the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Opinion: Papers that shaped tropospheric chemistry
A. R. Ravishankara
Erika von Schneidemesser
Roberto Sommariva
Which published papers have transformed our understanding of the chemical processes in the troposphere and shaped the field of atmospheric chemistry? By way of expert solicitation and interactive peer review, this paper explores the influence of the ideas in peer-reviewed articles based on input from our community of atmospheric scientists. We explore how these papers have shaped the development of the field of atmospheric chemistry and identify the major landmarks in the field of atmospheric chemistry through the lens of those papers' impact on science, legislation and environmental events. We also explore the ways in which one can identify the papers that have most impacted the field and discuss the advantages and disadvantages of the various approaches. Our work highlights the difficulty of creating a simple list, and we explore the reasons for this difficulty. The paper also provides a history of the development of our understanding of tropospheric chemistry and points some ways for the future.
- Article
(5268 KB) - Full-text XML
- BibTeX
- EndNote
Air quality and anthropogenic climate change are two environmental issues of current importance to society. Atmospheric composition is central to both these issues. The atmosphere, along with its components, supports life on Earth. In turn, the atmosphere is affected by human population growth and industrialization, as well as all the consequences of those changes. The changes in atmospheric composition also influence the ecosystem on which humans rely.
Air pollution (née composition) and its impacts have a history stretching back to antiquity – see, for example, the expositions in Brimblecombe (1987), Fuller (2018), Jacobson (2002), Stern (1968), Sportisse (2010), Preining and Davis (1999), Fowler et al. (2020), and others. Changes in atmospheric composition, with particularly negative impacts on human health (Lelieveld et al., 2015; Landrigan et al., 2018), ecosystems (Fowler et al., 2009) and climate (see Fiore et al., 2012; von Schneidemesser et al., 2015), have become primary global concerns during the latter part of the 20th and the 21st centuries. As an academic subject, air pollution has mostly been systematically studied only since the middle to late 20th century. There have been several recent reviews (e.g. Brasseur et al., 2003; Monks et al., 2009; Ravishankara et al., 2015; Ravishankara, 2003), which have mapped the growth of atmospheric chemistry, but it is not only peer-reviewed papers that provide relevant overviews. It is important to note that when dealing with the development of this subject (or any scientific subject for that matter), much of the baseline knowledge is embodied in textbooks, which for many are the entry point to and the primary reference for the topic (e.g. Jacob, 1999; Wayne, 2000; Finlayson-Pitts and Pitts, 2000; Seinfeld and Pandis, 2006; Brasseur et al., 1999).
Figure 1 shows the number of peer-reviewed papers by year that mentioned the phrase “atmospheric chemistry” in the text, as catalogued by the Scopus bibliographic database (https://www.scopus.com/, last access: 20 June 2020). It shows growth in the later 1970s from around 100 papers a year to approximately 4000 a year currently, with an especially large increase over the past 2 decades. Of course, many more papers discuss atmospheric chemistry or are relevant to it without explicitly mentioning these words!
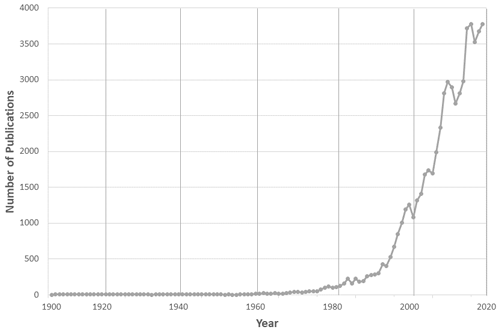
Figure 1Number of publications per year in a SCOPUS search on the phrase “atmospheric chemistry” (compiled in June 2020).
This paper aims to recognize and highlight some of the most influential peer-reviewed articles that have shaped this field. There were many pivotal scientific discoveries and there were many papers that spurred action and further research. What were the significant discoveries that shaped the atmospheric chemistry of today? And how do we narrow down the list of contributions to highlight the most impactful ones?
There are many ways to choose the papers that described discoveries and influenced atmospheric composition and chemistry. Here we have assembled a compilation of influential articles. Our goal is not to show what makes a “great” paper, which depends not only on the science, but also the quality of the writing, readability, structure of the written work and the point of view of the reader – all criteria that are highly subjective. Nor are we aiming only for those papers that led to policy and management actions. Instead, we try to reflect on the papers' science and content and the influence of the ideas in those papers on our community of scientists and on the field's development. Our approach is to present our thoughts – informed by the solicitation for input from colleagues in the field – and share what we think are the fundamental discoveries and developments, start a discussion, and allow others to build on, reinforce or critique our work.
In addition to peer-reviewed papers and the above-mentioned textbooks, we of course have other media through which we communicate (or have communicated) with our peers. These include scientific reports, conferences and meetings. In addition, we have scientific assessments and evaluations, which often get more scrutiny and review than the peer-reviewed papers they include. These days, other communication media, such as social media, have also become prevalent as formats for exchange both within the scientific community and with a broader audience. However, the entire community cannot attend all conferences and meetings, the scientific reports are not always accessible and are often not peer reviewed, and the assessments are often driven more by policy needs rather than by scientific discoveries. Publishing peer-reviewed papers is the closest we come to reaching the whole community. We do note that, despite its known issues, the peer-reviewed literature is still considered the gold standard for quality and reliability. For these reasons, we discuss only peer-reviewed papers here, although we aim to communicate the overarching scientific advances that shaped the field.
1.1 How were the papers selected?
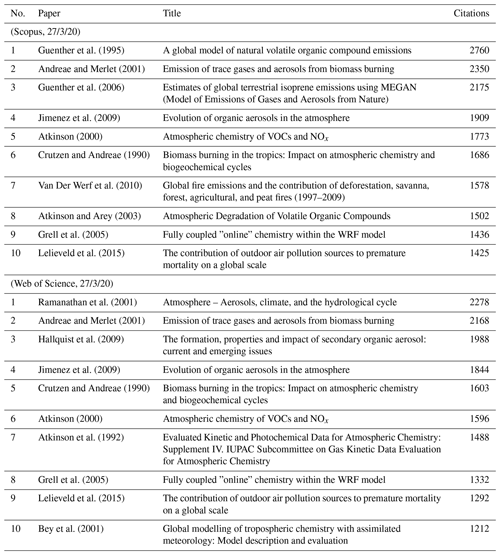
Easily measurable criteria, like the number of citations, are one metric. However, this approach favours papers of a particular vintage and not necessarily the earlier or later papers. Besides, there are several drawbacks to these simple and objective criteria. Citations tend to go down when something is assumed to be common knowledge and makes it into textbooks or compilations. For example, nobody cites Priestley for discovering oxygen or Schönbein for discovering ozone whenever atmospheric composition is mentioned. Indeed, some of the central concepts of atmospheric chemistry and physics are considered to be common knowledge, and their origins are taken for granted. The number of citations will also be influenced by the journal in which a paper is published and quite often (we hate to say this) also depends on who else cited them and in which journal they were cited. Citations also depend on how many people are otherwise researching a particular subject. Furthermore, critical assessments and expert data evaluations suppress the citation of the original papers. This is particularly the case, for example, for papers on chemical kinetics and photochemistry, whereby people tend to simply cite the data evaluations such as National Aeronautics and Space Administration Jet Propulsion Laboratory (NASA/JPL, https://jpldataeval.jpl.nasa.gov/, last access: 6 August 2021) or International Union of Pure and Applied Chemistry (IUPAC, http://iupac.pole-ether.fr/, last access: 6 August 2021) panel reports. Similarly, people often cite the quadrennial Scientific Assessment of Ozone Depletion and Intergovernmental Panel on Climate Change (IPCC) assessments, thereby obfuscating the underlying original papers. Other types of papers, such as reviews, tend to get an excessive number of citations (for understandable reasons). Lastly, we cannot overlook the influence of journal availability in different parts of the world. This availability is exacerbated when journal costs go up, and not everybody can access new papers. Nevertheless, there is still a relevance to the number of citations of a paper. We show, for example, the 10 most-cited papers when we searched for the combination of words “atmospheric” and “chemistry” in Table 1.
For all the above reasons, we decided to use a different approach here. We solicited the scientific community to obtain input from the experts in the field. To accomplish this, we put out a call through the International Global Atmospheric Chemistry (IGAC) (Melamed et al., 2015) project to its contacts and thereby engaged a broad audience. Despite the broad audience of IGAC, the vast majority of responses came from scientists in North America and Europe. An initial list of influential papers was established by combining the replies received from the expert solicitation to evaluate the most-nominated papers. In addition, a variety of perspectives were assembled for the writing team, including different career stages, nationalities and genders. Despite all these efforts, the selection methods will still inevitably create bias that cannot be escaped. Therefore, in many respects, the chosen papers are not supposed to be a definitive list, but rather a compilation that allows researchers to discuss and reflect on what makes impactful science and maybe ponder the landmarks in our subject. Furthermore, we hope that the end product can provide an interesting history and context to those who are joining the community and document the current “perception” of the most important papers.
We have noted the drawbacks in our methodology simply to present some of the limitations of what we did in this paper up front. However, we hope that others will find this work relevant and engaging. Through an open and active peer-review process, we were able to obtain the perspective of a broader community more reflective of the global composition of the field. To facilitate this, the paper was published first as an open-access discussion paper that included a public comment period. We hope that this approach overcame some of the limitations and reservations we expressed earlier. We thank all reviewers and contributors for their help in determining the final shape of this overview.
1.2 Scope of work
As with the selection method, one can debate the scope and the methodology for a work such as this. Still, the boundaries we have drawn encompass studies that have shaped our understanding of the atmosphere and the underlying chemical and physical processes, focusing mostly on the troposphere. This includes modelling, field measurements, remote sensing and laboratory studies (Abbatt et al., 2014). We have also included atmospheric interactions with the biosphere, cryosphere and hydrosphere.
We selected 2010 as the cut-off year. Our rationale is that for a paper to have been influential in the whole field it must be at least 10 years old and thus had time to accrue recognition. We recognize that important papers in newer areas of endeavour are disadvantaged by this criterion. Examples include the subjects of secondary organic aerosol (SOA) formation (Ehn et al., 2014; Crounse et al., 2013), the chemistry of Criegee intermediates (Welz et al., 2012; Mauldin III et al., 2012), galactic-ray-induced aerosol particle nucleation (Kirkby et al., 2011) and air pollution–climate connections (Shindell et al., 2012). Influential assessments such as bounding black carbon (Bond et al., 2013) are also missed. However, these areas will undoubtedly be recognized in the coming decades. The 10-year window has also allowed the scientific community to have extensive input on a paper's validity, i.e. meeting the criterion of “standing the test of time”.
The papers have been grouped into the following general categories and are presented as such in Sect. 2.
- 2.1
Foundations
- 2.2
Aerosols and clouds
- 2.3
Secondary organic aerosols
- 2.4
Chemical kinetics, laboratory data and chemical mechanisms
- 2.5
Heterogeneous and multiphase chemistry
- 2.6
Chemical models
- 2.7
Tropospheric ozone
- 2.8
Nitrogen chemistry
- 2.9
HOx chemistry
- 2.10
Nighttime chemistry
- 2.11
Halogen chemistry
- 2.12
Volatile organic compounds
- 2.13
Biogenic emissions and chemistry
- 2.14
Biomass burning
- 2.15
Emissions and deposition
- 2.16
Chemical transport
- 2.17
Satellites and the troposphere
- 2.18
Stratospheric chemistry
- 2.19
Other issues that influenced tropospheric chemistry
The groups were chosen to reflect the main areas of research or endeavour, recognizing that this division could be done in several different ways. There is no assumed equivalence in these groups regarding their perceived or real importance or impacts. In the following, we discuss the papers in each group to show why they have been nominated and to put them in the historical context of the development of atmospheric chemistry as a discipline.
2.1 Foundations
Atmospheric chemistry has some long-standing and deep roots. However, it blossomed in the second half of the 20th century following concerns about ozone layer depletion and various forms of tropospheric pollution, such as the Los Angeles smog, London smog and acid precipitation (Table 2). Many note John Dalton's early contributions on the proportion of gases in the atmosphere (Dalton, 1805) and John Tyndall's Bakerian lecture on radiation and gases (Tyndall, 1861) as among the first studies in this field. The work of Arrhenius, “On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground” (Arrhenius, 1896), and the subsequent paper of Callendar, “The artificial production of carbon dioxide and its influence on temperature” (Callendar, 1938), laid the groundwork for the linkage between atmospheric chemistry and climate. Concerning aerosols, the seminal work of John Aitken (Aitken, 1888), “On the number of dust particles in the atmosphere”, details early work to count the number per cubic centimetre in various indoor and outdoor environments. It is interesting to note that physiologists looking at the number of live germs in the air stimulated Aitken's work. The later work of Köhler (1936), which explored cloud droplet nucleation, remains the basis for later work (see Sect. 2.2). The start of atmospheric chemistry as a distinct discipline probably arrived with Chapman's chemical theory of the stratospheric ozone layer in 1930 (Chapman, 1930), which will be further discussed in Sect. 2.18. This study heralded the importance of atmospheric chemistry on a global scale.
Table 2Science, regulatory and environmental landmarks of the 20th and early 21st centuries.
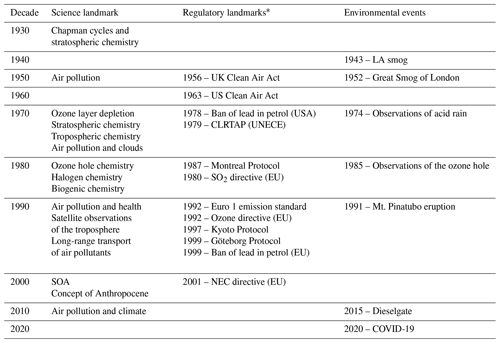
* For more details on the UK/EU perspective see Williams (2004) and Maynard and Williams (2018), and for the USA perspective see Jacobson (2002); see also Monks and Williams (2020).
In analysing the influential papers on atmospheric composition, one cannot help but note the relationship between these papers and the most significant contemporary environmental issues (Table 2). The first of these was the Los Angeles smog, which had its European counterpart, the London “pea soup” (Brimblecombe, 1987). The two events, which in chemical terms have no equivalence, had comparable impacts on public health and opinion. The oft recognized work of Haagen-Smit (Haagen-Smit and Fox, 1954; Haagen-Smit, 1952; Haagen-Smit et al., 1953) in the early 1950s on the Los Angeles smog was the first to coin the term “air pollution” in the modern era. Haagen-Smit showed that automobile exhaust gases can form ozone in the air and should therefore be considered a definite source of smog. Figure 2, redrawn from Haagen-Smit (1952), shows a schematic presentation of the reactions in polluted air leading to smog. Notably, the basic features of tropospheric chemical processes, as we understand them today, were already recognized in these early papers, and they showed how ozone could be chemically produced in the troposphere. Brasseur has documented these findings in a very thorough review (Brasseur et al., 2003).
It is widely recognized that both Crutzen (1973a, b) and Chameides and Walker (1973) found that similar “smog reactions” oxidize methane (CH4) and carbon monoxide (CO) to produce substantial amounts of ozone in remote regions of the troposphere. They estimated chemically produced ozone to be much greater than that transported from the stratosphere, which was believed to be the primary source of this chemical in the troposphere at that point. A few years earlier, in 1970, Hiram Levy II suggested that the hydroxyl radical, which provides the dominant oxidation mechanism in the troposphere, was formed in unpolluted air by the same mechanism that had been described as occurring in polluted air (Levy, 1971). This paper is recognized by many as the first description of the chemistry of the lower atmosphere involving hydroxyl radical reactions of methane and carbon monoxide, hydroperoxyl radicals, and the photolysis of ozone and formaldehyde as radical sources. In particular, he found that the very short-lived electronically excited oxygen atom (O1D) is a possible source of the hydroxyl radical (OH), an idea now well established.
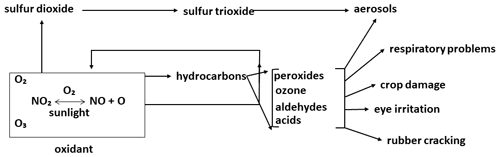
Figure 2Schematic representation of the reactions in polluted air leading to smog symptoms (adapted from Haagen-Smit et al., 1953).
Around the same time, Weinstock (1969) explained how cosmic rays lead to the production of radiocarbon dioxide (14CO2), which is incorporated into living plants. This process requires a rapid turnover of radiocarbon monoxide (14CO), which was unexpected because the lower atmosphere was thought to be a “chemical desert”. Instead, carbon monoxide appeared to have a turnover time of about 1 10 of a year, primarily driven by hydroxyl radical oxidation. To some, this paper kicked off the research which led to our present understanding of the atmospheric chemistry of the lower atmosphere.
It has been claimed that “acid rain was one of the most important environmental issues during the last decades of the twentieth century” (Grennfelt et al., 2019) (see Table 2). One of the reasons is that acid rain first demonstrated that air quality was not merely a local issue but a regional issue and showed that the atmosphere has no definite boundaries (Fowler et al., 2020). Although the case of acid rain and its effects had been noted and reported by some earlier papers (e.g. Odèn, 1968), for some, it is the paper by Likens and Bormann (1974) that made this issue known to the science community at large. Other early papers (for example, from Urone and Schroeder, 1969; Penkett et al., 1979) also recognized the vital role of liquid-phase oxidation of sulfur dioxide (SO2) by oxidants such as hydrogen peroxide (H2O2) and ozone (O3). Current estimates suggest that roughly 50 % of the SO2 oxidation in the lower troposphere occurs through liquid-phase reactions.
The story of lead in the atmosphere is a complex interplay between science, policy and economics (Monks and Williams, 2020); observations in snow (Murozumi et al., 1969) underpinned the alarming growth and spread of lead pollution as well as its demise (Boutron et al., 1991).
There is little doubt that one of the most impactful series of papers is that on the eponymous curve by Keeling (Keeling, 1960; Keeling et al., 1979; Pales and Keeling, 1965), showing the steady rise in carbon dioxide (CO2) measured at Mauna Loa observatory (this work has continued uninterrupted by NOAA/ESRL/GML over the past few decades). Keeling's work was built on the previously mentioned work of Callendar (1938), who compared measurements of CO2 at Kew, UK (1891–1901), with those in the eastern USA (1936–1938) and noted an increase in concentration. Although the gas in question is CO2, which is often seen only as a gas important only for climate, changes in its levels reflect the changing composition of the atmosphere and the effects that it can have, showing that the two subjects cannot be easily separated. Furthermore, the increase in CO2 is central to ocean acidification, a topic not touched upon here but nevertheless very important. The seminal paper by Ramanathan et al. (1985) that highlighted the role of CH4, chlorofluorocarbons (CFCs) and nitrous oxide (N2O) for climate strengthened the case for the inclusion of chemistry in the discussion of the climate issue. In many respects, this close coupling between atmospheric chemistry and climate change was brought to the forefront with the 1995 Nobel Prize being awarded to Paul Crutzen, Mario Molina and F. Sherwood Rowland “for their work in atmospheric chemistry, particularly concerning the formation and decomposition on ozone” (https://www.nobelprize.org/prizes/chemistry/1995/press-release/, last access: 6 August 2021) (see Sect. 2.18) and, later, with the Nobel Prize to the IPCC.
The role of field campaigns, observations and the attendant models in shaping our understanding of atmospheric chemistry should be recognized as foundational. In general, the adage that the atmosphere is under-observed is still true. Every time a new instrument has been developed to detect a new chemical in the atmosphere, there have been significant advances (Heard, 2006). One could posit that the entire field of atmospheric science started because of detection and quantification of oxygen and ozone in the Earth's atmosphere. Some recent major advances in our field has been through field measurements. For example, observations of ozone depletion (including the ozone hole), aerosol particles, free radicals and stable molecules (including ozone layer depleting substances, CO and methane) fundamentally changed the course of the field. Organized systematic probing of the atmosphere has been critical over the past 4 or so decades. Here again, the introduction of new instruments (optical, mass spectrometric, etc.) has been game-changing. It is also important to note and highlight the enormous contributions of satellite observations to provide global coverage. Often, as field campaigns and their impact are spread across many papers, it is difficult to pull out their specific contributions. Many of the early experiments, encompassing long-range transport, biomass burning and aerosols, particularly using aircraft, have been detailed in Melamed et al. (2015). Assembling a large number of instruments on a large aircraft to simultaneously measure an array of chemicals was pioneered by Davis (1980) and has been a paradigm for field studies ever since.
In addition to organized episodic field measurements, continual measurements of chemicals (often called monitoring) have produced some of the most significant findings about the atmosphere. For example, continual monitoring of surface ozone from Paris or similar stations going back over 100 years or more has shown the trends in tropospheric pollution due to human activities (Volz and Kley, 1988). The continual monitoring of Antarctic ozone led to the discovery of the ozone hole. Long-term monitoring of CO2 is the poster child for climate change! Much of this continual monitoring has been carried out by national agencies and international partnerships. Examples include the US National Oceanic and Atmospheric Administration (NOAA) Earth Systems Research Laboratory (ESRL) Global Monitoring Laboratory (GML) contributions (Montzka et al., 2007) and international efforts, such as the Advanced Global Atmospheric Gases Experiment (AGAGE) network (Prinn et al., 1995, 2001), the World Meteorological Organization–Global Atmospheric Watch (WMO-GAW) (WMO, 2017) and the Network for the Detection of Atmospheric Composition Change (NDACC) (De Mazière et al., 2018).
2.2 Aerosols and clouds
Aerosols in the atmosphere greatly influence both air quality and climate change; they are also significant media for composition change in the atmosphere. In this section we discuss three main areas of research related to aerosols: (1) understanding the mechanisms and atmospheric chemistry processes that influence aerosol particle formation, nucleation, and growth as well as how aerosols affect composition; (2) the role of aerosols as cloud condensation nuclei and the influence that this process has on climate; and (3) the impact of particulate matter on human health. These areas are, however, related and there is not always a clear division. Secondary organic aerosol (SOA) and heterogeneous and multiphase chemistry are discussed in the corresponding sections (Sect. 2.3 and 2.5).
The roots of modern aerosol science lie, as previously discussed (see Sect. 2.1), in the works of Aitken (1888) and Köhler (1936) on the cloud droplet. Approximately 20 years after Köhler's research, Junge (1955) provided the power law describing aerosol particle number and identified the stratospheric aerosol layer, now dubbed the “Junge layer”. Junge concluded: “A real step forward in the understanding of the basic processes in air chemistry can be gained only if aerosol particles and gases are measured simultaneously but separately, and if the aerosol particles, in turn, are separated according to size”. This suggestion has been a clarion call for atmospheric scientists ever since.
Junge and Ryan (1958) attempted to elucidate the formation of particles from gas-phase reactants, particularly SO2 and ammonia (NH3), while Fitzgerald (1974) investigated the variation in aerosol particle composition with particle size. They showed that cloud droplet size distribution was insensitive to the specific soluble constituents. Twomey (1977, 1974) suggested that air pollution gives rise to the whitening of clouds and influences the planet's radiative balance. He also indicated that there is a connection between pollution aerosols and cloud reflectance (albedo). This concept is now often referred to as the “Twomey effect”. Twomey (1977) expanded on the 1974 work by exploring the balance between the scattering versus absorption effect on incoming solar radiation. It is on this basis that much of the current research on the role of aerosols via their direct and indirect effects on climate has been built. Bolin and Charlson (1976) estimated that anthropogenic sulfate aerosol from the US and Europe would lead to a global temperature decrease of 0.03–0.06 ∘C. They recognized early on that “we are already approaching the time when the magnitude of the indirect effects of increasing use of fossil fuel may be comparable to the natural changes of the climate over decades and centuries”.
In the early 1970s, Whitby and Knutson developed an instrument to measure particle size distribution in the nanometre to micrometre range (Knutson and Whitby, 1975) – the well-known aerosol particle mobility analyser. They used the measurements from this instrument to introduce a new formulation of the formation and growth of atmospheric aerosol particle size modes – the “Whitby diagram”, which is now a common textbook figure and is shown in Fig. 3. The outcomes of this work show the importance and influence of the development of new instruments that probe the atmosphere.
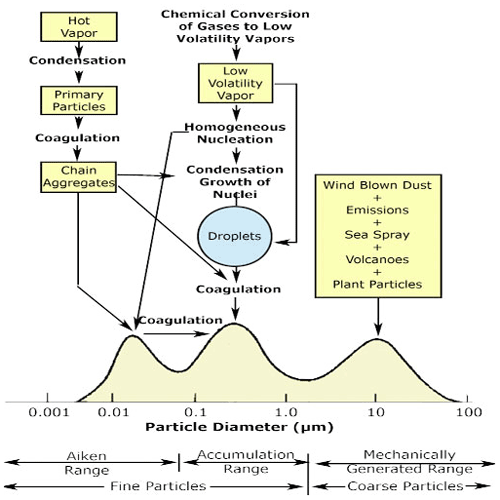
Figure 3Schematic of atmospheric aerosol particle size distribution showing the three modes, the main source of mass for each mode, and the principal processes involved in inserting mass to each mode along with the principal removal mechanisms (Whitby, 1978) (taken from https://serc.carleton.edu/NAGTWorkshops/metacognition/largeclasses.html, last access: 30 August 2021., under CCC).
The CLAW hypothesis (the acronym taken from the surnames of the proposers Charlson, Lovelock, Andreae and Warren) (Charlson et al., 1987) further connected aerosol science to gas-phase chemistry, specifically focused on the feedback loop between ocean ecosystems and Earth's climate. This hypothesis built on earlier work by Lovelock et al. (1972) on the oxidation of marine dimethylsulfide. Although the conclusions of Charlson et al. (1987) have been questioned (Quinn and Bates, 2011), this paper highlighted the interconnections within atmospheric sciences and environmental sciences in general.
The work of Friedlander and co-workers (Stelson et al., 1979) further highlighted the role of liquid-phase chemistry leading to aerosol particles. A key milestone in our understanding of sulfate formation was the recognition that the reaction of the hydroxysulfonyl radical (HOSO2) with oxygen (O2) is exothermic (Calvert et al., 1978) and leads to gas-phase sulfur trioxide (SO3), contrary to what was accepted at the time. Prior to this finding, there were major difficulties in understanding the formation of gas-phase sulfuric acid (H2SO4) (Davis et al., 1979) from gas-phase SO2 oxidation, an essential step for the nucleation of new particles from the gas phase in the atmosphere.
This area of research was further developed by Robbin and Damschen (1981), who investigated the role of hydrogen peroxide in the liquid phase in oxidizing SO2, which was key to understanding the phenomenon of acid rain. Graedel and Weschler (1981) reviewed the chemical transformations in atmospheric aerosol particles and raindrops and extended the idea of Martin and Damschen. Stelson and Seinfeld (1982) evaluated the thermodynamics of ammonium, nitrate and sulfate aerosols, which was a significant step in understanding particle formation and growth. Pankow's 1994 work (Pankow, 1994a, b) on the absorption model of gas–particle partitioning of organic compounds in the atmosphere is of fundamental importance for models to calculate the amounts of particulate matter (PM) formed and their growth in the urban and regional air, as well as in the global atmosphere.
Charlson et al. (1990, 1991) produced the first global estimate of the direct aerosol effect that subsequently had a large impact on climate modelling. The role that aerosols play in cloud condensation nuclei (CCN) and cloud albedo was also acknowledged, concluding that it may be substantial. How substantial, however, was not quantified at that point because of lack of knowledge of the relationships involved. A few years later, Boucher and Lohmann (1995) provided an estimate of the indirect effect of anthropogenic aerosols on climate. After many additional years of study based on these foundations and analyses of radiative balance, the total radiative forcing by anthropogenic aerosol is now estimated to be roughly −1.1 W m−2 (IPCC, 2013), thereby solidifying the importance of aerosols in climate change.
Building on the work of Whitby, Mäkelä et al. (1997) conducted continuous monitoring of particles at a forest site in Finland. Beyond confirming the existence of three submicron particle size modes (the nucleation, Aitken and accumulation modes; see also Covert et al., 1996), they also observed new particle formation events. These events have been subsequently observed by others and are often depicted in the literature using the famous “banana plots” (Fig. 4).
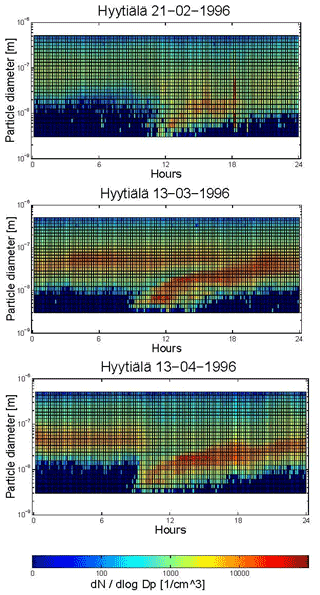
Figure 4Contour plots of a particle formation event occurring in the morning, followed by a subsequent growing process of the nucleation mode during the afternoon. (a) 21 February 1996, (b) 13 March 1996 and (c) 13 April 1996 (Mäkelä et al., 1997). Thought to be the origin of the “banana plot” (provided from original data by Markku Kulmalu).
There has been ample recognition of research on process representations, such as mole-fraction-based thermodynamic models (Clegg et al., 1998a, b) and the one-parameter model for hygroscopic growth (Petters and Kreidenweis, 2007) and CCN. Facchini et al. (1999) presented experimental work aimed at understanding the role of surface tension in droplet growth and the subsequent effect on cloud albedo and radiative forcing (RF), while Knipping et al. (2000) used a simplified experiment to investigate the role of reactions of gases with ions at the air–water interface.
More specifically, the role of organics in the formation and growth of aerosol particles has been a significant area of research (Kulmala et al., 2000). In addition to the natural hydrocarbons noted above, it has become clear that anthropogenic hydrocarbons such as aromatic compounds are also involved in new particle formation and growth (Odum et al., 1997).
Jaenicke (2005) was the first to suggest, to the best of our knowledge, that biological particles are an important fraction of atmospheric aerosol particles. This paper prompted the development of a new and exciting field within atmospheric sciences. Fröhlich-Nowoisky et al. (2016) reviewed the role of bioaerosols in health, climate and ecosystems.
2.3 Secondary organic aerosols
Since the mid-2000s, secondary organic aerosol (SOA) has been the focus of much research addressing abundance, sources and production pathways. One of the foundational works in this area is the recognition of the role of natural and anthropogenic hydrocarbons, in particular isoprene chemistry, in the formation of SOA (Claeys et al., 2004).
The chemical composition of SOA across the globe is still poorly understood (Zhang et al., 2007), although ways to describe the growth of SOA have advanced significantly (Kalberer et al., 2004). Donahue et al. (2006) developed an approach based on the volatility of organics, a concept termed the “volatility basis set”. This concept has been extended to a host of volatilities and their classifications. For example, as shown in Fig. 5, Robinson et al. (2007) postulated that a large amount of SOA mass is unexplained by current models, and methods used to estimate SOA production do not capture what is measured in the field.
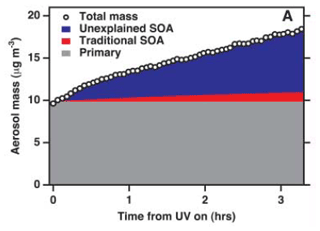
Figure 5Aerosol particle formation from the photochemical oxidation of diesel exhaust in an environmental chamber. The grey area indicates the primary aerosol particle (POA + other species). The red area shows the upper-bound estimate of the contribution of known SOA precursors to the suspended aerosol particle mass, leaving behind a large fraction that is not accounted for (blue area) (Robinson et al., 2007).
The introduction of the aerosol mass spectrometer (AMS) by Worsnop and colleagues (Canagaratna et al., 2007) along with the pioneering instruments of Prather (Gard et al., 1997) and Murphy et al. (2006) that built on the early work of Sinha (1984) have helped determine aerosol composition. Studies using these instruments have established that organic compounds are ubiquitous in aerosol particles. Zhang et al. (2007) and later Jimenez et al. (2009) explored the chemical composition of PM at different sites across a part of the globe (Fig. 6), and their work has now been extended by a large number of groups.
Aimed at addressing some of the “missing urban SOA” in models, Surratt et al. (2010) investigated SOA production from isoprene, and Virtanen et al. (2010) showed the amorphous solid state of biogenic secondary organic aerosol particles, challenging the traditional views of the kinetics and thermodynamics of SOA formation and transformation that assumed that low-viscosity, liquid-like particles exchanged chemicals rapidly with the gas phase.
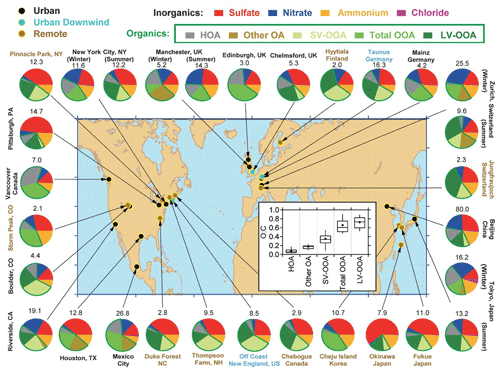
Figure 6Total mass concentration (in micrograms per cubic metre) and mass fractions of nonrefractory inorganic species and organic components in submicrometer aerosol particles measured with the AMS at multiple surface locations in the Northern Hemisphere at mid-latitudes. The organic components were obtained with FA-AMS methods (Zhang et al., 2007). In some studies, the FA-AMS methods identified one OOA factor, whereas in other locations, two types, SV-OOA and LV-OOA, were identified. HOA is a surrogate for urban primary OA, and “other OA” includes primary OAs other than HOA that have been identified in several studies, including BBOA. Inset: distributions of O:C for the OA components identified at the different sites (Jimenez et al., 2009).
2.4 Chemical kinetics, laboratory data and chemical mechanisms
Chemical kinetics is one of the foundations of atmospheric chemistry (Abbatt et al., 2014). This includes a number of different areas: investigation of individual chemical reactions, theoretical understanding of elementary reactions, evaluations and compilation of kinetics data, development and compilation of chemical mechanisms for use in models (see Sect. 2.6), and testing and simplification of the mechanisms for use in numerical models. The work by Demerjian et al. (1974) is considered by many in the community to be one of the cornerstones of chemical mechanism development, and it has been influential in a number of other research areas as well. This paper provided an explicit chemical mechanism for the troposphere in which all the chemical reactions were written as numerically integrated stoichiometric equations to predict photochemical ozone production rates. Previously, all chemical mechanisms had been highly “reduced” (into simple mechanisms) and/or parameterized with non-stoichiometric equations. Using Demerjian's approach, many explicit atmospheric chemical mechanisms have been derived, including one of the most widely used, the Master Chemical Mechanism (MCM, Fig. 7) (Jenkin et al., 1997, 2003; Saunders et al., 2003). Currently, there are a variety of tropospheric chemistry mechanisms that capture the scope of chemical reactions that are used in a number of models including the 1990 Carter mechanism (Carter, 1990), the regional acid deposition model/regional atmospheric chemistry mechanisms (RADM/RACM) (Stockwell et al., 1990, 1997), SAPRC-07 (Carter, 2010) and the Chemical Aqueous Phase Radical Mechanism (CAPRAM) (Ervens et al., 2003). One of the key foundational techniques for estimating rate constants is that of structure–activity relationships (Kwok and Atkinson, 1995). In the near future, calculations of rate coefficients based on ab initio quantum calculations will likely be common.
There is no doubt that chemical kinetic data compilations have been the backbone of providing much needed experimental data to all chemical mechanisms and models (see Sect. 2.6). The comprehensive reviews of Atkinson starting in the mid-1980s (Atkinson, 1986), followed by many others, provided a consistent description of the reaction pathways of the alkyl, peroxy and alkoxy radicals produced by reactions of hydroxyl radicals with a wide range of organic compounds. These papers led the way for the compilation of the IUPAC and NASA/JPL chemical kinetic data evaluation of tropospheric reactions (Atkinson et al., 1989; Crowley et al., 2010; Atkinson et al., 1992, 2004, 2006; DeMore et al., 1997; Burkholder et al., 2020). (Note that compilation of kinetics data for stratospheric reactions dates back to the mid-1970s: Hudson and Reed, 1979.) These works are the foundation for the development of all chemical mechanisms and have led to the standardization and improvement of condensed mechanisms used in all chemical models.
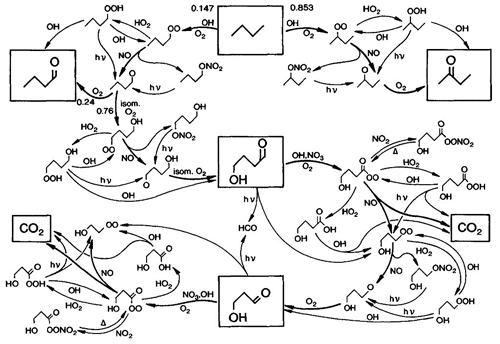
Figure 7Oxidation mechanism of butane in the Master Chemical Mechanism (Jenkin et al., 1997) (under CCC).
The recognition of reactions with negative activation energies and the role of weakly bound adducts were other key steps in improving our understanding of chemical kinetics. When the adduct is strong, we term it an association reaction, which exhibits negative activation energies and pressure dependence. Understanding and representing these type of reactions in atmospheric chemistry constitute a major step. In particular, the pioneering work of Troe and co-workers has enabled realistic and simpler representation of these reactions based on the Rice–Ramsperger–Kassel–Marcus (RRKM) theory (Troe, 1979, 1994).
Atmospheric chemistry is often termed atmospheric photochemistry since the initiator for many of the reactions is the production of free radicals, which are directly or indirectly the result of solar radiation. Over the decades, the representation of photochemical processes has been well established. A key element is the calculation of the “j value” (i.e. the photolysis rate) for a photochemical process, which depends on radiative transfer to obtain the solar flux and laboratory measurements of absorption cross sections and quantum yields. The pioneering works on methods for quickly and accurately calculating j values are those of Madronich and Flocke (1999) and of Prather and colleagues (Wild et al., 2000).
Moving towards individual reactions, the work of Howard and Evenson (1977) on the reaction between the hydroperoxyl radical and nitric oxide, HO2+ NO, has been recognized as a scrupulously careful study that overturned conventional wisdom on this key reaction in photochemical smog and ozone formation (and in stratospheric chemistry). The work of Vaghjiani and Ravishankara (1991) demonstrated the importance of operating at low [OH] to reduce secondary reactions and extended measurements down to low and atmospherically relevant temperatures.
Three papers nearly a decade apart address the fundamental importance of robust laboratory measurements to underpin model-led interpretation of experimental data. The seminal work demonstrating the long-wavelength tail on the ozone photodissociation quantum yield (Ball et al., 1993; Ravishankara et al., 1998) and the related work on the O1D + N2 O2 reactions (Ravishankara et al., 2002) identified key processes in the formation of OH radicals in the troposphere. Prompted by the findings of Lelieveld et al. (2008) (see Sect. 2.13) and of Hofzumahaus et al. (2009) (see Sect. 2.9), a pair of laboratory papers published in 2009 about HOx radical regeneration in the oxidation of isoprene (Peeters et al., 2009) and unexpected epoxide formation in the gas-phase photooxidation of isoprene (Paulot et al., 2009) have changed the way we understand the gas and aerosol products and impacts of isoprene chemistry (Kleindienst, 2009). It is worth noting that Peeters et al. (2009), using theoretical electronic structure calculations, showed the major role of autoxidation chemistry (peroxy–hydroperoxy isomerization). This work changed the traditional view of peroxy radical chemistry and introduced the ideas of isomerization and more complex pathways to atmospheric chemistry.
2.5 Heterogeneous and multiphase chemistry
Earth's atmosphere contains various amounts of condensed matter suspended in air. The most visible condensed matter is, of course, clouds. One can also see aerosols when the particle numbers and sizes are large; examples include smog, wildfires and volcanic eruptions. In addition to clouds, snow and ice provide different mediums that can alter gas-phase chemistry.
Many chemical reactions occur on the surfaces of particles suspended in air, ice and snow on the ground, and within liquid drops. In general, these processes catalyse reactions that would be very slow in the gas phase, such as those between closed-shell molecules, and/or can produce products that do not form in the gas phase. For these reasons, heterogeneous and multiphase reactions are of immense interest, although the distinction between heterogeneous and multiphase chemistry is not always clear-cut (Ravishankara, 1997). Often, “heterogeneous” is taken to mean reactions at surfaces and “multiphase” to mean reactions involving the uptake of gases into (and reaction in) the liquid phase.
The unique contribution of reactions in and on condensed matter burst into the limelight owing to their role in the depletion of stratospheric ozone (Solomon et al., 1986). However, such reactions were recognized to be important before ozone hole research, for example, in the oxidation of SO2 (Urone and Schroeder, 1969; Penkett et al., 1979). Since the 1990s, the roles of heterogeneous and multiphase reactions have been highlighted in many tropospheric processes, as noted here in various sections (see, for instance, Sect. 2.1).
Chameides and Davis (1982) studied the free radical chemistry of cloud droplets and its impact upon the composition of rain, showing that the radical chemistry in water droplets could drive production of peroxides, which have the ability to rapidly oxidize sulfur species – a strong link to acid rain. The work of Akimoto et al. (1987) on the photoenhancement of nitrous acid formation in the surface reaction of nitrogen dioxide and water vapour demonstrated the existence of an additional radical source in smog chamber experiments. This built on the earlier work of Pitts et al. (1984) and challenged our understanding of the role of such heterogeneous reactions in the atmosphere. The work of Mozurkewich et al. (1987), Hanson et al. (1992), and subsequently Thornton and Abbatt (2005) on the measurements of HO2 uptake to aqueous aerosol particles was highly influential in the debate on the aerosol loss of HOx, a question that had vexed many modelling studies.
A pioneering paper in tropospheric cloud chemistry is the study by Jacob et al. (1986) in the San Joaquin valley that used a multiphase measurement and modelling approach to study the formation of acid fog. Two further papers have brought heterogeneous chemistry to the fore: Dentener et al. (1996), in their original paper on the role of mineral aerosol as a reactive surface in the global troposphere, showed the potential role of mineral dust in sulfur oxides (SOx), NOy (NOy includes nitrogen oxide – NO – and nitrogen dioxide – NO2, as well as the compounds produced from the oxidation of those) and O3 chemistry; and Jacob (2000), who reviewed in more detail the chemistry of ozone via HOx and NOy at the interface of gas-phase and suspended particles (including clouds), led to a highly regarded series of recommendations for future studies.
Another similar area is that of chemical reactions on and in snow and ice. Such reactions were highlighted by Barrie et al. (1988), specifically with regard to the production of halogens on the ice surface, hinting at the role of the cryosphere as a source of chemical species to the troposphere (see Sect. 2.11). Given the extent of the cryosphere and in particular of snow (Grannas et al., 2007), findings in the late 1990s demonstrated its role in promoting heterogeneous and multiphase reactions as a significant source of unusual and unexpected chemical species to the atmosphere. One of the most-nominated works in this area was that by Honrath et al. (1999) investigating NOx production from the illuminated snowpack. The pioneering work of Davis et al. (2001) on the unexpected production of NOx in pristine Antarctica is also worthy of note.
2.6 Chemical models
Up front, we want to acknowledge that we are not doing full justice to the important role played by chemical models in the understanding and development of tropospheric chemistry, informing policy making, and deciphering field observations. We do, however, note some of the key developments in modelling, which is the way we couple atmospheric motion with chemical processes.
Chemical models are the conduit to represent our knowledge of the chemical and physical processes in the atmosphere within a mathematical (numerical) framework that allows prediction and testing against observations (in the laboratory and the atmosphere). Therefore, models are the tools upon which atmospheric environmental policies are developed. Indeed, the efforts in modelling are vast and they are pivotal tools of tropospheric chemistry. Policies pertaining to climate, air quality and acid precipitation, for example, are based on such model predictions and projections. Further, models represent the tool that has enabled quantification of emissions (the quantity of most interest to policy makers), identification of sources and evaluation of impacts. One could argue that our knowledge would be incomplete without models.
Early, simple chemical models (with no chemical transport) were useful tools to elucidate and test the basic theory of photochemical ozone formation (Levy, 1971). The recognition that one cannot treat chemical transformation without considering atmospheric transport and mixing came early. The original simple 1-D models, often designed with a parameterized vertical transport in terms of an “eddy diffusion” concept, were superseded by 2-D models and have now been largely replaced by complex 3-D models. Two-dimensional models of the stratosphere (which are zonal averages with latitude and height being the variable dimensions) have been extremely useful and are still used in assessment activities (see, for example, Garcia and Solomon, 1983; Fleming et al., 1999.). The NASA conference publication 3042 (Jackman et al., 1989) provides an excellent review of 16 2-D and a few 3-D stratospheric models that were used in the 1980s and 1990s. Also, chemical transport models (CTMs), which use analysed winds, are often used to separate transport from chemistry. Such models are extremely powerful in accounting for observations and atmospheric budget calculations, as well as deciphering the roles of various chemical processes taking place in the atmosphere. However, projections and predictions in a changing climate require coupling of chemistry to meteorological prediction models. Now, free-running, on-line 3-D models, which include chemistry, have been implemented, and the continued enhancements in computing capabilities have greatly improved our modelling capabilities. Logan and co-authors are recognized by many contributors as providing the basic model description of global tropospheric chemistry (Logan et al., 1981). Bey et al. (2001) first described GEOS-Chem, a global, three-dimensional, tropospheric chemical transport model. Though not the only global tropospheric model, as an open-source model with a large user community and flexibility, it has become a very influential global model. In recent years the Weather Research and Forecasting (WRF-Chem) model has also been used extensively (Grell et al., 2005). There are now numerous 3-D models developed by various organizations. We refer readers to two excellent articles that describe the role of models and their development in detail (Brasseur, 2020; Zhang, 2008). Inverse modelling, especially for source and sink attribution, has been shown to be a powerful tool by Hein et al. (1997).
In addition to the global models, regional and airshed models were critical for air quality predictions and are still employed for regulatory use. A series of three papers from the Seinfeld group (Reynolds et al., 1973, 1974; Roth et al., 1974) provided the earliest complete descriptions of an air quality policy model. They linked together emission inventories, meteorological data, chemical mechanisms and air quality network data to evaluate model performance. All subsequent air quality policy models have followed the same general approach and their basic formulation.
Another major use of models is to interpret large-scale field measurements. One of the earliest detailed tropospheric chemical modelling studies that integrated highly instrumented intensive field campaign data was that of Harriss (1988) for the ABLE 2A (Amazon Boundary-Layer Experiment) campaign in the Amazon boundary layer. Now, use of multiple models to interpret field data is a common feature of modern tropospheric chemistry research.
Multi-model ensembles of the troposphere as epitomized by Stevenson et al. (2006) and Fiore et al. (2009) (see Sect. 2.16) are a powerful tool for generalizing the model “understanding” of the atmosphere. This modelling approach makes use of many different models to achieve a more accurate representation of the observations than would be possible by using only one model, thus producing more reliable outcomes for assessments and policies on a global scale. In addition, multiple runs of the same models with slightly different initial conditions are often used to examine the range of outcomes. This approach is akin to the use of multiple models and model runs in weather predictions.
2.7 Tropospheric ozone
Ozone is one of the central molecules of atmospheric chemistry and runs through much of the foundations of the discipline, from its role in the stratosphere as a UV shield, to its role as a major greenhouse gas, to its pivotal part in the troposphere as the start and end product of oxidation chemistry, and its detrimental influence as an air pollutant harmful to human health and ecosystems. Much of the early thinking on ozone was focused on the question of whether tropospheric ozone was a small subset of stratospheric ozone; see, for example, Galbally (1968) and Fabian and Pruchniewicz (1977). The latter paper showed the value of observational networks based on standardized instrumentation and calibration techniques, together with consistent siting criteria, and raised the issue of seasonal variations in tropospheric ozone and the nature of the processes that drive them. The vertical structure of a layer of high O3 concentrations in the stratosphere, where O2 could be directly photolysed to make oxygen atoms and hence ozone, and declining concentration in the troposphere were indicative of a stratospheric source and a tropospheric sink, and this was the prevalent theory prior to the late 1970s (see also Sect. 2.18). A major breakthrough is represented by the two papers by Chameides and Walker (1973) and Crutzen (1973b) that showed that ozone can be photochemically generated in the troposphere, just like it is made in smog via reactions involving hydrocarbons and nitrogen oxides.
The importance of ozone as a radiative gas has been known for a long time, with a significant fraction of heating in the stratosphere coming from ozone photolysis followed by its reformation, thus converting sunlight to heat. In 1979, Fishman et al. (1979) identified tropospheric ozone as a greenhouse gas. Hence, a change in tropospheric ozone will perturb the radiative energy budget of the Earth–atmosphere system, which will in turn perturb the climate system.
Large-scale mapping of global tropospheric ozone was first undertaken by Logan (1985), who looked at seasonal behaviour and trends with a view to understanding anthropogenic influence. This was later complemented by a paper exploring the photochemical origins of tropospheric, rather than stratospheric, ozone in the rural United States (Logan, 1989). As the understanding of the photochemistry of ozone developed, measurements at Niwot Ridge, Colorado (Liu, 1987), aimed to quantify the elements of the ozone budget by season, bringing forward the concept of ozone production efficiency. Lin et al. (1988) explored the non-linearity of tropospheric ozone production with respect to non-methane hydrocarbons (NMHCs) and NOx. Though this chemistry had been outlined much earlier – e.g. Demerjian et al. (1974) – this work explored it in the background atmosphere with models and measurements. A powerful demonstration of the low-NOx ozone destruction chemistry came from measurements made at Cape Grim, Australia, a background station where Ayers and Penkett (Ayers et al., 1992) and their team(s) used measurements of ozone and peroxides (Fig. 8) to show further experimental proof for the photochemical control of ozone in remote locations.
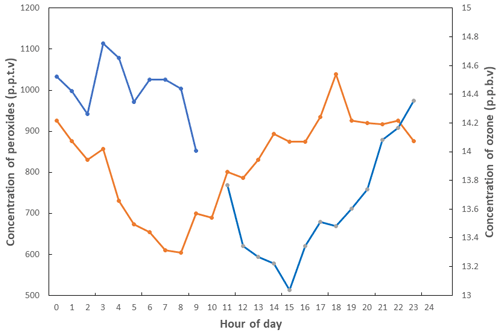
Figure 8Average diurnal cycles of peroxide (orange line) and ozone (blue line) in baseline (low NOx) air at Cape Grim (Tasmania, Australia) in January 1992. Adapted from Ayers et al. (1992).
Measurements have always been a critical driver in tropospheric chemistry, and the idea to use in-service commercial aircraft as a platform for programmes such as MOZAIC/IAGOS (Thouret et al., 1998) has been recognized for the enormous amount of high-quality data, which would otherwise be difficult to regularly obtain from the upper troposphere and lower stratosphere. Using such measurements Newell et al. (1999) combined dynamical and chemical tracers to further delineate ozone origin and budgets. In the same year, Logan published a synthesis of ozonesonde data (Logan, 1999) which gave an unprecedented look at the seasonal and vertical distribution of ozone and became a reference point for the subject. A year later, Thompson et al. (2000) used a combination of shipboard and satellite views of a tropospheric ozone maximum to suggest the occurrence of a tropical Atlantic ozone “paradox”. The Atlantic paradox refers to a greater tropospheric ozone column amount over the South Atlantic than the North Atlantic during the West African biomass burning season. This phenomenon was further explored using an expanded network of ozonesondes in the Southern Hemisphere (SHADOZ) (Thompson et al., 2003). In combination with the earlier work of Logan, these observations became the basis for the measurement-based description of ozone in the troposphere.
A decade's worth of knowledge of the relationship between ozone and its precursors was pulled together by Sillman (Sillman, 1999), cementing the concepts of NOx- and VOC-sensitive (or NOx-saturated) chemical regimes. The paper introduced a generation of researchers to isopleth diagrams (the famous Sillman plot, Fig. 9) and ozone production efficiencies (OPEs).
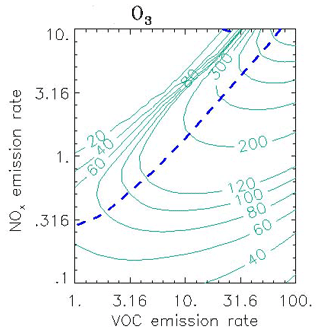
Figure 9Ozone isopleths (ppb) as a function of the average emission rate for NOx and VOC (in 1012 molec. cm−2 s−1) in 0-D calculations. The isopleths (solid green lines) represent conditions during the afternoon following 3 d calculations with a constant emission rate at the hour corresponding to maximum O3. The short blue dashed line represents the transition from VOC-sensitive to NOx-sensitive conditions. Adapted from Sillman and He (2002).
The power of models to explore global tropospheric ozone distributions, budgets and radiative forcing was fully demonstrated in the study by Stevenson et al. (2006) that brought together 26 atmospheric chemistry models to explore both the air quality and the climate roles of ozone (see also Sect. 2.6). As discussed in Sect. 2.16, a similar approach was used by Fiore et al. (2009) to explore the relationship between intercontinental transport and ozone.
2.8 Nitrogen chemistry
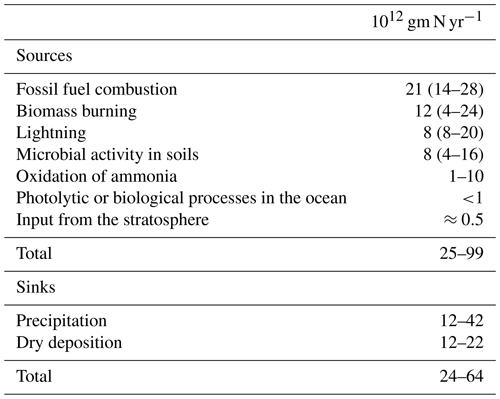
Nitrogen oxides are an integral part of tropospheric processes. Nitrogen oxides are released into the troposphere from a variety of biogenic and anthropogenic sources including fossil fuel combustion, biomass burning, microbial activity in soils and lightning. The concept of the Leighton photostationary state (Leighton, 1961) between NO, NO2 and O3 was well established by the mid-1990s, and early work from Singh and Hanst (1981) highlighted the potential role of peroxyacetyl nitrate (PAN) as a reservoir for NOx in the unpolluted atmosphere. The measurement of by chemiluminescence was critical to the widespread measurement of NOx (Kley and McFarland, 1980). A landmark paper in the area of nitrogen chemistry was that of Logan (1983) that brought together global and regional budgets for the nitrogen oxides (Table 3). Later, a paper that focused more narrowly on a specific source of NOx was that of Yienger and Levy II (1995), who produced an empirical model of global soil–biogenic NOx emissions. Higher up in the atmosphere, the work on sources and chemistry of NOx by Jaeglé et al. (1998) is recognized for its contribution to the understanding of the NOx cycle in the upper troposphere.
Nitrous acid (HONO), which is somewhat of a “Cinderella” molecule whose photolysis can be a major OH source, especially during the early morning, was first identified in the troposphere by Perner and Platt (1979), the heterogeneous nature of which has always attracted a lot of interest (Kurtenbach et al., 2001).
These works were complemented by a more holistic view of the nitrogen cycle, in particular the concept of reactive nitrogen (Nr) by Galloway et al. (2004) that clearly showed the linkages between the terrestrial ecosystem and the atmosphere as well as how the nitrogen budget had and would change, leading to the important concept of nitrogen cascade (Sutton et al., 2011). In more recent times, extensive work on vehicle NOx sources from exhaust remote sensing data (Bishop and Stedman, 1996), as epitomized in Carslaw (2005), should be highlighted. This paper pointed out the trends that can be said to have led to the denouement of the Volkswagen emissions scandal.
2.9 HOx chemistry
There is no doubt that the chemistry of OH and HO2 (known together as HOx) has a central role in the atmosphere and holds a certain fascination for atmospheric scientists owing to the significant challenges involved in measurement and understanding its impact locally to globally. Much of the history of the measurements of OH and HO2 is covered in the review of Heard and Pilling (2003). As they wrote, “clearly, OH plays a central role in tropospheric chemistry. The in situ measurement of its concentration has long been a goal, but its short lifetime and consequently low concentration provide a serious challenge”.
In order to assess the global impact of OH chemistry in the absence of direct measurements, reactive proxies have been used. Singh (1977) used methyl chloroform to estimate OH abundance since methyl chloroform is exclusively anthropogenic and its emissions are known. This type of work provided a comprehensive picture of the global distribution of OH and, hence, a first overall look at the oxidative capacity of the atmosphere. It was followed, using halocarbon measurements by the AGAGE network, by a global OH determination, which also introduced the atmospheric chemistry community to formal inverse modelling (Prinn et al., 1995). Spivakovsky and co-workers expanded on this work to derive 3-D distributions of OH and used this information to assess the wider impact on the lifetimes of halocarbons, which have implications for stratospheric ozone (Spivakovsky et al., 2000), and of methane, which is currently the second most important greenhouse gas. Thanks to the availability of long-term observations of halocarbons from the AGAGE and NOAA networks, later work using a similar approach found evidence for substantial variations of atmospheric hydroxyl radicals in the previous 2 decades (Prinn et al., 2001), thus providing a broad overview not only of the global distribution but also of the temporal variability of this crucial species. Such estimates allowed for the quantification of the lifetime of important chemicals such as methane and CFC substitutes such as the hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs).
In situ OH detection in the troposphere has proven elusive for a long time. The use of laser-induced fluorescence provided some of the first clues to its atmosphere concentrations in the 1970s and early 1980s (Davis et al., 1976; Wang et al., 1975), but many of these early measurements were found to have significant artefacts. Long-path UV absorption in Germany showed the OH abundances in the German boundary layer to be around 1–4×106 cm−3 (Perner et al., 1976b). The study by Eisele et al. (1994) at the Fritz Peak Observatory in Colorado was the first intercomparison experiment of different measurement techniques and provided much needed confidence in the observations of this key molecule. Hard et al. (1984) and then Stevens et al. (1994) developed the low-pressure laser-induced fluorescence (LIF) instrument, which quickly became one of the most successful and widely used techniques for ambient measurements of OH and HO2. As ambient observations of HOx became available, they were found useful to test our understanding of tropospheric chemical processes by comparing them with the results of chemical models (see Sect. 2.6). Recognized as a foundational paper in this area, Ehhalt (1999) explained with clarity the role of radicals in tropospheric oxidation and what controls their concentrations using both ambient measurements and calculated concentrations of OH. The OH radical is particularly suited to test our understanding of chemical processes, and this was clearly demonstrated in 2009 when the discrepancies between observed and calculated OH and HO2 in the polluted region of southern China led Hofzumahaus and co-workers to propose a regeneration pathway for OH, which does not involve NOx and thus does not produce O3 (Hofzumahaus et al., 2009). This, together with the work by Lelieveld and co-workers (Lelieveld et al., 2008), prompted a major reassessment of the isoprene oxidation mechanism by Peeters et al. (2009), who suggested that isomerization of hydroxyperoxy radicals from isoprene oxidation could be fast enough to regenerate HOx in highly forested, low-NOx environments (see Sect. 2.4), leading to a major revision of isoprene chemistry and its role in the troposphere (see Sect. 2.13).
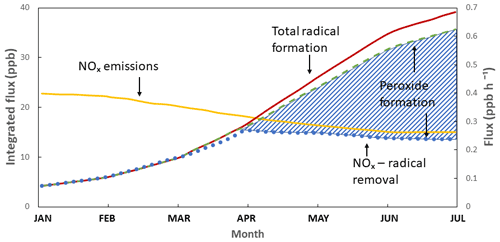
The sources and sinks of HOx radicals have always been a major research focus (Finlayson and Pitts, 1976), and the work of Paulson and Orlando (1996) on the reactions of ozone with alkenes as a source of HOx in the boundary layer is widely recognized. Radical chemistry is highly sensitive to the levels of NO and NO2, and Kleinman's modelling work on hydrogen peroxide (H2O2) concentrations in the boundary layer is recognized for its simple elegance in describing how the HOx cycle chemistry is influenced by NOx and in giving insight into the differing fates of OH and HO2 radicals under different NOx regimes (Kleinman, 1991) (Fig. 10).
The first direct measurements of OH lifetime (Di Carlo et al., 2004) provided evidence of missing reactivity, i.e. the existence of unknown sinks of the OH radical, which relates to earlier work by Lewis et al. (2000) on unmeasured volatile organic compounds (see Sect. 2.12).
2.10 Nighttime chemistry
There is widespread recognition that the atmosphere's oxidative chemistry is active during the night as well as during the day. Evidence of nighttime chemistry driven by the nitrate radical (NO3) and ozone was first observed in the polluted troposphere in 1980 by Platt and co-workers (Platt et al., 1980). Much of the early NO3 work, including laboratory and field studies, is summarized in Wayne's seminal review (Wayne et al., 1991). The ground-breaking work of Platt and colleagues and Plane and colleagues based on long-path absorption has shown the importance of NO3 in the troposphere (Allan et al., 1999; Platt et al., 1979).
Two papers that have been highly influential in shaping our view of nocturnal chemistry are “Nitrogen oxides in the nocturnal boundary layer: Simultaneous in situ measurements of NO3” (Brown et al., 2003) and “Variability in Nocturnal Nitrogen Oxide Processing and Its Role in Regional Air Quality” (Brown et al., 2006). Both these papers showed the power of state-of-the-art measurements coupled with models to assess the impact of nocturnal and heterogeneous chemistry on regional air quality. In particular, the paper by Brown et al. (2006) was a powerful demonstration of the role of heterogeneous chemistry and aerosol particle composition in controlling dinitrogen pentoxide (N2O5) and therefore NO3 concentrations (Fig. 11).
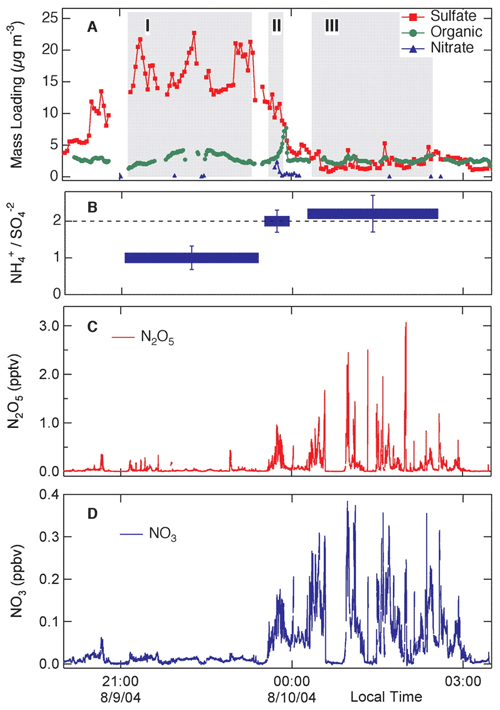
Figure 11Measurements during a flight of the NOAA research aircraft P3 for 9 and 10 August 2004, showing the relationship between NO3 and N2O5 concentrations and aerosol particle composition (Brown et al., 2006).
The area of NO3 chemistry is very active, and there have been significant further studies since Brown et al. (2003). Another area of particular note for nighttime processes involves those due to the Criegee intermediate. The role of Criegee intermediates has been known for awhile. However, recent ability to isolate and measure the reactivity of this intermediate is showing the importance of this radical.
2.11 Halogen Chemistry
In comparison to the atmospheric chemistry in the stratosphere, where halogen chemistry has been well known and characterized for a long time (see Sect. 2.18), the recognition of the role of halogen species in the oxidative chemistry of the troposphere occurred much later. Reviews of the earlier work can be found in Cicerone (1981), Platt and Hönninger (2003), Monks (2005), and the extensive review by von Glasow and Crutzen (2007).
The role of halogens in the troposphere has been discussed going back to the 1970s (e.g. Graedel, 1979). The potential importance of iodine in the troposphere was highlighted by a seminal paper by Chameides and Davis in 1980 (Chameides and Davis, 1980). An important early paper is that from Barrie in 1988 (Barrie et al., 1988) that demonstrated the dramatic impact of bromine chemistry on Arctic boundary layer ozone (Fig. 12). The occurrence of ozone depletion events in the polar boundary layer suggested that halogens could have a significant impact on atmospheric chemistry at low altitudes and not just in the stratosphere. This work brought together halogen and heterogeneous chemistry and led to the discovery of bromine-catalysed ozone depletion on ice-covered surfaces (see Sect. 2.5).
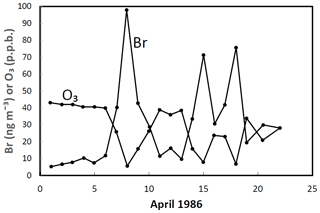
Figure 12A comparison of daily mean ground-level O3 and filterable Br− concentrations at Alert, Canada, in April 1986 illustrating the strong inverse correlation between the two chemicals. Adapted from Barrie et al. (1988).
One of the foundational papers in the area of halogen chemistry is the modelling study by Vogt et al. (1996), which set the theoretical framework of the sea-salt activation mechanism for halogen release and linked halogen chemistry with the sulfur cycle. While the initial research focus was on chlorine and bromine, Alicke et al. (1999) reported the first iodine oxide observations in the marine boundary layer at Mace Head, Ireland, and proved that iodine can also be an important player in the chemistry of the troposphere. Further investigation found evidence that biogenic iodine species can be responsible for the formation of marine aerosol and cloud condensation nuclei (O'Dowd et al., 2002). Recognizing the potential for the wide-scale impact of iodine chemistry in particle formation, Finlayson-Pitts and her colleagues suggested the importance of chlorine in tropospheric chemistry based on laboratory data (Finlayson-Pitts et al., 1989) as seen earlier by Schroeder and Urone (1974), but it was not until 2008 that Osthoff and co-workers (Osthoff et al., 2008) – and the related comment “When air pollution meets sea salt” by von Glasow (2008) – brought attention to the potential for nitryl chloride (ClNO2) chemistry to impact ozone formation, nitrogen recycling and VOC oxidation with the first ambient observations of this molecule. Also in 2008, the work from Read et al. (2008) clearly showed the global importance of halogens for tropospheric ozone using long-term observations of iodine and bromine oxides (IO, BrO) made at the Cape Verde Atmospheric Observatory. High concentrations of tropospheric Cl2, first reported by Spicer et al. (1998), have been found in other places.
2.12 Volatile organic compounds
Volatile organic compounds (VOCs) embrace a wide variety of species emitted from man-made and natural sources. In many respects VOCs are the fuel of the oxidative chemistry in the atmosphere involved in many gas- and particle-phase processes.
Ehhalt (1974) brought together the details of the methane sources and sinks and put them into a consistent framework that described the life cycle of methane. This conceptual framework has subsequently been expanded to a wide range of trace organic gases. The original understanding of the life cycle of methane has remained largely unchanged over the subsequent 40 years and has formed the basis of the IPCC science assessments on the role of methane in global warming and climate change. Methane itself has long been recognized as important for tropospheric chemistry, but also for climate change as a greenhouse gas and as a source of water vapour to the stratosphere (Jones et al., 1986). Specifically, the work of Blake and Rowland (1986) documented the global increase in methane and its implications for climate change.
The large differences in reactivity among the individual VOCs have always been a feature of their chemistry. Darnall et al. (1976) produced a reactivity scale for atmospheric hydrocarbons1 based on their reaction with the hydroxyl radical, an idea that is still influential to the present day. The concept was further advanced by Carter and Atkinson (1989), who looked at incremental hydrocarbon reactivity; knowledge of the reactivities of organics with respect to ozone formation in the atmosphere can provide a useful basis for developing appropriate control strategies to reduce ambient ozone levels. It was the beginning of an approach that is often now used in regulation to determine which organic compounds would have the greatest effect in reducing ozone.
VOC transformation can be important in a number of different atmospheric processes. One highly cited early example is the work of Pitts et al. (1978) on the atmospheric reactions of polycyclic aromatic hydrocarbons and their ability to form mutagenic nitro derivatives under typical atmospheric conditions.
While measurement techniques rarely seem to get a mention as being influential, the discipline relies on observations as a critical part of the oeuvre. Already mentioned was the huge impact that accurate techniques to measure the OH radicals had on the development of the field (see Sect. 2.9). Other examples include the development of proton-transfer-reaction mass spectrometry, which has revolutionized the measurement, in particular, of VOCs (Lindinger et al., 1998), and the earlier work of Lovelock and Lipsky (1960) on the development and application of electron capture detectors that allowed the measurement of VOCs such as dimethyl sulfide and the halocarbons in the troposphere.
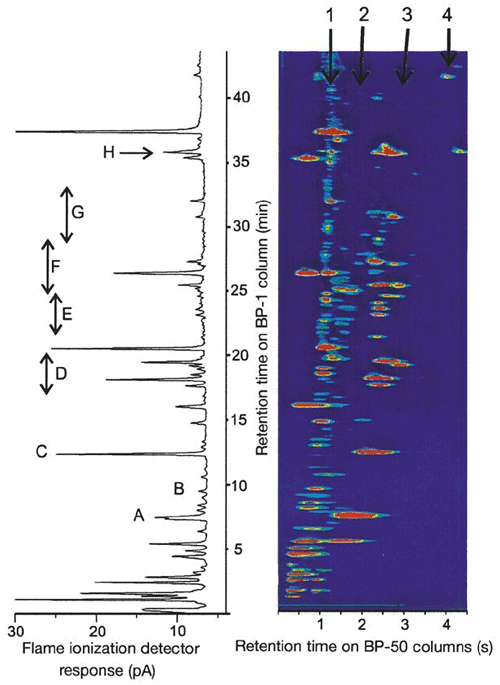
Research is ongoing as to how many VOCs there are in the atmosphere and what the consequences are of not being able to measure and/or quantify them all. The work of Lewis et al. (2000) used a novel VOC measurement technique (GC × GC) to find that there was a larger pool of ozone-forming carbon compounds in urban atmospheres than previously posited (Fig. 13). The later paper by Goldstein and Galbally (2007) expanded on this work by hypothesizing that thousands of VOCs are still unmeasured and unknown, with potentially huge consequences for the carbon budget of the atmosphere.
Continuing work in this area, de Gouw et al. (2005) produced a landmark study that combined analysis of organic carbon in both the gas and particle phase in the polluted atmosphere as part of the New England Air Quality study by looking at the evolution of VOCs from their emission sources. The study showed that most of the organic carbon in the particle phase was formed by secondary anthropogenic processes and that an increasing fraction of the total organic mass was composed of oxygenated VOCs as a result of the air masses being processed and/or aged.
2.13 Biogenic emissions and chemistry
Although it has been known for a long time that plants emit organic compounds, the relevance of biogenic VOCs for atmospheric chemical processes was not immediately recognized. The first report that plants emit volatile organic compounds into the atmosphere was made in 1957 by the Georgian scientist Guivi Sanadze (Sanadze, 1957). Unaware of Sanadze's work in the USSR, Rasmussen and Went independently discovered isoprene emissions in 1964 (Rasmussen and Went, 1964). Sanadze was also the first to show that isoprene emission rates are temperature-dependent (Sanadze and Kursanov, 1966). However, the relevance of biogenic VOCs for atmospheric chemical processes was not immediately recognized. Went (1960) hypothesized that “volatilisation of terpenes and other plant products results in the production of, first, blue haze, then veil clouds …”. Although Tingey et al. (1979) at the US Environmental Protection Agency did note the potential for isoprene to play a role in regional air quality in 1978, this was not formalized until the ground-breaking work of Chameides and colleagues in 1988 (Chameides et al., 1988) and MacKenzie and colleagues in 1991 (MacKenzie et al., 1991).
In 1992 the seminal review of Fehsenfeld et al. (1992) brought the importance of isoprene and a wide range of other VOCs of biological origin to the attention of the atmospheric chemistry community, opening up an entirely new branch of atmospheric chemistry. Other influential reviews of biogenic VOC emissions include the physiology of plants (Kesselmeier and Staudt, 1999), and more recently Sharkey and Monson (2017) reviewed the enigmatic nature of isoprene emissions.
Over time, biogenic chemistry became pivotal for major policy formulations to abate ozone pollution. Underpinning the atmospheric chemistry research that Fehsenfeld et al. (1992) promoted, plant physiologists began working on understanding the biological and environmental controls on biogenic VOC emission rates. This work allowed the development of relatively simple functions to predict the emissions of biogenic VOCs, which resulted in the first spatially and temporally resolved global model of biogenic emissions (Guenther et al., 1993). These soon evolved into more sophisticated high-resolution global models (Guenther et al., 1995, 2000), allowing the emissions of biogenic compounds to be included in atmospheric chemistry models across all scales. Eventually, this work took the form of the widely used MEGAN (Model of Emissions of Gases and Aerosols from Nature) (Guenther et al., 2006), which is still used in modern Earth system models today (Table 1 and Fig. 14).
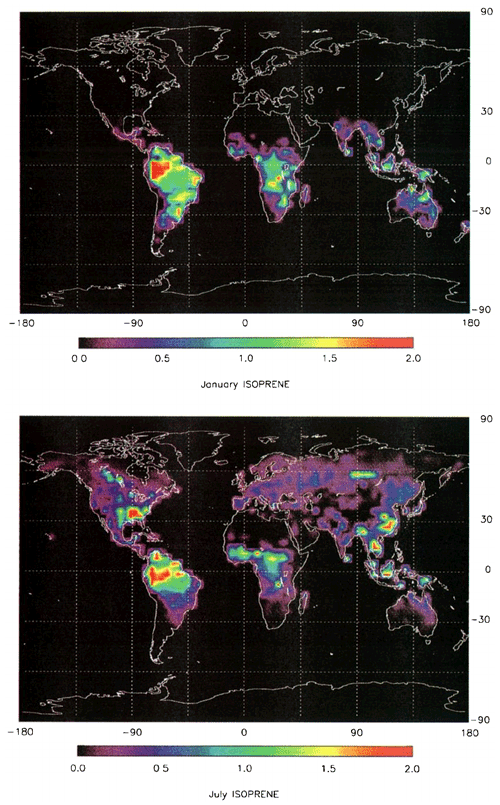
Figure 14Global distribution of isoprene emission rate estimates (g C m−2 month−1) for (top) January and (bottom) July (from Guenther et al., 1995).
Terrestrial vegetation is not the only source of biogenic emissions. Aneja et al. (1979) discussed the importance of biogenic sulfur compounds and their role in stratospheric chemistry, while Charlson et al. (1987) connected marine biology, atmospheric chemistry and climate to the already mentioned CLAW hypothesis (see Sect. 2.2).
Marine aerosol formation was thought for a long time to be dominated by inorganic components, mainly sea salt and non-sea-salt sulfate, but O'Dowd and co-workers (O'Dowd et al., 2004) showed that biological activity of plankton blooms can enhance the concentration of cloud condensation nuclei, a key aspect of the chemistry–climate feedback mechanism. A similar mechanism is also active in relation to biogenic halogen compounds (O'Dowd et al., 2002), which also affects aerosol formation as well as the ozone, nitrogen and sulfur cycles, as discussed in Sect. 2.2.
A paper that has been defined as “controversial” but that “set off a huge amount of activity” is “Atmospheric oxidation capacity sustained by a tropical forest” by Lelieveld et al. (2008), which proposed a new chemical mechanism for low-NOx, high-VOC regions (such as tropical forests) based on modelling studies of a field dataset. Although further studies contradicted this hypothesis, Lelieveld et al. (2008) were instrumental in prompting a large number of laboratory, theoretical and field studies in the past 10 years. These studies resulted in a major revision of our understanding of biogenic VOC chemistry (see Sect. 2.4 and 2.9).
2.14 Biomass burning
Biomass burning, particularly in the tropics, affects terrestrial vegetation dynamics, soil erosion, movement of organic carbon, hemispheric atmospheric composition, air quality and more broadly radiative forcing via emissions of trace gases and aerosols (Monks et al., 2009). Crutzen et al. (1979) were the first to highlight biomass burning in the tropics as an important source of atmospheric gases, such as molecular hydrogen (H2), CO, N2O, NO, chloromethane (CH3Cl) and carbonyl sulfide (COS). The importance of biomass burning, based on the observations of a small set of fires, and the appreciation of its potential role represent a major step in our understanding of the role of biomass burning in air quality, climate change and the composition of the troposphere. It is, however, the later paper “Biomass Burning in the Tropics: Impact on Atmospheric Chemistry and Biogeochemical Cycles” by Crutzen and Andreae (1990), one of the top 10 most-cited atmospheric chemistry papers (Table 1), that has had the greatest impact on this research area by providing quantitative estimates of the amounts of biomass burning taking place around the world and the resulting emissions as well as recognizing the critical role of biomass burning emissions in the tropics and from activities in developing countries that were not well documented. Hao and Liu (1994) made a further advance by looking at where and when biomass burning and thereby the related emissions occur. They developed an improved database of the amount of biomass burned owing to deforestation, shifting cultivation, savannah fires, fuel wood use and clearing of agricultural residues focused on tropical America, Africa and Asia during the late 1970s.
Simoneit et al. (1999) introduced the important concept that “the monosaccharide derivatives (e.g. levoglucosan) are proposed as specific indicators for cellulose in biomass burning emissions”. They showed that levoglucosan is emitted at such high concentrations that it can be detected in air pollution filter samples at considerable distances from the original combustion source, allowing for source apportionment.
The 2001 paper “Emission of trace gases and aerosols from biomass burning” by Andreae and Merlet (2001), which is also one of the top 10 most-cited papers (Table 1 and Fig. 15), pulled together emission factors for a large variety of species emitted from biomass fires and is considered a key reference for biomass burning emission factors. Further work in the biomass burning area was later presented by Reid et al. (2005) in a review paper wherein they looked at measurements of smoke particle size, chemistry, thermodynamic properties and emission factors from a variety of sources, including laboratory burns, in situ experiments, remote sensing and modelling. They brought together information from the “milieu of small pieces of the biomass-burning puzzle” and showed that there are large differences in measured particle properties and particle carbon budgets across the literature. Van der Werf et al. (2006) investigated interannual variability and the underlying mechanisms regulating variability at continental to global scales using a time series of 8 years of satellite and model data. Total carbon emissions were driven by burning in forested areas, while the amount of burned area was driven by savannah fires, which are influenced by different environmental and human factors than forest fires.
Figure 15Vertical profiles of O3 in the tropical troposphere. The profile over the equatorial Pacific shows no influence from biomass burning, whereas the profile over the Pacific off South America suggests O3 enhancement due to long-range transport from the tropical continents. The O3 profiles over Brazil and the Congo show high O3 concentrations at altitudes between 1 and 4 km due to photochemical production in biomass burning plumes. At higher altitudes, O3 concentrations are also substantially enhanced, possibly also because of O3 production by reactions in the emissions of biomass burning (Crutzen and Andreae, 1990).
2.15 Emissions and deposition
Non-chemical sources and sinks of various species are critical components of atmospheric processes and are therefore essential for global and regional models. Early advocates included the work of Olivier and the team that created EDGAR (Emission Database for Global Atmospheric Research) (Olivier et al., 1994).
Emissions from vehicles and power plants have always been an essential aspect of air-quality-related policies and therefore an area where more focused inventory work was needed and done. This approach was pioneered in California in the early 1990s, and the studies by Calvert et al. (1993), Lawson (1993), and Singer and Harley (1996) helped define and verify the California Smog Control Program, providing a solid scientific basis with reliable emissions data. Techniques such as the remote monitoring of traffic-generated carbon monoxide (Chaney, 1983; Bishop and Stedman, 1996) must also be recognized for the role they played in understanding vehicle emissions.
Agricultural emissions (from both crop and animal production) play an important role in several atmospherically mediated processes of environmental and public health concerns (Chameides et al., 1999). These atmospheric reactions and processes affect local and regional environmental quality, including odour, particulate matter (PM) exposure, eutrophication, acidification, exposure to toxins, climate and pathogens (Erisman et al., 2008; Aneja et al., 2009). Agricultural emissions also contribute to the global problems caused by greenhouse gas emissions, specifically nitrous oxide and methane.
The deposition of gases and aerosol particles to the surface is another critical process in the atmosphere. Chamberlain (1966) is credited with the first exposition of the resistance network approach to describe the uptake of gases on surfaces and the identification of transport through the atmospheric boundary layer, through the surface layer and through the stomata on plants as important elements of surface uptake. Building upon this work, a comprehensive and widely adopted parameterization of the dry deposition process for regional and global models was presented in the late 1980s by Wesely (1989). Currently, there is renewed interest in quantifying and understanding deposition processes. Yet, a systematic description based on fundamental independently measurable physico-chemical parameters is lacking. A complication can arise from a range of oxygenated VOCs that can exhibit bidirectional exchange above vegetation (Karl et al., 2010).
2.16 Chemical transport
Transport is an integral part of atmospheric processes and influences atmospheric composition across a range of spatial scales. As early as 1975, Junge (1975) pointed out the importance of the atmospheric residence time of a constituent with respect to global transport and dispersion. Prather's work (Prather, 1994, 1996) provided new insights into timescales for atmospheric oxidation chemistry.
Stratospheric–tropospheric exchange (STE) has always been recognized as a key mechanism in determining tropospheric composition. Early chemical dynamics were demonstrated by Danielsen (1968), who laid the foundations for three-dimensional modelling of chemical transport looking at stratosphere–troposphere exchange based on radioactivity, ozone and potential vorticity. Later, Holton and co-authors (Holton et al., 1995) proposed an approach that placed stratosphere–troposphere exchange in the framework of general circulation and helped clarify the roles of the different mechanisms involved and the interplay between large and small scales through the use of dynamical tracers and potential vorticity. This work is recognized as a big step forward for the understanding of the tropospheric ozone budget. Stohl and colleagues (Stohl et al., 2003) brought together what has been viewed by many as the authoritative work on stratosphere–troposphere exchange.
While the regional nature of air pollution has always been recognized, that is less the case for the impact of transcontinental emissions on air quality. Jacob et al. (1999) showed that there was a need for a global outlook for understanding regional air quality and meeting pollution reduction objectives. This perspective spawned a decade of intense work on intercontinental air pollution and transport. Well recognized in this area is the work by Stohl and colleagues (Stohl et al., 2002), who mapped out the pathways and timescale of intercontinental air pollution transport and brought life to the subjects of atmospheric dynamics and transport of air pollution (Fig. 16). Observational studies such as those by Merrill et al. (1985), Moody et al. (1995), Stohl and Trickl (1999), and Forster et al. (2001) showed the range of mechanisms and the impact of long-range transport of air pollution.
Figure 16Total columns (a, c) and zonally averaged mixing ratios (b, d), both divided by the respective time interval, of an Asian tracer for ages of 6–8 d (a, b) and 25–30 d (c, d) during DJF. The plots shows the horizontal and vertical impact of a pollution tracer (Stohl et al., 2002).
Moody et al. (1998) explored atmospheric transport history using back trajectories for the Harvard Forest experiment, demonstrating the power of trajectory methods at the regional scale. Key tools in the development of this area were the early simplistic isentropic trajectory methods (Merrill et al., 1985), the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model (Draxler and Hess, 1998), a forerunner of various particle trajectory and dispersion models which developed into a widely used particle dispersion model, and the FLEXible PARTicle dispersion (FLEXPART) model, a Lagrangian particle dispersion model designed for calculating the long-range and mesoscale dispersion of air pollutants (Stohl et al., 2005).
The ideas of intercontinental source–receptor relationships were embodied in the aforementioned works by Jacob et al. (1999) and Stohl et al. (2002). The long-range transport concept was developed in an effort to explore the source–transport relationships that drive observed ozone concentration in regions farther away from the emission regions (Fiore et al., 2009). This large community collaboration provided valuable insights into the sensitivities of the hemispheric regional background of ozone and how this is controlled by emissions from continental source regions.
Another critical area for atmospheric chemistry is boundary layer dynamics and meteorology. This is particularly important since most emissions occur in the boundary layer. Atmospheric dynamics in this important region have been mostly expressed as parameterizations in numerical models (Stull, 1988). The spatial and temporal scales involved in the processes in this region cover a wide range. The understanding of this region has been mostly based on meteorological and energy–water vapour balance points of view. However, the chemical transformation and dispersion in this region are crucial for what quantity of chemicals actually gets out of the region to influence the regional and global atmosphere. Furthermore, the process of dry deposition (see Sect. 2.15), a critical loss process for chemicals, is mostly limited to the boundary layer.
2.17 Satellites and the troposphere
The importance of satellites for the discipline of atmospheric chemistry centres on the ability to give a self-consistent global view of a selected set of tropospheric trace species (Burrows et al., 2011; Martin, 2008; Prospero et al., 2002). The beginning and first demonstration of the effective application of these attributes for the troposphere were the data and the retrievals from the GOME instrument on ERS-2 (Burrows et al., 1999) and SCIAMACHY on Envisat (Bovensmann et al., 1999). Historically, the roots of these early instruments are in stratospheric chemistry, with GOME being deployed to track stratospheric ozone and its key controlling chemical species. Much effort has followed with measurements from instruments such as OMI, MOPITT, TES, MODIS and ACE as well as Space-Shuttle-borne instrumentation, e.g. CRISTA (Burrows et al., 2011; Martin, 2008).
The most-nominated paper in this area, and one that demonstrated the power of such observations for tropospheric composition research, was “Increase in tropospheric nitrogen dioxide over China observed from space” by Richter et al. (2005), which showed the capability of satellites to track the build-up of air pollution over vast regions from space (Fig. 17). The importance of the work by Palmer and co-workers in establishing a method to convert satellite observations to vertical columns for comparison with e.g. models was also widely recognized (Palmer et al., 2001).
Figure 17The mean annual NO2 column amount normalized to that in 1996 for the geographical regions of the USA, the central east coast of the USA, western Europe, Poland, Japan, eastern central China and Hong Kong, showing a marked increase in NO2 over China and a decrease over Europe (Richter et al., 2005).
Satellite-based instrumentation can measure not only gas-phase trace species but also dust and aerosol particles; mapping of the global distribution of dust (Prospero et al., 2002), the combination of SeaWIFS and TOMS to track Asian dust events (Husar et al., 2001), and the development of the MODIS aerosol algorithm (Remer et al., 2005) provided convincing demonstrations of this capability. A step change in this area was made with the paper by Wang and Christopher (2003), “Intercomparison between satellite-derived aerosol optical thickness and PM2.5 mass: Implications for air quality studies”, which was the first description of the derivation of surface PM2.5 from satellite aerosol optical depth (AOD) and was built on by Liu et al. (2004) for the USA. Later, a global picture was developed by van Donkelaar et al. (2006). These types of observations have been extensively used to estimate the global impact of particulate matter (both PM2.5 and PM10) on health.
2.18 Stratospheric chemistry
Tropospheric chemistry has always been influenced by the study of stratospheric chemistry. At the same time, tropospheric chemistry has been pivotal in determining what surface emissions get to the stratosphere. As mentioned earlier (see Sect. 2.1), the basis of stratospheric ozone chemistry was laid in the 1930s (Chapman, 1930) (for a full history see Brasseur, 2019), whereas tropospheric chemistry followed a couple of decades later. Interest in stratospheric ozone chemistry increased substantially following the works of Johnston and Crutzen on the role of nitrogen oxides in the stratosphere (Crutzen, 1970; Johnston, 1971). The impact of supersonic transport (Johnston, 1971) and chlorofluorocarbons (CFCs) (Molina and Rowland, 1974) was important for stratospheric ozone chemistry. While Crutzen (1970) showed that the nitrogen oxides in the stratosphere come mostly from the nitrous oxides from the ground, Johnson suggested that a fleet of supersonic aircraft could release large amounts of nitrogen oxides into the lower stratosphere, causing substantial ozone loss (Johnston, 1971). The potential threat of supersonic transport highlighted the importance of gas-phase catalysis in the atmosphere and, in particular, the catalytic ozone destruction by HOx and NOx. These works opened the world's eyes to the potential for global environmental change from human activities. Soon after, Lovelock (1974) identified CFCs in the troposphere and showed that practically all the CFCs emitted to date were still in the atmosphere. The significant contributions of Hampson (1964), Crutzen and Johnston, as well as the recognition of chlorine-catalysed ozone destruction by Stolarski and Cicerone (1974), paved the way for the seminal work of Molina and Rowland (Molina and Rowland, 1974) linking chlorofluorocarbons to ozone layer depletion. The recognition that bromine compounds can also destroy stratospheric ozone (McElroy et al., 1986) further refined the story. The potential role of iodine in stratospheric ozone depletion has also been raised (Solomon et al., 1994), but it is still somewhat unsettled.
The ozone hole (Farman et al., 1985) was an unanticipated shock that awoke the world to the global nature of ozone layer depletion. The origin of the ozone hole was understood in a historic set of studies over a relatively short 5-year period. First was the insightful and seminal work of Solomon et al. (1986) that showed that chlorofluorocarbons and other ozone-depleting gases were the key anthropogenic ingredient for the ozone hole. The confluence of cold temperatures that led to the formation of polar stratospheric clouds (PSCs) and the winter vortex formation over Antarctica provided the opportunity for the massive ozone depletion that resulted in the ozone hole. This work confirmed the suggestion of Farman (Farman et al., 1985) that the ozone hole was due to the increasing abundances of CFCs. In particular, Solomon and co-workers (Solomon et al., 1986) recognized that stable molecules such as ClONO2 and HCl could react on solids (and indeed liquids). Along the way, during this intense investigative period, the detection and quantification of the role of ClO as a catalyst by Anderson et al. (1991), as well as De Zafra et al. (1988), provided the “smoking gun” that linked CFCs with the ozone hole. The entire set of field measurements, from the ground, aircraft and balloons, solidified this linkage.
Less heralded, but equally important, were the laboratory studies that showed that chlorine nitrate (ClONO2) and HCl did indeed react on PSCs (Hanson and Ravishankara, 1994; Tolbert et al., 1987; Leu, 1988; Molina, 1991; Molina et al., 1987) and determined the critical rate coefficients for the self-reaction of ClO, the rate-limiting step in the unique catalytic cycles in Antarctica (Cox and Hayman, 1988; Sander et al., 1989; Trolier et al., 1990). Much was learned in later years by studying the Arctic and from continued observations over the Antarctic. It should be noted that the termolecular reaction of ClO was suggested to be important for chlorine chemistry by Molina and Molina (1987), and the history of this reaction goes back to work at Cambridge by Norrish and Neville (1934) and Porter and Wright (1953). One of the lessons from this episode is that natural factors, in this case the formation of a vortex and the occurrence of polar stratospheric clouds, can lead to unexpected consequences when an anthropogenic ingredient (ozone-depleting chemicals) is added to the mix.
The numerical modelling of the stratosphere was an important ingredient for the success of mitigating polar ozone loss, along with the theories of ozone layer depletion and the ozone hole, laboratory studies of key processes, and measurements in the atmosphere. Over the years, these models have enabled a great deal of understanding of the coupling between chemistry and climate. The development of stratospheric chemical transport models (Chipperfield and Pyle, 1988) was a pivotal advancement that enabled quantitative understanding of ozone layer depletion, including the ozone hole (see Sect. 2.6).
The weight of science led to the Vienna convention, the Montreal Protocol, and the protocol's many amendments and adjustments that are leading to the phasing-out of ozone-depleting gases. The Montreal Protocol is the first international treaty on an environmental issue to be universally ratified and is regarded as one of the most successful. That said, the ozone layer depletion story is not complete. For example, the recognition that nitrous oxide is the remaining major ozone-depleting gas emission (Ravishankara et al., 2009) has connected food production (tropospheric nitrogen cycles) to ozone layer depletion and highlighted the importance of a holistic approach to environmental issues.
2.19 Other issues that influence tropospheric chemistry
Atmospheric chemistry advances have been influenced by growth in other areas. In particular, the importance of anthropogenic climate change has been instrumental in invigorating atmospheric chemistry studies because many of the major climate forcing agents are chemically active, and climate change, in turn, influences tropospheric chemistry (von Schneidemesser et al., 2015). In addition to climate change, other adjacent discoveries and findings have influenced tropospheric chemistry studies.
The global atmospheric and climatic consequences of nuclear war were investigated by both Crutzen and Birks (1982) and Turco et al. (1983). Using models developed for looking at the impact of volcanic eruptions, Turco et al. (1983) concluded that “enhancement of solar ultraviolet radiation due to ozone depletion, long-term exposure to cold, dark, and radioactivity could pose a serious threat to human survivors and to other species”. Similarly, Crutzen and Birks (1982) concluded that “the screening of sunlight by the fire-produced aerosol over extended periods during the growing season would eliminate much of the food production in the Northern Hemisphere”.
Air quality has an obvious direct impact on people, and this connection was recognized very early (it was in fact the primary motivation behind the fundamental work of Haagen-Smit). In 1993, Dockery et al. (1993) presented a study of six US cities, showing a direct association between air pollution and mortality rates. This paper is a great example of how an adjacent field influences another, in this case atmospheric chemistry and public health. Though association between air pollution and health stretches back to the Los Angeles and London smog, the “Six Cities Study” was a landmark as it demonstrated that the association between air pollution and mortality extended to much lower concentrations than those observed in the smog days.
A mixture of the history of the discipline and its landmark ideas emerges from this exercise of asking the community what they consider to have shaped their research field. Table 2 seeks to bring these elements together to look at the evolution of the leading scientific concepts, their relevance to the environmental legislation (in this sense, we acknowledge a Euro- and US-centric bias) and the most notable environmental events that have shaped the discipline. Atmospheric science often sits at an interesting intersection between societal interests (e.g. acid precipitation, air quality, ozone layer depletion and climate) and scientific venture. Monks and Williams (2020) have recently explored how environmental events in air quality drive policy and how a scientific and societal paradigm shift occurs once the emergency phase has passed.
From an overview of all the nominated papers, several general features are apparent. Ambient measurements are one of the cornerstones of atmospheric science (Abbatt et al., 2014). It is clear that the atmosphere is under-sampled, but over time we have found many ingenious ways to build different measurement strategies from the ground, ships, aircraft, balloons, sondes and satellites. With a focus on chemistry, it is clear that one needs to be able to measure with surety, sensitivity, specificity and speed in the troposphere. Many of the nominated papers reflect the importance of instrument development. Examples include the electrostatic sizers in aerosol science (Knutson and Whitby, 1975), various techniques to measure the hydroxyl radical (Eisele et al., 1994; Stevens et al., 1994; Perner et al., 1976a), chemiluminescence for (Kley and McFarland, 1980), the development of chemical ionization mass spectrometry (Lindinger et al., 1998), the application of the GC × GC-MS technique (Lewis et al., 2000) and aerosol mass spectrometry measurements (e.g. Zhang et al., 2007). Often, the science underlying the development of these instruments, such as ion-molecule chemistry, is not necessarily acknowledged in the community. The paradigm for field instruments has been the development of analytical methods in the laboratory that are then adopted and/or adapted for field studies. Advances in associated fields such lasers, optics, optical detectors (e.g. camera and diode arrays), mass spectrometry (such as ion traps and high-resolution time-of-flight mass spectrometry), separation methods (such as various chromatography methods) and meteorological instruments have fundamentally altered our understanding of atmospheric chemistry in general and tropospheric chemistry in particular. For example, recent developments in chemical ionization mass spectrometry have led to its pervasive use in our science. It is fair to say that the ability to separate and measure constituents in the part-per-quadrillion and part-per-trillion mixing ratio range has led to the detection of minuscule amounts of chemicals and their variations. Similarly, the recent revolution in low-cost sensors coupled with evolution in telecommunications is already changing and will continue to change our field in the near future. Yet another important area is the development of the details of the chemistry through observations of intermediates and products, for example. These studies have provided some critical information regarding the details of the chemistry (Cohen and Murphy, 2003).
Another common theme is the critical importance and impact of long-term observations, often termed monitoring, of key atmospheric components from CO2 (the “Keeling” curve) to chemically active molecules such as halocarbons (the Antarctic ozone hole, ozone layer depletion and climate change), methane (the changes in the global OH field, background ozone production) and NOx (catalyst for tropospheric ozone production, vehicle emissions and acid precipitation). On the other hand, many breakthroughs in understanding the observations emerged because of basic laboratory information on kinetics and photochemistry (e.g. the reaction of HO2+ NO, the determination of O(1D) quantum yields, and the reactions of ClONO2 and HCl on PSCs). It is noteworthy that both laboratory studies and long-term observations are currently under funding stress, a situation that is already worrying the community (see, for example, the discussion in Burkholder et al., 2017).
There is no doubt that atmospheric chemistry is an integrative science: one of the recurring themes in the papers discussed here is the tight relationship between ambient observations, laboratory experiments and modelling. The integrative power of models has been recognized from the early studies by Levy (1971) to the development of highly sophisticated global transport models by Chipperfield and Pyle (1988) and Bey et al. (2001) up to the more recent demonstrations of the power of model ensembles (Stevenson et al., 2006; Fiore et al., 2009). Much of this progress has parallels in stratospheric chemistry. It is evident in the community that models are a powerful tool to map, test and predict the atmosphere's past, present and future. The predictions and projections from these models play essential roles in policy, planning and management of environmental issues.
Another form of integrative or meta-analysis brings together a range of individual studies to produce a more significant outcome, such as new insights or models. There are some notable examples of this approach in the works on biomass burning by Crutzen and Andreae (1990) and more recently by Van Der Werf et al. (2010). Similarly, the work to produce isoprene emissions models brought together several global isoprene flux measurements (Guenther et al., 1995, 2006). Other examples include the work of Zhang et al. (2007) and Jimenez et al. (2009), who integrated various sets of AMS observations to give insight into the distributions of SOA.
There have been developments in fields adjacent to atmospheric chemistry that have shaped atmospheric chemistry progress. Examples include developments in epidemiology (e.g. the Six Cities Study by Dockery et al., 1993), atmospheric dynamics (the role of transport in determining chemical composition, the role of the Antarctic vortex), ocean science (pertaining to deposition to the ocean surface and emissions from the oceans), and biological and plant science (e.g. Kesselmeier and Staudt, 1999). Integrating atmospheric chemistry with these adjacent fields is not only essential but also fruitful and was for many years embodied in the IGBP (Seitzinger et al., 2015), the WCRP and Earth system science (now future Earth) programmes. Wider contexts, such as paleoclimate, have allowed an understanding of climate and atmospheric history over 100 000-year timescales (e.g. Petit et al., 1999). That work set the framework for understanding that the present-day atmospheric burdens of carbon dioxide and methane as important greenhouse gases are unprecedented during the past 420 000 years. They also allow us to estimate the composition of the troposphere in those ancient times.
Similarly, there are concepts that have their roots in tropospheric chemistry and have gone on to have a wider impact. The concept of the Anthropocene, most recently highlighted by Crutzen and Stroemer in 2000, indicates that we are in a new geological epoch driven by human activities. The idea was more fully expounded in Crutzen (2002) as “the geology of mankind”. There is little doubt that this is a key idea that has influenced much thinking as well as work far beyond atmospheric science (Table 2).
As discussed in detail in the Introduction, we opted here to assemble this compilation of papers by reaching out to the community for nominations rather than using the number of citations as a primary measure. We will not go into the details again here; suffice it to say that there are inherent advantages and drawbacks to any method one might consider for such a work. In that sense, we would like to acknowledge and thank all those who provided feedback during the open peer-review phase of the paper.
We would be remiss in not noting that many areas of tropospheric chemistry remain in their infancy. The chemistry in the boundary layer and the dynamics of this region represent such an example. This is particularly noteworthy since humans live and therefore emit mostly in the boundary layer. Another noteworthy understudied area is deposition, which still largely consists of parameterizations. Developing an understanding of the fundamental steps that are independently measured and understood would be a laudable goal for atmospheric chemists. The state and development of emission inventories, so key to models, represent an area in need of work. New measurements will provide insights into the ever-changing nature of our atmosphere across differing spatial and temporal scales.
The papers highlighted capture a substantial scope of the atmospheric research endeavour over the last 60 years. The challenge now for you, the reader, is to continue to reflect on the papers included here and continue this discussion not only with respect to tropospheric chemistry but also to its related areas.
| ABLE | Amazon Boundary-Layer Experiment |
| ACE | Advanced Composition Explorer |
| AGAGE | Advanced Global Atmospheric Gases Experiment |
| AMS | Aerosol mass spectrometer |
| AOD | Aerosol optical depth |
| BBOA | Biomass burning organic aerosol |
| CAPRAM | Chemical Aqueous Phase Radical Mechanism |
| CCN | Cloud condensation nuclei |
| CFC | Chlorofluorocarbon |
| CLAW | Charlson–Lovelock–Andreae–Warren |
| CLRTAP | Convention on Long-range Transboundary Air Pollution |
| CRISTA | Cryogenic Infrared Spectrometers and Telescopes for the Atmosphere |
| CTM | Chemical transport model |
| EDGAR | Emission Database for Global Atmospheric Research |
| EPA | United States Environmental Protection Agency |
| ESRL | Earth System Research Laboratories |
| FA-AMS | Factor analysis of aerosol mass spectrometry |
| FLEXPART | FLEXible PARTicle dispersion model |
| GC | Gas chromatography |
| GC-MS | Gas chromatography–mass spectrometry |
| GEOS-Chem | Goddard Earth Observing System–Chemical Transport Model |
| GML | Global Monitoring Laboratory |
| GOME | Global Ozone Monitoring Experiment |
| HCFC | Hydrochlorofluorocarbon |
| HOA | Hydrocarbon-like organic aerosol |
| HYSPLIT | Hybrid Single-Particle Lagrangian Integrated Trajectory model |
| IAGOS | In-service Aircraft for a Global Observing System |
| IGAC | International Global Atmospheric Chemistry |
| IGBP | International Geosphere–Biosphere Programme |
| IPCC | Intergovernmental Panel on Climate Change |
| IUPAC | International Union of Pure and Applied Chemistry |
| JPL | NASA Jet Propulsion Laboratory |
| LIF | Laser-induced fluorescence |
| LV-OOA | Low-volatility oxygenated organic aerosol |
| MCM | Master Chemical Mechanism |
| MEGAN | Model of Emissions of Gases and Aerosols from Nature |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| MOPITT | Measurements Of Pollution In The Troposphere |
| MOZAIC | Measurements of OZone and water vapour by in-service Airbus airCraft |
| NASA | National Aeronautics and Space Administration |
| NECs | National Emissions reduction Commitments |
| NMHC | Non-methane hydrocarbon |
| NOAA | National Oceanic and Atmospheric Administration |
| OA | Organic aerosol |
| OMI | Ozone Monitoring Instrument |
| OOA | Oxygenated organic aerosol |
| OPE | Ozone production efficiency |
| PAN | Peroxyacetyl nitrate |
| PM | Particulate matter |
| POA | Primary organic aerosol |
| PSCs | Polar stratospheric clouds |
| RACM | Regional Atmospheric Chemistry Mechanism |
| RADM | Regional Acid Deposition Model |
| RF | Radiative forcing |
| RRKM | Rice–Ramsperger–Kassel–Marcus |
| SAPRC | Statewide Air Pollution Research Center |
| SCIAMACHY | SCanning Imaging Absorption spectroMeter for Atmospheric CartograpHY |
| SeaWIFS | Sea-Viewing Wide Field-of-View Sensor |
| SHADOZ | Southern Hemisphere ADditional OZonesondes |
| SOA | Secondary organic aerosol |
| STE | Stratospheric–tropospheric exchange |
| SV-OOA | Semi-volatile oxygenated organic aerosol |
| TES | Technology Experiment Satellite |
| TOMS | Total Ozone Mapping Spectrometer |
| UNECE | United Nations Economic Commission for Europe |
| UV | Ultraviolet |
| VOC | Volatile organic compound |
| WCRP | World Climate Research Programme |
| WRF | Weather Research and Forecasting model |
| WRF-Chem | Weather Research and Forecasting model coupled to Chemistry |
Data that were used in Fig. 1 can be found on the Scopus bibliographic database (https://www.scopus.com/, last access: 20 June 2020).
PSM developed the concept and led the writing. PSM, ARR, RS and EvS solicited input. ARR, RS and EvS contributed to writing and editing.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to thank everybody who sent their contributions following our call, as well as those who participated in the ACP discussion and provided further input for this paper.
The work of A. R. Ravishankara was supported by Colorado State University. The work of Erika von Schneidemesser was supported by the IASS Potsdam, with financial support provided by the Federal Ministry of Education and Research of Germany (BMBF) and the Ministry for Science, Research and Culture of the State of Brandenburg (MWFK).
This paper was edited by Barbara Ervens and reviewed by two anonymous referees.
Abbatt, J., George, C., Melamed, M., Monks, P., Pandis, S., and Rudich, Y.: New Directions: Fundamentals of atmospheric chemistry: Keeping a three-legged stool balanced, Atmos. Environ., 84, 390–391, https://doi.org/10.1016/j.atmosenv.2013.10.025, 2014.
Aitken, J.: On the Number of Dust Particles in the Atmosphere, Nature, 37, 428–430, https://doi.org/10.1038/037428a0, 1888.
Akimoto, H., Takagi, H., and Sakamaki, F.: Photoenhancement of the nitrous acid formation in the surface reaction of nitrogen dioxide and water vapor: Extra radical source in smog chamber experiments, Int. J. Chem. Kinet., 19, 539–551, https://doi.org/10.1002/kin.550190606, 1987.
Alicke, B., Hebestreit, K., Stutz, J., and Platt, U.: Iodine oxide in the marine boundary layer, Nature, 397, 572–573, 1999.
Allan, B. J., Carslaw, N., Coe, H., Burgess, R. A., and Plane, J. M. C.: Observations of the nitrate radical in the marine boundary layer, J. Atmos. Chem., 33, 129–154, https://doi.org/10.1023/A:1005917203307, 1999.
Anderson, J. G., Toohey, D. W., and Brune, W. H.: Free Radicals Within the Antarctic Vortex: The Role of CFCs in Antarctic Ozone Loss, Science, 251, 39–46, https://doi.org/10.1126/science.251.4989.39, 1991.
Andreae, M. O. and Merlet, P.: Emission of trace gases and aerosols from biomass burning, Global Biogeochem. Cy., 15, 955–966, https://doi.org/10.1029/2000gb001382, 2001.
Aneja, V. P., Overton, J. H., Cupitt, L. T., Durham, J. L., and Wilson, W. E.: Carbon disulphide and carbonyl sulphide from biogenic sources and their contributions to the global sulphur cycle, Nature, 282, 493–496, https://doi.org/10.1038/282493a0, 1979.
Aneja, V. P., Schlesinger, W. H., and Erisman, J. W.: Effects of agriculture upon the air quality and climate: Research, policy, and regulations, Environ. Sci. Technol., 43, 4234–4240, https://doi.org/10.1021/es8024403, 2009.
Arrhenius, S.: On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground, Philosophical Magazine and Journal of Science, 41, 237–276, 1896.
Atkinson, R.: Kinetics and Mechanisms of the Gas-Phase Reactions of the Hydroxyl Radical with Organic Compounds under Atmospheric Conditions, Chem. Rev., 86, 69–201, https://doi.org/10.1021/cr00071a004, 1986.
Atkinson, R.: Atmospheric chemistry of VOCs and NO(x), Atmos. Environ., 34, 2063–2101, https://doi.org/10.1016/S1352-2310(99)00460-4, 2000.
Atkinson, R. and Arey, J.: Atmospheric Degradation of Volatile Organic Compounds, Chem. Rev., 103, 4605–4638, https://doi.org/10.1021/cr0206420, 2003.
Atkinson, R., Baulch, D. L., Cox, R. A., Hampson, R. F., Jr., Kerr, J. A., and Troe, J.: Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement III. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry, J. Phys. Chem. Ref. Data, 18, 881–1097, https://doi.org/10.1063/1.555832, 1989.
Atkinson, R., Baulch, D. L., Cox, R. A., Hampson, R. F., Jr., Kerr, J. A., and Troe, J.: Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement IV. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry, J. Phys. Chem. Ref. Data, 21, 1125–1568, https://doi.org/10.1063/1.555918, 1992.
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., and Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I – gas phase reactions of Ox, HOx, NOx and SOx species, Atmos. Chem. Phys., 4, 1461–1738, https://doi.org/10.5194/acp-4-1461-2004, 2004.
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., and Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II – gas phase reactions of organic species, Atmos. Chem. Phys., 6, 3625–4055, https://doi.org/10.5194/acp-6-3625-2006, 2006.
Ayers, G. P., Penkett, S. A., Gillett, R. W., Bandy, B., Galbally, I. E., Meyer, C. P., Elsworth, C. M., Bentley, S. T., and Forgan, B. W.: Evidence for photochemical control of ozone concentrations in unpolluted marine air, Nature, 360, 446–449, https://doi.org/10.1038/360446a0, 1992.
Ball, S. M., Hancock, G., Murphy, I. J., and Rayner, S. P.: The relative quantum yields of O2(a1Δg) from the photolysis of ozone in the wavelength range 270 nm 329 nm, Geophys. Res. Lett., 20, 2063–2066, https://doi.org/10.1029/93gl02494, 1993.
Barrie, L. A., Bottenheim, J. W., Schnell, R. C., Crutzen, P. J., and Rasmussen, R. A.: Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere, Nature, 334, 138–140, 1988.
Bey, I., Jacob, D. J., Yantosca, R. M., Logan, J. A., Field, B. D., Fiore, A. M., Li, Q., Liu, H. Y., Mickley, L. J., and Schultz, M. G.: Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation, J. Geophys. Res. Atmos., 106, 23073–23095, https://doi.org/10.1029/2001JD000807, 2001.
Bishop, G. A. and Stedman, D. H.: Measuring the Emissions of Passing Cars, Accounts Chem. Res., 29, 489–495, https://doi.org/10.1021/ar950240x, 1996.
Blake, D. R. and Rowland, F. S.: World-wide increase in tropospheric methane, 1978–1983, J. Atmos. Chem., 4, 43–62, https://doi.org/10.1007/BF00053772, 1986.
Bolin, B. and Charlson, R. J.: On the role of the tropospheric sulfur cycle in the shortwave radiative climate of the earth, Ambio, 5, 47–54, 1976.
Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., DeAngelo, B. J., Flanner, M. G., Ghan, S., Kärcher, B., Koch, D., Kinne, S., Kondo, Y., Quinn, P. K., Sarofim, M. C., Schultz, M. G., Schulz, M., Venkataraman, C., Zhang, H., Zhang, S., Bellouin, N., Guttikunda, S. K., Hopke, P. K., Jacobson, M. Z., Kaiser, J. W., Klimont, Z., Lohmann, U., Schwarz, J. P., Shindell, D., Storelvmo, T., Warren, S. G., and Zender, C. S.: Bounding the role of black carbon in the climate system: A scientific assessment, J. Geophys. Res.-Atmos., 118, 5380–5552, https://doi.org/10.1002/jgrd.50171, 2013.
Boucher, O. and Lohmann, U.: The sulfate-CCN-cloud albedo effect, Tellus B, 47, 281–300, https://doi.org/10.3402/tellusb.v47i3.16048, 1995.
Boutron, C. F., Görlach, U., Candelone, J.-P., Bolshov, M. A., and Delmas, R. J.: Decrease in anthropogenic lead, cadmium and zinc in Greenland snows since the late 1960s, Nature, 353, 153–156, https://doi.org/10.1038/353153a0, 1991.
Bovensmann, H., Burrows, J. P., Buchwitz, M., Frerick, J., Noel, S., Rozanov, V. V., Chance, K. V., and Goede, A. P. H.: SCIAMACHY: Mission objectives and measurement modes, J. Atmos. Sci., 56, 127–150, 1999.
Brasseur, G.: The Ozone Layer – from Discovery to Recovery, American Meteorological Society, Boston, USA, 2019.
Brasseur, G. P.: The Importance of Fundamental Science for Society: The Success Story of Ozone Research, Perspect. Earth Space Sci., 1, e2020CN000136, https://doi.org/10.1029/2020CN000136, 2020.
Brasseur, G. P., Orlando, J. J., and Tyndall, G. S.: Atmospheric Chemistry and Global Change, OUP, 1999.
Brasseur, G. P., Prinn, R. G., and Pszenny, A. A. P.: Atmospheric Chemistry in a Changing World, Global Change – IGBP Series, Springer, 2003.
Brimblecombe, P.: The Big Smoke: The History of Air Pollution in London since Medieval Times Methuen & Co., New York, 1987.
Brown, S. S., Stark, H., Ryerson, T. B., Williams, E. J., Nicks Jr, D. K., Trainer, M., Fehsenfeld, F. C., and Ravishankara, A. R.: Nitrogen oxides in the nocturnal boundary layer: Simultaneous in situ measurements of NO3, N2O5, NO2, NO, and O3, J. Geophys. Res., 108, 4299, https://doi.org/10.1029/2002JD002917, 2003.
Brown, S. S., Ryerson, T. B., Wollny, A. G. C., Brock, A., Peltier, R., Sullivan, A. P., Weber, R. J., Dube, W. P., Trainer, M., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. R.: Variability in Nocturnal Nitrogen Oxide Processing and Its Role in Regional Air Quality, Science, 311, 67–70, 2006.
Burkholder, J. B., Abbatt, J. P. D., Barnes, I., Roberts, J. M., Melamed, M. L., Ammann, M., Bertram, A. K., Cappa, C. D., Carlton, A. G., Carpenter, L. J., Crowley, J. N., Dubowski, Y., George, C., Heard, D. E., Herrmann, H., Keutsch, F. N., Kroll, J. H., McNeill, V. F., Ng, N. L., Nizkorodov, S. A., Orlando, J. J., Percival, C. J., Picquet-Varrault, B., Rudich, Y., Seakins, P. W., Surratt, J. D., Tanimoto, H., Thornton, J. A., Tong, Z., Tyndall, G. S., Wahner, A., Weschler, C. J., Wilson, K. R., and Ziemann, P. J.: The Essential Role for Laboratory Studies in Atmospheric Chemistry, Environ. Sci. Technol., 51, 2519–2528, https://doi.org/10.1021/acs.est.6b04947, 2017.
Burkholder, J. B., Abbatt, J. P. D., Cappa, C., Dibble, T. S., Colb, C. E., Orkin, C. L., Wilmouth, D. M., Sander, S. P., Barker, J. R., Crounse, J. D., Huie, R. E., Kurylo, M. J., Percival, C. J., and Wine, P. H.: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, JPL, JPL, 2020.
Burrows, J. P., Weber, M., Buchwitz, M., Rozanov, V., Ladstatter-Weissenmayer, A., Richter, A., DeBeek, R., Hoogen, R., Bramstedt, K., Eichmann, K. U., and Eisinger, M.: The global ozone monitoring experiment (GOME): Mission concept and first scientific results, J. Atmos. Sci., 56, 151–175, 1999.
Burrows, J. P., Platt, U., and Borrell, P.: The Remote Sensing of Tropospheric Composition from Space, Physics of Earth and Space Environments, Springer-Verlag Berlin, Heidelberg, 551 pp., 2011.
Callendar, G. S.: The artificial production of carbon dioxide and its influence on temperature, Q. J. Roy. Meteorol. Soc., 64, 223–240, https://doi.org/10.1002/qj.49706427503, 1938.
Calvert, J. G., Su, F., Bottenheim, J. W., and Strausz, O. P.: Mechanism of the homogeneous oxidation of sulfur dioxide in the troposphere, Atmos. Environ. (1967), 12, 197–226, https://doi.org/10.1016/0004-6981(78)90201-9, 1978.
Calvert, J. G., Heywood, J. B., Sawyer, R. F., and Seinfeld, J. H.: Achieving acceptable air quality: Some reflections on controlling vehicle emissions, Science, 261, 37–45, https://doi.org/10.1126/science.261.5117.37, 1993.
Canagaratna, M. R., Jayne, J. T., Jimenez, J. L., Allan, J. D., Alfarra, M. R., Zhang, Q., Onasch, T. B., Drewnick, F., Coe, H., Middlebrook, A., Delia, A., Williams, L. R., Trimborn, A. M., Northway, M. J., DeCarlo, P. F., Kolb, C. E., Davidovits, P., and Worsnop, D. R.: Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer, Mass Spectrom. Rev., 26, 185–222, https://doi.org/10.1002/mas.20115, 2007.
Carslaw, D. C.: Evidence of an increasing NO2 NOX emissions ratio from road traffic emissions, Atmos. Environ., 39, 4793–4802, https://doi.org/10.1016/j.atmosenv.2005.06.023, 2005.
Carter, W. P. L.: A detailed mechanism for the gas-phase atmospheric reactions of organic compounds, Atmos. Environ. Pt. A, 24, 481–518, https://doi.org/10.1016/0960-1686(90)90005-8, 1990.
Carter, W. P. L.: Development of the SAPRC-07 chemical mechanism, Atmos. Environ., 44, 5324–5335, https://doi.org/10.1016/j.atmosenv.2010.01.026, 2010.
Carter, W. P. L. and Atkinson, R.: A Computer Modeling Study of Incremental Hydrocarb. Reactiv. Environ. Sci. Technol., 23, 864–880, 1989.
Chamberlain, A. C.: Transport of gases to and from grass and grass-like surfaces, Proc. R. Soc. Lond. A, 290, 236–265, 1966.
Chameides, W. L. and Walker, J. C. G.: A photochemical theory for tropospheric ozone, J. Geophys. Res., 78, 8751–8760, 1973.
Chameides, W. L. and Davis, D. D.: Iodine: Its possible role in tropospheric photochemistry, J. Geophys. Res.-Ocean., 85, 7383–7398, https://doi.org/10.1029/JC085iC12p07383, 1980.
Chameides, W. L. and Davis, D. D.: The free radical chemistry of cloud droplets and its impact upon the composition of rain, J. Geophys. Res., 87, 4863–4877, https://doi.org/10.1029/JC087iC07p04863, 1982.
Chameides, W. L., Lindsay, R. W., Richardson, J., and Kiang, C. S.: The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study, Science, 241, 1473–1475, https://doi.org/10.1126/science.3420404, 1988.
Chameides, W. L., Yu, H., Liu, S. C., Bergin, M., Zhou, X., Mearns, L., Wang, G., Kiang, C. S., Saylor, R. D., Luo, C., Huang, Y., Steiner, A., and Giorgi, F.: Case study of the effects of atmospheric aerosols and regional haze on agriculture: An opportunity to enhance crop yields in China through emission controls?, P. Natl. Acad. Sci. USA, 96, 13626–13633, https://doi.org/10.1073/pnas.96.24.13626, 1999.
Chaney, L. W.: The remote measurement of traffic generated carbon monoxide, J. Air Pollut. Control Assoc., 33, 220–222, https://doi.org/10.1080/00022470.1983.10465568, 1983.
Chapman, S.: On ozone and atomic oxygen in the upper atmosphere, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 10, 369–383, https://doi.org/10.1080/14786443009461588, 1930.
Charlson, R. J., Lovelock, J. E., Andreae, M. O., and Warren, S. G.: Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate, Nature, 326, 655–661, https://doi.org/10.1038/326655a0, 1987.
Charlson, R. J., Langner, J., and Rodhe, H.: Sulphate aerosol and climate, Nature, 348, p. 22, https://doi.org/10.1038/348022a0, 1990.
Charlson, R. J., Langner, J., Rodhe, H., Leovy, C. B., and Warren, S. G.: Perturbation of the Northern Hemisphere radiative balance by backscattering from anthropogenic sulfate aerosols, Tellus A, 43, 152–163, https://doi.org/10.3402/tellusa.v43i4.11944, 1991.
Chipperfield, M. P. and Pyle, J. A.: Two-dimensional modelling of the Antarctic lower stratosphere, Geophys. Res. Lett., 15, 875–878, https://doi.org/10.1029/GL015i008p00875, 1988.
Cicerone, R. J.: Halogens in the atmosphere, Rev. Geophys., 19, 123–139, https://doi.org/10.1029/RG019i001p00123, 1981.
Claeys, M., Graham, B., Vas, G., Wang, W., Vermeylen, R., Pashynska, V., Cafmeyer, J., Guyon, P., Andreae, M. O., Artaxo, P., and Maenhaut, W.: Formation of Secondary Organic Aerosols Through Photooxidation of Isoprene, Science, 303, 1173–1176, https://doi.org/10.1126/science.1092805, 2004.
Clegg, S. L., Brimblecombe, P., and Wexler, A. S.: Thermodynamic model of the system H+-NH-SO-NO-H2O at tropospheric temperatures, J. Phys. Chem. A, 102, 2137–2154, 1998a.
Clegg, S. L., Brimblecombe, P., and Wexler, A. S.: Thermodynamic model of the system H+-NH-Na+-SO-NO-Cl−-H2O at 298.15 K, J. Phys. Chem. A, 102, 2155–2171, 1998b.
Cohen, R. C. and Murphy, J. G.: Photochemistry of NO2 in Earth's Stratosphere: Constraints from Observations, Chem. Rev., 103, 4985–4998, https://doi.org/10.1021/cr020647x, 2003.
Covert, D. S., Wiedensohler, A., Aalto, P., Heintzenberg, J., McMurry, P. H., and Leck, C.: Aerosol number size distributions from 3 to 500 nm diameter in the arctic marine boundary layer during summer and autumn, Tellus B, 48, 197–212, https://doi.org/10.3402/tellusb.v48i2.15886, 1996.
Cox, R. A. and Hayman, G. D.: The stability and photochemistry of dimers of the ClO radical and implications for Antarctic ozone depletion, Nature, 332, 796–800, https://doi.org/10.1038/332796a0, 1988.
Crounse, J. D., Nielsen, L. B., Jørgensen, S., Kjaergaard, H. G., and Wennberg, P. O.: Autoxidation of organic compounds in the atmosphere, J. Phys. Chem. Lett., 4, 3513–3520, https://doi.org/10.1021/jz4019207, 2013.
Crowley, J. N., Ammann, M., Cox, R. A., Hynes, R. G., Jenkin, M. E., Mellouki, A., Rossi, M. J., Troe, J., and Wallington, T. J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume V – heterogeneous reactions on solid substrates, Atmos. Chem. Phys., 10, 9059–9223, https://doi.org/10.5194/acp-10-9059-2010, 2010.
Crutzen, P. J.: A discussion of the chemistry of some minor constituents in the stratosphere and troposphere, Pure Appl. Geophys., 106, 1385–1399, https://doi.org/10.1007/BF00881092, 1973a.
Crutzen, P. J.: Photochemical reactions initiated by and influencing ozone in the unpolluted troposphere, Tellus, 26, 47–57, 1973b.
Crutzen, P. J.: The influence of nitrogen oxides on the atmospheric ozone content, Q. J. Roy. Meteorol. Soc., 96, 320–325, https://doi.org/10.1002/qj.49709640815, 1970.
Crutzen, P. J.: Geology of mankind, Nature, 415, p. 23, https://doi.org/10.1038/415023a, 2002.
Crutzen, P. J. and Birks, J. W.: The atmosphere after a nuclear war: twilight at noon, Ambio, 11, 114–125, 1982.
Crutzen, P. J. and Andreae, M. O.: Biomass burning in the tropics: Impact on atmospheric chemistry and biogeochemical cycles, Science, 250, 1669–1678, 1990.
Crutzen, P. J., Heidt, L. E., Krasnec, J. P., Pollock, W. H., and Seiler, W.: Biomass burning as a source of atmospheric gases CO, H2, N2O, NO, CH3Cl and COS, Nature, 282, 253–256, https://doi.org/10.1038/282253a0, 1979.
Dalton, J.: Experimental enquiry into the proportion of the several gases or elastic fluids, constituting the atmosphere, Memoirs of the Literary and Philosophical Society of Manchester, 1, 244–258, 1805.
Danielsen, E. F.: Stratospheric-Tropospheric Exchange Based on Radioactivity, Ozone and Potential Vorticity, J. Atmos. Sci., 25, 502–518, 1968.
Darnall, K. R., Lloyd, A. C., Winer, A. M., and Pitts, J. N.: Reactivity scale for atmospheric hydrocarbons based on reaction with hydroxyl radical, Environ. Sci. Technol., 10, 692–696, https://doi.org/10.1021/es60118a008, 1976.
Davis, D., Nowak, J. B., Chen, G., Buhr, M., Arimoto, R., Hogan, A., Eisele, F., Mauldin, L., Tanner, D., Shetter, R., Lefer, B., and McMurry, P.: Unexpected high levels of NO observed at South Pole, Geophys. Res. Lett., 28, 3625–3628, https://doi.org/10.1029/2000GL012584, 2001.
Davis, D. D.: Project Gametag: An overview, J. Geophys. Res.-Ocean., 85, 7285–7292, https://doi.org/10.1029/JC085iC12p07285, 1980.
Davis, D. D., Heaps, W., and McGee, T.: Direct measurements of natural tropospheric levels of OH via an aircraft borne tunable dye laser, Geophys. Res. Lett., 3, 331–333, https://doi.org/10.1029/GL003i006p00331, 1976.
Davis, D. D., Ravishankara, A. R., and Fischer, S.: SO2 oxidation via the hydroxyl radical: Atmospheric fate of HSOx radicals, Geophys. Res. Lett., 6, 113–116, https://doi.org/10.1029/GL006i002p00113, 1979.
de Gouw, J. A., Middlebrook, A. M., Warneke, C., Goldan, P. D., Kuster, W. C., Roberts, J. M., Fehsenfeld, F. C., Worsnop, D. R., Canagaratna, M. R., Pszenny, A. A. P., Keene, W. C., Marchewka, M., Bertman, S. B., and Bates, T. S.: Budget of organic carbon in a polluted atmosphere: Results from the New England Air Quality Study in 2002, J. Geophys. Res.-Atmos., 110, 1–22, https://doi.org/10.1029/2004JD005623, 2005.
De Mazière, M., Thompson, A. M., Kurylo, M. J., Wild, J. D., Bernhard, G., Blumenstock, T., Braathen, G. O., Hannigan, J. W., Lambert, J. C., Leblanc, T., McGee, T. J., Nedoluha, G., Petropavlovskikh, I., Seckmeyer, G., Simon, P. C., Steinbrecht, W., and Strahan, S. E.: The Network for the Detection of Atmospheric Composition Change (NDACC): history, status and perspectives, Atmos. Chem. Phys., 18, 4935–4964, https://doi.org/10.5194/acp-18-4935-2018, 2018.
Demerjian, K. L., Kerr, J. A., and Calvert, J. G.: Mechanism of photochemical smog formation, Adv. Environ. Sci. Technol., 4, 1–262, 1974.
DeMore, W. B., Sander, S. P., Golden, D. M., Hampson, R. F., Kurylo, M. J., Howard, C. J., Ravishankara, A. R., Kolb, C. E., and Molina, M. J.: Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling, NASA, JPL, 1997.
Dentener, F. J., Carmichael, G. R., Zhang, Y., Lelieveld, J., and Crutzen, P. J.: Role of mineral aerosol as a reactive surface in the global troposphere, J. Geophys. Res.-Atmos., 101, 22869–22889, https://doi.org/10.1029/96JD01818, 1996.
De Zafra, R. L., Jaramillo, M., Parrish, A., Solomon, P., Connor, B., and Barrett, J.: High concentrations of chlorine monoxide at low altitudes in the Antarctic spring stratosphere: Diurnal variation, Nature, 328, 408–411, 1988.
Di Carlo, P., Brune, W. H., Martinez, M., Harder, H., Lesher, R., Ren, X. R., Thornberry, T., Carroll, M. A., Young, V., Shepson, P. B., Riemer, D., Apel, E., and Campbell, C.: Missing OH reactivity in a forest: Evidence for unknown reactive biogenic VOCs, Science, 304, 722–725, 2004.
Dockery, D. W., Pope, C. A., Xu, X., Spengler, J. D., Ware, J. H., Fay, M. E., Ferris, B. G., and Speizer, F. E.: An association between air pollution and mortality in six US cities, New Engl. J. Med., 29, 1753–1759, 1993.
Donahue, N. M., Robinson, A. L., Stanier, C. O., and Pandis, S. N.: Coupled partitioning, dilution, and chemical aging of semivolatile organics, Environ. Sci. Technol., 40, 2635–2643, https://doi.org/10.1021/es052297c, 2006.
Draxler, R. R.,and Hess, G. D.: An overview of the HYSPLIT_4 modelling system for trajectories, dispersion and deposition, Austr. Meteorol. Mag., 47, 295–308, 1998.
Ehhalt, D. H.: The atmospheric cycle of methane, Tellus, 26, 58–70, https://doi.org/10.1111/j.2153-3490.1974.tb01952.x, 1974.
Ehhalt, D. H.: Photooxidation of trace gases in the troposphere, Phys. Chem. Chem. Phys., 1, 5401–5408, 1999.
Ehn, M., Thornton, J. A., Kleist, E., Sipilä, M., Junninen, H., Pullinen, I., Springer, M., Rubach, F., Tillmann, R., Lee, B., Lopez-Hilfiker, F., Andres, S., Acir, I. H., Rissanen, M., Jokinen, T., Schobesberger, S., Kangasluoma, J., Kontkanen, J., Nieminen, T., Kurtén, T., Nielsen, L. B., Jørgensen, S., Kjaergaard, H. G., Canagaratna, M., Maso, M. D., Berndt, T., Petäjä, T., Wahner, A., Kerminen, V. M., Kulmala, M., Worsnop, D. R., Wildt, J., and Mentel, T. F.: A large source of low-volatility secondary organic aerosol, Nature, 506, 476–479, https://doi.org/10.1038/nature13032, 2014.
Eisele, F. L., Mount, G. H., Fehsenfeld, F. C., Harder, J., Marovich, E., Parrish, D. D., Roberts, J., Trainer, M., and Tanner, D.: Intercomparison of tropospheric OH and ancillary trace gas measurements at Fritz Peak Observatory, Colorado, J. Geophys. Res., 99, 18605–18626, 1994.
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z., and Winiwarter, W.: How a century of ammonia synthesis changed the world, Nat. Geosci., 1, 636–639, https://doi.org/10.1038/ngeo325, 2008.
Ervens, B., George, C., Williams, J. E., Buxton, G. V., Salmon, G. A., Bydder, M., Wilkinson, F., Dentener, F., Mirabel, P., Wolke, R., and Herrmann, H.: CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application, J. Geophys. Res.-Atmos., 108, 4426, https://doi.org/10.1029/2002JD002202, 2003.
Fabian, P. and Pruchniewicz, P. G.: Meridional distribution of ozone in the troposphere and its seasonal variations, J. Geophys. Res. , 82, 2063–2073, 1977.
Facchini, M. C., Mircea, M., Fuzzi, S., and Charlson, R. J.: Cloud albedo enhancement by surface-active organic solutes in growing droplets, Nature, 401, 257–259, https://doi.org/10.1038/45758, 1999.
Farman, J. C., Gardiner, B. G., and Shanklin, J. D.: Large losses of total ozone in Antarctica reveal seasonal ClOx NOx interaction, Nature, 315, 207–210, https://doi.org/10.1038/315207a0, 1985.
Fehsenfeld, F., Calvert, J., Fall, R., Goldan, P., Guenther, A. B., Hewitt, C. N., Lamb, B., Liu, S., Trainer, M., Westberg, H., and Zimmerman, P.: Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry, Global Biogeochem. Cy., 6, 389–430, https://doi.org/10.1029/92GB02125, 1992.
Finlayson, B. J. and Pitts, J. N.: Photochemistry of the Polluted Troposphere, Science, 192, 111–119, https://doi.org/10.1126/science.192.4235.111, 1976.
Finlayson-Pitts, B. J. and Pitts, J. N.: Chemistry of the upper and lower atmosphere – theory, experiments, and applications, Academic Press, San Diego, 969 pp., 2000.
Finlayson-Pitts, B. J., Ezell, M. J., and Pitts, J. N.: Formation of chemically active chlorine compounds by reactions of atmospheric NaCl particles with gaseous N2O5 and ClONO2, Nature, 337, 241–244, https://doi.org/10.1038/337241a0, 1989.
Fiore, A. M., Dentener, F. J., Wild, O., Cuvelier, C., Schultz, M. G., Hess, P., Textor, C., Schulz, M., Doherty, R. M., Horowitz, L. W., MacKenzie, I. A., Sanderson, M. G., Shindell, D. T., Stevenson, D. S., Szopa, S., Van Dingenen, R., Zeng, G., Atherton, C., Bergmann, D., Bey, I., Carmichael, G., Collins, W. J., Duncan, B. N., Faluvegi, G., Folberth, G., Gauss, M., Gong, S., Hauglustaine, D., Holloway, T., Isaksen, I. S. A., Jacob, D. J., Jonson, J. E., Kaminski, J. W., Keating, T. J., Lupu, A., Marmer, E., Montanaro, V., Park, R. J., Pitari, G., Pringle, K. J., Pyle, J. A., Schroeder, S., Vivanco, M. G., Wind, P., Wojcik, G., Wu, S., and Zuber, A.: Multimodel estimates of intercontinental source-receptor relationships for ozone pollution, J. Geophys. Res.-Atmos., 114, D04301, https://doi.org/10.1029/2008jd010816, 2009.
Fiore, A. M., Naik, V., Spracklen, D. V., Steiner, A., Unger, N., Prather, M., Bergmann, D., Cameron-Smith, P. J., Cionni, I., Collins, W. J., Dalsoren, S., Eyring, V., Folberth, G. A., Ginoux, P., Horowitz, L. W., Josse, B., Lamarque, J. F., MacKenzie, I. A., Nagashima, T., O'Connor, F. M., Righi, M., Rumbold, S. T., Shindell, D. T., Skeie, R. B., Sudo, K., Szopa, S., Takemura, T., and Zeng, G.: Global air quality and climate, Chem. Soc. Rev., 41, 6663–6683, https://doi.org/10.1039/c2cs35095e, 2012.
Fishman, J., Ramanathan, V., Crutzen, P. J., and Liu, S. C.: Tropospheric ozone and climate, Nature, 282, 818–820, https://doi.org/10.1038/282818a0, 1979.
Fitzgerald, J. W.: Effect of Aerosol Composition on Cloud Droplet Size Distribution: A Numerical Study, J. Atmos. Sci., 31, 1358–1367, 1974.
Fleming, E. L., Jackman, C. H., Stolarski, R. S., and Considine, D. B.: Simulation of stratospheric tracers using an improved empirically based two-dimensional model transport formulation, J. Geophys. Res.-Atmos., 104, 23911–23934, https://doi.org/10.1029/1999JD900332, 1999.
Forster, C., Wandinger, U., Wotawa, G., James, P., Mattis, I., Althausen, D., Simmonds, P., O'Doherty, S., Jennings, S. G., Kleefeld, C., Schneider, J., Trickl, T., Kreipl, S., Jäger, H., and Stohl, A.: Transport of boreal forest fire emissions from Canada to Europe, J. Geophys. Res., 106, 22887–22906, 2001.
Fowler, D., Pilegaard, K., Sutton, M. A., Ambus, P., Raivonen, M., Duyzer, J., Simpson, D., Fagerli, H., Fuzzi, S., Schjoerring, J. K., Granier, C., Neftel, A., Isaksen, I. S. A., Laj, P., Maione, M., Monks, P. S., Burkhardt, J., Daemmgen, U., Neirynck, J., Personne, E., Wichink-Kruit, R., Butterbach-Bahl, K., Flechard, C., Tuovinen, J. P., Coyle, M., Gerosa, G., Loubet, B., Altimir, N., Gruenhage, L., Ammann, C., Cieslik, S., Paoletti, E., Mikkelsen, T. N., Ro-Poulsen, H., Cellier, P., Cape, J. N., Horvath, L., Loreto, F., Niinemets, U., Palmer, P. I., Rinne, J., Misztal, P., Nemitz, E., Nilsson, D., Pryor, S., Gallagher, M. W., Vesala, T., Skiba, U., Brueggemann, N., Zechmeister-Boltenstern, S., Williams, J., O'Dowd, C., Facchini, M. C., de Leeuw, G., Flossman, A., Chaumerliac, N., and Erisman, J. W.: Atmospheric composition change: Ecosystems-Atmosphere interactions, Atmos. Environ., 43, 5193–5267, https://doi.org/10.1016/j.atmosenv.2009.07.068, 2009.
Fowler, D., Brimblecombe, P., Burrows, J., Heal, M. R., Grennfelt, P., Stevenson, D. S., Jowett, A., Nemitz, E., Coyle, M., Lui, X., Chang, Y., Fuller, G. W., Sutton, M. A., Klimont, Z., Unsworth, M. H., and Vieno, M.: A chronology of global air quality, Philos. T. Roy. Soc. A, 378, 20190314, https://doi.org/10.1098/rsta.2019.0314, 2020.
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., Lang-Yona, N., Burrows, S. M., Gunthe, S. S., Elbert, W., Su, H., Hoor, P., Thines, E., Hoffmann, T., Després, V. R., and Pöschl, U.: Bioaerosols in the Earth system: Climate, health, and ecosystem interactions, Atmos. Res., 182, 346–376, https://doi.org/10.1016/j.atmosres.2016.07.018, 2016.
Fuller, G. W.: The Invisible Killer: The Rising Threat of Global Air Pollution – and how we can fight back, Melville House, UK 2018.
Galbally, I.: Some measurements of ozone variation and destruction in the atmospheric surface layer, Nature, 218, 456–457, https://doi.org/10.1038/218456a0, 1968.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., Asner, G. P., Cleveland, C. C., Green, P. A., Holland, E. A., Karl, D. M., Michaels, A. F., Porter, J. H., Townsend, A. R., and Vorosmarty, C. J.: Nitrogen cycles: past, present, and future, Biogeochemistry, 70, 153–226, https://doi.org/10.1007/s10533-004-0370-0, 2004.
Garcia, R. R. and Solomon, S.: A numerical model of the zonally averaged dynamical and chemical structure of the middle atmosphere, J. Geophys. Res.-Ocean., 88, 1379–1400, https://doi.org/10.1029/JC088iC02p01379, 1983.
Gard, E., Mayer, J. E., Morrical, B. D., Dienes, T., Fergenson, D. P., and Prather, K. A.: Real-Time Analysis of Individual Atmospheric Aerosol Particles: Design and Performance of a Portable ATOFMS, Anal. Chem., 69, 4083–4091, https://doi.org/10.1021/ac970540n, 1997.
Goldstein, A. H. and Galbally, I. E.: Known and Unexplored Organic Constituents in the Earth's Atmosphere, Environ. Sci. Technol., 41, 1515–1521, 2007.
Graedel, T. E.: Kinetic photochemistry of the marine atmosphere, J. Geophys. Res., 84, 273–286, 1979.
Graedel, T. E. and Weschler, C. J.: Chemistry within aqueous atmospheric aerosols and raindrops, Rev. Geophys., 19, 505–539, https://doi.org/10.1029/RG019i004p00505, 1981.
Grannas, A. M., Jones, A. E., Dibb, J., Ammann, M., Anastasio, C., Beine, H. J., Bergin, M., Bottenheim, J., Boxe, C. S., Carver, G., Chen, G., Crawford, J. H., Dominé, F., Frey, M. M., Guzmán, M. I., Heard, D. E., Helmig, D., Hoffmann, M. R., Honrath, R. E., Huey, L. G., Hutterli, M., Jacobi, H. W., Klán, P., Lefer, B., McConnell, J., Plane, J., Sander, R., Savarino, J., Shepson, P. B., Simpson, W. R., Sodeau, J. R., von Glasow, R., Weller, R., Wolff, E. W., and Zhu, T.: An overview of snow photochemistry: evidence, mechanisms and impacts, Atmos. Chem. Phys., 7, 4329–4373, https://doi.org/10.5194/acp-7-4329-2007, 2007.
Grell, G. A., Peckham, S. E., Schmitz, R., McKeen, S. A., Frost, G., Skamarock, W. C., and Eder, B.: Fully coupled ”online” chemistry within the WRF model, Atmos. Environ., 39, 6957–6975, https://doi.org/10.1016/j.atmosenv.2005.04.027, 2005.
Grennfelt, P., Engleryd, A., Forsius, M., Hov, Ø., Rodhe, H., and Cowling, E.: Acid rain and air pollution: 50 years of progress in environmental science and policy, Ambio, 49, 849–864, https://doi.org/10.1007/s13280-019-01244-4, 2019.
Guenther, A., Hewitt, C., Erickson, D., Fall, R., Geron, C., Graedel, T., Harley, P., Klinger, L., Lerdau, M., McKay, W., Pierce, T., Scholes, R., Steinbrecher, R., Tallamraju, R., Taylor, J., and Zimmerman, P.: A global model of natural volatile organic compound emissions, J. Geophys. Res., 100, 8873–8892, 1995.
Guenther, A., Geron, C., Pierce, T., Lamb, B., Harley, P., and Fall, R.: Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America, Atmos. Environ., 34, 2205–2230, https://doi.org/10.1016/S1352-2310(99)00465-3, 2000.
Guenther, A., Karl, T., Harley, P., Wiedinmyer, C., Palmer, P. I., and Geron, C.: Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature), Atmos. Chem. Phys., 6, 3181–3210, https://doi.org/10.5194/acp-6-3181-2006, 2006.
Guenther, A. B., Zimmerman, P. R., Harley, P. C., Monson, R. K., and Fall, R.: Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses, J. Geophys. Res.-Atmos., 98, 12609–12617, https://doi.org/10.1029/93jd00527, 1993.
Haagen-Smit, A. J.: Chemistry and Physiology of Los Angeles Smog, Industr. Eng. Chem., 44, 1342–1346, https://doi.org/10.1021/ie50510a045, 1952.
Haagen-Smit, A. J. and Fox, M. M.: Photochemical ozone formation with hydrocarbons and automobile exhaust, Air Repair, 4, 105–136, https://doi.org/10.1080/00966665.1954.10467649, 1954.
Haagen-Smit, A. J., Bradley, C. E., and Fox, M. M.: Ozone Formation in Photochemical Oxidation of Organic Substances, Industr. Eng. Chem., 45, 2086–2089, https://doi.org/10.1021/ie50525a044, 1953.
Hallquist, M., Wenger, J. C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., Dommen, J., Donahue, N. M., George, C., Goldstein, A. H., Hamilton, J. F., Herrmann, H., Hoffmann, T., Iinuma, Y., Jang, M., Jenkin, M., Jimenez, J. L., Kiendler-Scharr, A., Maenhaut, W., McFiggans, G., Mentel, T., Monod, A., Prevot, A. S. H., Seinfeld, J. H., Surratt, J. D., Szmigielski, R., and Wildt, J.: The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues, Atmos. Chem. Phys. , 9, 5155–5236, https://doi.org/10.5194/acp-9-5155-2009 2009.
Hampson, J.: Photochemical behavior of the ozone layer, Can. Armament Res. and Dev. Estab., Valcartier, Quebec, Canada, 1964.
Hanson, D. R. and Ravishankara, A. R.: Reactive uptake of CIONO2 onto sulfuric acid due to reaction with HCl and H2O, J. Phys. Chem., 98, 5728–5735, https://doi.org/10.1021/j100073a026, 1994.
Hanson, D. R., Burkholder, J. B., Howard, C. J., and Ravishankara, A. R.: Measurement of OH and HO2 radical uptake coefficients on water and sulfuric acid surfaces, J. Phys. Chem., 96, 4979–4985, https://doi.org/10.1021/j100191a046, 1992.
Hao, W. M. and Liu, M. H.: Spatial and temporal distribution of tropical biomass burning, Global Biogeochem. Cy., 8, 495–504, 1994.
Harriss, R. C.: The Amazon Boundary Layer Experiment (ABLE 2A): dry season 1985, J. Geophys. Res., 93, 1351–1360, https://doi.org/10.1029/JD093iD02p01351, 1988.
Hard, T. M., O'Brien, R. J., Chan, C. Y., and Mehrabzadeh, A. A.: Tropospheric free radical determination by fluorescence assay with gas expansion, Environ. Sci. Technol., 18, 768–777, https://doi.org/10.1021/es00128a009, 1984.
Heard, D. E. and Pilling, M. J.: Measurement of OH and HO2 in the Troposphere, Chem. Rev., 103, 5163–5198, 2003.
Hein, R., Crutzen, P. J., and Heimann, M.: An inverse modeling approach to investigate the global atmospheric methane cycle, Global Biogeochem. Cy., 11, 43–76, https://doi.org/10.1029/96GB03043, 1997.
Hofzumahaus, A., Rohrer, F., Lu, K. D., Bohn, B., Brauers, T., Chang, C. C., Fuchs, H., Holland, F., Kita, K., Kondo, Y., Li, X., Lou, S. R., Shao, M., Zeng, L. M., Wahner, A., and Zhang, Y. H.: Amplified Trace Gas Removal in the Troposphere, Science, 324, 1702–1704, https://doi.org/10.1126/science.1164566, 2009.
Holton, J. R., Haynes, P. H., McIntyre, M. E., Douglass, A. R., Rood, R. B., and Pfister, L.: Stratosphere-troposphere exchange, Rev. Geophys., 33, 403–439, https://doi.org/10.1029/95rg02097, 1995.
Honrath, R. E., Peterson, M. C., Guo, S., Dibb, J. E., Shepson, P. B., and Campbell, B.: Evidence of NOx production within or upon ice particles in the Greenland snow-pack, Geophys. Res. Lett., 26, 695–698, 1999.
Howard, C. J. and Evenson, K. M.: Kinetics of the reaction of HO2 with NO, Geophys. Res. Lett., 4, 437–440, https://doi.org/10.1029/GL004i010p00437, 1977.
Hudson, R. D. and Reed, E. I.: Stratosphere: present and future, NASA Reference Publication 1049, available at: https://ntrs.nasa.gov/api/citations/19800006383/downloads/19800006383.pdf (last access: 30 August 2021), 1979.
Husar, R. B., Tratt, D. M., Schichtel, B. A., Falke, S. R., Li, F., Jaffe, D., Gassó, S., Gill, T., Laulainen, N. S., Lu, F., Reheis, M. C., Chun, Y., Westphal, D., Holben, B. N., Gueymard, C., McKendry, I., Kuring, N., Feldman, G. C., McClain, C., Frouin, R. J., Merrill, J., DuBois, D., Vignola, F., Murayama, T., Nickovic, S., Wilson, W. E., Sassen, K., Sugimoto, N., and Malm, W. C.: Asian dust events of April 1998, J. Geophys. Res.-Atmos., 106, 18317–18330, https://doi.org/10.1029/2000JD900788, 2001.
IPCC: Climate Change 2013 – The Physical Science Basis, Cambridge University Press, Cambridge, 1552 pp., 2013.
Jackman, C., Seals, R., and Prather, M.: Two-dimensional intercomparison of stratospheric models, NASA Conference Publication 3042, available at: https://ntrs.nasa.gov/api/citations/19900002089/downloads/19900002089.pdf (last access: 30 August 2021), 1989.
Jacob, D. J.: Introduction to Atmospheric Chemistry, Princeton University Press, Princeton, USA, 1999.
Jacob, D. J., Munger, J. W., Waldman, J. M., and Hoffmann, M. R.: The H2SO4 – HNO3 – NH3 system at high humidities and in fogs, 1. Spatial and temporal patterns in the San Joaquin Valley of California, J. Geophys. Res., 91, 1073–1088, https://doi.org/10.1029/JD091iD01p01073, 1986.
Jacob, D. J.: Heterogeneous chemistry and tropospheric ozone, Atmos. Environ., 34, 2131–2159, https://doi.org/10.1016/S1352-2310(99)00462-8, 2000.
Jacob, D. J., Logan, J. A., and Murti, P. P.: Effect of rising Asian emissions on surface ozone in the United States, Geophys. Res. Lett., 26, 2175–2178, https://doi.org/10.1029/1999GL900450, 1999.
Jacobson, M. Z.: Atmospheric Pollution: History, Science and Regulation, Cambridge University Press, Cambridge, UK, 2002.
Jaeglé, L., Jacob, D. J., Wang, Y., Weinheimer, A. J., Ridley, B. A., Campos, T. L., Sachse, G. W., and Hagen, D. E.: Sources and chemistry of NOx in the upper troposphere over the United States, Geophys. Res. Lett., 25, 1705–1708, https://doi.org/10.1029/97gl03591, 1998.
Jaenicke, R.: Abundance of cellular material and proteins in the atmosphere, Science, 308, p. 73, https://doi.org/10.1126/science.1106335, 2005.
Jenkin, M. E., Saunders, S. M., and Pilling, M. J.: The tropospheric degradation of volatile organic compounds: A protocol for mechanism development, Atmos. Environ., 31, 81–104, 1997.
Jenkin, M. E., Saunders, S. M., Wagner, V., and Pilling, M. J.: Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic compounds, Atmos. Chem. Phys., 3, 181–193, https://doi.org/10.5194/acp-3-181-2003, 2003.
Jimenez, J. L., Canagaratna, M. R., Donahue, N. M., Prevot, A. S. H., Zhang, Q., Kroll, J. H., DeCarlo, P. F., Allan, J. D., Coe, H., Ng, N. L., Aiken, A. C., Docherty, K. S., Ulbrich, I. M., Grieshop, A. P., Robinson, A. L., Duplissy, J., Smith, J. D., Wilson, K. R., Lanz, V. A., Hueglin, C., Sun, Y. L., Tian, J., Laaksonen, A., Raatikainen, T., Rautiainen, J., Vaattovaara, P., Ehn, M., Kulmala, M., Tomlinson, J. M., Collins, D. R., Cubison, M. J., Dunlea, J., Huffman, J. A., Onasch, T. B., Alfarra, M. R., Williams, P. I., Bower, K., Kondo, Y., Schneider, J., Drewnick, F., Borrmann, S., Weimer, S., Demerjian, K., Salcedo, D., Cottrell, L., Griffin, R., Takami, A., Miyoshi, T., Hatakeyama, S., Shimono, A., Sun, J. Y., Zhang, Y. M., Dzepina, K., Kimmel, J. R., Sueper, D., Jayne, J. T., Herndon, S. C., Trimborn, A. M., Williams, L. R., Wood, E. C., Middlebrook, A. M., Kolb, C. E., Baltensperger, U., and Worsnop, D. R.: Evolution of Organic Aerosols in the Atmosphere, Science, 326, 1525, https://doi.org/10.1126/science.1180353, 2009.
Johnston, H.: Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust, Science, 173, 517–522, https://doi.org/10.1126/science.173.3996.517, 1971.
Junge, C.: The size distribution and aging of natural aerosols as determined from electrical and optical data on the atmosphere, J. Meteorol., 12, 13–25, 1955.
Junge, C. E.: Basic considerations about trace constituents in the atmosphere as related to the fate of global pollutants, Adv. Environ. Sci. Technol., 8, 7–25, 1975.
Junge, C. E. and Ryan, T. G.: Study of the SO2 oxidation in solution and its role in atmospheric chemistry, Q. J. Roy. Meteorol. Soc., 84, 46–55, https://doi.org/10.1002/qj.49708435906, 1958.
Kalberer, M., Paulsen, D., Sax, M., Steinbacher, M., Dommen, J., Prevot, A. S. H., Fisseha, R., Weingartner, E., Frankevich, V., Zenobi, R., and Baltensperger, U.: Identification of Polymers as Major Components of Atmospheric Organic Aerosols, Science, 303, 1659–1662, https://doi.org/10.1126/science.1092185, 2004.
Karl, T., Harley, P., Emmons, L., Thornton, B., Guenther, A., Basu, C., Turnipseed, A., and Jardine, K.: Efficient Atmospheric Cleansing of Oxidized Organic Trace Gases by Vegetation, Science, 1192534, https://doi.org/10.1126/science.1192534, 2010.
Keeling, C. D.: The Concentration and Isotopic Abundances of Carbon Dioxide in the Atmosphere, Tellus, 12, 200–203, https://doi.org/10.1111/j.2153-3490.1960.tb01300.x, 1960.
Keeling, C. D., Mook, W. G., and Tans, P. P.: Recent trends in the 13C 12C ratio of atmospheric carbon dioxide, Nature, 277, 121–123, https://doi.org/10.1038/277121a0, 1979.
Kesselmeier, J. and Staudt, M.: Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology, J. Atmos. Chem., 33, 23–88, https://doi.org/10.1023/A:1006127516791, 1999.
Kirkby, J., Curtius, J., Almeida, J., Dunne, E., Duplissy, J., Ehrhart, S., Franchin, A., Gagné, S., Ickes, L., Kürten, A., Kupc, A., Metzger, A., Riccobono, F., Rondo, L., Schobesberger, S., Tsagkogeorgas, G., Wimmer, D., Amorim, A., Bianchi, F., Breitenlechner, M., David, A., Dommen, J., Downard, A., Ehn, M., Flagan, R. C., Haider, S., Hansel, A., Hauser, D., Jud, W., Junninen, H., Kreissl, F., Kvashin, A., Laaksonen, A., Lehtipalo, K., Lima, J., Lovejoy, E. R., Makhmutov, V., Mathot, S., Mikkilä, J., Minginette, P., Mogo, S., Nieminen, T., Onnela, A., Pereira, P., Petäjä, T., Schnitzhofer, R., Seinfeld, J. H., Sipilä, M., Stozhkov, Y., Stratmann, F., Tomé, A., Vanhanen, J., Viisanen, Y., Vrtala, A., Wagner, P. E., Walther, H., Weingartner, E., Wex, H., Winkler, P. M., Carslaw, K. S., Worsnop, D. R., Baltensperger, U., and Kulmala, M.: Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation, Nature, 476, 429–435, https://doi.org/10.1038/nature10343, 2011.
Kleindienst, T. E.: Epoxying Isoprene Chemistry, Science, 325, 687–688, https://doi.org/10.1126/science.1178324, 2009.
Kleinman, L. I.: Seasonal dependence of boundary layer peroxide concentration: the low and high NOx regimes, J. Geophys. Res., 96, 20721–720733, 1991.
Kley, D. and McFarland, M.: Chemiluminescence detector for NO and NO2, Atmos. Technol., 12, 63–69, 1980.
Knipping, E. M., Lakin, M. J., Foster, K. L., Jungwirth, P., Tobias, D. J., Gerber, R. B., Dabdub, D., and Finlayson-Pitts, B. J.: Experiments and simulations of ion-enhanced interfacial chemistry on aqueous NaCl aerosols, Science, 288, 301–306, https://doi.org/10.1126/science.288.5464.301, 2000.
Knutson, E. O. and Whitby, K. T.: Aerosol classification by electric mobility: apparatus, theory, and applications, J. Aerosol Sci., 6, 443–451, https://doi.org/10.1016/0021-8502(75)90060-9, 1975.
Köhler, H.: The nucleus in and the growth of hygroscopic droplets, Trans. Farad. Soc., 32, 1152–1161, https://doi.org/10.1039/TF9363201152, 1936.
Kulmala, M., Pirjola, L., and Mäkelä, J. M.: Stable sulphate clusters as a source of new atmospheric particles, Nature, 404, 66–69, https://doi.org/10.1038/35003550, 2000.
Kurtenbach, R., Becker, K. H., Gomes, J. A. G., Kleffmann, J., Lörzer, J. C., Spittler, M., Wiesen, P., Ackermann, R., Geyer, A., and Platt, U.: Investigations of emissions and heterogeneous formation of HONO in a road traffic tunnel, Atmos. Environ., 35, 3385–3394, https://doi.org/10.1016/S1352-2310(01)00138-8, 2001.
Kwok, E. S. C. and Atkinson, R.: Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: An update, Atmos. Environ., 29, 1685–1695, https://doi.org/10.1016/1352-2310(95)00069-B, 1995.
Landrigan, P. J., Fuller, R., Acosta, N. J. R., Adeyi, O., Arnold, R., Basu, N. N., Baldé, A. B., Bertollini, R., Bose-O'Reilly, S., Boufford, J. I., Breysse, P. N., Chiles, T., Mahidol, C., Coll-Seck, A. M., Cropper, M. L., Fobil, J., Fuster, V., Greenstone, M., Haines, A., Hanrahan, D., Hunter, D., Khare, M., Krupnick, A., Lanphear, B., Lohani, B., Martin, K., Mathiasen, K. V., McTeer, M. A., Murray, C. J. L., Ndahimananjara, J. D., Perera, F., Potočnik, J., Preker, A. S., Ramesh, J., Rockström, J., Salinas, C., Samson, L. D., Sandilya, K., Sly, P. D., Smith, K. R., Steiner, A., Stewart, R. B., Suk, W. A., van Schayck, O. C. P., Yadama, G. N., Yumkella, K., and Zhong, M.: The Lancet Commission on pollution and health, The Lancet, 391, 462–512, https://doi.org/10.1016/S0140-6736(17)32345-0, 2018.
Lawson, D. R.: “Passing the test” – human behavior and California's smog check program, Air Waste, 43, 1567–1575, https://doi.org/10.1080/1073161X.1993.10467226, 1993.
Leighton, P. A.: Photochemistry of Air Pollution, Academic Press, New York, 1961.
Lelieveld, J., Butler, T. M., Crowley, J. N., Dillon, T. J., Fischer, H., Ganzeveld, L., Lawrence, M. G., Martinez, M., Taraborrelli, D., and Williams, J.: Atmospheric oxidation capacity sustained by a tropical forest, Nature, 452, 737–740 2008.
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., and Pozzer, A.: The contribution of outdoor air pollution sources to premature mortality on a global scale, Nature, 525, 367–371, https://doi.org/10.1038/nature15371, 2015.
Leu, M. T.: Laboratory studies of sticking coefficients and heterogeneous reactions important in the Antarctic stratosphere, Geophys. Res. Lett., 15, 17-20, https://doi.org/10.1029/GL015i001p00017, 1988.
Levy, H.: Normal atmosphere – large radical and formaldehyde concentrations predicted, Science, 173, 141-&, https://doi.org/10.1126/science.173.3992.141, 1971.
Lewis, A. C., Carslaw, N., Marriott, P. J., Kinghorn, R. M., Morrison, P., Lee, A. L., Bartle, K. D., and Pilling, M. J.: A larger pool of ozone-forming carbon compounds in urban atmospheres, Nature, 405, 778–781, https://doi.org/10.1038/35015540, 2000.
Likens, G. E. and Bormann, F. H.: Acid rain: A serious regional environmental problem, Science, 184, 1176–1179, https://doi.org/10.1126/science.184.4142.1176, 1974.
Lin, X., Trainer, M., and Liu, S. C.: On the nonlinearity of the tropospheric ozone production, J. Geophys. Res.-Atmos., 93, 15879–15888, https://doi.org/10.1029/JD093iD12p15879, 1988.
Lindinger, W., Hansel, A., and Jordan, A.: On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) – Medical applications, food control and environmental research, Int. J. Mass Spectrom., 173, 191–241, 1998.
Liu, S. C.: Ozone production in the rural troposphere and the implications for regional and global ozone distributions, J. Geophys. Res., 92, 4191–4207, https://doi.org/10.1029/JD092iD04p04191, 1987.
Liu, Y., Park, R. J., Jacob, D. J., Li, Q., Kilaru, V., and Sarnat, J. A.: Mapping annual mean ground-level PM2.5 concentrations using Multiangle Imaging Spectroradiometer aerosol optical thickness over the contiguous United States, J. Geophys. Res.-Atmos., 109, D22206, https://doi.org/10.1029/2004JD005025, 2004.
Logan, J. A.: Nitrogen oxides in the troposphere: global and regional budgets, J. Geophys. Res., 88, 10785–10807, https://doi.org/10.1029/JC088iC15p10785, 1983.
Logan, J. A.: Tropospheric ozone – seasonal behaviour, trends, and anthropogenic influence, J. Geophys. Res.-Atmos., 90, 10463–10482, 1985.
Logan, J. A.: Ozone in rural areas of the United States, J. Geophys. Res., 94, 8511–8532, https://doi.org/10.1029/JD094iD06p08511, 1989.
Logan, J. A.: An analysis of ozonesonde data for the troposphere: Recommendations for testing 3-D models and development of a gridded climatology for tropospheric ozone, J. Geophys. Res.-Atmos., 104, 16115–16149, https://doi.org/10.1029/1998JD100096, 1999.
Logan, J. A., Prather, M. J., Wofsy, S. C., and McElroy, M. B.: Tropospheric chemistry: a global perspective, J. Geophys. Res., 86, 7210–7254, https://doi.org/10.1029/JC086iC08p07210, 1981.
Lovelock, J. E.: Atmospheric halocarbons and stratospheric ozone, Nature, 252, 292–294, https://doi.org/10.1038/252292a0, 1974.
Lovelock, J. E. and Lipsky, S. R.: Electron Affinity Spectroscopy—A New Method for the Identification of Functional Groups in Chemical Compounds Separated by Gas Chromatography, J. Am. Chem. Soc., 82, 431–433, https://doi.org/10.1021/ja01487a045, 1960.
Lovelock, J. E., Maggs, R. J., and Rasmussen, R. A.: Atmospheric Dimethyl Sulphide and the Natural Sulphur Cycle, Nature, 237, 452–453, https://doi.org/10.1038/237452a0, 1972.
MacKenzie, R. A., Harrison, R. M., Colbeck, I., and Nicholas Hewitt, C.: The role of biogenic hydrocarbons in the production of ozone in urban plumes in southeast England, Atmos. Environ., Pt. A, 25, 351–359, https://doi.org/10.1016/0960-1686(91)90306-R, 1991.
Madronich, S. and Flocke, S.: The Role of Solar Radiation in Atmospheric Chemistry, The Handbook of Environmental Chemistry (Reactions and Processes), edited by: Boule, P., Springer-Verlag, Berlin, Heidelberg, 1999.
Mäkelä, J. M., Aalto, P., Jokinen, V., Pohja, T., Nissinen, A., Palmroth, S., Markkanen, T., Seitsonen, K., Lihavainen, H., and Kulmala, M.: Observations of ultrafine aerosol particle formation and growth in boreal forest, Geophys. Res. Lett., 24, 1219–1222, https://doi.org/10.1029/97GL00920, 1997.
Martin, R. V.: Satellite remote sensing of surface air quality, Atmos. Environ., 42, 7823–7843, https://doi.org/10.1016/j.atmosenv.2008.07.018, 2008.
Mauldin Iii, R. L., Berndt, T., Sipilä, M., Paasonen, P., Petäjä, T., Kim, S., Kurtén, T., Stratmann, F., Kerminen, V. M., and Kulmala, M.: A new atmospherically relevant oxidant of sulphur dioxide, Nature, 488, 193–196, https://doi.org/10.1038/nature11278, 2012.
Maynard, R. L. and Williams, M. L.: Regulation of Air Quality in the European Union, in: Regulatory Toxicology in the European Union, Roy. Soc. Chem., Cambridge, UK, 539–556, 2018.
McElroy, M. B., Salawitch, R. J., Wofsy, S. C., and Logan, J. A.: Reductions of Antarctic ozone due to synergistic interactions of chlorine and bromine, Nature, 321, 759–762, https://doi.org/10.1038/321759a0, 1986.
Melamed, M. L., Monks, P. S., Goldstein, A. H., Lawrence, M. G., and Jennings, J.: The international global atmospheric chemistry (IGAC) project: Facilitating atmospheric chemistry research for 25 years, Anthropocene, 12, 17–28, https://doi.org/10.1016/j.ancene.2015.10.001, 2015.
Merrill, J. T., Bleck, R., and Avila, L.: Modeling atmospheric transport to the Marshall Islands, J. Geophys. Res.-Atmos., 90, 12927–12936, https://doi.org/10.1029/JD090iD07p12927, 1985.
Molina, L. T. and Molina, M. J.: Production of chlorine oxide (Cl2O2) from the self-reaction of the chlorine oxide (ClO) radical, The J. Phys. Chem., 91, 433–436, https://doi.org/10.1021/j100286a035, 1987.
Molina, M. J.: Heterogeneous chemistry on polar stratospheric clouds, Atmos. Environ. Pt. A, 25, 2535–2537, https://doi.org/10.1016/0960-1686(91)90170-C, 1991.
Molina, M. J. and Rowland, F. S.: Stratospheric sink for chlorofluoromethanes: Chlorine atomic-atalysed destruction of ozone, Nature, 249, 810–812, https://doi.org/10.1038/249810a0, 1974.
Molina, M. J., Tso, T. L., Molina, L. T., and Wang, F. C. Y.: Antarctic stratospheric chemistry of chlorine nitrate, hydrogen chloride, and ice: Release of active chlorine, Science, 238, 1253–1257, https://doi.org/10.1126/science.238.4831.1253, 1987.
Monks, P. S.: Gas-phase radical chemistry in the troposphere, Chem. Soc. Rev., 34, 376–395, Doi https://doi.org/10.1039/B307982c, 2005.
Monks, P. S. and Williams, M. L.: What does success look like for air quality policy? A perspective, Philos. T. R. Soc. Math. Phys. Eng. Sci., 378, 20190326, https://doi.org/10.1098/rsta.2019.0326, 2020.
Monks, P. S., Granier, C., Fuzzi, S., Stohl, A., Williams, M. L., Akimoto, H., Amann, M., Baklanov, A., Baltensperger, U., Bey, I., Blake, N., Blake, R. S., Carslaw, K., Cooper, O. R., Dentener, F., Fowler, D., Fragkou, E., Frost, G. J., Generoso, S., Ginoux, P., Grewe, V., Guenther, A., Hansson, H. C., Henne, S., Hjorth, J., Hofzumahaus, A., Huntrieser, H., Isaksen, I. S. A., Jenkin, M. E., Kaiser, J., Kanakidou, M., Klimont, Z., Kulmala, M., Laj, P., Lawrence, M. G., Lee, J. D., Liousse, C., Maione, M., McFiggans, G., Metzger, A., Mieville, A., Moussiopoulos, N., Orlando, J. J., O'Dowd, C. D., Palmer, P. I., Parrish, D. D., Petzold, A., Platt, U., Poschl, U., Prevot, A. S. H., Reeves, C. E., Reimann, S., Rudich, Y., Sellegri, K., Steinbrecher, R., Simpson, D., ten Brink, H., Theloke, J., van der Werf, G. R., Vautard, R., Vestreng, V., Vlachokostas, C., and von Glasow, R.: Atmospheric composition change – global and regional air quality, Atmos. Environ., 43, 5268–5350, https://doi.org/10.1016/j.atmosenv.2009.08.021, 2009.
Montzka, S. A., Calvert, P., Hall, B. D., Elkins, J. W., Conway, T. J., Tans, P. P., and Sweeney, C.: On the global distribution, seasonality, and budget of atmospheric carbonyl sulfide (COS) and some similarities to CO2, J. Geophys. Res.-Atmos., 112, D09302, https://doi.org/10.1029/2006JD007665, 2007.
Moody, J. L., Oltmans, S. J., Levy, H., and Merrill, J. T.: Transport, climatology of tropospheric ozone – Bermuda, 1988–1991, J. Geophys. Res.-Atmos., 100, 7179–7194, https://doi.org/10.1029/94jd02830, 1995.
Moody, J. L., Munger, J. W., Goldstein, A. H., Jacob, D. J., and Wofsy, S. C.: Harvard Forest regional-scale air mass composition by Patterns in Atmospheric Transport History (PATH), J. Geophys. Res.-Atmos., 103, 13181–13194, https://doi.org/10.1029/98jd00526, 1998.
Mozurkewich, M., McMurry , P. H., Gupta, A., and Calvert, J. G.: Mass Accomodation Coefficient for HO2 Radicals on Aqueous Particles, J. Geophys. Res., 92, 4163–4170, 1987.
Murozumi, M., Chow, T. J., and Patterson, C.: Chemical concentrations of pollutant lead aerosols, terrestrial dusts and sea salts in Greenland and Antarctic snow strata, Geochim. Cosmochim. Ac., 33, 1247–1294, https://doi.org/10.1016/0016-7037(69)90045-3, 1969.
Murphy, D. M., Cziczo, D. J., Froyd, K. D., Hudson, P. K., Matthew, B. M., Middlebrook, A. M., Peltier, R. E., Sullivan, A., Thomson, D. S., and Weber, R. J.: Single-particle mass spectrometry of tropospheric aerosol particles, J. Geophys. Res.-Atmos., 111, D23S32, https://doi.org/10.1029/2006JD007340, 2006.
Newell, R. E., Thouret, V., Cho, J. Y. N., Stoller, P., Marenco, A., and Smit, H. G.: Ubiquity of quasi-horizontal layers in the troposphere, Nature, 398, 316–319, 1999.
Norrish, R. G. W. and Neville, G. H. J.: The decomposition of ozone photosensitised by chlorine, J. Chem. Soc., 1864–1872, 1934.
O'Dowd, C. D., Bahreini, R., Flagan, R. C., Seinfeld, J. H., Hämerl, K., Pirjola, L., Kulmala, M., and Hoffmann, T.: Marine aerosol formation from biogenic iodine emissions, Nature, 417, 632–636, https://doi.org/10.1038/nature00775, 2002.
O'Dowd, C. D., Facchini, M. C., Cavalli, F., Ceburnis, D., Mircea, M., Decesari, S., Fuzzi, S., Young, J. Y., and Putaud, J. P.: Biogenically driven organic contribution to marine aerosol, Nature, 431, 676–680, https://doi.org/10.1038/nature02959, 2004.
Odèn, S.: The acidification of air precipitation and its consequences in the natural environment, NFR, 1968.
Odum, J. R., Jungkamp, T. P. W., Griffin, R. J., Flagan, R. C., and Seinfeld, J. H.: The atmospheric aerosol forming potential of whole gasoline vapor, Science., 276, 96–99, 1997.
Olivier, J. G. J., Bouwman, A. F., van der Maas, C. W. M., and Berdowski, J. J. M.: Emission database for global atmospheric research (Edgar), Environ. Monit. Ass., 31, 93–106, https://doi.org/10.1007/BF00547184, 1994.
Osthoff, H. D., Roberts, J. M., Ravishankara, A. R., Williams, E. J., Lerner, B. M., Sommariva, R., Bates, T. S., Coffman, D., Quinn, P. K., Dibb, J. E., Stark, H., Burkholder, J. B., Talukdar, R. K., Meagher, J., Fehsenfeld, F. C., and Brown, S. S.: High levels of nitryl chloride in the polluted subtropical marine boundary layer, Nat. Geosci., 1, 324–328, https://doi.org/10.1038/ngeo177, 2008.
Pales, J. C. and Keeling, C. D.: The concentration of atmospheric carbon dioxide in Hawaii, J. Geophys. Res. (1896–1977), 70, 6053–6076, https://doi.org/10.1029/JZ070i024p06053, 1965.
Palmer, P. I., Jacob, D. J., Chance, K., Martin, R. V., Spurr, R. J. D., Kurosu, T. P., Bey, I., Yantosca, R., Fiore, A., and Li, Q.: Air mass factor formulation for spectroscopic measurements from satellites: Application to formaldehyde retrievals from the Global Ozone Monitoring Experiment, J. Geophys. Res.-Atmos., 106, 14539–14550, https://doi.org/10.1029/2000jd900772, 2001.
Pankow, J. F.: An absorption model of gas/particle partitioning of organic compounds in the atmosphere, Atmos. Environ., 28, 185–188, https://doi.org/10.1016/1352-2310(94)90093-0, 1994a.
Pankow, J. F.: An absorption model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol, Atmos. Environ., 28, 189–193, https://doi.org/10.1016/1352-2310(94)90094-9, 1994b.
Paulot, F., Crounse, J. D., Kjaergaard, H. G., Kürten, A., Clair, J. M. S., Seinfeld, J. H., and Wennberg, P. O.: Unexpected epoxide formation in the gas-phase photooxidation of isoprene, Science, 325, 730–733, https://doi.org/10.1126/science.1172910, 2009.
Paulson, S. E. and Orlando, J. J.: The reactions of ozone with alkenes: An important source of HOx in the boundary layer, Geophys. Res. Lett., 23, 3727–3730, https://doi.org/10.1029/96gl03477, 1996.
Peeters, J., Nguyen, T. L., and Vereecken, L.: HOx radical regneration in the oxidation of isoprene, Phys. Chem. Chem Phys., 11, 5935–5939, 2009.
Penkett, S. A., Jones, B. M. R., Brich, K. A., and Eggleton, A. E. J.: The importance of atmospheric ozone and hydrogen peroxide in oxidising sulphur dioxide in cloud and rainwater, Atmos. Environ. (1967), 13, 123–137, https://doi.org/10.1016/0004-6981(79)90251-8, 1979.
Perner, D. and Platt, U.: Detection of nitrous acid in the atmosphere by differential optical absorption, Geophys. Res. Lett., 6, 917–920, https://doi.org/10.1029/GL006i012p00917, 1979.
Perner, D., Ehhalt, D. H., Pätz, H. W., Platt, U., Röth, E. P., and Volz, A.: OH – Radicals in the lower troposphere, Geophys. Res. Lett., 3, 466–468, https://doi.org/10.1029/GL003i008p00466, 1976a.
Perner, D., Ehhalt, D. H., Pätz, H. W., Platt, U., Röth, E. P., and Volz, A.: OH – Radicals in the lower troposphere, Geophys. Res. Lett., 3, 466–468, https://doi.org/10.1029/GL003i008p00466, 1976b.
Petit, J. R., Jouzel, J., Raynaud, D., Barkov, N. I., Barnola, J. M., Basile, I., Bender, M., Chappellaz, J., Davis, M., Delaygue, G., Delmotte, M., Kotiyakov, V. M., Legrand, M., Lipenkov, V. Y., Lorius, C., Pépin, L., Ritz, C., Saltzman, E., and Stievenard, M.: Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica, Nature, 399, 429–436, https://doi.org/10.1038/20859, 1999.
Petters, M. D. and Kreidenweis, S. M.: A single parameter representation of hygroscopic growth and cloud condensation nucleus activity, Atmos. Chem. Phys., 7, 1961–1971, https://doi.org/10.5194/acp-7-1961-2007, 2007.
Pitts, J., Van Cauwenberghe, K., Grosjean, D., Schmid, J., Fitz, D., Belser, W., Knudson, G., and Hynds, P.: Atmospheric reactions of polycyclic aromatic hydrocarbons: facile formation of mutagenic nitro derivatives, Science, 202, 515–519, https://doi.org/10.1126/science.705341, 1978.
Pitts, J., N., Sanhueza, E., Atkinson, R., Carter, W. P. L., Winer, A. M., Harris, G. W., and Plum, C. N.: An investigation of the dark formation of nitrous acid in environmental chambers, Int. J. Chem. Kin., 16, 919–939, https://doi.org/10.1002/kin.550160712, 1984.
Platt, U. and Hönninger, G.: The role of halogen species in the troposphere, Chemosphere, 52, 325–338, 2003.
Platt, U., Perner, D., and Paetz, H. W.: Simultaneous measurement of atmospheric CH2O, O3, AND NO2 by differential optical absorption, J. Geophys. Res., 84, 6329–6335, https://doi.org/10.1029/JC084iC10p06329, 1979.
Platt, U., Perner, D., Winer, A. M., Harris, G. W., and Pitts Jr, J. N.: Detection of NO3 in the polluted troposphere by differential optical absorption, Geophys. Res. Lett., 7, 89–92, https://doi.org/10.1029/GL007i001p00089, 1980.
Porter, G. and Wright, F. J.: Studies of free radical reactivity by the methods of flash photolysis. The photochemical reaction between chlorine and oxygen, Discuss. Faraday Soc., 14, 23–34, https://doi.org/10.1039/DF9531400023, 1953.
Prather, M. J.: Lifetimes and eigenstates in atmospheric chemistry, Geophys. Res. Lett., 21, 801–804, https://doi.org/10.1029/94GL00840, 1994.
Prather, M. J.: Time scales in atmospheric chemistry: Theory, GWPs for CH4 and CO, and runaway growth, Geophys. Res. Lett., 23, 2597–2600, https://doi.org/10.1029/96GL02371, 1996.
Preining, O., and Davis, E. J.: History of Aerosol Science, Symposium on the History of Aerosol Science, Vienna, Austria, 1999.
Prinn, R. G., Weiss, R. F., Miller, B. R., Huang, J., Alyea, F. N., Cunnold, D. M., Fraser, P. J., Hartley, D. E., and Simmonds, P. G.: Atmospheric trends and lifetime of CH3CCl3 and global OH concentrations, Science, 269, 187–192, https://doi.org/10.1126/science.269.5221.187, 1995.
Prinn, R. G., Huang, J., Weiss, R. F., Cunnold, D. M., Fraser, P. J., Simmonds, P. G., McCulloch, A., Harth, C., Salameh, P., O'Doherty, S., Wang, R. H. J., Porter, L., and Miller, B. R.: Evidence for substantial variations of atmospheric hydroxyl radicals in the past two decades, Science, 292, 1882–1888, https://doi.org/10.1126/science.1058673, 2001.
Prospero, J. M., Ginoux, P., Torres, O., Nicholson, S. E., and Gill, T. E.: Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product, Rev. Geophys., 40, 1002, https://doi.org/10.1029/2000RG000095, 2002.
Quinn, P. K. and Bates, T. S.: The case against climate regulation via oceanic phytoplankton sulphur emissions, Nature, 480, 51–56, https://doi.org/10.1038/nature10580, 2011.
Ramanathan, V., Cicerone, R. J., Singh, H. B., and Kiehl, J. T.: Trace gas trends and their potential role in climate change, J. Geophys. Res., 90, 5547–5566, https://doi.org/10.1029/JD090iD03p05547, 1985.
Ramanathan, V., Crutzen, P. J., Kiehl, J. T., and Rosenfeld, D.: Atmosphere – Aerosols, climate, and the hydrological cycle, Science, 294, 2119–2124, https://doi.org/10.1126/science.1064034, 2001.
Rasmussen, R. and Went, F. W.: Volatile organic matter of plant origin in the atmosphere, Science, 144, p. 566, https://doi.org/10.1126/science.144.3618.566-a, 1964.
Ravishankara, A. R.: Heterogeneous and multiphase chemistry in the troposphere, Science, 276, 1058–1065, https://doi.org/10.1126/science.276.5315.1058, 1997.
Ravishankara, A. R.: Introduction: Atmospheric ChemistryLong-Term Issues, Chem. Rev., 103, 4505–4508, https://doi.org/10.1021/cr020463i, 2003.
Ravishankara, A. R., Hancock, G., Kawasaki, M., and Matsumi, Y.: Photochemistry of ozone: Surprises and recent lessons, Science, 280, 60–61, https://doi.org/10.1126/science.280.5360.60, 1998.
Ravishankara, A. R., Dunlea, E. J., Blitz, M. A., Dillon, T. J., Heard, D. E., Pilling, M. J., Strekowski, R. S., Nicovich, J. M., and Wine, P. H.: Redetermination of the rate coefficient for the reaction of O(1D) with N2, Geophys. Res. Lett., 29, 1745–1748, https://doi.org/10.1029/2002GL014850, 2002.
Ravishankara, A. R., Rudich, Y., and Pyle, J. A.: Role of Chemistry in Earth's Climate, Chem. Rev., 115, 3679–3681, https://doi.org/10.1021/acs.chemrev.5b00226, 2015.
Read, K. A., Mahajan, A. S., Carpenter, L. J., Evans, M. J., Faria, B. V. E., Heard, D. E., Hopkins, J. R., Lee, J. D., Moller, S. J., Lewis, A. C., Mendes, L., McQuaid, J. B., Oetjen, H., Saiz-Lopez, A., Pilling, M. J., and Plane, J. M. C.: Extensive halogen-mediated ozone destruction over the tropical Atlantic Ocean, Nature, 453, 1232–1235, 2008.
Reid, J. S., Koppmann, R., Eck, T. F., and Eleuterio, D. P.: A review of biomass burning emissions part II: intensive physical properties of biomass burning particles, Atmos. Chem. Phys., 5, 799–825, https://doi.org/10.5194/acp-5-799-2005, 2005.
Remer, L. A., Kaufman, Y. J., Tanré, D., Mattoo, S., Chu, D. A., Martins, J. V., Li, R. R., Ichoku, C., Levy, R. C., Kleidman, R. G., Eck, T. F., Vermote, E., and Holben, B. N.: The MODIS aerosol algorithm, products, and validation, J. Atmos. Sci., 62, 947–973, https://doi.org/10.1175/JAS3385.1, 2005.
Reynolds, S. D., Roth, P. M., and Seinfeld, J. H.: Mathematical modeling of photochemical air pollution – I: Formulation of the model, Atmos. Environ. (1967), 7, 1033–1061, https://doi.org/10.1016/0004-6981(73)90214-X, 1973.
Reynolds, S. D., Liu, M.-K., Hecht, T. A., Roth, P. M., and Seinfeld, J. H.: Mathematical modeling of photochemical air pollution – III. Evaluation of the model, Atmos. Environ. (1967), 8, 563–596, https://doi.org/10.1016/0004-6981(74)90143-7, 1974.
Richter, A., Burrows, J. P., Nuss, H., Granier, C., and Niemeier, U.: Increase in tropospheric nitrogen dioxide over China observed from space, Nature, 437, 129–132, https://doi.org/10.1038/nature04092, 2005.
Robbin, M. L. and Damschen, D. E.: Aqueous oxidation of sulfur dioxide by hydrogen peroxide at low pH, Atmos. Environ. (1967), 15, 1615–1621, https://doi.org/10.1016/0004-6981(81)90146-3, 1981.
Robinson, A. L., Donahue, N. M., Shrivastava, M. K., Weitkamp, E. A., Sage, A. M., Grieshop, A. P., Lane, T. E., Pierce, J. R., and Pandis, S. N.: Rethinking Organic Aerosols: Semivolatile Emissions and Photochemical Aging, Science, 315, 1259–1262, https://doi.org/10.1126/science.1133061, 2007.
Roth, P. M., Roberts, P. J. W., Mei-Kao, L., Reynolds, S. D., and Seinfeld, J. H.: Mathematical modeling of photochemical air pollution – II. A model and inventory of pollutant emissions, Atmos. Environ. (1967), 8, 97–130, https://doi.org/10.1016/0004-6981(74)90023-7, 1974.
Sanadze, G. A.: The nature of gaseous substances emitted by leaves of Robinia pseudoacacia, Soobshch Akad Nauk Gruz SSR, 19, 83–86, 1957.
Sanadze, G. A. and Kursanov, A. N.: Light and temperature curves of the evolution of C5H8, Fiziol. Rast., 13, 458–461, 1966.
Sander, S. P., Friedl, R. R., and Yung, Y. L.: Rate of formation of the ClO dimer in the polar stratosphere: Implications for ozone loss, Science, 245, 1095–1098, https://doi.org/10.1126/science.245.4922.1095, 1989.
Saunders, S. M., Jenkin, M. E., Derwent, R. G., and Pilling, M. J.: Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 3, 161–180, https://doi.org/10.5194/acp-3-161-2003, 2003.
Schroeder, W. H. and Urone, P.: Formation of nitrosyl chloride from salt particles in air, Environ. Sci. Technol., 8, 756–758, https://doi.org/10.1021/es60093a015, 1974.
Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd Edn., Wiley, 2006.
Seitzinger, S. P., Gaffney, O., Brasseur, G., Broadgate, W., Ciais, P., Claussen, M., Erisman, J. W., Kiefer, T., Lancelot, C., Monks, P. S., Smyth, K., Syvitski, J., and Uematsu, M.: International Geosphere–Biosphere Programme and Earth system science: Three decades of co-evolution, Anthropocene, 12, 3–16, https://doi.org/10.1016/j.ancene.2016.01.001, 2015.
Sharkey, T. D. and Monson, R. K.: Isoprene research – 60 years later, the biology is still enigmatic, Plant Cell Environ., 40, 1671–1678, https://doi.org/10.1111/pce.12930, 2017.
Shindell, D., Kuylenstierna, J. C. I., Vignati, E., van Dingenen, R., Amann, M., Klimont, Z., Anenberg, S. C., Muller, N., Janssens-Maenhout, G., Raes, F., Schwartz, J., Faluvegi, G., Pozzoli, L., Kupiainen, K., Höglund-Isaksson, L., Emberson, L., Streets, D., Ramanathan, V., Hicks, K., Oanh, N. T. K., Milly, G., Williams, M., Demkine, V., and Fowler, D.: Simultaneously Mitigating Near-Term Climate Change and Improving Human Health and Food Security, Science, 335, 183–189, https://doi.org/10.1126/science.1210026, 2012.
Sillman, S.: The relation between ozone, NOx and hydrocarbons in urban and polluted rural environments, Atmos. Environ., 33, 1821–1845, https://doi.org/10.1016/s1352-2310(98)00345-8, 1999.
Sillman, S. and He, D.: Some theoretical results concerning O3-NOx-VOC chemistry and NOx-VOC indicators, J. Geophys. Res.-Atmos., 107, 4659, https://doi.org/10.1029/2001JD001123, 2002.
Simoneit, B. R. T., Schauer, J. J., Nolte, C. G., Oros, D. R., Elias, V. O., Fraser, M. P., Rogge, W. F., and Cass, G. R.: Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles, Atmos. Environ., 33, 173–182, https://doi.org/10.1016/S1352-2310(98)00145-9, 1999.
Singer, B. C. and Harley, R. A.: A Fuel-Based Motor Vehicle Emission Inventory, J. Air Waste Manag. Assoc., 46, 581–593, https://doi.org/10.1080/10473289.1996.10467492, 1996.
Singh, H. B.: Preliminary estimation of average tropospheric HO concentrations in the northern and southern hemispheres, Geophys. Res. Lett., 4, 453–456, https://doi.org/10.1029/GL004i010p00453, 1977.
Singh, H. B. and Hanst, P. L.: Peroxyacetyl nitrate (PAN) in the unpolluted atmosphere: An important reservoir for nitrogen oxides, Geophys. Res. Lett., 8, 941–944, https://doi.org/10.1029/GL008i008p00941, 1981.
Sinha, M. P.: Laser-induced volatilization and ionization of microparticles, Rev. Sci. Instr., 55, 886–891, https://doi.org/10.1063/1.1137851, 1984.
Solomon, S., Garcia, R. R., Rowland, F. S., and Wuebbles, D. J.: On the depletion of Antarctic ozone, Nature, 321, 755–758, https://doi.org/10.1038/321755a0, 1986.
Spicer, C. W., Chapman, E. G., Finlayson-Pitts, B. J., Plastridge, R. A., Hubbe, J. M., Fast, J. D., and Berkowitz, C. M.: Unexpectedly high concentrations of molecular chlorine in coastal air, Nature, 394, 353–356, https://doi.org/10.1038/28584, 1998.
Spivakovsky, C. M., Logan, J. A., Montzka, S. A., Balkanski, Y. J., Foreman-Fowler, M., Jones, D. B. A., Horowitz, L. W., Fusco, A. C., Brenninkmeijer, C. A. M., Prather, M. J., Wofsy, S. C., and McElroy, M. B.: Three-dimensional climatological distribution of tropospheric OH: Update and evaluation, J. Geophys. Res.-Atmos., 105, 8931–8980, https://doi.org/10.1029/1999JD901006, 2000.
Sportisse, B.: Fundamentals in Air Pollution, Springer, 293 pp., 2010.
Stelson, A. W., Friedlander, S. K., and Seinfeld, J. H.: A note on the equilibrium relationship between ammonia and nitric acid and particulate ammonium nitrate, Atmos. Environ. (1967), 13, 369–371, https://doi.org/10.1016/0004-6981(79)90293-2, 1979.
Stelson, A. W. and Seinfeld, J. H.: Thermodynamic prediction of the water activity, NH4NO3 dissociation constant, density and refractive index for the NH4NO3-(NH4)2SO4-H2O system at 25 ∘C, Atmos. Environ. (1967), 16, 2507–2514, https://doi.org/10.1016/0004-6981(82)90142-1, 1982.
Stern, A. C.: Air Pollution, 2nd Edn., Academic Press, New York, 1968.
Stevens, P. S., Mather, J. H., and Brune, W. H.: Measurement of tropospheric OH and HO2 by laser-induced fluorescence at low pressure, J. Geophys. Res., 99, 3543–3557, https://doi.org/10.1029/93JD03342, 1994.
Stevenson, D. S., Dentener, F. J., Schultz, M. G., Ellingsen, K., van Noije, T. P. C., Wild, O., Zeng, G., Amann, M., Atherton, C. S., Bell, N., Bergmann, D. J., Bey, I., Butler, T., Cofala, J., Collins, W. J., Derwent, R. G., Doherty, R. M., Drevet, J., Eskes, H. J., Fiore, A. M., Gauss, M., Hauglustaine, D. A., Horowitz, L. W., Isaksen, I. S. A., Krol, M. C., Lamarque, J. F., Lawrence, M. G., Montanaro, V., Muller, J. F., Pitari, G., Prather, M. J., Pyle, J. A., Rast, S., Rodriguez, J. M., Sanderson, M. G., Savage, N. H., Shindell, D. T., Strahan, S. E., Sudo, K., and Szopa, S.: Multimodel ensemble simulations of present-day and near-future tropospheric ozone, J. Geophys. Res.-Atmos., 111, D08301, https://doi.org/10.1029/2005jd006338, 2006.
Stockwell, W. R., Middleton, P., Chang, J. S., and Tang, X.: The second generation regional acid deposition model chemical mechanism for regional air quality modeling, J. Geophys. Res.-Atmos., 95, 16343–16367, https://doi.org/10.1029/JD095iD10p16343, 1990.
Stockwell, W. R., Kirchner, F., Kuhn, M., and Seefeld, S.: A new mechanism for regional atmospheric chemistry modeling, J. Geophys. Res.-Atmos., 102, 25847–25879, https://doi.org/10.1029/97jd00849, 1997.
Stohl, A. and Trickl, T.: A text book example of long range transport: simultaneous observation of ozone maxima of stratospheric and North American origin in the FT over Europe, J. Geophys. Res., 104, 30445–430462, 1999.
Stohl, A., Eckhardt, S., Forster, C., James, P., and Spichtinger, N.: On the pathways and timescales of intercontinental air pollution transport, J. Geophys. Res.-Atmos., 107, 4684, https://doi.org/10.1029/2001JD001396, 2002.
Stohl, A., Bonasoni, P., Cristofanelli, P., Collins, W., Feichter, J., Frank, A., Forster, C., Gerasopoulos, E., Gäggeler, H., James, P., Kentarchos, T., Kromp-Kolb, H., Krüger, B., Land, C., Meloen, J., Papayannis, A., Priller, A., Seibert, P., Sprenger, M., Roelofs, G. J., Scheel, H. E., Schnabel, C., Siegmund, P., Tobler, L., Trickl, T., Wernli, H., Wirth, V., Zanis, P., and Zerefos, C.: Stratosphere-troposphere exchange: A review, and what we have learned from STACCATO, J. Geophys. Res.-Atmos., 108, 8516, https://doi.org/10.1029/2002jd002490, 2003.
Stohl, A., Forster, C., Frank, A., Seibert, P., and Wotawa, G.: Technical note: The Lagrangian particle dispersion model FLEXPART version 6.2, Atmos. Chem. Phys., 5, 2461–2474, https://doi.org/10.5194/acp-5-2461-2005, 2005.
Stolarski, R. S. and Cicerone, R. J.: Stratospheric Chlorine: a Possible Sink for Ozone, Can. J. Chem., 52, 1610–1615, https://doi.org/10.1139/v74-233, 1974.
Stull, R. B.: An Introduction to Boundary Layer Meteorology, Kluwer Academic, Dordrecht, 1988.
Surratt, J. D., Chan, A. W. H., Eddingsaas, N. C., Chan, M., Loza, C. L., Kwan, A. J., Hersey, S. P., Flagan, R. C., Wennberg, P. O., and Seinfeld, J. H.: Reactive intermediates revealed in secondary organic aerosol formation from isoprene, P. Natl. Acad. Sci. USA, 107, 6640–6645, https://doi.org/10.1073/pnas.0911114107, 2010.
Sutton, M. A., Howard, C. M., Erisman, J. W., Billen, G., Bleeker, A., Grenfelt, P., Grinsven, H. v., and Grizzetti, B.: The European Nitrogen Assessment: Sources, Effects and Policy Perspectives, Cambridge University Press, Cambridge, 664 pp., 2011.
Thompson, A. M., Doddridge, B. G., Witte, J. C., Hudson, R. D., Luke, W. T., Johnson, J. E., Johnson, B. J., Oltmans, S. J., and Weller, R.: A tropical atlantic paradox: Shipboard and satellite views of a tropospheric ozone maximum and wave-one in January-February 1999, Geophys. Res. Lett., 27, 3317–3320, https://doi.org/10.1029/1999GL011273, 2000.
Thompson, A. M., Witte, J. C., Oltmans, S. J., Schmidlin, F. J., Logan, J. A., Fujiwara, M., Kirchhoff, V. W. J. H., Posny, F., Coetzee, G. J. R., Hoegger, B., Kawakami, S., Ogawa, T., Fortuin, J. P. F., and Kelder, H. M.: Southern Hemisphere Additional Ozonesondes (SHADOZ) 1998–2000 tropical ozone climatology 2. Tropospheric variability and the zonal wave-one, J. Geophys. Res.-Atmos., 108, 8241, https://doi.org/10.1029/2002JD002241, 2003.
Thornton, J. and Abbatt, J. P. D.: Measurements of HO2 uptake to aqueous aerosol: Mass accomodation coefficients and net reactive loss, J. Geophys. Res.-Atmos., 110, 1-12, https://doi.org/10.1029/2004JD005402, 2005.
Thouret, V., Marenco, A., Logan, J. A., Nédélec, P., and Grouhel, C.: Comparisons of ozone measurements from the MOZAIC airborne program and the ozone sounding network at eight locations, J. Geophys. Res.-Atmos., 103, 25695–25720, https://doi.org/10.1029/98JD02243, 1998.
Tingey, D. T., Manning, M., Grothaus, L. C., and Burns, W. F.: The Influence of Light and Temperature on Isoprene Emission Rates from Live Oak, Physiol. Plantarum, 47, 112–118, https://doi.org/10.1111/j.1399-3054.1979.tb03200.x, 1979.
Tolbert, M. A., Rossi, M. J., Malhotra, R., and Golden, D. M.: Reaction of chlorine nitrate with hydrogen chloride and water at antarctic stratospheric temperatures, Science, 238, 1258–1260, https://doi.org/10.1126/science.238.4831.1258, 1987.
Troe, J.: Predictive possibilities of unimolecular rate theory, J. Phys. Chem., 83, 114–126, https://doi.org/10.1021/j100464a019, 1979.
Troe, J.: The Polanyi lecture. The colourful world of complex-forming bimolecular reactions, Journal of the Chemical Society, Farad. Trans., 90, 2303–2317, https://doi.org/10.1039/FT9949002303, 1994.
Trolier, M., Mauldin Iii, R. L., and Ravishankara, A. R.: Rate coefficient for the termolecular channel of the self-reaction of ClO, J. Phys. Chem., 94, 4896–4907, https://doi.org/10.1021/j100375a027, 1990.
Turco, R. P., Toon, O. B., Ackerman, T. P., Pollack, J. B., and Sagan, C.: Nuclear winter: Global consequences of multiple nuclear explosions, Science, 222, 1283–1292, https://doi.org/10.1126/science.222.4630.1283, 1983.
Twomey, S.: Pollution and the planetary albedo, Atmos. Environ. (1967), 8, 1251-1256, https://doi.org/10.1016/0004-6981(74)90004-3, 1974.
Twomey, S.: The Influence of Pollution on the Shortwave Albedo of Clouds, J. Atmos. Sci., 34, 1149–1152, 1977.
Tyndall, J.: The Bakerian Lecture: On the Absorption and Radiation of Heat by Gases and Vapours, and on the Physical Connexion of Radiation, Absorption, and Conduction, Philos. T. Ro. Soc. Lond., 151, 1–36, 1861.
Urone, P. and Schroeder, W. H.: SO2 in the atmosphere: A wealth of monitoring data, but few reaction rate studies, Environ. Sci. Technol., 3, 436–445, https://doi.org/10.1021/es60028a006, 1969.
Vaghjiani, G. L. and Ravishankara, A. R.: New measurement of the rate coefficient for the reaction of OH with methane, Nature, 350, 406–409, https://doi.org/10.1038/350406a0, 1991.
van der Werf, G. R., Randerson, J. T., Giglio, L., Collatz, G. J., Kasibhatla, P. S., and Arellano Jr., A. F.: Interannual variability in global biomass burning emissions from 1997 to 2004, Atmos. Chem. Phys., 6, 3423–3441, https://doi.org/10.5194/acp-6-3423-2006, 2006.
Van Der Werf, G. R., Randerson, J. T., Giglio, L., Collatz, G. J., Mu, M., Kasibhatla, P. S., Morton, D. C., Defries, R. S., Jin, Y., and Van Leeuwen, T. T.: Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009), Atmos. Chem. Phys., 10, 11707–11735, https://doi.org/10.5194/acp-10-11707-2010, 2010.
van Donkelaar, A., Martin, R. V., and Park, R. J.: Estimating ground-level PM2.5 using aerosol optical depth determined from satellite remote sensing, J. Geophys. Res.-Atmos., 111, D21201, https://doi.org/10.1029/2005jd006996, 2006.
Virtanen, A., Joutsensaari, J., Koop, T., Kannosto, J., Yli-Pirilä, P., Leskinen, J., Mäkelä, J. M., Holopainen, J. K., Pöschl, U., Kulmala, M., Worsnop, D. R., and Laaksonen, A.: An amorphous solid state of biogenic secondary organic aerosol particles, Nature, 467, 824–827, https://doi.org/10.1038/nature09455, 2010.
Vogt, R., Crutzen, P. J., and Sander, R.: A mechanism for halogen release from sea-salt aerosol in the remote marine boundary layer, Nature, 383, 327–330, 1996.
Volz, A. and Kley, D.: Evaluation of the Montsouris Series of Ozone Measurements Made in the 19th-Century, Nature, 332, 240–242, 1988.
von Glasow, R.: Pollution meets sea salt, Nat. Geosci., 1, 292, https://doi.org/10.1038/ngeo192, 2008.
von Glasow, R. and Crutzen, P. J.: Tropospheric halogen chemistry in: Treatise on Geochemistry, edited by: Holland, H. D. and Turekian, K. K., Elsevier-Pergamon, Oxford, 1–67, 2007.
von Schneidemesser, E., Monks, P. S., Allan, J. D., Bruhwiler, L., Forster, P., Fowler, D., Lauer, A., Morgan, W. T., Paasonen, P., Righi, M., Sindelarova, K., and Sutton, M. A.: Chemistry and the Linkages between Air Quality and Climate Change, Chem. Rev., 115, 3856–3897, https://doi.org/10.1021/acs.chemrev.5b00089, 2015.
Wang, C. C., Davis, L. I., Wu, C. H., Japar, S., Niki, H., and Weinstock, B.: Hydroxyl Radical Concentrations Measured in Ambient Air, Science, 189, 797–800, https://doi.org/10.1126/science.189.4205.797, 1975.
Wang, J. and Christopher, S. A.: Intercomparison between satellite-derived aerosol optical thickness and PM2.5 mass: Implications for air quality studies, Geophys. Res. Lett., 30, 2095, https://doi.org/10.1029/2003GL018174, 2003.
Wayne, R. P.: Chemistry of Atmospheres, 3rd Edn., Oxford University Press, Oxford, 2000.
Wayne, R. P., Barnes, I., Biggs, P., Burrows, J. P., Canosa-mas, C. E., Hjorth, J., LeBras, G., Moortgat, G. K., Perner, D., Poulet, G., Restelli, G., and Sidebottom, H.: The Nitrate Radical – Physics, Chemistry, and the Atmosphere, Atmos. Environ. Pt. A, 25, 1–203, 1991.
Weinstock, B.: Carbon monoxide: Residence time in the atmosphere, Science, 166, 224–225, https://doi.org/10.1126/science.166.3902.224, 1969.
Welz, O., Savee, J. D., Osborn, D. L., Vasu, S. S., Percival, C. J., Shallcross, D. E., and Taatjes, C. A.: Direct kinetic measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2, Science, 335, 204–207, 2012.
Went, F. W.: Blue Hazes in the Atmosphere, Nature, 187, 641–643, https://doi.org/10.1038/187641a0, 1960.
Wesely, M. L.: Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models, Atmos. Environ. (1967), 23, 1293–1304, https://doi.org/10.1016/0004-6981(89)90153-4, 1989.
Whitby, K. T.: The physical characteristics of sulfur aerosols, Atmos. Environ. (1967), 12, 135–159, https://doi.org/10.1016/0004-6981(78)90196-8, 1978.
Wild, O., Zhu, X., and Prather, M. J.: Fast-J: Accurate simulation of in- and below-cloud photolysis in tropospheric chemical models, J. Atmos. Chem., 37, 245–282, https://doi.org/10.1023/A:1006415919030, 2000.
Williams, M.: Air pollution and policy – 1952–2002, Sci. Total Environ., 334/335, 15–20, https://doi.org/10.1016/j.scitotenv.2004.04.026, 2004.
WMO: WMO Global Atmosphere Watch (GAW) Implementation Plan: 2016–2023, WMO, Geneva, 2017.
Yienger, J. J. and Levy II, H.: Empirical model of global soil-biogenic NOχ emissions, J. Geophys. Res.-Atmos., 100, 11447–11464, https://doi.org/10.1029/95jd00370, 1995.
Zhang, Q., Jimenez, J. L., Canagaratna, M. R., Allan, J. D., Coe, H., Ulbrich, I., Alfarra, M. R., Takami, A., Middlebrook, A. M., Suni, Y. L., Dzepina, K., Dunlea, E., Docherty, K., DeCarlo, P. F., Salcedo, D., Onasch, T., Jayne, J., Miyoshi, T., Shimono, A., Hatakeyama, S., Takegawa, N., Kondo, Y., Schneider, J., Drewnick, F., Borrmann, S., Weimer, S., Demerjian, K., Williams, P., Bower, K., Bahreini, R., Cottrell, L., Griffin, R. J., Rautiainen, J., Sun, J. Y., Zhang, Y. M., and Worsnop, D. R.: Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes, Geophys. Res. Lett., 34, L13801, https://doi.org/10.1029/ 2007GL029979, 2007.
Zhang, Y.: Online-coupled meteorology and chemistry models: history, current status, and outlook, Atmos. Chem. Phys., 8, 2895–2932, https://doi.org/10.5194/acp-8-2895-2008, 2008.
The term “hydrocarbons”, although often used interchangeably with VOCs, does not describe the same group of compounds. Hydrocarbons are a subset of VOCs that exclusively contain hydrogen and carbon, thereby including no, for instance, oxygenated VOC species.