the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Large simulated future changes in the nitrate radical under the CMIP6 SSP scenarios: implications for oxidation chemistry
Scott Archer-Nicholls
Rachel Allen
Nathan L. Abraham
Paul T. Griffiths
The nitrate radical (NO3) plays an important role in the chemistry of the lower troposphere, acting as the principle oxidant during the night together with ozone. Previous model simulations suggest that the levels of NO3 have increased dramatically since the preindustrial period. Here, we show projections of the evolution of the NO3 radical from 1850–2100 using the United Kingdom Earth System Model (UKESM1) under the Coupled Model Intercomparison Project Phase 6 (CMIP6) shared socioeconomic pathway (SSP) scenarios. Our model results highlight diverse trajectories for NO3, with some scenarios and regions undergoing rapid growth of NO3 to unprecedented levels over the course of the 21st century and others seeing sharp declines. The local increases in NO3 (up to 40 ppt above the preindustrial base line) are driven not only by local changes in emissions of nitrogen oxides but have an important climate component, with NO3 being favoured in warmer future climates. The changes in NO3 lead to changes in the oxidation of important secondary organic aerosol precursors, with potential impacts on particulate matter pollution regionally and globally. This work highlights the potential for substantial future growth in NO3 and the need to better understand the formation of secondary organic aerosol (SOA) from NO3 to accurately predict future air quality and climate implications.
- Article
(3381 KB) - Full-text XML
-
Supplement
(7258 KB) - BibTeX
- EndNote
Whilst nitrogen is ubiquitous in the atmosphere, the majority of gaseous nitrogen-containing molecules are chemically inert. Reactive nitrogen species (NOy) make up a much smaller fraction but encompass a diverse set of molecules that play a paramount role in the chemistry of the atmosphere. Nitrogen oxide (NO) and nitrogen dioxide (NO2), collectively known as NOx, are essential for the catalytic formation of ozone (O3) in the troposphere, a key air pollutant and greenhouse gas (Monks et al., 2015). The reaction of NO2 with O3 produces the nitrate radical (NO3):
During the daytime, NO3 can undergo rapid photolysis or reaction with NO, resulting in a very short lifetime, typically in the order of seconds (Wayne et al., 1991). However, at nighttime, in regions of high NOx, NO3 is able to persist and become the major oxidant of volatile organic compounds (VOCs) (the hydroxyl radical (OH) and ozone (O3) dominate this oxidation during the daytime), acting as the most important oxidant during the night (Brown and Stutz, 2012). NO3 also undergoes a reversible reaction with NO2, forming a thermal equilibrium with dinitrogen pentoxide (N2O5):
N2O5 has a short lifetime with respect to decomposition at typical atmospheric boundary layer temperatures. As temperature increases, the rate of decomposition of N2O5 increases resulting in a greater fraction of reactive nitrogen in the form of NO3. This temperature dependence in the equilibrium between NO2, NO3 and N2O5 is further driven by the extreme temperature dependence in the formation of NO3 through Reaction (R1). Due to their tight chemical coupling, NO3 and N2O5 have been termed the NxOy family (NxOy = NO3 + N2O5) (Stone et al., 2014). In this sense, sinks of N2O5 lead to corresponding indirect loss of NO3. Loss of NxOy has important implications for tropospheric ozone as it is an important nighttime reservoir of NOx and Ox (O + O3 + NO2 + others) (Archibald et al., 2020a). In the daytime, any remaining NxOy is converted back to NOx; therefore nighttime sinks of NxOy reduce the levels of NOx available to photochemically form ozone in the troposphere. The heterogeneous hydrolysis of N2O5, which occurs readily on aerosol surfaces, is one of the major nighttime sinks of reactive nitrogen in the troposphere (e.g. Riemer et al., 2003).
NO3 reactions with alkenes proceed rapidly via the addition of NO3 to the double bond (Wayne et al., 1991; Brown and Stutz, 2012). As a result, the oxidation of biogenic volatile organic compounds (BVOCs), in particular terpenes, which are emitted in vast quantities by the world's forests (Sakulyanontvittaya et al., 2008), is sensitive to the levels of NO3 present (Ng et al., 2017). BVOC oxidation by NO3 can lead to the formation of secondary organic aerosol (SOA) (Fry et al., 2009; Ng et al., 2017), fine aerosols that have implications for the planetary radiation budget and human health (Pöschl, 2005). The extent that NO3-initiated chemistry contributes to SOA formation (relative to O3 and OH-initiated production) depends on the molecular structure of the BVOC (and therefore their rate coefficients for reaction with NO3), the rate of their emissions and the levels of NO3 present.
Ng et al. (2017) highlight that there are significant gaps in our understanding of the NO3-initiated oxidation of BVOCs. BVOC oxidation chemistry has been studied for many decades (e.g. Brewer et al., 1984); however there are relatively few mechanistic studies of the products of NO3-initiated oxidation (e.g. Boyd et al., 2015; Fry et al., 2009; Faxon et al., 2018; Ehn et al., 2017) compared to OH and O3 (e.g. Atkinson and Arey, 2003; McGillen et al., 2020). Nonetheless there is mounting evidence that NO3 oxidation could be a significant contributor to SOA formation. Kiendler-Scharr et al. (2016) highlight the ubiquity of organic nitrates in submicron aerosol collected in European nighttime urban conditions, whilst Hamilton et al. (2021) recently found NO3 oxidation of isoprene to be a significant source of SOA production in Beijing. Recent studies show that highly oxidised molecules (HOMs), formed from the auto-oxidation and accretion of monoterpene oxidation products, are capable of nucleating new aerosol particles without sulfate seeds (Ehn et al., 2014; Tröstl et al., 2016; Bianchi et al., 2019) with important implications for large-scale feedbacks between the climate and the biosphere (Scott et al., 2017; Gordon et al., 2017). Recently, Zhao et al. (2021) have shown that HOMs from NO3-initiated oxidation of isoprene may contribute to a significant fraction of the isoprene SOA yield. But these complex processes are so far lacking from Earth system and global chemistry-transport models.
Oxidation of BVOCs is dependent on changes to the oxidant budget (with respect to OH, O3 and NO3) and to the changes in the emissions of BVOCs themselves. Our understanding of the processes which control emissions of BVOCs highlights that these have important dependences on climatic parameters including CO2 concentrations (Arneth et al., 2007), land use, temperature, humidity and precipitation (Arneth et al., 2008). Whilst BVOC emissions models are reasonably well constrained in the present day, past and future trends are less certain, with some models indicating large increases in BVOC emissions in the future (Pacifico et al., 2012) and others small to no increases (Hantson et al., 2017).
Estimates of changes in the levels of NO3 from the preindustrial period to the present day indicate significant increases, on the order of 100 %–1000 % (Khan et al., 2015), driven by changes in emissions of NOx. The formation of NO3 is also dependent on ozone burdens which are likely to change in the future (Archibald et al., 2020a; Griffiths et al., 2021). In addition, the fraction of NxOy in the form of NO3 increases with temperature, while the main sinks are determined by the amount of N2O5 which decreases with temperature. Ng et al. (2017) postulated that NO3 would increase with increasing NOx emissions, but given the complex chemical processes at work NO3 is likely to vary nonlinearly with NOx emissions. To our knowledge, no study has investigated how the NO3 radical may change in the future under changing NOx emissions and a changing climate or how these changes will affect BVOC oxidation and SOA formation given changing terpene emissions.
This study presents results on the historic and future evolution of NO3 using simulations from 1850–2100 made with the United Kingdom Earth System Model (UKESM1) (Sellar et al., 2019; Archibald et al., 2020b). The simulations, based on the Coupled Model Intercomparison Project Phase 6 (CMIP6) Historic and ScenarioMIP scenarios (O'Neill et al., 2016), indicate that there are substantial changes in NO3 simulated in the future at both the global mean and regional level, depending on the emission scenarios and resulting climate considered. Using these simulations we show that future trends in NO3 have an impact on the regional oxidation of BVOCs and as a result the SOA budget and burden. Taking a particular focus on changes occurring over South Asia, a region with severe present-day air quality issues and large variation in how emissions will evolve in this region under different scenarios, we highlight an important role for enhanced levels of NO3-initiated BVOC oxidation in the future.
2.1 Description of UKESM1
The model simulations make use of the new UK Earth System Model (UKESM1) (Sellar et al., 2019). This is a fully coupled Earth system model using the Global Atmosphere 7.1/Global Land 7.0 (GA7.1/GL7.1; Walters et al., 2019). The dynamical model is coupled with the United Kingdom Chemistry and Aerosol model (UKCA), using the StratTrop mechanism for gas-phase chemistry (Archibald et al., 2020b) and the two-moment GLOMAP-mode aerosol scheme (Mulcahy et al., 2020). UKCA and UKESM1 are designed to quantify the response of the Earth system to forcings and simulate nascent feedbacks that exist in the coupled chemistry–aerosol–climate system. As such, pragmatic decisions have been made to ensure that as complete a representation as possible of many processes is included in the model. For the chemistry of N2O5, this includes a fairly basic description of its chemistry in the gas phase and its loss to aerosol surfaces (heterogeneous chemistry) is simplified, with a fixed uptake coefficient being used (γ = 0.1). Jones et al. (2021) suggest that the simplified treatment of heterogeneous uptake of N2O5 leads to an overestimate of the loss of N2O5 based on a comparison of modelled and measured HNO3 across the USA. McDuffie et al. (2018) have determined a median γ = 0.0143 (range: 2 × 10−5 to 0.1751) based on constrained modelling of aircraft observations. The inclusion of this median uptake coefficient would be likely to lead to an increase in the atmospheric mixing ratio of NxOy, and thus our results can be seen as lower bounds on the potential changes in [NO3]. UKCA does not include any in-particle chemistry for N2O5 and also lacks aerosol nitrate in the present implementation, similar to some other CMIP6 class models. The omission of aerosol nitrate is a weakness in the model, but recent work including this process (Jones et al., 2021) suggests that the effects of it are small on NxOy.
The experimental setup is that of the simulations conducted as part of the Coupled Model Intercomparison Project Phase 6 (CMIP6; Eyring et al., 2016) DECK and ScenarioMIP experiments. Historical emissions are from the Community Emissions Data System (CEDS; Hoesly et al., 2018). Future emissions progress along one of four benchmark shared socioeconomic pathways (SSPs; Gidden et al., 2019). The analysis focuses on four representative pathways: SSP1-2.6, SSP2-4.5, SSP3-7.0 and SSP5-8.5. Each SSP has different assumptions about the amount of emissions of air pollutant precursors and climate forcers (Gidden et al., 2019). We perform our analyses focused on 5-year periods (preindustrial, PI: 1850–1854; present day, PD: 2010–2014; end of century: 2090–2094). These were conducted by performing new simulations using re-start files from the start dumps from the core CMIP6 simulations contributed by UKESM1. It was necessary to re-run the CMIP6 simulations with UKESM1 in order to (i) provide high temporal-resolution output with additional diagnostics for the NO3 reactions which did not exist in the CMIP6 runs, needed to assess the oxidation of Monoterp and isoprene, and (ii) include a correction to the NO3 + Monoterp reaction rate coefficient.
2.2 Description of relevant chemical reactions
The representation of BVOC chemistry in the StratTrop chemical mechanism used in UKESM1 is similar to other Earth system models in being very simplified. Isoprene is treated as an individual compound undergoing reactions with OH, O3 and NO3, while monoterpenes are represented by a surrogate species, Monoterp, which can undergo oxidation via OH, NO3 or O3 with rate coefficients equal to the equivalent oxidation reactions of α-pinene to form an inert species, SEC_ORG, which irreversibly condenses to form SOA (Mann et al., 2010). This simplification has been shown to broadly capture the relation between BVOC emissions, SOA formation and the climate impacts well (Scott et al., 2017; Mulcahy et al., 2020).
where F is some factor between 0 and 1 representing the yield of SOA production from Monoterp. The assumed yield of SEC_ORG from Monoterp is 0.13 but is doubled to 0.26 in order to account for the missing production from isoprene oxidation (Mann et al., 2010; Mulcahy et al., 2020). The rate coefficients kOH, and are equal to the rate coefficients for the reactions between α-pinene and OH, O3 and NO3, respectively, from Atkinson et al. (1989). Due to an error, the reaction rate coefficient activation energy for the NO3 + Monoterp reaction was incorrect in the original version of UKESM1 and has been corrected for these experiments. The rate coefficient used in all previous studies using StratTrop with the GLOMAP aerosol was
whereas the correct form is
At T = 298 K, this difference in activation energy results in being 4.3 times faster than . This correction results in a smaller fraction of Monoterp being oxidised by NO3 compared to OH and O3, partly compensated for by an increase in NO3 burden (see Supplement further details).
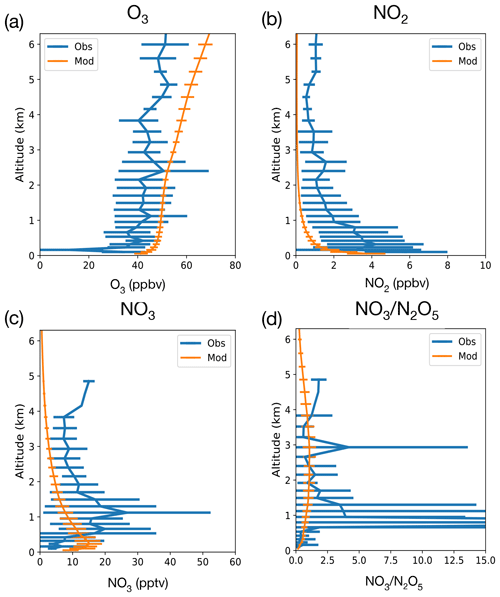
Figure 1Comparison of vertical profile of nighttime concentrations of O3 (a), NO2 (b), NO3 (c) and the NO3:N2O5 ratio (d) from the RONOCO July 2010 flight campaign and 2010–2014 July nighttime values from UKESM1 simulations from grid cells corresponding to flight regions. Lines show means, and error bars show standard deviation.
3.1 Comparison of present-day modelled and observed NO3
Our analysis begins by evaluating the performance of UKESM1 against aircraft observations and pre-compiled ground-based observations presented in Khan et al. (2015). Figure 1 shows the comparison of UKESM1 modelled vertical profiles of O3, NO2, NO3 and NO3:N2O5 against observations made in the eastern UK, over the North Sea, as part of the RONOCO summer campaign that took place in July 2010 (Stone et al., 2014). UKESM1 output is not constrained by meteorological forcing, so we sample the model during July 2010–2014, outputting model data once every 27 h and sampling data between 21:00 and 03:00 LT to generate a nighttime climatology for July, which is plotted in orange in Fig. 1 with the error bars reflecting the variability (1σ) in the model climatology. In general the model simulates the vertical profile of the RONOCO observations well. There is a positive bias in modelled O3 and a negative bias in modelled NO2 (consistent with previous analyses with UKCA the chemistry component of UKESM1; e.g. Archibald et al., 2020b). These biases partly offset each other; the vertical profile of the rate of production of NO3 (Reaction R1) is in good qualitative agreement between the model and observations, with maxima found above the surface below 1 km, but the absolute magnitude of the NO3 production rate is underestimated in the model by a factor of ∼ 2 in this region. One reason for the model disagreement is likely the resolution, whereby UKESM1 is unable to simulate the fine plumes of NO2 that drive NO3 production that were observed during the RONOCO campaign (Stone et al., 2014). In addition to the poor horizontal resolution, models like UKESM1 also suffer from biases in vertical resolution and mixing. The simulation of boundary layer height in models like UKESM1 is difficult, and Fig. 1 suggests that the model boundary layer height is much lower than the observations. This would make sense if in the model there is a significant land fraction in the grid boxes being analysed, as is the case.
Table 1Monthly average values from of NO3 mixing ratios (ppt) from UKESM1 compared with a review of NO3 measurements and STOCHEM model output adapted from Table 3 in Khan et al. (2015).
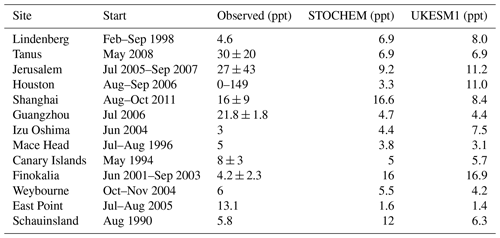
Table 1 compares results from the Historical simulation of UKESM1 against the STOCHEM-CRI model data from Khan et al. (2015) and a synthesis of surface observations; see Khan et al. (2015) for details and references for observational data. Our model data are averages from the 2010–2014 period corresponding to the months of each campaign and are in good qualitative and quantitative agreement with the observations (which come from a range of different techniques and cover a range of different dates over the period of ca. the 1990s–2010s; see Khan et al. (2015) for details). The largest disagreement in Table 1 is at Guangzhou, China, where both models predict much lower levels of NO3 than were observed using a long-path DOAS (differential optical absorption spectroscopy) instrument (Li et al., 2012). Despite being of low horizontal and vertical resolution and in light of the caveats already discussed through the analysis of Fig. 1, we find that UKESM1 reproduces well current observations of NO3 suggesting it is a suitable tool for simulating past and future changes in the NO3 burden and distribution.
3.2 Modelled past and future changes in O3, NOx and NO3
Our analysis focuses on 5-year periods from the simulations, comparing present-day (PD; 2010–2014) and preindustrial (PI; 1850–1854) periods with end of century (2090–2094) predictions under four shared socioeconomic pathways (SSPs) as used for CMIP6. Data for these 5-year periods were collected by re-running the UKESM1 CMIP6 simulations with additional diagnostics for analysis and corrected NO3 + Monoterp reaction rate coefficient (see the Methods section for details). The future emission pathways we studied, described in more detail in Gidden et al. (2019), are in short the following: SSP1-2.6, a 2 ∘C world with a focus on sustainability, economic growth and connectivity and low population growth driven by low-carbon technology and energy efficiency; SSP2-4.5, a middle-of-the-road scenario with slower convergence of income levels, greater population growth and reliance on fossil fuels further into the century; SSP3-7.0, a regional rivalry scenario with increased inequality and high population growth in low- to middle-income countries; and SSP5-8.5, similar to SSP1 in terms of population and economic growth but driven by increasing unabated use of energy and fossil fuels. Although SSP5-8.5 leads to the greatest increase in long-lived greenhouse gas emissions, and therefore global warming, by the end of the century, SSP3-7.0 has the highest emissions of short-lived air pollutants (Rao et al., 2017). Emissions of BVOCs also change between these scenarios as they are interactive in UKESM1 (Sellar et al., 2019). BVOC emissions are higher in the PI compared to the PD due to changing land use, reducing forested area over the 20th century. In the future SSP scenarios, BVOC emissions are predicted to increase as temperatures increase, in spite of the increasing CO2 levels (which, in the absence of other changes, would cause BVOC emissions to decrease; Arneth et al., 2008).
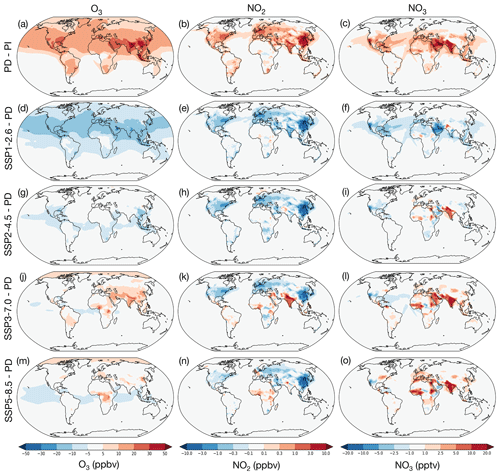
Figure 2Changes in O3, NO2 and NO3 mixing ratios averaged over lowest 1 km of the atmosphere, showing difference between the present-day (2010–2014) and the preindustrial period (1850–1854) (a–c), SSP1-2.6 (2090–2094) – PD (d–f), SSP2-4.5 (2090–2094) – PD (g–i), SSP3-7.0 (2090–2094) – PD (j–l), and SSP5-8.5 (2090–2094) – PD (m–o).
The results from the UKESM1 simulations show that large changes to monthly average O3, NO2 and NO3 levels have taken place in the lowest kilometre of the atmosphere from the PI to the PD (Fig. 2a–c). The region of the lowest kilometre is analysed here, rather than the surface level, because nighttime O3 and NO3 levels tend to be low at the surface due to rapid reaction with fresh emissions of NO and the low nighttime boundary layer. In both observations and the model, peak NO3 tends to occur a few hundred metres above the surface (e.g. Fish et al., 1999).
The main driver for the changes between the PI and PD periods is attributed to the increase in anthropogenic emissions, with emissions of NOx increasing by orders of magnitude over this period. The largest increases in NO2 and O3 occur over populated regions (Fig. 2 a and b). The changes in O3 simulated with UKESM1 are in good agreement with other CMIP6 models (Griffiths et al., 2021) and show an increase in the tropospheric burden of around 40 % from the PI–PD. NO3 is similarly much higher in the PD compared to the PI (Fig. 2c), an increase in tropospheric burden of approximately 75 %, in good agreement with previous global modelling studies (Khan et al., 2015) and with observations (see Sect. 2.1). To first order, NO3 is proportional to O3 × NO2 as these species drive its production, but there are some important deviations from this relationship. Firstly, concentrations are lower at high latitudes because colder climates mean more NO3 is partitioned into N2O5. Changes in NO3 are also suppressed in regions with high BVOC emissions, such as the south-east USA, South America and South-East Asia/South China, due to the high reactivity with these species. The greatest increases are over the Middle East and the Indian subcontinent – regions with high local temperatures, NOx emissions and O3 as well as relatively low BVOC emissions.
The simulated future changes in O3, NO2 and NO3 in the lowest 1 km depend greatly on the future SSP scenario. Focusing on the change between the future and the PD, the SSP1-2.6 scenario shows large reductions in O3, NO2 and NO3 across the world, especially in the Northern Hemisphere (Fig. 2d–f). These reductions are similar to but smaller than the increases in NO3 from the PI to the PD, leading to a tropospheric NO3 burden under SSP1-2.6 that is 26 % lower than the PD burden. In SSP2-4.5 (Fig. 2g–i) NO2 decreases in most regions, although to a lesser degree in some regions such as India. However, reductions in O3 are muted, with similar concentrations across most of the Northern Hemisphere, likely due to competing trends from changes to stratosphere-to-troposphere transport of ozone (driven by increases in the Brewer–Dobson circulation under climate change), lower NOx emissions (decreasing ozone), and higher temperatures and BVOC emissions (increasing ozone) (e.g. Archibald et al., 2020a). Changes in NO3 are spatially variable, with small reductions in North America and China but increasing levels in northern Europe, India and parts of Africa. In the SSP3-7.0 scenario (Fig. 2j–l), which simulates the greatest increase in emissions of short-lived pollutants, near-surface O3 is predicted to increase over most populated regions, particularly in South Asia and West Africa. In contrast, NO2 shows diverging trends, decreasing in North America, Europe and China but increasing considerably in India, the Middle East and West Africa. Over most parts of the land and oceans NO3 is predicted to not change significantly, but specific regions show dramatic increases: by over 10 ppt in West Africa, the Middle East and India (Fig. 2l). These large increases in NO3 are of a similar order to the PI–PD changes shown in Fig. 2c, effectively doubling near-surface NO3 concentrations in the future over many populated regions, whilst O3 increases are only in the order of 50 % greater than the PD. In SSP5-8.5 (Fig. 2m–o), the warmest future scenario, the increases in O3 in Asia are small and NO2 is predicted to remain similar to the present day in India whilst decreasing significantly in China. However, there is still a considerable increase in NO3 over India as well as West Africa and Europe predicted under the SSP5-8.5 scenario (in the order of 10–20 ppt increases above PD levels). These variations in trends between scenarios show that it is not sufficient to assume that lower NOx emissions in the future will result in reduced NO3. Rather, it is a complicated outcome also depending on other changes in the chemical environment and climate.
To investigate the role of changes in climate on NO3, through changes in temperature over the lowest 1 km of the atmosphere, the logarithm of Keq (Reaction R3) is plotted against the corresponding temperature values in Fig. 3, using data from each of the scenarios. This shows the strong dependence of Keq, in accordance with the Van 't Hoff relationship. As the climate warms the thermal equilibrium shifts such that more NxOy is found as NO3, increasing the amount of NO3 available for oxidation and reducing the sink due to the N2O5 heterogeneous reactions. UKESM1 simulates levels of warming by the end of the 21st century that are at the higher end of the CMIP6 multi-model mean but within the ensemble spread (Tebaldi et al., 2021).
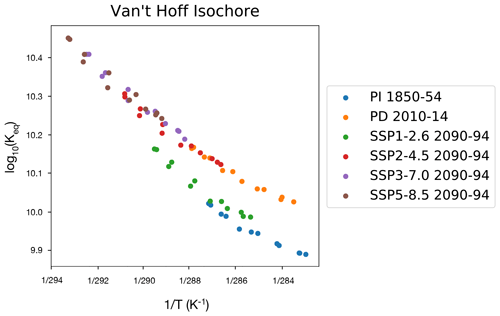
Figure 3Van 't Hoff isochore of the thermal equilibrium between NO3 and N2O5 (a) and change in temperature in UKESM1 simulations from 1850 to 2100 in four representative SSP scenarios and (b) changes in temperature from the historical simulation from 1850 to 2014 and along SSP scenarios from 2014 to 2100.
3.3 Changes to BVOC oxidation
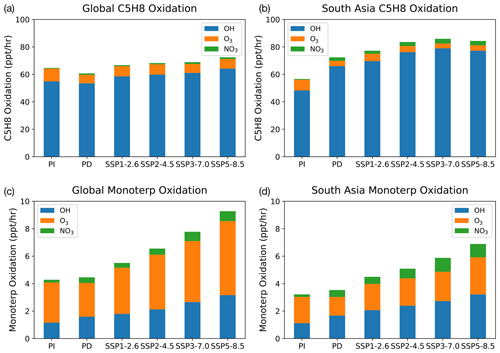
Figure 4 shows vertically integrated modelled oxidation fluxes for isoprene and Monoterp (the lumped monoterpene species in UKESM1) averaged across the globe and over southern Asia (SA; defined as 5∘ N, 50∘ E to 35∘ N, 95∘ E, based on the source–receptor region used by the Task Force on Hemispheric Transport of Air Pollution, TF HTAP, https://www.htap.org, last access: 1 March 2022; plots for the other TF HTAP regions are included in the Supplement). The modelled oxidation flux is calculated at each model chemistry time step (1 h), thereby taking into account the concentrations of the BVOC, oxidants and the temperature-dependent rate coefficients for their reactions, which are then averaged over each month for analysis. The fluxes are further averaged over the lowest 1 km of the atmosphere to reflect boundary layer oxidation in Fig. 4. In the PI atmosphere, NO3-initiated oxidation accounts for less than 1 % of isoprene and 4 % of Monoterp, due to the low NOx emissions in this period (Fig. 2b). This fraction of oxidation increases to 3 % and 13 % for isoprene and Monoterp, respectively, for the present day (i.e. a factor of 3 increase). UKESM1 predicts that monoterpene emissions increase in a warming climate in all scenarios, particularly in SSP3-7.0 and SSP5-8.5. Across the whole troposphere, the relative amount via NO3 stays the same in SSP3-7.0 relative to the PD, although the absolute amount increases as BVOC emissions increase. However, over the South Asian region the fraction of Monoterp oxidised by NO3 increases from 13 % to 15 %. In SSP1-2.6, the Monoterp oxidation fraction by NO3 decreases in all regions evaluated.
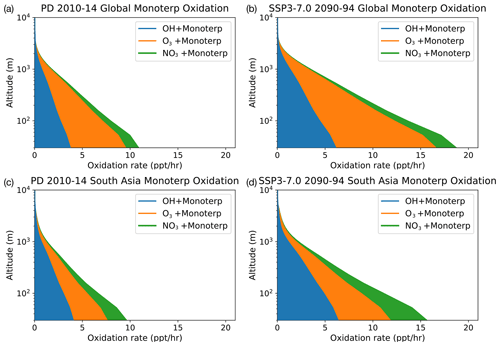
Figure 5Vertical profile of Monoterp oxidation via OH, O3 and NO3. Panels (a) and (c) show the model-calculated present-day global and South Asian oxidation fluxes, respectively. Panels (b) and (d) show these fluxes at the end of the 21st century under the SSP3-7.0 scenario. The future scenario results in increases in all oxidation fluxes, with the NO3-initiated oxidation flux increasing by a factor of 2 compared with the present-day oxidation rates in South Asia.
Focusing on Monoterp oxidation, which in UKESM1 leads to the formation of SOA, Fig. 5 shows the vertical profiles of the oxidation of this compound under PD conditions (Fig. 5a and c) and the SSP3-7.0 future scenario (Fig. 5b and d). Similar figures for the other SSP scenarios are included in the Supplement. As with Fig. 4, Fig. 5 shows that the future scenario results in an increase in oxidation of Monoterp (an increase in SOA production in the model) for all oxidants. The vertical profiles show a sharp drop in the oxidation flux away from the surface as a result of the relatively short lifetime of the Monoterp species, which is emitted in the model at the surface and decays away in the vertical rapidly. Focusing on the near surface, where the rate of SOA production via Monoterp oxidation is highest, Fig. 5 shows that over southern Asia UKESM1 predicts an increase in near-surface NO3 oxidation of Monoterp of a factor of 2 under the SSP3-7.0 scenario. This implies potential for enhanced production of organic nitrogen containing aerosols under future climate and emission scenarios, compounds whose health and climatic effects have been hitherto less well studied but which observational evidence suggests are ubiquitous in the atmosphere (Kiendler-Scharr et al., 2016).
Whilst we have demonstrated large potential changes in NO3 in the future under the SSP scenarios, we acknowledge that there are some limitations to the state-of-the-art CMIP6 models, like UKESM1. Faithful simulation of NO3 requires models which capture a wide range of processes as discussed in Ng et al. (2017). These include the ability to simulate faithfully the diurnal variability in the boundary layer, which has been shown to be a challenge for these types of models (for example in East Asia; Yue et al. (2021) have shown that CMIP6 models cannot reproduce observed trends in the boundary layer height), and simulating the diurnal emissions of important NO3 sinks, like isoprene (Cao et al., 2021) – which almost all interactive chemistry CMIP6 models do – and NO3 sources, like NOx, which no CMIP6 models do. Further work is required to improve the representation of these key emissions sources and processes in global chemistry–climate models. In addition, the simplistic treatment of SOA in UKESM1 prevents us from being able to explore how the composition of SOA may change under the changing climate and emission scenarios that have been explored for CMIP6. Many previous studies have highlighted how the formation of SOA is highly sensitive to the conditions it is being formed under and how the composition of SOA will change concomitantly (e.g. Hoyle et al., 2011; Pye et al., 2010; Schwantes et al., 2019). These feedbacks between changes in SOA formation mechanism and SOA composition are being explored through future work in UKESM1.
In this work we have demonstrated the potential pathways for future evolution of NO3, the most important oxidant at night, in the lower troposphere. Analysis of our model simulations against historic measurements highlights that in spite of the relatively low resolution of UKESM1, it is able to capture the magnitude and variability in observations of NO3 and its precursors (O3 and NO2). We have assessed four different future scenarios which span a wide range of possible NOx and VOC emission pathways and levels of climate change, simulated using UKESM1. This is, to our knowledge, the first assessment of future simulations of NO3, and this work highlights the potential for significant increases in this major nighttime oxidant. In particular we have shown that, depending on the emission scenario, regions of southern Asia are an area of particular interest with potential increases in NO3 to unprecedented levels, more than double their present-day values. We also demonstrate the importance of climate change for NO3 and show that the ratio of NO3:N2O5 is predicted to increase under the higher climate forcing future scenarios. The impacts of an increase in NO3-dominated BVOC oxidation are, as yet, uncertain. An increasing body of literature is examining the mechanistic pathways through which BVOCs and NO3 react and the impacts of BVOC + NO3-derived SOA. He et al. (2021) have shown through detailed laboratory experiments using cavity-enhanced absorption spectroscopy that some of the organic nitrates in BVOC + NO3-derived SOA may serve as atmosphere-stable NOx sinks or reservoirs and will absorb and scatter incoming solar radiation during the daytime leading to an anthropogenic radiative forcing component (given that NO3 is primarily an anthropogenic species).
The potential increases in NO3 are shown to be important in the context of enhancing the oxidation of BVOCs in the atmosphere. Due to the poorer understanding of SOA formation from NO3 oxidation (Ng et al., 2017) than from OH or O3 oxidation, these findings highlight the need for further lab and field studies to better understand the SOA-forming potential of BVOC oxidation initiated via NO3 and the incorporation of these data into global chemistry and Earth system models. Our simulated changes in NO3 are likely dependent on the following:
-
model structure; more work investigating the impacts of vertical and horizontal resolution is required. Our estimates are that low-resolution Earth system models like UKESM1 will underestimate the peak concentrations of NO3 owing to poor vertical mixing.
-
emissions, including soil NOx emissions, which are highly uncertain still, and the diurnal and hebdomadal variation in anthropogenic ones – as with most CMIP6 models the emissions in UKESM1 are prescribed without diurnal variations, which are likely to impact NO3 more than other oxidants. Here we estimate that including vertical profiles of emissions in Earth system models will increase the lifetime of NOx and hence the rate of NO3 production, but detailed studies are needed to assess this highly non-linear system.
-
chemical processes, with simplifications in the treatment of N2O5 heterogeneous chemistry being a potentially key area (as discussed by Archibald et al. (2020a) in the context of ozone budgets). As eluded to in Sect. 4, a key missing component in UKESM1 is the state dependence of SOA formation. Whilst previous studies have used chamber studies to derive conditional state dependence for the yield of SOA as a function of NOx (e.g. Pye et al., 2010), future work should more holistically treat the suite of low-volatility SOA precursors and their formation in the gas phase and coupling to the aerosol phase, as discussed by, e.g., Schwantes et al. (2019).
-
the chemical mechanism used; Archer-Nicholls et al. (2021) and Weber et al. (2021) show that the NOy budget is highly sensitive to the use of a more comprehensive chemical mechanism in the UKESM1 framework.
In this work we assessed the impacts of changing (see the Supplement S1 and S2 for details). We found that it is very difficult to predict how even a relatively simple change, like changing the rate constant for BVOC + NO3 reactions, will modify the output of an Earth system model. Decreasing results in vary spatially variable changes in NO3, NO2 and O3. In spite of the large change in the changes in the fluxes of BVOC + NO3 oxidation were much more muted. Dedicated uncertainty analysis looking at points (1) to (4) above are needed. Finally, almost all CMIP6 models that have simulated interactive chemistry have simulated changes in NO3, and we encourage the modelling community to undertake a multi-model analysis similar to those focused on OH (Stevenson et al., 2020) and O3 (Griffiths et al., 2021). Further multi-model analyses are required to constrain the level of model uncertainty in predictions of NO3 and the potential impacts this could have on future air quality and climate. Co-ordination and partnership between the NO3 observational community and the modelling community could then allow the future changes in NO3 to be observationally constrained.
Due to intellectual property rights restrictions, we cannot provide the source code or documentation papers for the Unified Model (UM). The Met Office Unified Model is available for use under licence. A number of research organisations and national meteorological services use the UM in collaboration with the Met Office to undertake basic atmospheric process research, produce forecasts, develop the UM code, and build and evaluate Earth system models. For further information on how to apply for a licence, see http://www.metoffice.gov.uk/research/modelling-systems/unified-model (last access: 1 October 2021).
The UM data used to produce the figures are available from the Centre of Environmental Data Analysis (CEDA) (see Supplement for details; Archer-Nicholls et al., 2023). Data from five experiments are included in the archive: ScenarioMIP SSP1-2.6 (ssp126), SSP2-4.5 (ssp245), SSP3-7.0 (ssp370), SSP5-8.5(ssp585) experiments and the CMIP Historical experiment (hist) (https://doi.org/10.5285/b70e6ae10a9f463d88819eb981cd4d0f; Archer-Nicholls et al., 2023). For the ScenarioMIP experiments, data are archived for the years 2090–2094. For the CMIP Historical experiment, data are archived for the years 1850–1854. The data comprise monthly mean output from both environmental variables, tracer mass mixing ratio and chemical reaction tendencies in moles per grid box per second. Other relevant data may be found in the CMIP6 archive for CMIP and ScenarioMIP hosted at the Centre for Environmental Data Analysis (CEDA) and at the Earth System Federation Grid.
The supplement related to this article is available online at: https://doi.org/10.5194/acp-23-5801-2023-supplement.
ATA designed the research and supervised the analysis and led the revision of the paper. SAN and RA led the analysis. NLA and SAN performed the re-runs of UKESM1. PTG contributed to the analysis. All authors contributed to writing the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors would like to thank the UK Met Office Clean Air SPF and the JWCRP UKCA project. UKCA is a collaborative programme supported by NCAS and the Met Office, and we wish to acknowledge them for their continuing support of the project. This work used JASMIN, the UK collaborative data analysis facility.
This research has been supported by the Natural Environment Research Council (grant no. NE/P016383/1).
This paper was edited by Kostas Tsigaridis and reviewed by two anonymous referees.
Archer-Nicholls, S., Abraham, N. L., Shin, Y. M., Weber, J., Russo, M. R., Lowe, D., Utembe, S. R., O'Connor, F. M., Kerridge, B., Latter, B., Siddans, R., Jenkin, M., Wild, O., and Archibald, A. T.: The Common Representative Intermediates Mechanism Version 2 in the United Kingdom Chemistry and Aerosols Model, J. Adv. Model. Earth Sy., 13, 1–37, https://doi.org/10.1029/2020MS002420, 2021.
Archer-Nicholls, S., Allen, R., Shin, Y., Abraham, N. L., Griffiths, P., and Archibald, A.: UKESM1 diagnostics for CMIP6 ScenarioMIP and CMIP historical experiments, NERC EDS Centre for Environmental Data Analysis [data set], https://doi.org/10.5285/b70e6ae10a9f463d88819eb981cd4d0f, 2023.
Archibald, A. T., Neu, J. L., Elshorbany, Y. F., Cooper, O. R., Young, P. J., Akiyoshi, H., Cox, R. A., Coyle, M., Derwent, R. G., Deushi, M., and Finco, A.: Tropospheric Ozone Assessment Report: A critical review of changes in the tropospheric ozone burden and budget from 1850 to 2100, Elementa: Science of the Anthropocene, 8, 1–53, https://doi.org/10.1525/elementa.2020.034, 2020a.
Archibald, A. T., O'Connor, F. M., Abraham, N. L., Archer-Nicholls, S., Chipperfield, M. P., Dalvi, M., Folberth, G. A., Dennison, F., Dhomse, S. S., Griffiths, P. T., Hardacre, C., Hewitt, A. J., Hill, R. S., Johnson, C. E., Keeble, J., Köhler, M. O., Morgenstern, O., Mulcahy, J. P., Ordóñez, C., Pope, R. J., Rumbold, S. T., Russo, M. R., Savage, N. H., Sellar, A., Stringer, M., Turnock, S. T., Wild, O., and Zeng, G.: Description and evaluation of the UKCA stratosphere–troposphere chemistry scheme (StratTrop vn 1.0) implemented in UKESM1, Geosci. Model Dev., 13, 1223–1266, https://doi.org/10.5194/gmd-13-1223-2020, 2020b.
Arneth, A., Niinemets, Ü., Pressley, S., Bäck, J., Hari, P., Karl, T., Noe, S., Prentice, I. C., Serça, D., Hickler, T., Wolf, A., and Smith, B.: Process-based estimates of terrestrial ecosystem isoprene emissions: incorporating the effects of a direct CO2−isoprene interaction, Atmos. Chem. Phys., 7, 31–53, https://doi.org/10.5194/acp-7-31-2007, 2007.
Arneth, A., Monson, R. K., Schurgers, G., Niinemets, Ü., and Palmer, P. I.: Why are estimates of global terrestrial isoprene emissions so similar (and why is this not so for monoterpenes)?, Atmos. Chem. Phys., 8, 4605–4620, https://doi.org/10.5194/acp-8-4605-2008, 2008.
Atkinson, R. and Arey, J.: Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review, Atmos. Environ., 37, 197–219. https://doi.org/10.1016/S1352-2310(03)00391-1, 2003.
Atkinson, R., Baulch, D. L., Cox, R. A., Hampson, R. F., Kerr Chairman, J. A., and Troe, J.: Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement III, IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry, J. Phys. Chem. Ref. Data, 18, 881–1097, https://doi.org/10.1063/1.555832, 1989.
Bianchi, F., Kurtén, T., Riva, M., Mohr, C., Rissanen, M. P., Roldin, P., Berndt, T., Crounse, J. D., Wennberg, P. O., Mentel, T. F., Wildt, J., Junninen, H., Jokinen, T., Kulmala, M., Worsnop, D. R., Thornton, J. A., Donahue, N., Kjaergaard, H. G., and Ehn, M.: Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol, Chem. Rev., 119, 3472–3509, https://doi.org/10.1021/acs.chemrev.8b00395, 2019.
Boyd, C. M., Sanchez, J., Xu, L., Eugene, A. J., Nah, T., Tuet, W. Y., Guzman, M. I., and Ng, N. L.: Secondary organic aerosol formation from the system: effect of humidity and peroxy radical fate, Atmos. Chem. Phys., 15, 7497–7522, https://doi.org/10.5194/acp-15-7497-2015, 2015.
Brewer, D. A., Ogliaruso, M. A., Augustsson, T. R., and Levine, J. S.: The oxidation of isoprene in the troposphere: Mechanism and model calculations, Atmos. Environ. (1967), 18, 2723–2744, https://doi.org/10.1016/0004-6981(84)90338-X, 1984.
Brown, S. S. and Stutz, J.: Nighttime radical observations and chemistry, Chem. Soc. Rev., 41, 6405–6447, 2012.
Cao, Y., Yue, X., Liao, H., Yang, Y., Zhu, J., Chen, L., Tian, C., Lei, Y., Zhou, H., and Ma, Y.: Ensemble projection of global isoprene emissions by the end of 21st century using CMIP6 models, Atmos. Environ., 267, 118766, https://doi.org/10.1016/j.atmosenv.2021.118766, 2021.
Ehn, M., Thornton, J. A., Kleist, E., Sipilä, M., Junninen, H., Pullinen, I., Springer, M., Rubach, F., Tillmann, R., Lee, B., Lopez-Hilfiker, F., Andres, S., Acir, I.-H., Rissanen, M., Jokinen, T., Schobesberger, S., Kangasluoma, J., Kontkanen, J., Nieminen, T., and Mentel, T. F.: A large source of low-volatility secondary organic aerosol, Nature, 506, 476–479, https://doi.org/10.1038/nature13032, 2014.
Ehn, M., Berndt, T., Wildt, J., and Mentel, T.: Highly oxygenated molecules from atmospheric autoxidation of hydrocarbons: a prominent challenge for chemical kinetics studies, Int. J. Chem. Kinet., 49, 821–831, 2017.
Eyring, V., Bony, S., Meehl, G. A., Senior, C. A., Stevens, B., Stouffer, R. J., and Taylor, K. E.: Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization, Geosci. Model Dev., 9, 1937–1958, https://doi.org/10.5194/gmd-9-1937-2016, 2016.
Faxon, C., Hammes, J., Le Breton, M., Pathak, R. K., and Hallquist, M.: Characterization of organic nitrate constituents of secondary organic aerosol (SOA) from nitrate-radical-initiated oxidation of limonene using high-resolution chemical ionization mass spectrometry, Atmos. Chem. Phys., 18, 5467–5481, https://doi.org/10.5194/acp-18-5467-2018, 2018.
Fish, D. J., Shallcross, D. E., and Jones, R. L.: The vertical distribution of NO3 in the atmospheric boundary layer, Atmos. Environ., 33, 687–691, https://doi.org/10.1016/S1352-2310(98)00332-X, 1999.
Fry, J. L., Kiendler-Scharr, A., Rollins, A. W., Wooldridge, P. J., Brown, S. S., Fuchs, H., Dubé, W., Mensah, A., dal Maso, M., Tillmann, R., Dorn, H.-P., Brauers, T., and Cohen, R. C.: Organic nitrate and secondary organic aerosol yield from NO3 oxidation of β-pinene evaluated using a gas-phase kinetics/aerosol partitioning model, Atmos. Chem. Phys., 9, 1431–1449, https://doi.org/10.5194/acp-9-1431-2009, 2009.
Gidden, M. J., Riahi, K., Smith, S. J., Fujimori, S., Luderer, G., Kriegler, E., van Vuuren, D. P., van den Berg, M., Feng, L., Klein, D., Calvin, K., Doelman, J. C., Frank, S., Fricko, O., Harmsen, M., Hasegawa, T., Havlik, P., Hilaire, J., Hoesly, R., Horing, J., Popp, A., Stehfest, E., and Takahashi, K.: Global emissions pathways under different socioeconomic scenarios for use in CMIP6: a dataset of harmonized emissions trajectories through the end of the century, Geosci. Model Dev., 12, 1443–1475, https://doi.org/10.5194/gmd-12-1443-2019, 2019.
Gordon, H., Kirkby, J., Baltensperger, U., Bianchi, F., Breitenlechner, M., Curtius, J., Dias, A., Dommen, J., Donahue, N. M., Dunne, E. M., Duplissy, J., Ehrhart, S., Flagan, R. C., Frege, C., Fuchs, C., Hansel, A., Hoyle, C. R., Kulmala, M., Kürten, A., and Carslaw, K. S.: Causes and importance of new particle formation in the present-day and preindustrial atmospheres, J. Geophys. Res.-Atmos., 122, 8739–8760, https://doi.org/10.1002/2017JD026844, 2017.
Griffiths, P. T., Murray, L. T., Zeng, G., Shin, Y. M., Abraham, N. L., Archibald, A. T., Deushi, M., Emmons, L. K., Galbally, I. E., Hassler, B., Horowitz, L. W., Keeble, J., Liu, J., Moeini, O., Naik, V., O'Connor, F. M., Oshima, N., Tarasick, D., Tilmes, S., Turnock, S. T., Wild, O., Young, P. J., and Zanis, P.: Tropospheric ozone in CMIP6 simulations, Atmos. Chem. Phys., 21, 4187–4218, https://doi.org/10.5194/acp-21-4187-2021, 2021.
Hamilton, J. F., Bryant, D. J., Edwards, P. M., Ouyang, B., Bannan, T. J., Mehra, A., Mayhew, A. W., Hopkins, J. R., Dunmore, R. E., Squires, F. A., Lee, J. D., Newland, M. J., Worrall, S. D., Bacak, A., Coe, H., Percival, C., Whalley, L. K., Heard, D. E., Slater, E. J., Jones, R. L., Cui, T., Surratt, J. D., Reeves, C. E., Mills, G. P., Grimmond, S., Sun, Y., Xu, W., Shi, Z., and Rickard, A. R.: Key Role of NO3 Radicals in the Production of Isoprene Nitrates and Nitrooxyorganosulfates in Beijing, Environ. Sci. Technol., 55, 842–853, https://doi.org/10.1021/acs.est.0c05689, 2021.
Hantson, S., Knorr, W., Schurgers, G., Pugh, T. A. M., and Arneth, A.: Global isoprene and monoterpene emissions under changing climate, vegetation, CO2 and land use, Atmos. Environ., 155, 35–45, https://doi.org/10.1016/j.atmosenv.2017.02.010, 2017.
He, Q., Tomaz, S., Li, C., Zhu, M., Meidan, D., Riva, M., Laskin, A., Brown, S. S., George, C., Wang, X., and Rudich, Y.: Optical properties of secondary organic aerosol produced by nitrate radical oxidation of biogenic volatile organic compounds, Environ. Sci. Technol., 55, 2878–2889, https://doi.org/10.1021/acs.est.0c06838, 2021.
Hoesly, R. M., Smith, S. J., Feng, L., Klimont, Z., Janssens-Maenhout, G., Pitkanen, T., Seibert, J. J., Vu, L., Andres, R. J., Bolt, R. M., Bond, T. C., Dawidowski, L., Kholod, N., Kurokawa, J.-I., Li, M., Liu, L., Lu, Z., Moura, M. C. P., O'Rourke, P. R., and Zhang, Q.: Historical (1750–2014) anthropogenic emissions of reactive gases and aerosols from the Community Emissions Data System (CEDS), Geosci. Model Dev., 11, 369–408, https://doi.org/10.5194/gmd-11-369-2018, 2018.
Hoyle, C. R., Boy, M., Donahue, N. M., Fry, J. L., Glasius, M., Guenther, A., Hallar, A. G., Huff Hartz, K., Petters, M. D., Petäjä, T., Rosenoern, T., and Sullivan, A. P.: A review of the anthropogenic influence on biogenic secondary organic aerosol, Atmos. Chem. Phys., 11, 321–343, https://doi.org/10.5194/acp-11-321-2011, 2011.
Jones, A. C., Hill, A., Remy, S., Abraham, N. L., Dalvi, M., Hardacre, C., Hewitt, A. J., Johnson, B., Mulcahy, J. P., and Turnock, S. T.: Exploring the sensitivity of atmospheric nitrate concentrations to nitric acid uptake rate using the Met Office's Unified Model, Atmos. Chem. Phys., 21, 15901–15927, https://doi.org/10.5194/acp-21-15901-2021, 2021.
Khan, M. A. H., Cooke, M. C., Utembe, S. R., Archibald, A. T., Derwent, R. G., Xiao, P., Percival, C. J., Jenkin, M. E., Morris, W. C., and Shallcross, D. E.: Global modeling of the nitrate radical (NO3) for present and pre-industrial scenarios, Atmos. Res., 164–165, 347–357, https://doi.org/10.1016/j.atmosres.2015.06.006, 2015.
Kiendler-Scharr, A., Mensah, A. A., Friese, E., Topping, D., Nemitz, E., Prévôt, A. S., Äijälä, M., Allan, J., Canonaco, F., Canagaratna, M., and Carbone, S.: Ubiquity of organic nitrates from nighttime chemistry in the European submicron aerosol, Geophys. Res. Lett., 43, 7735–7744, https://doi.org/10.1002/2016GL069239, 2016.
Li, S., Liu, W., Xie, P., Qin, M., and Yang, Y.: Observation of nitrate radical in the nocturnal boundary layer during a summer field campaign in pearl river delta China, Terr. Atmos. Ocean. Sci., 23, 39–48, https://doi.org/10.3319/TAO.2011.07.26.01(A), 2012.
Mann, G. W., Carslaw, K. S., Spracklen, D. V., Ridley, D. A., Manktelow, P. T., Chipperfield, M. P., Pickering, S. J., and Johnson, C. E.: Description and evaluation of GLOMAP-mode: a modal global aerosol microphysics model for the UKCA composition-climate model, Geosci. Model Dev., 3, 519–551, https://doi.org/10.5194/gmd-3-519-2010, 2010.
McDuffie, E. E., Fibiger, D. L., Dubé, W. P., Lopez-Hilfiker, F., Lee, B. H., Thornton, J. A., Shah, V., Jaeglé, L., Guo, H., Weber, R. J., Reeves, J. M., Weinheimer, A. J., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Dibb, J. E., Veres, P., Ebben, C., Sparks, T. L., Wooldridge, P. J., Cohen, R. C., Hornbrook, R. S., Apel, E. C., Campos, T., Hall, S. R., Ullmann, K., and Brown, S. S.: Heterogeneous N2O5 uptake during winter: Aircraft measurements during the 2015 WINTER campaign and critical evaluation of current parameterizations, J. Geophys. Res.-Atmos., 123, 4345–4372, https://doi.org/10.1002/2018JD028336, 2018.
McGillen, M. R., Carter, W. P. L., Mellouki, A., Orlando, J. J., Picquet-Varrault, B., and Wallington, T. J.: Database for the kinetics of the gas-phase atmospheric reactions of organic compounds, Earth Syst. Sci. Data, 12, 1203–1216, https://doi.org/10.5194/essd-12-1203-2020, 2020.
Monks, P. S., Archibald, A. T., Colette, A., Cooper, O., Coyle, M., Derwent, R., Fowler, D., Granier, C., Law, K. S., Mills, G. E., Stevenson, D. S., Tarasova, O., Thouret, V., von Schneidemesser, E., Sommariva, R., Wild, O., and Williams, M. L.: Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer, Atmos. Chem. Phys., 15, 8889–8973, https://doi.org/10.5194/acp-15-8889-2015, 2015.
Mulcahy, J. P., Johnson, C., Jones, C. G., Povey, A. C., Scott, C. E., Sellar, A., Turnock, S. T., Woodhouse, M. T., Abraham, N. L., Andrews, M. B., Bellouin, N., Browse, J., Carslaw, K. S., Dalvi, M., Folberth, G. A., Glover, M., Grosvenor, D. P., Hardacre, C., Hill, R., Johnson, B., Jones, A., Kipling, Z., Mann, G., Mollard, J., O'Connor, F. M., Palmiéri, J., Reddington, C., Rumbold, S. T., Richardson, M., Schutgens, N. A. J., Stier, P., Stringer, M., Tang, Y., Walton, J., Woodward, S., and Yool, A.: Description and evaluation of aerosol in UKESM1 and HadGEM3-GC3.1 CMIP6 historical simulations, Geosci. Model Dev., 13, 6383–6423, https://doi.org/10.5194/gmd-13-6383-2020, 2020.
Ng, N. L., Brown, S. S., Archibald, A. T., Atlas, E., Cohen, R. C., Crowley, J. N., Day, D. A., Donahue, N. M., Fry, J. L., Fuchs, H., Griffin, R. J., Guzman, M. I., Herrmann, H., Hodzic, A., Iinuma, Y., Jimenez, J. L., Kiendler-Scharr, A., Lee, B. H., Luecken, D. J., Mao, J., McLaren, R., Mutzel, A., Osthoff, H. D., Ouyang, B., Picquet-Varrault, B., Platt, U., Pye, H. O. T., Rudich, Y., Schwantes, R. H., Shiraiwa, M., Stutz, J., Thornton, J. A., Tilgner, A., Williams, B. J., and Zaveri, R. A.: Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol, Atmos. Chem. Phys., 17, 2103–2162, https://doi.org/10.5194/acp-17-2103-2017, 2017.
O'Neill, B. C., Tebaldi, C., van Vuuren, D. P., Eyring, V., Friedlingstein, P., Hurtt, G., Knutti, R., Kriegler, E., Lamarque, J.-F., Lowe, J., Meehl, G. A., Moss, R., Riahi, K., and Sanderson, B. M.: The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6, Geosci. Model Dev., 9, 3461–3482, https://doi.org/10.5194/gmd-9-3461-2016, 2016.
Pacifico, F., Folberth, G. A., Jones, C. D., Harrison, S. P., and Collins, W. J.: Sensitivity of biogenic isoprene emissions to past, present, and future environmental conditions and implications for atmospheric chemistry, J. Geophys. Res.-Atmos., 117, D22302, https://doi.org/10.1029/2012JD018276, 2012.
Pöschl, U.: Atmospheric aerosols: composition, transformation, climate and health effects, Angew. Chem. Int. Edit., 44, 7520–7540, https://doi.org/10.1002/anie.200501122, 2005.
Pye, H. O. T., Chan, A. W. H., Barkley, M. P., and Seinfeld, J. H.: Global modeling of organic aerosol: the importance of reactive nitrogen (NOx and NO3), Atmos. Chem. Phys., 10, 11261–11276, https://doi.org/10.5194/acp-10-11261-2010, 2010.
Rao, S., Klimont, Z., Smith, S. J., Van Dingenen, R., Dentener, F., Bouwman, L., Riahi, K., Amann, M., Bodirsky, B. L., van Vuuren, D. P., Aleluia Reis, L., Calvin, K., Drouet, L., Fricko, O., Fujimori, S., Gernaat, D., Havlik, P., Harmsen, M., Hasegawa, T., and Tavoni, M.: Future air pollution in the Shared Socio-economic Pathways, Glob. Environ. Chang., 42, 346–358, https://doi.org/10.1016/j.gloenvcha.2016.05.012, 2017.
Riemer, N., Vogel, H., Vogel, B., Schell, B., Ackermann, I., Kessler, C., and Hass, H.: Impact of the heterogeneous hydrolysis of N2O5 on chemistry and nitrate aerosol formation in the lower troposphere under photosmog conditions, J. Geophys. Res., 108, 4144, https://doi.org/10.1029/2002JD002436, 2003.
Sakulyanontvittaya, T., Duhl, T., Wiedinmyer, C., Helmig, D., Matsunaga, S., Potosnak, M., Milford, J., and Guenther, A.: Monoterpene and sesquiterpene emission estimates for the United States, Environ. Sci. Technol., 42, 1623–1629, https://doi.org/10.1021/es702274e, 2008.
Schwantes, R. H., Charan, S. M., Bates, K. H., Huang, Y., Nguyen, T. B., Mai, H., Kong, W., Flagan, R. C., and Seinfeld, J. H.: Low-volatility compounds contribute significantly to isoprene secondary organic aerosol (SOA) under high-NOx conditions, Atmos. Chem. Phys., 19, 7255–7278, https://doi.org/10.5194/acp-19-7255-2019, 2019.
Schwantes, R. H., Emmons, L. K., Orlando, J. J., Barth, M. C., Tyndall, G. S., Hall, S. R., Ullmann, K., St. Clair, J. M., Blake, D. R., Wisthaler, A., and Bui, T. P. V.: Comprehensive isoprene and terpene gas-phase chemistry improves simulated surface ozone in the southeastern US, Atmos. Chem. Phys., 20, 3739–3776, https://doi.org/10.5194/acp-20-3739-2020, 2020.
Scott, C. E., Arnold, S. R., Monks, S. A., Asmi, A., Paasonen, P., and Spracklen, D. V.: Substantial large-scale feedbacks between natural aerosols and climate, Nat. Geosci., 11, 1–6, https://doi.org/10.1038/s41561-017-0020-5, 2017.
Sellar, A. A., Jones, C. G., Mulcahy, J., Tang, Y., Yool, A., Wiltshire, A., O'Connor, F. M., Stringer, M., Hill, R., Palmieri, J., Woodward, S., Mora, L., Kuhlbrodt, T., Rumbold, S., Kelley, D. I., Ellis, R., Johnson, C. E., Walton, J., Abraham, N. L., and Zerroukat, M.: UKESM1: Description and evaluation of the UK Earth System Model, J. Adv. Model. Earth Sy., 11, 4513–4558, https://doi.org/10.1029/2019ms001739, 2019.
Stevenson, D. S., Zhao, A., Naik, V., O'Connor, F. M., Tilmes, S., Zeng, G., Murray, L. T., Collins, W. J., Griffiths, P. T., Shim, S., Horowitz, L. W., Sentman, L. T., and Emmons, L.: Trends in global tropospheric hydroxyl radical and methane lifetime since 1850 from AerChemMIP, Atmos. Chem. Phys., 20, 12905–12920, https://doi.org/10.5194/acp-20-12905-2020, 2020.
Stone, D., Evans, M. J., Walker, H., Ingham, T., Vaughan, S., Ouyang, B., Kennedy, O. J., McLeod, M. W., Jones, R. L., Hopkins, J., Punjabi, S., Lidster, R., Hamilton, J. F., Lee, J. D., Lewis, A. C., Carpenter, L. J., Forster, G., Oram, D. E., Reeves, C. E., Bauguitte, S., Morgan, W., Coe, H., Aruffo, E., Dari-Salisburgo, C., Giammaria, F., Di Carlo, P., and Heard, D. E.: Radical chemistry at night: comparisons between observed and modelled HOx, NO3 and N2O5 during the RONOCO project, Atmos. Chem. Phys., 14, 1299–1321, https://doi.org/10.5194/acp-14-1299-2014, 2014.
Tebaldi, C., Debeire, K., Eyring, V., Fischer, E., Fyfe, J., Friedlingstein, P., Knutti, R., Lowe, J., O'Neill, B., Sanderson, B., van Vuuren, D., Riahi, K., Meinshausen, M., Nicholls, Z., Tokarska, K. B., Hurtt, G., Kriegler, E., Lamarque, J.-F., Meehl, G., Moss, R., Bauer, S. E., Boucher, O., Brovkin, V., Byun, Y.-H., Dix, M., Gualdi, S., Guo, H., John, J. G., Kharin, S., Kim, Y., Koshiro, T., Ma, L., Olivié, D., Panickal, S., Qiao, F., Rong, X., Rosenbloom, N., Schupfner, M., Séférian, R., Sellar, A., Semmler, T., Shi, X., Song, Z., Steger, C., Stouffer, R., Swart, N., Tachiiri, K., Tang, Q., Tatebe, H., Voldoire, A., Volodin, E., Wyser, K., Xin, X., Yang, S., Yu, Y., and Ziehn, T.: Climate model projections from the Scenario Model Intercomparison Project (ScenarioMIP) of CMIP6, Earth Syst. Dynam., 12, 253–293, https://doi.org/10.5194/esd-12-253-2021, 2021.
Tröstl, J., Chuang, W. K., Gordon, H., Heinritzi, M., Yan, C., Molteni, U., Ahlm, L., Frege, C., Bianchi, F., Wagner, R., Simon, M., Lehtipalo, K., Williamson, C., Craven, J. S., Duplissy, J., Adamov, A., Almeida, J., Bernhammer, A.-K., Breitenlechner, M., and Baltensperger, U.: The role of low-volatility organic compounds in initial particle growth in the atmosphere, Nature, 533, 527–531, https://doi.org/10.1038/nature18271, 2016.
Wayne, R. P., Barnes, I., Biggs, P., Burrows, J. P., Canosa-Mas, C. E., Hjorth, J., Bras, G. L., Moortgat, G. K., Perner, D., Poulet, G., Restelli, G., and Sidebottom, H.: The Nitrate Radical: Physics, Chemistry, and the Atmosphere, Atmos. Environ., 25A, 1–203, https://doi.org/10.1016/0960-1686(91)90192-A, 1991.
Walters, D., Baran, A. J., Boutle, I., Brooks, M., Earnshaw, P., Edwards, J., Furtado, K., Hill, P., Lock, A., Manners, J., Morcrette, C., Mulcahy, J., Sanchez, C., Smith, C., Stratton, R., Tennant, W., Tomassini, L., Van Weverberg, K., Vosper, S., Willett, M., Browse, J., Bushell, A., Carslaw, K., Dalvi, M., Essery, R., Gedney, N., Hardiman, S., Johnson, B., Johnson, C., Jones, A., Jones, C., Mann, G., Milton, S., Rumbold, H., Sellar, A., Ujiie, M., Whitall, M., Williams, K., and Zerroukat, M.: The Met Office Unified Model Global Atmosphere 7.0/7.1 and JULES Global Land 7.0 configurations, Geosci. Model Dev., 12, 1909–1963, https://doi.org/10.5194/gmd-12-1909-2019, 2019.
Weber, J., Archer-Nicholls, S., Abraham, N. L., Shin, Y. M., Bannan, T. J., Percival, C. J., Bacak, A., Artaxo, P., Jenkin, M., Khan, M. A. H., Shallcross, D. E., Schwantes, R. H., Williams, J., and Archibald, A. T.: Improvements to the representation of BVOC chemistry–climate interactions in UKCA (v11.5) with the CRI-Strat 2 mechanism: incorporation and evaluation, Geosci. Model Dev., 14, 5239–5268, https://doi.org/10.5194/gmd-14-5239-2021, 2021.
Yue, M., Wang, M., Guo, J., Zhang, H., Dong, X., and Liu, Y.: Long-Term Trend Comparison of Planetary Boundary Layer Height in Observations and CMIP6 Models over China, J. Climate, 34, 8237–8256. 2021.
Zhao, D., Pullinen, I., Fuchs, H., Schrade, S., Wu, R., Acir, I.-H., Tillmann, R., Rohrer, F., Wildt, J., Guo, Y., Kiendler-Scharr, A., Wahner, A., Kang, S., Vereecken, L., and Mentel, T. F.: Highly oxygenated organic molecule (HOM) formation in the isoprene oxidation by NO3 radical, Atmos. Chem. Phys., 21, 9681–9704, https://doi.org/10.5194/acp-21-9681-2021, 2021.