the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Contrasting solubility and speciation of metal ions in total suspended particulate matter and fog from the coast of Namibia – Part 1
Chiara Giorio
Anne Monod
Valerio Di Marco
Pierre Herckes
Denise Napolitano
Amy Sullivan
Gautier Landrot
Daniel Warnes
Marika Nasti
Sara D'Aronco
Agathe Gérardin
Nicolas Brun
Karine Desboeufs
Sylvain Triquet
Servanne Chevaillier
Claudia Di Biagio
Francesco Battaglia
Frédéric Burnet
Stuart J. Piketh
Andreas Namwoonde
Jean-François Doussin
Paola Formenti
The western coast of southern Africa is a region of particular climate interest and crossroads for aerosols of different origins as well as fog occurrences. In this study, we present a comparison between the concentration of dissolved trace metals in pairs of total suspended particulate (TSP) and fog water samples collected in Henties Bay, Namibia, during the AErosols, Radiation and CLOuds in southern Africa (AEROCLO-sA) field campaign in September 2017. From inductively coupled plasma mass spectrometry measurements, we found that the concentration of dissolved Al, Fe, Ni, Cu, and Cr is enhanced in fog samples compared to the TSP samples. We found that thermodynamic modelling predicts the formation of soluble complexes with inorganic and organic ligands in fog for Cu, Cr, and Ni, but it would predict Al and Fe to precipitate as hydroxides given the neutral pH of fog. In contrast, X-ray absorption near edge structure measurements showed the presence of oxalate of Fe complexes that could explain its enhanced dissolved concentration in fog samples, despite a neutral pH. In addition, transmission electron microscopy and dynamic light scattering measurements revealed the presence of nano-sized colloidal particles containing Fe and Al in filtered fog samples that may appear soluble in inductively coupled plasma mass spectrometer (ICP-MS) measurements. We hypothesise that these complexes are formed in the early stages of particle activation into droplets when water content and, therefore, pH are expected to be lower and then remain in fog in a kinetically stable form or lead to the formation of colloidal nanoparticles.
- Article
(3057 KB) - Full-text XML
- Companion paper
-
Supplement
(956 KB) - BibTeX
- EndNote
Aerosols contribute the largest uncertainty to global radiative forcing budget estimates (IPCC, 2021) due to the lack of information related to global aerosol distributions, composition, and ageing effects in the atmosphere, all of which affect aerosol radiative properties. Natural aerosols from aeolian erosion of arid and semi-arid continental surfaces, marine emissions, and forest fires constitute an essential piece of the puzzle, without which our ability to predict Earth's climate evolution over time diminishes, and the development of adaptation strategies for future climate change remains limited (Bauer et al., 2022).
The western coast of southern Africa is a region of particular climate interest but is still understudied. It is the crossroads for aerosols of different origins and chemical compositions, including mineral dust emitted predominantly from the coastal riverbeds and gravel plains in Namibia (Dansie et al., 2018; von Holdt and Eckardt, 2018; Shikwambana and Kganyago, 2022; Vickery and Eckardt, 2013), sea spray and marine biogenic emissions from the Benguela upwelling system (Giorio et al., 2022a; Klopper et al., 2020), ship traffic emissions and dust from mining (Klopper et al., 2020; Tournadre, 2014), and long-range-transported biomass burning aerosols from central Africa (Flamant et al., 2022; Formenti et al., 2018, 2019; Gupta et al., 2021; Hecobian et al., 2011; Redemann et al., 2021). It is also an area of the globe where future climate projections predict severe warming and drought (Engelbrecht et al., 2024; Trisos et al., 2023), affecting the most sensitive regional environmental features, such as the oceanic Benguela upwelling system and stratocumulus cloud deck (Jarre et al., 2015; Louw et al., 2016; Tyson and Preston-Whyte, 2000), but also the coastal fog resulting from the advection of low-stratus and moist cold air masses from the ocean (Andersen et al., 2020) and the vector of water to the hyperarid Namibian ecosystems (Gottlieb et al., 2019; Henschel et al., 2019).
The western coast of southern Africa appears henceforth as an ideal natural laboratory for investigating the composition of aerosols and their metal chemical speciation, i.e. the chemical form in which metals, and in particular iron, are present in the aerosol and dissolved in a solution and the way in which they condition their ability to form fog and cloud droplets.
In this study, we take advantage of the first simultaneous measurements of aerosol and fog composition conducted at a ground-based site in Henties Bay, Namibia, during the AErosols, Radiation and CLOuds in southern Africa (AEROCLO-sA) field campaign in August–September 2017 (Formenti et al., 2019). In particular, we focus on the period 3–11 September, which was characterised by the occurrence of several fog events as well as by a synoptic-scale circulation that brought north-easterly winds and continental air masses to the site, thus transporting mining dust from northern Namibia and possibly farther north from the Zambian Copper Belt (Formenti et al., 2025). A previous study in this area found that the fractional solubility of iron can reach values of up to 20 % due to the photo-reduction processes of marine biogenic emissions (Desboeufs et al., 2024). Such processes can involve the formation of photoactive complexes of Fe(III) and methanesulfinic acid, which absorb and photolyse in the visible range to produce Fe(II) and methanesulfonic acid, among other species (Johansen and Key, 2006). Similarly, Fe(III)–carboxylate complexes can undergo photochemical redox reactions where the Fe(III) is reduced to Fe(II) (Siefert et al., 1994). In this paper, we present a comparison between the concentration of dissolved trace metals in pairs of total suspended particulate (TSP) and fog water samples, aiming to understand the role of particulate matter in fog droplet formation and the chemical processes facilitating the transfer of those aerosol particles to the aqueous phase in fog. In this respect, we explore the formation of metal–ligand complexes (compounds consisting of a central metal ion coordinated by molecules or ions, i.e. ligands), which could be promoted by the strong oceanic biogenic activities of the Benguela upwelling (Giorio et al., 2022a) as well as by fog events. Indeed, many water-soluble organic and inorganic compounds already present in the aerosol have coordinating properties towards metal ions, and they can increase their solubility, bioavailability, and light-absorption characteristics, as demonstrated by previous studies in urban, rural, and desert environments (Deguillaume et al., 2005, 2010; Desboeufs et al., 2005; Giorio et al., 2017, 2022b; Paris et al., 2011; Paris and Desboeufs, 2013; Tapparo et al., 2020). In addition, it has already been demonstrated that atmospheric processing can lead to increasing solubility of iron in mineral dusts (Buck et al., 2006; Cwiertny et al., 2008; Gao et al., 2019; Longo et al., 2016; Rodríguez et al., 2021; Shi et al., 2012; Winton et al., 2015).
The AEROCLO-sA field campaign took place in August–September 2017 at the Sam Nujoma Marine and Coastal Resources Research Centre (SANUMARC) of the University of Namibia in Henties Bay (22°6′ S, 14°30′ E; 20 m above sea level) (Formenti et al., 2019). The aerosol measurements were conducted by the PortablE Gas and Aerosols Sampling Units (PEGASUS) mobile facility as detailed in the companion paper by Formenti et al. (2025). A detailed description of the chemical composition of the TSP samples can be found in Formenti et al. (2025), while a detailed description of the fog microphysics and chemical composition is given in Sect. 4.2.
Four aerosol samples including particles up to 40 µm in aerodynamic diameter (Rajot et al., 2008), hereafter called TSP matter, were collected in parallel during daytime and nighttime (from approximately 07:00–17:00 UTC and 17:30–06:30 UTC, respectively): one on 47 mm diameter Teflon membranes (Zefluor®, 2 µm diameter pore size), two on Nuclepore® polycarbonate filters (one of 37 mm diameter and one of 47 mm diameter, 0.4 µm pore size), and the fourth on 47 mm diameter quartz membranes (Pall, 2500QAT-UP Tissuquartz). Field blanks were also collected (filters mounted on the sampling lines without activating the airflow). Details of the preparation and storage as well as analysis of the elemental composition, water-soluble inorganic and organic species, concentration of dissolved metals, and organic and elemental carbon are described in detail in Formenti et al. (2025).
The list of aerosol samples and concomitant fog samples relevant to this paper is presented in Table 1, while in the following paragraphs we describe the strategy of fog and seawater collection and analysis.
Table 1Dates and times of collection of concurrent aerosol and fog samples in Henties Bay during the AEROCLO-sA campaign in September 2017. Dates are reported as “dd/mm/yy”, time is in UTC.
2.1 Fog and seawater sample collection
Fog samples were collected using two pre-cleaned Caltech Active Strand Cloud Water Collectors (CASCCs) with a droplet size collection efficiency of 50 % at 3.5 µm in diameter (Demoz et al., 1996). One of the collectors was a stainless-steel version, ss-CASCC, described by Herckes et al. (2002), while the other was a “classic” Teflon CASCC developed by Demoz et al. (1996). The collectors were run in parallel, at an airflow rate of about 25 m3 min−1, and were thoroughly cleaned before sampling using ultrapure water (>18 MΩ cm, Millipore) for the CASCC and acetone (HPLC grade 99.9 %), followed by ultrapure water for the ss-CASCC. Blank samples were taken routinely between each fog event by nebulising ultrapure water on the CASCC and ss-CASCC. Samples collected with the ss-CASCC were analysed for organic acids, while samples collected with the CASCC were analysed for pH, inorganic ions, and elements. Samples for major ion analysis were prepared immediately after collection from the CASCC by pouring 1 mL into a plastic vial and were stored at −18 °C. Samples for organic acid analysis were prepared immediately after collection from the ss-CASCC by pouring a 950 µL sample of non-filtered fog water and 50 µL chloroform into a 1.5 mL glass vial and storing it at 4 °C. Aliquots for dissolved trace metal analysis were prepared by filtering the samples through a 0.22 µm filter (PES, Millipore) and acidifying them with high-purity nitric acid (EMD Chemicals, 67 %–70 % Omni Trace Ultra) to a pH of less than 2.
Nine fog samples were collected (Table 1) through five fog episodes (see Sect. 4.1 for details of the fog occurrences). In addition to these fog samples, two samples of coastal seawater were collected on the seashore in pre-cleaned high-density polyethylene (HDPE) bottles on 10 September (at 16:30 UTC) and 11 September (at 13:17 UTC). They were aliquoted and analysed in the same way as the fog samples. To determine the amount of water collected with the CASCC and ss-CASCC, the sample bottles were weighed before and after sampling.
2.2 Fog microphysics and chemical analysis
Cloud droplet measurements were conducted with a fog monitor 100 (FM-100, Droplet Measurement Technologies Inc., Boulder, CO, USA) located on a scaffolding about 2.5 m high. This forward-scattering spectrometer probe provides the droplet number concentration, size distribution, and liquid water content for particle sizes with an optical equivalent diameter of 2–50 µm. Droplet size is calculated on the basis of laser-scattered light, employs Mie theory for spherical water particles with a refractive index of 1.33, and is classified into 32 bin sizes of width 2 or 3 µm. The instrument was calibrated with glass beads of known diameters and refractive indices. Despite large uncertainties (Guyot et al., 2015; Spiegel et al., 2012), it is one of the most commonly used instruments for documenting the microphysical properties of fog (Gonser et al., 2012; Gultepe et al., 2006, 2009; Mazoyer et al., 2022; Niu et al., 2012; Wagh et al., 2023). Data were recorded every second and subsequently aggregated to 1 min averages.
The pH was measured by a pH meter (9110DJWP pH probe from Thermo Scientific) as soon as possible after sampling on non-filtered samples. The pH meter was calibrated daily using pH 4.01, 7.00, and 10.01 buffers.
Water-soluble ions were analysed by ion chromatography (IC, Dionex Corporation). A dual-channel Dionex ICS-3000 ion chromatograph equipped with conductivity detectors was used to measure the cations and anions. For the cation analysis, an eluent generator provided a concentration of 20 mM methanesulfonic acid at a flow rate of 0.5 mL min−1 to perform the separation on a Dionex IonPac CS12A analytical column (3 mm×150 mm). The anion analysis was performed on a Dionex IonPac AS14A analytical column (4 mm×250 mm) using 1 mM sodium bicarbonate8 mM sodium carbonate eluent at a flow rate of 1 mL min−1. The complete runtime for both was 17 min. No fog samples showed concentrations below the detection limits, except for phosphate (two fog samples, all blanks, and the sea samples).
Organic acids including acetate, propionate, formate, methanesulfonate (MSA), glutarate, succinate, malonate, maleate, and oxalate were analysed using a Dionex DX-500 IC with a gradient pump, conductivity detector, and self-regenerating anion suppressor. A 20-fold dilution was used for formate quantification. Separation was performed using a Dionex AS-11HC analytical (4 mm×250 mm) column using a sodium hydroxide gradient at a flow rate of 1.5 mL min−1. The complete run time was 65 min.
For dissolved trace metal determination, fog samples were aliquoted in the field into pre-cleaned (with nitric acid) HDPE bottles and stored at 4 °C until analysis. Prior to analysis, samples were filtered through a 0.22 µm filter (PES, Millipore) and acidified using high-purity nitric acid (EMD Chemicals, 67 %–70 % Omni Trace Ultra) to a pH of less than 2. The samples were analysed for 40 trace metals using a Thermo Electron X-Series 2 quadrupole inductively coupled plasma mass spectrometer (ICP-MS). All elements were analysed using the kinetic-energy-discrimination (KED) mode and, as the collision cell gas, a hydrogen (7 %) and helium mixture. Scandium, germanium, yttrium, indium, and bismuth were used as the internal standards. As for the TSP samples, dissolved metals are defined operationally as passing through a 0.2 µm filter, which may include nano-sized particles as reported in previous studies (Giorio et al., 2025; Shi et al., 2009). One difference between the concentrations of dissolved metals in fog and TSP samples may arise from the pH of the extraction solution, which was in both cases autogenic but may be more acidic for TSP samples (not measured) compared to fog samples (measured as being close to neutral).
2.3 XANES analysis
Iron speciation, including the identification of the presence of iron–organic ligand complexes and the partitioning of iron species in the II and III oxidation states, was investigated by XANES at the FeK edge. The XANES analysis was performed at the SAMBA (Spectroscopies Applied to Materials based on Absorption) line at the SOLEIL synchrotron facility in Saint-Aubin, France (Briois et al., 2011). A Si(220) double-crystal monochromator was used to produce a monochromatic X-ray beam, which was 4000 µm×1000 µm in size at the focal point. The energy range was scanned from 7050 to 7350 eV at a step size of 0.2 eV.
Standards of Fe(II) and Fe(III) oxides, hydroxides, salts, and oxalate complexes (see Formenti et al., 2025, for details) were prepared as round pellets of 10 mm in diameter in a matrix of boron nitride (BN, molar mass 24.819 g mol−1) using an automatic hydraulic press (Atlas Power T8, Specac) available at the chemistry laboratory at SOLEIL and operated at a pressure of 5 t. The pellets were analysed in transmission mode. The TSP and fog samples were analysed in fluorescence mode in the same energy range and at the step resolution of the standard references. As in Formenti et al. (2014) and Caponi et al. (2017), samples were mounted in an external setup mode. The TSP samples were analysed as detailed in Formenti et al. (2025). For the fog samples, several aliquots of 100 µL solution were deposited on a Kapton sticky film and left to evaporate until a significant deposit was observed visually and the minimum Fe concentration of 0.4 µg cm−2 was obtained. The deposit was finally protected by sticking another portion of Kapton film on top of it, forming a pellet ready for analysis.
Data analysis was conducted with the FASTOSH software package developed at SOLEIL. The Fe speciation was obtained by the least-squares fit of the measured XANES spectra based on the linear combination of reference spectra of oxides, clays, oxalates of Fe(II) and Fe(III), and pyrite. Fits were conducted on the first derivative of the normalised spectral absorbance in the energy region between 7100 and 7180 eV, corresponding to −30 and +50 eV of the K edge. The results of the fits were evaluated on the basis of the resulting chi square (χ2). Additional details of the reference standards and data analysis are reported in Formenti et al. (2025).
2.4 Transmission electron microscopy (TEM) and dynamic light scattering (DLS) measurements of fog samples
The coarse fraction of a fog sample was filtered out by aspirating a fog sample through a 0.2 µm filter. The supernatant was then allowed to sediment in order to remove any small but colloidally unstable particulate matter by resting an Eppendorf tube upright for 24 h. The resultant suspension was then sampled for analysis.
A Malvern Panalytical Zetasizer Nano was utilised to perform DLS on the prepared fog samples. The angle and intensity of light scattering caused by suspended particles can be correlated with particle size, thereby investigating the hydrodynamic particle size distribution of the suspension and confirming the presence of a colloidally stable phase.
Fog samples were prepared in the same manner for TEM. A 35 µL droplet of the suspension was deposited onto a TEM grid (Cu–C, 300 mesh) before blotting off the excess with filter paper. A Thermo Scientific (FEI Company) Talos F200X G2 TEM was used to capture images of the nanoparticles in the fog samples. Energy dispersive X-ray spectroscopy (EDS) was used to identify chemical elements occurring within the aforementioned nanoparticles and to perform elemental mapping within a nanoparticle to understand the distribution of elements. A Super-X EDS detector system with four windowless silicon-drift detectors was utilised for EDS analysis.
2.5 Ancillary measurements
A weather detector (Vaisala PWD 22/52) provided 1 min average horizontal visibility using the forward-scatter measurement principle.
Standard meteorological parameters (wind speed and direction, air temperature, and relative humidity) were measured by a compact weather station (models CE155N, CE157N, and BA711; Cimel Electronique, Paris, France) installed 10 m above ground level on an extendable mast on the roof of the PEGASUS facility.
3.1 Calculation of the pH and liquid water content of aerosols
The average aerosol liquid water (ALW) content and pH for the aerosol fraction were calculated using the comprehensive version of the Extended Aerosol Inorganics Model E-AIM IV (Clegg et al., 1992, 1998; Wexler, 2002). The averaged relative humidity (RH) and temperature over the sampling period and aerosol concentrations of , Na+, Cl−, , and were used as input for the calculation. Ion balance was achieved by adding an appropriate amount of H+ or converting an appropriate amount of into NH3. Acetic acid and oxalic acid were also included in the calculation and allowed to dissociate in solution (Clegg et al., 2003). Partitioning equilibria with gas components and solid precipitation were also considered. K+, Ca2+, Mg2+, and were not considered in the calculation as these species are not included in E-AIM. This can lead to an underestimation of the real pH of aerosols. The ISORROPIA II model could be used to consider these species, but a few studies have pointed out that ISORROPIA does not perform well at very high relative humidity (such as in this campaign) when run in reverse mode (i.e. when only particle-phase measurements are available) (Haskins et al., 2018; Hull et al., 2025; Peng et al., 2019). In addition, the absence of carbonate measurements can contribute to the uncertainty around the real aerosol pH. Including formic acid in the calculations did not show any difference, and therefore it was ignored.
3.2 Speciation of metal ions in deliquescent aerosol
Chemical speciation of metal ions in deliquescent aerosol was computed using the Visual MinteQ 3.1 model (available online at https://vminteq.com, last access: 2 September 2025). For the speciation, the following species were considered: Na+, , K+, Mg2+, Ca2+, Cl−, , , , oxalate, acetate, Al, Cu, Ni, Mn, Cr, Fe, Pb, and Zn. For metal ions, the dissolved concentration measured with ICP-MS was used. The oxidation state of the latter metal ions was assumed to be the following: Al(III), Cu(II), Ni(II), Mn(II), Cr(III), Pb(II), and Zn(II). In the case of Fe, we assumed that Fe(II) and Fe(III) were present in a 1:1 ratio as used in previous studies (Giorio et al., 2022b; Scheinhardt et al., 2013; Shahpoury et al., 2024a). This 1:1 ratio was used to obtain an average picture of Fe speciation, considering the dynamic nature of the Fe2+Fe3+ couple in atmospheric aerosol with expected higher Fe2+ concentrations during the day and higher Fe3+ concentrations during the night (Mao et al., 2017). For the speciation, liquid-phase concentrations of the dissolved complex-forming compounds were calculated using the ALW obtained from E-AIM IV, assuming initially a complete dissolution. The pH was fixed at the value calculated by E-AIM IV, and the mean temperature over the sampling time was used. The speciation model was run according to the procedure described in previous studies (Giorio et al., 2022b; Scheinhardt et al., 2013). When specific chemical species were predicted to be present under oversaturation conditions, the calculation was repeated, allowing precipitation.
3.3 Speciation of metal ions in fog
Chemical speciation of metal ions in fog was calculated at the measured pH value by means of the PITMAP software (Di Marco, 1998; Tapparo et al., 2020). Briefly, mass balance equations are solved. That is, the species concentration at equilibrium is obtained using the Newton–Raphson method (Press et al., 2007). Speciation was done considering metal ions and ligands quantified in the fog samples: formate, acetate, propionate, oxalate, malonate, succinate, glutarate, maleate, methanesulfonate, , Cl−, , , Mg(II), Ca(II), Sr(II), Ba(II), V (in the form ), Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), and Al(III). For all metal ions, the thermodynamically stable oxidation state was considered. The input thermodynamic data (stoichiometry and stability constant of the complexes, including metal–aqua complexes) were obtained from the literature (The IUPAC Stability Constants Database, 2006).
Unlike Visual MinteQ, PITMAP does not explicitly calculate the amount of precipitated solids. Speciation of metals obtained by Visual MinteQ and PITMAP for three fog samples (H603, H901, and H1002) was qualitatively equal, with minor differences being observed only for species at very low concentrations () (see Sect. S1 in the Supplement).
4.1 Meteorological conditions and fog occurrence
Figure 1 illustrates the meteorological conditions during the campaign, which were characterised by remarkable stability in terms of temperature (around 12 °C) with a narrow range (<5 °C) and humidity (RH 95±4 %, minimum 78.5 %, and maximum 100 %), which is a clear indication of maritime influence. At the same time, a persistent stratocumulus cloud deck kept solar irradiance below 600 W m−2 (Giorio et al., 2022a).
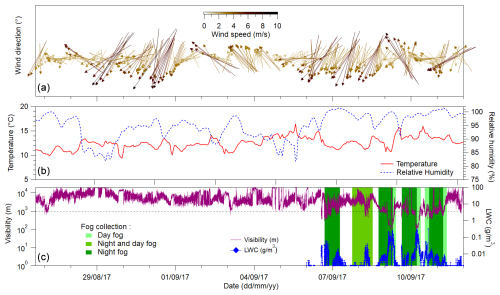
Figure 1Time series of wind direction and speed (a). Hourly average relative humidity and temperature (b). Visibility and liquid water content (LWC) of the fog (c). The green shades in panel (c) correspond to the fog collection periods. Fog events are generally classified as visibility<1 km.
The visibility data show the presence of fog events (classified as visibility<1 km) starting from 3 September 2017 and becoming persistent for extended periods from the night of 6–7 September 2017 (Fig. 1). Two fog events were particularly intense over 3 consecutive days, 9–11 September, with visibility reduced to below 100 m and with the liquid water content up to 0.3 g m−3.
Wind patterns were characterised by a gentle () occasional land or sea breeze superimposed with moderate to strong (up to around 10 m s−1) breezes coming predominantly from the NW and NNW and from the SW (Fig. 1). Notable exceptions were 4 September (NE and NNW winds), 5 September (SWW winds), 6–9 September (SSW winds), and 11 September (SW winds). Air masses predominantly travelled along the coastline (NNW–SSE) before reaching the sampling site in Henties Bay. These air masses often travelled above the ocean or the Namib, transporting sea spray, marine biogenic emissions, and mineral dusts. When the winds blow from S and SE, sporadic anthropogenic influences from the towns of Henties Bay and Walvis Bay can be expected (Giorio et al., 2022a). During fog events, the wind speed is almost always lower than 4 m s−1 from the SSW to WNW. The observed fog was henceforth mostly due to the advection of marine stratocumulus or was locally formed, as confirmed by the fog microphysics (Weston et al., 2022) and chemical composition as shown in the sections below.
4.2 Fog chemical composition
The major ions showed extremely high concentrations in fogs compared to other studies in coastal areas (Table 2), but this was in agreement with the findings of Gottlieb et al. (2019) for various sites in Namibia, while Fe, Zn, and Cu concentrations were of the same order or even reduced compared to other remote sites (Table 2) due to scarce vegetation sources in the hyperarid Namib region, where there is hardly any vegetation.
Table 2The pH, concentrations of major ions, organic acids, and elements measured in fogs and seawater at the coastal site in Henties Bay, together with comparisons with previous coastal and marine studies.
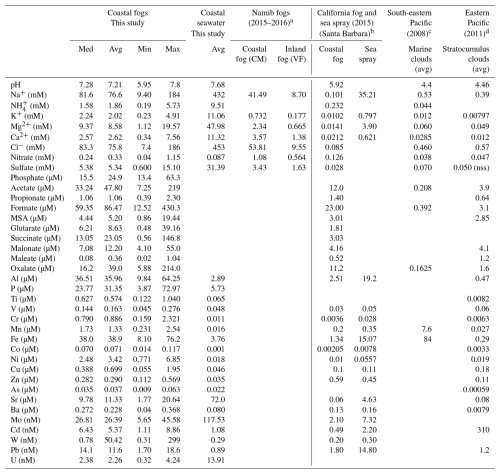
a Gottlieb et al. (2019) (CM: coastal metals, 15 km inland; VF: Vogelfederberg, 60 km inland). b Boris et al. (2018) and Herckes (personal communication) for metals.
c Benedict et al. (2012) (VOCALS-Rex campaign). d Wang et al. (2014) and Sorooshian et al. (2013) for organic acids (E-PEACE campaign).
nss: non-sea salt. Additional elements are reported in Table S1 in the Supplement.
Table 2 shows that the fogs were more diluted than local seawater in terms of major ions (Na+, Cl, K+, , Mg2+, Ca2+, , and ). This result is also corroborated by microphysics measurements that show rapid changes between fog, mist, and sea spray, the latter contributing significantly to reducing visibility (Formenti et al., 2019; Weston et al., 2022). Conversely, several elements and compounds, such as Al, Ti, Ga, Fe, P, nitrate, and all rare Earth elements (see Table S1 in the Supplement for elements not reported in Table 2), were more concentrated in coastal fog than in seawater. The elevated nitrate concentrations can be attributed to the atmospheric processes affecting the important marine phytoplanktonic emissions of reactive nitrogen species (organic nitrogen and/or NO), ending, by photooxidation, in particulate nitrate formation (Altieri et al., 2021) and henceforth explaining the presence of nitrate in the remote oceanic atmosphere. The correlation of the nitrate concentrations with markers of secondary chemistry, such as oxalate and MSA (R2=0.89 and 0.53, respectively), in our coastal fog samples supports this hypothesis. Carboxylic and dicarboxylic acids, i.e. notably highly oxidised species such as formate, acetate, succinate, and oxalate, also showed high concentrations in fogs. A remarkable correlation between formate and acetate (R2>0.99) suggests that they have similar sources or formation pathways. The high formate-to-acetate ratio (2.02) indicates the more important secondary formation of carboxylic acids in fog water as compared to other possible sources (Li et al., 2020). Surprisingly, U was more concentrated in seawater than in fog. A source attribution analysis is summarised in the Supplement in the form of enrichment factors (see Figs. S4 and S5 in the Supplement). Besides the local seawater, to represent the influence of the marine source, we present the enrichment factors calculated with respect to Al concentrations in the aerosol phase measured concurrently with the fog and representing the local dust content during the campaign (Formenti et al., 2025).
Generally, the chemical composition of the fog samples shows a large marine and mineral dust influence accompanied by highly processed organics which may have been formed in situ or in clouds and then advected on the coast. Indeed, more and more studies show atmospheric volatile organic compound (VOC) emissions from marine planktonic microorganisms. These compounds can then undergo atmospheric photochemistry inducing the formation of new ultrafine particles which may grow in size and contribute to cloud condensation nuclei (Giorio et al., 2022a; Halsey et al., 2017; Mungall et al., 2017; Peltola et al., 2022).
4.3 Comparison of metal solubility in coincident aerosol and fog samples
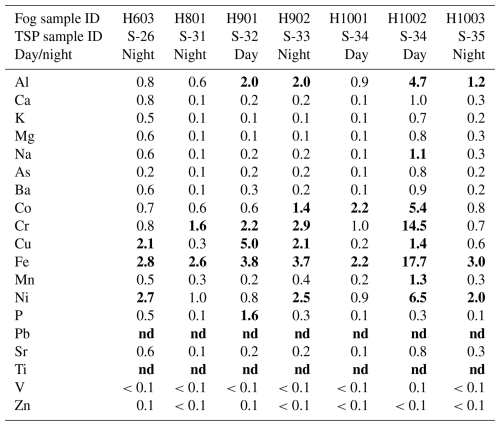
Solubility of metals in TSP samples is discussed for the whole campaign in Formenti et al. (2025). Here, we focus the discussion on the comparison between dissolved metals in pairs of TSP and fog samples. For the comparison, fog water-dissolved metal concentrations (µM) were transformed into air concentrations (ng m−3) using the liquid water content (g H2O m−3). This allows for comparison of fog concentrations with dissolved metal concentrations in TSP samples. The ratio of fog concentrations to TSP concentrations is shown in Table 3. When concentrations of dissolved metals in TSP samples are compared with concentrations in fog concurrently collected, higher concentrations of Al, Fe, Cu, and Ni are found in fog (Table 3). The Al, Fe, Cu, and Ni concentrations in fog are up to a factor of 4.7, 17, 5, and 6.5 higher than dissolved concentrations in TSP samples. Similarly, the Cr concentration in fog was up to 14.5 times higher than the dissolved concentration in TSP samples and Ti was detected in fog but not in the corresponding samples of the dissolved fractions of TSP samples.
This result suggests that the chemical processing of airborne particles in fog would enhance metal solubility, such as the formation of metal–ligand complexes and/or photochemical processes, if they are both locally produced and TSP samples act as condensation nuclei for fog formation. Previous studies have shown that Fe solubility can be driven by the formation of metal–ligand complexes with oxalates (Giorio et al., 2022b; Paris et al., 2011; Paris and Desboeufs, 2013). Similar processes can also impact Cu and Al solubility (Giorio et al., 2022b). Other organics, such as malonate, tartrate, or humic-like substances (HULISs) (Paris and Desboeufs, 2013), can also influence Fe solubility. This is also the case for formate and acetate, which, despite being weaker complexing agents, are better pH buffers (Upadhyay et al., 2011). Fe solubility is strongly dependent on pH (Desboeufs et al., 1999, 2003). However, this pH dependency does not explain the observations in this study, as Fe is supposed to be more soluble at acidic pH than at the neutral pH of the fog samples (Fig. 2).
A previous study of the same site showed that Fe solubility in PM10 was correlated with methanesulfonic acid concentrations, suggesting that photochemical reduction may be responsible for the enhanced Fe solubility (Desboeufs et al., 2024). Such a process would be compatible with the observations of a higher Fe solubility in fog samples, with the largest differences in dissolved Fe concentrations observed in samples collected during daytime (Table 3). It is worth keeping in mind that liquid water content (LWC) may be underestimated in nighttime fog due to the continuous running of the fog collectors until morning, which leads to an overestimation of the volume of air sampled. Formenti et al. (2025) show that fluoride could also play a role in Al, Ti, Si, and Fe solubilities. However, as all fog samples were collected in a period of low fluoride concentrations, the possibility can be excluded that this species could play a role in the enhanced dissolved metal concentrations in the fog samples.
Table 3Ratio between the mass concentration of dissolved metals in fog samples and dissolved metals in the corresponding TSP samples collected in Henties Bay during the AEROCLO-sA campaign in August–September 2017. Values for which metal concentrations in the dissolved fraction of TSP were below detection limits while they were above detection limits in fog are reported as “nd” (not defined). Values above 1 indicate higher solubilities in fog and are highlighted in bold.
4.4 Speciation of metals in TSP and fog
The speciation of the most abundant metals (see Sects. 3.2 and 3.3) in the deliquescent TSP as well as in the fog samples is calculated to investigate which species are present in solution and could drive the solubility of each metal.
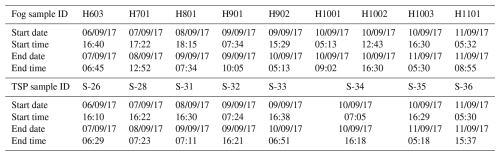
ALW content and pH, estimated using the E-AIM IV model, were in the ranges 0.122–412 mg m−3 and 0.1–3.2, respectively (Fig. 2). The high ALW indicates that the aerosol fraction, dominated by sea salt, was likely always in a deliquescent state due to the mild temperatures and elevated RH (observed from 6 September until the end of the campaign) (Fig. 1). Generally, the aerosol acidity was inversely proportional to the ALW content. It is worth noting that the calculated aerosol pH is very low (pH<3) compared to the fog samples (neutral pH) collected concurrently. The measured pH values for the fog samples ranged from 5.95 to 7.8, with a median value of 7.28, similar to that of seawater (Table 2). These pH values are toward the upper end of values reported in the literature, which, however, refer to urban and agricultural areas (Ge et al., 2022; Gundel et al., 1994; Paglione et al., 2021; Pye et al., 2020; Wen et al., 2005). The higher pH measured in this study in the fog samples is likely due to a high water content as well as the dominance of the sea spray component. The pH in fog might be at least partly driven by the presence of carbonates, as evidenced by the moderate correlation between −log ([Ca(II)]) and pH (r2=0.21), similar to what happens in seawater (Jiang et al., 2019). The E-AIM IV model does not consider K(I), Mg(II), Ca(II), and carbonates and therefore may underestimate aerosol pH compared to what is expected for sea spray aerosol (Pye et al., 2020).
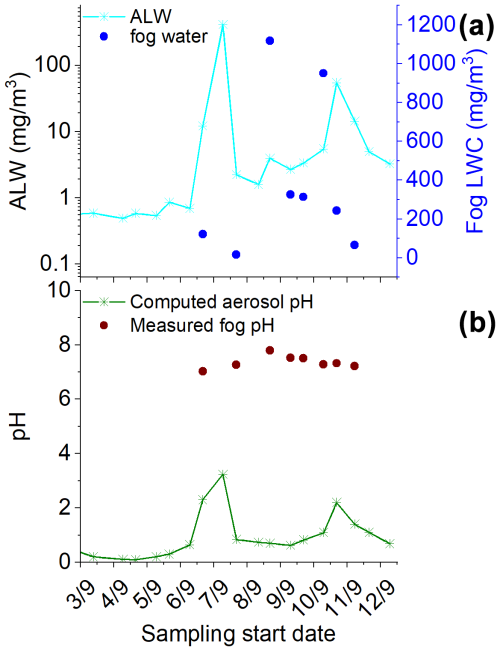
Figure 2Time series of (a) aerosol liquid water (ALW) content calculated with E-AIM and measured fog LWC retrieved from the measured water collected with the CASCC sampler and (b) pH in aerosol calculated with E-AIM and measured pH in fog. Fog samples H1001 and H1002 have been averaged over the TSP sample S-34 collection time. The TSP samples reported here are those corresponding to sample IDs S20 to S38.
Speciation of metal ions in deliquescent aerosol depended on ALW content, pH, and the number of inorganic and organic ligands in the samples. Due to the high ALW and low pH, a large fraction of the metal ions is in free form, with such a fraction being dominant during the second part of the campaign characterised by higher RH and fog events. For most of the metals, another significant fraction is bound to inorganic compounds such as for Al(III), Cr(III), Cu(II), and Ni(II) and Cl− for Ca(II), Mg(II), Mn(II), and Zn(II). Fe is the only exception, with Fe(II) being equally distributed between binding and Cl− and Fe(III) also being complexed with organics, in particular oxalate (Fig. S6 in the Supplement). In addition to Fe(III), a significant fraction of Al(III) and a minor fraction of Cu(II) are also predicted to bind oxalate. In addition, in the first part of the campaign when ALW was relatively lower, part of Ca(II) was calculated to precipitate as CaSO4. Given that pH was likely underestimated in our calculations, the real speciation picture may be shifted in favour of a larger proportion of hydroxide formation for some metal ions, which could limit their ability to dissolve in water.
Fog samples were characterised by much higher water content, ranging from 16–1117 mg m−3, and close to a neutral pH. Given the extremely different pH conditions, we would expect the speciation picture to be completely different compared to that of TSP.
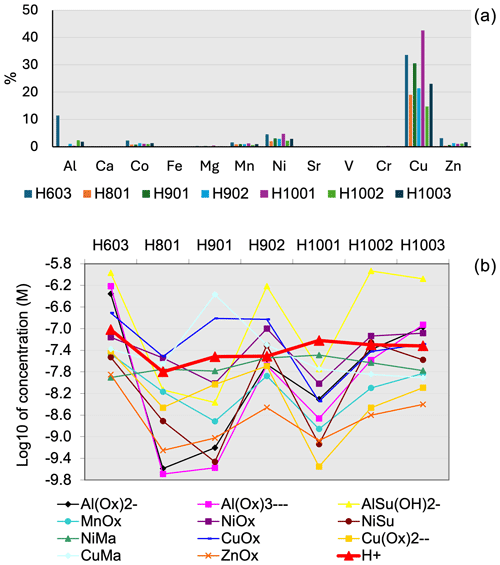
The most striking and surprising difference is observed for Al(III) and Fe(III), which, according to our calculation, would form insoluble hydroxides in fog water (Fig. 3) and therefore should not be in solution unless they are present in a colloidal form (Shi et al., 2009). This result runs counter to observations from ICP-MS analysis, which show an even higher concentration of Al and Fe in solution in the fog samples compared to the dissolved concentration in TSP. In the case of Al(III), only a minor component is predicted to be bound with organics such as oxalate and succinate (Fig. 4b). Ni(II) speciation in fog shows similar results compared with TSP (Fig. 3), with the only difference being that Ni(II) is partly present as soluble hydroxide in fog in addition to being present in free form and bound to as in TSP. Only a minor component of Ni(II) is predicted to be bound to organics such as oxalate, malonate, and succinate (Fig. 4b). A notable difference between the two types of samples is instead observed for Cu(II), which becomes bound to oxalate and malonate for a fraction between 19 % and 43 % (Fig. 4a) in the fog samples, while in TSP only a minor fraction (<7.3 %) was bound to the organics. In the case of Cr(III), speciation in TSP showed an almost equal distribution between free-form and bound for the only two samples in which Cr was detected in the dissolved fraction, while in fog it was mainly present as soluble hydroxide and a mixed –hydroxide complex (Fig. S7 in the Supplement).
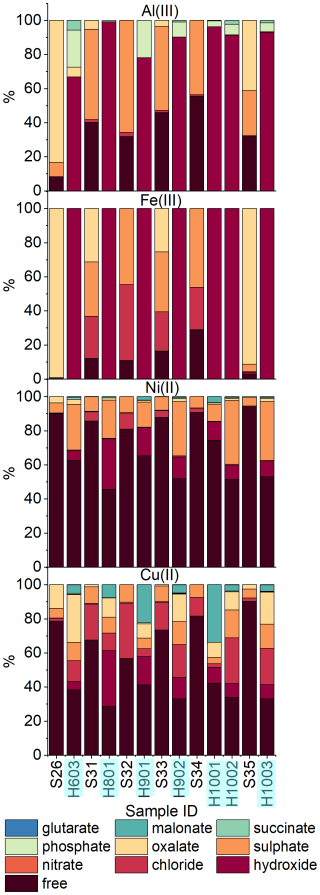
Figure 3Comparison of the detailed speciation of dissolved metals in TSP (sample IDs starting with “S”) and fog (sample IDs starting with “H”) for (top to bottom) Al(III), Fe(III), Ni(II), and Cu(II). Samples collected at approximately the same time are shown side by side. The sampling of TSP sample S34 covered the same times as both the H1001 and H1002 fog samples.
4.5 Investigation contrasting Fe solubility in TSP and fog using XANES and TEM
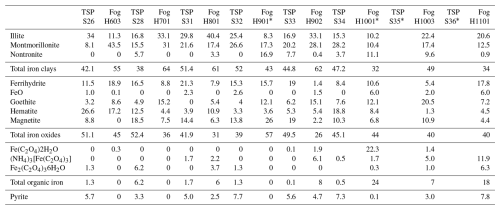
Iron speciation was measured experimentally in order to elucidate the processes that may increase iron solubility in the fog samples compared to the TSP samples. Table 4 and Fig. 5 show the results of the linear combination fitting of the XANES spectra of the TSP and fog samples using reference spectra that are representative of various iron oxides, clays, pyrite, and complexes with oxalate formed by either Fe(II) or Fe(III). The results show that, for both TSP and fog samples, iron oxides and clays are by far the major components, as expected for samples affected by the transport of mineral dust. In the case of TSP, iron oxides and hydroxides contribute 39 %–52 % and iron clays contribute 38 %–52 % (Table 4), making the majority of Fe insoluble. In fog samples, the contributions of these two classes of minerals are more variable, with iron oxides and hydroxides contributing 27 %–56 % and iron clays contributing 32 %–64 % (Table 4). Iron-oxalate complexes, in particular those of Fe(III), contributed to some samples of both TSP and fog. Excluding samples that were close to the detection limit or affected by diffraction, iron oxalate contributed up to 6 % in TSP samples and up to 8 % in fog samples. Pyrite was found to contribute up to 7.8 % of the total iron concentration, which was attributed to the transport of fugitive dust from smelting areas (Formenti et al., 2025).
Table 4Results of the spectral (XANES, FeK edge) deconvolution done on the TSP and fog samples using a linear combination fitting with Fe reference standards
of iron oxides, clays, organic complexes, and sulfide. Results are reported as the percentage contribution of each component used for the linear combination fitting.
∗ Noisy spectra affected by diffraction.
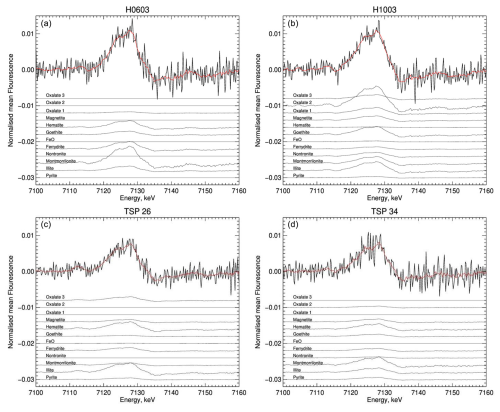
Figure 5XANES spectra at the FeK edge for two fog samples (top, H0603 and H1003) and two TSP samples (bottom, TSP 26 and TSP 34) showing the results of the spectral deconvolution using a linear combination fitting with Fe measured reference standards of oxides, clays, organic complexes, and sulfide. The individual contributions of the references to the total fit (red curve) are represented as stacked black lines. For the sake of clarity, the contributions of organic complexes (named “oxalate 1” for Fe(C2O4)2H2O, “oxalate 2” for (NH4)3[Fe(C2O4)3], and “oxalate 3” for Fe2(C2O4)36H2O in the figure) are multiplied by 10.
The presence of a significant amount of iron oxalate in more than one fog sample (Table 4) could explain the enhanced solubility of iron in fog compared to TSP samples. This amount is comparable to the dissolved fraction of iron (<2 % in TSP in these samples). The results of the XANES measurements run counter to the results of the thermodynamic modelling that predicted iron to precipitate in fog samples in the form of hydroxides, but they are consistent with ICP-MS measurements that showed that dissolved Fe was indeed present in the fog samples. On the other hand, the thermodynamic modelling results are qualitatively consistent with XANES measurements of TSP samples, for which oxalates of iron complexes were expected and indeed experimentally detected. We hypothesise that iron-oxalate complexes are formed through aqueous-phase processing of iron during particle activation into droplets when the water content is lower and the pH is also lower than in the collected fog samples. These complexes may then remain in the fog droplets in a kinetically stable form before being converted into insoluble hydroxides. Additionally, Marcotte et al. (2020) showed that iron-oxide solubility in mineral dust decreases with increasing particle size. Therefore, as activation to droplets is a size-dependent process, there may be fractionation of soluble iron whereby, for example, smaller particles with a higher soluble iron content contribute more to fog droplets compared to larger particles with a lower soluble iron content. The XANES measurements also showed that iron was predominantly present in the Fe(III) oxidation state. This result would suggest that photochemical reduction to Fe(II) was not a significant process in the enhanced iron solubility in the fog samples.
In addition to organic iron formation during droplet activation, colloidal iron may be present in fog (Gledhiir and Buck, 2012), which would be detected as being dissolved in ICP-MS measurements. To test this hypothesis, TEM and DLS measurements were performed on fog samples before and after filtration through a 0.22 µm pore-size filter.
Particles identified by DLS in the sub-100 nm range, after filtration and sedimentation, reveal the presence of a colloidal phase within the fog samples (Fig. 6a). TEM confirmed this hypothesis, as distinct particles with diameters ranging from 50 to 150 nm were identified (Fig. 6b). These are likely to be the same particles observed using DLS. EDS showed that these particles had cores containing silicon, aluminium, iron, and sulfur, among other less prominent elements (Fig. 6c).
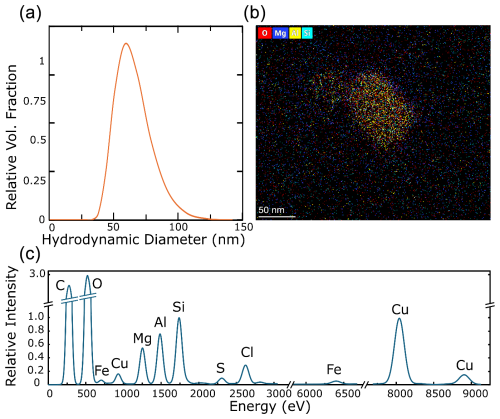
Figure 6Results of the analyses of filtered and sedimented fog samples showing (a) the normalised distribution of hydrodynamic diameters of suspended particulates, weighted by volume, from DLS measurements. (b) An elemental map of nanoscopic suspended particles, with the scale bar representing 50 nm and imaged by TEM, and (c) energy-dispersive X-ray spectra showing the relative intensity of elements occurring in the particle mapped in (b).
In this study, we compared TSP and fog samples collected in parallel on the Namibian coast in Henties Bay during the AEROCLO-sA campaign in September 2017.
We found contrasting concentrations of dissolved elements in TSP and fog samples, with higher-than-expected dissolved concentrations of Al, Fe, Cu, Ni, and Cr in fog. Thermodynamic modelling of aqueous-phase equilibria in deliquescent aerosols and fog suggests that Cu solubility in fog could be due to complexation with inorganic (i.e. chloride and sulfate) and organic (i.e. oxalate and malonate) ligands, which is favoured by the neutral pH observed in fog samples. For Cr, the enhanced solubility in fog could be due to the formation of soluble hydroxides and phosphate–hydroxide complexes in fog. For Ni, the thermodynamic modelling shows that it may be present partly in free form, partly as soluble hydroxide, and partly as sulfate, and only a minor component would be complexed by organics in fog.
Previous studies have shown that aqueous-phase processing can lead to an increase in the solubility of various elements, such as iron and aluminium (Giorio et al., 2022b; Li et al., 2024; Shi et al., 2015; Tapparo et al., 2020). However, the majority of the observations report an acidic pH for fogs and clouds (Pye et al., 2020), which would be consistent with increased element solubility. Here we show enhanced concentrations of dissolved Al and Fe in fog characterised by a neutral pH in which these elements would be predicted to precipitate as hydroxides. The thermodynamic modelling results run counter to experimental measurements that show that both Al and Fe are detected in fog (by ICP-MS) and that Fe can be present as an oxalate complex in fog (from XANES measurements) as well as in colloidal form (from DLS and TEM measurements).
We hypothesise that (1) Al and Fe undergo aqueous-phase processing during particle activation into droplets when the water content and pH are lower and the formation of metal–ligand complexes with organic acids is favoured and that (2) Al and Fe remain in fog water as kinetically stable metal–organic complexes or as a colloidal suspension of nanoparticles.
Our study considers the bulk composition of TSP and fog samples for thermodynamic calculations; however, single-particle composition may be very different from bulk composition. The single-particle composition would be the most relevant one for understanding the processing happening during particle activation into droplets, as the activation can be both size- and composition-dependent (Marcotte et al., 2020; Zhang et al., 2024). Nevertheless, the finding that Al and Fe solubility can be enhanced in fog even at neutral pH expands the breadth of the importance of aqueous-phase processing in the atmosphere. The enhanced solubility of Al and Fe, as well as their complexation with organics, can have impacts on their ability to produce reactive oxygen species (Campbell et al., 2023; Shahpoury et al., 2021, 2024a, b) and potentially modify particle optical properties.
All of the data are made freely available by the French national service for atmospheric data (AERIS-SEDOO) at https://baobab.sedoo.fr/AEROCLO/ (last access: 3 September 2025). The FASTOSH XANES data analysis package is available for download at https://www.synchrotron-soleil.fr/fr/lignes-de-lumiere/samba (last access: 2 March 2024; Landrot and Fonda, 2025).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-16107-2025-supplement.
CG and PF conceived the study, analysed the data, and drafted the manuscript. CG, KD, and PF collected the TSP samples. AM collected and analysed the fog samples. PH and DN analysed the fog samples. GL, PF, FB, and CDB performed the XANES measurements. DW performed the TEM and DLS measurements. CG, VDM, MN, and SD performed the thermodynamic modelling calculations. ST, SC, and KD analysed the TSP samples. FB provided the visibility data. JFD, SJP, and AN contributed to the sample and data collection and provided support for the field campaign. All of the authors revised and approved the manuscript before submission.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “New observations and related modelling studies of the aerosol–cloud–climate system in the Southeast Atlantic and southern Africa regions (ACP/AMT inter-journal SI)”. It is not associated with a conference.
The authors are grateful to the AEROCLO-sA consortium for their work in the field and during the preparation of the field campaign and to SANUMARC for hosting the field campaign. The authors are grateful to Andrea Osti for help with the calculations using PITMAP. The authors wish to thank AERIS (https://www.aeris-data.fr/, last access: 3 September 2025), the French centre for atmospheric data and service, for providing the campaign website and organising the curation and open distribution of the AEROCLO-sA data. Finally, SOLEIL is acknowledged for provision of the synchrotron radiation facilities (Proposal 20210043).
This work was supported by the French National Research Agency (grant no. ANR-15-CE01-0014-01), the French national programme LEFE/INSU, the French National Agency for Space Studies (CNES), and the South African National Research Foundation (NRF) (grant UID 105958). Chiara Giorio's work was supported by the Supporting TAlent in ReSearch@University of Padova STARS-StG “MOCAA” and a BP Next Generation fellowship awarded by the Yusuf Hamied Department of Chemistry at the University of Cambridge.
This paper was edited by Manabu Shiraiwa and reviewed by Akinori Ito and one anonymous referee.
Altieri, K. E., Fawcett, S. E., and Hastings, M. G.: Reactive Nitrogen Cycling in the Atmosphere and Ocean, Annu. Rev. Earth Pl. Sc., 49, 523–550, https://doi.org/10.1146/annurev-earth-083120-052147, 2021.
Andersen, H., Cermak, J., Fuchs, J., Knippertz, P., Gaetani, M., Quinting, J., Sippel, S., and Vogt, R.: Synoptic-scale controls of fog and low-cloud variability in the Namib Desert, Atmos. Chem. Phys., 20, 3415–3438, https://doi.org/10.5194/acp-20-3415-2020, 2020.
Bauer, S. E., Tsigaridis, K., Faluvegi, G., Nazarenko, L., Miller, R. L., Kelley, M., and Schmidt, G.: The Turning Point of the Aerosol Era, J. Adv. Model. Earth Sy., 14, e2022MS003070, https://doi.org/10.1029/2022MS003070, 2022.
Benedict, K. B., Lee, T., and Collett, J. L.: Cloud water composition over the southeastern Pacific Ocean during the VOCALS regional experiment, Atmos. Environ., 46, 104–114, https://doi.org/10.1016/j.atmosenv.2011.10.029, 2012.
Boris, A. J., Napolitano, D. C., Herckes, P., Clements, A. L., and Collett, J. L.: Fogs and air quality on the Southern California coast, Aerosol Air Qual. Res., 18, 224–239, https://doi.org/10.4209/aaqr.2016.11.0522, 2018.
Briois, V., Fonda, E., Belin, S., Barthe, L., La Fontaine, C., Langlois, F., Ribbens, M., and Villain, F.: SAMBA: The 4–40 keV X-ray Absorption spectroscopy beamline at SOLEIL, UVX 2010 – 10e Colloque sur les Sources Coherentes et Incoherentes UV, VUV et X: Applications et Developpements Recents, 41–47, https://doi.org/10.1051/uvx/2011006, 2011.
Buck, C. S., Landing, W. M., Resing, J. A., and Lebon, G. T.: Aerosol iron and aluminum solubility in the northwest Pacific Ocean: Results from the 2002 IOC cruise, Geochem. Geophy. Geosy., 7, Q04M07, https://doi.org/10.1029/2005GC000977, 2006.
Campbell, S. J., Utinger, B., Barth, A., Paulson, S. E., and Kalberer, M.: Iron and Copper Alter the Oxidative Potential of Secondary Organic Aerosol: Insights from Online Measurements and Model Development, Environ. Sci. Technol., 57, 13546–13558, https://doi.org/10.1021/acs.est.3c01975, 2023.
Caponi, L., Formenti, P., Massabó, D., Di Biagio, C., Cazaunau, M., Pangui, E., Chevaillier, S., Landrot, G., Andreae, M. O., Kandler, K., Piketh, S., Saeed, T., Seibert, D., Williams, E., Balkanski, Y., Prati, P., and Doussin, J.-F.: Spectral- and size-resolved mass absorption efficiency of mineral dust aerosols in the shortwave spectrum: a simulation chamber study, Atmos. Chem. Phys., 17, 7175–7191, https://doi.org/10.5194/acp-17-7175-2017, 2017.
Clegg, S. L., Pitzer, K. S., and Brimblecombe, P.: Thermodynamics of multicomponent, miscible, ionic solutions. Mixtures including unsymmetrical electrolytes, J. Phys. Chem., 96, 9470–9479, https://doi.org/10.1021/j100202a074, 1992.
Clegg, S. L., Brimblecombe, P., and Wexler, A. S.: Thermodynamic model of the system H+----H2O at tropospheric temperatures, J. Phys. Chem.-USA, 102, 2137–2154, https://doi.org/10.1021/jp973042r, 1998.
Clegg, S. L., Seinfeld, J. H., and Edney, E. O.: Thermodynamic modelling of aqueous aerosols containing electrolytes and dissolved organic compounds. II. An extended Zdanovskii-Stokes-Robinson approach, J. Aerosol. Sci., 34, 667–690, https://doi.org/10.1016/S0021-8502(03)00019-3, 2003.
Cwiertny, D. M., Baltrusaitis, J., Hunter, G. J., Laskin, A., Scherer, M. M., and Grassian, V. H.: Characterization and acid-mobilization study of iron-containing mineral dust source materials, J. Geophys. Res.-Atmos., 113, D05202, https://doi.org/10.1029/2007JD009332, 2008.
Dansie, A. P., Thomas, D. S. G., Wiggs, G. F. S., and Munkittrick, K. R.: Spatial variability of ocean fertilizing nutrients in the dust-emitting ephemeral river catchments of Namibia, Earth Surf. Proc. Land., 43, 563–578, https://doi.org/10.1002/esp.4207, 2018.
Deguillaume, L., Leriche, M., Desboeufs, K., Mailhot, G., George, C., and Chaumerliac, N.: Transition Metals in Atmospheric Liquid Phases: Sources, Reactivity, and Sensitive Parameters, Chem. Rev., 105, 3388–3431, https://doi.org/10.1021/cr040649c, 2005.
Deguillaume, L., Desboeufs, K. V., Leriche, M., Long, Y., and Chaumerliac, N.: Effect of iron dissolution on cloud chemistry: from laboratory measurements to model results, Atmos. Pollut. Res., 1, 220–228, https://doi.org/10.5094/APR.2010.029, 2010.
Demoz, B. B., Collett, J. L. B., and Daube, B. C.: On the Caltech Active Strand Cloudwater Collectors, Atmos. Res., 41, 47–62, 1996.
Desboeufs, K., Formenti, P., Torres-Sánchez, R., Schepanski, K., Chaboureau, J.-P., Andersen, H., Cermak, J., Feuerstein, S., Laurent, B., Klopper, D., Namwoonde, A., Cazaunau, M., Chevaillier, S., Feron, A., Mirande-Bret, C., Triquet, S., and Piketh, S. J.: Fractional solubility of iron in mineral dust aerosols over coastal Namibia: a link to marine biogenic emissions?, Atmos. Chem. Phys., 24, 1525–1541, https://doi.org/10.5194/acp-24-1525-2024, 2024.
Desboeufs, K. V., Losno, R., Vimeux, F., and Cholbi, S.: The pH-dependent dissolution of wind-transported Saharan dust, J. Geophys. Res.-Atmos., 104, 21287–21299, https://doi.org/10.1029/1999JD900236, 1999.
Desboeufs, K. V., Losno, R., and Colin, J. L.: Relationship between droplet pH and aerosol dissolution kinetics: Effect of incorporated aerosol particles on droplet pH during cloud processing, J. Atmos. Chem., 46, 159–172, https://doi.org/10.1023/A:1026011408748, 2003.
Desboeufs, K. V., Sofikitis, A., Losno, R., Colin, J. L., and Ausset, P.: Dissolution and solubility of trace metals from natural and anthropogenic aerosol particulate matter, Chemosphere, 58, 195–203, https://doi.org/10.1016/j.chemosphere.2004.02.025, 2005.
Engelbrecht, F. A., Steinkopf, J., Padavatan, J., and Midgley, G. F.: Projections of Future Climate Change in Southern Africa and the Potential for Regional Tipping Points, in: Sustainability of Southern African Ecosystems under Global Change. Ecological Studies, edited by: von Maltitz, G. P., Midgley, G. F., Veitch, J., Brümmer, C., Rötter, R. P., Viehberg, F. A., and Veste, M., vol. 248, Springer, Cham, 169–190, https://doi.org/10.1007/978-3-031-10948-5_7, 2024.
Flamant, C., Gaetani, M., Chaboureau, J.-P., Chazette, P., Cuesta, J., Piketh, S. J., and Formenti, P.: Smoke in the river: an Aerosols, Radiation and Clouds in southern Africa (AEROCLO-sA) case study, Atmos. Chem. Phys., 22, 5701–5724, https://doi.org/10.5194/acp-22-5701-2022, 2022.
Formenti, P., Caquineau, S., Chevaillier, S., Klaver, A., Desboeufs, K., Rajot, J. L., Belin, S., and Briois, V.: Dominance of goethite over hematite in iron oxides of mineral dust from Western Africa: Quantitative partitioning by X-ray absorption spectroscopy, J. Geophys. Res.-Atmos., 119, 12740–12754, https://doi.org/10.1002/2014JD021668, 2014.
Formenti, P., Piketh, S. J., Namwoonde, A., Klopper, D., Burger, R., Cazaunau, M., Feron, A., Gaimoz, C., Broccardo, S., Walton, N., Desboeufs, K., Siour, G., Hanghome, M., Mafwila, S., Omoregie, E., Junkermann, W., and Maenhaut, W.: Three years of measurements of light-absorbing aerosols over coastal Namibia: seasonality, origin, and transport, Atmos. Chem. Phys., 18, 17003–17016, https://doi.org/10.5194/acp-18-17003-2018, 2018.
Formenti, P., D'Anna, B., Flamant, C., Mallet, M., Piketh, S. J., Schepanski, K., Waquet, F., Auriol, F., Brogniez, G., Burnet, F., Chaboureau, J.-P., Chauvigné, A., Chazette, P., Denjean, C., Desboeufs, K., Doussin, J.-F., Elguindi, N., Feuerstein, S., Gaetani, M., Giorio, C., Klopper, D., Mallet, M. D., Nabat, P., Monod, A., Solmon, F., Namwoonde, A., Chikwililwa, C., Mushi, R., Welton, E. J., and Holben, B.: The Aerosols, Radiation and Clouds in Southern Africa Field Campaign in Namibia: Overview, Illustrative Observations, and Way Forward, B. Am. Meteorol. Soc., 100, 1277–1298, https://doi.org/10.1175/BAMS-D-17-0278.1, 2019.
Formenti, P., Giorio, C., Desboeufs, K., Zherebker, A., Gaetani, M., Baldo, C., Landrot, G., Montebello, S., Chevaillier, S., Triquet, S., Siour, G., Di Biagio, C., Battaglia, F., Doussin, J.-F., Feron, A., Namwoonde, A., and Piketh, S. J.: Elemental composition, iron mineralogy, and solubility of anthropogenic and natural mineral dust aerosols in Namibia: a case study analysis from the AEROCLO-sA campaign – Part 2, Atmos. Chem. Phys., 25, 16127–16145, https://doi.org/10.5194/acp-25-16127-2025, 2025.
Gao, Y., Marsay, C. M., Yu, S., Fan, S., Mukherjee, P., Buck, C. S., and Landing, W. M.: Particle-Size Variability of Aerosol Iron and Impact on Iron Solubility and Dry Deposition Fluxes to the Arctic Ocean, Sci. Rep.-UK, 9, 16653, https://doi.org/10.1038/s41598-019-52468-z, 2019.
Ge, P., Fan, S., Wang, Y., Zhang, S., Wu, H., Shao, N., and Zu, F.: Chemical characteristics of three-stage fog water in an agricultural city in China, Front. Environ. Sci., 10, 1–15, https://doi.org/10.3389/fenvs.2022.1003669, 2022.
Giorio, C., Marton, D., Formenton, G., and Tapparo, A.: Formation of Metal–Cyanide Complexes in Deliquescent Airborne Particles: A New Possible Sink for HCN in Urban Environments, Environ. Sci. Technol., 51, 14107–14113, https://doi.org/10.1021/acs.est.7b03123, 2017.
Giorio, C., Doussin, J. F., D'Anna, B., Mas, S., Filippi, D., Denjean, C., Mallet, M. D., Bourrianne, T., Burnet, F., Cazaunau, M., Chikwililwa, C., Desboeufs, K., Feron, A., Michoud, V., Namwoonde, A., Andreae, M. O., Piketh, S. J., and Formenti, P.: Butene Emissions From Coastal Ecosystems May Contribute to New Particle Formation, Geophys. Res. Lett., 49, e2022GL098770, https://doi.org/10.1029/2022GL098770, 2022a.
Giorio, C., D'Aronco, S., Di Marco, V., Badocco, D., Battaglia, F., Soldà, L., Pastore, P., and Tapparo, A.: Emerging investigator series: aqueous-phase processing of atmospheric aerosol influences dissolution kinetics of metal ions in an urban background site in the Po Valley, Environ. Sci. Process. Impacts., 24, 884–897, https://doi.org/10.1039/d2em00023g, 2022b.
Giorio, C., Borca, C. N., Zherebker, A., D'Aronco, S., Saidikova, M., Sheikh, H. A., Harrison, R. J., Badocco, D., Soldà, L., Pastore, P., Ammann, M., and Huthwelker, T.: Iron Speciation in Urban Atmospheric Aerosols: Comparison between Thermodynamic Modeling and Direct Measurements, ACS Earth and Space Chemistry, 9, 649–661, https://doi.org/10.1021/acsearthspacechem.4c00359, 2025.
Gledhiir, M. and Buck, K. N.: The organic complexation of iron in the marine environment: A review, Front. Microbiol., 3, 1–17, https://doi.org/10.3389/fmicb.2012.00069, 2012.
Gonser, S. G., Klemm, O., Griessbaum, F., Chang, S. C., Chu, H. Sen, and Hsia, Y. J.: The relation between humidity and liquid water content in fog: An experimental approach, Pure Appl. Geophys., 169, 821–833, https://doi.org/10.1007/s00024-011-0270-x, 2012.
Gottlieb, T. R., Eckardt, F. D., Venter, Z. S., and Cramer, M. D.: The contribution of fog to water and nutrient supply to Arthraerua leubnitziae in the central Namib Desert, Namibia, J. Arid Environ., 161, 35–46, https://doi.org/10.1016/j.jaridenv.2018.11.002, 2019.
Gultepe, I., Müller, M. D., and Boybeyi, Z.: A New Visibility Parameterization for Warm-Fog Applications in Numerical Weather Prediction Models, J. Appl. Meteorol. Clim., 45, 1469–1480, https://doi.org/10.1175/JAM2423.1, 2006.
Gultepe, I., Pearson, G., Milbrandt, J. A., Hansen, B., Platnick, S., Taylor, P., Gordon, M., Oakley, J. P., and Cober, S. G.: The Fog Remote Sensing and Modeling Field Project, B. Am. Meteorol. Soc., 90, 341–360, https://doi.org/10.1175/2008BAMS2354.1, 2009.
Gundel, L. A., Benner, W. H., and Hansen, A. D. A.: Chemical composition of fog water and interstitial aerosol in Berkeley, California, Atmos. Environ., 28, 2715–2725, https://doi.org/10.1016/1352-2310(94)90443-X, 1994.
Gupta, S., Mcfarquhar, G. M., Brien, J. R. O., Delene, D. J., Michael, R., Dobracki, A., Podolske, J. R., Redemann, J., Leblanc, S. E., Segal-Rozenhaimer, M., and Pistone, K.: Impact of the Variability in Vertical Separation between Biomass – Burning Aerosols and Marine Stratocumulus on Cloud Microphysical Properties over the Southeast Atlantic, Atmos. Chem. Phys., 21, 4615–4635, https://doi.org/10.5194/acp-21-4615-2021, 2021.
Guyot, G., Gourbeyre, C., Febvre, G., Shcherbakov, V., Burnet, F., Dupont, J.-C., Sellegri, K., and Jourdan, O.: Quantitative evaluation of seven optical sensors for cloud microphysical measurements at the Puy-de-Dôme Observatory, France, Atmos. Meas. Tech., 8, 4347–4367, https://doi.org/10.5194/amt-8-4347-2015, 2015.
Halsey, K. H., Giovannoni, S. J., Graus, M., Zhao, Y., Landry, Z., Thrash, J. C., Vergin, K. L., and de Gouw, J.: Biological cycling of volatile organic carbon by phytoplankton and bacterioplankton, Limnol. Oceanogr., 62, 2650–2661, https://doi.org/10.1002/lno.10596, 2017.
Haskins, J. D., Jaeglé, L., Shah, V., Lee, B. H., Lopez-Hilfiker, F. D., Campuzano-Jost, P., Schroder, J. C., Day, D. A., Guo, H., Sullivan, A. P., Weber, R., Dibb, J., Campos, T., Jimenez, J. L., Brown, S. S., and Thornton, J. A.: Wintertime Gas-Particle Partitioning and Speciation of Inorganic Chlorine in the Lower Troposphere Over the Northeast United States and Coastal Ocean, J. Geophys. Res.-Atmos., 123, 12897–12916, https://doi.org/10.1029/2018JD028786, 2018.
Hecobian, A., Liu, Z., Hennigan, C. J., Huey, L. G., Jimenez, J. L., Cubison, M. J., Vay, S., Diskin, G. S., Sachse, G. W., Wisthaler, A., Mikoviny, T., Weinheimer, A. J., Liao, J., Knapp, D. J., Wennberg, P. O., Kürten, A., Crounse, J. D., Clair, J. St., Wang, Y., and Weber, R. J.: Comparison of chemical characteristics of 495 biomass burning plumes intercepted by the NASA DC-8 aircraft during the ARCTAS/CARB-2008 field campaign, Atmos. Chem. Phys., 11, 13325–13337, https://doi.org/10.5194/acp-11-13325-2011, 2011.
Henschel, J. R., Wassenaar, T. D., Kanandjembo, A., Louw, M. K., Neef, G., Shuuya, T., and Soderberg, K.: Roots point to water sources of Welwitschia mirabilis in a hyperarid desert, Ecohydrology, 12, e2039, https://doi.org/10.1002/eco.2039, 2019.
Herckes, P., Lee, T., Trenary, L., Kang, G., Chang, H., and Collett, J. L.: Organic matter in central California radiation fogs, Environ. Sci. Technol., 36, 4777–4782, https://doi.org/10.1021/es025889t, 2002.
von Holdt, J. R. and Eckardt, F. D.: Dust activity and surface sediment characteristics of the dustiest river in southern Africa: the Kuiseb River, Central Namib, S. Afr. Geogr. J., 100, 104–121, https://doi.org/10.1080/03736245.2017.1339627, 2018.
Hull, T., D'Aronco, S., Crumeyrolle, S., Hanoune, B., Giammanco, S., La Spina, A., Salerno, G., Soldà, L., Badocco, D., Pastore, P., Sellitto, P., and Giorio, C.: Metal speciation of volcanic aerosols from Mt. Etna at varying aerosol water content and pH obtained by different thermodynamic models, Environmental Science: Atmospheres, 5, 8–24, https://doi.org/10.1039/D4EA00108G, 2025.
IPCC: Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I., Huang, M., Leitzell, K., Lonnoy, E., Matthews, J. B. R., Maycock, T. K., Waterfield, T., Yelekçi, O., Yu, R., and Zhou, B., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2391 pp., https://doi.org/10.1017/9781009157896, 2021.
Jarre, A., Hutchings, L., Kirkman, S. P., Kreiner, A., Tchipalanga, P. C. M., Kainge, P., Uanivi, U., van der Plas, A. K., Blamey, L. K., Coetzee, J. C., Lamont, T., Samaai, T., Verheye, H. M., Yemane, D. G., Axelsen, B. E., Ostrowski, M., Stenevik, E. K., and Loeng, H.: Synthesis: Climate effects on biodiversity, abundance and distribution of marine organisms in the Benguela, Fish Oceanogr., 24, 122–149, https://doi.org/10.1111/fog.12086, 2015.
Jiang, L. Q., Carter, B. R., Feely, R. A., Lauvset, S. K., and Olsen, A.: Surface ocean pH and buffer capacity: past, present and future, Sci. Rep.-UK, 9, 18624, https://doi.org/10.1038/s41598-019-55039-4, 2019.
Johansen, A. M. and Key, J. M.: Photoreductive dissolution of ferrihydrite by methanesulfinic acid: Evidence of a direct link between dimethylsulfide and iron-bioavailability, Geophys. Res. Lett., 33, L14818, https://doi.org/10.1029/2006GL026010, 2006.
Klopper, D., Formenti, P., Namwoonde, A., Cazaunau, M., Chevaillier, S., Feron, A., Gaimoz, C., Hease, P., Lahmidi, F., Mirande-Bret, C., Triquet, S., Zeng, Z., and Piketh, S. J.: Chemical composition and source apportionment of atmospheric aerosols on the Namibian coast, Atmos. Chem. Phys., 20, 15811–15833, https://doi.org/10.5194/acp-20-15811-2020, 2020.
Landrot, G. and Fonda, E.: a software for the treatment of XAFS datasets of environmental relevance or acquired in operando conditions, J. Synchrotron Rad., 32, 1085–1094, 2025.
Li, T., Wang, Z., Wang, Y., Wu, C., Liang, Y., Xia, M., Yu, C., Yun, H., Wang, W., Wang, Y., Guo, J., Herrmann, H., and Wang, T.: Chemical characteristics of cloud water and the impacts on aerosol properties at a subtropical mountain site in Hong Kong SAR, Atmos. Chem. Phys., 20, 391–407, https://doi.org/10.5194/acp-20-391-2020, 2020.
Li, W., Qi, Y., Liu, Y., Wu, G., Zhang, Y., Shi, J., Qu, W., Sheng, L., Wang, W., Zhang, D., and Zhou, Y.: Daytime and nighttime aerosol soluble iron formation in clean and slightly polluted moist air in a coastal city in eastern China, Atmos. Chem. Phys., 24, 6495–6508, https://doi.org/10.5194/acp-24-6495-2024, 2024.
Longo, A. F., Feng, Y., Lai, B., Landing, W. M., Shelley, R. U., Nenes, A., Mihalopoulos, N., Violaki, K., and Ingall, E. D.: Influence of Atmospheric Processes on the Solubility and Composition of Iron in Saharan Dust, Environ. Sci. Technol., 50, 6912–6920, https://doi.org/10.1021/acs.est.6b02605, 2016.
Louw, D. C., van der Plas, A. K., Mohrholz, V., Wasmund, N., Junker, T., and Eggert, A.: Seasonal and interannual phytoplankton dynamics and forcing mechanisms in the Northern Benguela upwelling system, J. Marine Syst., 157, 124–134, https://doi.org/10.1016/j.jmarsys.2016.01.009, 2016.
Mao, J., Fan, S., and Horowitz, L. W.: Soluble Fe in Aerosols Sustained by Gaseous HO2 Uptake, Environ. Sci. Tech. Let., 4, 98–104, https://doi.org/10.1021/acs.estlett.7b00017, 2017.
Di Marco, V. B.: Studio della formazione di complessi tra alluminio e molecole di interesse ambientale, biologico e farmaceutico, https://hdl.handle.net/11577/186050 (last access: 3 September 2025), 1998.
Marcotte, A. R., Anbar, A. D., Majestic, B. J., and Herckes, P.: Mineral dust and iron solubility: Effects of composition, particle size, and surface area, Atmosphere, 11, 533, https://doi.org/10.3390/atmos11050533, 2020.
Mazoyer, M., Burnet, F., and Denjean, C.: Experimental study on the evolution of droplet size distribution during the fog life cycle, Atmos. Chem. Phys., 22, 11305–11321, https://doi.org/10.5194/acp-22-11305-2022, 2022.
Mungall, E. L., Abbatt, J. P. D., Wentzell, J. J. B., Lee, A. K. Y., Thomas, J. L., Blais, M., Gosselin, M., Miller, L. A., Papakyriakou, T., Willis, M. D., and Liggio, J.: Microlayer source of oxygenated volatile organic compounds in the summertime marine Arctic boundary layer, P. Natl. Acad. Sci. USA, 114, 6203–6208, https://doi.org/10.1073/pnas.1620571114, 2017.
Niu, S. J., Liu, D. Y., Zhao, L. J., Lu, C. S., Lü, J. J., and Yang, J.: Summary of a 4 year fog field study in Northern Nanjing, Part 2: Fog microphysics, Pure Appl. Geophys., 169, 1137–1155, https://doi.org/10.1007/s00024-011-0344-9, 2012.
Paglione, M., Decesari, S., Rinaldi, M., Tarozzi, L., Manarini, F., Gilardoni, S., Facchini, M. C., Fuzzi, S., Bacco, D., Trentini, A., Pandis, S. N., and Nenes, A.: Historical Changes in Seasonal Aerosol Acidity in the Po Valley (Italy) as Inferred from Fog Water and Aerosol Measurements, Environ. Sci. Technol., 55, 7307–7315, https://doi.org/10.1021/acs.est.1c00651, 2021.
Paris, R. and Desboeufs, K. V.: Effect of atmospheric organic complexation on iron-bearing dust solubility, Atmos. Chem. Phys., 13, 4895–4905, https://doi.org/10.5194/acp-13-4895-2013, 2013.
Paris, R., Desboeufs, K. V., and Journet, E.: Variability of dust iron solubility in atmospheric waters: Investigation of the role of oxalate organic complexation, Atmos. Environ., 45, 6510–6517, https://doi.org/10.1016/j.atmosenv.2011.08.068, 2011.
Peltola, M., Rose, C., Trueblood, J. V., Gray, S., Harvey, M., and Sellegri, K.: New particle formation in coastal New Zealand with a focus on open-ocean air masses, Atmos. Chem. Phys., 22, 6231–6254, https://doi.org/10.5194/acp-22-6231-2022, 2022.
Peng, X., Vasilakos, P., Nenes, A., Shi, G., Qian, Y., Shi, X., Xiao, Z., Chen, K., Feng, Y., and Russell, A. G.: Detailed Analysis of Estimated pH, Activity Coefficients, and Ion Concentrations between the Three Aerosol Thermodynamic Models, Environ. Sci. Technol., 53, 8903–8913, https://doi.org/10.1021/acs.est.9b00181, 2019.
Press, W. H., Teukolsky, S. A., Vetterling, W. T., and Flannery, B. P.: Numerical recipes: the art of scientific computing, Cambridge University Press, 1235, ISBN 0521880688, 2007.
Pye, H. O. T., Nenes, A., Alexander, B., Ault, A. P., Barth, M. C., Clegg, S. L., Collett Jr., J. L., Fahey, K. M., Hennigan, C. J., Herrmann, H., Kanakidou, M., Kelly, J. T., Ku, I.-T., McNeill, V. F., Riemer, N., Schaefer, T., Shi, G., Tilgner, A., Walker, J. T., Wang, T., Weber, R., Xing, J., Zaveri, R. A., and Zuend, A.: The acidity of atmospheric particles and clouds, Atmos. Chem. Phys., 20, 4809–4888, https://doi.org/10.5194/acp-20-4809-2020, 2020.
Rajot, J. L., Formenti, P., Alfaro, S., Desboeufs, K., Chevaillier, S., Chatenet, B., Gaudichet, A., Journet, E., Marticorena, B., Triquet, S., Maman, A., Mouget, N., and Zakou, A.: AMMA dust experiment: An overview of measurements performed during the dry season special observation period (SOP0) at the Banizoumbou (Niger) supersite, J. Geophys. Res.-Atmos., 113, 1–18, https://doi.org/10.1029/2008JD009906, 2008.
Redemann, J., Wood, R., Zuidema, P., Doherty, S. J., Luna, B., LeBlanc, S. E., Diamond, M. S., Shinozuka, Y., Chang, I. Y., Ueyama, R., Pfister, L., Ryoo, J.-M., Dobracki, A. N., da Silva, A. M., Longo, K. M., Kacenelenbogen, M. S., Flynn, C. J., Pistone, K., Knox, N. M., Piketh, S. J., Haywood, J. M., Formenti, P., Mallet, M., Stier, P., Ackerman, A. S., Bauer, S. E., Fridlind, A. M., Carmichael, G. R., Saide, P. E., Ferrada, G. A., Howell, S. G., Freitag, S., Cairns, B., Holben, B. N., Knobelspiesse, K. D., Tanelli, S., L'Ecuyer, T. S., Dzambo, A. M., Sy, O. O., McFarquhar, G. M., Poellot, M. R., Gupta, S., O'Brien, J. R., Nenes, A., Kacarab, M., Wong, J. P. S., Small-Griswold, J. D., Thornhill, K. L., Noone, D., Podolske, J. R., Schmidt, K. S., Pilewskie, P., Chen, H., Cochrane, S. P., Sedlacek, A. J., Lang, T. J., Stith, E., Segal-Rozenhaimer, M., Ferrare, R. A., Burton, S. P., Hostetler, C. A., Diner, D. J., Seidel, F. C., Platnick, S. E., Myers, J. S., Meyer, K. G., Spangenberg, D. A., Maring, H., and Gao, L.: An overview of the ORACLES (ObseRvations of Aerosols above CLouds and their intEractionS) project: aerosol–cloud–radiation interactions in the southeast Atlantic basin, Atmos. Chem. Phys., 21, 1507–1563, https://doi.org/10.5194/acp-21-1507-2021, 2021.
Rodríguez, S., Prospero, J. M., López-Darias, J., García-Alvarez, M. I., Zuidema, P., Nava, S., Lucarelli, F., Gaston, C. J., Galindo, L., and Sosa, E.: Tracking the changes of iron solubility and air pollutants traces as African dust transits the Atlantic in the Saharan dust outbreaks, Atmos. Environ., 246, 118092, https://doi.org/10.1016/j.atmosenv.2020.118092, 2021.
Scheinhardt, S., Müller, K., Spindler, G., and Herrmann, H.: Complexation of trace metals in size-segregated aerosol particles at nine sites in Germany, Atmos. Environ., 74, 102–109, https://doi.org/10.1016/j.atmosenv.2013.03.023, 2013.
Shahpoury, P., Zhang, Z. W., Arangio, A., Celo, V., Dabek-Zlotorzynska, E., Harner, T., and Nenes, A.: The influence of chemical composition, aerosol acidity, and metal dissolution on the oxidative potential of fine particulate matter and redox potential of the lung lining fluid, Environ. Int., 148, 106343, https://doi.org/10.1016/j.envint.2020.106343, 2021.
Shahpoury, P., Lelieveld, S., Johannessen, C., Berkemeier, T., Celo, V., Dabek-Zlotorzynska, E., Harner, T., Lammel, G., and Nenes, A.: Influence of aerosol acidity and organic ligands on transition metal solubility and oxidative potential of fine particulate matter in urban environments, Sci. Total Environ., 906, 167405, https://doi.org/10.1016/j.scitotenv.2023.167405, 2024a.
Shahpoury, P., Lelieveld, S., Srivastava, D., Baccarini, A., Mastin, J., Berkemeier, T., Celo, V., Dabek-Zlotorzynska, E., Harner, T., Lammel, G., and Nenes, A.: Seasonal Changes in the Oxidative Potential of Urban Air Pollutants: The Influence of Emission Sources and Proton- and Ligand-Mediated Dissolution of Transition Metals, ACS ES&T Air, https://doi.org/10.1021/acsestair.4c00093, 2024b.
Shi, Z., Krom, M. D., Bonneville, S., Baker, A. R., Jickells, T. D., and Benning, L. G.: Formation of iron nanoparticles and increase in iron reactivity in mineral dust during simulated cloud processing, Environ. Sci. Technol., 43, 6592–6596, https://doi.org/10.1021/es901294g, 2009.
Shi, Z., Krom, M. D., Jickells, T. D., Bonneville, S., Carslaw, K. S., Mihalopoulos, N., Baker, A. R., and Benning, L. G.: Impacts on iron solubility in the mineral dust by processes in the source region and the atmosphere: A review, Aeolian Res., 5, 21–42, https://doi.org/10.1016/j.aeolia.2012.03.001, 2012.
Shi, Z., Krom, M. D., Bonneville, S., and Benning, L. G.: Atmospheric processing outside clouds increases soluble iron in mineral dust, Environ. Sci. Technol., 49, 1472–1477, https://doi.org/10.1021/es504623x, 2015.
Shikwambana, L. and Kganyago, M.: Meteorological Influence of Mineral Dust Distribution Over South-Western Africa Deserts Using Reanalysis and Satellite Data, Front. Environ. Sci., 10, 1–11, https://doi.org/10.3389/fenvs.2022.856438, 2022.
Siefert, R. L., Erel, Y., and Hoffmann, M. R.: Iron photochemistry of aqueous suspensions of ambient aerosol with added organic acids, Pergamon Geochimica et Cosmochimica Acta, 58, 3271–3279, 1994.
Sorooshian, A., Wang, Z., Coggon, M. M., Jonsson, H. H., and Ervens, B.: Observations of sharp oxalate reductions in stratocumulus clouds at variable altitudes: Organic acid and metal measurements during the 2011 E-PEACE campaign, Environ. Sci. Technol., 47, 7747–7756, https://doi.org/10.1021/es4012383, 2013.
Spiegel, J. K., Zieger, P., Bukowiecki, N., Hammer, E., Weingartner, E., and Eugster, W.: Evaluating the capabilities and uncertainties of droplet measurements for the fog droplet spectrometer (FM-100), Atmos. Meas. Tech., 5, 2237–2260, https://doi.org/10.5194/amt-5-2237-2012, 2012.
Tapparo, A., Di Marco, V., Badocco, D., D'Aronco, S., Soldà, L., Pastore, P., Mahon, B. M., Kalberer, M., and Giorio, C.: Formation of metal-organic ligand complexes affects solubility of metals in airborne particles at an urban site in the Po valley, Chemosphere, 241, 125025, https://doi.org/10.1016/j.chemosphere.2019.125025, 2020.
The IUPAC Stability Constants Database: Chemistry International – Newsmagazine for IUPAC, 28, 14–15, https://doi.org/10.1515/ci.2006.28.5.14, 2006.
Tournadre, J.: Anthropogenic pressure on the open ocean: The growth of ship traffic revealed by altimeter data analysis, Geophys. Res. Lett., 41, 7924–7932, https://doi.org/10.1002/2014GL061786, 2014.
Trisos, C. H., Adelekan, I. O., and Totin, E.: Africa, in: Climate Change 2022 – Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, vol. 1, Cambridge University Press, Cambridge, United Kingdom and New York, USA, 1285–1456, https://doi.org/10.1017/9781009325844.011, 2023.
Tyson, P. D. and Preston-Whyte, R. A.: The weather and climate of southern Africa, 2nd edn., Oxford University Press, Cape Town, 408, ISBN 0195718062, ISBN 9780195718065, 2000.
Upadhyay, N., Majestic, B. J., and Herckes, P.: Solubility and speciation of atmospheric iron in buffer systems simulating cloud conditions, Atmos. Environ., 45, 1858–1866, https://doi.org/10.1016/j.atmosenv.2011.01.010, 2011.
Vickery, K. J. and Eckardt, F. D.: Dust emission controls on the lower Kuiseb River valley, Central Namib, Aeolian Res., 10, 125–133, https://doi.org/10.1016/j.aeolia.2013.02.006, 2013.
Wagh, S., Kulkarni, R., Lonkar, P., Parde, A. N., Dhangar, N. G., Govardhan, G., Sajjan, V., Debnath, S., Gultepe, I., Rajeevan, M., and Ghude, S. D.: Development of visibility equation based on fog microphysical observations and its verification using the WRF model, Model. Earth Syst. Environ., 9, 195–211, https://doi.org/10.1007/s40808-022-01492-6, 2023.
Wang, Z., Sorooshian, A., Prabhakar, G., Coggon, M. M., and Jonsson, H. H.: Impact of emissions from shipping, land, and the ocean on stratocumulus cloud water elemental composition during the 2011 E-PEACE field campaign, Atmos. Environ., 89, 570–580, https://doi.org/10.1016/j.atmosenv.2014.01.020, 2014.
Wen, J. L., Yi, P. Z., Hong, M. L., Fan, R. M., Yu, H. L., and Wang, C. M.: Fog- and rainwater chemistry in the tropical seasonal rain forest of Xishuangbanna, southwest China, Water Air Soil. Pollut., 167, 295–309, https://doi.org/10.1007/s11270-005-0080-9, 2005.
Weston, M. J., Piketh, S. J., Burnet, F., Broccardo, S., Denjean, C., Bourrianne, T., and Formenti, P.: Sensitivity analysis of an aerosol-aware microphysics scheme in Weather Research and Forecasting (WRF) during case studies of fog in Namibia, Atmos. Chem. Phys., 22, 10221–10245, https://doi.org/10.5194/acp-22-10221-2022, 2022.
Wexler, A. S.: Atmospheric aerosol models for systems including the ions H+, , Na+, , , Cl−, Br−, and H2O, J. Geophys. Res., 107, 4207, https://doi.org/10.1029/2001JD000451, 2002.
Winton, V. H. L., Bowie, A. R., Edwards, R., Keywood, M., Townsend, A. T., van der Merwe, P., and Bollhöfer, A.: Fractional iron solubility of atmospheric iron inputs to the Southern Ocean, Mar. Chem., 177, 20–32, https://doi.org/10.1016/j.marchem.2015.06.006, 2015.
Zhang, G., Peng, X., Sun, W., Fu, Y., Yang, Y., Liu, D., Shi, Z., Tang, M., Wang, X., and Bi, X.: Fog/cloud processing of atmospheric aerosols from a single particle perspective: A review of field observations, Atmospheric Environment, 329, 120536, https://doi.org/10.1016/j.atmosenv.2024.120536, 15 July 2024.