the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Heterogeneous OH oxidation of isoprene-epoxydiol-derived organosulfates: kinetics, chemistry and formation of inorganic sulfate
Hoi Ki Lam
Kai Chung Kwong
Hon Yin Poon
James F. Davies
Zhenfa Zhang
Avram Gold
Jason D. Surratt
Man Nin Chan
Acid-catalyzed multiphase chemistry of epoxydiols formed from isoprene oxidation yields the most abundant organosulfates (i.e., methyltetrol sulfates) detected in atmospheric fine aerosols in the boundary layer. This potentially determines the physicochemical properties of fine aerosols in isoprene-rich regions. However, chemical stability of these organosulfates remains unclear. As a result, we investigate the heterogeneous oxidation of aerosols consisting of potassium 3-methyltetrol sulfate ester (C5H11SO7K) by gas-phase hydroxyl (OH) radicals at a relative humidity (RH) of 70.8 %. Real-time molecular composition of the aerosols is obtained by using a Direct Analysis in Real Time (DART) ionization source coupled to a high-resolution mass spectrometer. Aerosol mass spectra reveal that 3-methyltetrol sulfate ester can be detected as its anionic form () via direct ionization in the negative ionization mode. Kinetic measurements reveal that the effective heterogeneous OH rate constant is measured to be cm3 molecule−1 s−1 with a chemical lifetime against OH oxidation of 16.2±0.3 days, assuming an OH radical concentration of 1.5×106 molecules cm−3. Comparison of this lifetime with those against other aerosol removal processes, such as dry and wet deposition, suggests that 3-methyltetrol sulfate ester is likely to be chemically stable over atmospheric timescales. Aerosol mass spectra only show an increase in the intensity of bisulfate ion () after oxidation, suggesting the importance of fragmentation processes. Overall, potassium 3-methyltetrol sulfate ester likely decomposes to form volatile fragmentation products and aqueous-phase sulfate radial anion (). subsequently undergoes intermolecular hydrogen abstraction to form . These processes appear to explain the compositional evolution of 3-methyltetrol sulfate ester during heterogeneous OH oxidation.
- Article
(409 KB) - Full-text XML
-
Supplement
(708 KB) - BibTeX
- EndNote
Isoprene (2-methyl-1,3-butadiene, C5H8), emitted from terrestrial vegetation to the atmosphere, is the largest atmospheric source of non-methane volatile organic compounds. Apart from enhancing urban ozone levels via photochemical oxidation initiated by gas-phase hydroxyl (OH) radicals (Chameides et al., 1988), isoprene-derived oxidation products can also significantly contribute to the formation of secondary organic aerosol (SOA) (Carlton et al., 2009). Gas-phase photochemical oxidation of isoprene by OH radicals can produce isoprene-derived hydroxyhydroperoxides (ISOPOOH) in yields greater than 70 % under low nitrogen oxide (NOX) conditions (Paulot et al., 2009). Further reactions of ISOPOOH with OH radicals yield large quantities of isomeric isoprene epoxydiols (IEPOX), which partition into aqueous sulfate aerosols through acid-catalyzed ring-opening reactions. This multiphase chemical pathway is key for the substantial production of isoprene-derived SOA constituents (e.g., 2-methyltetrols, organosulfates, 3-methyltetrahydrofuran-3,4-diols and oligomers) within atmospheric fine particulate matter (PM2.5) (Carlton et al., 2009; Froyd et al., 2010; Surratt et al., 2010; Lin et al., 2012).
Among these SOA constituents, IEPOX-derived organosulfates (e.g., methyltetrol sulfates) have been widely detected in atmospheric aerosols and are estimated to account for 0.3 %–1.7 % of the total organic carbon (Chan et al., 2010; Froyd et al., 2010; Hatch et al., 2011; Lin et al., 2012; Stone et al., 2012; He et al., 2014; Budisulistiorini et al., 2015; Rattanavaraha et al., 2016; Meade et al., 2016; Hettiyadura et al., 2017). While the formation mechanisms of organosulfates have been extensively studied (Surratt et al., 2007, 2008; Minerath et al., 2009; Cole-Filipiak et al., 2010; Nozière et al., 2010; Lin et al., 2012; Nguyen et al., 2014), their chemical transformations and stability remain unclear. These low-volatility organosulfates are preferentially present in particle phase and can be oxidized by gas-phase oxidants (e.g., OH radicals, ozone and nitrate radicals) at or near the aerosol surface throughout their atmospheric lifetimes. The heterogeneous oxidative processes can change the size, composition and physicochemical properties (e.g., light scattering and absorption, water uptake and cloud condensation nuclei activity) of both laboratory-generated and atmospheric organic aerosols (Rudich et al., 2007; George and Abbatt, 2010; Kroll et al., 2015). However, the extent of heterogeneous oxidation of organosulfates has not been clearly examined to date. Therefore, a better understanding of particle-phase transformations of isoprene-derived organosulfates can provide more insights on their potential impacts on human health, air quality and climate.
Table 1Chemical structure, properties, effective heterogeneous OH rate constant and atmospheric lifetime against the OH radical of potassium 3-methyltetrol sulfate ester.
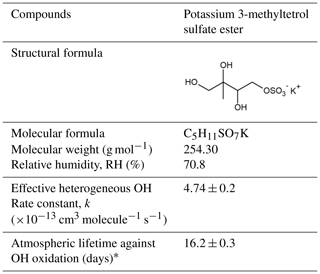
* Using a 24 h average OH concentration of 1.5×106 molecules cm−3.
In this work, we investigate the heterogeneous OH oxidation of potassium 3-methyltetrol sulfate ester (C5H11SO7K, Table 1) as a single-component aerosol system by using an aerosol flow tube reactor at 70.8 % RH in order to gain a more fundamental understanding of the kinetics and chemistry. The molecular composition of the aerosols before and after oxidation is characterized in real time using a soft atmospheric pressure ionization source (Direct Analysis in Real Time, DART) coupled to a high-resolution mass spectrometer. The 3-methyltetrol sulfate ester investigated in this study is one of the isomers of the methyltetrol sulfates found in atmospheric aerosols, which are collectively the most abundant particulate organosulfates (Budisulistiorini et al., 2015). On the basis of aerosol mass spectra and previously reported reaction pathways, oxidative kinetics and reaction products resulting from the heterogeneous OH oxidation of 3-methyltetrol sulfate ester are discussed. We acknowledge that although 3-methyltetrol sulfate ester derived from the reactive uptake of gas-phase δ-IEPOX onto sulfate seed aerosols is not the sole contributor to IEPOX-derived organosulfates (Cui et al., 2019), the findings of this work provide a basis for understanding the heterogeneous OH reactivity of other IEPOX-derived organosulfates (e.g., 2-methyltetrol sulfate esters) that predominate in atmospheric aerosols better.
The heterogeneous OH oxidation experiments were carried out in an aerosol flow tube reactor at 70.8 % RH. The synthesis of potassium 3-methyltetrol sulfate ester has been described in the literature (Bondy et al., 2018). The experimental details of the oxidation experiment have been explained elsewhere (Chim et al., 2017a, b). Briefly, 3-methyltetrol sulfate ester aerosols were generated by an atomizer (TSI, model 3076) and mixed with nitrogen, oxygen, ozone and hexane before entering the reactor. Inside the reactor, the aerosols were oxidized heterogeneously by gas-phase OH radicals, which were generated by the photolysis of ozone under ultraviolet light at 254 nm in the presence of water vapor. The RH within the reactor was controlled by varying the dry ∕ wet gas ratio. A water jacket was used to maintain a stable temperature of 20 ∘C inside the reactor. The OH concentration was controlled by varying the ozone concentration. By measuring the decay of hexane using a gas chromatograph coupled with a flame ionization detector, the OH exposure, which is an integral of gas-phase OH radical concentration and reaction time, can be calculated (Smith et al., 2009; Davies and Wilson, 2015):
where [Hex]o and [Hex] are the hexane concentration before and after OH oxidation, respectively, t is the reaction time (or aerosol residence time), which was measured to be 1.3 min, kHex is the rate constant for gas-phase OH reaction with hexane and [OH] is the time-averaged OH radical concentration. The OH exposure varied from 0 to molecules cm−3 s. An annular Carulite catalyst denuder and an activated charcoal denuder were used to remove ozone and gas-phase species from the aerosol stream leaving the reactor, respectively. As a result, only particle-phase products are detected. A fraction of the aerosol stream was sampled by a scanning mobility particle sizer (SMPS, TSI, CPC Model 3775, Classifier Model 3081) to measure the aerosol size and number distribution. The surface-weighted diameter of the aerosols was measured to be 225.9±1.4 nm before oxidation. The remaining flow was then directed into a stainless steel tube heater at 380–400 ∘C, where the temperature and aerosol residence time in the heater were sufficient to completely vaporize the aerosols. The gas-phase species were then directed into the ionization region, an open narrow space between a DART ionization source (IonSense: DART SVP) and an atmospheric inlet of a high-resolution mass spectrometer (ThermoFisher, Q Exactive Orbitrap) for real-time chemical characterization (Chan et al., 2013). The DART ionization source was operated in a negative ion mode, with helium as the ionizing gas (Cody et al., 2005). Metastable helium atoms generated were responsible for ionizing the gas-phase species in the ionization region. 3-methyltetrol sulfate ester can be ionized via direct ionization (Block et al., 2010; Hajslova et al., 2011). Most recently, Kwong et al. (2018) have detected the ionic form of two organosulfates (sodium salts of methyl sulfate (CH3SO4Na) and ethyl sulfate (C2H5SO4Na)) using the DART ionization source in negative ionization mode. Mass spectra were scanned over a range of m∕z 70–700. Each mass spectrum was averaged over a 2–3 min sampling time, with a mass resolution of about 140 000. The mass spectra were analyzed using Xcalibar software (Xcalibar Software, Inc., Herndon, VA, USA).
Particle phase state (e.g., solid or aqueous droplet) is known to play an important role in governing the heterogeneous kinetics and chemistry of organic aerosols (McNeil et al., 2008; Renbaum and Smith, 2009; Chan et al., 2014; Slade and Knopf, 2014; Zhang et al., 2018). The hygroscopicity of potassium 3-methyltetrol sulfate ester aerosols has not been experimentally determined. Recently, Estillore et al. (2016) have measured the hygroscopicity of a diverse set of organosulfates, including potassium salts of glycolic acid sulfate, hydroxyacetone sulfate, 4-hydroxy-2,3-epoxybutane sulfate and 2-butenediol sulfate as well as sodium salts of methyl sulfate, ethyl sulfate, propyl sulfate and benzyl sulfate. According to Estillore et al. (2016), these organosulfate aerosols did not show a distinct phase transition but absorbed or desorbed water reversibly when the RH increased or decreased, suggesting that these organosulfate aerosols were likely to be aqueous when RH was above 10 %. Based on the literature results, we assume that potassium 3-methyltetrol sulfate ester exhibits hygroscopicity similar to the potassium salts of organosulfates reported by Estillore et al. (2016) (e.g., potassium 4-hydroxy-2,3-epoxybutane sulfate, C4H7SO6K) and remains aqueous prior to oxidation.
Volatilization of 3-methyltetrol sulfate ester and the impact of ozone and UV light on the aerosol composition were investigated in the absence of OH. The intensity of parent ions with aerosols removed from the gas stream was less than 5 % of that measured in the presence of 3-methyltetrol sulfate ester aerosols, suggesting that the volatilization of 3-methyltetrol sulfate ester is insignificant. No reaction product was observed in the presence of ozone without UV light or in the absence of ozone with the UV light, suggesting that 3-methyltetrol sulfate ester is not likely to be photolyzed or react with ozone under our experimental conditions.
3.1 Aerosol mass spectra
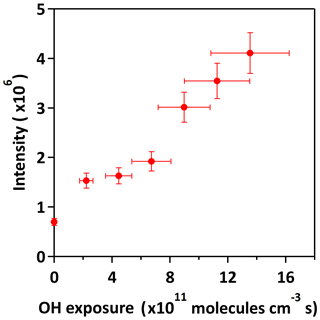
Figure 1 shows the data measured before and after oxidation at 70.8 % RH. Before oxidation (Fig. 1a), a dominant ion peak at m∕z 215 is observed, which corresponds to the ionic form of potassium 3-methyltetrol sulfate ester () (Table 1). After oxidation (Fig. 1b), the parent ion remains the most dominant ion peak at the maximum OH exposure of molecules cm−3 s. There is no significant change in the ion intensity except for bisulfate ion (; m∕z 97). Figure 2 shows the evolution of against OH exposure at 70.8 %, which indicates that the intensity of increases with the OH exposure. In the following sections, the kinetics and chemistry will be discussed based on the aerosol mass spectra and aerosol-phase reactions previously proposed in the literature.
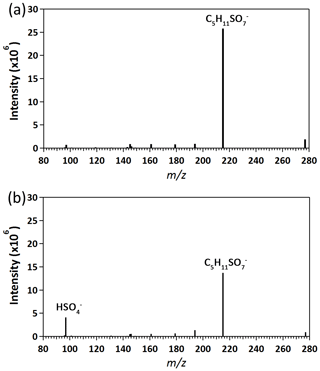
Figure 1Aerosol mass spectra of potassium 3-methyltetrol sulfate ester before (a) and after (b) OH oxidation at 70.8 % RH.
3.2 Oxidation kinetics
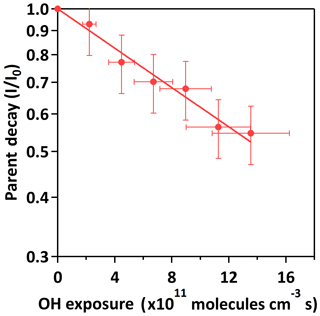
Oxidation kinetics can be quantified by analyzing the parent decay of 3-methyltetrol sulfate ester against the OH exposure. Figure 3 shows the normalized decay of 3-methyltetrol sulfate ester against OH exposure. At the maximum OH exposure ( molecules cm−3 s), ∼45 % of 3-methyltetrol sulfate ester is oxidized. The decay of the 3-methyltetrol sulfate ester can be fitted with an exponential function to obtain an effective second-order heterogeneous OH rate constant (k) through Eq. (2) (Smith et al., 2009):
where I is the ion signal at a given OH exposure, Io is the ion signal before oxidation, [OH] is the concentration of gas-phase OH radicals and t is the reaction time. The k is determined to be cm3 molecule−1 s−1 (Table 1). Based on the fitted k value, the chemical lifetime of 3-methyltetrol sulfate ester against heterogeneous OH oxidation (τ) can be estimated by Eq. (3):
where [OH] is the 24 h averaged OH radical concentration of 1.5×106 molecules cm−3. The chemical lifetime against oxidation is calculated to be 16.2±0.3 days. The estimated timescales are longer than those of other important aerosol removal processes, such as dry and wet deposition (∼7–10 days) (Seinfeld and Pandis, 2016). In addition to heterogeneous oxidation, organosulfates can undergo hydrolysis to form polyols and sulfuric acid, with rates depending on their molecular structure and aerosol acidity (Darer et al., 2011; Hu et al., 2011). According to Darer et al. (2011), primary isoprene-derived organosulfates are stable against hydrolysis, even at low pH, while secondary and tertiary organosulfates are less thermodynamically stable than primary organosulfates. Since 3-methyltetrol sulfate ester is a primary organosulfate (Table 1), it is unlikely to hydrolyze. With reference to the literature results and our new experimental observations, 3-methyltetrol sulfate ester may possibly be considered chemically stable against heterogeneous OH oxidation and hydrolysis over atmospheric timescales.
3.3 Proposed reaction mechanisms
Based on the aerosol mass spectra and well-known aerosol-phase reactions previously reported in the literature (George and Abbatt, 2010; Kroll et al., 2015), we tentatively propose reaction mechanisms for the heterogeneous OH oxidation of 3-methyltetrol sulfate ester. The reaction schemes proposed can be found in the Supplement (Schemes S1–S5). Briefly, potassium methyltetrol sulfate ester likely dissociates and exists in its ionic form in the droplets. In the first oxidation step, the OH radical abstracts a hydrogen atom to form an alkyl radical, which quickly reacts with oxygen to form a peroxy radical. We propose that the formation of alkoxy radical may be favored over the Russell mechanism (Russell, 1957) and Bennett–Summers reactions (Bennett and Summers, 1974) as functionalization products were not detected. Alkoxy radicals, once formed, may tend to undergo fragmentation due to the presence of vicinal hydroxyl groups, which lower the activation energy required for the decomposition of the alkoxy radicals (Cheng et al., 2015; Wiegel et al., 2015; Jimenez et al., 2009; Peeters et al., 2004; Vereecken and Peeters, 2009).
Sulfate radical anion () can be formed through the decomposition of the alkoxy radical and is a strong oxidant in aqueous phase (Neta et al., 1988; Clifton and Huie, 1989; Padmaja et al., 1993). can abstract a hydrogen atom from a neighboring organic molecule (e.g., unreacted 3-methyltetrol sulfate ester) to form (Reaction R1) or react with particle-phase water to yield a and an OH radical (Reaction R2) (Tang et al., 1988) as illustrated below. It is noted that or OH radical recycled from Reaction (R2) can react with 3-methyltetrol sulfate ester, contributing to the secondary chain reactions.
Since 3-methyltetrol sulfate ester is unlikely to hydrolyze (Darer et al., 2011), the formation of the upon OH oxidation could be best explained by the formation and subsequent reactions of .
Based on the proposed reaction mechanisms, the decomposition of alkoxy radicals can lead to formation fragmentation products (without sulfate group) and smaller organosulfates. We acknowledge that the ionization efficiency and detection limit of the reaction products are not fully understood. The absence of the potential products might attribute to the DART ionization and detection issues. More work is needed to investigate the formation and abundance of the reaction products formed upon oxidation in order to better understand the reaction pathways. It is also noted that the formation of second- or higher-generation products is possible due to the high OH concentrations used in this study. As the potential second- or higher-generation products have not been detected, possibly due to their low concentrations and/or ionization issues, for clarity, the formation and oxidation of these higher-generation products are not discussed further.
This work investigates the kinetics of oxidation and molecular transformations of potassium 3-methyltetrol sulfate ester resulting from heterogeneous OH oxidation. Kinetic measurements reveal that the chemical lifetime of 3-methyltetrol sulfate ester against heterogeneous OH oxidation and hydrolysis is longer than those against other aerosol removal processes, such as dry and wet deposition. 3-methyltetrol sulfate ester is potentially chemically stable over its atmospheric lifetime. In the atmosphere where particles may contain compounds with different surface-active properties, the chemical lifetime could be affected due to the inhomogeneity in surface concentration. If surface-active compounds are present, the chemical lifetime will likely be longer due to a lower surface concentration of the parent molecules. This reduces the collision probability between gas-phase OH radicals and parent molecules at the particle surface, leading to a smaller overall oxidation rate.
Aerosol mass spectra reveal that only the intensity of increases after oxidation, suggesting the dominance of fragmentation processes over functionalization processes. During oxidation, alkoxy radicals are likely to be formed following hydrogen abstraction of 3-methyltetrol sulfate ester by OH radicals. The alkoxy radicals subsequently fragment into volatile products and . undergoes intermolecular hydrogen abstraction to form in the aerosol phase, while the volatile fragmentation products may tend to partition into the gas phase. It is also noted that volatile fragmentation products likely contain polar functional groups. They may partition back to aerosols, for instance, aqueous droplets because of their high water solubilities or Henry's law constants. Additionally, they could be reactively uptaken by aerosols which contain reactive nitrogen or oxygen species through reactions. Smaller organosulfates have not been observed, possibly due to the rapid continuous decomposition of the reaction intermediates proposed in Schemes S1–S5. The absence of smaller organosulfates suggests that the oxidation of 3-methyltetrol sulfate ester is not a source of smaller organosulfates detected in atmospheric aerosols. Further investigations into whether large organosulfates yield smaller organosulfates upon heterogeneous OH oxidation are desirable. Aerosol mass spectra have revealed that the OH oxidation of 3-methyltetrol sulfate ester can lead to the formation of inorganic sulfate (e.g., ), in accord with our report that the heterogeneous OH oxidation of sodium methyl sulfate and sodium ethyl sulfate can lead to the formation of (Kwong et al., 2018). Given the high atmospheric abundance of organosulfates in atmospheric aerosols, further study of the contribution and transformation of organosulfates to inorganic sulfate through chemical reactions (e.g., heterogeneous oxidation, aqueous-phase oxidation and hydrolysis) is desirable. Methyltetrol sulfates are the most abundant isoprene-derived organosulfates measured in atmospheric PM2.5 samples collected from isoprene-rich regions influenced by anthropogenic emissions (Budisulistiorini et al., 2015; Hettiyadura et al., 2017). Additional studies are required to better understand the role of the molecular structure (i.e., position of the methyl and sulfate group) in the kinetics and chemistry of methyltetrol sulfates and other organosulfates upon heterogeneous OH oxidation, in particular the effect on the formation of smaller organosulfates, volatile fragmentation products and inorganic sulfate, since the 2-methyltetrol sulfate ester and its isomers, rather than the 3-methyltetrol sulfate ester investigated in this study, predominate in atmospheric aerosols (Cui et al., 2018). Future investigations on the transformation of other organosulfates, for instance, glycolic acid sulfate, which is the most abundant organosulfate in the overall atmosphere, are also desirable (Liao et al., 2015).
The underlying research data are available upon request from the corresponding author (mnchan@cuhk.edu.hk).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-19-2433-2019-supplement.
HKL and MNC designed and ran the experiments. HKL, MNC and KCK prepared and wrote the manuscript. All authors provided comments and suggestions for the manuscript.
The authors declare that they have no conflict of interest.
Hoi Ki Lam, Kai Chung Kwong, Hon Yin Poon and Man Nin Chan are supported by the CUHK
direct grant (4053281) and Hong Kong Research Grants Council (HKRGC) Project
ID: 2191111 (Ref 24300516) and 2130626 (Ref 14300118). Synthesis of
potassium methyltetrol sulfate was supported by the National Science
Foundation (NSF) under Atmospheric and Geospace (AGS) grant 1703535. We
would like to thank Kevin Wilson for his insightful comments on the reaction
mechanisms proposed for the OH reaction with 3-methyltetrol sulfate.
Edited by: Frank Keutsch
Reviewed by: three anonymous referees
Bennett, J. E. and Summers, R.: Product studies of the mutual termination reactions of sec-alkylperoxy radicals: Evidence for non-cyclic termination, Can. J. Chem., 52, 1377–1379, https://doi.org/10.1139/v74-209, 1974.
Block, E., Dane, A. J., Thomas, S., and Cody, R. B.: Applications of Direct Analysis in Real Time Mass Spectrometry (DART-MS) in Allium Chemistry, 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl Trisulfane S-Oxide, and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums, J. Agric. Food. Chem., 58, 4617–4625, https://doi.org/10.1021/jf1000106, 2010.
Bondy, A. L., Craig, R. L., Zhang, Z., Gold, A., Surratt, J. D., and Ault, A. P.: Isoprene-derived organosulfates: Vibrational mode analysis by Raman spectroscopy, acidity-dependent spectral modes, and observation in individual atmospheric particles, J. Phys. Chem. A, 122, 303–315, https://doi.org/10.1021/acs.jpca.7b10587, 2018.
Budisulistiorini, S. H., Li, X., Bairai, S. T., Renfro, J., Liu, Y., Liu, Y. J., McKinney, K. A., Martin, S. T., McNeill, V. F., Pye, H. O. T., Nenes, A., Neff, M. E., Stone, E. A., Mueller, S., Knote, C., Shaw, S. L., Zhang, Z., Gold, A., and Surratt, J. D.: Examining the effects of anthropogenic emissions on isoprene-derived secondary organic aerosol formation during the 2013 Southern Oxidant and Aerosol Study (SOAS) at the Look Rock, Tennessee ground site, Atmos. Chem. Phys., 15, 8871–8888, https://doi.org/10.5194/acp-15-8871-2015, 2015.
Carlton, A. G., Wiedinmyer, C., and Kroll, J. H.: A review of Secondary Organic Aerosol (SOA) formation from isoprene, Atmos. Chem. Phys., 9, 4987–5005, https://doi.org/10.5194/acp-9-4987-2009, 2009.
Chameides, W. L., Lindsay, R. W., Richardson, J., and Kiang, C. S.: The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study, Science, 241, 1473–1475, https://doi.org/10.1126/science.3420404, 1988.
Chan, M. N., Surratt, J. D., Claeys, M., Edgerton, E. S., Tanner, R. L., Shaw, S. L., Zheng, M., Knipping, E. M., Eddingsaas, N. C., Wennberg, P. O., and Seinfeld, J. H.: Characterization and quantification of isoprene-derived epoxydiols in ambient aerosol in the Southeastern United States, Environ. Sci. Technol., 44, 4590–4596, https://doi.org/10.1021/es100596b, 2010.
Chan, M. N., Nah, T., and Wilson, K. R.: Real time in situ chemical characterization of sub-micron organic aerosols using Direct Analysis in Real Time mass spectrometry (DART-MS): the effect of aerosol size and volatility, Analyst, 138, 3749–3757, https://doi.org/10.1039/c3an00168g, 2013.
Chan, M. N., Zhang, H., Goldstein, A. H., and Wilson, K. R.: Role of water and phase in the heterogeneous oxidation of solid and aqueous succinic acid aerosol by hydroxyl radicals, J. Phys. Chem. C, 118, 28978–28992, https://doi.org/10.1021/jp5012022, 2014.
Cheng, C. T., Chan, M. N., and Wilson, K. R.: The role of alkoxy radicals in the heterogeneous reaction of two structural isomers of dimethylsuccinic acid, Phys. Chem. Chem. Phys., 17, 25309–25321, https://doi.org/10.1039/c5cp03791c, 2015.
Chim, M. M., Chow, C. Y., Davie, J. F., and Chan, M. N.: Effects of relative humidity and particle phase water on the heterogeneous OH oxidation of 2-methylglutaric acid aqueous droplets, J. Phys. Chem. A, 121, 1666–1674, 2017a.
Chim, M. M., Cheng, C. T., Davies, J. F., Berkemeier, T., Shiraiwa, M., Zuend, A., and Chan, M. N.: Compositional evolution of particle-phase reaction products and water in the heterogeneous OH oxidation of model aqueous organic aerosols, Atmos. Chem. Phys., 17, 14415–14431, https://doi.org/10.5194/acp-17-14415-2017, 2017b.
Clifton, C. L. and Huie, R. E.: Rate constants for hydrogen abstraction reactions of the sulfate radical, alcohols, Int. J. Chem. Kinet., 21, 677–687, https://doi.org/10.1002/kin.550210807, 1989.
Cody, R. B., Laramée, J. A., and Durst, H. D.: Versatile new ion source for the analysis of materials in open air under ambient conditions, Anal. Chem., 77, 2297–2302, https://doi.org/10.1021/ac050162j, 2005.
Cole-Filipiak, N. C., O'Connor, A. E., and Elrod, M. J.: Kinetics of the hydrolysis of atmospherically relevant isoprene-derived hydroxy epoxides, Environ. Sci. Technol., 44, 6718–6723, https://doi.org/10.1021/es1019228, 2010.
Cui, T., Zeng, Z., dos Santos, E. O., Zhang, Z., Chen, Y., Zhang, Y., Rose, C. A., Budisulistiorini, S. H., Collins, L. B., Bodnar, W. M., de Souza, R. A. F., Martin, S. T., Machado, C. M. D., Turpin, B. J., Gold, A., Ault, A. P., and Surratt, J .D.: Development of a hydrophilic interaction liquid chromatography (HILIC) method for the chemical characterization of water-soluble isoprene epoxydiol (IEPOX)-derived secondary organic aerosol, Environ. Sci.-Proc. Imp., 20, 1524–1536, 2018.
Darer, A. I., Cole-Filipiak, N. C., O'Connor, A. E., and Elrod, M. J.: Formation and stability of atmospherically relevant isoprene-derived organosulfates and organonitrates, Environ. Sci. Technol., 45, 1895–1902, https://doi.org/10.1021/es103797z, 2011.
Davies, J. F. and Wilson, K. R.: Nanoscale interfacial gradients formed by the reactive uptake of OH radicals onto viscous aerosol surfaces, Chem. Sci., 6, 7020–7027, https://doi.org/10.1039/c5sc02326b, 2015.
Estillore, A. D., Hettiyadura, A. P. S., Qin, Z., Leckrone, E., Wombacher, B., Humphry, T., Stone, E. A., and Grassian, V. H.: Water uptake and hygroscopic growth of organosulfate aerosol, Environ. Sci. Technol., 50, 4259–4268, https://doi.org/10.1021/acs.est.5b05014, 2016.
Froyd, K. D., Murphy, S. M., Murphy, D. M., de Gouw, J. A., Eddingsaas, N. C., and Wennberg, P. O.: Contribution of isoprene-derived organosulfates to free tropospheric aerosol mass, P. Natl. Acad. Sci. USA, 107, 21360–21365, https://doi.org/10.1073/pnas.1012561107, 2010.
George, I. J. and Abbatt, J. P. D.: Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals, Nature Chem., 2, 713–722, https://doi.org/10.1038/nchem.806, 2010.
Hajslova, J., Cajka, T., and Vaclavik, L.: Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis, TrAC-Trend Anal. Chem., 30, 204–218, https://doi.org/10.1016/j.trac.2010.11.001, 2011.
Hatch, L. E., Creamean, J. M., Ault, A. P., Surratt, J. D., Chan, M. N., Seinfeld, J. H., Edgerton, E. S., Su, Y., and Prather, K. A.: Measurements of isoprene-derived organosulfates in ambient aerosols by aerosol time-of-flight mass spectrometry - Part 1: Single particle atmospheric observations in Atlanta, Environ. Sci. Technol., 45, 5105–5111, https://doi.org/10.1021/es103944a, 2011.
He, Q. F., Ding, X., Wang, X. M., Yu, J. Z., Fu, X. X., Liu, T. Y., Zhang, Z., Xue, J., Chen, D. H., Zhong, L. J., and Donahue, N. M.: Organosulfates from pinene and isoprene over the Pearl River Delta, south China: Seasonal variation and implication in formation mechanisms, Environ. Sci. Technol., 48, 9236–9245 https://doi.org/10.1021/es501299v, 2014.
Hettiyadura, A. P. S., Jayarathne, T., Baumann, K., Goldstein, A. H., de Gouw, J. A., Koss, A., Keutsch, F. N., Skog, K., and Stone, E. A.: Qualitative and quantitative analysis of atmospheric organosulfates in Centreville, Alabama, Atmos. Chem. Phys., 17, 1343–1359, https://doi.org/10.5194/acp-17-1343-2017, 2017.
Hu, K. S., Darer, A. I., and Elrod, M. J.: Thermodynamics and kinetics of the hydrolysis of atmospherically relevant organonitrates and organosulfates, Atmos. Chem. Phys., 11, 8307–8320, https://doi.org/10.5194/acp-11-8307-2011, 2011.
Jimenez, J. L., Canagaratna, M. R., Donahue, N. M., Prevot, A. S., Zhang, Q., Kroll, J. H., DeCarlo, P. F., Allan, J. D., Coe, H., Ng, N. L., Aiken, A. C., Docherty, K. S., Ulbrich, I. M., Grieshop, A. P., Robinson, A. L., Duplissy, J., Smith, J. D., Wilson, K. R., Lanz, V. A., Hueglin, C., Sun, Y. L., Tian, J., Laaksonen, A., Raatikainen, T., Rautiainen, J., Vaattovaara, P., Ehn, M., Kulmala, M., Tomlinson, J. M., Collins, D. R., Cubison, M. J., Dunlea, E. J., Huffman, J. A., Onasch, T. B., Alfarra, M. R., Williams, P. I., Bower, K., Kondo, Y., Schneider, J., Drewnick, F., Borrmann, S., Weimer, S., Demerjian, K., Salcedo, D., Cottrell, L., Griffin, R., Takami, A., Miyoshi, T., Hatakeyama, S., Shimono, A., Sun, J. Y., Zhang, Y. M., Dzepina, K., Kimmel, J. R., Sueper, D., Jayne, J. T., Herndon, S. C., Trimborn, A. M., Williams, L. R., Wood, E. C., Middlebrook. A. M., Kolb, C. E., Baltensperger, U., and Worsnop, D. R.: Evolution of organic aerosols in the atmosphere, Science, 326, 1525–1529, https://doi.org/10.1126/science.1180353, 2009.
Kroll, J. H., Lim, C. Y., Kessler, S. H., and Wilson, K. R.: Heterogeneous oxidation of atmospheric organic aerosol: Kinetics of changes to the amount and oxidation state of particle-phase organic carbon, J. Phys. Chem. A, 119, 10767–10783, https://doi.org/10.1021/acs.jpca.5b06946, 2015.
Kwong, K. C., Chim, M. M., Davies, J. F., Wilson, K. R., and Chan, M. N.: Importance of sulfate radical anion formation and chemistry in heterogeneous OH oxidation of sodium methyl sulfate, the smallest organosulfate, Atmos. Chem. Phys., 18, 2809–2820, https://doi.org/10.5194/acp-18-2809-2018, 2018.
Liao, J., Froyd, K. D., Murphy, D. M., Keutsch, F. N., Yu, G., Wennberg, P. O., St. Clair, J. M., Crounse, J. D., Wisthaler, A., Mikoviny, T., Jimenez, J. L., Campuzano-Jost, Pedro, Day, D. A., Hu, W., Ryerson, T. B., Pollack, I. B., Peischl, J., Anderson, B. E., Ziemba, L. D., Blake, D. R., Meinardi, S., and Diskin, G.: Airborne measurements of organosulfates over the continental U. S., J. Geophys. Res.-Atmos., 120, 2990–3005, 2015.
Lin, Y. H., Zhang, Z., Docherty, K. S., Zhang, H., Budisulistiorini, S. H., Rubitschun, C. L., Shaw, S. L., Knipping, E. M., Edgerton, E. S., Kleindienst, T. E., Gold, A., and Surratt, J. D.: Isoprene epoxydiols as precursors to secondary organic aerosol formation: Acid-catalyzed reactive uptake studies with authentic compounds, Environ. Sci. Technol., 46, 250–258, https://doi.org/10.1021/es202554c, 2012.
McNeill, V. F., Yatavelli, R. L. N., Thornton, J. A., Stipe, C. B., and Landgrebe, O.: Heterogeneous OH oxidation of palmitic acid in single component and internally mixed aerosol particles: vaporization and the role of particle phase, Atmos. Chem. Phys., 8, 5465–5476, https://doi.org/10.5194/acp-8-5465-2008, 2008.
Meade, L. E., Riva, M., Blomberg, M. Z., Brock, A. K., Qualters, E. M., Siejack, R. A., Ramakrishnan, K., Surratt, J. D., and Kautzman, K. E.: Seasonal variations of fine particulate organosulfates derived from biogenic and anthropogenic hydrocarbons in the mid-Atlantic United States, Atmos. Environ., 145, 405–414, https://doi.org/10.1016/j.atmosenv.2016.09.028, 2016.
Minerath, E. C., Schultz, M. P., and Elrod, M. J.: Kinetics of the reactions of isoprene-derived epoxides in model tropospheric aerosol solutions, Environ. Sci. Technol., 43, 8133–8139, https://doi.org/10.1021/es902304p, 2009.
Neta, P., Huie, R. E., and Ross, A. B.: Rate constants for reactions of inorganic radicals in aqueous solution, J. Phys. Chem. Ref. Data, 17, 1027–1284, https://doi.org/10.1063/1.555808, 1988.
Nguyen, T. B., Coggon, M. M., Bates, K. H., Zhang, X., Schwantes, R. H., Schilling, K. A., Loza, C. L., Flagan, R. C., Wennberg, P. O., and Seinfeld, J. H.: Organic aerosol formation from the reactive uptake of isoprene epoxydiols (IEPOX) onto non-acidified inorganic seeds, Atmos. Chem. Phys., 14, 3497–3510, https://doi.org/10.5194/acp-14-3497-2014, 2014.
Nozière, B., Ekström, S., Alsberg, T., and Holmström, S.: Radical-initiated formation of organosulfates and surfactants in atmospheric aerosols, Geophys. Res. Lett., 37, L05806, https://doi.org/10.1029/2009gl041683, 2010.
Padmaja, S., Alfassi, Z. B., Neta, P., and Huie, R. E.: Rate constants for reactions of SO4 radicals in acetonitrile, Int. J. Chem. Kinet., 25, 193–198, https://doi.org/10.1002/kin.550250307, 1993.
Paulot, F., Crounse, J. D., Kjaergaard, H. G., Kurten, A., St. Clair, J. M., Seinfeld, J. H., and Wennberg, P. O.: Unexpected epoxide formation in the gas-phase photooxidation of isoprene, Science, 325, 730–733, https://doi.org/10.1126/science.1172910, 2009.
Peeters, J., Fantechi, G., and Vereecken, L.: A generalized structure–activity relationship for the decomposition of (substituted) alkoxy radicals, J. Atmos. Chem., 48, 59–80, https://doi.org/10.1023/b:joch.0000034510.07694.ce, 2004.
Rattanavaraha, W., Chu, K., Budisulistiorini, S. H., Riva, M., Lin, Y.-H., Edgerton, E. S., Baumann, K., Shaw, S. L., Guo, H., King, L., Weber, R. J., Neff, M. E., Stone, E. A., Offenberg, J. H., Zhang, Z., Gold, A., and Surratt, J. D.: Assessing the impact of anthropogenic pollution on isoprene-derived secondary organic aerosol formation in PM2.5 collected from the Birmingham, Alabama, ground site during the 2013 Southern Oxidant and Aerosol Study, Atmos. Chem. Phys., 16, 4897–4914, https://doi.org/10.5194/acp-16-4897-2016, 2016.
Renbaum, L. H. and Smith G. D.: The importance of phase in the radical-initiated oxidation of model organic aerosols: reactions of solid and liquid brassidic acid particles, Phys. Chem. Chem. Phys., 11, 2441–2451, https://doi.org/10.1039/b816799k, 2009.
Rudich, Y., Donahue, N. M., and Mentel, T. F.: Aging of organic aerosol: Bridging the gap between laboratory and field studies, Annu. Rev. Phys. Chem., 58, 321–352, https://doi.org/10.1146/annurev.physchem.58.032806.104432, 2007.
Russell, G. A.: Deuterium-isotope effects in the autoxidation of aralkyl hydrocarbons, Mechanism of the interaction of peroxy radicals, J. Am. Chem. Soc, 79, 3871–3877, https://doi.org/10.1021/ja01571a068, 1957.
Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd edn., John Wiley & Sons, Hoboken, 2016.
Slade, J. H. and Knopf, D. A.: Multiphase OH oxidation kinetics of organic aerosol: The role of particle phase state and relative humidity, Geophys. Res. Lett., 41, 5297–5306, https://doi.org/10.1002/2014gl060582, 2014.
Smith, J. D., Kroll, J. H., Cappa, C. D., Che, D. L., Liu, C. L., Ahmed, M., Leone, S. R., Worsnop, D. R., and Wilson, K. R.: The heterogeneous reaction of hydroxyl radicals with sub-micron squalane particles: a model system for understanding the oxidative aging of ambient aerosols, Atmos. Chem. Phys., 9, 3209–3222, https://doi.org/10.5194/acp-9-3209-2009, 2009.
Stone, E. A., Yang, L., Yu, L. E., and Rupakheti, M.: Characterization of organosulfates in atmospheric aerosols at four Asian locations, Atmos. Environ., 47, 323–329, https://doi.org/10.1016/j.atmosenv.2011.10.058, 2012.
Surratt, J. D., Lewandowski, M., Offenberg, J. H., Jaoui, M., Kleindienst, T. E., Edney, E. O., and Seinfeld, J. H.: Effect of acidity on secondary organic aerosol formation from isoprene, Environ. Sci. Technol., 41, 5363–5369, https://doi.org/10.1021/es0704176, 2007.
Surratt, J. D., Gomez-González, Y., Chan, A. W. H., Vermeylen, R., Shahgholi, M., Kleindienst, T. E., Edney, E. O., Offenberg, J. H., Lewandowski, M., Jaoui, M., Maenhaut, W., Claeys, M., Flagan, R. C., and Seinfeld, J. H.: Organosulfate formation in biogenic secondary organic aerosol, J. Phys. Chem. A, 112, 8345–8378, https://doi.org/10.1021/jp802310p, 2008.
Surratt, J. D., Chan, A. W. H., Eddingsaas, N. C., Chan, M. N., Loza, C. L., Kwan, A. J., Hersey, S. P., Flagan, R. C., Wennberg, P. O., and Seinfeld, J. H.: Reactive intermediates revealed in secondary organic aerosol formation from isoprene, P. Natl. Acad. Sci. USA, 107, 6640–6645, https://doi.org/10.1073/pnas.0911114107, 2010.
Tang, Y., Thorn, R. P., Mauldin, R. L., and Wine, P. H.: Kinetics and spectroscopy of the radical in aqueous solution, J. Photoch. Photobio. A, 44, 243–258, https://doi.org/10.1016/1010-6030(88)80097-2, 1988.
Vereecken, L. and Petters, J.: Decomposition of substituted alkoxy radicals – part I: a generalized structure–activity relationship for reaction barrier heights, Phys. Chem. Chem. Phys., 11, 9062–9074, https://doi.org/10.1039/b909712k, 2009.
Wiegel, A. A., Wilson, K. R., Hinsberg, W. D., and Houle, F. A.: Stochastic methods for aerosol chemistry: a compact molecular description of functionalization and fragmentation in the heterogeneous oxidation of squalane aerosol by OH radicals, Phys. Chem. Chem. Phys., 17, 4398–4411, https://doi.org/10.1039/c4cp04927f, 2015.
Zhang, Y., Chen, Y., Lambe, A. T., Olson, N. E., Lei, Z., Craig, R. L., Zhang, Z., Gold, A., Onasch, T. B., Jayne, J. T., Worsnop, D. R., Gaston, C. J., Thornton, J. A., Vizuete, W., Ault, A. P., Surratt, J. D.: Effect of the aerosol-phase state on secondary organic aerosol formation from the reactive uptake of isoprene-derived epoxydiols (IEPOX), Environ. Sci. Tech. Lett., 5, 167–174, https://doi.org/10.1021/acs.estlett.8b00044, 2018.