, J.: Distinctions in source regions and formation mechanisms of secondary aerosol in Beijing from summer to winter, Atmos. Chem. Phys., 19, 10319–10334, https://doi.org/10.5194/acp-19-10319-2019, 2019.
Ervens, B.: Modeling the Processing of Aerosol and Trace Gases in Clouds and Fogs, Chem. Rev., 115, 4157–4198, https://doi.org/10.1021/cr5005887, 2015.
Fang, Y., Ye, C., Wang, J., Wu, Y., Hu, M., Lin, W., Xu, F., and Zhu, T.: Relative humidity and O3 concentration as two prerequisites for sulfate formation, Atmos. Chem. Phys., 19, 12295–12307, https://doi.org/10.5194/acp-19-12295-2019, 2019.
Fröhlich, R., Cubison, M. J., Slowik, J. G., Bukowiecki, N., Prévôt, A. S. H., Baltensperger, U., Schneider, J., Kimmel, J. R., Gonin, M., Rohner, U., Worsnop, D. R., and Jayne, J. T.: The ToF-ACSM: a portable aerosol chemical speciation monitor with TOFMS detection, Atmos. Meas. Tech., 6, 3225–3241, https://doi.org/10.5194/amt-6-3225-2013, 2013.
Guo, H., Sullivan, A. P., Campuzano-Jost, P., Schroder, J. C., Lopez-Hilfiker, F. D., Dibb, J. E., Jimenez, J. L., Thornton, J. A., Brown, S. S., Nenes, A., and Weber, R. J.: Fine particle pH and the partitioning of nitric acid during winter in the northeastern United States, J. Geophys. Res.-Atmos., 121, 10355–10376, https://doi.org/10.1002/2016JD025311, 2016.
Guo, S., Hu, M., Zamora, M. L., Peng, J., Shang, D., Zheng, J., Du, Z., Wu, Z., Shao, M., Zeng, L., Molina, M. J., and Zhang, R.: Elucidating severe urban haze formation in China, P. Natl. Acad. Sci. USA, 111, 17373–17378, https://doi.org/10.1073/pnas.1419604111, 2014.
Han, C., Liu, Y., and He, H.: Role of Organic Carbon in Heterogeneous Reaction of NO2 with Soot, Environ. Sci. Technol., 47, 3174–3181, https://doi.org/10.1021/es304468n, 2013.
He, H., Wang, Y., Ma, Q., Ma, J., Chu, B., Ji, D., Tang, G., Liu, C., Zhang, H., and Hao, J.: Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days, Sci. Rep., 4, 4172, https://doi.org/10.1038/srep04172, 2014.
He, P., Alexander, B., Geng, L., Chi, X., Fan, S., Zhan, H., Kang, H., Zheng, G., Cheng, Y., Su, H., Liu, C., and Xie, Z.: Isotopic constraints on heterogeneous sulfate production in Beijing haze, Atmos. Chem. Phys., 18, 5515–5528, https://doi.org/10.5194/acp-18-5515-2018, 2018.
Hollaway, M., Wild, O., Yang, T., Sun, Y., Xu, W., Xie, C., Whalley, L., Slater, E., Heard, D., and Liu, D.: Photochemical impacts of haze pollution in an urban environment, Atmos. Chem. Phys., 19, 9699–9714, https://doi.org/10.5194/acp-19-9699-2019, 2019.
Hu, D. W., Chen, J. M., Ye, X. N., Li, L., and Yang, X.: Hygroscopicity and evaporation of ammonium chloride and ammonium nitrate: Relative humidity and size effects on the growth factor, Atmos. Environ., 45, 2349–2355, https://doi.org/10.1016/j.atmosenv.2011.02.024, 2011.
Huang, L., Zhao, Y., Li, H., and Chen, Z.: Kinetics of Heterogeneous Reaction of Sulfur Dioxide on Authentic Mineral Dust: Effects of Relative Humidity and Hydrogen Peroxide, Environ. Sci. Technol., 49, 10797–10805, https://doi.org/10.1021/acs.est.5b03930, 2015.
Huang, L., An, J., Koo, B., Yarwood, G., Yan, R., Wang, Y., Huang, C., and Li, L.: Sulfate formation during heavy winter haze events and the potential contribution from heterogeneous SO2 + NO2 reactions in the Yangtze River Delta region, China, Atmos. Chem. Phys., 19, 14311–14328, https://doi.org/10.5194/acp-19-14311-2019, 2019.
Huang, R.-J., Zhang, Y., Bozzetti, C., Ho, K.-F., Cao, J.-J., Han, Y., Daellenbach, K. R., Slowik, J. G., Platt, S. M., Canonaco, F., Zotter, P., Wolf, R., Pieber, S. M., Bruns, E. A., Crippa, M., Ciarelli, G., Piazzalunga, A., Schwikowski, M., Abbaszade, G., Schnelle-Kreis, J., Zimmermann, R., An, Z., Szidat, S., Baltensperger, U., Haddad, I. E., and Prévôt, A. S. H.: High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 514, 218–222, https://doi.org/10.1038/nature13774, 2014.
Hung, H.-M., Hsu, M.-N., and Hoffmann, M. R.: Quantification of SO2 oxidation on interfacial surfaces of acidic micro-droplets: Implication for ambient sulfate formation, Environ. Sci. Technol., 52, 9079–9086, https://doi.org/10.1021/acs.est.8b01391, 2018.
Ji, D., Gao, W., Maenhaut, W., He, J., Wang, Z., Li, J., Du, W., Wang, L., Sun, Y., Xin, J., Hu, B., and Wang, Y.: Impact of air pollution control measures and regional transport on carbonaceous aerosols in fine particulate matter in urban Beijing, China: insights gained from long-term measurement, Atmos. Chem. Phys., 19, 8569–8590, https://doi.org/10.5194/acp-19-8569-2019, 2019.
Kong, L. D., Zhao, X., Sun, Z. Y., Yang, Y. W., Fu, H. B., Zhang, S. C., Cheng, T. T., Yang, X., Wang, L., and Chen, J. M.: The effects of nitrate on the heterogeneous uptake of sulfur dioxide on hematite, Atmos. Chem. Phys., 14, 9451–9467, https://doi.org/10.5194/acp-14-9451-2014, 2014.
Kortelainen, A., Hao, L., P. Tiitta, P., Jaatinen, A., Miettinen, P., Kulmala, M., Smith, J., Laaksonen, A., Worsnop, D., and Virtanen, A.: Sources of particulate organic nitrates in the boreal forest in Finland, Boreal Environ. Res., 22, 13–26, 2017.
Kulmala, M., and Wagner, P. E.: Mass accommodation and uptake coefficients – a quantitative comparison, J. Aerosol Sci., 32, 833–841, https://doi.org/10.1016/S0021-8502(00)00116-6, 2001.
Lang, J., Zhang, Y., Zhou, Y., Cheng, S., Chen, D., Guo, X., Chen, S., Li, X., Xing, X., and Wang, H.: Trends of PM2.5 and Chemical Composition in Beijing, 2000–2015, Aerosol Air Qual. Res., 17, 412–425, https://doi.org/10.4209/aaqr.2016.07.0307, 2017.
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., and Pozzer, A.: The contribution of outdoor air pollution sources to premature mortality on a global scale, Nature, 525, 367–371, https://doi.org/10.1038/nature15371, 2015.
Li, C., Martin, R. V., van Donkelaar, A., Boys, B. L., Hammer, M. S., Xu, J.-W., Marais, E. A., Reff, A., Strum, M., Ridley, D. A., Crippa, M., Brauer, M., and Zhang, Q.: Trends in chemical composition of global and regional population-weighted fine particulate matter estimated for 25 Years, Environ. Sci. Technol., 51, 11185–11195, https://doi.org/10.1021/acs.est.7b02530, 2017.
Li, H., Zhang, Q., Zheng, B., Chen, C., Wu, N., Guo, H., Zhang, Y., Zheng, Y., Li, X., and He, K.: Nitrate-driven urban haze pollution during summertime over the North China Plain, Atmos. Chem. Phys., 18, 5293–5306, https://doi.org/10.5194/acp-18-5293-2018, 2018.
Li, Y. J., Liu, P. F., Bergoend, C., Bateman, A. P., and Martin, S. T.: Rebounding hygroscopic inorganic aerosol particles: Liquids, gels, and hydrates, Aerosol Sci. Technol., 51, 388–396, https://doi.org/10.1080/02786826.2016.1263384, 2017.
Lightstone, J. M., Onasch, T. B., Imre, D., and Oatis, S.: Deliquescence, Efflorescence, and Water Activity in ammonium nitrate and mixed ammonium nitrate/succinic acid microparticles, J. Phys. Chem. A, 104, 9337–9346, https://doi.org/10.1021/jp002137h, 2000.
Liu, C., Ma, Q., Liu, Y., Ma, J., and He, H.: Synergistic reaction between SO2 and NO2 on mineral oxides: a potential formation pathway of sulfate aerosol, Phys. Chem. Chem. Phys., 14, 1668–1676, https://doi.org/10.1039/C1CP22217A, 2012.
Liu, F., Beirle, S., Zhang, Q., van der A, R. J., Zheng, B., Tong, D., and He, K.: NOx emission trends over Chinese cities estimated from OMI observations during 2005 to 2015, Atmos. Chem. Phys., 17, 9261–9275, https://doi.org/10.5194/acp-17-9261-2017, 2017.
Liu, H., He, K., Wang, Q., Huo, H., Lents, J., Davis, N., Nikkila, N., Chen, C., Osses, M., and He, C.: Comparison of vehicle activity and emission inventory between Beijing and Shanghai, J. Air Waste Manage. Assoc., 57, 1172–1177, https://doi.org/10.3155/1047-3289.57.10.1172, 2007.
Liu, L., Bei, N., Wu, J., Liu, S., Zhou, J., Li, X., Yang, Q., Feng, T., Cao, J., Tie, X., and Li, G.: Effects of stabilized Criegee intermediates (sCIs) on sulfate formation: a sensitivity analysis during summertime in Beijing–Tianjin–Hebei (BTH), China, Atmos. Chem. Phys., 19, 13341–13354, https://doi.org/10.5194/acp-19-13341-2019, 2019.
Liu, P., Ye, C., Xue, C., Zhang, C., Mu, Y., and Sun, X.: Formation mechanisms of atmospheric nitrate and sulfate during the winter haze pollution periods in Beijing: gas-phase, heterogeneous and aqueous-phase chemistry, Atmos. Chem. Phys., 20, 4153–4165, https://doi.org/10.5194/acp-20-4153-2020, 2020.
Liu, T., Clegg, S. L., and Abbatt, J. P. D.: Fast oxidation of sulfur dioxide by hydrogen peroxide in deliquesced aerosol particles, P. Natl. Acad. Sci. USA, 117, 1354–1359, https://doi.org/10.1073/pnas.1916401117, 2020.
Liu, T., Chan, A. W. H., and Abbatt, J. P. D.: Multiphase oxidation of sulfur dioxide in aerosol particles: implications for sulfate formation in polluted environments, Environ. Sci. Technol., 55, 4227–4242, https://doi.org/10.1021/acs.est.0c06496, 2021.
Liu, Y., Liggio, J., Harner, T., Jantunen, L., Shoeib, M., and Li, S.-M.: Heterogeneous OH initiated oxidation: A possible explanation for the persistence of organophosphate flame retardants in air, Environ. Sci. Technol., 48, 1041–1048, https://doi.org/10.1021/es404515k, 2014.
Liu, Y., Han, C., Ma, J., Bao, X., and He, H.: Influence of relative humidity on heterogeneous kinetics of NO2 on kaolin and hematite, Phys. Chem. Chem. Phys., 17, 19424–19431, https://doi.org/https://doi.org/10.1039/C5CP02223A, 2015.
Liu, Y., Wu, Z., Wang, Y., Xiao, Y., Gu, F., Zheng, J., Tan, T., Shang, D., Wu, Y., Zeng, L., Hu, M., Bateman, A. P., and Martin, S. T.: Submicrometer particles are in the liquid state during heavy haze episodes in the urban atmosphere of Beijing, China, Environ. Sci. Technol. Lett., 4, 427–432, https://doi.org/10.1021/acs.estlett.7b00352, 2017.
Liu, Y., Wu, Z., Huang, X., Shen, H., Bai, Y., Qiao, K., Meng, X., Hu, W., Tang, M., and He, L.: Aerosol phase state and its link to chemical composition and liquid water content in a subtropical coastal megacity, Environ. Sci. Technol., 53, 5027–5033, https://doi.org/10.1021/acs.est.9b01196, 2019.
Liu, Y., Ni, S., Jiang, T., Xing, S., Zhang, Y., Bao, X., Feng, Z., Fan, X., Zhang, L., and Feng, H.: Influence of Chinese New Year overlapping COVID-19 lockdown on HONO sources in Shijiazhuang, Sci. Total Environ., 745, 141025, https://doi.org/10.1016/j.scitotenv.2020.141025, 2020a.
Liu, Y., Yan, C., Feng, Z., Zheng, F., Fan, X., Zhang, Y., Li, C., Zhou, Y., Lin, Z., Guo, Y., Zhang, Y., Ma, L., Zhou, W., Liu, Z., Wei, Z., Dada, L., Dallenbach, K. R., Kontkanen, J., Cai, R., Chan, T., Chu, B., Du, W., Yao, L., Wang, Y., Cai, J., Kangasluoma, J., Kokkonen, T., Kujansuu, J., Rusanen, A., Deng, C., Fu, Y., Yin, R., Li, X., Lu, Y., Liu, Y., Lian, C., Yang, D., Wang, W., Ge, M., Wang, Y., Worsnop, D., Junninen, H., He, H., Kerminen, V. M., Zheng, J., Wang, L., Jiang, J., Petäjä, T., Bianchi, F., and Kulmala, M.: Continuous and comprehensive atmospheric observations in Beijing: A station to understand the complex urban atmospheric environment, Big Earth Data, 4, 295–321, https://doi.org/10.1080/20964471.2020.1798707, 2020b.
Liu, Y., Zhang, Y., Lian, C., Yan, C., Feng, Z., Zheng, F., Fan, X., Chen, Y., Wang, W., Chu, B., Wang, Y., Cai, J., Du, W., Daellenbach, K. R., Kangasluoma, J., Bianchi, F., Kujansuu, J., Petäjä, T., Wang, X., Hu, B., Wang, Y., Ge, M., He, H., and Kulmala, M.: The promotion effect of nitrous acid on aerosol formation in wintertime in Beijing: the possible contribution of traffic-related emissions, Atmos. Chem. Phys., 20, 13023–13040, https://doi.org/10.5194/acp-20-13023-2020, 2020c.
Ma, Q., Liu, Y., and He, H.: Synergistic effect between NO2 and SO2 in their adsorption and reaction on γ-alumina, J. Phys. Chem. A, 112, 6630–6635, https://doi.org/10.1021/jp802025z, 2008.
Maaß, F., Elias, H., and Wannowius, K. J.: Kinetics of the oxidation of hydrogen sulfite by hydrogen peroxide in aqueous solution: ionic strength effects and temperature dependence, Atmos. Environ., 33, 4413–4419, https://doi.org/10.1016/S1352-2310(99)00212-5, 1999.
Maahs, H. G.: Kinetics and mechanism of the oxidation of S(IV) by ozone in aqueous solution with particular reference to SO2 conversion in nonurban tropospheric clouds, J. Geophys. Res.-Oceans, 88, 10721–10732, https://doi.org/10.1029/JC088iC15p10721, 1983.
Marshall, F. H., Berkemeier, T., Shiraiwa, M., Nandy, L., Ohm, P. B., Dutcher, C. S., and Reid, J. P.: Influence of particle viscosity on mass transfer and heterogeneous ozonolysis kinetics in aqueous–sucrose–maleic acid aerosol, Phys. Chem. Chem. Phys., 20, 15560–15573, https://doi.org/10.1039/C8CP01666F, 2018.
Martin, L. R. and Good, T. W.: Catalyzed oxidation of sulfur dioxide in solution: The iron-manganese synergism, Atmos. Environ. A-Gen., 25, 2395–2399, https://doi.org/10.1016/0960-1686(91)90113-L, 1991.
Murphy, D. M. and Fahey, D. W.: Mathematical treatment of the wall loss of a trace species in denuder and catalytic converter tubes, Anal. Chem., 59, 2753–2759, https://doi.org/10.1021/ac00150a006, 1987.
Onasch, T. B., Siefert, R. L., Brooks, S. D., Prenni, A. J., Murray, B., Wilson, M. A., and Tolbert, M. A.: Infrared spectroscopic study of the deliquescence and efflorescence of ammonium sulfate aerosol as a function of temperature, J. Geophys. Res.-Atmos., 104, 21317–21326, https://doi.org/10.1029/1999jd900384, 1999.
Pye, H. O. T., Nenes, A., Alexander, B., Ault, A. P., Barth, M. C., Clegg, S. L., Collett Jr., J. L., Fahey, K. M., Hennigan, C. J., Herrmann, H., Kanakidou, M., Kelly, J. T., Ku, I.-T., McNeill, V. F., Riemer, N., Schaefer, T., Shi, G., Tilgner, A., Walker, J. T., Wang, T., Weber, R., Xing, J., Zaveri, R. A., and Zuend, A.: The acidity of atmospheric particles and clouds, Atmos. Chem. Phys., 20, 4809–4888, https://doi.org/10.5194/acp-20-4809-2020, 2020.
Qi, J., Zheng, B., Li, M., Yu, F., Chen, C., Liu, F., Zhou, X., Yuan, J., Zhang, Q., and He, K.: A high-resolution air pollutants emission inventory in 2013 for the Beijing-Tianjin-Hebei region, China, Atmos. Environ., 170, 156–168, https://doi.org/10.1016/j.atmosenv.2017.09.039, 2017.
Ravishankara, A. R.: Heterogeneous and multiphase chemistry in the troposphere, Science, 276, 1058–1065, https://doi.org/10.1126/science.276.5315.1058, 1997.
Seinfeld, J. H. and Pandis, S. N.: Atmospheric chemistry and physics: From air pollution to climate change, 2nd Edn., John Wiley and Sons, New Jersey, USA, 429–44, 2006.
Shao, J., Chen, Q., Wang, Y., Lu, X., He, P., Sun, Y., Shah, V., Martin, R. V., Philip, S., Song, S., Zhao, Y., Xie, Z., Zhang, L., and Alexander, B.: Heterogeneous sulfate aerosol formation mechanisms during wintertime Chinese haze events: air quality model assessment using observations of sulfate oxygen isotopes in Beijing, Atmos. Chem. Phys., 19, 6107–6123, https://doi.org/10.5194/acp-19-6107-2019, 2019.
Shi, G., Xu, J., Shi, X., Liu, B., Bi, X., Xiao, Z., Chen, K., Wen, J., Dong, S., Tian, Y., Feng, Y., Yu, H., Song, S., Zhao, Q., Gao, J., and Russell, A. G.: Aerosol pH dynamics during haze periods in an urban environment in China: Use of detailed, hourly, speciated observations to study the role of ammonia availability and secondary aerosol formation and urban environment, J. Geophys. Res.-Atmos., 124, 9730–9742, https://doi.org/10.1029/2018JD029976, 2019.
Shiraiwa, M., Ammann, M., Koop, T., and Pöschl, U.: Gas uptake and chemical aging of semisolid organic aerosol particles, P. Natl. Acad. Sci. USA, 108, 11003–11008, https://doi.org/10.1073/pnas.1103045108, 2011.
Shiraiwa, M., Li, Y., Tsimpidi, A. P., Karydis, V. A., Berkemeier, T., Pandis, S. N., Lelieveld, J., Koop, T., and Pöschl, U.: Global distribution of particle phase state in atmospheric secondary organic aerosols, Nat. Commun., 8, 15002, https://doi.org/10.1038/ncomms15002, 2017.
Song, Q. and Osada, K.: Seasonal variation of aerosol acidity in Nagoya, Japan and factors affecting it, Atmos. Environ. X, 5, 100062, https://doi.org/10.1016/j.aeaoa.2020.100062, 2020.
Spindler, G., Hesper, J., Brüggemann, E., Dubois, R., Müller, T., and Herrmann, H.: Wet annular denuder measurements of nitrous acid: laboratory study of the artefact reaction of NO2 with S(IV) in aqueous solution and comparison with field measurements, Atmos. Environ., 37, 2643–2662, https://doi.org/10.1016/S1352-2310(03)00209-7, 2003.
Sun, J., Liu, L., Xu, L., Wang, Y., Wu, Z., Hu, M., Shi, Z., Li, Y., Zhang, X., Chen, J., and Li, W.: Key Role of Nitrate in Phase Transitions of Urban Particles: Implications of Important Reactive Surfaces for Secondary Aerosol Formation, J. Geophys. Res.-Atmos., 123, 1234–1243, https://doi.org/10.1002/2017jd027264, 2018.
Sun, Y. L., Wang, Z. F., Du, W., Zhang, Q., Wang, Q. Q., Fu, P. Q., Pan, X. L., Li, J., Jayne, J., and Worsnop, D. R.: Long-term real-time measurements of aerosol particle composition in Beijing, China: seasonal variations, meteorological effects, and source analysis, Atmos. Chem. Phys., 15, 10149–10165, https://doi.org/10.5194/acp-15-10149-2015, 2015.
Tang, G., Zhang, J., Zhu, X., Song, T., Münkel, C., Hu, B., Schäfer, K., Liu, Z., Zhang, J., Wang, L., Xin, J., Suppan, P., and Wang, Y.: Mixing layer height and its implications for air pollution over Beijing, China, Atmos. Chem. Phys., 16, 2459–2475, https://doi.org/10.5194/acp-16-2459-2016, 2016.
Tian, M., Liu, Y., Yang, F. M., Zhang, L. M., Peng, C., Chen, Y., Shi, G. M., Wang, H. B., Luo, B., Jiang, C. T., Li, B., Takeda, N., and Koizumi, K.: Increasing importance of nitrate formation for heavy aerosol pollution in two megacities in Sichuan Basin, southwest China, Environ. Pollut., 250, 898–905, https://doi.org/10.1016/j.envpol.2019.04.098, 2019.
Tilgner, A., Schaefer, T., Alexander, B., Barth, M., Collett Jr., J. L., Fahey, K. M., Nenes, A., Pye, H. O. T., Herrmann, H., and McNeill, V. F.: Acidity and the multiphase chemistry of atmospheric aqueous particles and clouds, Atmos. Chem. Phys. Discuss. [preprint], https://doi.org/10.5194/acp-2021-58, in review, 2021.
Usher, C. R., Michel, A. E., and Grassian, V. H.: Reactions on mineral dust, Chem. Rev., 103, 4883–4939, https://doi.org/10.1021/cr020657y, 2003.
Wand, M. P. and Jones, M. C.: Comparison of smoothing parameterizations in bivariate kernel density estimation, J. Am. Stat. Assoc., 88, 520–528, https://doi.org/10.1080/01621459.1993.10476303, 1993.
Wang, G., Zhang, R., Gomez, M. E., Yang, L., Zamora, M. L., Hu, M., Lin, Y., Peng, J., Guoc, S., Meng, J., Li, J., Cheng, C., Hu, T., Ren, Y., Wang, Y., Gao, J., Cao, J., An, Z., Zhou, W., Li, G., Wang, J., Tian, P., Marrero-Ortiz, W., Secrest, J., Du, Z., Zheng, J., Shang, D., Zeng, L., Shao, M., Wang, W., Huang, Y., Wang, Y., Zhu, Y., Li, Y., Hu, J., Pan, B., Cai, L., Cheng, Y., Ji, Y., Zhang, F., Rosenfeld, D., Liss, P. S., Duce, R. A., Kolb, C. E., and Molina, M. J.: Persistent sulfate formation from London Fog to Chinese haze, P. Natl. Acad. Sci. USA, 113, 13630–13635, https://doi.org/10.1073/pnas.1616540113, 2016.
Wang, J. F., Li, J. Y., Ye, J. H., Zhao, J., Wu, Y. Z., Hu, J. L., Liu, D. T., Nie, D. Y., Shen, F. Z., Huang, X. P., Huang, D. D., Ji, D. S., Sun, X., Xu, W. Q., Guo, J. P., Song, S. J., Qin, Y. M., Liu, P. F., Turner, J. R., Lee, H. C., Hwang, S. W., Liao, H., Martin, S. T., Zhang, Q., Chen, M. D., Sun, Y. L., Ge, X. L., and Jacob, D. J.: Fast sulfate formation from oxidation of SO2 by NO2 and HONO observed in Beijing haze, Nat. Commun., 11, 2844, https://doi.org/10.1038/s41467-020-16683-x, 2020.
Wang, Q.-Q., Ma, Y.-L., Tan, J.-H., Yang, F.-M., Wei, L.-F., Duan, J.-C., and He, K.-B.: Characterization of water-soluble heavy metals of PM2.5 during winter in Beijing, China Environ. Sci., 34, 2204–2210, 2014 (in Chinese).
Wang, W., Liu, M., Wang, T., Song, Y., Zhou, L., Cao, J., Hu, J., Tang, G., Chen, Z., Li, Z., Xu, Z., Peng, C., Lian, C., Chen, Y., Pan, Y., Zhang, Y., Sun, Y., Li, W., Zhu, T., Tian, H., and Ge, M.: Sulfate formation is dominated by manganese-catalyzed oxidation of SO2 on aerosol surfaces during haze events, Nat. Commun., 12, 1993, https://doi.org/10.1038/s41467-021-22091-6, 2021.
Wang, Y., Zhuang, G., Tang, A., Yuan, H., Sun, Y., Chen, S., and Zheng, A.: The ion chemistry and the source of PM2.5 aerosol in Beijing, Atmos. Environ., 39, 3771–3784, https://doi.org/10.1016/j.atmosenv.2005.03.013, 2005.
Wang, Y., Chen, Y., Wu, Z., Shang, D., Bian, Y., Du, Z., Schmitt, S. H., Su, R., Gkatzelis, G. I., Schlag, P., Hohaus, T., Voliotis, A., Lu, K., Zeng, L., Zhao, C., Alfarra, M. R., McFiggans, G., Wiedensohler, A., Kiendler-Scharr, A., Zhang, Y., and Hu, M.: Mutual promotion between aerosol particle liquid water and particulate nitrate enhancement leads to severe nitrate-dominated particulate matter pollution and low visibility, Atmos. Chem. Phys., 20, 2161–2175, https://doi.org/10.5194/acp-20-2161-2020, 2020.
Wang, Y. S., Teter, J., and Sperling, D.: China's soaring vehicle population: Even greater than forecasted?, Energ. Policy, 39, 3296–3306, https://doi.org/10.1016/j.enpol.2011.03.020, 2011.
Warneck, P.: The oxidation of sulfur(IV) by reaction with iron(III): A critical review and data analysis, Phys. Chem. Chem. Phys., 20, 4020–4037, https://doi.org/10.1039/C7CP07584G, 2018.
World Health Organization (WHO): Air quality guidelines – global update 2005, World Health Organization, Geneva, Switzerland, 11, available at: https://www.euro.who.int (last access: 27 August 2021), 2006.
World Health Organization (WHO): Health effects of particulate matter, World Health Organization, Geneva, Switzerland, 7, available at: https://www.euro.who.int (last access: 27 August 2021), 2013.
Wu, L., Sun, J., Zhang, X., Zhang, Y., Wang, Y., Zhong, J., and Yang, Y.: Aqueous-phase reactions occurred in the PM2.5 cumulative explosive growth during the heavy pollution episode (HPE) in 2016 Beijing wintertime, Tellus B, 71, 1620079, https://doi.org/10.1080/16000889.2019.1620079, 2019.
Wu, Z., Wang, Y., Tan, T., Zhu, Y., Li, M., Shang, D., Wang, H., Lu, K., Guo, S., Zeng, L., and Zhang, Y.: Aerosol liquid water driven by anthropogenic inorganic salts: Implying its key role in haze formation over the North China Plain, Environ. Sci. Technol. Lett., 5, 160–166, https://doi.org/10.1021/acs.estlett.8b00021, 2018.
Xie, Y., Ding, A., Nie, W., Mao, H., Qi, X., Huang, X., Xu, Z., Kerminen, V.-M., Petäjä, T., Chi, X., Virkkula, A., Boy, M., Xue, L., Guo, J., Sun, J., Yang, X.-Q., Kulmala, M., and Fu, C.: Enhanced sulfate formation by nitrogen dioxide: Implications from in-situ observations at the SORPES Station, J. Geophys. Res.-Atmos., 120, 12679–12694, https://doi.org/10.1002/2015JD023607, 2015.
Xue, J., Yuan, Z., Griffith, S. M., Yu, X., Lau, A. K. H., and Yu, J. Z.: Sulfate formation enhanced by a cocktail of high NOx, SO2, particulate matter, and droplet pH during haze-fog events in megacities in China: An observation-based modeling investigation, Environ. Sci. Technol., 50, 7325–7334, https://doi.org/10.1021/acs.est.6b00768, 2016.
Yang, D., Zhang, S., Niu, T., Wang, Y., Xu, H., Zhang, K. M., and Wu, Y.: High-resolution mapping of vehicle emissions of atmospheric pollutants based on large-scale, real-world traffic datasets, Atmos. Chem. Phys., 19, 8831–8843, https://doi.org/10.5194/acp-19-8831-2019, 2019.
Yang, W., He, H., Ma, Q., Ma, J., Liu, Y., Liu, P., and Mu, Y.: Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust, Phys. Chem. Chem. Phys., 18, 956–964, https://doi.org/10.1039/C5CP06144J, 2016.
Yao, L., Fan, X., Yan, C., Kurtén, T., Daellenbach, K., Li, C., Wang, Y., Guo, Y., Dada, L., Rissanen, M. P., Cai, J., Tham, Y. J., Zha, Q., Zhang, S., Du, W., Yu, M., Zheng, F., Zhou, Y., Kontkanen, J., Chan, T., Shen, J., Kujansuu, J. T., Kangasluoma, J., Jiang, J., Wang, L., Worsnop, D. R., Petäjä, T., Kerminen, V.-M., Liu, Y., Chu, B., He, H., Kulmala, M., and Bianchi, F.: Unprecedented ambient sulphur trioxide (SO3) detection: Possible formation mechanism and atmospheric implications, Environ. Sci. Technol. Lett., 7, 809–818, https://doi.org/10.1021/acs.estlett.0c00615, 2020.
Ye, C., Liu, P., Ma, Z., Xue, C., Zhang, C., Zhang, Y., Liu, J., Liu, C., Sun, X., and Mu, Y.: High H2O2 concentrations observed during haze periods during the winter in Beijing: Importance of H2O2 oxidation in sulfate formation, Environ. Sci. Technol. Lett., 5, 757–763, https://doi.org/10.1021/acs.estlett.8b00579, 2018.
Ye, C., Chen, H., Hoffmann, E. H., Mettke, P., Tilgner, A., He, L., Mutzel, A., Brüggemann, M., Poulain, L., Schaefer, T., Heinold, B., Ma, Z., Liu, P., Xue, C., Zhao, X., Zhang, C., Zhang, F., Sun, H., Li, Q., Wang, L., Yang, X., Wang, J., Liu, C., Xing, C., Mu, Y., Chen, J., and Herrmann, H.: Particle-phase photoreactions of HULIS and TMIs establish a strong source of H2O2 and particulate sulfate in the winter North China Plain, Environ. Sci. Technol., 55, 7818–7830, https://doi.org/10.1021/acs.est.1c00561, 2021.
Yu, Z., Jang, M., and Park, J.: Modeling atmospheric mineral aerosol chemistry to predict heterogeneous photooxidation of SO2, Atmos. Chem. Phys., 17, 10001–10017, https://doi.org/10.5194/acp-17-10001-2017, 2017.
Zhang, F., Wang, Y., Peng, J., Chen, L., Sun, Y., Duan, L., Ge, X., Li, Y., Zhao, J., Liu, C., Zhang, X., Zhang, G., Pan, Y., Wang, Y., Zhang, A. L., Ji, Y., Wang, G., Hu, M., Molina, M. J., and Zhang, R.: An unexpected catalyst dominates formation and radiative forcing of regional haze, P. Natl. Acad. Sci. USA, 117, 3960–3966, https://doi.org/10.1073/pnas.1919343117, 2020.
Zhang, X., Zhong, J., Wang, J., Wang, Y., and Liu, Y.: The interdecadal worsening of weather conditions affecting aerosol pollution in the Beijing area in relation to climate warming, Atmos. Chem. Phys., 18, 5991–5999, https://doi.org/10.5194/acp-18-5991-2018, 2018.
Zhang, Y., Sun, J., Zhang, X., Shen, X., Wang, T., and Qin, M.: Seasonal characterization of components and size distributions for submicron aerosols in Beijing, Sci. China Earth Sci., 56, 890–900, https://doi.org/10.1007/s11430-012-4515-z, 2013.
Zhang, Y., Bao, F., Li, M., Chen, C., and Zhao, J.: Nitrate enhanced oxidation of SO2 on mineral dust: A vital role of proton, Environ. Sci. Technol., 53, 10139–10145, https://doi.org/10.1021/acs.est.9b01921, 2019.
Zhao, Y., Liu, Y., Ma, J., Ma, Q., and He, H.: Heterogeneous reaction of SO2 with soot: The roles of relative humidity and surface composition of soot in surface sulfate formation, Atmos. Environ., 152, 465–476, https://doi.org/10.1016/j.atmosenv.2017.01.005, 2017.
Zheng, B., Zhang, Q., Zhang, Y., He, K. B., Wang, K., Zheng, G. J., Duan, F. K., Ma, Y. L., and Kimoto, T.: Heterogeneous chemistry: a mechanism missing in current models to explain secondary inorganic aerosol formation during the January 2013 haze episode in North China, Atmos. Chem. Phys., 15, 2031–2049, https://doi.org/10.5194/acp-15-2031-2015, 2015.
Zhong, J., Zhang, X., Dong, Y., Wang, Y., Liu, C., Wang, J., Zhang, Y., and Che, H.: Feedback effects of boundary-layer meteorological factors on cumulative explosive growth of PM2.5 during winter heavy pollution episodes in Beijing from 2013 to 2016, Atmos. Chem. Phys., 18, 247–258, https://doi.org/10.5194/acp-18-247-2018, 2018.
Ziemke, J. R., Oman, L. D., Strode, S. A., Douglass, A. R., Olsen, M. A., McPeters, R. D., Bhartia, P. K., Froidevaux, L., Labow, G. J., Witte, J. C., Thompson, A. M., Haffner, D. P., Kramarova, N. A., Frith, S. M., Huang, L.-K., Jaross, G. R., Seftor, C. J., Deland, M. T., and Taylor, S. L.: Trends in global tropospheric ozone inferred from a composite record of TOMS/OMI/MLS/OMPS satellite measurements and the MERRA-2 GMI simulation , Atmos. Chem. Phys., 19, 3257–3269, https://doi.org/10.5194/acp-19-3257-2019, 2019.




