the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Seasonal variations in the production of singlet oxygen and organic triplet excited states in aqueous PM2.5 in Hong Kong SAR, South China
Yuting Lyu
Yin Hau Lam
Yitao Li
Nadine Borduas-Dedekind
Photooxidants drive many atmospheric chemical processes. The photoexcitation of light-absorbing organic compounds (i.e., brown carbon, BrC) in atmospheric waters can lead to the generation of reactive organic triplet excited states (3C∗), which can undergo further reactions to produce other photooxidants such as singlet oxygen (). To determine the importance of these aqueous photooxidants in secondary organic aerosol (SOA) formation and transformation, we must know their steady-state concentrations and quantum yields. However, there have been limited measurements of aqueous 3C∗ and in atmospheric samples outside of North America and Europe. In this work, we report the first measurements of the steady-state concentrations and quantum yields of 3C∗ and produced in aerosols in South China. We quantified the production of 3C∗ and in illuminated aqueous extracts of PM2.5 collected in different seasons at two urban sites and one coastal semi-rural site during a year-round study conducted in Hong Kong SAR, South China. The mass absorption coefficients at 300 nm for BrC in the aqueous PM2.5 extracts ranged from 0.49 to 2.01 m2 g-C−1 for the three sites. Both and 3C∗ were produced year-round. The steady-state concentrations of () in the illuminated aqueous extracts ranged from to M, with a study average of M. At nearly 2 orders of magnitude lower than , the steady-state concentrations of 3C∗ ([3C∗]ss) ranged from to M, with a study average of M. The quantum yields of and 3C∗ also spanned wide ranges across samples, with a range of 1.19 % to 13.74 % and an average of (5.19±2.63) % for and a range of 0.05 % to 3.24 % and an average of (0.56±0.66) % for 3C∗. The and [3C∗]ss correlated with the concentration and absorbance of BrC, thus implying that the amount of BrC drives the steady-state concentrations of these photooxidants. The locations (urban vs. semi-rural) did not have a significant effect on [3C∗]ss and , which indicated that BrC from local sources did not have a significant influence on the year-round 3C∗ and production. 3C∗ and production were found to be the highest in winter and the lowest in summer for all three sites. The observed seasonal trends of and 3C∗ production could be attributed to the seasonal variations in the long-range air mass transport. Our analysis highlighted the key role that regional sources play in influencing the composition and concentrations of water-soluble BrC in winter PM2.5 in Hong Kong SAR, which contributed to their highest 3C∗ and production. The current results will be useful for modeling seasonal aqueous organic aerosol photochemistry in the South China region.
- Article
(4885 KB) - Full-text XML
-
Supplement
(15217 KB) - BibTeX
- EndNote
Atmospheric aqueous phases (e.g., aqueous aerosol, cloud water, and fog droplets) serve as important media for chemical reactions of organic compounds. Many of the chemical transformations in atmospheric aqueous phases are driven by photochemically generated oxidants, particularly triplet excited states of organic matter (3C∗), singlet-state oxygen (), and hydroxyl radicals (•OH). Light-absorbing organic compounds, commonly known as brown carbon (BrC), serve as key precursors for the formation of photooxidants in atmospheric aqueous phases (Laskin et al., 2015; Hems et al., 2021).
Upon the absorption of sunlight, some BrC chromophores (e.g., aromatic carbonyls) can be promoted from their ground states to reactive 3C∗ with species-specific energy levels (Canonica et al., 1995; Yu et al., 2014). 3C∗ is not a single photooxidant. Instead, 3C∗ is comprised of a variety of species with a range of reactivities (McNeill and Canonica, 2016). Some 3C∗ species can react rapidly with organic compounds (e.g., phenolic compounds and anilines) through single-electron transfer and proton-coupled electron transfer reactions (Lathioor and Leigh, 2006; Erickson et al., 2015). Some 3C∗ species can also react with organic compounds (e.g., aromatic amino acids) through hydrogen abstraction reactions (Walling and Gibian, 1965; Tsentalovich et al., 2002). In addition, energy transfer from 3C∗ to molecular oxygen (3O2) leads to the formation of (Herzberg and Herzberg, 1947). This reaction occurs rapidly under ambient conditions for most 3C∗ species, since the energy required for 3O2→1Δg is only 94 kJ mol−1 (Zepp et al., 1985; Wilkinson et al., 1993; McNeill and Canonica, 2016). typically reacts with electron-rich or unsaturated species (e.g., alkenes, cyclic dienes, and polycyclic aromatic hydrocarbons) through addition reactions (Ghogare and Greer, 2016; Kaur and Anastasio, 2017; Nolte and Peijnenburg, 2018; Manfrin et al., 2019; Barrios et al., 2021). The production of 3C∗ and is influenced by both the concentrations (i.e., quantity) and quantum yields (i.e., quality) of BrC chromophores (Bogler et al., 2022). The quantum yield, which describes the efficiency of oxidant photosensitization, can be obtained from dividing the number of moles of oxidant generated by the number of moles of photons absorbed by the photosensitizer. The relative importance of the quantity vs. quality of BrC chromophores in the production of 3C∗ and depends on the BrC source.
Aqueous reactions between organic compounds and photooxidants play key roles in forming and transforming secondary organic aerosols (SOAs). Understanding the significance and contributions of these reactions to the SOA budget necessitates knowledge of the steady-state concentrations and quantum yields of the photooxidants. Out of all the photooxidants, •OH production in various atmospheric aqueous phases has been the most widely investigated (Arakaki and Faust, 1998; Arakaki et al., 1999, 2006, 2013; Anastasio and McGregor, 2001; Anastasio and Jordan, 2004; Anastasio and Newberg, 2007; Kaur and Anastasio, 2017; Kaur et al., 2019; Manfrin et al., 2019; Leresche et al., 2021; Ma et al., 2023b). •OH can be photochemically produced from BrC (Chen et al., 2021; Li et al., 2022) and other photolabile compounds such as inorganic nitrate, nitrite, and metal–organic complexes (Kaur and Anastasio, 2017; Kaur et al., 2019; Leresche et al., 2021; Ma et al., 2023b). There have been considerably fewer measurements of 3C∗ and production in atmospheric aqueous phases.
So far, several studies have measured production in cloud water (Faust and Allen, 1992), fog water (Anastasio and McGregor, 2001; Kaur and Anastasio, 2017), rainwater (Albinet et al., 2010), and particulate matter (PM) extracts (Cote et al., 2018; Kaur et al., 2019; Manfrin et al., 2019; Leresche et al., 2021; Bogler et al., 2022; Ma et al., 2023b). originates from a 3C∗ molecule, and therefore measuring both and its 3C∗ precursor is important. However, there have only been four investigations of 3C∗ production in atmospheric aqueous phases (Kaur and Anastasio, 2018; Kaur et al., 2019; Chen et al., 2021; Ma et al., 2023b). These studies showed that the concentrations of 3C∗ (10−16 to 10−13 M) and (10−15 to 10−12 M) produced are typically 2 to 4 orders of magnitude larger than the concentrations of •OH (10−17 to 10−15 M) produced. Thus, despite the reactivity of 3C∗ and being substantially lower than •OH, 3C∗ and can play important roles in aqueous SOA formation and transformation due to their large concentrations.
Spatiotemporal measurements of photooxidant production in atmospheric aqueous phases are important for understanding how aqueous reactions between organic compounds and photooxidants can change as a function of season and of location. Leresche et al. (2021) measured •OH and production in illuminated extracts of PM2.5 collected during the winter, spring, and summer seasons in urban and rural settings in Colorado, USA, while Bogler et al. (2022) measured production in illuminated extracts of PM10 collected year-round at a rural site and a suburban site in Switzerland. The two studies highlighted the roles that seasonality and/or local anthropogenic activities play in influencing photooxidant production. At present, investigations of photooxidant production in atmospheric aqueous phases have been restricted to North America and Europe. Given the important role that aqueous photochemistry plays in forming and transforming SOA in many regions, there is, therefore, a need to investigate the spatiotemporal variations in the photooxidant production in atmospheric aqueous phases in regions outside of North America and Europe.
In this work, we investigated the production of 3C∗ and in illuminated extracts of PM2.5 collected during different seasons at three sites (two urban and one semi-rural) in Hong Kong SAR (hereafter Hong Kong). Hong Kong is a densely populated coastal city located on the east of the Pearl River Delta (PRD) in South China. Its seasonal meteorological conditions and air quality are strongly influenced by the East Asian monsoon (Yihui and Chan, 2005). Clean marine air masses are transported from southwestern sea areas to Hong Kong in the summer, whereas polluted air mass are transported from northern continental areas to Hong Kong in mid-fall and winter (Tanner and Law, 2002). Local sources are the main contributors to summer PM2.5, whereas regional sources are the main contributors to winter PM2.5 (Pathak et al., 2003; Louie et al., 2005a, b; Huang et al., 2014; Li et al., 2015; Wong et al., 2020). The main objectives of this study are to (1) characterize the steady-state concentrations and quantum yields of 3C∗ and and (2) determine how location and seasonality influence 3C∗ and production in Hong Kong. This work presents the first spatiotemporal measurements of photooxidants produced in atmospheric aerosols in East Asia. Results from this study provide insights into the levels of 3C∗ and produced in PM2.5 in the South China region, which will be useful for improving our understanding of aqueous organic aerosol photochemical processes in this region.
2.1 PM2.5 filter sampling and extraction
2.1.1 Sampling locations
The year-round sampling campaign took place from December 2020 to December 2021 in Hong Kong. The three sites were the City University of Hong Kong campus (CU; 22∘20′05′′ N, 114∘10′23′′ E) and the air quality monitoring stations at Tsuen Wan (TW; 22∘20′17′′ N, 114∘06′52′′ E) and Hok Tsui (HT; 22∘12′33′′ N, 114∘15′12′′ E; Fig. 1). The CU and TW sites are located in urban areas with many residential and commercial (and industrial for TW) activities. Since the semi-rural coastal HT site is located away from local emission sources (approximately 6 km away from the closest urban area), it was mostly used as a receptor site to monitor air pollution originating from sources outside of Hong Kong in past studies (Tanner and Law, 2002; Li et al., 2018). In Hong Kong, winter nominally runs from December to February, spring runs from March to May, summer runs from June to August, and fall runs from September to November. Sampling activities at each site took place for approximately 1 month during each season (Table S1 in the Supplement).
2.1.2 Sampling and extraction protocols
PM2.5 was collected on three prebaked (550 ∘C for 12 h) 47 mm diameter quartz filters (Pall Tissuquartz™, 2500 QAT-UP), using a custom-built, medium-volume sampler with a PM2.5 inlet. Ambient air was sampled onto each quartz filter at 30 L min−1. The sampler was deployed at ground level at the CU and HT sites and on a 17 m a.g.l. (above ground level) rooftop at the TW site. PM2.5 samples were collected continuously for 72 h on every third day. The filter samples were stored in resealable bags at −25 ∘C until the day of extraction. Blank filter samples were generated in the same way as the ambient filter samples, except that the sampler pump for this channel was switched off during sampling.
Each filter was extracted in 7 mL Milli-Q water inside a 15 mL sterile centrifuge tube (Guangzhou Jet Bio-Filtration Co., Ltd.) by vortexing for 4 min (DLAB MX-S; medium-high power). The disintegrated filter parts were removed from the extracts by filtration, using 0.22 µm pore size nylon syringe filters (Nylon66; Tianjin Jinteng Experimental Equipment Co., Ltd.). The filtered extracts were stored in amber vials at 4 ∘C in a refrigerator until the day of photochemical experiments. The maximum amount of time for which the extracts were stored in the refrigerator (i.e., from the day of extraction to the day of project completion) is 6 months. We compared the water-soluble organic carbon (WSOC) and light absorption measurements performed on the extracts within a week of extraction to those done after the photochemical experiments were concluded and observed minimal changes in the WSOC and light absorption properties of the extracts.
Extracts from three consecutive sampling periods (nine filters in 9 d) were aggregated to minimize daily variability. This procedure resulted in roughly three aggregated extracts per season for each site and are referred to by the site and sampling start date. For example, sample CU041220 refers to extracts of filters collected from 4 to 13 December 2020 at the CU site. Due to sampler pump malfunction, filters were not collected at the CU site from 18 to 24 June 2020 and at the HT site from 18 to 27 April 2020. In addition, some aggregated extracts were comprised only of two consecutive sampling periods (six filters in 6 d) due to limited filter samples. It should be noted that all the aggregated extracts were further diluted with Milli-Q water by a factor of 2.22 for light absorption measurements and photochemical experiments. This was equivalent to extracting each filter with 15.54 mL Milli-Q water. The PM2.5 mass to water mass ratios (PM2.5 mass H2O mass) were calculated for each aggregated extract using the ambient PM2.5 mass concentrations measured at or near the sampling sites by the Hong Kong Environmental Protection Department. Detailed information about the sampling periods, allocation of aggregated extracts, and calculation of PM2.5 mass H2O mass values are shown in Table S1.
2.2 Light absorption measurements
The UV-visible (UV-VIS) absorbance spectra of the extracts were obtained in 1 nm increments, using a UV-VIS-NIR spectrophotometer (Shimadzu UV-3600), with Milli-Q water as the reference sample. The spectra were corrected by subtracting spectra from the field blanks and the average absorbance between 700 and 800 nm (Ossola et al., 2021). The decadic absorption coefficient (αλ; cm−1) was calculated using the following equation:
where Aλ is the dimensionless absorbance of extracts at wavelength λ, and l is the optical path length (1 cm) of the cuvette. The rate of light absorption (Rabs; mol-photons L−1 s−1) of each extract was calculated using the following equation:
where d is the path length of the light through the quartz tubes used in the photochemical experiments (cm), 103 is for units conversion (cm3 L−1), I0,λ (mol-photons cm−2 s−1 nm−1) is the absolute irradiance of the light source at wavelength λ, and Δλ is the interval of wavelength (1 nm). d was assumed to be equal to the inner diameter of the quartz tubes (1.25 cm). We acknowledge that the actual optical path length may be slightly different from the inner diameter of the quartz tubes used in our calculations. Nevertheless, we do not expect these differences to affect our Rabs and quantum yield calculations significantly (Ossola et al., 2021). For instance, using d = 1 cm will cause the calculated quantum yields to decrease, on average, only by 0.53 %, relative to quantum yields calculated using d = 1.25 cm. A wavelength range of 290 to 600 nm was used to cover both the output of the photoreactor lamps and light absorption range of all the extracts (Fig. S1 in the Supplement). Rabs was not corrected for light screening (i.e., inner-filter effect), since the absorbance coefficients of all the extracts were below 0.1 cm−1 in the UVA range. The wavelength-dependent mass absorption coefficients for the WSOC (MACλ; m2 g-C−1) in the extracts were calculated using the following equation:
where ln (10) is the base conversion factor, 10−2 is for unit conversion, and [WSOC] (in mg-C L−1) is the concentration of the WSOC in each extract (Table S2) measured by a total organic carbon (TOC) analyzer (Shimadzu TOC-VCSH). It should be noted that the mass ratio of the organic material (OM) to organic carbon (OC) in PM2.5 in Hong Kong is approximately 2.1 (Chen and Yu, 2007). Thus, the calculated MACλ values would be halved had they been normalized by [OM] instead of [WSOC]. Section S1 in the Supplement describes the detection methods of inorganic ions in the extracts.
Various light absorption properties were obtained for each extract, based on their absorbance and WSOC measurements (Table S3). The α300 value is the UV absorption coefficient at 300 nm. SUVA254 and SUVA365 are the specific UV absorbances obtained from dividing the UV absorption coefficients at 254 nm and at 365 nm (α254 and α365, respectively) by [WSOC]. The AAE is the absorption Ångström exponent, which can be calculated using the following equation:
The AAE values were obtained from the negative of the slope of the linear plot of ln(αλ) vs. ln(λ) in the range of 300 to 450 nm (26 extracts) or 300 to 350 nm (8 extracts). The narrower wavelength range was used for extracts that had very low absorbance at the long wavelengths to ensure good linearity.
2.3 Chemicals used in photochemical experiments
The chemical probe for , furfuryl alcohol (98 %), was purchased from Acros Organics (now Thermo Scientific Chemicals) and was distilled under vacuum conditions before being prepared into a 100 µM stock solution. Deuterium oxide (D2O, 99 % atom D) was purchased from Sigma-Aldrich. The chemical probe for 3C∗, 2,6-dimethoxyphenol (syringol, 98 %), was purchased from J&K Scientific. The chemical actinometer, 2-nitrobenzaldhyde (2-NB; 98 %), was purchased from J&K Scientific. Preparation of all chemical solutions and dilution of the extracts were performed using ultrapure Milli-Q water (Merck; resistivity of 18.2 MΩ cm).
2.4 Photochemical experiments
Irradiation experiments were conducted in a Rayonet photoreactor (RPR-200; Southern New England Ultraviolet Co.) equipped with 12 UVA lamps (RPR-3500A; Southern New England Ultraviolet Co.). The spectral irradiance is shown in Fig. S2. The procedure used to determine the photon flux is described in Sect. S2. In a typical photochemical experiment, quartz tubes containing 5 mL of extract spiked with a probe compound (10 µM) were placed on a merry-go-round sample holder (RMA-500; Southern New England Ultraviolet Co.) in the middle of the photoreactor for continuous illumination. The chemical probes for and 3C∗ were furfuryl alcohol (Appiani et al., 2017) and syringol (Kaur and Anastasio, 2018; Kaur et al., 2019; Ma et al., 2023b), respectively. The temperature inside the photoreactor during the experiment was maintained at 26±1 ∘C by a cooling fan positioned at the bottom of the photoreactor. Aliquots of the solutions were removed at different reaction times to monitor the loss of the chemical probe, using an ultrahigh-pressure liquid chromatography system coupled to a photodiode array detector (UPLC-PDA; Waters ACQUITY H-Class). Separation of furfuryl alcohol and syringol was achieved using a Phenomenex Kinetex polar C18 column (2.6 µm; 100×2.1 mm) and elution at 0.3 mL min−1 with Milli-Q water-to-acetonitrile ratios of 9:1 and 8:2, respectively. The PDA detection wavelengths for furfuryl alcohol and syringol were 216 and 210 nm, respectively. Control experiments showed that syringol and furfuryl alcohol loss in illuminated Milli-Q water and field blank extracts were mostly minimal, and the differences were within experimental errors (Fig. S3). This indicated that the production of 3C∗ and was negligible in the background samples.
2.5 Quantification of steady-state concentrations, formation rates, and quantum yields of 1O
Furfuryl alcohol was used as the chemical probe (Appiani et al., 2017). The kinetic solvent isotope effect (KSIE) was used to account for furfuryl alcohol degradation by oxidants other than in the quantification of the steady-state concentrations of () in the extracts (Davis et al., 2018). These experiments involved comparing the decay of furfuryl alcohol in pure water (H2O) to that in heavy water (D2O; Haag and Hoigne, 1986; Allen et al., 1996; Anastasio and McGregor, 2001; Kaur and Anastasio, 2017; Kaur et al., 2019; Ma et al., 2023b). The extracts were prepared in Milli-Q water or in a mixture of 1:1 Milli-Q water D2O (), in which they were spiked with 10 µM furfuryl alcohol. The furfuryl alcohol decay followed pseudo first-order kinetics (Fig. S4). Their rate constants were used to calculate as follows:
and are the pseudo first-order rate constants of furfuryl alcohol loss in the 1:1 Milli-Q water D2O () mixture and in Milli-Q water, respectively, as determined from the slopes of the linear plot of vs. irradiation time (Fig. S4). is the second-order rate constant of FFA with at 26 ∘C (1.084×108; Appiani et al., 2017), and and are the deactivation rates in pure H2O (2.81×105 s−1) and pure D2O (1.57×104 s−1), respectively (Davis et al., 2018). Since the furfuryl alcohol decay from direct photolysis was minimal, the photolysis rate ( s−1; Fig. S3) was not used to correct the and values.
The formation rate of () was calculated as follows:
The quantum yield of () was calculated as follows:
2.6 Quantification of steady-state concentrations, formation rates, and quantum yields of 3C∗
We used syringol as the sole 3C∗ chemical probe. However, we acknowledge that due to the chemical complexity of 3C∗ species, a single chemical probe has limitations with respect to quantifying all of the 3C∗ species (McNeill and Canonica, 2016; Maizel and Remucal, 2017). While some studies have used multiple probes (and thus performed multiple photochemical experiments) to better constrain 3C∗ measurements (Kaur and Anastasio, 2018; Kaur et al., 2019; Ma et al., 2023b), we were unable to do so in our study due to insufficient extract volumes for additional photochemical experiments. Thus, only a subset of 3C∗ species that oxidize syringol were quantified in this study (Kaur and Anastasio, 2018).
The syringol decays followed pseudo first-order kinetics (Fig. S5). The syringol decay rates were used to calculate the steady-state concentrations of 3C∗ ([3C∗]ss) as follows:
where is the pseudo first-order rate constant of syringol loss determined from the slope of the linear plot of vs. irradiation time (Fig. S5). is the second-order rate constant between syringol and (; Tratnyek and Hoigne, 1991), jSYR is the loss rate of syringol in field blank samples ( s−1, Fig. S3), and is the second-order rate constant between syringol and a model 3C∗ species (Table S4). Since 3C∗ is comprised of a variety of species with a range of reactivities, there is no single value for the rate constant of syringol with 3C∗. Thus, the [3C∗]ss value for each extract was calculated by taking the average of the [3C∗]ss values calculated using four model 3C∗ species (2-acetonaphthone, 32AN∗; 3'-methoxyacetophenone, 33MAP∗; 3,4-dimethoxybenzaldehyde, 3DMB∗; benzophenone, 3BP∗), which were chosen to cover the range of 3C∗ reactivities in atmospheric samples. While previous studies performed •OH photochemical experiments to correct for the reaction between syringol and •OH in their [3C∗]ss calculations (Kaur and Anastasio, 2018; Kaur et al., 2019; Ma et al., 2023c), we did not do so in our study due to insufficient extract volumes for additional photochemical experiments. However, previous studies have reported that the contribution of both •OH and to the loss of syringol were < 20 % for the measurement of [3C∗]ss in fog water (Kaur and Anastasio, 2018) and PM extracts (Kaur et al., 2019).
The formation rate of 3C∗ () was calculated as follows:
where is the average second-order rate constant for the four model 3C∗ species being quenched via energy transfer to dissolved O2 (2.8×109 M−1 s−1; Canonica et al., 2000; Kaur and Anastasio, 2018). [O2 (aq)] is the dissolved O2 concentration in water at 26 ∘C ( M; Rounds et al., 2013), is the estimated overall rate constant for 3C∗ loss (i.e., reaction and quenching) due to WSOC (9.3×107 L mol-C−1 s−1; Kaur et al., 2019), and [WSOC] (in mg-C L−1) is the concentration of WSOC in each extract (Table S2).
The quantum yield of 3C∗ () was calculated as follows:
Uncertainties were propagated from the measured decay kinetics of furfuryl alcohol and syringol in triplicate photochemical experiments and 1 standard deviation of the second-order rate constants in the literature. Statistics and linear regression analyses were performed using Prism 8 software.
3.1 Characteristics of the extracts
3.1.1 WSOC and light absorption properties
The same sampling flow rate (30 L min−1) and period (72 h) were used to collect all the filters, and the same dilution ratio (i.e., equivalent to extracting each filter in 15.54 mL Milli-Q water) was used to prepare all the extracts. This allowed us to compare the WSOC concentrations and light absorption properties across the extracts. The PM2.5 mass H2O mass ratios for the extracts (Table S1) ranged from to µg PM2.5 µg H2O−1, which were close to fog and cloud water conditions but were much more diluted compared to aerosol liquid water conditions (ca. 1 µg PM µg H2O−1; Liao and Seinfeld, 2005; Herrmann et al., 2015; Nguyen et al., 2016; Seinfeld and Pandis, 2016). The concentrations of WSOC in the extracts ranged from 3.8 to 25.7 mg-C L−1, with a study average of 13.7 mg-C L−1 (Table S2), which were close to the WSOC concentrations previously measured in fog and ground-based clouds (Herckes et al., 2013). The concentrations of WSOC in the extracts were linearly correlated (simple linear regression or SLR r2=0.93) with the PM2.5 mass H2O mass ratios (Fig. 2).
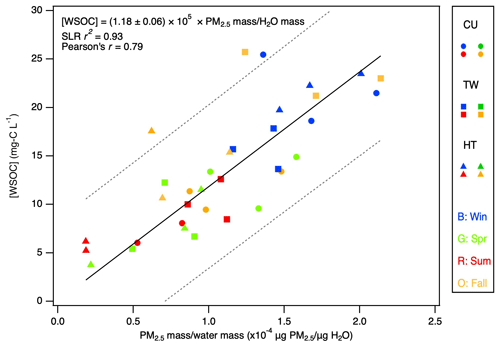
Figure 2The WSOC concentration as a function of the PM2.5 mass water mass ratio for the extracts. Blue, green, red, and orange symbols denote the winter, spring, summer, and fall samples, respectively. The dashed lines represent 95 % prediction bands. The SLR r2 and Pearson's r are the coefficient of determination for simple linear regression and the Pearson correlation coefficient, respectively.
Once converted to the carbon mass concentration in air, the study average WSOC concentration (1.7±0.8 µg m−3) was close to the previously reported values at another Hong Kong urban site (1.8±1.1 µg m−3) and the semi-rural HT site (1.3±1.1 µg m−3) for PM2.5 (Huang et al., 2014). The WSOC concentration had a noticeable seasonal trend in which the concentrations were higher in the fall and winter extracts, and the lowest concentrations were measured in the summer extracts (Table S2). The seasonal variations in the WSOC concentration in PM2.5 could be attributed to the seasonal variations in long-range air mass transport influenced by the East Asian monsoon system (Huang et al., 2014; Zhang et al., 2018; Chow et al., 2022). Air masses originating mainly from polluted continental areas located north of Hong Kong contributed to the high PM2.5 and WSOC concentrations in fall and winter (Figs. S6 to S8). In the summer, air masses originating from clean marine regions are located south of Hong Kong instead. These summer marine air masses generally have low PM2.5 and WSOC concentrations. This results in Hong Kong having substantially lower PM2.5 and WSOC concentrations in the summer when compared to the fall and winter. Consequently, regional sources are the main PM2.5 contributors in fall and winter, whereas local sources are the main PM2.5 contributors in the summer (Huang et al., 2014; Zhang et al., 2018; Chow et al., 2022).
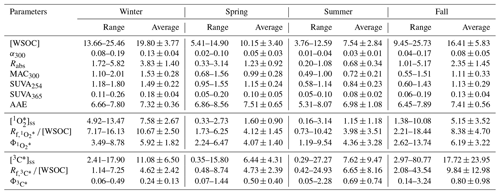
All the extracts had absorbance from the near-UV to the visible region, indicating the presence of BrC and the potential of generating and 3C∗ in all extracts. The absorption coefficient, αλ, and mass absorption coefficient, MACλ, declined exponentially with λ for all the extracts (Fig. 3). The average values of the absorption coefficient and mass absorption coefficient at 300 nm (α300 and MAC300) indicated that, on average, the absorbance for the urban CU and TW extracts was slightly higher than the absorbance for the semi-rural HT extracts (Table 1). Upon grouping the α300 and MAC300 data sets based on seasonality irrespective of the sampling location, we observed noticeable differences in the seasonal α300 and MAC300 values (Table 2). The average seasonal α300 and MAC300 values followed similar trends, namely winter > fall > spring > summer. Since the MAC300 accounts for WSOC dilution (Eq. 3), the higher MAC300 values in the winter extracts indicated that the water-soluble organic compounds in winter PM2.5 were more strongly absorbing and/or were less diluted with weakly absorbing water-soluble organic compounds when compared to the PM2.5 from the other three seasons.
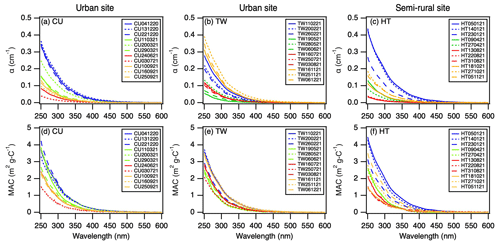
Figure 3(a–c) αλ and (d–f) MACλ of PM2.5 extracts from CU, TW, and HT, respectively. The lines in blue, green, red, and orange indicate samples collected during the winter, spring, summer, and fall seasons, respectively.
Table 1Summary of WSOC concentration, light absorption properties, steady-state concentrations, and quantum yields of and 3C∗ for the CU, TW, and HT sites.
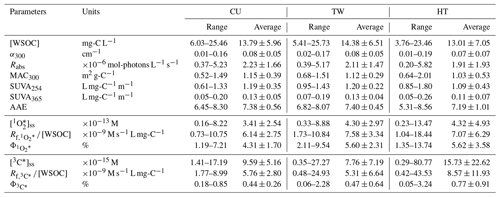
Note that the uncertainties given are 1 standard deviation.
Table 2Summary of WSOC concentration, light absorption properties, steady-state concentrations, and quantum yields of and 3C∗ for the four seasons.
Note that the unit for each parameter is the same as in Table 1. Uncertainties given are 1 standard deviation.
The AAE describes the spectral dependence of light absorption and is typically used to indicate the BrC contribution to the total absorption of aerosols (Helin et al., 2021). The AAE value for black carbon is typically close to 1, while AAE values larger than 1 indicate the presence of BrC (Kirchstetter et al., 2004). All the AAE values were larger than 1, thus indicating the omnipresence of BrC. The AAE values were fairly similar among the three sites (Table 1) and across the four seasons (Table 2). The Rabs values summarize the light absorption rates ranging from 290 to 600 nm. Rabs was linearly correlated with the WSOC concentration, with Pearson's r values between 0.88 and 0.97 for the three sites (Fig. S9). The good correlation between Rabs and the WSOC concentration implied that water-soluble BrC was likely the main contributor to the total light absorption.
SUVA254 and SUVA365, which are the specific UV absorbance obtained from dividing the absorption coefficients at 254 and at 365 nm by the WSOC concentration, are commonly used as proxies for organic matter aromaticity. Higher SUVA254 and SUVA365 values indicate enhanced aromaticity (Weishaar et al., 2003). As expected, the SUVA254 values for the three sites were higher than the SUVA365 values. These average SUVA254 and SUVA365 values for the three sites indicated that the organic matter in the urban CU and TW extracts, on average, had higher aromaticity than those in the semi-rural HT extracts (Table 1). It is possible that the observed higher absorbance and aromaticity in the urban CU and TW extracts were due to the presence of oxygenated aromatic compounds (e.g., highly substituted phenolic compounds) from local anthropogenic sources such as vehicle emissions, combustion-related (e.g., cooking, power generation, and power usage) activities, and solvent usage (Guo et al., 2003; Chen et al., 2017; Cui et al., 2018; Bilal et al., 2019). Upon grouping the SUVA254 and SUVA365 data sets based on seasonality irrespective of the sampling location, the average seasonal SUVA254 and SUVA365 values indicated that the organic matter in the fall and winter extracts, on average, had higher aromaticity than those in the spring and summer extracts (Table 2). The higher aromaticity in the fall and winter extracts was likely due to strong biomass burning contributions to ambient fall and winter PM2.5. Hong Kong generally has low levels of biomass burning activities. However, fall and winter PM2.5 in continental areas north of Hong Kong (e.g., parts of mainland China) can have substantial contributions from biomass burning, especially in rural areas in which residential biomass burning is used for intensive heating purposes (Chen et al., 2017). It is possible that biomass-burning-influenced air masses from these northern continental areas were transported to Hong Kong during fall and winter and consequently contributed to the higher aromaticity in these extracts.
3.1.2 Site and seasonal variations in WSOC and light absorption properties
We hypothesized that the site and seasonal variations in the WSOC concentration and light absorption properties of water-soluble BrC in the PM2.5 drove the site and seasonal variations in 3C∗ and production. Thus, we examined the site and seasonal variations in the WSOC and light absorption properties of the extracts. The above comparisons of the average WSOC concentration, α300, MAC300, SUVA254, and SUVA365 values of the urban CU and TW extracts vs. the semi-rural HT extracts indicated that, on average, PM2.5 at CU and TW had slightly higher concentrations of and/or more absorbing water-soluble BrC comprised of organic matter with high aromaticity compared to PM2.5 at HT. However, statistics performed on the WSOC concentration, α300, MAC300, AAE, Rabs, SUVA254, and SUVA365 data sets showed that their variations between the three sites were not significant (p>0.05; Table S3). These results indicated that the locations (i.e., urban vs. semi-rural) did not have a significant influence on the concentration of WSOC and light absorption properties of water-soluble BrC in PM2.5.
Since the locations did not have a significant influence on the WSOC concentration and light absorption properties of water-soluble BrC, we combined the data sets from the three sites and separated them based on seasonality. Despite the spread in their seasonal values, seasonal variations in the WSOC concentration, α300, MAC300, Rabs, SUVA254, and SUVA365 values were statistically significant (p<0.05; Fig. S10). This implied that the seasonal variations in long-range air mass transport had a significant influence on the WSOC concentration and light absorption properties of water-soluble BrC. The WSOC concentration, α300, MAC300, Rabs, SUVA254, and SUVA365 had noticeably similar trends, namely winter > fall > spring > summer. These seasonal trends indicated that winter and fall PM2.5 had higher concentrations of and/or more absorbing water-soluble BrC comprised of organic matter with high aromaticity compared to the summer and spring PM2.5. Based on the seasonal variations in long-range air mass transport during the study (Figs. S6 to S8), regional sources were important contributors to water-soluble BrC comprised of organic matter of high aromaticity in winter and fall PM2.5. Interestingly, seasonal variations in the AAE values were not statistically significant (p > 0.05). While it is unclear why seasonal trends were not observed for the AAE values in our study, other studies have similarly reported the lack of seasonal trends in the AAE values (Du et al., 2014; Ma et al., 2023b).
3.2 1O and 3C∗ production during extract illumination
3.2.1 1O
The pseudo first-order decay rate constants of furfuryl alcohol ( chemical probe) in photochemical experiments (Fig. S4) were used to determine (Eq. 5). The values spanned 2 orders of magnitude, ranging from to M, with a study average of M (Table S5). These values were in line with those previously measured in atmospheric samples (10−15 to 10−12 M; Table S7). The values were linearly correlated with two indicators of water-soluble BrC, namely WSOC concentration and α300, with Pearson's r values of 0.88 and 0.92, respectively (Fig. S11a and b). These correlations provided strong evidence that the production of was linked to water-soluble BrC. The large range in the values was likely due to the variations in the absorbance in the BrC chromophores (Fig. 3).
The values ranged from to M s−1 (Table S5). Across all extracts, the was linearly correlated with Rabs (Fig. S12a), which was consistent with water-soluble BrC being a source of . The study average WSOC-normalized ( M s−1 L mg-C−1) value was within a factor of 2 of the previously reported values for PM2.5 samples collected in urban and rural areas in Colorado, USA (Leresche et al., 2021), and for PM samples collected in biomass-burning-influenced areas in California, USA (Kaur et al., 2019; Ma et al., 2023a). The values ranged from 0.77 % to 13.74 %, with a study average of (5.12±2.66) %, which was noticeably higher than previously reported values for atmospheric PM samples (0.3 % to 4.5 %; Kaur and Anastasio, 2017; Manfrin et al., 2019; Kaur et al., 2019; Leresche et al., 2021; Bogler et al., 2022). This suggested that the water-soluble BrC in our extracts has higher photosensitization efficiencies compared to that in previous studies, which could be due to the different composition and age of water-soluble BrC in atmospheric PM in different locations. For instance, ozone is a major ground-level air pollutant in Hong Kong (Liao et al., 2021). Exposure to ambient ozone pollution could have led to higher values due to the formation of quinone-like moieties from the ozone aging of phenolic moieties present in water-soluble BrC (Leresche et al., 2019). It is also possible that the higher values observed in our study could be due to differences in experimental conditions. For instance, we used UVA light to illuminate the extracts in photochemical experiments, whereas previous studies used xenon arc lamps (Kaur et al., 2019) or a solar simulator instrument (Leresche et al., 2021). In addition, the different methodologies used to determine may have contributed to our study's higher values. While this study determined the values from the and Rabs measurements (Eq. 7), other studies used a reference sensitizer (e.g., perinaphthenone) to determine their values (Manfrin et al., 2019; Bogler et al., 2022).
3.2.2 3C∗
The pseudo first-order decay rate constants of syringol (3C∗ chemical probe) in photochemical experiments (Fig. S5) were used to determine [3C∗]ss (Eq. 8). The [3C∗]ss values were close to the values calculated using only the bimolecular rate constant for the model 3C∗ species 3DMB∗ (Table S6). This indicated that the 3C∗ species quantified in this study had reactivities close to 3DMB∗. Similar observations were reported for 3C∗ species in PM extracts from biomass-burning-influenced areas in California, USA (Kaur and Anastasio, 2018; Kaur et al., 2019). It is important to note that due to the chemical complexity of 3C∗ species, a single chemical probe cannot quantify all the 3C∗ species (Maizel and Remucal, 2017). Hence, only a subset of 3C∗ species that can oxidize syringol was quantified in our study (Kaur and Anastasio, 2018). The [3C∗]ss values spanned 2 orders of magnitude, ranging from to M, with a study average of M. While the range of [3C∗]ss values was in line with those previously measured in atmospheric samples (10−16 to 10−13 M; Table S7), not all of these previous studies used syringol as the 3C∗ chemical probe. The choice of the 3C∗ chemical probe can impact the [3C∗]ss measurements. This is because different 3C∗ chemical probes react with different subsets of 3C∗ species with different oxidizing abilities (Maizel and Remucal, 2017; Kaur and Anastasio, 2018; Ma et al., 2023c). In addition, the decay of oxidizing 3C∗ chemical probes (e.g., syringol and 2,4,6-trimethylphenol) can be inhibited by the co-presence of some atmospheric species (e.g., copper and water-soluble organic matter), especially under highly concentrated conditions (Canonica and Laubscher, 2008; Maizel and Remucal, 2017; McCabe and Arnold, 2017; Ma et al., 2023b, c). Using the equations provided by Ma et al. (2023b), we estimate that our reported [3C∗]ss values may be underestimated by as much as a factor of 2 due to water-soluble organic matter inhibiting the decay of syringol. In addition, water-soluble copper, another atmospheric species known to inhibit syringol decay (Ma et al., 2023c), can be present in substantial concentrations in PM2.5 in some urban areas in Hong Kong (Yang et al., 2023). However, the extent to which water-soluble copper will impact [3C∗]ss values is currently unknown. Nevertheless, the [3C∗]ss values were linearly correlated with the WSOC concentration and α300 (Fig. S11c and d), which was consistent with water-soluble BrC being a source of 3C∗. The correlations of [3C∗]ss with the WSOC concentration and α300 were noticeably weaker than the correlations of with the WSOC concentration and α300. The weaker [3C∗]ss correlations could be attributed to the chemical complexity of the 3C∗ pool. Even though water-soluble BrC is a key precursor of 3C∗, the sample-to-sample variability in the subset of 3C∗ species that were able to oxidize syringol likely caused the weaker [3C∗]ss correlations with the WSOC concentration and α300.
The values ranged from to M s−1, with a study average of M s−1 (Table S6). The study average WSOC-normalized ( M s−1 L mg-C−1) value was 3 to 7 times lower than the previously reported value for PM samples collected in biomass-burning-influenced areas in California, USA (Kaur et al., 2019; Ma et al., 2023a). Across all extracts, the value was linearly correlated with Rabs, with a Pearson's r value of 0.63 (Fig. S12b), which indicated that 3C∗ production was linked to water-soluble BrC. The correlation between and Rabs was weaker than the correlation between and Rabs. Kaur et al. (2019) similarly reported weaker linear correlations for Rabs vs. when compared to Rabs vs. for extracts of winter PM collected from areas influenced by biomass burning emissions in California, USA. Sample-to-sample variability in the subset of 3C∗ species that were able to oxidize syringol likely caused the weaker Rabs vs. correlations.
The values ranged from 0.05 % to 3.24 %, with a study average of (0.55±0.66) %, which was approximately 9 times lower than the study average of . The difference in 3C∗ and photosensitization efficiencies could be due to only a subset of 3C∗ species that can oxidize syringol being captured in our photochemical experiments, since different 3C∗ species may have different photosensitization efficiencies. Our study average was also lower than the average ((2.40±1.00) %) reported by Kaur et al. (2019) for extracts of PM collected from biomass-burning-influenced areas in California, USA. This suggested that the water-soluble BrC in our extracts have a lower fraction of oxidizing 3C∗ species compared to that in PM samples investigated by Kaur et al. (2019), which could be due to the different composition and age of water-soluble BrC in atmospheric PM.
3.3 Site and seasonal variations of 1O and 3C∗ production
The steady-state concentrations and quantum yields of and 3C∗ were fairly similar among the three sites (Fig. S13). Variations in these values across the three sites were not statistically significant (p>0.05). This indicated that the location (i.e., urban vs. semi-rural) did not have a significant effect on the steady-state concentrations and photosensitization efficiencies of 3C∗ and , which implied that water-soluble BrC from local PM2.5 sources did not have a significant influence on the year-round 3C∗ and production. The large spreads in the steady-state concentration and quantum yield values highlighted the broad range of BrC chromophores present in the PM2.5 at the three locations that are capable of photosensitizing and 3C∗.
Since the locations did not have a significant influence on the production of and 3C∗, we combined the and 3C∗ data sets from the three sites and separated them based on seasonality. We observed a distinct seasonal trend for (Fig. 4a). The values were generally the highest in the winter and lowest in the summer (Table 2). The seasonal variations in the values were also found to be statistically significant (p<0.05). The seasonal trend for [3C∗]ss was noticeably weaker and was not statistically significant (p>0.05; Fig. 4b). However, the [3C∗]ss values were mostly higher in the fall and winter and lower in the spring and summer (Table 2). The differences in the strengths of the seasonal trends of (i.e., strong and statistically significant) and [3C∗]ss (i.e., weak and statistically insignificant) could be attributed to sample-to-sample variations in 3C∗ species that can form . Even though 3C∗ is a precursor of , not all 3C∗ species will form . In addition, high-energy and strongly reducing 3C∗ species are not necessarily efficient photosensitizers (McNeill and Canonica, 2016). Sample-to-sample variability in the subset of 3C∗ species that were able to oxidize syringol could also have contributed to the weak seasonal [3C∗]ss trend. The fall [3C∗]ss average (Table 2) was noticeably high, and this was due to the inclusion of an abnormally high [3C∗]ss value ( M) obtained for the HT271021 sample, which was identified as a “far-out outlier” by Tukey's fences. Unlike the other samples, we observed fast photobleaching for the HT271021 sample during the photochemical experiments (Figs. S4 and S5), which likely resulted in overestimated steady-state concentrations (Sect. 2.4 and 2.5). It should be noted that, while a high value was also obtained for the HT271021 sample, it was not identified as an outlier by Tukey's fences.
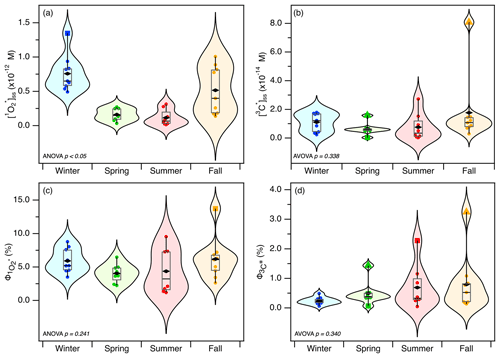
Figure 4Violin plots showing the seasonal variations of (a) , (b) [3C∗]ss, (c) , and (d) . For the box plots, the triangles indicate far-out outliers, and the squares indicate outliers identified by Tukey's fences. The whiskers denote the minimum and maximum values, the boxes denote the 25th and 75th percentile values, black diamonds indicate the mean values, and the midlines of the boxes denote the median values.
Overall, seasonality had noticeable effects on and (to a lesser extent) [3C∗]ss, as these values were the highest in the fall and winter and the lowest in the summer. The seasonal trends of and [3C∗]ss correlated with the seasonal trends of the WSOC concentration and light absorption properties of water-soluble BrC (Fig. S10). The fall and winter extracts had higher concentrations of and/or more absorbing water-soluble BrC comprised of organic matter with high aromaticity than the spring and summer extracts. Thus, the higher concentrations of and/or more absorbing water-soluble BrC in the winter and fall extracts likely enhanced and 3C∗ production. In particular, additional statistical analyses (Student's t test) performed on the seasonal values for , PM2.5 mass H2O mass ratio, WSOC concentration, and light absorption properties of water-soluble BrC (Table S8) suggested that the seasonal differences in the values were driven primarily by the PM2.5 mass concentration and WSOC concentration. Since the seasonal variations in PM2.5 and water-soluble BrC were due to the seasonal variations in long-range air mass transport, this implied that regional PM2.5 sources located in continental areas north of Hong Kong contributed to the higher photooxidant production in the fall and winter.
The seasonal trends of and (Fig. 4c and d) were noticeably weaker than the seasonal trends of and [3C∗]ss (Fig. 4a and b). The average for winter, spring, summer, and fall were (5.92±1.82) %, (4.07±1.40) %, (4.36±3.28) %, and (6.19±3.22) %, respectively, while the average for winter, spring, summer, and fall were (0.24±1.23) %, (0.50±0.40) %, (0.69±0.74) %, and (0.80±0.98) %, respectively. The average and values were noticeably the highest for the fall season. This was due to the inclusion of abnormally high quantum yield values obtained for the HT271021 sample (identified as a far-out outlier by Tukey's fences). Fast photobleaching for the HT271021 sample during the photochemical experiments (Figs. S4 and S5) likely resulted in overestimated quantum yields. The variations in and across the four seasons were not statistically significant (p > 0.05), which indicated that seasonality did not have a significant effect on the photosensitization efficiencies of and 3C∗. However, we cannot discount the possibility that the statistically insignificant variations in and across the four seasons could be due to photobleaching. Leresche et al. (2021) previously reported reduced photosensitization for the extracts of summer PM2.5 collected from Colorado, USA, due to enhanced photobleaching. Thus, it is possible that the summer BrC chromophores may have been more effective for the production of photooxidants, but the enhanced photobleaching caused by stronger solar irradiation led to their weakened photosensitization ability and consequently resulted in statistically insignificant variations in and across the four seasons.
We also compared the influence of seasonal variations in long-range air mass transport on the and [3C∗]ss values for the urban CU and TW sites vs. the semi-rural HT site. Since the spring sampling months could be viewed as a transition period during which the dominant air masses that arrive in Hong Kong gradually shifted from the polluted continental northern areas (fall and winter months) to the clean marine southern regions (summer months; Figs. S6 to S8), for simplicity, we excluded the spring data sets from this comparison. The fall and winter data sets were combined and the subsequent average value was compared to the average value of the summer data set. Larger contrasts in the and [3C∗]ss values were observed for the semi-rural HT site compared to the urban CU and TW sites (Tables S5 and S6), which were in line with the larger contrasts in the average WSOC concentrations and light absorption properties for HT compared to CU and TW (Tables S2 and S3). This could be attributed to the nature of the sites. Due to the seasonal variations in long-range air mass transport (Figs. S6 to S8), local sources are the main contributors to summer PM2.5, whereas regional sources located in continental areas north of Hong Kong are the main contributors to fall and winter PM2.5 (Pathak et al., 2003; Louie et al., 2005a, b; Huang et al., 2014; Li et al., 2015; Wong et al., 2020). In contrast to the urban CU and TW sites, the semi-rural HT site is located far from urban areas (approximately 6 km away from the nearest urban area). Thus, contributions of local anthropogenic emissions (e.g., traffic and combustion-related activities) to water-soluble BrC in summer PM2.5 at the semi-rural HT site are smaller compared to those at the urban CU and TW sites. This would result in larger contrasts between the average WSOC concentrations and light absorption properties from the combined fall plus winter data set vs. summer data set for the semi-rural HT site when compared to the urban CU and TW sites. Consequently, the higher concentrations of water-soluble BrC in summer PM2.5 from local anthropogenic emissions at the urban CU and TW sites contributed to their higher summer and [3C∗]ss values, and consequently smaller fall plus winter vs. summer and [3C∗]ss contrasts, compared to the semi-rural HT site.
3.4 Relating [3C∗]ss and [1O to water-soluble BrC concentration and light absorption properties
To examine more closely how water-soluble BrC contributed to and 3C∗ production, we first investigated how the and [3C∗]ss values changed as a function of MAC300, a light absorbance parameter that accounts for WSOC dilution. Both and [3C∗]ss showed positive correlations with MAC300 (Fig. 5a and c), which indicated that the production of and 3C∗ were governed by the quantity and absorption efficiency of water-soluble BrC. was noticeably more strongly linearly correlated with MAC300 compared to [3C∗]ss. The weaker [3C∗]ss correlations could be attributed to the chemical complexity of the 3C∗ pool, which cannot be quantified completely by syringol. Thus, even though water-soluble BrC is a key precursor of 3C∗, the sample-to-sample variability in the size of the population of 3C∗ species that were able to oxidize syringol likely caused the weaker [3C∗]ss correlations with MAC300.
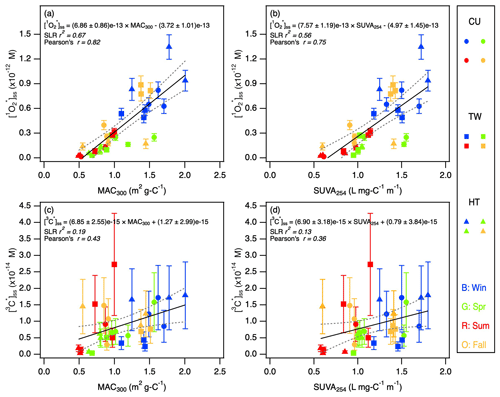
Figure 5(a, b) and (c, d) [3C∗]ss as a function of MAC300 and SUVA254. The outlier, HT271021, was excluded. Blue, green, red, and orange symbols denote the winter, spring, summer, and fall samples, respectively. Dashed lines represent 95 % confidence bands. SLR r2 and Pearson's r indicate coefficient of determination of simple linear regression and Pearson correlation coefficient, respectively.
The and [3C∗]ss depend on both the quality and quantity of the BrC chromophores. The quantity of the BrC chromophores is associated with their concentrations, whereas the quality is associated with their quantum yields and WSOC-normalized light absorption properties (e.g., MAC and SUVA values; Bogler et al., 2022). In other words, some BrC chromophores are more efficient at making photooxidants, and thus PM2.5 with higher quantum yields can be considered to have higher-quality BrC chromophores towards and 3C∗ formation. A high WSOC concentration in an extract will result in a high (and/or a high [3C∗]ss) only if a high concentration of water-soluble BrC chromophores is present in the extract. The relative importance in the quantity vs. quality of BrC chromophores in our study could be ascertained from the comparison of the seasonal trends of and [3C∗]ss (Fig. 4a and b) vs. the seasonal trends of and (Fig. 4c and d). Stronger seasonal trends were observed for and [3C∗]ss, which indicated that the quantity of BrC chromophores mainly governed and 3C∗ production in our study. The important role that the quantity of BrC chromophores plays in driving and 3C∗ production is further emphasized by the weakened seasonal trends of WSOC-normalized and [3C∗]ss values (Sect. S3 and Fig. S14).
Even though the quantity of BrC chromophores appeared to be the main driver of and 3C∗ production in our study, it is still worth investigating factors that affected the quality of BrC chromophores. We hypothesized that the quality of BrC chromophores was influenced by the presence of light-absorbing aromatic compounds (Laskin et al., 2015). To test this hypothesis, we evaluated the contributions of aromatic compounds to and 3C∗ production by plotting the and [3C∗]ss values as a function of two commonly used indicators of aromaticity, namely SUVA254 and SUVA365 (Figs. 5b, d, and S15). Both and [3C∗]ss generally showed positive correlations with SUVA254 and SUVA365. These correlations provided evidence that the production of and 3C∗ was enhanced by aromatic compounds. This enhancement likely occurred though a combination of enhanced rates of light absorption and photosensitization of water-soluble BrC chromophores (Manfrin et al., 2019; Chen et al., 2021). The linear correlations of [3C∗]ss with SUVA254 and SUVA365 were noticeably weaker compared to . The weaker [3C∗]ss correlations could be attributed to the sample-to-sample variability in the size of the population of 3C∗ species that were able to oxidize syringol.
It is important to note that even though our results (Figs. 5b, d, and S15) indicated that aromatic compounds were likely key water-soluble BrC constituents and photosensitizers that enhanced and 3C∗ production, there are other water-soluble BrC constituents and photosensitizers that can also promote and 3C∗ production. One such example is imidazoles, which are formed from aqueous reactions of dicarbonyls with reduced nitrogen-containing compounds such as amines, ammonium ions, and amino acids (Haan et al., 2009; De Haan et al., 2009, 2011; Kampf et al., 2012; Powelson et al., 2014). Recent studies have shown that imidazoles can also be formed from aqueous 3C∗-photosensitized reactions of phenolic compounds in the presence of ammonium ions (Mabato et al., 2022, 2023). To the best of our knowledge, there has not been a study that has investigated the concentrations of imidazoles in atmospheric PM in Hong Kong. However, imidazoles have been detected in atmospheric PM in urban Guangzhou (another city in South China; Lian et al., 2022) and at a background forest site in the Nanling Mountains of South China (He et al., 2022). Thus, future studies can focus on identifying other water-soluble BrC constituents and photosensitizers (e.g., imidazoles) in atmospheric PM in Hong Kong that can play potentially important roles in enhancing and 3C∗ production.
In this study, we reported the steady-state concentrations and quantum yields of 3C∗ and produced by PM2.5 in Hong Kong, South China. We quantified the production of 3C∗ and in illuminated aqueous extracts of PM2.5 collected in different seasons at two urban sites and one coastal semi-rural site during a year-round study. Variations in the WSOC concentrations and light absorption properties of water-soluble BrC across the three sites were found to be statistically insignificant. In contrast, variations in the WSOC concentrations and light absorption properties of water-soluble BrC across the four seasons were significant. Higher concentrations of WSOC and more light-absorbing water-soluble BrC were present in the PM2.5 during the fall and winter months. This could be attributed to monsoon-influenced seasonal variations in long-range air mass transport to Hong Kong. Air masses originating mainly from polluted continental areas located north of Hong Kong contributed to the higher concentrations of WSOC and more light-absorbing water-soluble BrC in the fall and winter PM2.5, whereas air masses originating mainly from clean marine regions located south of Hong Kong were responsible for the lower concentrations of WSOC and less light-absorbing water-soluble BrC in the summer PM2.5.
and 3C∗ were produced in all the illuminated aqueous extracts of PM2.5. The spanned 2 orders of magnitude, ranging from to M, with a study average of M. The [3C∗]ss spanned 2 orders of magnitude, ranging from to M, with a study average of M. These and [3C∗]ss values were in line with the steady-state concentrations previously reported for PM extracts, fog water, and rainwater (Table S7). The and [3C∗]ss correlated with the concentration of WSOC and the absorbance of water-soluble BrC, which indicated that water-soluble BrC was a key source of and 3C∗. Positive linear correlations between their steady-state concentrations and indicators of aromaticity (SUVA254 and SUVA365) implied that the production of and 3C∗ was enhanced by aromatic compounds, likely though a combination of enhanced rates of light absorption and photosensitization of water-soluble BrC chromophores. Location (i.e., urban vs. semi-rural) did not have a significant effect on and [3C∗]ss, which indicated that BrC amounts from local PM2.5 sources were likely not the primary drivers of year-round 3C∗ and production. In contrast, seasonality had a significant effect on and [3C∗]ss, with higher and [3C∗]ss observed in the fall and winter compared to the summer. This indicated that the seasonal trends of and 3C∗ production in PM2.5 in Hong Kong were governed by the seasonal variations in long-range air mass transport. Consequently, regional PM2.5 sources located in continental areas north of Hong Kong contributed to the higher and 3C∗ production in the fall and winter.
Even though the steady-state concentrations of •OH ([•OH]ss) were not measured in this study due to insufficient extract volumes, previous studies have reported that they are typically on the order of 10−17 to 10−15 M (Arakaki and Faust, 1998; Arakaki et al., 1999, 2006, 2013; Anastasio and McGregor, 2001; Anastasio and Jordan, 2004; Anastasio and Newberg, 2007; Kaur and Anastasio, 2017; Kaur et al., 2019; Manfrin et al., 2019). We hypothesize that the [•OH]ss in our illuminated extracts are also on the order of 10−17 to 10−15 M. The main precursors of •OH in Hong Kong are likely BrC and inorganic nitrate, both of which have the highest concentrations in the winter and the lowest concentrations in the summer (Table S2). Therefore, it is likely that •OH production will have a similar seasonal trend to that of the 3C∗ and production. Consequently, the concentrations of 3C∗ and can potentially be up to 103 and 105 higher than the concentrations of •OH in the extracts, respectively. Based on work by Kaur et al. (2019) and Ma et al. (2023b), the differences between the 3C∗ and concentrations vs. •OH concentrations are expected to be even larger under aerosol liquid water conditions. Thus, despite the lower reactivities of organic aerosol compounds with and 3C∗ compared to their corresponding reactivities with •OH, and 3C∗ will likely be present at high-enough concentrations that they can be competitive photooxidants to •OH under aerosol liquid water conditions (Kaur et al., 2019; Manfrin et al., 2019). This necessitates the inclusion of aqueous reactions involving and 3C∗ with organic aerosol compounds into atmospheric models, since these photooxidants may play important roles in the photochemical processing of organic aerosol compounds in atmospheric aqueous phases due to their high concentrations offsetting their lower reactivities.
The significance of our results lies foremost in the seasonal trends observed for and [3C∗]ss and how they correlated with the seasonal variations in the long-range air mass transport. Since many South China cities share similar monsoon-influenced seasonal air quality and aerosol pollution characteristics to that of Hong Kong, we anticipate that many South China cities will have similar seasonal trends of and 3C∗ production in atmospheric aerosols. In addition, given that their high concentrations will likely offset their lower reactivities, and 3C∗ seasonality in atmospheric aerosols can potentially influence the aqueous photochemical processing of organic aerosol compounds in South China, a region in which aqueous aerosol chemistry plays important roles in the formation and transformation of SOA (Y. J. Li et al., 2013; N. Li et al., 2013). It should be noted that although our results showed that the location (i.e., urban vs. semi-rural) did not have a significant effect on and 3C∗ production in PM2.5 in Hong Kong, this may not necessarily be the case for other South China cities, especially not those that are located close to areas with biomass burning activities (Yuan et al., 2015).
While this study reports the first measurements of the quantum yields and steady-state concentrations of 3C∗ and produced in atmospheric aerosols in South China, there are a number of caveats that should be noted. First, the and [3C∗]ss values reported in our study serve as lower limits, since they were measured using extracts comprised of only the water-soluble fraction of PM2.5. Water-insoluble BrC, which reportedly dominates the total BrC absorption in some parts of China (Bai et al., 2020; Huang et al., 2020; Wang et al., 2022), will likely produce and 3C∗ as well. Second, due to limited extract volumes for photochemical experiments and chemical analysis, only one 3C∗ chemical probe was used in our study to quantify 3C∗ quantum yields, formation rates, and steady-state concentrations. Hence, we only report concentrations of a subset of 3C∗ species. Measurements of 3C∗ quantum yields and steady-state concentrations can be better constrained with the use of multiple 3C∗ probes (Kaur and Anastasio, 2018; Kaur et al., 2019; Ma et al., 2023b, c). Third, photochemical experiments were performed using diluted extracts. These experimental conditions were substantially more diluted than atmospheric PM2.5 conditions. Thus, the concentrations of BrC chromophores in our extracts were substantially lower than those in atmospheric PM2.5, which would influence the reaction kinetics and consequently 3C∗ and production. Based on work by Kaur et al. (2019) and Ma et al. (2023b), higher and [3C∗]ss values in atmospheric PM2.5 are expected due to the higher concentrations of BrC chromophores, though extrapolation from diluted extraction conditions to concentrated PM2.5 conditions is complex and non-linear. Fourth, our extracts were not buffered, and their average pH was 4.68±0.29, whereas the pH of atmospheric PM2.5 in Hong Kong has been reported to be between 1.8 and 5.1 (Nah and Lam, 2022; Nah et al., 2023). pH can influence the composition of protonated vs. unprotonated BrC chromophores, which in turn will affect their absorption and reaction kinetics (Ma et al., 2021). Fifth, this work focuses on and 3C∗ production in PM2.5 extracts. Previous work on production in illuminated extracts of size-fractionated supermicron-sized road dust (< 45 to 500 µm) suggests that aerosol size may influence production (Cote et al., 2018). At present, it is unclear how aerosol size within atmospheric PM2.5 influences and 3C∗ production. Hence, the effects of dilution, pH, and aerosol size on photooxidant production from both water-soluble and water-insoluble BrC in atmospheric PM should be explored in future studies to further our understanding of aqueous organic aerosol photochemistry in the South China region.
Light absorption and kinetic data have been submitted to the Zenodo data repository (https://doi.org/10.5281/zenodo.7827983; Lyu et al., 2023). Data can also be made available upon request to the corresponding author (theodora.nah@cityu.edu.hk).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-23-9245-2023-supplement.
YuL and TN designed the study. YHL collected the field samples. YuL performed the chemical analysis and experiments. YuL, YLi, NBD, and TN analyzed the data. YuL and TN prepared the paper, with contributions from all co-authors.
At least one of the (co-)authors is a member of the editorial board of Atmospheric Chemistry and Physics. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We acknowledge the assistance of Yanhao Miao with the backward trajectory analysis, Jason Lam for his assistance with the furfuryl alcohol distillation, and Wing Chi Au and Chung Ming Tai for their help on filter extraction and/or chemical analysis.
This research has been supported by the Research Grants Council, University Grants Committee (grant no. 11303720).
This paper was edited by Sergey A. Nizkorodov and reviewed by Cort Anastasio and one anonymous referee.
Albinet, A., Minero, C., and Vione, D.: Photochemical generation of reactive species upon irradiation of rainwater: Negligible photoactivity of dissolved organic matter, Sci. Total Environ., 408, 3367–3373, 2010. a
Allen, J. M., Gossett, C. J., and Allen, S. K.: Photochemical formation of singlet molecular oxygen (1O2) in illuminated aqueous solutions of p-aminobenzoic acid (PABA), J. Photoch. Photobio. B, 32, 33–37, https://doi.org/10.1016/1011-1344(95)07185-7, 1996. a
Anastasio, C. and Jordan, A. L.: Photoformation of hydroxyl radical and hydrogen peroxide in aerosol particles from Alert, Nunavut: implications for aerosol and snowpack chemistry in the Arctic, Atmos. Environ., 38, 1153–1166, https://doi.org/10.1016/j.atmosenv.2003.11.016, 2004. a, b
Anastasio, C. and McGregor, K. G.: Chemistry of fog waters in California's Central Valley: 1. In situ photoformation of hydroxyl radical and singlet molecular oxygen, Atmos. Environ., 35, 1079–1089, 2001. a, b, c, d
Anastasio, C. and Newberg, J. T.: Sources and sinks of hydroxyl radical in sea-salt particles, J. Geophys. Res.-Atmos., 112, D10306, https://doi.org/10.1029/2006JD008061, 2007. a, b
Appiani, E., Ossola, R., Latch, D. E., Erickson, P. R., and McNeill, K.: Aqueous singlet oxygen reaction kinetics of furfuryl alcohol: effect of temperature, pH, and salt content, Environ. Sci.-Proc. Imp., 19, 507–516, https://doi.org/10.1039/C6EM00646A, 2017. a, b, c
Arakaki, T. and Faust, B. C.: Sources, sinks, and mechanisms of hydroxyl radical (•OH) photoproduction and consumption in authentic acidic continental cloud waters from Whiteface Mountain, New York: The role of the Fe(r) (r = II, III) photochemical cycle, J. Geophys. Res.-Atmos., 103, 3487–3504, https://doi.org/10.1029/97JD02795, 1998. a, b
Arakaki, T., Miyake, T., Shibata, M., and Sakugawa, H.: Photochemical formation and scavenging of hydroxyl radical in rain and dew waters, Nip. Kag. Kai., 5, 335–340, https://doi.org/10.14934/chikyukagaku.43.15, 1999. a, b
Arakaki, T., Kuroki, Y., Okada, K., Nakama, Y., Ikota, H., Kinjo, M., Higuchi, T., Uehara, M., and Tanahara, A.: Chemical composition and photochemical formation of hydroxyl radicals in aqueous extracts of aerosol particles collected in Okinawa, Japan, Atmos. Environ., 40, 4764–4774, https://doi.org/10.1016/j.atmosenv.2006.04.035, 2006. a, b
Arakaki, T., Anastasio, C., Kuroki, Y., Nakajima, H., Okada, K., Kotani, Y., Handa, D., Azechi, S., Kimura, T., Tsuhako, A., and Miyagi, Y.: A general scavenging rate constant for reaction of hydroxyl radical with organic carbon in atmospheric waters, Environ. Sci. Technol., 47, 8196–8203, https://doi.org/10.1021/es401927b, 2013. a, b
Bai, Z., Zhang, L., Cheng, Y., Zhang, W., Mao, J., Chen, H., Li, L., Wang, L., and Chen, J.: Water/Methanol-Insoluble Brown Carbon Can Dominate Aerosol-Enhanced Light Absorption in Port Cities, Environ. Sci. Technol., 54, 14889–14898, https://doi.org/10.1021/acs.est.0c03844, pMID: 32790286, 2020. a
Barrios, B., Mohrhardt, B., Doskey, P. V., and Minakata, D.: Mechanistic insight into the reactivities of aqueous-phase singlet oxygen with organic compounds, Environ. Sci. Technol., 55, 8054–8067, https://doi.org/10.1021/acs.est.1c01712, 2021. a
Bilal, M., Nichol, J. E., Nazeer, M., Shi, Y., Wang, L. C., Kumar, K. R., Ho, H. C., Mazhar, U., Bleiweiss, M. P., Qiu, Z. F., Khedher, K. M., and Lolli, S.: Characteristics of fine particulate matter (PM2.5) over urban, suburban, and rural areas of Hong Kong, Atmosphere, 10, 496, https://doi.org/10.3390/atmos10090496, 2019. a
Bogler, S., Daellenbach, K. R., Bell, D. M., Prévôt, A. S., El Haddad, I., and Borduas-Dedekind, N.: Singlet Oxygen Seasonality in Aqueous PM10 is Driven by Biomass Burning and Anthropogenic Secondary Organic Aerosol, Environ. Sci. Technol., 56, 15389–15397, https://doi.org/10.1021/acs.est.2c04554, 2022. a, b, c, d, e, f
Canonica, S. and Laubscher, H.-U.: Inhibitory effect of dissolved organic matter on triplet-induced oxidation of aquatic contaminants, Photoch. Photobio. Sci., 7, 547–551, https://doi.org/10.1039/b719982a, 2008. a
Canonica, S., Jans, U., Stemmler, K., and Hoigne, J.: Transformation kinetics of phenols in water: photosensitization by dissolved natural organic material and aromatic ketones, Environ. Sci. Technol., 29, 1822–1831, https://doi.org/10.1021/es00007a020, 1995. a
Canonica, S., Hellrung, B., and Wirz, J.: Oxidation of phenols by triplet aromatic ketones in aqueous solution, J. Phys. Chem. A, 104, 1226–1232, https://doi.org/10.1021/jp9930550, 2000. a
Chen, J., Li, C., Ristovski, Z., Milic, A., Gu, Y., Islam, M. S., Wang, S., Hao, J., Zhang, H., He, C., Guo, H., Fu, H., Miljevic, B., Morawska, L., Thai, P., LAM, Y. F., Pereira, G., Ding, A., Huang, X., and Dumka, U. C.: A review of biomass burning: Emissions and impacts on air quality, health and climate in China, Sci. Total Environ., 579, 1000–1034, https://doi.org/10.1016/j.scitotenv.2016.11.025, 2017. a, b
Chen, Q. C., Mu, Z., Xu, L., Wang, M. M., Wang, J., Shan, M., Fan, X. J., Song, J. Z., Wang, Y. Q., Lin, P. C., and Du, L.: Triplet-state organic matter in atmospheric aerosols: Formation characteristics and potential effects on aerosol aging, Atmos. Environ., 252, 118343, https://doi.org/10.1016/j.atmosenv.2021.118343, 2021. a, b, c
Chen, X. and Yu, J. Z.: Measurement of organic mass to organic carbon ratio in ambient aerosol samples using a gravimetric technique in combination with chemical analysis, Atmos. Environ., 41, 8857–8864, 2007. a
Chow, W. S., Liao, K., Huang, X. H. H., Leung, K. F., Lau, A. K. H., and Yu, J. Z.: Measurement report: The 10-year trend of PM2.5 major components and source tracers from 2008 to 2017 in an urban site of Hong Kong, China, Atmos. Chem. Phys., 22, 11557–11577, https://doi.org/10.5194/acp-22-11557-2022, 2022. a, b
Cote, C. D., Schneider, S. R., Lyu, M., Gao, S., Gan, L., Holod, A. J., Chou, T. H., and Styler, S. A.: Photochemical production of singlet oxygen by urban road dust, Environ. Sci. Technol. Letters, 5, 92–97, https://doi.org/10.1021/acs.estlett.7b00533, 2018. a, b
Cui, L., Wang, X. L., Ho, K. F., Gao, Y., Liu, C., Ho, S. S. H., Li, H. W., Lee, S. C., Wang, X. M., Jiang, B. Q., Huang, Y., Chow, J. C., Watson, J. G., and Chen, L. W.: Decrease of VOC emissions from vehicular emissions in Hong Kong from 2003 to 2015: Results from a tunnel study, Atmos. Environ., 177, 64–74, https://doi.org/10.1016/j.atmosenv.2018.01.020, 2018. a
Davis, C. A., McNeill, K., and Janssen, E. M.-L.: Non-singlet oxygen kinetic solvent isotope effects in aquatic photochemistry, Environ. Sci. Technol., 52, 9908–9916, https://doi.org/10.1021/acs.est.8b01512, 2018. a, b
De Haan, D. O., Tolbert, M. A., and Jimenez, J. L.: Atmospheric condensed-phase reactions of glyoxal with methylamine, Geophys. Res. Lett., 36, L11819, https://doi.org/10.1029/2009GL037441, 2009. a
De Haan, D. O., Hawkins, L. N., Kononenko, J. A., Turley, J. J., Corrigan, A. L., Tolbert, M. A., and Jimenez, J. L.: Formation of Nitrogen-Containing Oligomers by Methylglyoxal and Amines in Simulated Evaporating Cloud Droplets, Environ. Sci. Technol., 45, 984–991, https://doi.org/10.1021/es102933x, pMID: 21171623, 2011. a
Du, Z., He, K., Cheng, Y., Duan, F., Ma, Y., Liu, J., Zhang, X., Zheng, M., and Weber, R.: A yearlong study of water-soluble organic carbon in Beijing II: Light absorption properties, Atmos. Environ., 89, 235–241, https://doi.org/10.1016/j.atmosenv.2014.02.022, 2014. a
Erickson, P. R., Walpen, N., Guerard, J. J., Eustis, S. N., Arey, J. S., and McNeill, K.: Controlling factors in the rates of oxidation of anilines and phenols by triplet methylene blue in aqueous solution, J. Phys. Chem. A, 119, 3233–3243, https://doi.org/10.1021/jp511408f, 2015. a
Faust, B. C. and Allen, J. M.: Aqueous-phase photochemical sources of peroxyl radicals and singlet molecular oxygen in clouds and fog, J. Geophys. Res.-Atmos., 97, 12913–12926, https://doi.org/10.1029/92JD00843, 1992. a
Ghogare, A. A. and Greer, A.: Using singlet oxygen to synthesize natural products and drugs, Chem. Rev., 116, 9994–10034, 2016. a
Guo, H., Lee, S., Ho, K., Wang, X., and Zou, S.: Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong, Atmos. Environ., 37, 5307–5317, https://doi.org/10.1016/j.atmosenv.2003.09.011, 2003. a
Haag, W. R. and Hoigne, J.: Singlet oxygen in surface waters. 3. Photochemical formation and steady-state concentrations in various types of waters, Environ. Sci. Technol., 20, 341–348, 1986. a
Haan, D. O. D., Corrigan, A. L., Smith, K. W., Stroik, D. R., Turley, J. J., Lee, F. E., Tolbert, M. A., Jimenez, J. L., Cordova, K. E., and Ferrell, G. R.: Secondary Organic Aerosol-Forming Reactions of Glyoxal with Amino Acids, Environ. Sci. Technol., 43, 2818–2824, https://doi.org/10.1021/es803534f, pMID: 19475956, 2009. a
He, C., Wang, H., Gong, D., Lv, S., Wu, G., Wang, R., Chen, Y., Ding, Y., Li, Y., and Wang, B.: Insights into high concentrations of particle-bound imidazoles in the background atmosphere of southern China: Potential sources and influencing factors, Sci. Total Environ., 806, 150804, https://doi.org/10.1016/j.scitotenv.2021.150804, 2022. a
Helin, A., Virkkula, A., Backman, J., Pirjola, L., Sippula, O., Aakko-Saksa, P., Väätäinen, S., Mylläri, F., Järvi- nen, A., Bloss, M., Aurela, M., Jakobi, G., Karjalainen, P., Zimmermann, R., Jokiniemi, J., Saarikoski, S., Tissari, J., Rönkkö, T., Niemi, J. V., and Timonen, H.: Variation of absorption Ångström exponent in aerosols from different emission sources, J. Geophys. Res.-Atmos., 126, e2020JD034094, https://doi.org/10.1029/2020JD034094, 2021. a
Hems, R. F., Schnitzler, E. G., Liu-Kang, C., Cappa, C. D., and Abbatt, J. P.: Aging of atmospheric brown carbon aerosol, ACS Earth and Space Chemistry, 5, 722–748, https://doi.org/10.1021/acsearthspacechem.0c00346, 2021. a
Herckes, P., Valsaraj, K. T., and Collett Jr, J. L.: A review of observations of organic matter in fogs and clouds: Origin, processing and fate, Atmos. Res., 132, 434–449, https://doi.org/10.1016/j.atmosres.2013.06.005, 2013. a
Herrmann, H., Schaefer, T., Tilgner, A., Styler, S. A., Weller, C., Teich, M., and Otto, T.: Tropospheric aqueous-phase chemistry: kinetics, mechanisms, and its coupling to a changing gas phase, Chem. Rev., 115, 4259–4334, https://doi.org/10.1021/cr500447k, 2015. a
Herzberg, L. and Herzberg, G.: Fine Structure of the Infrared Atmospheric Oxygen Bands, Astrophys. J., 105, 353, https://doi.org/10.1086/144910, 1947. a
Huang, R.-J., Yang, L., Shen, J., Yuan, W., Gong, Y., Guo, J., Cao, W., Duan, J., Ni, H., Zhu, C., Dai, W., Li, Y., Chen, Y., Chen, Q., Wu, Y., Zhang, R., Dusek, U., O'Dowd, C., and Hoffmann, T.: Water-Insoluble Organics Dominate Brown Carbon in Wintertime Urban Aerosol of China: Chemical Characteristics and Optical Properties, Environ. Sci. Technol., 54, 7836–7847, https://doi.org/10.1021/acs.est.0c01149, pMID: 32479722, 2020. a
Huang, X. H. H., Bian, Q. J., Ng, W. M., Louie, P. K. K., and Yu, J. Z.: Characterization of PM2.5 major components and source investigation in suburban Hong Kong: a one year monitoring study, Aerosol Air Qual. Res., 14, 237–250, https://doi.org/10.4209/aaqr.2013.01.0020, 2014. a, b, c, d, e
Kampf, C. J., Jakob, R., and Hoffmann, T.: Identification and characterization of aging products in the glyoxal/ammonium sulfate system – implications for light-absorbing material in atmospheric aerosols, Atmos. Chem. Phys., 12, 6323–6333, https://doi.org/10.5194/acp-12-6323-2012, 2012. a
Kaur, R. and Anastasio, C.: Light absorption and the photoformation of hydroxyl radical and singlet oxygen in fog waters, Atmos. Environ., 164, 387–397, https://doi.org/10.1016/j.atmosenv.2017.06.006, 2017. a, b, c, d, e, f, g
Kaur, R. and Anastasio, C.: First measurements of organic triplet excited states in atmospheric waters, Environ. Sci. Technol., 52, 5218–5226, https://doi.org/10.1021/acs.est.7b06699, 2018. a, b, c, d, e, f, g, h, i, j, k
Kaur, R., Labins, J. R., Helbock, S. S., Jiang, W., Bein, K. J., Zhang, Q., and Anastasio, C.: Photooxidants from brown carbon and other chromophores in illuminated particle extracts, Atmos. Chem. Phys., 19, 6579–6594, https://doi.org/10.5194/acp-19-6579-2019, 2019. a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r, s, t, u, v, w
Kirchstetter, T. W., Novakov, T., and Hobbs, P. V.: Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon, J. Geophys. Res.-Atmos., 109, D21208, https://doi.org/10.1029/2004JD004999, 2004. a
Laskin, A., Laskin, J., and Nizkorodov, S. A.: Chemistry of atmospheric brown carbon, Chem. Rev., 115, 4335–4382, https://doi.org/10.1021/cr5006167, 2015. a, b
Lathioor, E. C. and Leigh, W. J.: Bimolecular hydrogen abstraction from phenols by aromatic ketone triplets, Photochem. Photobiol., 82, 291–300, https://doi.org/10.1562/2005-06-20-RA-581, 2006. a
Leresche, F., McKay, G., Kurtz, T., von Gunten, U., Canonica, S., and Rosario-Ortiz, F. L.: Effects of Ozone on the Photochemical and Photophysical Properties of Dissolved Organic Matter, Environ. Sci. Technol., 53, 5622–5632, https://doi.org/10.1021/acs.est.8b06410, pMID: 31022348, 2019. a
Leresche, F., Salazar, J. R., Pfotenhauer, D. J., Hannigan, M. P., Majestic, B. J., and Rosario-Ortiz, F. L.: Photochemical aging of atmospheric particulate matter in the aqueous phase, Environ. Sci. Technol., 55, 13152–13163, https://doi.org/10.1021/acs.est.1c00978, 2021. a, b, c, d, e, f, g, h
Li, J., Chen, Q., and Guan, D.: Insights into the triplet photochemistry of atmospheric aerosol and subfractions isolated with different polarity, Atmos. Environ., 290, 119375, https://doi.org/10.1016/j.atmosenv.2022.119375, 2022. a
Li, N., Fu, T.-M., Cao, J., Lee, S., Huang, X.-F., He, L.-Y., Ho, K.-F., Fu, J. S., and Lam, Y.-F.: Sources of secondary organic aerosols in the Pearl River Delta region in fall: Contributions from the aqueous reactive uptake of dicarbonyls, Atmos. Environ., 76, 200–207, https://doi.org/10.1016/j.atmosenv.2012.12.005, 2013. a
Li, Y. J., Lee, B. P., Su, L., Fung, J. C. H., and Chan, C. K.: Seasonal characteristics of fine particulate matter (PM) based on high-resolution time-of-flight aerosol mass spectrometric (HR-ToF-AMS) measurements at the HKUST Supersite in Hong Kong, Atmos. Chem. Phys., 15, 37–53, https://doi.org/10.5194/acp-15-37-2015, 2015. a, b
Li, Y. J., Lee, B. Y. L., Yu, J. Z., Ng, N. L., and Chan, C. K.: Evaluating the degree of oxygenation of organic aerosol during foggy and hazy days in Hong Kong using high-resolution time-of-flight aerosol mass spectrometry (HR-ToF-AMS), Atmos. Chem. Phys., 13, 8739–8753, https://doi.org/10.5194/acp-13-8739-2013, 2013. a
Li, Z., Xue, L., Yang, X., Zha, Q., Tham, Y. J., Yan, C., Louie, P. K., Luk, C. W., Wang, T., and Wang, W.: Oxidizing capacity of the rural atmosphere in Hong Kong, Southern China, Sci. Total Environ., 612, 1114–1122, https://doi.org/10.1016/j.scitotenv.2017.08.310, 2018. a
Lian, X. F., Tang, G. G., Dao, X., Hu, X. D., Xiong, X., Zhang, G. H., Wang, Z. H., Cheng, C. L., Wang, X. F., Bi, X. H., Li, L., Li, M., and Zhou, Z.: Seasonal variations of imidazoles in urban areas of Beijing and Guangzhou, China by single particle mass spectrometry, Sci. Total Environ., 844, 156995, https://doi.org/10.1016/j.scitotenv.2022.156995, 2022. a
Liao, H. and Seinfeld, J. H.: Global impacts of gas-phase chemistry-aerosol interactions on direct radiative forcing by anthropogenic aerosols and ozone, J. Geophys. Res.-Atmos., 110, D18208, https://doi.org/10.1029/2005JD005907, 2005. a
Liao, Z., Ling, Z., Gao, M., Sun, J., Zhao, W., Ma, P., Quan, J., and Fan, S.: Tropospheric Ozone Variability Over Hong Kong Based on Recent 20 years (2000–2019) Ozonesonde Observation, J. Geophys. Res.-Atmos., 126, e2020JD033054, https://doi.org/10.1029/2020JD033054, 2021. a
Louie, P. K. K., Chow, J. C., Chen, L.-W. A., Watson, J. G., Leung, G., and Sin, D. W. M.: PM2.5 chemical composition in Hong Kong: urban and regional variations, Sci. Total Environ., 338, 267–281, https://doi.org/10.1016/j.scitotenv.2004.07.021, 2005a. a, b
Louie, P. K. K., Watson, J. G., Chow, J. C., Chen, A., Sin, D. W., and Lau, A. K.: Seasonal characteristics and regional transport of PM2.5 in Hong Kong, Atmos. Environ., 39, 1695–1710, https://doi.org/10.1016/j.atmosenv.2004.11.017, 2005b. a, b
Lyu, Y., Lam, Y. H., Li, Y., Borduas-Dedekind, N., and Nah, T.: Efficient production of singlet oxygen and organic triplet excited states in aqueous PM2.5 in Hong Kong, South China, Version 1, Zenodo [data set], https://doi.org/10.5281/zenodo.7827983, 2023. a
Ma, L., Guzman, C., Niedek, C., Tran, T., Zhang, Q., and Anastasio, C.: Kinetics and mass yields of aqueous secondary organic aerosol from highly substituted phenols reacting with a triplet excited state, Environ. Sci. Technol., 55, 5772–5781, 2021. a
Ma, L., Worland, R., Heinlein, L., Guzman, C., Jiang, W., Niedek, C., Bein, K. J., Zhang, Q., and Anastasio, C.: Seasonal variations in photooxidant formation and light absorption in aqueous extracts of ambient particles, EGUsphere [preprint], https://doi.org/10.5194/egusphere-2023-861, 2023a. a, b
Ma, L., Worland, R., Jiang, W., Niedek, C., Guzman, C., Bein, K. J., Zhang, Q., and Anastasio, C.: Predicting photooxidant concentrations in aerosol liquid water based on laboratory extracts of ambient particles, Atmos. Chem. Phys., 23, 8805–8821, https://doi.org/10.5194/acp-23-8805-2023, 2023b. a, b, c, d, e, f, g, h, i, j, k, l, m
Ma, L., Worland, R., Tran, T., and Anastasio, C.: Evaluation of Probes to Measure Oxidizing Organic Triplet Excited States in Aerosol Liquid Water, Environ. Sci. Technol., 57, 6052–6062, https://doi.org/10.1021/acs.est.2c09672, 2023c. a, b, c, d, e
Mabato, B. R. G., Lyu, Y., Ji, Y., Li, Y. J., Huang, D. D., Li, X., Nah, T., Lam, C. H., and Chan, C. K.: Aqueous secondary organic aerosol formation from the direct photosensitized oxidation of vanillin in the absence and presence of ammonium nitrate, Atmos. Chem. Phys., 22, 273–293, https://doi.org/10.5194/acp-22-273-2022, 2022. a
Mabato, B. R. G., Li, Y. J., Huang, D. D., Wang, Y., and Chan, C. K.: Comparison of aqueous secondary organic aerosol (aqSOA) product distributions from guaiacol oxidation by non-phenolic and phenolic methoxybenzaldehydes as photosensitizers in the absence and presence of ammonium nitrate, Atmos. Chem. Phys., 23, 2859–2875, https://doi.org/10.5194/acp-23-2859-2023, 2023. a
Maizel, A. C. and Remucal, C. K.: The effect of probe choice and solution conditions on the apparent photoreactivity of dissolved organic matter, Environ. Sci.-Proc. Imp., 19, 1040–1050, 2017. a, b, c, d
Manfrin, A., Nizkorodov, S. A., Malecha, K. T., Getzinger, G. J., McNeill, K., and Borduas-Dedekind, N.: Reactive oxygen species production from secondary organic aerosols: the importance of singlet oxygen, Environ. Sci. Technol., 53, 8553–8562, https://doi.org/10.1021/acs.est.9b01609, 2019. a, b, c, d, e, f, g, h
McCabe, A. J. and Arnold, W. A.: Reactivity of triplet excited states of dissolved natural organic matter in stormflow from mixed-use watersheds, Environ. Sci. Technol., 51, 9718–9728, https://doi.org/10.1021/acs.est.7b01914, 2017. a
McNeill, K. and Canonica, S.: Triplet state dissolved organic matter in aquatic photochemistry: reaction mechanisms, substrate scope, and photophysical properties, Environ. Sci.-Proc. Imp., 18, 1381–1399, https://doi.org/10.1039/C6EM00408C, 2016. a, b, c, d
Nah, T. and Lam, Y. H.: Influence of urban heat islands on seasonal aerosol acidity and aerosol liquid water content in humid subtropical Hong Kong, South China, Atmos. Environ., 289, 119321, https://doi.org/10.1016/j.atmosenv.2022.119321, 2022. a
Nah, T., Lam, Y. H., Yang, J., and Yang, L.: Long-term trends and sensitivities of PM2.5 pH and aerosol liquid water to chemical composition changes and meteorological parameters in Hong Kong, South China: Insights from 10-year records from three urban sites, Atmos. Environ., 302, 119725, https://doi.org/10.1016/j.atmosenv.2023.119725, 2023. a
Nguyen, T. K. V., Zhang, Q., Jimenez, J. L., Pike, M., and Carlton, A. G.: Liquid water: Ubiquitous contributor to aerosol mass, Environ. Sci. Technol. Letters, 3, 257–263, https://doi.org/10.1021/acs.estlett.6b00167, 2016. a
Nolte, T. M. and Peijnenburg, W. J.: Aqueous-phase photooxygenation of enes, amines, sulfides and polycyclic aromatics by singlet (a1Δg) oxygen: prediction of rate constants using orbital energies, substituent factors and quantitative structure–property relationships, Environ. Chem., 14, 442–450, https://doi.org/10.1071/EN17155, 2018. a
Ossola, R., Jönsson, O. M., Moor, K., and McNeill, K.: Singlet oxygen quantum yields in environmental waters, Chem. Rev., 121, 4100–4146, 2021. a, b
Pathak, R. K., Yao, X., Lau, A. K., and Chan, C. K.: Acidity and concentrations of ionic species of PM2.5 in Hong Kong, Atmos. Environ., 37, 1113–1124, https://doi.org/10.1016/S1352-2310(02)00958-5, 2003. a, b
Powelson, M. H., Espelien, B. M., Hawkins, L. N., Galloway, M. M., and De Haan, D. O.: Brown Carbon Formation by Aqueous-Phase Carbonyl Compound Reactions with Amines and Ammonium Sulfate, Environ. Sci. Technol., 48, 985–993, https://doi.org/10.1021/es4038325, pMID: 24351110, 2014. a
Rounds, S. A., Wilde, F. D., and Ritz, G. F.: Dissolved oxygen (ver. 3.0), U.S. Geological Survey Techniques of Water-Resources Investigations, Reston, Virginia, book 9, chap. A6.2, https://doi.org/10.3133/twri09A6.2, 2013. a
Seinfeld, J. H. and Pandis, S. N.: Atmospheric chemistry and physics: from air pollution to climate change, John Wiley & Sons, New York, ISBN: 978-1-118-94740-1, 2016. a
Tanner, P. A. and Law, P.-T.: Effects of synoptic weather systems upon the air quality in an Asian megacity, Water Air Soil Poll., 136, 105–124, https://doi.org/10.1023/A:1015275404592, 2002. a, b
Tratnyek, P. G. and Hoigne, J.: Oxidation of substituted phenols in the environment: a QSAR analysis of rate constants for reaction with singlet oxygen, Environ. Sci. Technol., 25, 1596–1604, https://doi.org/10.1021/es00021a011, 1991. a
Tsentalovich, Y. P., Lopez, J., Hore, P., and Sagdeev, R.: Mechanisms of reactions of flavin mononucleotide triplet with aromatic amino acids, Spectrochim. Acta A, 58, 2043–2050, https://doi.org/10.1016/S1386-1425(01)00652-7, 2002. a
Walling, C. and Gibian, M. J.: Hydrogen Abstraction Reactions by the Triplet States of Ketones1, J. Am. Chem. Soc., 87, 3361–3364, https://doi.org/10.1021/ja01093a014, 1965. a
Wang, Q., Zhou, Y., Ma, N., Zhu, Y., Zhao, X., Zhu, S., Tao, J., Hong, J., Wu, W., Cheng, Y., and Su, H.: Review of Brown Carbon Aerosols in China: Pollution Level, Optical Properties, and Emissions, J. Geophys. Res.-Atmos., 127, e2021JD035473, https://doi.org/10.1029/2021JD035473, 2022. a
Weishaar, J. L., Aiken, G. R., Bergamaschi, B. A., Fram, M. S., Fujii, R., and Mopper, K.: Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon, Environ. Sci. Technol., 37, 4702–4708, https://doi.org/10.1021/es030360x, 2003. a
Wilkinson, F., Helman, W. P., and Ross, A. B.: Quantum yields for the photosensitized formation of the lowest electronically excited singlet state of molecular oxygen in solution, J. Phys. Chem. Ref. Data, 22, 113–262, 1993. a
Wong, Y. K., Huang, X. H. H., Louie, P. K. K., Yu, A. L. C., Chan, D. H. L., and Yu, J. Z.: Tracking separate contributions of diesel and gasoline vehicles to roadside PM2.5 through online monitoring of volatile organic compounds and PM2.5 organic and elemental carbon: a 6-year study in Hong Kong, Atmos. Chem. Phys., 20, 9871–9882, https://doi.org/10.5194/acp-20-9871-2020, 2020. a, b
Yang, J., Ma, L., He, X., Au, W. C., Miao, Y., Wang, W.-X., and Nah, T.: Measurement report: Abundance and fractional solubilities of aerosol metals in urban Hong Kong – insights into factors that control aerosol metal dissolution in an urban site in South China, Atmos. Chem. Phys., 23, 1403–1419, https://doi.org/10.5194/acp-23-1403-2023, 2023. a
Yihui, D. and Chan, J. C.: The East Asian summer monsoon: an overview, Meteorol. Atmos. Phys., 89, 117–142, https://doi.org/10.1007/s00703-005-0125-z, 2005. a
Yu, L., Smith, J., Laskin, A., Anastasio, C., Laskin, J., and Zhang, Q.: Chemical characterization of SOA formed from aqueous-phase reactions of phenols with the triplet excited state of carbonyl and hydroxyl radical, Atmos. Chem. Phys., 14, 13801–13816, https://doi.org/10.5194/acp-14-13801-2014, 2014. a
Yuan, J.-F., Huang, X.-F., Cao, L.-M., Cui, J., Zhu, Q., Huang, C.-N., Lan, Z.-J., and He, L.-Y.: Light absorption of brown carbon aerosol in the PRD region of China, Atmos. Chem. Phys., 16, 1433–1443, https://doi.org/10.5194/acp-16-1433-2016, 2016. a
Zepp, R. G., Schlotzhauer, P. F., and Sink, R. M.: Photosensitized transformations involving electronic energy transfer in natural waters: role of humic substances, Environ. Sci. Technol., 19, 74–81, https://doi.org/10.1021/es00131a008, 1985. a
Zhang, X., Yuan, Z., Li, W., Lau, A. K., Yu, J. Z., Fung, J. C., Zheng, J., and Alfred, L.: Eighteen-year trends of local and non-local impacts to ambient PM10 in Hong Kong based on chemical speciation and source apportionment, Atmos. Res., 214, 1–9, https://doi.org/10.1016/j.atmosres.2018.07.004, 2018. a, b