the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Atmospheric organosulfate formation regulated by continental outflows and marine emissions over East Asian marginal seas
Shubin Li
Yujue Wang
Yiwen Zhang
Yizhe Yi
Yuchen Wang
Yuqi Guo
Chao Yu
Yue Jiang
Jinhui Shi
Chao Zhang
Jialei Zhu
Jianzhen Yu
Xiaohong Yao
Huiwang Gao
Organosulfates (OSs) represent an unrecognized fraction and a potentially important source of marine organic aerosols. Based on shipboard observations over East Asian marginal seas, we characterized OSs in marine aerosols during spring, summer, and autumn. The C2–C3 OSs and isoprene-/monoterpenes-derived OSs were quantified using synthesized standards. The total quantified OSs concentrations ranged from 4.5 to 109.1 ng m−3, contributing 0.1 %–3.2 % of the mass concentration of marine organic aerosols. The highest OSs concentrations, dominated by C2–C3 OSs and isoprene-OSs, were observed in summer, which surpassed the abundance of methane sulfonic acid, a key component in climate regulation by oceanic phytoplankton sulphur emissions. Abundant OSs formation in summer was mainly attributed to the increased isoprene emissions from the ocean. During the spring and autumn cruises, transported continental pollutants resulted in the higher fraction of monoterpene-derived (nitrooxy-)OSs, as well as the elevated OSs concentrations over regions surrounded by the continent. This work highlights the joint effects of marine emissions and continental outflows on the formation and distribution of atmospheric OSs over marginal seas.
- Article
(1757 KB) - Full-text XML
-
Supplement
(1659 KB) - BibTeX
- EndNote
Marine atmospheric aerosols play a vital role in climate change through influencing cloud formation and solar radiative balance (Li et al., 2022). Marine phytoplankton could generate abundant dimethylsulfide (DMS), which further be oxidized in the atmosphere, forms methane sulphonic acid (MSA) or sulfate aerosols and then regulates the cloud condensation nuclei (CCN) formation and climate in the marine boundary layer (Andreae and Rosenfeld, 2008; Kettle and Andreae, 2000; Kloster et al., 2006). This is named the CLAW hypothesis, proposed by Charlson et al. (1987) and Ayers and Gras (1991). However, the following observational evidence and modelling studies indicated that CCN formation in marine atmospheres is far more complex than had been recognized by the CLAW hypothesis (Quinn and Bates, 2011). This is mainly attributed to the unknown organic fractions in marine aerosols, including those primarily emitted by sea spray and secondarily formed organic aerosols (SOA) via the oxidation of volatile organic compounds (VOCs).
Traditional SOA tracers, including those from the oxidation of isoprene and monoterpene, etc., could explain only < 10 % of marine organic aerosols (Fu et al., 2011; Guo et al., 2020). The majority of the marine SOA components remain unknown till now. Abundant isoprene could be emitted from the ocean, and isoprene SOA has been proved to be one of the most important fractions in marine organic aerosols (Hu et al., 2013). Another important SOA formation pathway from isoprene and monoterpene oxidation is facilitated by acidic sulfate particles under high humidity conditions, resulting in the formation of organosulfates (OSs) (Brüggemann et al., 2020). In the marine boundary layer, sulfate aerosols could largely be formed via the oxidation of DMS emitted by marine phytoplankton (Andreae, 1990; Li et al., 2018; Yan et al., 2024). Sulfate aerosols are generally abundant over various marine environments (Li et al., 2018; Shank et al., 2012), which serve as a key precursor for the OSs formation and provide an ideal condition for the reactive uptake of VOCs oxidation products. Laboratory studies suggested that the reactive uptake of isoprene epoxydiols (IEPOX) or monoterpene oxides onto aerosol particles and ring-opening epoxide reactions could be catalyzed by acidic sulfate aerosols, resulting in the formation of IEPOX-OS and monoterpene-derived OSs (Surratt et al., 2010; Schindelka et al., 2013; Riva et al., 2016a). Biogenic organosulfur formation via the acidic sulfate-catalyzed aqueous reactions with VOCs has been proved to represent an important source of atmospheric organic aerosols (Riva et al., 2019). Recent studies also indicated the existence and importance of organic sulfur compounds, including OSs, in marine aerosols (Bao et al., 2018; Ye et al., 2021).
Atmospheric OSs constitute a large portion of organic aerosols (OA) in the environments with substantial interactions of biogenic and anthropogenic emissions (Hettiyadura et al., 2019; Meade et al., 2016; Surratt et al., 2008; Wang et al., 2018). A recent cruise observation over Asian marginal seas suggested that OSs derived from isoprene and monoterpenes could contribute about 7 % of the OA mass concentration (Wang et al., 2023b). Wang et al. (2023b) also indicated that isoprene/monoterpene-derived OSs could surpass the traditionally identified SOA tracers generated from isoprene or monoterpene oxidation (e.g., methylglyceric acid, alkene triols, hydroxyglutaric acid, pinic acid etc.). Besides sulfate and MSA, atmospheric OSs could be a potential key species in the sulfur cycle in the marine boundary layer. Atmospheric OSs are generally with larger molecular weights than MSA, and both are less hygroscopic taking up water compared to the inorganic ammonium sulfate (Brüggemann et al., 2020; Hansen et al., 2015; Peng et al., 2021; Rosati et al., 2022). Previous studies reported the hygroscopicity parameter (κ) of 0.46 for sodium methanesulfonate, and 0.46, 0.40, 0.21 for methyl-, ethyl- and octyl-OS, and the hygroscopicity of OSs decreased with the alkyl chain length (Peng et al., 2021; Tang et al., 2019). The OSs molecules have the hydrophilic sulfate group and the hydrophobic organic group, making them surface-active compounds. It has been suggested that OSs could lower the surface tension of particles, affect the particles' ability to absorb water, and to act as CCN (Brüggemann et al., 2020; Hansen et al., 2015). The roles of OSs in regulating the CCN formation and climate may be different from MSA, which needs further elaboration via field observations and laboratory studies. We noted that the atmospheric OSs derived from isoprene or monoterpenes were rarely detected in the marine aerosols collected at a remote island site located in the Southern Ocean or the southern Indian Ocean (Claeys et al., 2010; Cui et al., 2019). These could be attributed to the low biogenic VOCs emission/flux or the degradation of OSs over long-term storage (Claeys et al., 2010; Cui et al., 2019).
The existence or abundance of atmospheric OSs in marine aerosols have not been well evaluated or quantified till now (Hawkins et al., 2010; Wang et al., 2023b; Ye et al., 2021), which limited the understanding on their formation processes or their roles in the sulfur cycle and aerosol climate effects in marine atmospheres. In this study, atmospheric OSs over East Asian marginal seas were quantified using synthesized OSs standards. We characterized the particulate OSs derived from isoprene and monoterpenes, and investigated their spatial distributions, seasonal variations, as well as the dominant environmental factors of OSs formation. Our results suggested that, over marginal seas, the spatiotemporal distribution of OSs abundance and composition was dependent on the relative importance of marine emissions and continental outflows. This work highlights the vital roles of OSs in altering the sulfur cycle in marine boundary layer, and further studies in open ocean are needed to understand the influence of OSs on the climate effects of marine aerosols.
2.1 Cruise observation and sample collection
Marine aerosol samples were collected during summer (16–26 July) and autumn (21 October–2 November) in 2021, and during spring (14–25 April) in 2022 over the Yellow Sea and Bohai Sea (YBS). The YBS are marginal seas surrounded by the East Asian continents (Fig. 1), where there are active interactions between transported continent outflows (e.g. Asian dust, anthropogenic pollutants) and marine emissions. The atmosphere over the YBS is dominantly influenced by the marine emissions or the continental outflows in different seasons, making it an ideal region to understand the roles of marine emissions and continental pollutants in the marine aerosol formation. The fine particle (PM2.5) and total suspended particulate (TSP) samples were collected using high-volume aerosol samplers (KB-1000, Qingdao Genstar Electronic Technology, China). The quartz fiber filters were pre-baked at 500 °C for 4.5 h and wrapped in pre-baked aluminum foil after sampling. Aerosol samplers were placed on the top deck of the vessel “Lanhai 101”, approximately 8 m above the sea surface. Each aerosol sample was collected for 10–24 h, and a field blank sample was collected during each cruise. The field blank sample was used to correct the potential sampling artifacts for the quantified OSs and other species in the marine aerosol samples.
During the observation, wind speed (WS), air temperature, and relative humidity (RH) were simultaneously measured by a shipboard meteorological observatory, with a time resolution of 10 s. The surface seawater (2–5 m) samples were collected by a conductivity–temperature–depth (CTD) assembly (Seabird 9/11), and the chlorophyll-a (Chl-a) concentration in surface seawater was measured using a CE Turner Designs fluorometer. There were 2–12 CTD sites during the sampling period of each filter sample. Concentrations of seawater isoprene were then estimated by empirical formulas based on previous studies (Ooki et al., 2015; Wang et al., 2023b). The 72 h backward trajectories of air masses from an altitude of 500 m above ground level were calculated using the HYSPLIT model (Version 5.2.1, NOAA), starting every 6 h (Fig. S1 in the Supplement). Trajectories at the center site of the observation region were calculated to represent the air masses during each cruise over the YBS.
2.2 HPLC-MS analysis and OSs quantification
An aliquot of each filter sample was extracted by methanol. The solutions were filtered using PTFE syringe filter (0.22 µm), and evaporated to dryness under a gentle stream of N2 gas. The dried residues were redissolved in methanol containing 0.1 % formic acid (100 µL). The OSs compounds were quantified using a QTRAP 4500 mass spectrometer (AB Sciex) coupled with an UHPLC system (Ultimate 3000, Thermo Scientific, DE) for the low-molecular-weight OSs, and an Exactive Plus-Orbitrap mass spectrometer (Thermo Scientific Inc.) with an UHPLC system (Ultimate 3000) for monoterpene-derived compounds. Mass spectrometry was operated using a negative-mode electrospray ionization. The monoterpene NOSs (C10H16NO7S− and C9H14NO8S−) were identified in the extracted ion chromatogram mode, and other OSs compounds were quantified in multiple-reaction monitoring (MRM) mode. In this work, C2–C3 OSs (HAS, GAS, and LAS), isoprene-OSs (IEPOX-OS, MAE-OS, and C5H7O7S−), monoterpene-OSs, and nitrooxy-OSs (NOSs) were quantified using synthesized OSs standards (Tables S1, S2 in the Supplement) (Wang et al., 2018).
Chromatographic separation of the low-molecular-weight OSs, including C2–C3 OSs and isoprene-OSs/NOSs, was optimized using an ethylene bridged hybrid (BEH) Amide column (2.1 mm × 100 mm, 1.7 µm, Waters, USA) equipped with a pre-column. Hydrophilic interaction liquid chromatography (HILIC) separation is an accurate analytical method for quantifying the low-molecular-weight OSs (Hettiyadura et al., 2015). The injection volume was 2.0 µL. The column was maintained at 35 °C. Mobile eluents were solvent A: ammonium acetate buffer (10 mM, pH 9) in ultrapure water and solvent B: 10 mM ammonium acetate buffer (10 mM, pH 9) in acetonitrile water (95 : 5). The flow rate was 0.4 mL min−1 at 0–2.5 min, then decreased to 0.35 mL min−1 from 2.5 to 11.5 min, and increased back to 0.4 mL min−1 from 11.5 to 18 min. The gradient elution was set as follows: 100 % B at 0–0.4 min; reduced to 88 % B at 0.4–2.4 min and maintained until 11 min; increased to 100 % B at 11–11.5 min, and maintained at 100 % B until 18 min to re-equilibrate the column. Monoterpene OSs/NOSs were analyzed using an Acquity UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 µm, Waters, USA) with a pre-column. The mobile eluents were solvent A (0.1 % acetic acid in ultrapure water) and solvent B (0.1 % acetic acid in methanol) at a flow rate of 0.3 mL min−1. The gradient elution procedure was performed as follows: 5 % B at 0–1.5 min; increased to 54 % B over 13.7 min and held for 1.0 min; then increased to 90 % B over 1.8 min and held for 5 min; decreased to 5 % B over 0.5 min and held for 1.5 min to re-equilibrate the column for next injection. The column temperature was maintained at 45 °C, and the injection volume was 5.0 µL.
As shown in Table S1, the UHPLC and MS/MS conditions produce highly linear calibration curves for the quantified OSs compounds (R2>0.99). The limit of detection (LOD) and limit of quantification (LOQ) of C2–C3 OSs range 0.07–0.79 and 0.24–2.62 µg L−1. The LOD and LOQ of monoterpene-OSs range 0.73–2.65 µg L−1 and 2.42–8.85 µg L−1. The relative standard deviation (RSD) of the quantified OSs is <12.1 % based on ten replicate injections of standards. Spike recoveries of the OSs standards on the blank filter are 94 %–105 %. The measurement uncertainty of OSs concentrations is 5.5 %–13.2 % considering the relative errors in air volume (5 %), extraction efficiency (recovery), and instrumental analysis (Hettiyadura et al., 2017). Atmospheric OSs are primarily present in the particle phase under ambient conditions due to their low volatilities. Laboratory studies suggested that hydrolysis could be an atmospheric removal process for some OSs (Chen et al., 2020; Hu et al., 2011; Lam et al., 2019). The quantified OSs in this study are likely to be chemically stable over the atmospheric time scales (Chen et al., 2020; Hu et al., 2011; Lam et al., 2019). A previous study showed a potential positive bias of atmospheric OSs during the filter sampling and subsequent offline analysis (Kristensen et al., 2016). This sampling artifact is because that the gas-phase epoxides or SO2 might absorb onto the filter substrates during the sampling. Subsequent on-filter oxidation and sulfation of the absorbed epoxides may form OSs, leading to a positive bias in the sampling and quantification of OSs (Kristensen et al., 2016; Brüggemann et al., 2020). In the present study, the field blanks were analyzed following the same procedures and used to correct the potential sampling artifacts. The mass loadings of the quantified OSs compounds in the field blank samples were <0.1 % of those in the marine aerosol samples. All the reported OSs concentrations have been corrected by subtracting the background values in the corresponding field blank sample.
2.3 Measurements of aerosol chemical composition
The filter samples, with a time resolution of 10–24 h, were used for the analysis of organic carbon (OC), elemental carbon (EC), water-soluble ions, and MSA in the marine aerosols. The concentrations of OC and EC were measured using a carbon analyzer (Model RT-3131, Sunset Laboratory, OR). The OA concentration was then calculated by multiplying OC by 1.6 (Wang et al., 2023b). Water-soluble cations (Na+, NH, K+, Mg2+, Ca2+), anions (Cl−, NO, SO), and MSA were measured using ion chromatography systems (ICS-2100 and ICS-Aquion RFIC, Thermo Scientific). The concentrations of non-sea-salt potassium ion (nss-K+) and non-sea-salt sulfate (nss-SO) were respectively calculated by [K+] − 0.037 × [Na+] and [SO] − 0.2516 × [Na+] (Millero and Sohn, 1992; Jung et al., 2020; Balasubramanian et al., 2003; Behera et al., 2013). The mass loadings of OC and sulfate in the field blank samples were <8 % and <1.3 % of those in the collected marine aerosol samples. All the reported concentrations of the aerosol compounds have been corrected by subtracting the background values measured in the corresponding field blank sample. The concentrations of PM2.5 or TSP were reconstructed by summing the concentrations of inorganic ions, OA, and EC in each aerosol sample.
3.1 Concentration and composition of marine atmospheric OSs
The total quantified OSs and nitrooxy-OSs ranged from 4.5 to 109.1 ng m−3 in marine aerosols during the shipboard observations over the YBS (Fig. 1, Table S2). The eleven quantified OSs and NOSs compounds contributed 0.1 %–3.2 % of the OA mass concentrations over the YBS. The observed OSs concentrations here were generally higher than the wintertime concentrations at inland sites, and lower than those in coastal regions (Kanellopoulos et al., 2022; Meade et al., 2016; Nguyen et al., 2014; Wang et al., 2020, 2021, 2022b) (Fig. 2). This was due to the active interactions between biogenic VOCs and sulfate aerosols under high RH conditions in coastal areas, which favored the aqueous-phase formation of OSs in the atmosphere. Acid sulfate-catalyzed reactions with isoprene-derived epoxide are widely adopted as the most important pathway for atmospheric OSs formation (Liao et al., 2015; Surratt et al., 2008; Schindelka et al., 2013; Brüggemann et al., 2020). Under the high-humidity conditions, OSs could also be formed via the heterogeneous reactions between SO2 and monoterpene ozonolysis intermediates or organic peroxides (Ye et al., 2018). The OSs formation may be limited by the low biogenic VOCs emissions or ambient RH in the wintertime inland environments (Wang et al., 2020). It is noted that, taking the autumn observation as an example, we compared the OSs concentrations in the PM2.5 and the TSP samples simultaneously collected during the cruise (Fig. S2). The majority of the data points fall along the 1 : 1 line (Fig. S2). The presence of OSs is dominant in fine particles, and thus our further discussion is focused on the results of the PM2.5 samples.
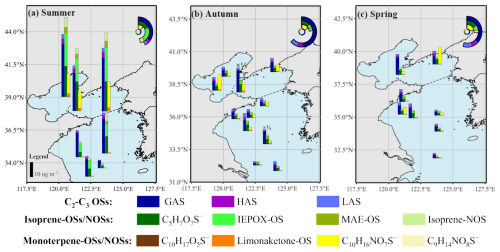
Figure 1Spatial distributions of OSs in PM2.5 over the YBS during (a) summer, (b) autumn in 2021, and (c) spring in 2022. The inserted charts in panels (a), (b), (c) are the contribution of different OSs compounds. The dotted lines in the panels are the dividing line of the North Yellow Sea (nYS) and the South Yellow Sea (sYS).
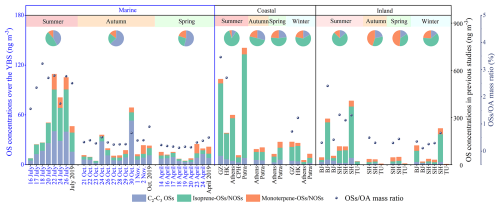
Figure 2Atmospheric OSs concentrations and mass ratios of (OSs + NOSs) OA over the YBS in this study and in inland and coastal atmospheres reported in previous studies (Kanellopoulos et al., 2022; Meade et al., 2016; Nguyen et al., 2014; Wang et al., 2020, 2021, 2022b). The data labels in this work are denoted in blue, and those from previous studies are in black. The pie charts represent the average contribution of OSs compound groups in each season. It is noted that the OSs abundance over the YBS and at coastal or inland sites are represented by different y-axes concentration ranges.
The C2–C3 OSs, including glycolic acid sulfate (GAS), hydroxyacetone sulfate (HAS), and lactic acid sulfate (LAS), were the most abundant compound group across the observed seasons (Figs. 1, 2). The C2–C3 OSs concentrations were respectively 7.2 ± 3.1, 24.2 ± 12.4, and 12.8 ± 14.4 ng m−3 in spring, summer, and autumn, comparable to the concentration levels at inland sites and lower than those in coastal areas (Fig. 2). In autumn and spring, the fraction of C2–C3 OSs, especially GAS, was much higher than other compound groups. The highest GAS concentration (47.8 ng m−3) over YBS was observed on 30 October during the autumn cruise. We noted that, in marine atmospheres, the contribution of C2–C3 OSs among the quantified OSs was much higher than those observed in various continental environments (Fig. 2). These low-molecular-weight OSs could be formed via the oxidation of VOCs precursors from both biogenic and anthropogenic origins (Wang et al., 2023a), and have been frequently observed as one of the most abundant OSs groups in previous studies (Wang et al., 2018, 2020; Cai et al., 2020).
The total concentration of quantified isoprene-OSs and NOSs ranged from 1.3 to 56.9 ng m−3, which were the most abundant group in summer over the YBS (Fig. 1). The predominance of isoprene OSs has been well documented at both coastal and inland sites (Fig. 2), which is attributed to the substantial biogenic isoprene emissions, especially during warmer seasons. Wu et al. (2021) reported abundant emission of isoprene from coastal and shelf seas (Wu et al., 2021), and isoprene OSs would then form via the interaction between sulfate aerosols and isoprene oxidation products (Surratt et al., 2010; Cooke et al., 2022). In the marine atmosphere over YBS, isoprene-derived OSs displayed a dominance by IEPOX-OS and C5H7O7S− during summer, and by C5H7O7S− during spring and autumn (Fig. 1). The C5H7O7S− compound has been suggested as a further oxidized or aged form of IEPOX-OS (Armstrong et al., 2022; Chen et al., 2020). The abundant presence of C5H7O7S− in marine aerosols across seasons indicated the rapid oxidation and aging processes of isoprene SOA in marine atmospheres. The high contribution of C5H7O7S− molecule among isoprene-derived OSs has been reported in marine aerosols, as well as in coastal and inland atmospheres (Hettiyadura et al., 2015; Kanellopoulos et al., 2022; Surratt et al., 2008; Wang et al., 2018, 2022b, 2023b).
The IEPOX-OS was one of the dominant OSs compounds during the summer cruise (Fig. 1), which is a typical low-NO oxidation product of isoprene formed via the acid-catalyzed ring opening of isoprene epoxydiols and subsequent nucleophilic addition of inorganic sulfate (Surratt et al., 2010; Lin et al., 2012). The dominance of IEPOX-OS among the biogenic OSs observed here is consistent with previous field observations under low-NO and high-RH conditions (Cooke et al., 2022; Lam et al., 2019; Liao et al., 2015). For the summertime samples, the contribution of IEPOX-OS among isoprene-OSs here is higher than that in a previous study conducted in 2019 over the YBS (Wang et al., 2023b). This could be due to the reduction of NOx emissions in the North China Plain (Li et al., 2024), resulting in a lower NO condition in 2022 than in 2019. It is also noted that a BEH Amide column and synthesized OSs standards were employed to separate and quantify the C2–C3 OSs and isoprene OSs in this study. The HILIC provides better separation and retention for the low-molecular-weight C2–C3 OSs and isoprene OSs, reflected by the retention time listed in Table S2. The OSs quantification here was more accurate than our previous study in 2019, in which a reversed-phase column and surrogate standard were used to separate and quantified the low-molecular-weight and highly polar OSs (Wang et al., 2023b). Separation of polar C2–C3 OSs and isoprene OSs using the reversed-phase chromatography could result in measurement bias due to the coelution and matrix effects (Hettiyadura et al., 2015; Liang et al., 2025). This could be an additional reason for the different OSs proportions between the two studies. The concentrations of methacrylic acid epoxide (MAE)-OS and isoprene-NOS, usually originated via NO/NO2 pathway or under high-NO conditions (Worton et al., 2013), were much lower than those of IEPOX-OS and its aged product (C5H7O7S−) in the marine atmospheres (Fig. 1, Table S2).
The mass concentration and contribution of monoterpene-derived (nitrooxy-)OSs were lower than those of C2–C3 OSs and isoprene-derived OSs over the YBS (Figs. 1, 2). This compound group was dominated by monoterpene NOSs (C10H16NO7S−), which were formed via the oxidation of monoterpenes in the presence of anthropogenic NOx (Surratt et al., 2008; Wang et al., 2018). The formation of monoterpene OSs/NOSs in marine atmospheres was driven by the transported continental pollutants. The concentration levels of monoterpene OSs/NOSs over the YBS were generally lower than those observed in continental atmospheres (Fig. 2) (He et al., 2014; Meade et al., 2016; Nguyen et al., 2014; Wang et al., 2020, 2021, 2022b).
3.2 Importance of OSs in marine atmospheres
The OSs concentrations and mass contribution among marine OA were the highest in summer, followed by those in autumn and spring (Figs. 1, 2). The average OSs concentration was 57.8 ± 38.9, 20.4 ± 19.7, and 13.3 ± 8.3 ng m−3 in summer, autumn, and spring, respectively. During the summer cruise, OSs occupied 1.6 %–3.2 % (2.5 % on average) of the marine OA mass concentrations, which were comparable to those observed in coastal regions and higher than those at the inland sites (Fig. 2). The elevated concentration levels and contributions of biogenic OSs, especially isoprene OSs and C2–C3 OSs, in summer were attributed to the increased biogenic VOCs emissions from marine phytoplankton or photochemical reactions in surface microlayer (Conte et al., 2020). The filter-sampling-averaged Chl-a concentrations were 0.6–5.3 mg m−3 (n=7, 2.1 ± 1.7 mg m−3 on average) during summer, 1.0–2.4 mg m−3 (n=8, 1.7±0.5 mg m−3 on average) during spring and 0.5–2.2 mg m−3 (n=11, 1.4 ± 0.6 mg m−3 on average) during autumn. High seawater Chl-a conditions (5.3 and 3.6 mg m−3) were observed during the summer cruise. In addition, the air temperature in summer was significantly (p<0.001) higher than that in other seasons (Fig. S3), and the summertime high temperature favored the sea-to-air transfer process of isoprene. The vital importance of biogenic OSs to OA formation in summer has been highlighted in previous observations at both marine and continental sites (Hettiyadura et al., 2017; Kanellopoulos et al., 2022; Meade et al., 2016; Nguyen et al., 2014; Wang et al., 2020, 2021, 2022b). We cannot exclude the potential influence of terrestrial biogenic VOCs emissions based on the observational evidence. The air masses were dominantly from the open ocean in summer (Fig. S1), indicating limited impacts from the continental outflows. During spring or autumn, the lower Chl-a and air temperature resulted in the decrease of biogenic OSs formation (Fig. 2). Though the seawater Chl-a was at similar concentration levels in spring and autumn (Fig. S3), the OSs abundance was lower in spring. The ambient temperature was lower in spring, and the oceanic phytoplankton had not revived from the low temperature conditions throughout winter. Thus, the biological activity and biogenic VOCs production were likely at low levels during the spring cruise.
During summer, the active interactions between biogenic VOCs, especially isoprene, and acidic sulfate converted notable fractions of inorganic sulfate aerosols to OSs in marine atmospheres. The abundance of OSs was comparable to that of MSA in summer, and their mass ratios were higher than those in autumn and spring (Fig. 3). During the summer cruise, the ratio of OSs-S SO-S and MSA-S SO-S were respectively 0.2 %–1.9 % (1.1 % on average) and 0.9 %–2.5 % (1.6 % on average) in terms of the molar mass of sulfur. Previous studies have suggested that atmospheric MSA formed via the oxidation of DMS contributes to the CCN formation in the marine boundary layer, which is a vital species relevant to the CLAW hypothesis of oceanic phytoplankton-controlled climate regulation (Ayers et al., 1997; Charlson et al., 1987; Quinn and Bates, 2011). Atmospheric OSs could modify the aerosol morphology, suppress the surface tension, and might play roles in altering the cloud formation (Estillore et al., 2016; Riva et al., 2019). The relevance of MSA and OSs in climate regulation and the CLAW hypothesis should be assessed considering their abundance in the atmosphere as well as their distinct physicochemical properties.
We noted that the OSs and MSA displayed strong correlations (r=0.86, p<0.01) in autumn (Fig. S4). This suggested that the atmospheric OSs and MSA formation were limited by the same environmental factors in autumn, which could be the lower marine biological activities indicated by the seawater Chl-a and temperatures (Fig. S3). The increase of marine phytoplankton emissions (e.g., DMS) may drive the formation and elevation of OSs during autumn over the YBS. Atmospheric OSs and MSA did not show an obvious correlation in summer. The seawater Chl-a and SST were higher during the summer cruise than during the other cruises (Fig. S3), indicating abundant marine biogenic emissions and sea-to-air exchange processes in summer. We proposed that the marine biogenic emitted precursors (e.g., DMS, isoprene) were abundant and in excess for the MSA and OSs formation in summer. The formation of MSA or OSs might be limited by different atmospheric oxidation or subsequent reaction processes of these precursors, and the environmental conditions driving their formation in summer need further investigation. The cruise observations indicated that OSs, besides MSA, should be taken into consideration when studying the sulfur cycle in marine atmospheres. The roles of atmospheric OSs in altering cloud formation need further investigation through shipboard observations, especially over oceanic regions with high phytoplankton biomass and high temperature.
3.3 Seasonal variation of atmospheric OSs composition
For the seasonal variations of OSs composition, the chemical spaces of the autumn and spring samples are highly overlapped, which are different from that of the summer samples (Fig. 4a). The fraction of isoprene-derived (nitrooxy-)OSs was higher during the summer cruise than those observed during the other two seasons. The autumn and spring samples generally showed a higher contribution by monoterpene-derived OSs compounds. The seasonal variation was attributed to the relatively lower isoprene emissions, indicated by the lower seawater Chl-a (Fig. S3), and the more severe influence of anthropogenic pollutants transported from the continent in spring and autumn (Fig. S1). In addition to the air mass back trajectories, the more severe impacts of continental outflows in spring and autumn were also indicated by elevated elemental carbon (EC) concentrations (0.5 and 0.4 µgC m−3 compared to 0.2 µgC m−3 in summer). In marine atmospheres over the YBS, the relative contribution of monoterpene-derived (nitrooxy-)OSs was lower than that in continental atmospheres under more severe impacts of anthropogenic pollutants (Fig. 4a).
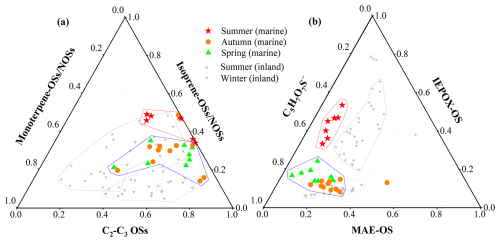
Figure 4(a) Relative abundance of isoprene OSs, monoterpene OSs, and C2–C3 OSs, and (b) composition of isoprene OSs over the YBS in summer (red), autumn (orange) and spring (green). The results previously reported at the inland urban site (Wang et al., 2020) are colored gray.
In marine atmospheres over the YBS, different influences of marine emissions versus continental outflows across seasons resulted in the variation of C2–C3 OSs isoprene-OSs mass ratios (Fig. S5). Strong correlations (r = 0.79–0.97, p≤0.05) between isoprene-OSs and C2–C3 OSs suggested their consistent biogenic sources dominated by isoprene oxidation, which has been reported in previous studies (Schindelka et al., 2013; Surratt et al., 2008; Wang et al., 2020). In summer, the abundance of C2–C3 OSs was comparable to that of isoprene OSs. However, during autumn and spring, we observed higher mass ratios of C2–C3 OSs versus isoprene-OSs due to the additional sources of C2–C3 OSs contributed by anthropogenic sources (Fu et al., 2008; Huang et al., 2018; Liao et al., 2015).
The chemical space distributions of isoprene OSs also displayed obvious seasonal variations. The fraction of IEPOX-OS among the isoprene-derived OSs was substantially higher, and that of MAE-OS was relatively lower in summer compared with those in spring and autumn (Fig. 4b). The low-NO conditions in summer favored the IEPOX formation from isoprene oxidation via HO2 pathway, and the formation of MAE via NO/NO2 pathway increased under the influence of continental pollutants in autumn and spring (Wang et al., 2020; Worton et al., 2013). The average mass ratio of IEPOX-OS MAE-OS was 4.7 during the summer cruise, much higher than those observed during spring (1.53) or autumn (0.49). The seasonal variations of IEPOX-OS MAE-OS mass ratios indicated that the isoprene oxidation pathways were dominated by the HO2 pathway in summer, and the importance of NO/NO2 pathway elevated during the other seasons over the YBS. During summer, the relative contribution of MAE-OS among isoprene-OSs in marine aerosols over the YBS was lower than those observed in continental atmospheres, indicated by the gray markers in Fig. 4b. This was due to the lower anthropogenic pollutants and NO conditions in marine atmospheres than in continental atmospheres. The proportions of C5H7O7S−, a further oxidation or aged forms of IEPOX-OS (Armstrong et al., 2022; Chen et al., 2020), were also higher in autumn and spring than in summer. The dominant presence of C5H7O7S− compared to IEPOX-OS indicated a highly oxidized state of marine SOA in spring and autumn.
3.4 Spatial distribution of OSs regulated by continental outflows
As shown in Fig. 5, atmospheric OSs concentrations over the Bohai Sea and the North Yellow Sea (nYBS, 51.3 ± 37.4 ng m−3) were notably higher than those over the South Yellow Sea (sYS, 16.1 ± 11.9 ng m−3). Surrounded by the continent, the nYBS region was under more severe impacts of transported anthropogenic pollutants compared with the relatively open sYS. This is also indicated by the variation of EC concentrations in atmospheric aerosols over nYBS and sYS areas (Fig. S6). Marine emissions dominated the biogenic OSs formation over the YBS in summer. However, we cannot exclude the potential influence of transported continental air masses, especially over the nYBS. This could be a reason for the higher OSs concentrations over the nYBS than those over the sYS.
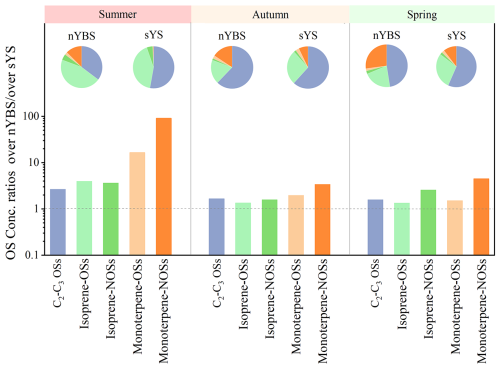
Figure 5Concentration ratios of atmospheric OSs over the nYBS versus those over the sYS. The pie charts show the relative contribution of OSs compound groups over the nYBS and the sYS during each season.
The concentration levels and compositions of OSs in atmospheric aerosols over the nYBS and the sYS are compared in Fig. 5. Among the quantified OSs derived from different VOCs precursors, monoterpene-NOSs displayed the most obvious enhancement ratios over the nYBS compared to those over the sYS (Fig. 5). During the summer cruise, monoterpene-NOSs over the nYBS elevated to nearly two orders of magnitude higher than those over the sYS. The mass contributions of monoterpene-NOSs among the total OSs over nYBS were higher than those over the sYS, as shown in the pie charts of Fig. 5. Monoterpene-NOSs are usually formed via the interactions between anthropogenic NOx, sulfate, and monoterpenes (Bryant et al., 2021, 2023; Wang et al., 2018). A recent study also suggested monoterpenes could be generated by biomass burning, besides the biogenic emissions (Wang et al., 2022a). The spatial difference of monoterpene-NOSs further indicated the more severe influence of anthropogenic pollutants over the nYBS.
The OSs abundance displayed the most obvious enhancement over the nYBS in the summer samples, in which the concentrations of C2–C3 OSs and isoprene-OSs NOSs over the nYBS elevated to 2.4 and 3.9 times of those over the sYS. The biogenic emissions from marine phytoplankton were more abundant in summer than in the other seasons. Transported anthropogenic pollutants over the nYBS would promote the formation of biogenic OSs via anthropogenic-biogenic interactions in marine atmospheres. Previous observation has suggested that the formation of biogenic SOA, including isoprene OSs, could be obviously mediated by anthropogenic sulfate and NOx in regions with substantial anthropogenic-biogenic interactions (Xu et al., 2015). We noted that isoprene-OSs were not observed in remote marine aerosols over the Southern Ocean or the southern Indian Ocean, where the influence of transported anthropogenic pollutants was likely limited (Claeys et al., 2010; Cui et al., 2019). Our results suggested the universal existence of biogenic OSs in marine aerosols over regions with anthropogenic-marine interactions. Further observation evidences are needed to understand the presence of OSs in different marine environments.
3.5 Origins and influence factors of atmospheric OSs
Principal Component Analysis (PCA) was performed using 26 aerosol samples to further understand the sources of atmospheric OSs over the YBS (Fig. 6, Table S3). A total of 18 particulate components, including OSs, water-soluble ions, EC, and MSA, were chosen to carry out the statistics. Three factors could explain 83 % of the measurements. Majority of the OSs and NOSs compounds showed high loadings in Factor 1, which explained 52 % of the measurements. Characterized by high loadings of nss-sulfate, Cl−, and low loadings of anthropogenic species (e.g., EC, nss-K+), Factor 1 represented the sulfate-catalyzed reactions with VOCs dominated by marine emissions. Factor 2 shows high loadings of EC, nss-K+, and NO, suggesting the transported anthropogenic origins dominated by combustion emissions, which explained 21 % of the measurements (Table S3). Factor 3, dominated by MSA, EC, and sea salts, was a mixed source of marine-anthropogenic interaction, which explained 10 % of the variance (Table S3). In this work, each aerosol sample was collected for 10–24 h, and the time-averaged aerosol component concentrations were used for the PCA analysis. The PCA factors reflected the overall variations of the atmospheric OSs sources across seasons over the YBS. The diurnal patterns of atmospheric OSs or their variation during some short-term episodes cannot be captured based on the filter-based analysis in this study. For example, the diurnal variations of marine boundary layer heights or atmospheric oxidation conditions may influence the OSs concentrations or formation pathways. Marine aerosol sampling and analysis with high time resolution are needed to gain insight into the day-night variations of OSs in marine aerosols in the future studies.
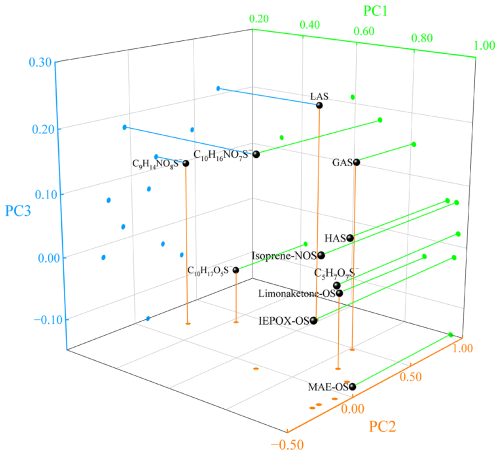
Figure 6PCA statistics of the measured OSs and NOSs during the cruise observations. PC1, PC2, and PC3 represent the source of sulfate-catalyzed reactions with biogenic VOCs, transported anthropogenic origin, and a mixed source of marine-anthropogenic interaction, respectively.
The majority of quantified OSs compounds, especially the isoprene-derived ones (IEPOX-OS, MAE-OS, C5H7O7S−, isoprene-NOS, GAS, and HAS), were dominated by the source of sulfate-catalyzed reactions with biogenic VOCs (Factor 1), as displayed in Fig. 6 and Table S3. The homogeneous origin of C2–C3 OSs and isoprene-OSs NOSs from the oxidation of isoprene has been approved in this work and previous observations (Surratt et al., 2008; Riva et al., 2016b). This source factor was more related to the marine emissions, rather than anthropogenic pollutants, indicated by the low loadings of anthropogenic EC or nss-K+. Isoprene could be largely emitted by phytoplankton and from photochemical processes in surface seawater, and then released into marine atmospheres (Brüggemann et al., 2018; Cui et al., 2023). The reactive uptake of isoprene by sulfate aerosols could be a vital reaction pathway for OSs formation in marine aerosols (Wang et al., 2023b). OSs concentrations elevated with increasing air temperature in summer and increasing wind speed in spring (Fig. 7). Higher temperature or wind speed would promote the sea-to-air exchange of isoprene and favored the OSs formation in marine atmospheres.
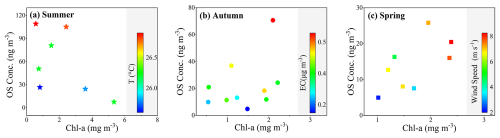
Figure 7Variations of OSs concentrations as a function of chlorophyll-a (Chl-a) in (a) summer, (b) autumn, and (c) spring. The markers are colored by air temperature, EC, and wind speed, respectively.
The loadings of monoterpene-OSs NOSs in anthropogenic-related sources (Factor 2 and Factor 3) cannot be neglected, which was different from the main source of isoprene OSs from marine-dominated sulfate-biogenic VOCs interaction (Factor 1). Lactic acid sulfate over the YBS showed comparable loadings in the transported anthropogenic origin (Factor 2, 0.70) and the marine-dominated sulfate-biogenic VOCs interaction source (Factor 1, 0.59). The loadings of LAS in the mixed source of marine-anthropogenic interaction (Factor 3) were higher than other identified OSs species (Fig. 6). A relatively high loading of GAS (0.48) was also observed in Factor 2 (Table S3). The PCA result provided observational evidence on the additional sources of monoterpene-OSs NOSs and C2–C3 OSs from transported anthropogenic pollutants over marginal seas. During the autumn cruise, higher OSs concentration levels were observed when higher EC concentrations occurred, which also indicated the additional contribution of OSs by anthropogenic sources (Fig. 7b).
This work quantified and characterized the atmospheric OSs derived from isoprene and monoterpenes over the Asia marginal seas. The chemical nature and distribution of OSs were modified by the joint influence of oceanic biological emissions and transported continental pollutants. The results highlight the abundant formation of airborne OSs in summer, which is promoted by the elevated biogenic VOCs emissions from the surface ocean. During high biological activity periods, atmospheric OSs levels could surpass the MSA concentrations in marine aerosols, which is a vital species in the well-known climate regulation via oceanic phytoplankton sulphur emissions (CLAW hypothesis). In the future studies, isoprene-derived OSs are suggested to be included as the molecular tracers of marine SOA related to phytoplankton emissions, especially during summer or over oceanic regions with high phytoplankton activities and high SST. Shipboard observations over open ocean areas are needed to gain further understanding on the roles of OSs in modifying the sulfur cycle, biogenic VOCs oxidation and regulating climate in marine boundary layer.
The dataset is available upon request from the corresponding author.
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-12585-2025-supplement.
YW designed and supervised the research. MH supervised and provided the instrumentations. SL, YZ, YY, YG, CY and YJ conducted the measurements. SL analyzed the data. YCW synthesized the standards. SL and YW wrote the manuscript with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Also, please note that this paper has not received English language copy-editing. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
Data acquisition and filter sample collection were performed onboard Lanhai No. 101, implementing the open research cruise NORC2021-01 and NORC2022-01 supported by the NSFC Shiptime Sharing Project (Project Number: 42049901 and 42149901).
This study was supported by the National Key Research and Development Program of China (2022YFF0803000; 2024YFC2815800), the National Natural Science Foundation of China (42205103; 42411540229; 22306059), the Taishan Scholars of Shandong Province, China (tsqn202306101), the Shandong Provincial Natural Science Foundation (ZR2022QD105), the Fundamental Research Funds for the Central Universities (202441011), special fund of State Key Joint Laboratory of Environmental Simulation and Pollution Control (22K01ESPCP), and Science and Technology Planning Project of Hunan Province (2023JJ40128).
This paper was edited by Jason Surratt and reviewed by two anonymous referees.
Andreae, M. O.: Ocean-atmosphere interactions in the global biogeochemical sulfur cycle, Mar. Chem., 30, 1–29, https://doi.org/10.1016/0304-4203(90)90059-L, 1990.
Andreae, M. O. and Rosenfeld, D.: Aerosol–cloud–precipitation interactions. Part 1. The nature and sources of cloud-active aerosols, Earth-Sci. Rev., 89, 13–41, https://doi.org/10.1016/j.earscirev.2008.03.001, 2008.
Armstrong, N. C., Chen, Y., Cui, T., Zhang, Y., Christensen, C., Zhang, Z., Turpin, B. J., Chan, M. N., Gold, A., Ault, A. P., and Surratt, J. D.: Isoprene epoxydiol-derived sulfated and nonsulfated oligomers suppress particulate mass loss during oxidative aging of secondary organic aerosol, Environ. Sci. Technol., 56, 16611–16620, https://doi.org/10.1021/acs.est.2c03200, 2022.
Ayers, G. P. and Gras, J. L.: Seasonal relationship between cloud condensation nuclei and aerosol methanesulphonate in marine air, Nature, 353, 834–835, https://doi.org/10.1038/353834a0, 1991.
Ayers, G. P., Cainey, J. M., Gillett, R. W., and Ivey, J. P.: Atmospheric sulphur and cloud condensation nuclei in marine air in the Southern Hemisphere, Philos. Trans. R. Soc. B, 352, 203–211, https://doi.org/10.1098/rstb.1997.0015, 1997.
Balasubramanian, R., Qian, W.-B., Decesari, S., Facchini, M. C., and Fuzzi, S.: Comprehensive characterization of PM2.5 aerosols in Singapore, J. Geophys. Res., 108, 4523, https://doi.org/10.1029/2002JD002517, 2003.
Bao, H., Niggemann, J., Luo, L., Dittmar, T., and Kao, S.-J.: Molecular composition and origin of water-soluble organic matter in marine aerosols in the Pacific off China, Atmos. Environ., 191, 27–35, https://doi.org/10.1016/j.atmosenv.2018.07.059, 2018.
Behera, S. N., Betha, R., and Balasubramanian, R.: Insights into chemical coupling among acidic gases, ammonia and secondary inorganic aerosols, Aerosol Air Qual. Res., 13, 1282–1296, https://doi.org/10.4209/aaqr.2012.11.0328, 2013.
Brüggemann, M., Hayeck, N., and George, C.: Interfacial photochemistry at the ocean surface is a global source of organic vapors and aerosols, Nat. Commun., 9, 2101, https://doi.org/10.1038/s41467-018-04528-7, 2018.
Brüggemann, M., Xu, R., Tilgner, A., Kwong, K. C., Mutzel, A., Poon, H. Y., Otto, T., Schaefer, T., Poulain, L., Chan, M. N., and Herrmann, H.: Organosulfates in ambient aerosol: State of knowledge and future research directions on formation, abundance, fate, and importance, Environ. Sci. Technol., 54, 3767–3782, https://doi.org/10.1021/acs.est.9b06751, 2020.
Bryant, D. J., Elzein, A., Newland, M., White, E., Swift, S., Watkins, A., Deng, W., Song, W., Wang, S., Zhang, Y., Wang, X., Rickard, A. R., and Hamilton, J. F.: Importance of oxidants and temperature in the formation of biogenic organosulfates and nitrooxy organosulfates, ACS Earth Space Chem., 5, 2291–2306, https://doi.org/10.1021/acsearthspacechem.1c00204, 2021.
Bryant, D. J., Nelson, B. S., Swift, S. J., Budisulistiorini, S. H., Drysdale, W. S., Vaughan, A. R., Newland, M. J., Hopkins, J. R., Cash, J. M., Langford, B., Nemitz, E., Acton, W. J. F., Hewitt, C. N., Mandal, T., Gurjar, B. R., Shivani, Gadi, R., Lee, J. D., Rickard, A. R., and Hamilton, J. F.: Biogenic and anthropogenic sources of isoprene and monoterpenes and their secondary organic aerosol in Delhi, India, Atmos. Chem. Phys., 23, 61–83, https://doi.org/10.5194/acp-23-61-2023, 2023.
Cai, D., Wang, X., Chen, J., and Li, X.: Molecular characterization of organosulfates in highly polluted atmosphere using ultra-high-resolution mass spectrometry, J. Geophys. Res., 125, e2019JD032253, https://doi.org/10.1029/2019JD032253, 2020.
Charlson, R. J., Lovelock, J. E., Andreae, M. O., and Warren, S. G.: Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate, Nature, 326, 655–661, https://doi.org/10.1038/326655a0, 1987.
Chen, Y., Zhang, Y., Lambe, A. T., Xu, R., Lei, Z., Olson, N. E., Zhang, Z., Szalkowski, T., Cui, T., Vizuete, W., Gold, A., Turpin, B. J., Ault, A. P., Chan, M. N., and Surratt, J. D.: Heterogeneous hydroxyl radical oxidation of isoprene-epoxydiol-derived methyltetrol sulfates: Plausible formation mechanisms of previously unexplained organosulfates in ambient fine aerosols, Environ. Sci. Technol. Lett., 7, 460–468, https://doi.org/10.1021/acs.estlett.0c00276, 2020.
Claeys, M., Wang, W., Vermeylen, R., Kourtchev, I., Chi, X., Farhat, Y., Surratt, J. D., Gómez-González, Y., Sciare, J., and Maenhaut, W.: Chemical characterisation of marine aerosol at Amsterdam Island during the austral summer of 2006–2007, J. Aerosol Sci., 41, 13–22, https://doi.org/10.1016/j.jaerosci.2009.08.003, 2010
Conte, L., Szopa, S., Aumont, O., Gros, V., and Bopp, L.: Sources and sinks of isoprene in the global open ocean: Simulated patterns and emissions to the atmosphere, J. Geophys. Res., 125, e2019JC015946, https://doi.org/10.1029/2019JC015946, 2020.
Cooke, M. E., Armstrong, N. C., Lei, Z., Chen, Y., Waters, C. M., Zhang, Y., Buchenau, N. A., Dibley, M. Q., Ledsky, I. R., Szalkowski, T., Lee, J. Y., Baumann, K., Zhang, Z., Vizuete, W., Gold, A., Surratt, J. D., and Ault, A. P.: Organosulfate formation in proxies for aged sea spray aerosol: Reactive uptake of isoprene epoxydiols to acidic sodium sulfate, ACS Earth Space Chem., 6, 2790–2800, https://doi.org/10.1021/acsearthspacechem.2c00156, 2022.
Cui, L., Xiao, Y., Hu, W., Song, L., Wang, Y., Zhang, C., Fu, P., and Zhu, J.: Enhanced dataset of global marine isoprene emissions from biogenic and photochemical processes for the period 2001–2020, Earth Syst. Sci. Data, 15, 5403–5425, https://doi.org/10.5194/essd-15-5403-2023, 2023.
Cui, T., Green, H. S., Selleck, P. W., Zhang, Z., O'Brien, R. E., Gold, A., Keywood, M., Kroll, J. H., and Surratt, J. D.: Chemical characterization of isoprene- and monoterpene-derived secondary organic aerosol tracers in remote marine aerosols over a quarter century, ACS Earth Space Chem., 3, 935–946, https://doi.org/10.1021/acsearthspacechem.9b00061, 2019.
Estillore, A. D., Hettiyadura, A. P. S., Qin, Z., Leckrone, E., Wombacher, B., Humphry, T., Stone, E. A., and Grassian, V. H.: Water uptake and hygroscopic growth of organosulfate aerosol, Environ. Sci. Technol., 50, 4259–4268, https://doi.org/10.1021/acs.est.5b05014, 2016.
Fu, P., Kawamura, K., and Miura, K.: Molecular characterization of marine organic aerosols collected during a round-the-world cruise, J. Geophys. Res., 116, D13302, https://https://doi.org/10.1029/2011JD015604, 2011.
Fu, T.-M., Jacob, D. J., Wittrock, F., Burrows, J. P., Vrekoussis, M., and Henze, D. K.: Global budgets of atmospheric glyoxal and methylglyoxal, and implications for formation of secondary organic aerosols, J. Geophys. Res., 113, D15303, https://doi.org/10.1029/2007JD009505, 2008.
Guo, T., Guo, Z., Wang, J., Feng, J., Gao, H., and Yao, X.: Tracer-based investigation of organic aerosols in marine atmospheres from marginal seas of China to the northwest Pacific Ocean, Atmos. Chem. Phys., 20, 5055–5070, https://doi.org/10.5194/acp-20-5055-2020, 2020.
Hansen, A. M. K., Hong, J., Raatikainen, T., Kristensen, K., Ylisirniö, A., Virtanen, A., Petäjä, T., Glasius, M., and Prisle, N. L.: Hygroscopic properties and cloud condensation nuclei activation of limonene-derived organosulfates and their mixtures with ammonium sulfate, Atmos. Chem. Phys., 15, 14071–14089, https://doi.org/10.5194/acp-15-14071-2015, 2015.
Hawkins, L. N., Russell, L. M., Covert, D. S., Quinn, P. K., and Bates, T. S.: Carboxylic acids, sulfates, and organosulfates in processed continental organic aerosol over the southeast Pacific Ocean during VOCALS-REx 2008, J. Geophys. Res., 115, D13201, https://doi.org/10.1029/2009JD013276, 2010.
He, Q.-F., Ding, X., Wang, X.-M., Yu, J.-Z., Fu, X.-X., Liu, T.-Y., Zhang, Z., Xue, J., Chen, D.-H., Zhong, L.-J., and Donahue, N. M.: Organosulfates from pinene and isoprene over the Pearl River Delta, South China: Seasonal variation and implication in formation mechanisms, Environ. Sci. Technol., 48, 9236–9245, https://doi.org/10.1021/es501299v, 2014.
Hettiyadura, A. P. S., Stone, E. A., Kundu, S., Baker, Z., Geddes, E., Richards, K., and Humphry, T.: Determination of atmospheric organosulfates using HILIC chromatography with MS detection, Atmos. Meas. Tech., 8, 2347–2358, https://doi.org/10.5194/amt-8-2347-2015, 2015.
Hettiyadura, A. P. S., Jayarathne, T., Baumann, K., Goldstein, A. H., de Gouw, J. A., Koss, A., Keutsch, F. N., Skog, K., and Stone, E. A.: Qualitative and quantitative analysis of atmospheric organosulfates in Centreville, Alabama, Atmos. Chem. Phys., 17, 1343–1359, https://doi.org/10.5194/acp-17-1343-2017, 2017.
Hettiyadura, A. P. S., Al-Naiema, I. M., Hughes, D. D., Fang, T., and Stone, E. A.: Organosulfates in Atlanta, Georgia: anthropogenic influences on biogenic secondary organic aerosol formation, Atmos. Chem. Phys., 19, 3191–3206, https://doi.org/10.5194/acp-19-3191-2019, 2019.
Hu, K. S., Darer, A. I., and Elrod, M. J.: Thermodynamics and kinetics of the hydrolysis of atmospherically relevant organonitrates and organosulfates, Atmos. Chem. Phys., 11, 8307–8320, https://doi.org/10.5194/acp-11-8307-2011, 2011.
Hu, Q.-H., Xie, Z.-Q., Wang, X.-M., Kang, H., He, Q.-F., and Zhang, P.: Secondary organic aerosols over oceans via oxidation of isoprene and monoterpenes from Arctic to Antarctic, Sci. Rep., 3, 2280, https://doi.org/10.1038/srep02280, 2013.
Huang, R.-J., Cao, J., Chen, Y., Yang, L., Shen, J., You, Q., Wang, K., Lin, C., Xu, W., Gao, B., Li, Y., Chen, Q., Hoffmann, T., O'Dowd, C. D., Bilde, M., and Glasius, M.: Organosulfates in atmospheric aerosol: synthesis and quantitative analysis of PM2.5 from Xi'an, northwestern China, Atmos. Meas. Tech., 11, 3447–3456, https://doi.org/10.5194/amt-11-3447-2018, 2018.
Jung, J., Hong, S.-B., Chen, M., Hur, J., Jiao, L., Lee, Y., Park, K., Hahm, D., Choi, J.-O., Yang, E. J., Park, J., Kim, T.-W., and Lee, S.: Characteristics of methanesulfonic acid, non-sea-salt sulfate and organic carbon aerosols over the Amundsen Sea, Antarctica, Atmos. Chem. Phys., 20, 5405–5424, https://doi.org/10.5194/acp-20-5405-2020, 2020.
Kanellopoulos, P. G., Kotsaki, S. P., Chrysochou, E., Koukoulakis, K., Zacharopoulos, N., Philippopoulos, A., and Bakeas, E.: PM2.5-bound organosulfates in two Eastern Mediterranean cities: The dominance of isoprene organosulfates, Chemosphere, 297, 134103, https://doi.org/10.1016/j.chemosphere.2022.134103, 2022.
Kettle, A. J. and Andreae, M. O.: Flux of dimethylsulfide from the oceans: A comparison of updated data sets and flux models, J. Geophys. Res., 105, 26793–26808, https://doi.org/10.1029/2000JD900252, 2000.
Kloster, S., Feichter, J., Maier-Reimer, E., Six, K. D., Stier, P., and Wetzel, P.: DMS cycle in the marine ocean-atmosphere system – a global model study, Biogeosciences, 3, 29–51, https://doi.org/10.5194/bg-3-29-2006, 2006.
Kristensen, K., Bilde, M., Aalto, P. P., Petäjä, T., and Glasius, M.: Denuder/filter sampling of organic acids and organosulfates at urban and boreal forest sites: Gas/particle distribution and possible sampling artifacts, Atmos. Environ., 130, 36–53, https://doi.org/10.1016/j.atmosenv.2015.10.046, 2016.
Lam, H. K., Kwong, K. C., Poon, H. Y., Davies, J. F., Zhang, Z., Gold, A., Surratt, J. D., and Chan, M. N.: Heterogeneous OH oxidation of isoprene-epoxydiol-derived organosulfates: kinetics, chemistry and formation of inorganic sulfate, Atmos. Chem. Phys., 19, 2433–2440, https://doi.org/10.5194/acp-19-2433-2019, 2019.
Li, H., Zheng, B., Lei, Y., Hauglustaine, D., Chen, C., Lin, X., Zhang, Y., Zhang, Q., and He, K.: Trends and drivers of anthropogenic NOx emissions in China since 2020, Environ. Sci. Ecotechnology, 21, 100425, https://doi.org/10.1016/j.ese.2024.100425, 2024.
Li, J., Michalski, G., Davy, P., Harvey, M., Katzman, T., and Wilkins, B.: Investigating source contributions of size-aggregated aerosols collected in Southern Ocean and Baring Head, New Zealand using sulfur isotopes, Geophys. Res. Lett., 45, 3717–3727, https://doi.org/10.1002/2018GL077353, 2018.
Li, J., Carlson, B. E., Yung, Y. L., Lv, D., Hansen, J., Penner, J. E., Liao, H., Ramaswamy, V., Kahn, R. A., Zhang, P., Dubovik, O., Ding, A., Lacis, A. A., Zhang, L., and Dong, Y.: Scattering and absorbing aerosols in the climate system, Nat. Rev. Earth Environ., 3, 363–379, https://doi.org/10.1038/s43017-022-00296-7, 2022.
Liao, J., Froyd, K. D., Murphy, D. M., Keutsch, F. N., Yu, G., Wennberg, P. O., St. Clair, J. M., Crounse, J. D., Wisthaler, A., Mikoviny, T., Jimenez, J. L., Campuzano-Jost, P., Day, D. A., Hu, W., Ryerson, T. B., Pollack, I. B., Peischl, J., Anderson, B. E., Ziemba, L. D., Blake, D. R., Meinardi, S., and Diskin, G.: Airborne measurements of organosulfates over the continental U.S., J. Geophys. Res., 120, 2990–3005, https://doi.org/10.1002/2014JD022378, 2015.
Liang, S., Wang, Y., Chen, H., Chan, W., and Yu, J. Z.: Accurate quantification of multifunctional C2−3 organosulfates in atmospheric aerosols using liquid chromatography-electrospray ionization mass spectrometry: Overcoming matrix effects and underestimation, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.5c01846, 2025.
Lin, Y.-H., Zhang, Z., Docherty, K. S., Zhang, H., Budisulistiorini, S. H., Rubitschun, C. L., Shaw, S. L., Knipping, E. M., Edgerton, E. S., Kleindienst, T. E., Gold, A., and Surratt, J. D.: Isoprene epoxydiols as precursors to secondary organic aerosol formation: Acid-catalyzed reactive uptake studies with authentic compounds, Environ. Sci. Technol., 46, 250–258, https://doi.org/10.1021/es202554c, 2012.
Meade, L. E., Riva, M., Blomberg, M. Z., Brock, A. K., Qualters, E. M., Siejack, R. A., Ramakrishnan, K., Surratt, J. D., and Kautzman, K. E.: Seasonal variations of fine particulate organosulfates derived from biogenic and anthropogenic hydrocarbons in the mid-Atlantic United States, Atmos. Environ., 145, 405–414, https://doi.org/10.1016/j.atmosenv.2016.09.028, 2016.
Millero, F. J. and Sohn, M. L.: Chemical Oceanography, CRC Press, Boca Raton, FL, 521 pp., ISBN 0849384238, 1992.
Nguyen, Q. T., Christensen, M. K., Cozzi, F., Zare, A., Hansen, A. M. K., Kristensen, K., Tulinius, T. E., Madsen, H. H., Christensen, J. H., Brandt, J., Massling, A., Nøjgaard, J. K., and Glasius, M.: Understanding the anthropogenic influence on formation of biogenic secondary organic aerosols in Denmark via analysis of organosulfates and related oxidation products, Atmos. Chem. Phys., 14, 8961–8981, https://doi.org/10.5194/acp-14-8961-2014, 2014.
Ooki, A., Nomura, D., Nishino, S., Kikuchi, T., and Yokouchi, Y.: A global-scale map of isoprene and volatile organic iodine in surface seawater of the Arctic, Northwest Pacific, Indian, and Southern Oceans, J. Geophys. Res., 120, 4108–4128, https://doi.org/10.1002/2014JC010519, 2015.
Peng, C., Razafindrambinina, P. N., Malek, K. A., Chen, L., Wang, W., Huang, R.-J., Zhang, Y., Ding, X., Ge, M., Wang, X., Asa-Awuku, A. A., and Tang, M.: Interactions of organosulfates with water vapor under sub- and supersaturated conditions, Atmos. Chem. Phys., 21, 7135–7148, https://doi.org/10.5194/acp-21-7135-2021, 2021.
Quinn, P. K. and Bates, T. S.: The case against climate regulation via oceanic phytoplankton sulphur emissions, Nature, 480, 51–56, https://doi.org/10.1038/nature10580, 2011.
Riva, M., Budisulistiorini, S. H., Zhang, Z., Gold, A., and Surratt, J. D.: Chemical characterization of secondary organic aerosol constituents from isoprene ozonolysis in the presence of acidic aerosol, Atmos. Environ., 130, 5–13, https://doi.org/10.1016/j.atmosenv.2015.06.027, 2016a.
Riva, M., Da Silva Barbosa, T., Lin, Y.-H., Stone, E. A., Gold, A., and Surratt, J. D.: Chemical characterization of organosulfates in secondary organic aerosol derived from the photooxidation of alkanes, Atmos. Chem. Phys., 16, 11001–11018, https://doi.org/10.5194/acp-16-11001-2016, 2016b.
Riva, M., Chen, Y., Zhang, Y., Lei, Z., Olson, N. E., Boyer, H. C., Narayan, S., Yee, L. D., Green, H. S., Cui, T., Zhang, Z., Baumann, K., Fort, M., Edgerton, E., Budisulistiorini, S. H., Rose, C. A., Ribeiro, I. O., de Oliveira, R. L., dos Santos, E. O., Machado, C. M. D., Szopa, S., Zhao, Y., Alves, E. G., de Sá, S. Z., Hu, W., Knipping, E. M., Shaw, S. L., Junior, S. D., de Souza, R. A. F., Palm, B. B., Jimenez, J. L., Glasius, M., Goldstein, A. H., Pye, H. O. T., Gold, A., Turpin, B. J., Vizuete, W., Martin, S. T., Thornton, J. A., Dutcher, C. S., Ault, A. P., and Surratt, J. D.: Increasing isoprene epoxydiol-to-inorganic sulfate aerosol ratio results in extensive conversion of inorganic sulfate to organosulfur forms: implications for aerosol physicochemical properties, Environ. Sci. Technol., 53, 8682–8694, https://doi.org/10.1021/acs.est.9b01019, 2019.
Rosati, B., Isokääntä, S., Christiansen, S., Jensen, M. M., Moosakutty, S. P., Wollesen de Jonge, R., Massling, A., Glasius, M., Elm, J., Virtanen, A., and Bilde, M.: Hygroscopicity and CCN potential of DMS-derived aerosol particles, Atmos. Chem. Phys., 22, 13449–13466, https://doi.org/10.5194/acp-22-13449-2022, 2022.
Schindelka, J., Iinuma, Y., Hoffmann, D., and Herrmann, H.: Sulfate radical-initiated formation of isoprene-derived organosulfates in atmospheric aerosols, Faraday Discuss., 165, 237–259, https://doi.org/10.1039/C3FD00042G, 2013.
Shank, L. M., Howell, S., Clarke, A. D., Freitag, S., Brekhovskikh, V., Kapustin, V., McNaughton, C., Campos, T., and Wood, R.: Organic matter and non-refractory aerosol over the remote Southeast Pacific: oceanic and combustion sources, Atmos. Chem. Phys., 12, 557–576, https://doi.org/10.5194/acp-12-557-2012, 2012.
Surratt, J. D., Gómez-González, Y., Chan, A. W. H., Vermeylen, R., Shahgholi, M., Kleindienst, T. E., Edney, E. O., Offenberg, J. H., Lewandowski, M., Jaoui, M., Maenhaut, W., Claeys, M., Flagan, R. C., and Seinfeld, J. H.: Organosulfate formation in biogenic secondary organic aerosol, J. Phys. Chem. A, 112, 8345–8378, https://doi.org/10.1021/jp802310p, 2008.
Surratt, J. D., Chan, A. W. H., Eddingsaas, N. C., Chan, M., Loza, C. L., Kwan, A. J., Hersey, S. P., Flagan, R. C., Wennberg, P. O., and Seinfeld, J. H.: Reactive intermediates revealed in secondary organic aerosol formation from isoprene, Proc. Natl. Acad. Sci., 107, 6640–6645, https://doi.org/10.1073/pnas.0911114107, 2010.
Tang, M., Guo, L., Bai, Y., Huang, R.-J., Wu, Z., Wang, Z., Zhang, G., Ding, X., Hu, M., and Wang, X.: Impacts of methanesulfonate on the cloud condensation nucleation activity of sea salt aerosol, Atmos. Environ., 201, 13–17, https://doi.org/10.1016/j.atmosenv.2018.12.034, 2019.
Wang, H., Ma, X., Tan, Z., Wang, H., Chen, X., Chen, S., Gao, Y., Liu, Y., Liu, Y., Yang, X., Yuan, B., Zeng, L., Huang, C., Lu, K., and Zhang, Y.: Anthropogenic monoterpenes aggravating ozone pollution, Natl. Sci. Rev., 9, nwac103, https://doi.org/10.1093/nsr/nwac103, 2022a.
Wang, Y., Hu, M., Guo, S., Wang, Y., Zheng, J., Yang, Y., Zhu, W., Tang, R., Li, X., Liu, Y., Le Breton, M., Du, Z., Shang, D., Wu, Y., Wu, Z., Song, Y., Lou, S., Hallquist, M., and Yu, J.: The secondary formation of organosulfates under interactions between biogenic emissions and anthropogenic pollutants in summer in Beijing, Atmos. Chem. Phys., 18, 10693–10713, https://doi.org/10.5194/acp-18-10693-2018, 2018.
Wang, Y., Hu, M., Wang, Y.-C., Li, X., Fang, X., Tang, R., Lu, S., Wu, Y., Guo, S., Wu, Z., Hallquist, M., and Yu, J. Z.: Comparative study of particulate organosulfates in contrasting atmospheric environments: Field evidence for the significant influence of anthropogenic sulfate and NOx, Environ. Sci. Technol. Lett., 7, 787–794, https://doi.org/10.1021/acs.estlett.0c00550, 2020.
Wang, Y., Zhao, Y., Wang, Y., Yu, J.-Z., Shao, J., Liu, P., Zhu, W., Cheng, Z., Li, Z., Yan, N., and Xiao, H.: Organosulfates in atmospheric aerosols in Shanghai, China: seasonal and interannual variability, origin, and formation mechanisms, Atmos. Chem. Phys., 21, 2959–2980, https://doi.org/10.5194/acp-21-2959-2021, 2021.
Wang, Y., Ma, Y., Kuang, B., Lin, P., Liang, Y., Huang, C., and Yu, J. Z.: Abundance of organosulfates derived from biogenic volatile organic compounds: Seasonal and spatial contrasts at four sites in China, Sci. Total Environ., 806, 151275, https://doi.org/10.1016/j.scitotenv.2021.151275, 2022b.
Wang, Y., Liang, S., Le Breton, M., Wang, Q. Q., Liu, Q., Ho, C. H., Kuang, B. Y., Wu, C., Hallquist, M., Tong, R., and Yu, J. Z.: Field observations of C2 and C3 organosulfates and insights into their formation mechanisms at a suburban site in Hong Kong, Sci. Total Environ., 904, 166851, https://doi.org/10.1016/j.scitotenv.2023.166851, 2023a.
Wang, Y., Zhang, Y., Li, W., Wu, G., Qi, Y., Li, S., Zhu, W., Yu, J. Z., Yu, X., Zhang, H.-H., Sun, J., Wang, W., Sheng, L., Yao, X., Gao, H., Huang, C., Ma, Y., and Zhou, Y.: Important roles and formation of atmospheric organosulfates in marine organic aerosols: Influence of Phytoplankton Emissions and Anthropogenic Pollutants, Environ. Sci. Technol., 57, 10284–10294, https://doi.org/10.1021/acs.est.3c01422, 2023b.
Worton, D. R., Surratt, J. D., LaFranchi, B. W., Chan, A. W. H., Zhao, Y., Weber, R. J., Park, J.-H., Gilman, J. B., de Gouw, J., Park, C., Schade, G., Beaver, M., Clair, J. M. St., Crounse, J., Wennberg, P., Wolfe, G. M., Harrold, S., Thornton, J. A., Farmer, D. K., Docherty, K. S., Cubison, M. J., Jimenez, J.-L., Frossard, A. A., Russell, L. M., Kristensen, K., Glasius, M., Mao, J., Ren, X., Brune, W., Browne, E. C., Pusede, S. E., Cohen, R. C., Seinfeld, J. H., and Goldstein, A. H.: Observational insights into aerosol formation from isoprene, Environ. Sci. Technol., 47, 11403–11413, https://doi.org/10.1021/es4011064, 2013.
Wu, Y.-C., Li, J.-L., Wang, J., Zhuang, G.-C., Liu, X.-T., Zhang, H.-H., and Yang, G.-P.: Occurance, emission and environmental effects of non-methane hydrocarbons in the Yellow Sea and the East China Sea, Environ. Pollut., 270, 116305, https://doi.org/10.1016/j.envpol.2020.116305, 2021.
Xu, L., Guo, H., Boyd, C. M., Klein, M., Bougiatioti, A., Cerully, K. M., Hite, J. R., Isaacman-VanWertz, G., Kreisberg, N. M., Knote, C., Olson, K., Koss, A., Goldstein, A. H., Hering, S. V., de Gouw, J., Baumann, K., Lee, S.-H., Nenes, A., Weber, R. J., and Ng, N. L.: Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States, Proc. Natl. Acad. Sci., 112, 37–42, https://doi.org/10.1073/pnas.1417609112, 2015.
Yan, S.-B., Xu, G.-B., Zhang, H.-H., Wang, J., Xu, F., Gao, X.-X., Zhang, J.-W., Wu, J.-W., and Yang, G.-P.: Factors controlling DMS emission and atmospheric sulfate aerosols in the western Pacific continental sea, J. Geophys. Res., 129, e2024JC020886, https://doi.org/10.1029/2024JC020886, 2024.
Ye, J., Abbatt, J. P. D., and Chan, A. W. H.: Novel pathway of SO2 oxidation in the atmosphere: reactions with monoterpene ozonolysis intermediates and secondary organic aerosol, Atmos. Chem. Phys., 18, 5549–5565, https://doi.org/10.5194/acp-18-5549-2018, 2018.
Ye, Y., Zhan, H., Yu, X., Li, J., Wang, X., and Xie, Z.: Detection of organosulfates and nitrooxy-organosulfates in Arctic and Antarctic atmospheric aerosols, using ultra-high resolution FT-ICR mass spectrometry, Sci. Total Environ., 767, 144339, https://doi.org/10.1016/j.scitotenv.2020.144339, 2021.





