the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Characterization of water-soluble brown carbon chromophores from wildfire plumes in the western USA using size-exclusion chromatography
Lisa Azzarello
Rebecca A. Washenfelder
Michael A. Robinson
Alessandro Franchin
Caroline C. Womack
Christopher D. Holmes
Steven S. Brown
Ann Middlebrook
Tim Newberger
Colm Sweeney
Wildfires are an important source of carbonaceous aerosol in the atmosphere. Organic aerosol that absorbs light in the ultraviolet to visible spectral range is referred to as brown carbon (BrC), and its impact on Earth's radiative budget has not been well characterized. We collected water-soluble brown carbon using a particle-into-liquid sampler (PILS) on board a Twin Otter aircraft during the Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) campaign. Samples were collected downwind of wildfires in the western United States from August to September 2019. We applied size-exclusion chromatography (SEC) with ultraviolet-visible spectroscopy to characterize the molecular size distribution of BrC chromophores. The wildfire plumes had transport ages of 0 to 5 h, and the absorption was dominated by chromophores with molecular weights <500 Da. With BrC normalized to a conserved biomass burning tracer, carbon monoxide, a consistent decrease in BrC absorption with plume age was not observed during FIREX-AQ. These findings are consistent with the variable trends in BrC absorption with plume age reported in recent studies. While BrC absorption trends were broadly consistent between the offline SEC analysis and the online PILS measurements, the absolute values of absorption and their spectral dependence differed. We investigate plausible explanations for the discrepancies observed between the online and offline analyses. This included solvent effects, pH, and sample storage. We suspect that sample storage impacted the absorption intensity of the offline measurements without impacting the molecular weight distribution of BrC chromophores.
- Article
(1563 KB) - Full-text XML
-
Supplement
(1507 KB) - BibTeX
- EndNote
The wildfire season across the western United States has greatly intensified over the past century. The U.S. Forest Service reports that the amount of western USA land burned by “high severity” wildfires (i.e., fires that destroy more than 95 % of vegetation) has increased 8-fold since 1985 (Parks and Abatzoglou, 2020). A variety of factors influences the number and intensity of wildfires, including fuel availability, temperature, drought conditions, location of lightning strikes, and direct human influence. During the 20th century, fire suppression tactics were applied throughout the western USA, and these efforts caused fuel to accumulate (Marlon et al., 2012). The combination of dry conditions, warmer temperatures, and fuel availability contributes to the intensity of present-day wildfires in the western USA. Consequently, the impact that these climatic conditions have on wildfire activity has been established. However, the feedback effects that wildfires have on climate is an ongoing area of research.
Wildfires emit carbonaceous particulate matter into the atmosphere (Bond et al., 2004; van der Werf et al., 2010). Based on volatility and optical properties, carbonaceous aerosol particles emitted from biomass burning are categorized as elemental carbon (EC) and organic carbon (OC; Turpin et al., 1990). Elemental carbon, referred to as black carbon (BC), is refractory and is characterized by broad absorbance across the ultraviolet (UV) to infrared wavelengths (Seinfeld and Pankow, 2003; Andreae and Gelencsér, 2006; Laskin et al., 2015). The light-absorbing components of organic aerosols are referred to as brown carbon (BrC; Laskin et al., 2015). The direct absorption and scattering of solar radiation by these aerosol particles impacts the global radiative budget (Boucher et al., 2013; Forster et al., 2007), but there is uncertainty about the magnitude of this effect. Currently, more information is known about BC and its impact on climate than BrC, as BrC is more chemically complex and more reactive (Buis, 2021; Di Lorenzo et al., 2017). The direct radiative forcing of BC has been estimated to be the second-largest anthropogenic climate forcing species (Ramanathan and Carmichael, 2008), and studies have suggested that BrC can contribute between 20 % and 40 % to positive radiative forcing from total carbonaceous absorbing aerosol (Feng et al., 2013; Zhang et al., 2017; L. Zeng et al., 2020).
Wildfire emissions are a dominant primary source of BrC (Washenfelder et al., 2015). The brown colour results from a combination of species with varying abilities to absorb light in the UV-visible region (from highly to weakly absorbing; Hems et al., 2021). The pyrolysis of lignin and cellulose contributes to the major chemical constituents in wildfire plumes, such as phenolic compounds and organic acids (Simoneit, 2002; Xie et al., 2019; Smith et al., 2014). Lignin pyrolysis products with aromatic functionalities absorb visible light and may contribute to the absorption properties of BrC (Hems et al., 2021). Secondary processes also contribute to BrC formation. The generation of secondary organic aerosol (SOA) stemming from gas-phase reaction products includes nitration of aromatic compounds in the presence of NOx or NO3 (Harrison et al., 2005; Finewax et al., 2018; Xie et al., 2017). For example, catechol can react with either the NO3 or OH radical to form 4-nitrocatechol (Finewax et al., 2018), and oxidation of toluene under elevated NOx conditions has been observed to form over 15 absorbing compounds, with nitroaromatics contributing up to 60 % of absorption in the visible region (Liu et al., 2016). Although there are hypotheses about the identity of BrC chromophores, up to 40 % of BrC constituents remain unidentified (Lin et al., 2017; Bluvshtein et al., 2017).
To characterize the absorbing constituents that contribute to BrC absorption, reverse-phase high-performance liquid chromatography (HPLC) coupled to high-resolution mass spectrometry has been applied (Fleming et al., 2020). However, fresh and aged BrC consist of extremely low volatile organic compounds (ELVOCs) that may be irreversibly retained on a traditional C18 reverse-phase HPLC column (Di Lorenzo and Young, 2016). Size-exclusion chromatography coupled to UV-visible absorption spectroscopy (SEC-UV) has been demonstrated as an alternative that successfully measures the absorption properties of high and low molecular weight (MW) ELVOCs in fresh and aged biomass-burning-derived samples (Di Lorenzo and Young, 2016; Di Lorenzo et al., 2017; Wong et al., 2019). Analysis by SEC-UV has been previously applied to samples collected during ground-based field measurement campaigns, where the method has established the quantification of BrC absorbance as a function of MW and provided insight into the composition of BrC. High-MW (>400 Da) compounds with unknown structural identities have been determined to contribute to BrC absorption, and the relative contribution to BrC absorption by high-MW species increases with smoke age (Di Lorenzo et al., 2017; Wong et al., 2017, 2019). These findings suggested that lower-MW species are less persistent in biomass burning smoke relative to higher-MW species, likely due to volatilization, oxidation, polymerization, or other processes (Di Lorenzo et al., 2017; Hems et al., 2021).
The Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ) field campaign examined the impact of wildfires on atmospheric chemistry and air quality in the western United States. In this work, we present the SEC-UV analysis of water-soluble BrC that was collected on board the National Oceanic and Atmospheric Administration (NOAA) Twin Otter aircraft during plume transects downwind of western USA forest fires. These represent the first aircraft samples analyzed by SEC-UV to characterize BrC. We compare the total absorption measured in online and offline samples and assign the BrC absorption to different MW classes. Finally, we examine how the composition of the mobile phase used in the SEC-UV analysis impacts elution time and spectral features. This provides cautionary information about interpreting absorption results in studies that apply chromatographic separation in an aqueous–organic matrix.
2.1 Overview of the FIREX-AQ field campaign
FIREX-AQ was a multi-platform field campaign that investigated wildfire emissions in the western United States from July to September 2019. Instrumented aircraft and mobile laboratories were used to intercept and sample smoke plumes throughout multiple western USA states. These included a DC-8, ER-2, and two Twin Otter aircraft. This study focuses on smoke sampled by the “Chemistry” Twin Otter aircraft, which was based in Boise, Idaho, from 29 July to 5 September 2019, and briefly in Cedar City, Utah, from 19 to 23 August 2019. The Twin Otter payload included gas and aerosol instruments to measure smoke composition, transport, and transformation. This included CO measurements by near-infrared cavity ring-down spectroscopy (G2401-m; Picarro Inc., Santa Clara, CA, USA; Crosson, 2008; Karion et al., 2013). A complete description of the payload installed on the Twin Otter can be found in Warneke et al. (2023). The payload weight limited the duration of in-flight sampling to 2.5–3 h, with a typical schedule of two or three flights per day during the afternoon, evening, or night. A total of 40 flights was completed in Arizona, Idaho, Nevada, Oregon, and Utah. Air mass back-trajectory analyses were used to estimate the plume age of each transect, as described in Liao et al. (2021) and Washenfelder et al. (2022). Briefly, the smoke age was calculated by summing the horizontal advection and vertical plume rise ages between the time of emission and the aircraft interception of the smoke plume. For the Twin Otter flights, many plume intercepts by the aircraft were approximately Lagrangian (Washenfelder et al., 2022).
2.2 Online measurement of water-soluble absorption and offline sample collection
The brown carbon–particle-into-liquid sampler (BrC–PILS) collected online absorption data and offline aqueous samples for the SEC-UV analysis. A complete description of the BrC–PILS instrument and sampling can be found in Zeng et al. (2021) and Washenfelder et al. (2022). Briefly, the BrC–PILS sampled smoke through a shared aerosol inlet on the Twin Otter. A parallel-plate carbon filter denuder removed volatile organic compounds prior to the aerosol entering the PILS. The PILS consisted of a steam generator and droplet impactor to collect aerosols into aqueous solution. The liquid flow then entered a liquid waveguide capillary cell (LWCC) to measure absorption. The instrument precision (3σ) for absorption at 365 nm was ±0.02 Mm−1 for 10 s in-flight data, with an uncertainty of ±11 % (Zeng et al., 2021). The flow exiting the LWCC was split between a total organic carbon (TOC) analyzer and an automated 14-port valve. The valve directed aqueous sample flow to 1 of 12 polypropylene sample tubes for offline SEC-UV analysis (Fig. S1 in the Supplement). Prior to deployment, each polypropylene tube was rinsed with 18.2 MΩ cm deionized water (DIW; Thermo Fisher Scientific Barnstead™ Smart2Pure™,) 8 to 10 times. The sample flow rate was monitored by a liquid mass flow meter prior to the flow diverting between the automated valve and the TOC analyzer. The sample flow was 1.53 mL min−1 during in-flight sampling, and the excess 0.43 mL min−1 was collected into an individual polypropylene tube for 12 s to 10 min. During in-flight sampling, the flight scientist actively controlled the sample collection into each polypropylene tube to target transects of the smoke plume (example shown in Fig. S2). For each flight, 6 to 12 aqueous samples were collected, with 201 total samples from 39 science flights. Field blanks of the DIW used to operate the BrC–PILS were stored similarly in clean polypropylene sample tubes at the beginning, halfway point, and end of the campaign. Once collected in the field, the samples and blanks were stored on ice for several hours prior to refrigeration until analysis.
2.3 Offline analysis by SEC-UV
Measurement by SEC-UV provides information about size-dependent light absorption properties of BrC chromophores. The offline aerosol samples were separated using an aqueous-gel-filtration column, with a MW range of 250 Da to 75 kDa (Polysep-GFC-P 3000, Phenomenex, Torrance, CA). Size-resolved components were detected using a diode-array detector from 190 to 800 nm (UltiMate 3000, Thermo Fisher Scientific, Sunnyvale, CA) coupled to an ion chromatograph (ICS 6000; Thermo Fisher Scientific) pump with a Dionex AS autosampler (Thermo Fisher Scientific). The isocratic method was run using a mobile phase that contained a 1:1 mixture of acetonitrile and 25 mM ammonium acetate in solution at a flow rate of 1 mL min−1 and a sample injection volume of 100 µL. A solution of Suwannee River Humic Acid (SRHA II; International Humic Substances Society, Saint Paul, MN, USA) was run prior to the FIREX-AQ samples to ensure proper operation of the SEC-UV set up.
The aqueous samples collected by the BrC–PILS did not require post-sampling processing and were injected onto the SEC column under mobile-phase flow to the diode-array detector. The uncertainty for the offline total absorption measurements considers the uncertainty in the liquid flow and PILS collection efficiency for a total uncertainty of ±10.5 %. Discussion of the SEC-UV method development and details of the conversion of SEC-UV signal to ambient absorption in units of inverse megameters can be found in the Supplement. We calculated BrC absorption as a function of MW by applying the calibration method described by Di Lorenzo and Young (2016; Fig. S3). Sample measurements were blank subtracted. The detection limit of the total integrated absorption (equivalent to 3σ of n=6 field blanks) was 2.5±0.2 and 0.70±0.02 mAU min at 250 and 300 nm, respectively. This corresponds to a 3σ detection limit of approximately 525 Mm−1 at 250 nm and 150 Mm−1 at 300 nm.
2.4 Absorption in different mobile phases
To assess the impact of pH and mobile-phase composition on wavelength-dependent absorption, the ammonium acetate solution was adjusted to pH 5 and pH 9 with acetic acid and ammonium hydroxide, respectively, prior to combining with acetonitrile. A 15 µg mL−1 in DIW solution of Suwannee River Fulvic Acid (SRFA II; International Humic Substances Society, Saint Paul, MN, USA) and a FIREX-AQ aqueous sample were injected onto the diode-array detector without the SEC column in line with the following mobile phases of DIW only: 25 mM ammonium acetate solution, the default mobile phase (described in Sect. 2.3), 25 mM ammonium acetate solution adjusted to pH 5, and 25 mM ammonium acetate solution adjusted to pH 9. Solutions of 4-nitrocatechol, 4-hydroxy-3-methoxycinnamaldehyde, vanillin, and 7-hydroxycoumarin in DIW with concentrations of , , , mol mL−1, respectively, were prepared and injected onto the diode-array detector to observe differences in their absorption profiles. To confirm the diode-array detector results, measurements of the SRFA solution were also made with UV-visible spectroscopy (8453; Agilent Technologies, Inc., Santa Clara, CA, USA), where the solution was mixed (1:1 ratio) with the various mobile phases prior to transferring to a cuvette for absorption measurements (Fig. S7).
3.1 Trends in absorption with plume age
We present molecular-size-resolved absorption for flights that met the following criteria: (1) maximum CO concentrations greater than 0.2 ppmv, (2) three or more downwind plume transects, (3) three or more aqueous samples collected, and (4) consistent wind direction. Of the 201 aqueous samples collected, 47 samples from six flights met the criteria and are summarized in Table S1 in the Supplement. Each aqueous sample had a measurable absorption signal in the deep UV region (250 to 300 nm), while the absorption signal above 300 nm was nearly indistinguishable from the blanks because the samples were relatively dilute. The average (± standard deviation) integrated absorption of the 47 samples that met the criteria was 10.4±4.9 mAU min (8134±3857 Mm−1) and 0.36±0.28 mAU min (316±214 Mm−1) for 250 and 300 nm, respectively.
To account for plume dilution, we follow the convention of normalizing BrC absorption to a conserved tracer to calculate (Forrister et al., 2015; Di Lorenzo et al., 2017; Washenfelder et al., 2022; Zeng et al., 2021; Sullivan et al., 2022), where ΔCO is the average CO mixing ratio measured during each aqueous sample collection subtracted by the average CO background outside the plume. Background BrC absorption at 365 nm (a common wavelength to report BrC absorption) was less than 0.2 Mm−1, and no background correction was made to determine ΔAbsλ,BrC (Washenfelder et al., 2022). The average CO and variation in the CO measured for each flight are shown in Fig. S9. Figure 1 shows as a function of plume age for the six selected flights, with a linear fit to each flight. The fitted slopes for vs. plume age vary from −0.21 to 0.88 and show different trends between flights. This indicates that BrC absorption increased downwind in some plumes and decreased downwind in others.
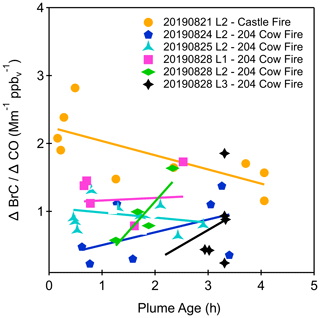
Figure 1Ratio of absorption measured by SEC-UV at 300 nm to CO enhancement as a function of plume age for aqueous samples collected for six flights during FIREX-AQ 2019.
Previous studies of normalized BrC absorption with plume age have reported conflicting results. In the earliest aircraft study, Forrister et al. (2015) collected filter samples from two fires in the western USA and measured the BrC absorption from water and methanol extracts. They observed that BrC absorption at 365 nm decayed exponentially over a plume age range spanning 0 to 50 h (Fig. S10; Forrister et al., 2015). Di Lorenzo et al. (2017) reported total absorption of size-resolved BrC chromophores using SEC-UV from three locations that were influenced to varying degrees by biomass burning smoke and observed minimal change as a function of transport times from 10 to >72 h (Fig. S10). In contrast to these measurements of relatively aged biomass burning aerosol, two studies from other FIREX-AQ instruments showed different trends for relatively fresh plumes. Using BrC–PILS measurements from the Twin Otter, Washenfelder et al. (2022) showed variable trends in slope values ranging from −0.02 to 0.02 over 0 to 5 h. Using filter samples from the DC-8 aircraft (Zeng et al., 2022) showed that BrC increased, decreased, or was unchanged as a function of plume age over 0 to 8 h. In another study of fresh plumes, aircraft-based measurements during the Western wildfire Experiment for Cloud chemistry, Aerosol absorption and Nitrogen (WE-CAN; Sullivan et al., 2022) investigated the evolution of water-soluble BrC at 405 nm normalized to CO and observed BrC depletion with a smoke age of <2 h and PILS water-soluble BrC absorption that broadly remained stable for a smoke age up to 9 h (Sullivan et al., 2022).
Our results are broadly consistent with measurements from FIREX-AQ and WE-CAN that sampled fresh plumes and differ from the studies that sampled more aged smoke. The relatively limited plume age range of the FIREX-AQ sampling makes it challenging to deduce long-term trends associated with changes in total absorption as a function of transport time. In addition, the disparity in time dependence between FIREX-AQ observations and those reported by Forrister et al. (2015) may be attributed to (i) FIREX-AQ having sampled a greater number of western USA forest fires and (ii) the younger age of the FIREX-AQ plumes. More in-flight sampling would be required to observe BrC absorption of plume ages from 5 to 50 h to determine if the results observed by Forrister et al. (2015) would also show variability with a greater number of fires or if the BrC lifetime would converge to a similar value.
3.2 Chemical evolution of brown carbon with plume age
Chromophores <500 Da were responsible for most of the absorption at 250–300 nm measured in the aqueous samples (Figs. 2 and S11). For the 47 samples, molecular species >500 Da contributed an average of 3.0±1.9 % to total measured absorption at 250 nm, while molecular species <500 Da contributed an average of 72±4.5 %. Absorption past the exclusion volume represents an unidentified MW, as elution past this retention time (Fig. S3) indicates that non-SEC analyte–column interactions were occurring. The average contribution to total measured absorption by undefined MW species was 25.1±5.7 %. Previous SEC-UV analyses have observed elution beyond the exclusion volume and non-size-exclusion effects (Wong et al., 2017; Lyu et al., 2021). Elution at later retention times has also been observed for fresh BrC separated in a mobile phase containing 50 % acetonitrile (Lyu et al., 2021). This result was attributed to non-size-exclusion effects, such as hydrophobic interactions of BrC with the SEC stationary phase, which may also have contributed to elution past the exclusion volume in our samples. The absorption density plots of the aqueous samples from the flights listed in Table S1 had similar size-resolved features with varying magnitude in absorption (Fig. S5).
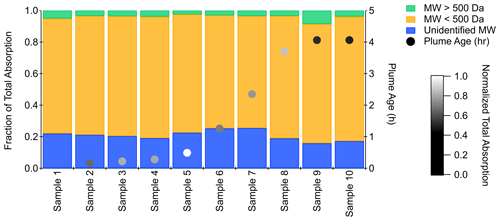
Figure 2Absorption contribution at 300 nm of high (>500 Da), low (<500 Da), and unidentified molecular weight species for aqueous samples collected during the second flight leg on 21 August 2019.
These results are the first reported SEC-UV measurements of very fresh (0–5 h) field samples of biomass burning smoke, and they confirm some of the observations from field studies that measured more aged smoke, as well as laboratory studies that generated fresh or aged smoke. Previous studies that examined biomass burning BrC using SEC-UV have similarly concluded that fresh, less aged smoke contains a large fraction of lower-MW absorbing species (Di Lorenzo et al., 2017; Wong et al., 2017; Lyu et al., 2021). In the examination of field samples, Di Lorenzo et al. (2017) collected ambient biomass burning aerosols that had been aged 10 to >72 h. They observed that low-MW (<500 Da) chromophores contributed more to total absorption than higher-MW (>500 Da) compounds in the least aged (10 to 15 h) biomass-burning-derived filter extracts (Di Lorenzo et al., 2017). These findings resemble the absorption features of our FIREX-AQ samples, which span a plume age range from 0 to 5 h. Wong et al. (2019) used SEC-UV to analyze filter extracts collected during fire seasons in Greece, with atmospheric ages of 1 to ∼70 h, and observed that high-MW species dominated the total BrC absorption of the fresh and aged smoke. Differences between the FIREX-AQ aqueous samples and the results presented by Wong et al. (2019) can be driven by varying types of fuel emissions, photochemical conditions, meteorology, and differences in back-trajectory analyses.
Two studies have applied SEC-UV analysis to lab-generated or lab-aged smoke samples. Wong et al. (2017) pyrolyzed dry hardwood and aged the samples from 0 to 10 h with UV light. They found that low-MW chromophores dominated the total absorption compared to high-MW species, which is generally consistent with our observations. Lyu et al. (2021) generated biomass burning aerosol from laboratory combustion of boreal peat and also analyzed the aerosol by SEC-UV. Under the same SEC-UV separation conditions, the FIREX-AQ water aqueous samples parallel the findings of Lyu et al. (2021), with low-W BrC chromophores dominating total absorption for unaged fresh smoke and smoke aged between 0 and 5 h in the atmosphere.
3.3 Comparison of SEC-UV and BrC–PILS absorption
Online and offline absorption sampling are complementary. The online sampling by the BrC–PILS provides continuous data with a much higher time resolution (reported at 10 s), but it is limited to two measurements, namely water-soluble absorption as a function of wavelength and water-soluble organic carbon concentration. In contrast, offline samples can be examined using SEC-UV, C18 chromatography, and other analytical techniques that are not feasible on board an aircraft. During FIREX-AQ, the BrC–PILS measured online water-soluble absorption in the same aqueous flow that was collected for offline sampling. These are the only BrC samples whose absorption was measured online and then subsequently offline during FIREX-AQ. We observe differences between the online and offline samples. First, absorption by the offline SEC-UV at wavelengths greater than 300 nm did not exceed its detection limit. To facilitate comparison of absorption magnitudes, a power law fit was applied to the BrC–PILS absorption between 315 and 395 nm to extrapolate absorption to 300 nm (Fig. 3). The total absorption by the SEC-UV measurements is approximately an order of magnitude greater than the BrC–PILS measurements at 300 nm (Fig. 3). To determine if the differences could be attributed to differences in the diode-array detector from the SEC-UV analysis and the BrC–PILS spectrometer, a standard solution of 4-nitrocatechol was run on both systems in pure water (without the SEC column in line and bypassing the PILS). At 350 nm, the agreement between the online, offline, and literature measurements of 4-nitrocatechol absorption was ±2.1 % (Fig. S13; Hinrichs et al., 2016).
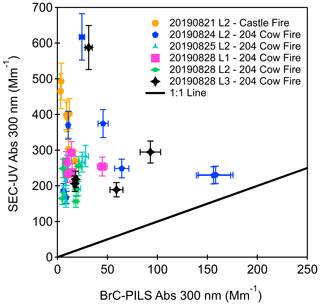
Figure 3Total absorption measured offline by the SEC-UV (at 300 nm) compared to the total absorption measured online by the BrC–PILS (extrapolated to 300 nm using a power law fit). Each colour represents a different flight leg, and each marker represents the integrated absorption at 300 nm for each aqueous sample measured by SEC-UV. The online BrC–PILS absorption measurement was averaged over the collection time of each aqueous sample. The error bars represent the total uncertainty in the online and offline measurements.
The comparison between the online and offline work presented here can be compared to previous non-colocated online–offline intercomparison studies. Di Lorenzo et al. (2017) compared offline absorption measurements of filter extracts by SEC-UV to PILS-LWCC online measurements during the Southern Oxidant and Aerosol Study (SOAS). Although the SEC-UV offline samples and online measurements during SOAS were not colocated, a median ratio (SEC-UV offline at 300 nm to PILS-LWCC online at 365 nm) of 0.9 and r2 of 0.53 (Fig. S12) were found. Zeng et al. (2021) also present an online–offline absorption comparison of water-soluble BrC collected on board the NASA DC-8 aircraft during FIREX-AQ. Online absorption measurements by a LWCC and aqueous filter extracts injected onto a LWCC offline showed good agreement at 365 nm (r2=0.84). The correlation suggested that the filter measurement of BrC is not significantly influenced by possible sampling artifacts associated with absorption of gases or evaporative loss of BrC components associated with filter collection (Zeng et al., 2021).
Differences observed in the FIREX-AQ aqueous samples indicate the necessity to investigate a potential explanation for these inconsistencies. Since the online BrC–PILS and offline SEC-UV samples represent the same samples, solubilization differences between aerosol collection methods cannot explain the variability observed between our measurements. Although reasonable agreement between online measurements and offline filter analyses has been demonstrated (Di Lorenzo et al., 2017; Zeng et al., 2021), Resch et al. (2023) indicated that filter extracts that are not refrigerated immediately or extracts that remain refrigerated for an extended storage time are susceptible to compositional changes. For logistical reasons, our aqueous samples were collected into polypropylene tubes in the field and were not immediately subjected to controlled refrigeration or to the SEC-UV analysis. The greater absorption observed by the SEC-UV analysis (Fig. 3) could reflect the possible hydrolysis of oligomeric compounds stored in aqueous solution, resulting in an increase the intensity of precursor monomers as decomposition products (Resch et al., 2023). We consider this, as well as other potential causes for the differences, in the next section.
3.4 Investigating the impact of solvent effects, pH, and storage effects on absorption spectra
The analysis of fresh biomass burning samples by SEC-UV may be affected by mobile-phase solvation effects, pH, and sample storage conditions. We analyze and discuss these variables below and make recommendations for SEC-UV analysis. Plausible chemical structures of chromophores responsible for BrC absorption have been identified and consist of conjugated systems functionalized with hydroxyls, amines, nitro, carbonyls, and carboxylic acid groups (Laskin et al., 2015; Hems et al., 2021; Lin et al., 2017; Fleming et al., 2020; Y. Zeng et al., 2020; Hettiyadura et al., 2021; Marrero-Ortiz et al., 2019; De Haan et al., 2018; Ji et al., 2022). The molecular complexities of BrC species may be susceptible to changes in the absorption spectra, depending on the analysis conditions.
First, we assess solvation effects due to changes in the mobile-phase composition. The PILS solubilizes BrC in pure water for the online measurements to facilitate absorption measurements (Weber et al., 2001). In contrast, the mobile phase used for the offline SEC-UV analysis was a mixture of acetonitrile and DIW with 25 mM ammonium acetate. Chromatographic packing materials are often incompatible with pure water and require a mixture with an organic solvent to elute compounds from the stationary phase or, in SEC separations, to prevent sorption to the stationary phase. For this reason, chromatographic-partitioning-based separations occur in aqueous–organic mixtures, where the composition can be deliberately modified to optimize interactions of the target molecules between the stationary phase and mobile phase. In SEC, non-size-exclusion interactions between the analyte and stationary phase are dominated by electrostatic and hydrophobic interactions (Hong et al., 2012). If the analyte and stationary phase are identically charged, ion exclusion effects can occur, resulting in an earlier elution time, as the analyte is prevented from entering the pores. If the analyte and stationary phase are oppositely charged, adsorption can result, leading to a later elution time. Hydrophobic effects can occur if the analyte interacts with hydrophobic sites of the column matrix (Hong et al., 2012). The purpose of adding ammonium acetate to the mobile phase is to increase the ionic strength of the mobile phase and facilitate ion pairing, which suppresses electrostatic interactions between the stationary phase and the polar and charged functional groups. The organic solvent used in our mobile phase was acetonitrile, which has been shown to be unreactive towards typical BrC components and has been recommended as an inert solvent for BrC extraction and analysis (Walser et al., 2008; Bateman et al., 2008; Chen et al., 2022). Therefore, we do not expect the mobile phase to chemically alter BrC compounds while being effective at mitigating column stationary phase–analyte interactions.
While chemical changes caused by our mobile phase are unlikely, it is possible that other solvent effects on absorption could be occurring. Effects of solvent on molecular absorption are well established in the photochemistry literature (Lignell et al., 2014; Mo et al., 2017; Zheng et al., 2018; Lyu et al., 2021; Chen et al., 2022; Dalton et al., 2023). The polarity of the solvent affects the absorption wavelength by changing stabilization of the ground and/or excited states. With a decrease in solvent polarity (acetonitrile water is less polar relative to pure water), this can lead to a decrease in the stabilization of the ground state of BrC compounds (such as 4-nitrocatechol), but this effect is molecule dependent. The impact of solvation on red and blue spectral shifting will likely be several nanometers, which could contribute to the observed differences in the offline and online absorption measurements. Previous work has shown that acetonitrile could disrupt π−π interactions between BrC molecules, which could cause the liberation of adsorbed low-MW BrC chromophores from larger chromophores or disrupt BrC aggregates (Lyu et al., 2021). Smaller, less conjugated systems typically absorb in the ultraviolet blue wavelength region, and their transition to red shifts when more conjugated systems are fused together (Gorkowski et al., 2022). Thus, we would expect absorption measurements in the presence of acetonitrile to be blue-shifted relative to those in pure water. This represents a possible explanation for greater absorption intensity at lower wavelengths measured in the offline SEC-UV analysis compared to the online analysis.
Second, we assess the pH of the sample matrix, which is known to affect the absorption profile of BrC compounds. Multiple studies have investigated the impact of pH on wavelength-dependent absorption. For example, Phillips et al. (2017) directly adjusted the pH of SRFA and biomass-burning-derived aqueous extracts (with sodium hydroxide or hydrochloric acid) and observed no measurable shift in the spectra to shorter or longer wavelengths; however, they did observe that as the pH increased, there was an increase in the magnitude of absorption, which was more pronounced at higher wavelengths. The pH of the default mobile-phase solution was 7.2, while the pH of the deionized water solutions in the PILS was approximately 5 (due to carbon dioxide dissolution). To investigate the impact pH has on BrC absorption, we measured several compounds that have been shown to contribute to BrC absorption (4-nitrocatechol, vanillin, 7-hydroxycoumarin, 4-hydroxy-3-methoxycinnamaldehyde, and mixture of the four compounds) under different solvent and pH conditions, such as DIW and DIW with 25 mM ammonium acetate, as well as the mobile phase at pH 5, 7.2, and 9. When the matrix conditions have a pH greater than the pKa of the compound in question, the species will deprotonate, resulting in a shift to longer wavelengths (Hinrichs et al., 2016). For compounds with a pKa between 5 and 9 (i.e., 4-nitrocatechol, 7-hydroxycoumarin, and vanillin), we observed this phenomenon (Fig. S6). To assess the impact of pH and mobile phase on a complex mixture, we also measured the absorption of a SRFA aqueous solution and a FIREX-AQ aqueous sample with the abovementioned mobile phases (Figs. 4 and S8). In contrast to the individual BrC compounds, no major changes in the spectral shape were observed under different mobile-phase conditions. To confirm these results, we measured the absorption of SRFA in each solvent, using a separate spectrophotometer (Fig. S7). This suggests that the pKa values of the majority of functional groups in the absorbing compounds present were less than 5 or above 9. Nitroaromatic compounds typically have pKa values between 5 and 8, thus suggesting low levels of this class of compound present in the aqueous samples. This observation is comparable to the online BrC–PILS analysis; for aqueous absorption, Washenfelder et al. (2022) observed that the average absorption contribution at 365 nm of 4-nitrocatechol was less than 1.1 %, and the summed contribution to absorption by 2-nitrophenol, 4-nitrophenol, 4-nitrocatechol, 4-nitroguaiacol, and 2,4-dinitrophenolate was less than 3.6 %. Since the absorption profile of SRFA and the FIREX-AQ sample appear similar in all mobile-phase conditions, we have no evidence that pH of the mobile phase in the SEC separation conditions impacts the wavelength-dependent absorption of the FIREX-AQ aqueous samples.
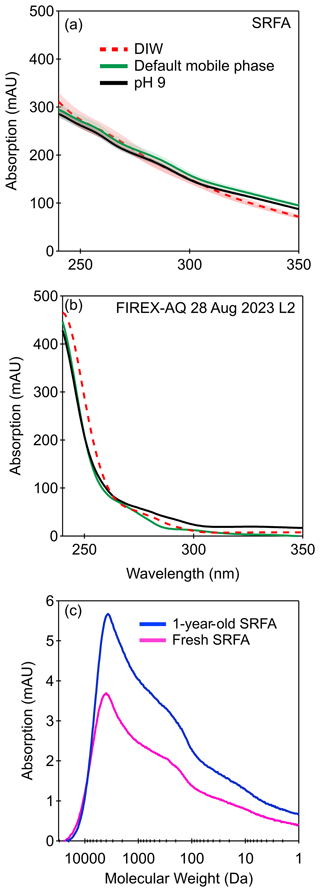
Figure 4Absorption as a function of wavelength of (a) SRFA and (b) a FIREX-AQ aqueous sample collected on 28 August 2019 (L3) with varying mobile phases. (c) Molecular weight profile of a freshly made 15 µg mL−1 SRFA solution and the same solution 1 year later. The shaded region represents the coefficient of variation for absorption at each wavelength using n=3 DIW.
Third, we assess the potential effect of storage on the aqueous samples measured by SEC-UV. A recent study by Resch et al. (2023) observed that biomass-burning-derived filter extracts stored at temperatures above freezing may undergo compositional changes that can increase in signal for various compounds. Hydrolysis reactions include converting alkenes to alcohols and esters to carboxylic acids and the breakdown of oligomers. The hydrolysis of oligomers such as dimer esters stored in an aqueous solution can result in an increase in precursor monomers as decomposition products, leading to an increase in signal (Zhao et al., 2018; Resch et al., 2023). Furthermore, ammonium and alkylamines have been observed in high levels in biomass burning aerosols (Di Lorenzo et al., 2018); aqueous reactions between dicarbonyls (e.g., glyoxal and methylglyoxal) with ammonium and amines may also contribute to an increase in absorption intensity at pH 4 to 7 (Powelson et al., 2014; Yang et al., 2023). The FIREX-AQ aqueous samples had a pH of 5 and were stored at 4 ∘C for 2 years prior to analysis. Assuming that they contained dicarbonyl compounds and reduced nitrogenous species, it is possible that reactions leading to products that can contribute to greater absorption during storage occurred. To further investigate the impacts of storage on a complex aqueous mixture, we measured the absorption spectra of two SRFA solutions, namely one freshly made and one stored for 1 year at 4 ∘C. We observed an increase in absorption in the aged SRFA solution in which integrated absorption was 39 % higher than the freshly made solution. This same effect was also observed with SRHA solutions (Fig. S14). Thus, it is possible that processes during storage could have led to increased absorption measured in the offline SEC samples.
Among the three processes discussed here, we conclude that the storage of aqueous extracts is the most plausible explanation for the higher absorption observed in the offline samples from FIREX-AQ. If hydrolysis reactions are occurring, we might expect this to impact the MW profile (i.e., SEC elution times). We examined the MW profile of freshly made and 1-year-aged SRFA solutions (Fig. 4c). The increase in absorption with storage does not measurably affect the molecular-size-resolved absorption of the mixtures. The same effect was observed for SRHA (Fig. S14). This demonstrates that any storage-induced changes in these complex mixtures of organic molecules have a minimal impact on the molecular weight relative to the wide MW range of the SEC column. The MW of the BrC species would have to change by ∼100 Da to be noticeable on the MW scale of our separation (250 Da to 75 kDa). Such a drastic change in MW is unlikely the case in most hydrolysis reactions. Thus, our results above in which we broadly categorize MW species to be less than or greater than 500 Da are likely robust. The SEC separation of the aqueous samples signify that low-MW (<500 Da) chromophores contribute more to total absorption than higher MW (>500 Da). This finding is supported by previous SEC-UV analyses of BrC aged less than 10 h (Di Lorenzo et al., 2017; Lyu et al., 2021). The consistent MW profiles between the freshly made and stored solutions of SRFA and SRHA reasonably suggest that storage did not have a major impact on the MW of BrC.
During FIREX-AQ, instruments onboard the NOAA Twin Otter aircraft sampled smoke plumes from wildfires in the western United States with plume ages of 0 to 5 h. The BrC–PILS measured water-soluble BrC absorption online and collected aerosol in aqueous solution for offline SEC-UV analysis. The aqueous samples were collected during downwind plume transects, and the online data were collected continuously during in-flight sampling. SEC-UV analysis shows that BrC absorption was dominated by chromophores <500 Da. This finding is consistent with reports of laboratory-generated fresh smoke samples. Integrated absorption at 300 nm from the SEC-UV analysis was used to calculate trends in normalized BrC absorption as a function of plume age. These trends were variable and did not show an exponential decay, which is consistent with recently published results from the FIREX-AQ field campaign that examined normalized BrC absorption trends for plumes over 0 to 10 h. Comparison of the online and offline analyses of the same aqueous extracts reveals discrepancies, specifically higher absorption intensity and absorption at lower wavelengths. These discrepancies between online and offline samples demonstrate the importance of considering the conditions in which the absorption measurements are made. The inconsistencies between the offline SEC-UV analysis and the online measurements are not explained by pH or solvent effects but may be due to reactions occurring during storage. Although increases in absorption may occur during storage of aqueous solutions, it is less likely to impact the MW of the FIREX-AQ BrC species. This highlights that the BrC species are more stable when collected on filters rather than in an aqueous solution and the importance of intercomparison absorption measurements by multiple methods.
The data used in the study are publicly available at https://csl.noaa.gov/groups/csl7/measurements/2019firex-aq/TwinOtter/DataDownload/ (FIREX-AQ Platform, 2023).
The supplement related to this article is available online at: https://doi.org/10.5194/acp-23-15643-2023-supplement.
LA and RAW collected the online absorption measurements and samples for the offline analysis. RAW designed the BrC-PILS and processed the online measurements. LA and CJY developed and completed the SEC-UV analysis. MAR, AF, and CCW contributed to the collection of the water samples. CDH contributed to the plume age analysis. TN and CS contributed to the carbon monoxide measurements. SSB, AM, RAW, MAR, and AF contributed to the overall success of inflight sampling and the editing this paper.
At least one of the (co-)authors is a member of the editorial board of Atmospheric Chemistry and Physics. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank Carsten Warneke, Joshua Schwarz, James Crawford, and Jack Dibb for organizing the FIREX-AQ field campaign. We thank the NOAA Aircraft Operations Center for support during the field mission. Lisa Azzarello has been supported by a Mitacs Globalink Research Internship. The FIREX-AQ project has been supported by the NOAA Atmospheric Chemistry, Carbon, and Climate Program (AC4). We thank Robert Di Lorenzo and Trevor VandenBoer for the helpful discussions. We thank the three anonymous reviewers for their insightful feedback.
This research has been supported by the Natural Sciences and Engineering Research Council of Canada (grant no. RGPIN-2018-05990).
This paper was edited by Jason Surratt and reviewed by three anonymous referees.
Andreae, M. O. and Gelencsér, A.: Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols, Atmos. Chem. Phys., 6, 3131–3148, https://doi.org/10.5194/acp-6-3131-2006, 2006.
Bateman, A. P., Walser, M. L., Desyaterik, Y., Laskin, J., Laskin, A., and Nizkorodov, S. A.: The effect of solvent on the analysis of secondary organic aerosol using electrospray ionization mass spectrometry, Environ. Sci. Technol., 42, 7341–7346, https://doi.org/10.1021/es801226w, 2008.
Bluvshtein, N., Lin, P., Flores, M., Segev, L., Mazar, Y., Tas, E., Snider, G., Weagle, C., Brown, S., Laskin, A., and Rudich, Y.: Broadband optical properties of biomass burning aerosol and identification of brown carbon chromophores, J. Geophys. Res.-Atmos., 122, 5441–5456, https://doi.org/10.1002/2016JD026230, 2017.
Bond, T. C., Streets, D. G., Yarber, K. F., Nelson, S. M., Woo, J.-H., and Klimont, Z.: A technology-based global inventory of black and organic carbon emissions from combustion, J. Geophys. Res.-Atmos., 109, D14203, https://doi.org/10.1029/2003JD003697, 2004.
Boucher, O., Randall, D., Artaxo, P., Bretherton, C., Feingold, G., Forster, P., Kerminen, V. M., Kondo, Y., Liao, H., Lohmann, U., Rasch, P., Satheesh, S. K., Sherwood, S., Stevens, B., and Zhang, X. Y.: Clouds and Aerosols, in: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York, NY, https://doi.org/10.1017/CBO9781107415324.016, 2013.
Buis, A.: The climate connections of a record fire year in the U.S. West – climate change vital Signs of the planet, https://climate.nasa.gov/blog/3066/the-climate-connections-of-a-record-fire-year-in-the-us-west/ (last access: 10 October 2023), 2021.
Chen, K., Raeofy, N., Lum, M., Mayorga, R., Woods, M., Bahreini, R., Zhang, H., and Lin, Y.-H.: Solvent effects on chemical composition and optical properties of extracted secondary brown carbon constituents, Aerosol Sci. Tech., 56, 917–930, https://doi.org/10.1080/02786826.2022.2100734, 2022.
Crosson, E. R.: A cavity ring-down analyzer for measuring atmospheric levels of methane, carbon dioxide, and water vapor, Appl. Phys. B, 92, 403–408, https://doi.org/10.1007/s00340-008-3135-y, 2008.
Dalton, A. B., Le, S. M., Karimova, N. V., Gerber, R. B., and Nizkorodov, S. A.: Influence of solvent on the electronic structure and the photochemistry of nitrophenols, Environ. Sci.-Atmos., 3, 257–267, https://doi.org/10.1039/D2EA00144F, 2023.
De Haan, D. O., Tapavicza, E., Riva, M., Cui, T., Surratt, J. D., Smith, A. C., Jordan, M.-C., Nilakantan, S., Almodovar, M., Stewart, T. N., de Loera, A., De Haan, A. C., Cazaunau, M., Gratien, A., Pangui, E., and Doussin, J.-F.: Nitrogen-containing, light-absorbing oligomers produced in aerosol particles exposed to methylglyoxal, photolysis, and cloud cycling, Environ. Sci. Technol., 52, 4061–4071, https://doi.org/10.1021/acs.est.7b06105, 2018.
Di Lorenzo, R. A. and Young, C. J.: Size separation method for absorption characterization in brown carbon: Application to an aged biomass burning sample, Geophys. Res. Lett., 43, 458–465, https://doi.org/10.1002/2015GL066954, 2016.
Di Lorenzo, R. A., Washenfelder, R. A., Attwood, A. R., Guo, H., Xu, L., Ng, N. L., Weber, R. J., Baumann, K., Edgerton, E., and Young, C. J.: Molecular-size-separated brown carbon absorption for biomass-burning aerosol at multiple field sites, Environ. Sci. Technol., 51, 3128–3137, https://doi.org/10.1021/acs.est.6b06160, 2017.
Di Lorenzo, R. A., Place, B. K., VandenBoer, T. C., and Young, C. J.: Composition of Size-Resolved Aged Boreal Fire Aerosols: Brown Carbon, Biomass Burning Tracers, and Reduced Nitrogen, ACS Earth and Space Chemistry, 2, 278–285, https://doi.org/10.1021/acsearthspacechem.7b00137, 2018.
Feng, Y., Ramanathan, V., and Kotamarthi, V. R.: Brown carbon: a significant atmospheric absorber of solar radiation?, Atmos. Chem. Phys., 13, 8607–8621, https://doi.org/10.5194/acp-13-8607-2013, 2013.
Finewax, Z., de Gouw, J. A., and Ziemann, P. J.: Identification and quantification of 4-nitrocatechol formed from OH and NO3 radical-initiated reactions of catechol in air in the presence of NOx: implications for secondary organic aerosol formation from biomass burning, Environ. Sci. Technol., 52, 1981–1989, https://doi.org/10.1021/acs.est.7b05864, 2018.
FIREX-AQ Platform: NOAA-CHEM Twin Otter Data Download, https://csl.noaa.gov/groups/csl7/measurements/2019firex-aq/TwinOtter/DataDownload/, last access: 18 December 2023.
Fleming, L. T., Lin, P., Roberts, J. M., Selimovic, V., Yokelson, R., Laskin, J., Laskin, A., and Nizkorodov, S. A.: Molecular composition and photochemical lifetimes of brown carbon chromophores in biomass burning organic aerosol, Atmos. Chem. Phys., 20, 1105–1129, https://doi.org/10.5194/acp-20-1105-2020, 2020.
Forrister, H., Liu, J., Scheuer, E., Dibb, J., Ziemba, L., Thornhill, K. L., Anderson, B., Diskin, G., Perring, A. E., Schwarz, J. P., Campuzano-Jost, P., Day, D. A., Palm, B. B., Jimenez, J. L., Nenes, A., and Weber, R. J.: Evolution of brown carbon in wildfire plumes, Geophys. Res. Lett., 42, 4623–4630, https://doi.org/10.1002/2015GL063897, 2015.
Forster, P., Ramaswamy, V., Artaxo, P., Berntsen, T., Betts, R. , Fahey, D. W., Haywood, J., Lean, J., Lowe, D. C., Myhre, G., Nganga, J., Prinn, R., Raga, G., Schulz, M., and Van Dorland, R.: Changes in atmospheric constituents and in radiative forcing, in: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K. B., Tignor, M., and Miller, H. L., Cambridge University Press: Cambridge, United Kingdom and New York, NY, 129-−243, ISBN: 9780521880091, 2007.
Gorkowski, K., Benedict, K. B., Carrico, C. M., and Dubey, M. K.: Complexities in Modeling Organic Aerosol Light Absorption, J. Phys. Chem. A, 126, 4827–4833, https://doi.org/10.1021/acs.jpca.2c02236, 2022.
Harrison, M. A. J., Barra, S., Borghesi, D., Vione, D., Arsene, C., and Olariu, R. I.: Nitrated phenols in the atmosphere: a review, Atmos. Environ., 39, 231–248, https://doi.org/10.1016/j.atmosenv.2004.09.044, 2005.
Hems, R. F., Schnitzler, E. G., Liu-Kang, C., Cappa, C. D., and Abbatt, J. P. D.: Aging of atmospheric brown carbon aerosol, ACS Earth and Space Chemistry, 5, 722–748, https://doi.org/10.1021/acsearthspacechem.0c00346, 2021.
Hettiyadura, A. P. S., Garcia, V., Li, C., West, C. P., Tomlin, J., He, Q., Rudich, Y., and Laskin, A.: Chemical composition and molecular-specific optical properties of atmospheric brown carbon associated with biomass burning, Environ. Sci. Technol., 55, 2511–2521, https://doi.org/10.1021/acs.est.0c05883, 2021.
Hinrichs, R. Z., Buczek, P., and Trivedi, J. J.: Solar absorption by aerosol-bound nitrophenols compared to aqueous and gaseous nitrophenols, Environ. Sci. Technol., 50, 5661–5667, https://doi.org/10.1021/acs.est.6b00302, 2016.
Hong, P., Koza, S., and Bouvier, E. S. P.: a review size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates, J. Liq. Chromatogr. R. T., 35, 2923–2950, https://doi.org/10.1080/10826076.2012.743724, 2012.
Ji, Y., Shi, Q., Ma, X., Gao, L., Wang, J., Li, Y., Gao, Y., Li, G., Zhang, R., and An, T.: Elucidating the critical oligomeric steps in secondary organic aerosol and brown carbon formation, Atmos. Chem. Phys., 22, 7259–7271, https://doi.org/10.5194/acp-22-7259-2022, 2022.
Karion, A., Sweeney, C., Wolter, S., Newberger, T., Chen, H., Andrews, A., Kofler, J., Neff, D., and Tans, P.: Long-term greenhouse gas measurements from aircraft, Atmos. Meas. Tech., 6, 511–526, https://doi.org/10.5194/amt-6-511-2013, 2013.
Laskin, A., Laskin, J., and Nizkorodov, S. A.: Chemistry of Atmospheric Brown Carbon, Chem. Rev., 115, 4335–4382, https://doi.org/10.1021/cr5006167, 2015.
Liao, J., Wolfe, G. M., Hannun, R. A., St. Clair, J. M., Hanisco, T. F., Gilman, J. B., Lamplugh, A., Selimovic, V., Diskin, G. S., Nowak, J. B., Halliday, H. S., DiGangi, J. P., Hall, S. R., Ullmann, K., Holmes, C. D., Fite, C. H., Agastra, A., Ryerson, T. B., Peischl, J., Bourgeois, I., Warneke, C., Coggon, M. M., Gkatzelis, G. I., Sekimoto, K., Fried, A., Richter, D., Weibring, P., Apel, E. C., Hornbrook, R. S., Brown, S. S., Womack, C. C., Robinson, M. A., Washenfelder, R. A., Veres, P. R., and Neuman, J. A.: Formaldehyde evolution in US wildfire plumes during the Fire Influence on Regional to Global Environments and Air Quality experiment (FIREX-AQ), Atmos. Chem. Phys., 21, 18319–18331, https://doi.org/10.5194/acp-21-18319-2021, 2021.
Lignell, H., Hinks, M. L., and Nizkorodov, S. A.: Exploring matrix effects on photochemistry of organic aerosols, P. Natl. Acad. Sci. USA, 111, 13780–13785, https://doi.org/10.1073/pnas.1322106111, 2014.
Lin, P., Bluvshtein, N., Rudich, Y., Nizkorodov, S. A., Laskin, J., and Laskin, A.: Molecular chemistry of atmospheric brown carbon inferred from a nationwide biomass burning event, Environ. Sci. Technol., 51, 11561–11570, https://doi.org/10.1021/acs.est.7b02276, 2017.
Liu, J., Lin, P., Laskin, A., Laskin, J., Kathmann, S. M., Wise, M., Caylor, R., Imholt, F., Selimovic, V., and Shilling, J. E.: Optical properties and aging of light-absorbing secondary organic aerosol, Atmos. Chem. Phys., 16, 12815–12827, https://doi.org/10.5194/acp-16-12815-2016, 2016.
Lyu, M., Thompson, D. K., Zhang, N., Cuss, C. W., Young, C. J., and Styler, S. A.: Unraveling the complexity of atmospheric brown carbon produced by smoldering boreal peat using size-exclusion chromatography with selective mobile phases, Environ. Sci.-Atmos., 1, 241–252, https://doi.org/10.1039/D1EA00011J, 2021.
Marlon, J. R., Bartlein, P. J., Gavin, D. G., Long, C. J., Anderson, R. S., Briles, C. E., Brown, K. J., Colombaroli, D., Hallett, D. J., Power, M. J., Scharf, E. A., and Walsh, M. K.: Long-term perspective on wildfires in the western USA, P. Natl. Acad. Sci. USA, 109, E535–E543, https://doi.org/10.1073/pnas.1112839109, 2012.
Marrero-Ortiz, W., Hu, M., Du, Z., Ji, Y., Wang, Y., Guo, S., Lin, Y., Gomez-Hermandez, M., Peng, J., Li, Y., Secrest, J., Zamora, M. L., Wang, Y., An, T., and Zhang, R.: Formation and optical properties of brown carbon from small α-dicarbonyls and amines, Environ. Sci. Technol., 53, 117–126, https://doi.org/10.1021/acs.est.8b03995, 2019.
Mo, Y., Li, J., Liu, J., Zhong, G., Cheng, Z., Tian, C., Chen, Y., and Zhang, G.: The influence of solvent and pH on determination of the light absorption properties of water-soluble brown carbon, Atmos. Environ., 161, 90–98, https://doi.org/10.1016/j.atmosenv.2017.04.037, 2017.
Parks, S. A. and Abatzoglou, J. T.: Warmer and Drier Fire Seasons Contribute to Increases in Area Burned at High Severity in Western US Forests From 1985 to 2017, Geophys. Res. Lett., 47, e2020GL089858, https://doi.org/10.1029/2020GL089858, 2020.
Phillips, S. M., Bellcross, A. D., and Smith, G. D.: Light Absorption by Brown Carbon in the Southeastern United States is pH-dependent, Environ. Sci. Technol., 51, 6782–6790, https://doi.org/10.1021/acs.est.7b01116, 2017.
Powelson, M. H., Espelien, B. M., Hawkins, L. N., Galloway, M. M., and De Haan, D. O.: Brown Carbon Formation by Aqueous-Phase Carbonyl Compound Reactions with Amines and Ammonium Sulfate, Environ. Sci. Technol., 48, 985–993, https://doi.org/10.1021/es4038325, 2014.
Ramanathan, V. and Carmichael, G.: Global and regional climate changes due to black carbon, Nat. Geosci., 1, 221–227, https://doi.org/10.1038/ngeo156, 2008.
Resch, J., Wolfer, K., Barth, A., and Kalberer, M.: Effects of storage conditions on the molecular-level composition of organic aerosol particles, Atmos. Chem. Phys., 23, 9161–9171, https://doi.org/10.5194/acp-23-9161-2023, 2023.
Seinfeld, J. H. and Pankow, J. F.: Organic Atmospheric Particulate Material, Annu. Rev. Phys. Chem., 54, 121–140, https://doi.org/10.1146/annurev.physchem.54.011002.103756, 2003.
Simoneit, B. R. T.: Biomass burning – a review of organic tracers for smoke from incomplete combustion, Appl. Geochem., 17, 129–162, https://doi.org/10.1016/S0883-2927(01)00061-0, 2002.
Smith, J. D., Sio, V., Yu, L., Zhang, Q., and Anastasio, C.: Secondary organic aerosol production from aqueous reactions of atmospheric phenols with an organic triplet excited state, Environ. Sci. Technol., 48, 1049–1057, https://doi.org/10.1021/es4045715, 2014.
Sullivan, A. P., Pokhrel, R. P., Shen, Y., Murphy, S. M., Toohey, D. W., Campos, T., Lindaas, J., Fischer, E. V., and Collett Jr., J. L.: Examination of brown carbon absorption from wildfires in the western US during the WE-CAN study, Atmos. Chem. Phys., 22, 13389–13406, https://doi.org/10.5194/acp-22-13389-2022, 2022.
Turpin, B. J., Cary, R. A., and Huntzicker, J. J.: An in situ, time-resolved analyzer for aerosol organic and elemental carbon, Aerosol Sci. Tech., 12, 161–171, https://doi.org/10.1080/02786829008959336, 1990.
van der Werf, G. R., Randerson, J. T., Giglio, L., Collatz, G. J., Mu, M., Kasibhatla, P. S., Morton, D. C., DeFries, R. S., Jin, Y., and van Leeuwen, T. T.: Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009), Atmos. Chem. Phys., 10, 11707–11735, https://doi.org/10.5194/acp-10-11707-2010, 2010.
Walser, M. L., Desyaterik, Y., Laskin, J., Laskin, A., and Nizkorodov, S. A.: High-resolution mass spectrometric analysis of secondary organic aerosol produced by ozonation of limonene, Phys. Chem. Chem. Phys., 10, 1009–1022, https://doi.org/10.1039/B712620D, 2008.
Warneke, C., Schwarz, J. P., Dibb, J., Kalashnikova, O., Frost, G., Al-Saad, J., Brown, S. S., Brewer, Wm. A., Soja, A., Seidel, F. C., Washenfelder, R. A., Wiggins, E. B., Moore, R. H., Anderson, B. E., Jordan, C., Yacovitch, T. I., Herndon, S. C., Liu, S., Kuwayama, T., Jaffe, D., Johnston, N., Selimovic, V., Yokelson, R., Giles, D. M., Holben, B. N., Goloub, P., Popovici, I., Trainer, M., Kumar, A., Pierce, R. B., Fahey, D., Roberts, J., Gargulinski, E. M., Peterson, D. A., Ye, X., Thapa, L. H., Saide, P. E., Fite, C. H., Holmes, C. D., Wang, S., Coggon, M. M., Decker, Z. C. J., Stockwell, C. E., Xu, L., Gkatzelis, G., Aikin, K., Lefer, B., Kaspari, J., Griffin, D., Zeng, L., Weber, R., Hastings, M., Chai, J., Wolfe, G. M., Hanisco, T. F., Liao, J., Campuzano Jost, P., Guo, H., Jimenez, J. L., Crawford, J., and the FIREX-AQ Science Team: Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ), J. Geophys. Res.-Atmos., 128, e2022JD037758, https://doi.org/10.1029/2022JD037758, 2023.
Washenfelder, R., Attwood, A., Brock, C., Guo, H., Xu, L., Weber, R., Ng, N., Allen, H., Ayres, B., Baumann, K., Cohen, R., Draper, D., Duffey, K., Edgerton, E., Fry, J., Hu, W., Jimenez, J., Palm, B., Romer, P., and Brown, S.: Biomass burning dominates brown carbon absorption in the rural southeastern United States, Geophys. Res. Lett., 42, 653–664, https://doi.org/10.1002/2014GL062444, 2015.
Washenfelder, R. A., Azzarello, L., Ball, K., Brown, S. S., Decker, Z. C. J., Franchin, A., Fredrickson, C. D., Hayden, K., Holmes, C. D., Middlebrook, A. M., Palm, B. B., Pierce, R. B., Price, D. J., Roberts, J. M., Robinson, M. A., Thornton, J. A., Womack, C. C., and Young, C. J.: Complexity in the evolution, composition, and spectroscopy of brown carbon in aircraft measurements of wildfire plumes, Geophys. Res. Lett., 49, e2022GL098951, https://doi.org/10.1029/2022GL098951, 2022.
Weber, R. J., Orsini, D., Daun, Y., Lee, Y.-N., Klotz, P. J., and Brechtel, F.: A particle-into-liquid collector for rapid measurement of aerosol bulk chemical composition, Aerosol Sci. Tech., 35, 718–727, https://doi.org/10.1080/02786820152546761, 2001.
Wong, J. P. S., Nenes, A., and Weber, R. J.: Changes in light absorptivity of molecular weight separated brown carbon due to photolytic aging, Environ. Sci. Technol., 51, 8414–8421, https://doi.org/10.1021/acs.est.7b01739, 2017.
Wong, J. P. S., Tsagkaraki, M., Tsiodra, I., Mihalopoulos, N., Violaki, K., Kanakidou, M., Sciare, J., Nenes, A., and Weber, R. J.: Atmospheric evolution of molecular-weight-separated brown carbon from biomass burning, Atmos. Chem. Phys., 19, 7319–7334, https://doi.org/10.5194/acp-19-7319-2019, 2019.
Xie, M., Chen, X., Hays, M. D., Lewandowski, M., Offenberg, J., Kleindienst, T. E., and Holder, A. L.: Light Absorption of secondary organic aerosol: composition and contribution of nitroaromatic compounds, Environ. Sci. Technol., 51, 11607–11616, https://doi.org/10.1021/acs.est.7b03263, 2017.
Xie, M., Chen, X., Hays, M. D., and Holder, A. L.: Composition and light absorption of N-containing aromatic compounds in organic aerosols from laboratory biomass burning, Atmos. Chem. Phys., 19, 2899–2915, https://doi.org/10.5194/acp-19-2899-2019, 2019.
Yang, L., Huang, R.-J., Shen, J., Wang, T., Gong, Y., Yuan, W., Liu, Y., Huang, H., You, Q., Huang, D. D., and Huang, C.: New Insights into the Brown Carbon Chromophores and Formation Pathways for Aqueous Reactions of α-Dicarbonyls with Amines and Ammonium, Environ. Sci. Technol., 57, 12351–12361, https://doi.org/10.1021/acs.est.3c04133, 2023.
Zeng, L., Zhang, A., Wang, Y., Wagner, N. L., Katich, J. M., Schwarz, J. P., Schill, G. P., Brock, C., Froyd, K. D., Murphy, D. M., Williamson, C. J., Kupc, A., Scheuer, E., Dibb, J., and Weber, R. J.: Global Measurements of Brown Carbon and Estimated Direct Radiative Effects, Geophys. Res. Lett., 47, e2020GL088747, https://doi.org/10.1029/2020GL088747, 2020.
Zeng, L., Sullivan, A. P., Washenfelder, R. A., Dibb, J., Scheuer, E., Campos, T. L., Katich, J. M., Levin, E., Robinson, M. A., and Weber, R. J.: Assessment of online water-soluble brown carbon measuring systems for aircraft sampling, Atmos. Meas. Tech., 14, 6357–6378, https://doi.org/10.5194/amt-14-6357-2021, 2021.
Zeng, L., Dibb, J., Scheuer, E., Katich, J. M., Schwarz, J. P., Bourgeois, I., Peischl, J., Ryerson, T., Warneke, C., Perring, A. E., Diskin, G. S., DiGangi, J. P., Nowak, J. B., Moore, R. H., Wiggins, E. B., Pagonis, D., Guo, H., Campuzano-Jost, P., Jimenez, J. L., Xu, L., and Weber, R. J.: Characteristics and evolution of brown carbon in western United States wildfires, Atmos. Chem. Phys., 22, 8009–8036, https://doi.org/10.5194/acp-22-8009-2022, 2022.
Zeng, Y., Shen, Z., Takahama, S., Zhang, L., Zhang, T., Lei, Y., Zhang, Q., Xu, H., Ning, Y., Huang, Y., Cao, J., and Rudolf, H.: Molecular absorption and evolution mechanisms of PM2.5 brown carbon revealed by electrospray ionization fourier transform–ion cyclotron resonance mass spectrometry during a severe winter pollution episode in Xi'an, China, Geophys. Res. Lett., 47, e2020GL087977, https://doi.org/10.1029/2020GL087977, 2020.
Zhang, Y., Forrister, H., Liu, J., Dibb, J., Anderson, B., Schwarz, J. P., Perring, A. E., Jimenez, J. L., Campuzano-Jost, P., Wang, Y., Nenes, A., and Weber, R. J.: Top-of-atmosphere radiative forcing affected by brown carbon in the upper troposphere, Nat. Geosci., 10, 486–489, https://doi.org/10.1038/ngeo2960, 2017.
Zhao, R., Kenseth, C. M., Huang, Y., Dalleska, N. F., Kuang, X. M., Chen, J., Paulson, S. E., and Seinfeld, J. H.: Rapid Aqueous-Phase Hydrolysis of Ester Hydroperoxides Arising from Criegee Intermediates and Organic Acids, J. Phys. Chem. A, 122, 5190–5201, https://doi.org/10.1021/acs.jpca.8b02195, 2018.
Zheng, D., Yuan, X.-A., Ma, H., Li, X., Wang, X., Liu, Z., and Ma, J.: Unexpected solvent effects on the UV/Vis absorption spectra of o-cresol in toluene and benzene: in contrast with non-aromatic solvents, Roy. Soc. Open Sci., 5, 171928, https://doi.org/10.1098/rsos.171928, 2018.





