the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Measurement report: The ice-nucleating activity of lichen sampled in a northern European boreal forest
Michael P. Adams
Grace C. E. Porter
Mark A. Holden
Jaana Bäck
Benjamin J. Murray
Ice-nucleating particles (INPs) facilitate the heterogeneous freezing of cloud droplets and thus modify cloud properties. Hence, it is important to understand the sources of INPs. During the HyICE-2018 campaign, which took place in the boreal forest of Hyytiälä, substantial concentrations of airborne heat-sensitive biological INPs were observed, despite many potential biological sources of INPs being snow-covered. A potential source of INPs that were not covered in snow was lichens that grow on trees; hence, we investigated these lichens as a potential source of biological INPs in this boreal forest environment. INPs derived from lichen sampled during HyICE-2018 are shown to nucleate ice at temperatures as warm as −5 °C with 103 INPs per gram of lichen. Successive filtration to smaller sizes removes some of the most active INPs in suspension, but substantial activity remains, even when filtering to 0.1 µm. The small size of the INPs from lichen means they have the potential to either be emitted directly into the atmosphere or be associated with larger particles, such as lichenous reproductive aerosol types (spores or diaspores). We also show that the INPs from lichens from Hyytiälä are sensitive to heat, which is similar to the INPs sampled from the atmosphere of Hyytiälä and consistent with the presence of ice-active proteins. Adding to previous evidence of lichenous INPs, this study shows that lichens from a European boreal forest in Hyytiälä harbour INPs. This novel finding may be especially important in this snow-covered habitat where few, if any, other biological INP sources are available. The great terrestrial abundance of lichens in Hyytiälä, and around the world, calls for further research to combine their ice-nucleating ability with dispersal studies to evaluate the flux of lichenous INPs into the atmosphere, as well as to what extent these particles reach heights and locations where they might influence cloud properties.
- Article
(6429 KB) - Full-text XML
- BibTeX
- EndNote
Clouds are a crucial part of the hydrological cycle and strongly affect Earth's radiative balance (Forster et al., 2021). Clouds properties are affected by a range of dynamical and microphysical processes, and it is becoming increasingly apparent that the formation of ice in clouds is amongst one of the least well-understood of these processes (Murray et al., 2021; Tan et al., 2016). Heterogeneous freezing of cloud droplets on ice-nucleating particles (INPs) influences precipitation, cloud lifetime, and the radiative effect of tropospheric clouds (DeMott et al., 2010). However, the identity, sources, and transport and therefore global distribution of INPs are poorly constrained (Murray et al., 2021). This is especially so for biological INPs, which are known to be active at relatively high temperatures but are highly variable in concentration (O'Sullivan et al., 2018).
Organic particles from different primary biological origins have been shown to exhibit ice nucleation ability, including biological particles in soil (Conen et al., 2011; O'Sullivan et al., 2014; Hill et al., 2016), on plants (Hill et al., 2014), in sea spray aerosol (DeMott et al., 2016), and in the sea surface microlayer (Irish et al., 2017; Wilson et al., 2015). Primary biological particles including bacteria, pollen, fungal spores, plankton, and diatoms have been shown to nucleate ice (Alpert et al., 2011; Schnell and Vali, 1976; Lindow, 1989; Pouleur et al., 1992; Pummer et al., 2012). Subcomponents of living matter have also been shown to nucleate ice, including cellulose and lignins (Hiranuma et al., 2019; Bogler and Borduas-Dedekind, 2020). Furthermore, it has been shown that nanometre-scale entities washed off fungus and pollen can be potent INPs (O'Sullivan et al., 2016, 2015; Pummer et al., 2012). In bioaerosols, an ice-nucleating ability is a selective property, and only a few bacterial strains and fungal species have been found to nucleate ice at high temperatures (Hoose and Möhler, 2012; Murray et al., 2012). Globally, more abundant INPs such as desert dust particles dominate the INP distribution at temperatures below about −15 °C (Vergara-Temprado et al., 2017). However, biological INPs are thought to have an influence on the hydrological cycle and climate – at least on regional scales (Prenni et al., 2009; Spracklen and Heald, 2014; Vergara-Temprado et al., 2017).
While a substantial effort has been made to understand the ice-nucleating activity of bacteria, pollen, and fungus, much less effort has been made to understand the ice-nucleating ability of lichens, despite their ubiquity in a variety of environments around the world (Hale, 1974). Several studies have shown that lichens from a range of environments and across multiple lichen species nucleate ice (Kieft, 1988; Kieft and Ahmadjian, 1989; Ashworth and Kieft, 1992; Moffett et al., 2015; Eufemio et al., 2023). In an early study, Kieft (1988) examined 15 lichens. Nearly all of them showed ice-nucleating activity at −8 °C, with −2.3 °C as the highest onset temperature. The bacteria that could be cultivated from the lichens showed no ice nucleation activity. In a recent study, Eufemio et al. (2023) tested lichens collected across Alaska for their ice-nucleating ability, pointing to their possible impact on cloud glaciation in a warming Arctic. Between them, Moffett et al. (2015) and Eufemio et al. (2023) surveyed the ice nucleation activity of 86 lichen samples and found that while ice nucleation was ubiquitous, these lichens had remarkably varied ice-nucleating abilities. Moffett et al. (2015) report onset freezing ranging from −5.1 to −20 °C, while Eufemio et al. (2023) report median freezing temperatures between −5.2 and −14.5 °C. In addition, there is substantial variability in ice nucleation between different samples of the same species of lichen. For example, one sample of Evernia prunastri nucleated ice at −5.6 °C, while another nucleated ice at −10 °C (Moffett et al., 2015). These studies show the ubiquity of ice nucleation in lichens, but given the observed variability in ice-nucleating activity, we cannot simply infer that lichens in one environment possess the same ice-nucleating activity as the same lichen genus or species in other environments.
For many years, lichens were thought to be symbiotic organisms composed of a fungal partner, the mycobiont, and a photobiont partner (Nash, 2008). However, it is now recognized that in addition to the mycobiont and photobiont (algae/cyanobacteria), lichen species can accommodate several additional symbionts, including yeasts and bacteria, associated with the fungus or locally living in the microhabitats of lichen thalli (Aschenbrenner et al., 2016; Cernava et al., 2017; Grimm et al., 2021). The symbiosis might be seen as a successful one as lichens are found worldwide from the tropics to the polar regions (Nash, 2008).
Kieft (1988) concluded that the INPs from the lichens are nonbacterial in origin and suspected them to be either membrane-bound proteins similar to those in bacteria or secondary metabolites. Kieft and Ahmadjian (1989) concluded that lichenous INPs are produced primarily by the mycobiont rather than the photobiont as the former showed ice nucleation activity at warmer temperatures. Kieft and Ruscetti (1990) argued that the sensitivity to proteases, guanidine hydrochloride, and urea could be taken as evidence of the proteinaceous nature of the INPs. In addition, heat treatment has been shown to remove the ice-nucleating activity of lichens (Kieft and Ruscetti, 1990; Daily et al., 2022; Henderson-Begg et al., 2009; Kieft, 1988), which is consistent with the presence of ice-nucleating proteins (Daily et al., 2022; Eufemio et al., 2023). However, ice-nucleating proteins from lichens appear to be more resistant to heat than proteins from bacteria as they are stable up to 70 °C (Kieft, 1988). We also know that some fungal materials produce proteins that nucleate ice effectively, and these proteins can become separated from the mycelia (O'Sullivan et al., 2015; Schwidetzky et al., 2023). Ashworth and Kieft (1992) demonstrated ice nucleation activity in whole lichen thalli, whereas in the studies before, the lichen had been ground and brought into suspension. Using a relationship between molecular size and the likelihood of becoming deactivated on exposure to gamma radiation, Kieft and Ruscetti (1992) found a logarithmic relationship between freezing temperature and protein size. This size dependence is consistent with the idea that larger aggregates of proteins have the potential to nucleate ice at higher temperatures (Schwidetzky et al., 2023).
There are two hypotheses as to why ice-nucleating activity might have evolved in lichens (Kieft, 1988). First, as proposed for ice-nucleation-active bacteria, lichen might benefit from the nucleation of ice at relatively modest supercooling and the more gradual formation of ice as it is less stressful to an organism than a rapid crystallization experience in greater supercooling. During rapid freezing at great supercooling, intracellular ice formation becomes more likely, and this is usually lethal to cells (Clarke et al., 2013; Daily et al., 2020, 2023). Second, ice nucleation might be a water-harvesting mechanism (Kieft, 1988; Henderson-Begg et al., 2009). Once a small amount of water is frozen on the thallus, more water may preferentially deposit on it. Later, when the temperature increases, this ice may melt, and the liquid water would become available to the lichen. This process is all the more important since lichens lack stomata and are therefore not able to actively control water loss as many plants do (Kappen and Valladares, 2007).
The distribution of aerosol particles originating from lichen in the atmosphere is poorly constrained. Lichens are complex and varied organisms that have evolved to produce entities that can become airborne. To appreciate which components of lichens might become airborne (and therefore to design an appropriate ice-nucleation study), we need some understanding of the forms and structures of lichens. Three growth forms of lichens are traditionally distinguished (Hale, 1974). Crustose lichens are in intimate contact with their substrate and cannot readily be separated from that substrate. Foliose lichen has a leafy plant body up to 0.3 m in diameter. Fruticose lichens appear hair-like. Lichens reproduce either sexually or asexually, producing spermatia (1 to 5 µm), spores (1 to 510 µm), or vegetative diaspores (10 to 3000 µm; e.g. isidia or soredia; Hale, 1974). Soredia are powdery granules of algae cells enveloped by fungal threads, whereas isidia are spiny outgrowths that are easily broken off the thallus (Hale, 1974). While spores are forcibly ejected, vegetative diaspores rely on external forces to be removed from the thallus (Bowler and Rundel, 1975). The vegetative strategy allows the invasion of new habitats, and species using this strategy often have a greater world distribution than their sexual counterparts (Hale, 1974; Bowler and Rundel, 1975). Clearly, these particles also have the potential to contribute to the INP population of the atmosphere if they harbour ice-nucleating entities.
As of now, little is known about the atmospheric abundance of ice-nucleation-active particles that stem from lichen (Després et al., 2012). However, the wide distribution of lichen indicates that they have a successful mechanism for dispersal (Hale, 1974), which in turn suggests high concentrations of propagules (e.g. spores, soredia, and isidia). Wind removal of soredia has been successfully demonstrated by Bailey (1966). It was found that while more soredia were removed at a higher moisture content of the thallus, the wind speed necessary for removal increased with the higher moisture content as well. Armstrong (1993) conducted a wind tunnel experiment and found humidity to lead to a substantial decrease in soredia dispersal. Armstrong (1991) identified humidity to be the most significant variable for soredia dispersal, stating that more soredia dispersed at lower humidity. It has been suggested that a high moisture content promotes soredia production but that a low moisture content facilitates release (Marshall, 1996). In a year-long aerobiological monitoring programme over Antarctica, Marshall (1996) found lichen soredia to be more abundant than spores. Soredia were collected every month, suggesting that soredia are produced year-round. The release was found to be independent of any specific meteorological variable. In a field study in an old-growth forest in Finland, samples of the lichen Lobaria pulmonaria were genetically analysed (Ronnås et al., 2017). Symbiotic propagules had a maximum dispersal range of 100 m, while ascospores dispersed several kilometres. Tormo et al. (2001) placed traps 6 m above the ground level in Spain. The mean soredia concentration was 0.4 m−3, with a daily maximum of 5 m−3. The concentration was higher during the day. Positive correlations with temperature and again negative correlations with humidity were found.
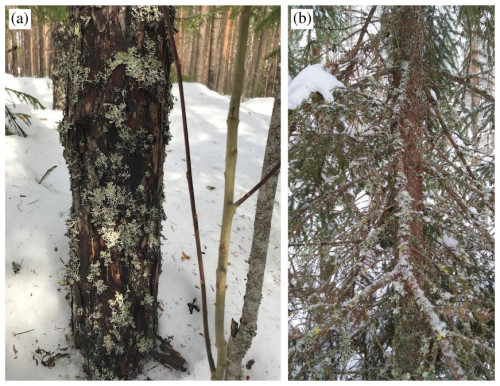
In this paper, we report the ice-nucleating ability of lichens that were present and exposed in the boreal forest at the Station for Measuring Ecosystem–Atmosphere Relations (SMEAR) II located in Hyytiälä, Finland, during the HyICE-2018 campaign. The HyICE-2018 campaign was focused on measuring atmospheric INPs between February and June 2018 (Brasseur et al., 2022). Schneider et al. (2021) report the presence of heat-sensitive biological INPs between −25 and −6 °C during the campaign. In addition, Vogel et al. (2024) suggested that INPs active below −24 °C are related to a biological source. However, the source of these INPs was unclear since the surface was snow-covered, which rules out leaf litter or bare soil as sources of INPs. We hypothesize that the trees which bore lichen and were exposed, even in the winter when the canopy and the ground were snow-covered (see Fig. 1), might have been a source of INPs. In this paper, we tackle the first part of the hypothesis, namely the question of if the lichens in the forest during the winter of HyICE-2018 contained ice-nucleating entities. Eufemio et al. (2023) have made this possibility obvious since they showed that lichens from across Alaska harbour INPs. It remains up to us to confirm this for the Hyytiälä boreal forest and to investigate the size of the lichenous INPs that we find. A positive outcome would provide motivation to address the question of whether sufficient quantities of INPs are released into the atmosphere to influence the INP population and subsequent cloud formation.
In order to quantify the ice-nucleating ability of lichens which are potentially relevant as sources of atmospheric INPs, we collected samples and analysed their ice-nucleating activity in the immersion mode using a droplet-freezing assay. Samples were collected and, as discussed below, were sampled as mixtures of multiple lichen species. Our experimental approach was to subsample from these mixtures of lichen and test the activity of the mixtures of species. We examined mixed samples of lichen for their ice-nucleating ability, the size of the ice-nucleating species, and the heat sensitivity rather than solely focusing on single species in order to reveal if the lichens in Hyytiäla harboured ice-nucleating entities and obtain an indication of their activity. This allowed us to address our stated objective of determining if there is a potential source of biological INPs associated with the prolific lichen population in Hyytiäla. In addition, we performed a set of experiments in which we attempted to separate the lichen species.
2.1 Sample collection and identification
The lichen was taken from Scots pine trees in a boreal forest environment in Hyytiälä with clean tweezers and placed in resealable plastic bags in March and April 2018 during the HyICE-2018 campaign. Lichen samples were imaged using a stereomicroscope (Stemi 508, Zeiss) and were identified with these pictures (see Figs. 2 and 3). One plastic bag contained specimens of Evernia prunastri (foliose), Bryoria sp. (fruticose), and Platismatia glauca (foliase), and two other plastic bags were filled with Hypogymnia physodes (foliose). The species were not collected separately because they tended to grow in the same locations, and clear separation in the field by eye was challenging (or impossible). These bags were sealed and transported back to the University of Leeds on a passenger flight at room temperature. Experiments were conducted in April and May 2018. By storing at room temperature, the samples were preserved at low relative humidity, conditions under which biological activity is inhibited. Nevertheless, we note that the storage at room temperature was pragmatic, and it is possible that the activity of the samples might be somewhat dependent upon the storage conditions.
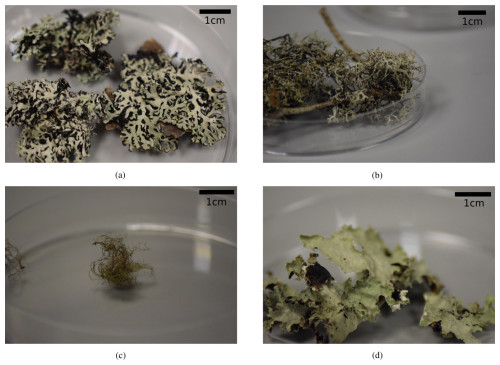
Figure 2Pictures of the sampled lichen species that were tested for ice nucleation activity in this study. (a) Hypogymnia physodes; (b) Evernia prunastri; (c) Bryoria sp.; (d) Platismatia glauca.
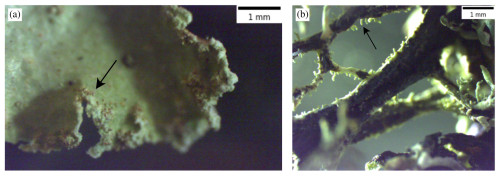
Figure 3Images that were obtained by the stereomicroscopy of the sampled lichens that were immersed in water for the species-specific testing. (a) Soralia (see arrow) of Platismatia glauca that produces soredia. (b) Isidia (see arrow) of Evernia prunastri.
Examples of the structures that can become aerosolized are shown in Fig. 3. Figure 3a shows soredia on Platismatia glauca, while Fig. 3b shows isidia on Evernia prunastri. These vegetative diaspores can be broken off the thallus through the action of wind, rain droplets, or even animals. The recognition that it is likely that these fragile structures on the surface of the lichen which preferentially become airborne helped us to design a droplet-freezing assay that is appropriate. In some previous ice nucleation studies, the lichens were ground with some water to produce a pulp that was then suspended in water (Kieft, 1988; Eufemio et al., 2023). This approach might be appropriate for studying the water harvesting properties of lichens but may be less relevant for understanding atmospheric implications. In addition, the practice of washing lichen samples to remove non-lichen ice-nucleating entities may inadvertently remove the soredia and isidia, which are the very entities of particular interest. Hence, we used an approach in which the lichen was exposed to water and gently agitated in order that fragile structures, like the soredia or isidia, might be removed (details in Sect. 2.2). The large pieces of lichen were allowed to settle to the bottom of the vial, and then the aqueous supernatant, which was clear to the eye, was sampled for the droplet-freezing assay. While this approach is clearly different from bioaerosol production from lichen via wind, it does bias the analysis towards the entities associated with lichen that are likely to become aerosolized.
2.2 Sample preparation
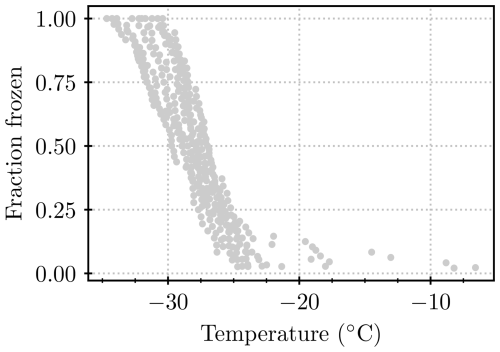
In order to generate clean water with minimal ice-nucleating contamination, 50 mL of nanopure water was filtered through a 0.2 µm filter (Minisart®, Sartorius) and deposited into a 50 mL polypropylene test tube. From this water, a blank was run on the Microlitre Nucleation by Immersed Particle Instrument (µL-NIPI; see Sect. 2.5; Whale et al., 2015) each morning to establish the baseline in the lab. In this way, the background signal could be evaluated, which determines the limit of detection for the instrument. The INPs examined in this study had freezing temperatures between −5 and −28 °C (except for a few dilutions, where the freezing temperature was somewhat lower), with T50 approximately between −15 and −20 °C. Therefore, a blank was regarded as acceptable if the first droplet froze at below −22 °C, T50 was at below −26 °C, and the last droplet froze at below −30 °C. The water from which the blank had been taken was then used further for immersing the lichen or for dilutions of suspensions. Samples that froze below −25 °C and thereby went into the range of considerable blank freezing (see Fig. A1) were excluded from the analysis.
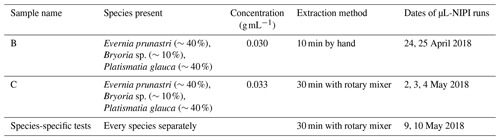
To prepare the aqueous lichen extracts, lichen was taken from its plastic bag with tweezers, separated from any bark or twigs, and placed in 50 mL polypropylene centrifuge tubes (Falcon tubes, Fisher Scientific). The lichen's mass was noted, as well as the amount of water which was then added to the tube. Lichen samples were taken from a mixture of lichen species stored in a single sealed plastic bag. Both samples B and C had a similar proportion of the different lichen species, but the sensitivity to the mixing method was explored (see Appendix B and Fig. B1 for results). The composition of the mixtures and the dates on which the samples were immersed in water and run on the µL-NIPI are given in Table 1. The composition was meant to mimic the mixture in the bag. For the species-specific runs, samples of only one species of lichen were put into each centrifuge tube. Sample B was rotated carefully by hand for 10 min, while sample C and the species-specific samples were left on a rotation mixer for 30 min and set at about 30 rotations per minute. In all procedures, care was taken to use relatively gentle approaches so as to minimize the break up of any structures in the lichen, since the atmospherically relevant INPs should be on its surface and readily removable. Sample B is split into B1 and B2 in the paper; B2 was sampled from the same suspension 1 d later, so it had more time to release INPs into the suspension/solution.
2.3 Filtration
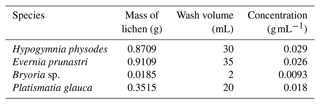
In order to learn more about the size of the ice-nucleating entities, samples were also filtered prior to testing. In total, 10 µm (NY10; Merck Millipore), 2 µm (Isopore™; Merck Millipore), 0.1 µm (6809-6002; Anodisc; Whatman®), and 0.02 µm (6809-6012; Anodisc; Whatman®) filters were used and placed within a 45 mm Advantec 301000 stainless-steel filter holder. The filters were employed in front of a syringe filled with a sample. Only for sample C were all filter sizes used, as it was realized after the processing of sample B that further size differentiation would be desirable. For the species-specific tests, the samples were partly too small to use all filters, so only the 2 µm filter was used. It should be noted that the size of the particles immersed in water may be different from the size of the dry particles that might become aerosolized.
2.4 Heat test
To test whether the ice-nucleating particles are heat-labile, a polypropylene test tube with 1 mL of sample solution was placed in a boiling water bath for 30 min. Different temperature heat treatments have been shown to have a different effect on biological INPs, and more deactivation happens with higher temperatures (Hara et al., 2016). In most studies, heat treatments that are meant to test for biological INPs involve heating at about 90 °C for 10 min (Hara et al., 2016; Christner et al., 2008; Moffett et al., 2015; O'Sullivan et al., 2014). Longer periods of 20 and 45 min have been used as well (Garcia et al., 2012; O'Sullivan et al., 2015). In this study, the sample containers were placed in a bath of boiling water; hence, the sample was warmed to above 90 °C for 30 min, as recommended by Daily et al. (2022). The sample was left to cool for a few minutes before being run on the µL-NIPI. Daily et al. (2022) have shown that not only biological INPs but also some minerals are affected by the wet heat tests. But since our samples are clearly biological in nature, we regard it as a valid method for the qualitative detection of protein-based biogenic INPs.
2.5 Ice nucleation measurements with the Microlitre Nucleation by Immersed Particle Instrument
Droplet-freezing techniques are widely used to study immersion-mode freezing (Vali, 1971; Murray et al., 2012). In these techniques, droplets of a suspension are cooled, and freezing events are recorded as a function of temperature. The volume of the droplets determines the temperature range that can be investigated. In a bigger volume of the same concentration, there are more INPs, and therefore it is more likely that rarer INPs are present in the droplet which are active at higher temperatures and dominate the freezing. Hence, multiple instruments with different droplet sizes and dilutions are needed to investigate ice-nucleating particles and their range of freezing temperatures.
The Microlitre Nucleation by Immersed Particle Instrument (µL-NIPI) is used for droplet-freezing experiments with 1 µL droplets. As such, it was first described by Atkinson et al. (2013) and has since been employed in a range of studies (O'Sullivan et al., 2014, 2015). It has been part of an intercomparison between 17 ice nucleation measurement techniques in Hiranuma et al. (2015) and DeMott et al. (2018). In Whale et al. (2015), one can find a detailed instrument description.
For the freezing experiments, a 22 mm diameter silanized glass slide (Hampton Research; HR3-231) is put on the aluminium plate after being rinsed thoroughly with methanol and nanopure water. If the samples had been prepared hours before being tested, then they were vortexed before being run on the µL-NIPI in order to homogenize the sample and stir up any particles that might have sedimented. Samples that were run in Leeds were vortexed for at least 10 s before each run. In total, 30 to 50 1 µL droplets were then pipetted directly onto the slide using a multi-dispense pipette (Sartorius eLINE®). Suboptimal mixing before pipetting is visible in the results where, e.g., dilutions do not line up, as discussed in Sect. 3. The aluminium plate was covered with a perspex chamber. The chamber has openings for a camera (Microsoft LifeCam HD) and two pipes for flushing with dry nitrogen. The nitrogen flow prevents frost growth and freezing due to contact with frost. Another benefit is that the nitrogen flow reduces the potential contamination with aerosol particles from laboratory air.
The cold stage (EF600 Stirling engine chiller; Grant Asymptote) was directed to cool with a temperature ramp of 1 °C min−1. The starting temperature was between 10 °C and room temperature, and the run was stopped as soon as the last droplet was observed to be frozen. When the EF600 was set to cool, the data-logger software was started. The temperature of the aluminium plate as a function of time was logged, and images of the droplets on the glass slide were recorded with the digital camera at a rate of one frame per second. The frames of the video were manually looked through, noting for each droplet the frame in which it showed the first signs of freezing so that the fraction of droplets frozen, f, could be calculated. The ice active site density per mass of lichen, nm, was calculated following Murray et al. (2012) (who reference Vali, 1971):
where the factor d is the dilution of the droplets, Vd is the droplet volume, Vw is the wash volume, and mlichen is the mass of lichen per sample. We normalize the result to the initial mass of lichen so that we can quantify the relative changes in activity on dilution, heat tests, and filtration. Dilutions of samples were made in order to reach lower-temperature ranges in the measurements. The errors in the INP concentration measurements were calculated following the procedure described in Harrison et al. (2016), which in turn is based on Wright and Petters (2013), and the temperature uncertainty is ±0.4 °C.
Previous studies have shown that a substantial fraction of the INPs observed during the HyICE-2018 campaign was of biological origin, based on a heat test (Schneider et al., 2021). Our hypothesis is that these biological INPs originate from the lichen that is abundant in the boreal forest ecosystem even when there is snow cover. However, because INPs typically make up a small portion of aerosol particles, their identification in aerosol samples is challenging. Thus, samples of lichen were taken in Hyytiälä and tested for their ice nucleation activity.
3.1 Ice-nucleating ability of the individual lichen species
Figure 4 shows the ice nucleation activity of each lichen species that was tested individually. The species-specific ice nucleation activities look very similar to each other. Only the spectrum of Hypogymnia physodes has a shape distinct from the others, with greater activity below −7 °C and less activity above −7 °C. One might conclude that the three species that were stored in one bag (Evernia prunastri, Bryoria sp., and Platismatia glauca) and were in close proximity to one another in the forest simply show the same INP concentrations because entities such as the soredia and isidia may have been spread throughout the sample. However, the 2 µm filtered size fractions shown in Fig. 4 are inconsistent with this idea. Evernia prunastri and Platismatia glauca are the species that were present the most in the bag, as well as in the mixed samples (see Table 2). The INP concentration of the Platismatia glauca sample in the 2 µm size fraction is half an order of magnitude higher than that of the Evernia prunastri sample at for example −10 °C, while the spectra of the unfiltered samples lie within error found in each other. So, in fact, the different lichen species seem to harbour differently sized INPs.
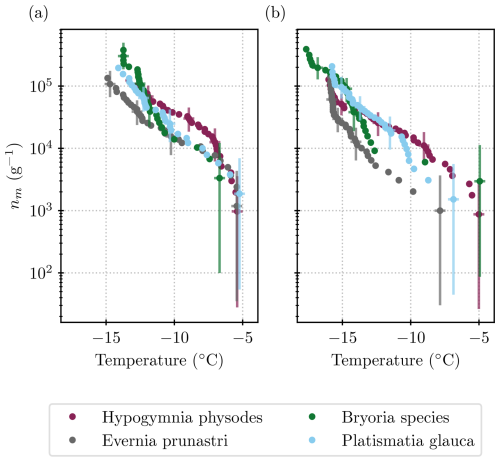
Figure 4INP measurements that were conducted for each species of lichen separately. Comparison of the (a) unfiltered samples and (b) those that were filtered through a 2 µm filter. For a comparison with the literature values, see Fig. 7.
3.2 Mixed lichen samples
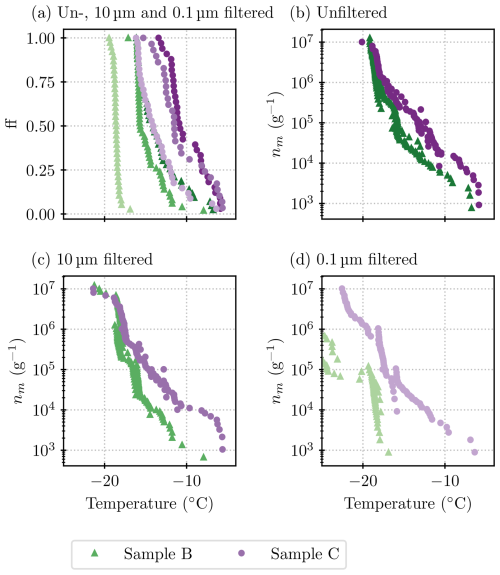
Further filtration and heat tests were done on mixed samples of the lichen species. Figure 5 shows all measurements that were made on lichen sample B, a mixture of Evernia prunastri and Platismatia glauca with only a small fraction of Bryoria sp. (see Table 1).
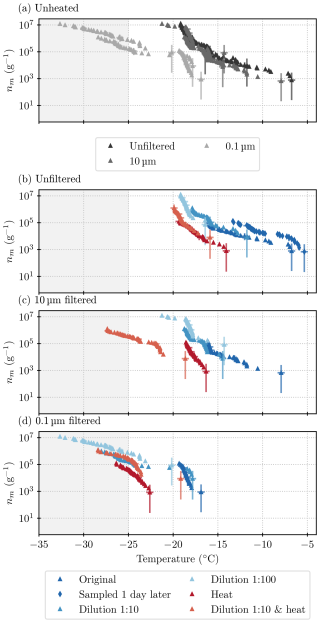
Figure 5Results acquired from all ice nucleation measurements on lichen sample B (INPs per gram of lichen sample). (a) All unheated samples (summarizes all blue points from the plots below), (b) unfiltered samples, (c) samples filtered through a 10 µm filter, and (d) samples filtered through a 0.1 µm filter. Higher dilutions are depicted as lighter shades of blue, and heat-tested samples are in red. The grey shading indicates the temperature at which the blanks started to freeze and thus results are deemed unreliable. The majority of results are for sample B1, but there is one run with B2 in panel (b). Uncertainties are included for every 10th data point and deduced as described in Sect. 2.5.
In Fig. 5a, the ice nucleation measurements that were conducted on different size fractions of sample B1 are plotted together for comparison. The spectra for the unfiltered sample and the sample that had been filtered through a 10 µm filter look similar in terms of the qualitative and quantitative (within 2 °C) characteristics. This indicates that the ice-nucleating entities can become independent of the lichen and that these entities are mostly smaller than 10 µm. The ice-nucleation activity drops by 1 to 2 orders of magnitude (a shift in temperature of about 4 to 5 °C) at respective temperatures for the sample that had been filtered through a 0.1 µm filter. This suggests that the size of most of the entities that nucleate ice above −18°C falls between 0.1 and 10 µm. In order to better constrain this size, a 2 µm filter sample was included in the next set of experiments (sample C; see below).
A striking feature that is present in all three size fractions of sample B1 is the “steps” in the spectra (sections that are almost vertical) that are visible at −16 and −18 °C (see also the differential spectra in Fig. C1). A sharp rise in INP concentrations at a specific temperature suggests that there is a single INP species with a specific temperature of freezing onset present in the sample. As there are no data for the concentration of the INP species active at or above −16 °C for the 0.1 µm size fraction, it can be concluded that this entity is between 0.1 and 10 µm in size. The species active at −18 °C, however, is also detected in the 0.1 µm size fraction.
All heat tests conducted on these samples show a striking loss of activity with a decrease in the temperatures where ice nucleation was observed. The onset freezing temperature that could be observed with 1 µL droplets dropped by 6 to 8 °C, depending on the size fraction. In fact, the heat test decreased the concentration of INPs active at temperatures greater than −14 °C so much that they could not be detected with the µL-NIPI technique. The loss of activity is greatest for INPs active at temperatures above about −16 °C, which is more than an order of magnitude. INPs that induce freezing at lower temperatures seem to be less heat-labile. In the unfiltered and 10 µm size fraction, after the heat test, activity is centred around −18 °C, just below the step at −18 °C (see Fig. 5b and c). However, in the 0.1 µm size fraction (see Fig. 5d), the heat test diminishes activity at −18 °C. Therefore, the INPs that are active around −18 °C and dominate the heated unfiltered samples, and 10 µm samples are concluded to be larger than 0.1 µm. The spectrum of the heat test in the smallest size fraction, 0.1 µm, is very close to the unheated sample spectrum below −24 °C, indicating that the INPs that are smaller than 0.1 µm and active below −24 °C are heat-stable (although these results are very close to our background). Overall, the heat tests show that the INPs that are active at temperatures warmer than −18 °C, including those species responsible for the steps at −16 °C in the unheated spectra, are heat-labile. In the heated samples larger than 0.1 µm, INPs that are active at around −18 °C dominate. It seems likely that the species active at −18 °C is only partially heat-labile or in fact consists of two species; it dominates in the heated samples of size fractions larger than 0.1 µm, but its activity diminishes upon heating the 0.1 µm size fraction.
In order to test if the activity of sample B changed with time, we took a sample of aqueous solution (B2) from the original centrifuge tube that contained water and lichen. This sample had been rotated by hand prior to B1 being sampled and then left for 1 d in a fridge (at 4 °C) before sample B2 was taken. The activity in this sample was up to about an order of magnitude greater than in sample B1 (see Fig. 5b). A potential explanation for this is that the lichen shed additional INPs into the solution while the lichen was in water. However, we note that precipitation samples have been seen to become more active with time, possibly related to the formation of ice-active protein aggregates (Stopelli et al., 2014). While this is in itself interesting and warrants future work, it also informed us that we could not perform more freezing assays with the original lichen–water mixture for further tests (such as additional filter tests). Hence, it was necessary to make fresh suspensions.
In order to investigate the size of these INPs present in the lichen samples further, a new sample of lichen (sample C) was taken out of the same bag as sample B. When sampling from the bag of lichen, we aimed to obtain the same mix of lichen species in this sample as in sample B (see Table 1). Figure 6 shows the results of the experiments conducted with sample C. An inspection of Fig. 6a shows that while the freezing characteristics are qualitatively similar to sample B, there are also some important differences that we discuss here.
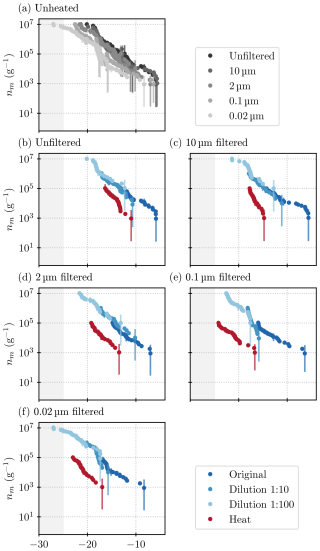
Figure 6Results acquired from all ice nucleation measurements on lichen sample C. (a) All unheated samples; (b) unfiltered samples; and samples filtered through a (c) 10 µm (d) 2 µm, (e) 0.1 µm, and (f) 0.02 µm filter. Higher dilutions are depicted as lighter shades of blue, and heat-tested samples are in red. The grey shading indicates the temperature at which the blanks started to freeze, and thus, results are deemed unreliable.
Again, warm-temperature INPs are present, and as in sample B, INP concentrations decrease substantially when the sample is filtered through a 0.1 µm filter (Figs. 6a and C1). Meanwhile, the 2 µm size fraction gives about the same signal as the 10 µm fraction. The bulk of the INPs present in the mixture of lichen species in lichen samples B and C are therefore concluded to be between 0.1 and 2 µm in size. The steps that can be clearly distinguished in all three size fractions of sample B1 are less prominent in sample C (but visible in the differential spectra in Fig. C1). The step at −18 °C can be seen in all size fractions, although naturally at lower concentrations in the 0.1 and 0.02 µm size fraction (because higher-temperature INPs are removed by filtration, and this reduces the concentration across the full spectrum since this is a cumulative quantity). The step at −16 °C, however, can only be identified in the 0.1 and 0.02 µm size fraction (see Fig. 6e and f). Only when the larger INPs are removed can the activity of these INPs be seen in the spectrum because their concentrations are lower than that of the INPs larger than 0.1 µm. That the step at −16 °C was visible in the spectra of sample B1 might be explained by the differing composition of the samples.
The heat tests on sample C again show a clear decrease in activity by about 1 to 2 orders of magnitude (a shift of about 5 to 10 °C. The higher-temperature INPs are more heat-labile than the ones active at colder temperatures, just like in sample B1. Also, as before, in the 10 µm size fraction in Fig. 6c), an INP species active at −18 °C is evident in the spectrum of the heated sample. However, heat tests of the smaller size fractions diminish activity at −18 °C.
Taking the results from lichen samples B1 and C together (where the extraction methods were different; see Sect. 2, Appendix B, and Fig. B1 for results), there is an INP species active at −18 °C present in all samples, whose concentration decreases when filtered through 2 µm pore size filters or smaller but remains distinguishable in the spectra of the 0.02 µm size fraction. The species active at −18 °C is partially heat-labile because a step at −18 °C is also visible in the heated samples but only in the size fractions larger than 2 µm. These different characteristics (activity and heat sensitivity) for different size fractions point to different states of the INP species, for example, as either attached to a larger particle or freely in a solution, as has been proposed for nano-INP (O'Sullivan et al., 2015), or as different aggregates as proposed for Pseudomonas syringae (Turner et al., 1990). The INPs that are active at around −16 °C are heat-labile and smaller than 0.02 µm for sample C (see Fig. 6). The identification of two INP species, active at certain temperatures and of a determined size, allows the relation of these findings to future studies that might find similar species.
We contrast the results from the present study with those from the literature in Fig. 7. In the literature studies, lichen samples were broken up by grinding and/or homogenizing to release ice-nucleating entities prior to testing in a freezing assay (Kieft, 1988; Kieft and Ahmadjian, 1989; Kieft and Ruscetti, 1990; Ashworth and Kieft, 1992; Eufemio et al., 2023). In contrast, we placed lichen in water, agitated the samples, and then tested the INP content of the resulting solution/suspension without breaking up the main body of the lichens. Hence, it is not a surprise that the concentration of INPs per unit mass of original lichen is at the low end of the range defined by the literature data. But it is striking that, even with our much more gentle INP extraction approach than those employed in the literature, still very active INPs are released into suspension. It is noteworthy that the earlier studies (from the 1980s and 1990s) quote their blanks as freezing between −10 and −15 °C, while blanks in this study showed that background freezing mostly occurred below about −25 °C, allowing measurements at lower temperatures in our study (see Appendix C).
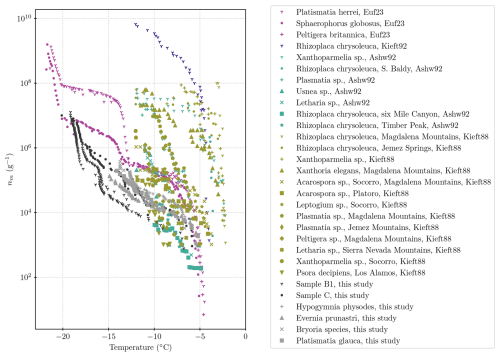
Figure 7Compilation of the literature values of INPs per gram of lichen in comparison with values from the present study. The literature data are from Eufemio et al. (2023), as well as Kieft (1988), Ashworth and Kieft (1992), and Kieft and Ruscetti (1992).
Lichens in the genus Platismatia were tested in the present study and by Ashworth and Kieft (1992) and Kieft (1988). The samples of Platismatia sp. from the Magdalena Mountains in New Mexico had very similar INP content to our sample at around −5 °C, with a concentration of 103 INPs per gram of lichen. However, the INP concentrations of Platismatia sp. from the Magdalena Mountains rise much more rapidly when the temperature is decreasing, resulting in a concentration of nearly 108 INPs per gram of lichen at −11 °C, whereas in this study, the Platismatia glauca sample showed a concentration of 105 INPs per gram of lichen at −11 °C. It is also evident that samples in the same genus have very different activities, even when the same techniques are applied. Kieft (1988) shows that a Platismatia sample from the Jemez Mountains had a much lower activity, with 106 INPs per gram at −11 °C, which is much closer to the values reported for our Platismatia sample.
Eufemio et al. (2023) very recently presented a study of 29 lichen species from Alaska, some of which were sampled from boreal forests (in addition to the three full spectra reproduced in Fig. 7; Eufemio et al., 2023, report T50 for additional samples). They also found high variability in ice-nucleating activity between species of lichen (T50 of −8 and −15 °C for the boreal samples; compare to Fig. B1), as well as sensitivity to heat. Their detailed analysis on three lichen species (see Fig. 7) demonstrates that there are two populations of ice-active material, with one active at around −7 °C and one at around −14 °C. They also showed that while the samples were generally sensitive to heat, these different populations of ice-active material responded differently to their heat treatment. They interpreted this as being evidence that there are different molecular compositions of ice-nucleating materials in lichens.
The size and heat sensitivity of ambient INPs during HyICE-2018 have some consistency with the properties of the lichenaceous INPs we studied here. Schneider et al. (2021) report that the ambient INPs were strongly heat-sensitive, with all activity above −13 °C being removed on heating. The size of INPs during HyICE-2018 is also reported by Porter et al. (2020), who revealed that the 0.25 to 0.5 µm fraction contained more INPs (above −22 °C) than any of the larger size fractions in their tests. Porter et al. (2020) comment that the more normal dependency, based on literature data, is that larger aerosol particles contribute more INPs than smaller aerosol particles; hence, their finding was unexpected. Alternatively, the biological INPs observed during HyICE-2018 might have come from a different source. Possibilities include the release of INPs from the needles or other surfaces of pine trees (Seifried et al., 2023) or perhaps from blowing snow that might release aerosol if snow particles sublime (Frey et al., 2020).
Kieft and Ruscetti (1990) is the only literature study that looks at the size of the lichenous INPs in droplet assays. Their samples were filtered through a 0.2 µm pore size filter. The samples had been centrifuged, and only the supernatant was used for testing, whereas in the present study, the whole suspension was filtered. Kieft and Ruscetti (1990) mention that their “extraction procedure did not remove all of the nuclei from the lichens” (Kieft and Ruscetti, 1990, p. 3521). This supports the results and the hypothesis brought forth in the present study. As the present study has found the bulk of lichenous INPs to be between 0.1 and 2 µm in size, it seems likely that these are not whole spores or diaspores. The very smallest recorded lichen spore is 1 µm in size, and vegetative diaspores are even larger. Hence, as proposed earlier, the INPs might be smaller particles (nano-INPs) or fragments of dispersal particles. As pointed out in O'Sullivan et al. (2015), for example, pollen harbours nanoscale entities that are attached to the pollen grains and are more numerous than the whole pollen grains. Similarly, the INPs found in this study are smaller than whole spores, soredia, or isidia. They could become airborne when attached to those larger propagules, or wind might pick up these smaller particles by coincidence. Alternatively, the INPs may also be bacteria living symbiotically with the lichen. As mentioned in Sect. 1, the bacteria that Kieft (1988) cultivated from lichen showed no ice-nucleating activity, but not all ice-nucleating bacteria are easily cultured.
However, when looking at their atmospheric relevance, it is not only important to know the size fraction of INPs, but the structure of the lichen that the particles stem from is bound to be important as well. For example, looking at Fig. 2, one can imagine that wind blowing over these lichens, as a possible way of dispersal for the INPs, interacts with each of them very differently. For example, Bryoria sp. is found to be hanging from trees and sways in the wind. Hence, particles that stem from this lichen species seem likely to be dispersed and lifted into the atmosphere more easily than particles on species that are tightly bound to trunks and branches. As discussed in Sect. 1, propagule dispersal mechanisms have been related to meteorological conditions. The lichen tested in the present study were sampled at subzero temperatures (see Fig. 5 in Schneider et al., 2021). Correlating meteorological variables (temperature, relative humidity, and wind speed) with the INP concentrations obtained from air filter measurements (Brasseur et al., 2022) could shed light on the hypothesis that lichenous INPs could be a local source in Hyytiälä.
A possible local source of INPs in Hyytiälä was explored with the thorough examination of the ice-nucleating ability of lichen sampled during the HyICE-2018 campaign. In accordance with the literature (Kieft, 1988; Ashworth and Kieft, 1992; Moffett et al., 2015), the mixtures of three lichen species sampled in Hyytiälä were shown to harbour INP-active components at temperatures as warm as −5 °C with concentrations of 103 g−1 of lichen. These findings were expanded through size segregation and heat tests. Many of the lichenous INPs were found to be between 0.1 and 2 µm in size when immersed in water, and those active at temperatures higher than −18 °C were heat-labile.
As mentioned in the Introduction, during HyICE-2018, the forest floor was covered in snow, thus preventing emissions of bioaerosol associated with leaf litter or soil, whereas copious quantities of lichen were exposed to the air. Thus, a viable explanation for the heat sensitivity and the size of ambient atmospheric INPs during HyICE-2018 is that they are derived from lichens.
Out of the cumulative nucleus spectra, two species of INPs could be identified. One species, active at −16 °C, was found to be heat-labile and smaller than 0.02 µm; the other species was active at −18 °C. The latter's concentration decreased upon filtering through a 2 µm pore size filter, but it was still detectable in the 0.02 µm size fraction. In the smaller size fractions <2 µm, this INP species was heat-labile, but in the larger size fractions, it was not. These differing sensitivities to heat across different size ranges suggest that the INP species responsible for freezing at −18 °C was present in two different states, attached to a larger particle or freely in a solution or in different states of aggregation. If it was attached to larger entities or in large aggregates, then it would be lost on filtration but is also apparently heat-stable. In contrast, when it is attached to small particles or free in a solution, it is more sensitive to heat.
In the species-specific experiments, the four species of lichen showed similar ice-nucleating activity, ranging from 103 INPs per gram of lichen at about −5 °C to 4×105 INPs per gram of lichen at about −14 °C. However, the species harbour differently sized INPs, as the activity decrease seen upon filtration through a 2 µm pore size filter varied by about 1 order of magnitude between species. This implies that some species of lichen may be more important as a source of INPs than others.
The size of INPs found in this study suggests that whole spores or soredia are not required for ice-nucleating activity but rather that smaller entities nucleate ice. This is analogous to pollen and fungal materials, where nanoscale ice-nucleating entities can become separate from their host (O'Sullivan et al., 2014). This supports the idea that these INPs could become airborne and either attached to (or part of) spores, soredia, or isidia or simply carried aloft by wind as independent particles. As the lichen species investigated here were sampled in Hyytiälä without any knowledge about their ice nucleation activity, and all were found to harbour INPs, these findings hold promise (in combination with the literature data) that lichen generally represent a source of atmospheric INPs, as they populate many terrestrial environments in great abundance. This source may be especially relevant in winter when the ground and with it other sources of biological INPs are snow-covered in boreal forests. Expanding upon lichen dispersal studies with emphasis on INPs, for example, wind tunnel experiments could be employed in combination with an online INP counter, such as the Portable Ice Nucleation Experiment (Möhler et al., 2021), controlling environmental factors such as temperature, relative humidity, and wind speed. Furthermore, it would need to be evaluated if and in which concentrations lichenaceous INPs reach atmospheric heights and locations where they might nucleate ice in clouds.
The comparison of samples B1 and C can be seen in Fig. B1. Generally, greater concentrations of INPs were present in sample C than in sample B1. As outlined in Sect. 2.2, lichens from sample B were mixed by hand for 10 min, and sample C was mixed on a rotary mixer for 30 min. The different procedures might contribute to the greater concentrations of INPs being released with the rotary mixer. This is consistent with the results for B1 and B2, where we saw more INPs being released with time, indicating sensitivity to the exact experimental procedure.
The “steps” discussed in Sect. 3 refer to different populations of ice-nucleating particles. These can be visualized in a differential spectrum computed from nm. We employed the heterogeneous underlying-based (HUB) package from de Almeida Ribeiro et al. (2023) to do this backward calculation. The settings of the script were left at the default ones, except for the number of points for the spline fit that was set to 50. However, we noticed that in our case, the algorithm was sensitive to the random seed and the other assumptions. Thus, the results are used here for illustrative purposes only. Figure C1 shows the results for those spectra which support the conclusions of the “steps” or different ice-nucleating species drawn in Sect. 3.
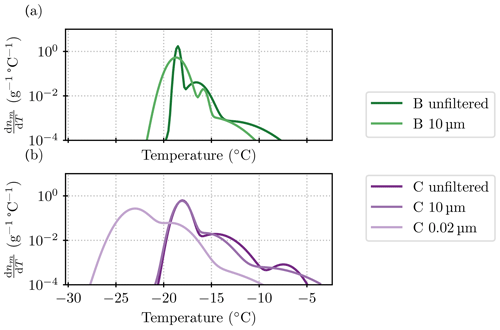
Figure C1Differential spectra for the filtered samples B and C (a and b, respectively). Note that other size fractions are not shown because the data from different dilutions did not line up well enough to allow for a sensible interpolation between them. Similarly, for the 10 µm size fraction of sample B, the point at −14 °C and 8.4×104 g−1 needed to be excluded to allow for a smooth fit.
Processed measurement data and plotting scripts are archived at https://doi.org/10.5281/zenodo.13886560 (Proske et al., 2024). The data processing for Fig. C1 was done with the HUB code from de Almeida Ribeiro et al. (2023).
UP conducted the experiments and analysis and wrote the paper together with BJM and with the support of the other co-authors. MPA and BJM conceived the idea for the study. MPA, GCEP, MAH, and BJM contributed to the design of the study and the analysis of the results. BJM sampled the lichen. MAH and UP took the lichen images. JB identified the lichen species sampled. All authors provided comments on and edits to the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Ice nucleation in the boreal atmosphere”. It is not associated with a conference.
We thank the team of the SMEAR II station for their efforts during the HyICE-2018 intensive campaign. We are grateful for support from and fruitful discussions with the HyICE-2018 team, in particular Jonathan Duplicy, and the members of the atmospheric ice nucleation group at Leeds University, in particular Mark Tarn and Tom Whale. We thank three anonymous reviewers and Hinrich Grothe for their constructive comments which improved the paper substantially.
This research has been supported by the European Research Council (grant no. 648661); the EU Horizon 2020 (grant no. 654109); and the UK Research and Innovation, Engineering and Physical Sciences Research Council (grant no. EP/M003027/1).
This paper was edited by Paul Zieger and reviewed by Hinrich Grothe and three anonymous referees.
Alpert, P. A., Aller, J. Y., and Knopf, D. A.: Ice nucleation from aqueous NaCl droplets with and without marine diatoms, Atmos. Chem. Phys., 11, 5539–5555, https://doi.org/10.5194/acp-11-5539-2011, 2011. a
Armstrong, R.: The Influence of Climate on the Dispersal of Lichen Soredia, Environ. Exp. Bot., 31, 239–245, https://doi.org/10.1016/0098-8472(91)90076-Z, 1991. a
Armstrong, R. A.: Dispersal of Soredia from Individual Soralia of the Lichen Hypogymnia Physodes (L.) NYL. in a Simple Wind Tunnel, Environ. Exp. Bot., 34, 39–45, 1993. a
Aschenbrenner, I. A., Cernava, T., Berg, G., and Grube, M.: Understanding Microbial Multi-Species Symbioses, Front. Microbiol., 7, 180, https://doi.org/10.3389/fmicb.2016.00180, 2016. a
Ashworth, E. N. and Kieft, T. L.: Measurement of Ice Nucleation in Lichens Using Thermal Analysis, Cryobiology, 29, 400–406, 1992. a, b, c, d, e, f
Atkinson, J. D., Murray, B. J., Woodhouse, M. T., Whale, T. F., Baustian, K. J., Carslaw, K. S., Dobbie, S., O'Sullivan, D., and Malkin, T. L.: The Importance of Feldspar for Ice Nucleation by Mineral Dust in Mixed-Phase Clouds, Nature, 498, 355–358, https://doi.org/10.1038/nature12278, 2013. a
Bailey, R. H.: Studies on the Dispersal of Lichen Soredia, Bot. J. Linn. Soc., 59, 479–490, https://doi.org/10.1111/j.1095-8339.1966.tb00074.x, 1966. a
Bogler, S. and Borduas-Dedekind, N.: Lignin's ability to nucleate ice via immersion freezing and its stability towards physicochemical treatments and atmospheric processing, Atmos. Chem. Phys., 20, 14509–14522, https://doi.org/10.5194/acp-20-14509-2020, 2020. a
Bowler, P. A. and Rundel, P. W.: Reproductive Strategies in Lichens, Bot. J. Linn. Soc., 70, 325–340, https://doi.org/10.1111/j.1095-8339.1975.tb01653.x, 1975. a, b
Brasseur, Z., Castarède, D., Thomson, E. S., Adams, M. P., Drossaart van Dusseldorp, S., Heikkilä, P., Korhonen, K., Lampilahti, J., Paramonov, M., Schneider, J., Vogel, F., Wu, Y., Abbatt, J. P. D., Atanasova, N. S., Bamford, D. H., Bertozzi, B., Boyer, M., Brus, D., Daily, M. I., Fösig, R., Gute, E., Harrison, A. D., Hietala, P., Höhler, K., Kanji, Z. A., Keskinen, J., Lacher, L., Lampimäki, M., Levula, J., Manninen, A., Nadolny, J., Peltola, M., Porter, G. C. E., Poutanen, P., Proske, U., Schorr, T., Silas Umo, N., Stenszky, J., Virtanen, A., Moisseev, D., Kulmala, M., Murray, B. J., Petäjä, T., Möhler, O., and Duplissy, J.: Measurement report: Introduction to the HyICE-2018 campaign for measurements of ice-nucleating particles and instrument inter-comparison in the Hyytiälä boreal forest, Atmos. Chem. Phys., 22, 5117–5145, https://doi.org/10.5194/acp-22-5117-2022, 2022. a, b
Cernava, T., Erlacher, A., Aschenbrenner, I. A., Krug, L., Lassek, C., Riedel, K., Grube, M., and Berg, G.: Deciphering Functional Diversification within the Lichen Microbiota by Meta-Omics, Microbiome, 5, 82, https://doi.org/10.1186/s40168-017-0303-5, 2017. a
Christner, B. C., Cai, R., Morris, C. E., McCarter, K. S., Foreman, C. M., Skidmore, M. L., Montross, S. N., and Sands, D. C.: Geographic, Seasonal, and Precipitation Chemistry Influence on the Abundance and Activity of Biological Ice Nucleators in Rain and Snow, P. Natl. Acad. Sci. USA, 105, 18854–18859, https://doi.org/10.1073/pnas.0809816105, 2008. a
Clarke, A., Morris, G. J., Fonseca, F., Murray, B. J., Acton, E., and Price, H. C.: A Low Temperature Limit for Life on Earth, PLoS ONE, 8, e66207, https://doi.org/10.1371/journal.pone.0066207, 2013. a
Conen, F., Morris, C. E., Leifeld, J., Yakutin, M. V., and Alewell, C.: Biological residues define the ice nucleation properties of soil dust, Atmos. Chem. Phys., 11, 9643–9648, https://doi.org/10.5194/acp-11-9643-2011, 2011. a
Daily, M. I., Whale, T. F., Partanen, R., Harrison, A. D., Kilbride, P., Lamb, S., Morris, G. J., Picton, H. M., and Murray, B. J.: Cryopreservation of Primary Cultures of Mammalian Somatic Cells in 96-Well Plates Benefits from Control of Ice Nucleation, Cryobiology, 93, 62–69, https://doi.org/10.1016/j.cryobiol.2020.02.008, 2020. a
Daily, M. I., Tarn, M. D., Whale, T. F., and Murray, B. J.: An evaluation of the heat test for the ice-nucleating ability of minerals and biological material, Atmos. Meas. Tech., 15, 2635–2665, https://doi.org/10.5194/amt-15-2635-2022, 2022. a, b, c, d
Daily, M. I., Whale, T. F., Kilbride, P., Lamb, S., Morris, G. J., Picton, H. M., and Murray, B. J.: A Highly Active Mineral-Based Ice Nucleating Agent Supports in Situ Cell Cryopreservation in a High Throughput Format, J. R. Soc. Interface, 20, 20220682, https://doi.org/10.1098/rsif.2022.0682, 2023. a
de Almeida Ribeiro, I., Meister, K., and Molinero, V.: HUB: a method to model and extract the distribution of ice nucleation temperatures from drop-freezing experiments, Atmos. Chem. Phys., 23, 5623–5639, https://doi.org/10.5194/acp-23-5623-2023, 2023. a, b
DeMott, P. J., Prenni, A. J., Liu, X., Kreidenweis, S. M., Petters, M. D., Twohy, C. H., Richardson, M. S., Eidhammer, T., and Rogers, D. C.: Predicting Global Atmospheric Ice Nuclei Distributions and Their Impacts on Climate, P. Natl. Acad. Sci. USA, 107, 11217–11222, 2010. a
DeMott, P. J., Hill, T. C. J., McCluskey, C. S., Prather, K. A., Collins, D. B., Sullivan, R. C., Ruppel, M. J., Mason, R. H., Irish, V. E., Lee, T., Hwang, C. Y., Rhee, T. S., Snider, J. R., McMeeking, G. R., Dhaniyala, S., Lewis, E. R., Wentzell, J. J. B., Abbatt, J., Lee, C., Sultana, C. M., Ault, A. P., Axson, J. L., Diaz Martinez, M., Venero, I., Santos-Figueroa, G., Stokes, M. D., Deane, G. B., Mayol-Bracero, O. L., Grassian, V. H., Bertram, T. H., Bertram, A. K., Moffett, B. F., and Franc, G. D.: Sea Spray Aerosol as a Unique Source of Ice Nucleating Particles, P. Natl. Acad. Sci. USA, 113, 5797–5803, https://doi.org/10.1073/pnas.1514034112, 2016. a
DeMott, P. J., Möhler, O., Cziczo, D. J., Hiranuma, N., Petters, M. D., Petters, S. S., Belosi, F., Bingemer, H. G., Brooks, S. D., Budke, C., Burkert-Kohn, M., Collier, K. N., Danielczok, A., Eppers, O., Felgitsch, L., Garimella, S., Grothe, H., Herenz, P., Hill, T. C. J., Höhler, K., Kanji, Z. A., Kiselev, A., Koop, T., Kristensen, T. B., Krüger, K., Kulkarni, G., Levin, E. J. T., Murray, B. J., Nicosia, A., O'Sullivan, D., Peckhaus, A., Polen, M. J., Price, H. C., Reicher, N., Rothenberg, D. A., Rudich, Y., Santachiara, G., Schiebel, T., Schrod, J., Seifried, T. M., Stratmann, F., Sullivan, R. C., Suski, K. J., Szakáll, M., Taylor, H. P., Ullrich, R., Vergara-Temprado, J., Wagner, R., Whale, T. F., Weber, D., Welti, A., Wilson, T. W., Wolf, M. J., and Zenker, J.: The Fifth International Workshop on Ice Nucleation phase 2 (FIN-02): laboratory intercomparison of ice nucleation measurements, Atmos. Meas. Tech., 11, 6231–6257, https://doi.org/10.5194/amt-11-6231-2018, 2018. a
Després, V. R., Huffman, J., Burrows, S. M., Hoose, C., Safatov, A., Buryak, G., Fröhlich-Nowoisky, J., Elbert, W., Andreae, M., Pöschl, U., and Jaenicke, R.: Primary Biological Aerosol Particles in the Atmosphere: A Review, Tellus B, 64, 15598, https://doi.org/10.3402/tellusb.v64i0.15598, 2012. a
Eufemio, R. J., de Almeida Ribeiro, I., Sformo, T. L., Laursen, G. A., Molinero, V., Fröhlich-Nowoisky, J., Bonn, M., and Meister, K.: Lichen species across Alaska produce highly active and stable ice nucleators, Biogeosciences, 20, 2805–2812, https://doi.org/10.5194/bg-20-2805-2023, 2023. a, b, c, d, e, f, g, h, i, j, k
Forster, P., Storelvmo, T., Armour, K., Collins, W., Dufresne, J.-L., Frame, D., Lunt, D., Mauritsen, T., Palmer, M., Watanabe, M., Wild, M., and Zhang, H.: The Earth's Energy Budget, Climate Feedbacks, and Climate Sensitivity, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 923–1054, https://doi.org/10.1017/9781009157896.009, 2021. a
Frey, M. M., Norris, S. J., Brooks, I. M., Anderson, P. S., Nishimura, K., Yang, X., Jones, A. E., Nerentorp Mastromonaco, M. G., Jones, D. H., and Wolff, E. W.: First direct observation of sea salt aerosol production from blowing snow above sea ice, Atmos. Chem. Phys., 20, 2549–2578, https://doi.org/10.5194/acp-20-2549-2020, 2020. a
Garcia, E., Hill, T. C. J., Prenni, A. J., DeMott, P. J., Franc, G. D., and Kreidenweis, S. M.: Biogenic Ice Nuclei in Boundary Layer Air over Two U.S. High Plains Agricultural Regions, J. Geophys. Res.-Atmos., 117, D18209, https://doi.org/10.1029/2012JD018343, 2012. a
Grimm, M., Grube, M., Schiefelbein, U., Zühlke, D., Bernhardt, J., and Riedel, K.: The Lichens' Microbiota, Still a Mystery?, Front. Microbiol., 12, 623839, https://doi.org/10.3389/fmicb.2021.623839, 2021. a
Hale, M. E.: The Biology of Lichens, Contemporary Biology, in: 2nd Edn., Edward Arnold, London, ISBN 978-0-7131-2456-9, 1974. a, b, c, d, e, f
Hara, K., Maki, T., Kakikawa, M., Kobayashi, F., and Matsuki, A.: Effects of Different Temperature Treatments on Biological Ice Nuclei in Snow Samples, Atmos. Environ., 140, 415–419, https://doi.org/10.1016/j.atmosenv.2016.06.011, 2016. a, b
Harrison, A. D., Whale, T. F., Carpenter, M. A., Holden, M. A., Neve, L., O'Sullivan, D., Vergara Temprado, J., and Murray, B. J.: Not all feldspars are equal: a survey of ice nucleating properties across the feldspar group of minerals, Atmos. Chem. Phys., 16, 10927–10940, https://doi.org/10.5194/acp-16-10927-2016, 2016. a
Henderson-Begg, S. K., Hill, T., Thyrhaug, R., Khan, M., and Moffett, B. F.: Terrestrial and Airborne Non-Bacterial Ice Nuclei, Atmos. Sci. Lett., 10, 215–219, https://doi.org/10.1002/asl.241, 2009. a, b
Hill, T. C. J., Moffett, B. F., DeMott, P. J., Georgakopoulos, D. G., Stump, W. L., and Franc, G. D.: Measurement of Ice Nucleation-Active Bacteria on Plants and in Precipitation by Quantitative PCR, Appl. Environ. Microb., 80, 1256–1267, https://doi.org/10.1128/AEM.02967-13, 2014. a
Hill, T. C. J., DeMott, P. J., Tobo, Y., Fröhlich-Nowoisky, J., Moffett, B. F., Franc, G. D., and Kreidenweis, S. M.: Sources of organic ice nucleating particles in soils, Atmos. Chem. Phys., 16, 7195–7211, https://doi.org/10.5194/acp-16-7195-2016, 2016. a
Hiranuma, N., Augustin-Bauditz, S., Bingemer, H., Budke, C., Curtius, J., Danielczok, A., Diehl, K., Dreischmeier, K., Ebert, M., Frank, F., Hoffmann, N., Kandler, K., Kiselev, A., Koop, T., Leisner, T., Möhler, O., Nillius, B., Peckhaus, A., Rose, D., Weinbruch, S., Wex, H., Boose, Y., DeMott, P. J., Hader, J. D., Hill, T. C. J., Kanji, Z. A., Kulkarni, G., Levin, E. J. T., McCluskey, C. S., Murakami, M., Murray, B. J., Niedermeier, D., Petters, M. D., O'Sullivan, D., Saito, A., Schill, G. P., Tajiri, T., Tolbert, M. A., Welti, A., Whale, T. F., Wright, T. P., and Yamashita, K.: A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: a comparison of 17 ice nucleation measurement techniques, Atmos. Chem. Phys., 15, 2489–2518, https://doi.org/10.5194/acp-15-2489-2015, 2015. a
Hiranuma, N., Adachi, K., Bell, D. M., Belosi, F., Beydoun, H., Bhaduri, B., Bingemer, H., Budke, C., Clemen, H.-C., Conen, F., Cory, K. M., Curtius, J., DeMott, P. J., Eppers, O., Grawe, S., Hartmann, S., Hoffmann, N., Höhler, K., Jantsch, E., Kiselev, A., Koop, T., Kulkarni, G., Mayer, A., Murakami, M., Murray, B. J., Nicosia, A., Petters, M. D., Piazza, M., Polen, M., Reicher, N., Rudich, Y., Saito, A., Santachiara, G., Schiebel, T., Schill, G. P., Schneider, J., Segev, L., Stopelli, E., Sullivan, R. C., Suski, K., Szakáll, M., Tajiri, T., Taylor, H., Tobo, Y., Ullrich, R., Weber, D., Wex, H., Whale, T. F., Whiteside, C. L., Yamashita, K., Zelenyuk, A., and Möhler, O.: A comprehensive characterization of ice nucleation by three different types of cellulose particles immersed in water, Atmos. Chem. Phys., 19, 4823–4849, https://doi.org/10.5194/acp-19-4823-2019, 2019. a
Hoose, C. and Möhler, O.: Heterogeneous ice nucleation on atmospheric aerosols: a review of results from laboratory experiments, Atmos. Chem. Phys., 12, 9817–9854, https://doi.org/10.5194/acp-12-9817-2012, 2012. a
Irish, V. E., Elizondo, P., Chen, J., Chou, C., Charette, J., Lizotte, M., Ladino, L. A., Wilson, T. W., Gosselin, M., Murray, B. J., Polishchuk, E., Abbatt, J. P. D., Miller, L. A., and Bertram, A. K.: Ice-nucleating particles in Canadian Arctic sea-surface microlayer and bulk seawater, Atmos. Chem. Phys., 17, 10583–10595, https://doi.org/10.5194/acp-17-10583-2017, 2017. a
Kappen, L. and Valladares, F.: Opportunistic Growth and Desiccation Tolerance: The Ecological Success of Poikilohydrous Autotrophs, in: Handbook of Functional Plant Ecology, edited by: Pugnaire, F. I. and Valladares, F., CRC Press, Boca Raton, 7–65, ISBN 9780429122477, 007. a
Kieft, T. L.: Ice Nucleation Activity in Lichens, Appl. Environ. Microb., 54, 1678–1681, 1988. a, b, c, d, e, f, g, h, i, j, k, l, m, n
Kieft, T. L. and Ahmadjian, V.: Biological Ice Nucleation Activity in Lichen Mycobionts and Photobionts, Lichenologist, 21, 355–362, https://doi.org/10.1017/S0024282989000599, 1989. a, b, c
Kieft, T. L. and Ruscetti, T.: Characterization of Biological Ice Nuclei from a Lichen, J. Bacteriol., 172, 3519–3523, https://doi.org/10.1128/jb.172.6.3519-3523.1990, 1990. a, b, c, d, e, f
Kieft, T. L. and Ruscetti, T.: Molecular Sizes of Lichen Ice Nucleation Sites Determined by Gamma Radiation Inactivation Analysis, Cryobiology, 29, 407–413, https://doi.org/10.1016/0011-2240(92)90042-Z, 1992. a, b
Lindow, S. E.: Localization of Ice Nucleation Activity and the iceC Gene Product in Pseudomonas Syringae and Escherichia Coli, Mol. Plant Microbe In., 2, 262, https://doi.org/10.1094/MPMI-2-262, 1989. a
Marshall, W. A.: Aerial Dispersal of Lichen Soredia in the Maritime Antarctic, New Phytol., 134, 523–530, https://doi.org/10.1111/j.1469-8137.1996.tb04370.x, 1996. a, b
Moffett, B. F., Getti, G., Henderson-Begg, S. K., and Hill, T. C. J.: Ubiquity of Ice Nucleation in Lichen – Possible Atmospheric Implications, Lindbergia, 3, 39–43, https://doi.org/10.25227/linbg.01070, 2015. a, b, c, d, e, f
Möhler, O., Adams, M., Lacher, L., Vogel, F., Nadolny, J., Ullrich, R., Boffo, C., Pfeuffer, T., Hobl, A., Weiß, M., Vepuri, H. S. K., Hiranuma, N., and Murray, B. J.: The Portable Ice Nucleation Experiment (PINE): a new online instrument for laboratory studies and automated long-term field observations of ice-nucleating particles, Atmos. Meas. Tech., 14, 1143–1166, https://doi.org/10.5194/amt-14-1143-2021, 2021. a
Murray, B. J., O'Sullivan, D., Atkinson, J. D., and Webb, M. E.: Ice Nucleation by Particles Immersed in Supercooled Cloud Droplets, Chem. Soc. Rev., 41, 6519, https://doi.org/10.1039/c2cs35200a, 2012. a, b, c
Murray, B. J., Carslaw, K. S., and Field, P. R.: Opinion: Cloud-phase climate feedback and the importance of ice-nucleating particles, Atmos. Chem. Phys., 21, 665–679, https://doi.org/10.5194/acp-21-665-2021, 2021. a, b
Nash, T. H.: Lichen Biology, Cambridge University Press, Leiden, ISBN 978-0-511-41407-7, 2008. a, b
O'Sullivan, D., Murray, B. J., Malkin, T. L., Whale, T. F., Umo, N. S., Atkinson, J. D., Price, H. C., Baustian, K. J., Browse, J., and Webb, M. E.: Ice nucleation by fertile soil dusts: relative importance of mineral and biogenic components, Atmos. Chem. Phys., 14, 1853–1867, https://doi.org/10.5194/acp-14-1853-2014, 2014. a, b, c, d
O'Sullivan, D., Murray, B. J., Ross, J. F., Whale, T. F., Price, H. C., Atkinson, J. D., Umo, N. S., and Webb, M. E.: The Relevance of Nanoscale Biological Fragments for Ice Nucleation in Clouds, Sci. Rep.-UK, 5, 1–7, https://doi.org/10.1038/srep08082, 2015. a, b, c, d, e, f
O'Sullivan, D., Murray, B. J., Ross, J. F., and Webb, M. E.: The adsorption of fungal ice-nucleating proteins on mineral dusts: a terrestrial reservoir of atmospheric ice-nucleating particles, Atmos. Chem. Phys., 16, 7879–7887, https://doi.org/10.5194/acp-16-7879-2016, 2016. a
O'Sullivan, D., Adams, M. P., Tarn, M. D., Harrison, A. D., Vergara-Temprado, J., Porter, G. C. E., Holden, M. A., Sanchez-Marroquin, A., Carotenuto, F., Whale, T. F., McQuaid, J. B., Walshaw, R., Hedges, D. H. P., Burke, I. T., Cui, Z., and Murray, B. J.: Contributions of Biogenic Material to the Atmospheric Ice-Nucleating Particle Population in North Western Europe, Sci. Rep.-UK, 8, 13821, https://doi.org/10.1038/s41598-018-31981-7, 2018. a
Porter, G. C. E., Sikora, S. N. F., Adams, M. P., Proske, U., Harrison, A. D., Tarn, M. D., Brooks, I. M., and Murray, B. J.: Resolving the size of ice-nucleating particles with a balloon deployable aerosol sampler: the SHARK, Atmos. Meas. Tech., 13, 2905–2921, https://doi.org/10.5194/amt-13-2905-2020, 2020. a, b
Pouleur, S., Richard, C., Martin, J. G., and Antoun, H.: Ice Nucleation Activity in Fusarium Acuminatum and Fusarium Avenaceum, Appl. Environ. Microb., 58, 2960–2964, 1992. a
Prenni, A. J., Petters, M. D., Kreidenweis, S. M., Heald, C. L., Martin, S. T., Artaxo, P., Garland, R. M., Wollny, A. G., and Pöschl, U.: Relative Roles of Biogenic Emissions and Saharan Dust as Ice Nuclei in the Amazon Basin, Nat. Geosci., 2, 402–405, https://doi.org/10.1038/ngeo517, 2009. a
Proske, U., Adams, M. P., Porter, G. C. E., Holden, M., Bäck, J., and Murray, B. J.: Data and Scripts for the Publication Measurement Report: The Ice-Nucleating Activity of Lichen Sampled in a Northern European Boreal Forest, version 2, Zenodo [code and data set], https://doi.org/10.5281/zenodo.13886560, 2024. a
Pummer, B. G., Bauer, H., Bernardi, J., Bleicher, S., and Grothe, H.: Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen, Atmos. Chem. Phys., 12, 2541–2550, https://doi.org/10.5194/acp-12-2541-2012, 2012. a, b
Ronnås, C., Werth, S., Ovaskainen, O., Várkonyi, G., Scheidegger, C., and Snäll, T.: Discovery of Long-Distance Gamete Dispersal in a Lichen-Forming Ascomycete, New Phytol., 216, 216–226, https://doi.org/10.1111/nph.14714, 2017. a
Schneider, J., Höhler, K., Heikkilä, P., Keskinen, J., Bertozzi, B., Bogert, P., Schorr, T., Umo, N. S., Vogel, F., Brasseur, Z., Wu, Y., Hakala, S., Duplissy, J., Moisseev, D., Kulmala, M., Adams, M. P., Murray, B. J., Korhonen, K., Hao, L., Thomson, E. S., Castarède, D., Leisner, T., Petäjä, T., and Möhler, O.: The seasonal cycle of ice-nucleating particles linked to the abundance of biogenic aerosol in boreal forests, Atmos. Chem. Phys., 21, 3899–3918, https://doi.org/10.5194/acp-21-3899-2021, 2021. a, b, c, d
Schnell, R. and Vali, G.: Biogenic Ice Nuclei: Part I. Terrestrial and Marine Sources, J. Atmos. Sci., 33, 1554–1564, 1976. a
Schwidetzky, R., de Almeida Ribeiro, I., Bothen, N., Backes, A. T., DeVries, A. L., Bonn, M., Fröhlich-Nowoisky, J., Molinero, V., and Meister, K.: Functional aggregation of cell-free proteins enables fungal ice nucleation, P. Natl. Acad. Sci. USA, 120, e2303243120, https://doi.org/10.1073/pnas.2303243120, 2023. a, b
Seifried, T. M., Reyzek, F., Bieber, P., and Grothe, H.: Scots Pines (Pinus sylvestris) as Sources of Biological Ice-Nucleating Macromolecules (INMs), Atmosphere-Basel, 14, 266, https://doi.org/10.3390/atmos14020266, 2023. a
Spracklen, D. V. and Heald, C. L.: The contribution of fungal spores and bacteria to regional and global aerosol number and ice nucleation immersion freezing rates, Atmos. Chem. Phys., 14, 9051–9059, https://doi.org/10.5194/acp-14-9051-2014, 2014. a
Stopelli, E., Conen, F., Zimmermann, L., Alewell, C., and Morris, C. E.: Freezing nucleation apparatus puts new slant on study of biological ice nucleators in precipitation, Atmos. Meas. Tech., 7, 129–134, https://doi.org/10.5194/amt-7-129-2014, 2014. a
Tan, I., Storelvmo, T., and Zelinka, M. D.: Observational Constraints on Mixed-Phase Clouds Imply Higher Climate Sensitivity, Science, 352, 224–227, https://doi.org/10.1126/science.aad5300, 2016. a
Tormo, R., Recio, D., Silva, I., and Muñoz, A.: A Quantitative Investigation of Airborne Algae and Lichen Soredia Obtained from Pollen Traps in South-West Spain, Eur. J. Phycol., 36, 385–390, https://doi.org/10.1080/09670260110001735538, 2001. a
Turner, M. A., Arellano, F., and Kozloff, L. M.: Three Separate Classes of Bacterial Ice Nucleation Structures, J. Bacteriol., 172, 2521–2526, https://doi.org/10.1128/jb.172.5.2521-2526.1990, 1990. a
Vali, G.: Quantitative Evaluation of Experimental Results on the Heterogeneous Freezing Nucleation of Supercooled Liquids, J. Atmos. Sci., 28, 402–409, 1971. a, b
Vergara-Temprado, J., Murray, B. J., Wilson, T. W., O'Sullivan, D., Browse, J., Pringle, K. J., Ardon-Dryer, K., Bertram, A. K., Burrows, S. M., Ceburnis, D., DeMott, P. J., Mason, R. H., O'Dowd, C. D., Rinaldi, M., and Carslaw, K. S.: Contribution of feldspar and marine organic aerosols to global ice nucleating particle concentrations, Atmos. Chem. Phys., 17, 3637–3658, https://doi.org/10.5194/acp-17-3637-2017, 2017. a, b
Vogel, F., Adams, M. P., Lacher, L., Foster, P. B., Porter, G. C. E., Bertozzi, B., Höhler, K., Schneider, J., Schorr, T., Umo, N. S., Nadolny, J., Brasseur, Z., Heikkilä, P., Thomson, E. S., Büttner, N., Daily, M. I., Fösig, R., Harrison, A. D., Keskinen, J., Proske, U., Duplissy, J., Kulmala, M., Petäjä, T., Möhler, O., and Murray, B. J.: Ice-nucleating particles active below −24 °C in a Finnish boreal forest and their relationship to bioaerosols, Atmos. Chem. Phys., 24, 11737–11757, https://doi.org/10.5194/acp-24-11737-2024, 2024. a
Whale, T. F., Murray, B. J., O'Sullivan, D., Wilson, T. W., Umo, N. S., Baustian, K. J., Atkinson, J. D., Workneh, D. A., and Morris, G. J.: A technique for quantifying heterogeneous ice nucleation in microlitre supercooled water droplets, Atmos. Meas. Tech., 8, 2437–2447, https://doi.org/10.5194/amt-8-2437-2015, 2015. a, b
Wilson, T. W., Ladino, L. A., Alpert, P. A., Breckels, M. N., Brooks, I. M., Browse, J., Burrows, S. M., Carslaw, K. S., Huffman, J. A., Judd, C., Kilthau, W. P., Mason, R. H., McFiggans, G., Miller, L. A., Nájera, J. J., Polishchuk, E., Rae, S., Schiller, C. L., Si, M., Temprado, J. V., Whale, T. F., Wong, J. P. S., Wurl, O., Yakobi-Hancock, J. D., Abbatt, J. P. D., Aller, J. Y., Bertram, A. K., Knopf, D. A., and Murray, B. J.: A Marine Biogenic Source of Atmospheric Ice-Nucleating Particles, Nature, 525, 234–238, https://doi.org/10.1038/nature14986, 2015. a
Wright, T. P. and Petters, M. D.: The Role of Time in Heterogeneous Freezing Nucleation, J. Geophys. Res.-Atmos., 118, 3731–3743, https://doi.org/10.1002/jgrd.50365, 2013. a
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Conclusions
- Appendix A: Blanks
- Appendix B: Comparison of the extraction techniques
- Appendix C: Differential spectra
- Code and data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Conclusions
- Appendix A: Blanks
- Appendix B: Comparison of the extraction techniques
- Appendix C: Differential spectra
- Code and data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References