the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Estimation of rate coefficients for the reactions of O3 with unsaturated organic compounds for use in automated mechanism construction
Michael E. Jenkin
Richard Valorso
Bernard Aumont
Mike J. Newland
Andrew R. Rickard
Reaction with ozone (O3) is an important removal process for unsaturated volatile organic compounds (VOCs) in the atmosphere. Rate coefficients for reactions of O3 with VOCs are therefore essential parameters for chemical mechanisms used in chemistry transport models. Updated and extended structure–activity relationship (SAR) methods are presented for the reactions of O3 with mono- and poly-unsaturated organic compounds. The methods are optimized using a preferred set of data including reactions of O3 with 221 unsaturated compounds. For conjugated dialkene structures, site-specific rates are defined, and for isolated poly-alkenes rates are defined for each double bond to determine the branching ratios for primary ozonide formation. The information can therefore guide the representation of the O3 reactions in the next generation of explicit detailed chemical mechanisms.
- Article
(1456 KB) - Full-text XML
-
Supplement
(397 KB) - BibTeX
- EndNote
Volatile organic compounds (VOCs) are emitted to the atmosphere from both biogenic and anthropogenic sources. Many of these compounds are unsaturated (i.e. contain at least one double bond), including the ubiquitous biogenic VOCs isoprene and monoterpenes (Sindelarova et al., 2014). Chemical degradation of these compounds in the atmosphere leads to a variety of secondary pollutants including ozone and secondary organic aerosol (SOA). Unsaturated compounds are generally highly reactive and react with the oxidant ozone (O3), which is typically present in the troposphere at mixing ratios in the range 10–200 ppb. The ozonolysis reaction involves the concerted addition of O3 to the double bond, followed by decomposition of the short-lived primary ozonide to yield a carbonyl compound and a carbonyl oxide, commonly referred to as a Criegee intermediate (Criegee, 1975). The reaction is important as a non-photolytic source of radicals and reactive intermediates, including the hydroxyl radical (e.g. Johnson and Marston, 2008; Cox et al., 2020). Ozonolysis of large alkenes (e.g. monoterpenes and sesquiterpenes) is also particularly efficient at producing SOA (Hallquist et al., 2009), including as a result of the formation of low-volatility products from reactions of Criegee intermediates with atmospheric trace gases (e.g. Heaton et al., 2007; Sakamoto et al., 2013; Zhao et al., 2015; Mackenzie-Rae et al., 2018; Chhantyal-Pun et al., 2018), and from auto-oxidation mechanisms involving peroxy radicals formed from decomposition of the Criegee intermediates (e.g. Ehn et al., 2014; Jokinen et al., 2015).
Previous assessments using explicit organic degradation mechanisms have demonstrated that the atmosphere contains an almost limitless number of organic compounds (e.g. Aumont et al., 2005), for which it is impractical to carry out experimental kinetics studies. This has resulted in the development of estimation methods for rate coefficients (e.g. see Calvert et al., 2000, 2011; McGillen et al., 2008; Vereecken et al., 2018, and references therein), which have been applied widely in chemical mechanisms and impact assessments. As part of the present work, a set of preferred kinetic data has been assembled for the reactions of O3 with 221 unsaturated organic compounds, based on reported experimental studies (see Sect. 2 for further details). Updated structure–activity relationship (SAR) methods are presented for the initial reactions of O3 with unsaturated organic compounds. In the cases of poly-alkenes, the rate coefficient is defined in terms of a summation of partial rate coefficients for O3 reaction at each relevant site in the given organic compound, so that the attack distribution is also defined. Application of the methods is illustrated with examples in the Supplement.
The information is currently being used to guide the representation of the O3-initiation reactions in the next generation of explicit detailed chemical mechanisms, based on the Generator for Explicit Chemistry and Kinetics of Organics in the Atmosphere (GECKO-A; Aumont et al., 2005) and the Master Chemical Mechanism (MCM; Saunders et al., 2003). It therefore contributes to a revised and updated set of rules that can be used in automated mechanism construction and provides formal documentation of the methods. This paper is part of a series of publications, including rules for the estimation of rate coefficients and branching ratios for the reactions of OH with aliphatic (Jenkin et al., 2018a) and aromatic (Jenkin et al., 2018b) organic compounds, and for peroxy radical reactions (Jenkin et al., 2019). Rules governing the decomposition of the primary ozonides, formed initially from the O3-initiation reactions, and the subsequent chemistry of the Criegee intermediates formed are considered in a further paper (Mouchel-Vallon et al., 2020).
A set of preferred kinetic data has been assembled from which to develop and validate the estimation methods for the O3 rate coefficients. The complete set includes 298 K data for 221 compounds, comprising 111 alkenes and 110 unsaturated oxygenated compounds. Temperature dependences are also defined for a subset of 39 compounds. In three cases, the preferred rate coefficient is an upper-limit value, and in three cases a lower-limit value. The information is provided as a part of the Supplement (spreadsheets SI_1 and SI_2). As described in more detail in Sect. 4, the oxygenates include both monofunctional and multifunctional compounds containing a variety of functional groups that are prevalent in both emitted VOCs and their degradation products, namely -OH, -C(=O)H, -C(=O)-, -O-, -C(=O)OH, -C(=O)O-, -OC(=O)-, -ONO2 and -C(=O)OONO2. For a core set of 30 reactions, preferred kinetic data are based on the evaluations of the IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation (Cox et al., 2020; http://iupac.pole-ether.fr/, last access: September 2020). The remaining values are informed by recommendations from other key evaluations with complementary coverage (e.g. Atkinson and Arey, 2003; Calvert et al., 2011, 2015) and have been revised and expanded following review and evaluation of additional data not included in those studies (as identified in spreadsheets SI_1 and SI_2).
As discussed in detail previously (e.g. Calvert et al., 2000; Vereecken et al., 2018, and references therein), the data indicate that the rate coefficients are highly sensitive to alkene structure and depend on the degree of alkyl substitution of the unsaturated bond(s), on steric effects and on ring strain effects in cyclic compounds. The set of preferred kinetic data has been used to update and extend a SAR method that can be used to estimate the rate coefficients when no experimental determinations are available. Similar to previous appraisals (e.g. Jenkin et al., 1997; Calvert et al., 2000), reference rate coefficients (k) are defined for addition of O3 to a series of alkene and conjugated dialkene structures, based on the preferred data for relevant sets of alkenes and conjugated dialkenes.
Table 1Arrhenius parameters () for the reference rate coefficients for O3 addition to generic monoalkenes containing linear alkyl groups (i.e. R can be -CH3 or -CH2R′), and the rate coefficient values at 298 Ka.
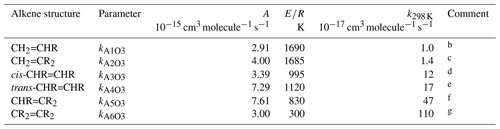
a k298K and E∕R based on rounded averages for
the sets of compounds identified in subsequent comments, with . Rate coefficients are also default values
for alkenes possessing remote substituents (i.e. β or higher),
including isolated double bonds.
b k298 K based on preferred data for propene,
but-1-ene, pent-1-ene, hex-1-ene, hept-1-ene, oct-1-ene and dec-1-ene; E∕R
based on preferred data for propene, but-1-ene, pent-1-ene, hex-1-ene and
hept-1-ene.
c k298 K based on preferred data for 2-methyl-propene,
2-methyl-but-1-ene, 2-methyl-pent-1-ene, 2-methyl-hept-1-ene,
2-methyl-oct-1-ene, 2-methyl-dec-1-ene, 2-methyl-undec-1-ene and
2-ethyl-but-1-ene; E∕R based on preferred data for 2-methyl-propene and
2-methyl-but-1-ene.
d k298 K based on preferred data for
cis-but-2-ene, cis-pent-2-ene, cis-hex-2-ene,
cis-hex-3-ene, cis-oct-4-ene and cis-dec-5-ene;
E∕R based on preferred data for cis-but-2-ene,
cis-pent-2-ene and cis-hex-2-ene.
e k298 K based on preferred data for
trans-but-2-ene, trans-pent-2-ene, trans-hex-2-ene,
trans-hex-3-ene and trans-oct-4-ene; E∕R based on
preferred data for trans-but-2-ene, trans-pent-2-ene and
trans-hex-2-ene.
f k298 K based on preferred data for
2-methyl-but-2-ene, cis-3-methyl-pent-2-ene and
trans-3-methyl-pent-2-ene; E∕R based on preferred data for
2-methyl-but-2-ene; where applicable, parameters are assumed to apply to both
cis- and trans-isomers.
g k298 K and E∕R based on preferred data for 2,3-dimethyl-but-2-ene; k298K is also consistent with reported lower-limit k for 3,4-dimethyl-hex-3-ene (cis- and trans-); where applicable, parameters are assumed to apply to both cis- and trans-isomers.
3.1 Acyclic monoalkenes
The set of preferred values contains data for the reactions of O3 with 43 acyclic monoalkenes. The generic rate coefficients for O3 addition to C=C bonds in acyclic monoalkene structures with differing extents of alkyl (R) substitution are given in Table 1 (kA1O3–kA6O3). These rate coefficients are based on averages of the preferred values of k at 298 K and of the preferred temperature coefficients, E∕R, for the identified sets of alkenes (as described in detail in the Table 1 comments), and are defined for “R” being a linear alkyl group (i.e. -CH3 or -CH2R′). In practice, reported data for sets of alk-1-enes (CH2=CHR) and 2-methylalk-1-enes (the majority of the CH2=CR2 dataset) show small systematic increases in k with the size of the alkyl group (Mason et al., 2009), although the preferred data for the other structural alkene groups do not apparently show such dependences (see Fig. 1). In view of the high sensitivity of k to the degree of alkyl substitution of the double bond, the use of single size-independent values of k for each of these structural groups is considered acceptable for the present SAR.
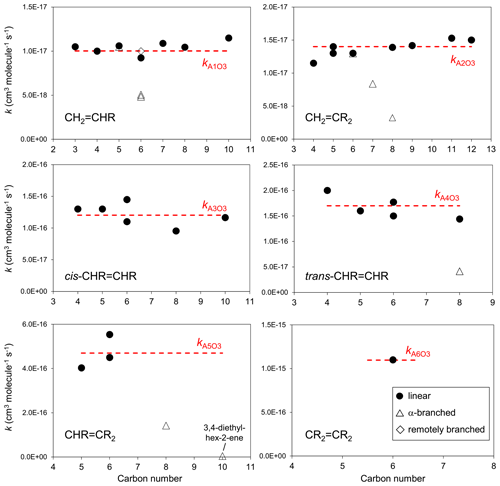
Figure 1Preferred kinetic data for the reactions of O3 with acyclic monoalkenes at 298 K as a function of carbon number, and the assigned rate coefficients for the generic monoalkene structures with linear alkyl substituents, as given in Table 1 (NB the α-branched R data in the trans-CHR=CHR panel consist of two co-incident data points).
Reported rate coefficients for monoalkenes possessing branched alkyl groups tend to be lower than those for alkenes possessing the corresponding linear alkyl groups, as is generally apparent from the data in Fig. 1. In most reported cases, branching occurs at the carbon atom adjacent (α) to the alkene structure, which may influence O3 attack through steric hindrance (e.g. Calvert et al., 2000; Johnson et al., 2000). Rate coefficients for alkenes with substituents at the α position are determined from the following expression,
where kAO3 is the appropriate reference rate coefficient in Table 1, and a value of Fα(X) is applied for each α substituent in the molecule. A factor, Fα(alkyl), describing the effect of each (acyclic) alkyl group at the α carbon atom was determined by minimizing the summed square deviation, , for the set of relevant branched alkenes (the resultant value is given in Table 2, along with those for selected oxygenated groups discussed below). It should be noted that the reported value of kobs for 3,4-diethyl-hex-2-ene is substantially lower than the reference value of kA5O3 for the CHR=CR2 structure (i.e. by 2 orders of magnitude; see Table 1), and this compound was therefore excluded from the optimization procedure for Fα(alkyl). Confirmatory measurements of that rate coefficient, and data for other α-branched alkenes, are therefore required to test and refine the method proposed here. In the absence of reported rate coefficients as a function of temperature for α-branched alkenes, the temperature dependence of Fα(alkyl) is assumed to be described by ; further temperature-dependent data are also required for this assumption to be fully tested. The limited data for more remotely branched alkenes suggest no significant effect on the rate coefficient. In those cases, the rate coefficients in Table 1 are applied as a default.
Table 2Substituent factors, Fα (298)(X), describing the effect of the given substituent at the α carbon atom in R groups in alkenes and in allylic oxygenated compounds at 298 Ka.
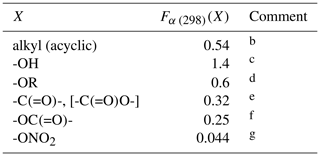
a The temperature dependence is assumed to be described by
.
b Based on data for 3-methyl-but-1-ene, 3-methyl-pent-1-ene,
trans-2,5-dimethyl-hex-3-ene, 2,3-dimethyl-but-1-ene and
3-methyl-2-isopropyl-but-1-ene, 3,3-dimethyl-but-1-ene,
trans-2,2-dimethyl-hex-3-ene, 2.3.3-trimethyl-but-1-ene and
2.4.4-trimethyl-pent-2-ene. Also applied to organic groups containing remote
substituents. The definition “acyclic” here is taken to mean that the first
carbon atom in the substituent is not part of a cycle.
c Based on data for 12 C3-C15 acyclic
allylic alcohols.
d Based on data for 3-ethoxypropene, 3-methyl-3,4-epoxy-1-butene
and 5-methyl-5-vinyl tetrahydrofuranol.
e Based on data for 3(Z-)4-methylhex-3,5-dienal,
3(E-)4-methylhex-3,5-dienal and 4-methyl-cyclohex-3-ene-1-one. Also applied
to -C(=O)O- substituents in the absence of data.
f Based on data for allyl acetate.
g Based on data for 2-methyl-4-nitrooxy-but-2-en-1-ol, and consistent with lower-limit value for 3-methyl-2-nitrooxy-but-3-en-1-ol.
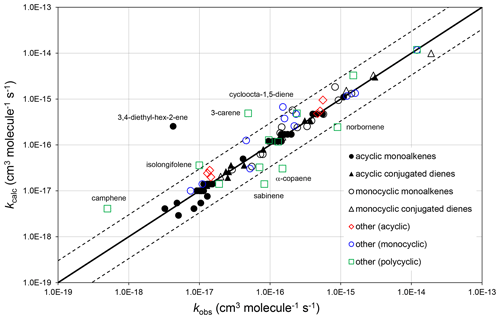
Figure 2Scatter plot of observed and calculated values of k at 298 K for the reactions of O3 with monoalkenes and poly-alkenes, based on the parameters determined in the present work. The broken lines show the factor-of-3 range, within which the majority of data fall.
The corresponding absolute deviations, (, at 298 K for 40 acyclic monoalkenes in the set of preferred values indicate that the estimation method reproduces the observed values to within about (see also Fig. 2). The three monoalkenes excluded from the procedure were ethene (a unique structure, for which no value needs to be calculated), 3,4-diethyl-hex-2-ene (as indicated above) and 3,4-dimethyl-hex-3-ene (for which only a lower-limit preferred value is available). In the final case, kcalc is a factor of 3 higher than the lower-limit value.
Table 3Optimized ring factors at 298 K, Fring (298), for the reactions of O3 with cyclic monoalkenes, and their temperature dependences described by .
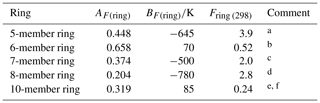
a Based on data for cyclopentene, 1-methylcyclopentene and
3-methylcyclopentene.
b Based on data for cyclohexene, 1-methylcyclohexene,
3-methylcyclohexene, 4-methylcyclohexene and 1,2-dimethylcyclohexene.
c Based on data for cycloheptene and 1-methylcycloheptene.
d Based on data for cis-cyclooctene,
1-methylcyclooctene and 3-methylcyclooctene.
e Based on data for cis-cyclodecene.
f Tentative values of Fring (298) of 2.1 and 12 can be derived for 9- and 11-member rings, respectively, based on limited data for structurally complex sesquiterpenes (see Sect. 3.2). These can be applied with an approximate average value of AF(ring)=0.3, and BF(ring) values of −580 and −1100 K, respectively.
3.2 Cyclic monoalkenes
The set of preferred values contains data for reactions of O3 with 14 simple monocyclic monoalkenes containing endocyclic double bonds, including cyclopentenes, cyclohexenes, cycloheptenes, cyclooctenes and cyclodecenes, with the temperature dependence also defined in seven cases. The values of k for these sets of compounds show systematic deviations from those observed for acyclic monoalkenes with the same level of substitution, likely resulting from the effects of ring strain (e.g. Calvert et al., 2000). Table 3 provides a series of ring factors, Fring, based on optimization to the 298 K rate coefficients and E∕R values within this dataset (as described in detail in the Table 3 comments). The rate coefficients for cyclic alkenes with endocyclic double bonds are therefore determined from the following expression,
where kAO3 is the appropriate reference rate coefficient (kA3O3–kA6O3) in Table 1. For polycyclic alkenes, a value of Fring needs to be applied for each ring for which the given C=C bond is a component. In addition to the Fring values given in Table 3, it is also possible to infer a tentative value of 12 for Fring (298) for 11-member rings, based on the reported rate coefficient for the sesquiterpene α-humulene (which contains a 1,4,8-cycloundecatriene ring), with the assumption that the values for Fring can be applied to cyclic systems with unconjugated multiple double bonds. The reported rate coefficient for the sesquiterpene β-caryophyllene (which contains a trans-cyclononene ring) then allows a tentative value of Fring (298)=2.1 for nine-member rings, although it is noted that the level of ring strain, and therefore Fring, likely depends on the cis-∕trans-conformation. Clearly, additional data for cyclononenes and cycloundecenes are required to confirm these tentative values of Fring.
As with the acyclic alkenes above, a value of Fα(alkyl) is also applied for each (acyclic) alkyl group at the α carbon atom in both monocyclic and polycyclic alkenes, where appropriate. For this procedure, the term “acyclic” is taken to mean that the first carbon atom in the substituent group is not part of a cycle that also contains the α carbon atom. To avoid ambiguity in defining the number of α acyclic alkyl groups, the base structure is taken to be cyclic as a default, and values of Fα(alkyl) are applied as appropriate to each acyclic alkyl group. This rule applies whether the double bond is endocyclic or exocyclic and has the effect of maximizing the number of acyclic alkyl groups (e.g. see example calculations B2–B5 in the Supplement).
Table 4Arrhenius parameters ( for the rate coefficients for O3 addition to generic conjugated dialkene structures, and the rate coefficient values at 298 Ka.
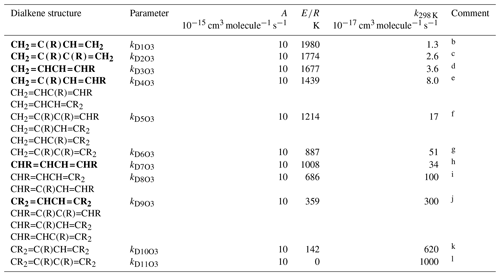
a k298 K for bold structures based on data for
the compounds identified in subsequent comments, with other values based on
trends in the data. A value of adopted in all cases, based on
the reported parameters for buta-1,3-diene, trans-penta-1,3-diene,
isoprene (2-methyl-buta-1,3-diene) and 2,3-dimethyl-buta-1,3-diene. ). Rate coefficients are also default
values for conjugated dialkenes possessing remote substituents (i.e. β
or higher), including isolated double bonds.
b k298 K based on data for isoprene
(2-methyl-buta-1,3-diene).
c k298 K based on data
2,3-dimethyl-buta-1,3-diene.
d k298 K based on rounded average of data for
cis-penta-1,3-diene and trans-penta-1,3-diene; parameters
are assumed to apply to both cis- and trans-isomers.
e k298 K based on data for
2-methyl-penta-1,3-diene. The same value of k298K is also adopted for
and .
f k298 K for taken to be
a factor of 2.1 greater than that for , based on the
trend in k observed on going from buta-1,3-diene (0.63) to
and , and from
to . The same value of
k298 K is also adopted for and
.
g k298 K for taken to
be a factor of 3 greater than that for , based on
the trend in k observed on going from to
.
h k298 K based on average of data for
cis-,trans-hexa-2,4-diene and
trans-,trans-hexa-2,4-diene; parameters are assumed to apply
to all cis- and trans- isomer combinations.
i k298 K for taken to be a
factor of 3 lower than that for , based on the trend
in k observed on going from to
; the same value of k298 K is also
adopted for .
j k298 K based on data for
2,5-dimethyl-hexa-2,4-diene; the same value of k298 K is also
adopted for , and
.
k k298 K for taken to
be a factor of 2.1 greater than that for , based on
the trend in k observed on going from buta-1,3-diene (0.63) to
and , and from
to .
l k298 K for assigned the same value as A, this being compatible with expected increase in k298 K relative to that for .
3.3 Acyclic conjugated dialkenes
The generic rate coefficients for O3 addition to C=C-C=C bond structures in acyclic conjugated dialkenes with differing extents of alkyl (R) substitution are given in Table 4, based on reported data for nine compounds (kD1O3−kD11O3). These rate coefficients are based on the preferred values of k for the identified dialkenes, with those for some structural groups being inferred from the observed trends in the impact of successive alkyl substitution on k, as described in detail in the Table 4 comments. The values are generally based on data for conjugated dialkenes for which “R” is a linear alkyl group (these making up almost all of the reported data). The limited information on dialkenes possessing branched substituent groups (5-methyl-hexa-1,3-diene and 5,5-dimethylhexa-1,3-diene) suggests that there is a less pronounced reducing effect on k, compared with that observed for the monoalkenes above. Similar to the approach used for the reactions of OH with conjugated dialkenes (Jenkin et al., 2018a), the following expression is therefore applied,
where kDO3 is the appropriate reference rate coefficient in Table 4, and a value of Fα(X) (Table 2) is applied for each α substituent in the molecule.
Temperature-dependent recommendations are available for four acyclic conjugated dialkenes (see Table 4 comments), with the recommended pre-exponential factor, A, being close to 10−14 in each case. This value of A is therefore adopted for all of the generic rate coefficients kD1O3–kD11O3, with E∕R given by .
Table 5Optimized ring factors at 298 K, , for the reactions of O3 with cyclic conjugated dialkenesa.

a These factors apply to conjugated dialkene systems
that are completely within the given ring structure. In cases where the
conjugated dialkene is only partially within the ring (e.g. as in the case of
β-phellandrene), the appropriate value of Fring given in
Table 3 should be applied. In the absence of data, the temperature dependence
is assumed to be described by .
b Based on data for cyclohexa-1,3-diene,
5-isopropyl-2-methyl-cyclohexa-1,3-diene (α-phellandrene) and
1-isopropyl-4-methyl-cyclohexa-1,3-diene (α-terpinene).
c Based on data for cyclohepta-1,3-diene.
d Based on data for based on data for cis-,cis-cycloocta-1,3-diene.
3.4 Cyclic conjugated dialkenes
The set of preferred values contains 298 K data for the reactions of O3 with five monocyclic conjugated dialkenes, including cyclohexa-1,3-dienes, cyclohepta-1,3-diene and cycloocta-1,3-diene. The values of k for these sets of compounds all show systematic deviations from those observed for acyclic conjugated dialkenes with the same level of substitution, again likely resulting from the effects of ring strain (e.g. Calvert et al., 2000; Lewin et al., 2001). In each case, these also differ substantially from those for the same sized cyclic monoalkenes (as shown in Table 3), and Table 5 shows a series of ring factors, , based on optimization to the cyclic conjugated dialkene dataset. The rate coefficients for cyclic conjugated dialkenes are therefore determined from the following expression:
In the absence of data, the temperature dependence is assumed to be described by . For polycyclic systems, a value of needs to be applied for each ring for which the given C=C-C=C bond structure is a component. As for acyclic conjugated dialkenes, a value of Fα(X) is applied for each α substituent in the molecule, where appropriate. In the cases of cyclic (or polycyclic) conjugated dialkenes with α acyclic alkyl substituents, the base structure is once again taken to be cyclic, and values of Fα(alkyl) are applied as appropriate (e.g. see example calculations D1 and D2 in the Supplement). Note that for the special case of conjugated dialkenes for which only one of the double bonds is within the ring (e.g. β-phellandrene: example D2 in the Supplement), a modified version of Eq. (4) is applied, in which is replaced by the appropriate value of Fring.
3.5 Other alkenes and poly-alkenes
The remainder of the alkene dataset consists of preferred values for 30 acyclic and cyclic compounds containing various combinations of isolated double bonds and conjugated dialkene structures, for which the methods described above can be used to estimate rate coefficients. There are also preferred values for a limited set of three conjugated poly-alkenes (one acyclic and two cyclic) and four alk-1-enyl-substituted aromatics (styrenes), for which there are insufficient data to attempt development of a SAR method.
The observed and calculated rate coefficients for 100 alkenes and poly-alkenes are compared in the correlation plot in Fig. 2. These are all the compounds for which preferred values are available in the reference database (spreadsheet SI_1), less those not covered by the SAR methods, i.e. the three conjugated poly-alkenes and four styrenes referred to above, and ethene and buta-1,3-diene, which are unique structures. As shown in Fig. 2, the SAR methods perform well for the sets of acyclic monoalkenes and conjugated dialkenes, and for the monocyclic monoalkenes and conjugated dialkenes. This is because the data show well-defined variations with structure, and because most of those rate coefficients were used as the basis of the SAR methods (as identified in Tables 1–5 and in spreadsheet SI_1).
The data for the remaining 30 compounds are subdivided into acyclic (6 compounds), monocyclic (11 compounds) and polycyclic (13 compounds) in Fig. 2, with the observed data from the first two categories also generally well described by the SAR methods. The observed values of k for the remaining polycyclic compounds are also reasonably well correlated, although with much more scatter than for the simpler structures. This is almost certainly due to a combination of ring strain and steric effects in these complex structures that cannot be fully accounted for by the SAR methods developed here. The alkenes in all these categories are identified in spreadsheet SI_1.
The set of preferred values contains data for the reactions of O3 with 110 unsaturated oxygenated compounds, possessing -OH, -C(=O)H, -C(=O)-, -O-, -C(=O)OH, -C(=O)O-, -OC(=O)-, -ONO2 and -C(=O)OONO2 substituents. The SAR methods applied to these compounds depend on the location of the substituent oxygenated group relative to the C=C bond and fall into three categories. For those possessing oxygenated substituents at the α position (i.e. allylic oxygenates), the methods described above for alkenes and dialkenes are modified to take account of the effect of the given substituent (see Sect. 4.1). More remote oxygenated substituents are assumed to have no effect, and the appropriate alkene or dialkene rate coefficient is applied unmodified in these cases (see Sect. 4.2). When the oxygenated group (including -C(=O)H, -C(=O)- and -C(=O)O-) is a substituent of the C=C group itself (i.e. vinylic oxygenates), the method is based on a series of reference rate coefficients for those specific structures, which are derived from the preferred data for the relevant sets of oxygenated compounds (see Sect. 4.3).
4.1 Allylic oxygenated compounds
Preferred kinetics data at 298 K are available for 25 allylic oxygenated compounds, containing the following substituents at the α carbon atom: -OH (14 compounds), -C(=O)H (3 compounds), -C(=O)R (1 compound), -OR (4 compounds, 1 possessing a remote -OH group), -OC(=O)R (1 compound), and both -ONO2 and -OH (2 compounds, with 1 having only a lower-limit recommendation). These data were used, in conjunction with the methods described for alkenes and dialkenes in Sect. 3, to optimize the corresponding values of Fα(X) given in Table 2. It was found that the effect of the -C(=O)H and -C(=O)R could reasonably be described by a single factor, Fα(-C(=O)-), and the further assumption was made that the same factor applies to groups containing the -C(=O)O- sub-structure. It is noted that several of the factors are based on data for limited sets of compounds (in some cases a single compound), and further data are clearly required to test the approach fully. As shown in Fig. 3, however, the method appears to work very well for most of the relevant compounds containing -OH groups (the largest subset of allylic oxygenates), providing some support for the approach.
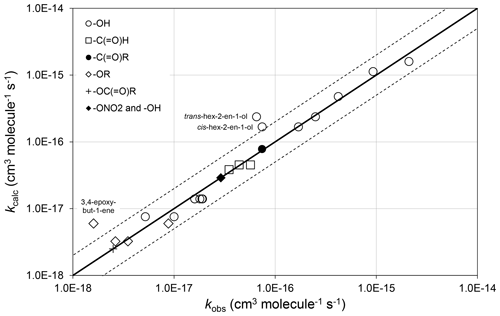
Figure 3Scatter plot of observed and calculated values of k at 298 K for the reactions of O3 with allylic oxygenated compounds possessing the groups shown (see Sect. 4.1). The broken lines show the factor-of-2 range.
There are almost no temperature-dependent data for allylic oxygenated compounds, and the temperature dependence of Fα(X) is therefore assumed to be described by . The recent study of Kalalian et al. (2020) reports temperature dependences for the reactions of O3 with cis-pent-2-en-1-ol and pent-1-en-3-ol. In the former case, the reported value of K is very well described using the above assumption, which leads to K, whereas in the latter case the observed and calculated values differ by about a factor of 2, K and K. Clearly, further temperature-dependent data are required for a variety of allylic oxygenated compounds for the method to be fully tested and refined.
4.2 Unsaturated compounds containing remote oxygenated substituents
The preferred data include rate coefficients for 22 unsaturated compounds possessing remote oxygenated substituents. In these cases, the oxygenated substituent is assumed to have no effect, and the corresponding alkene or dialkene rate coefficient, calculated as described in Sect. 3, is applied unmodified. As shown in Fig. 4, this assumption provides 298 K values of kcalc that are generally within about a factor of 2 of the values of kobs, and therefore within the scatter of the methods when applied to unsubstituted alkenes and dialkenes.
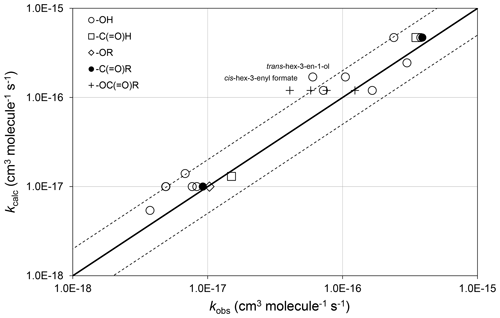
Figure 4Scatter plot of observed and calculated values of k at 298 K for the reactions of O3 with oxygenates containing remote oxygenated groups as shown (see Sect. 4.2). The broken lines show the factor-of-2 range.
In the majority of cases, the presence of the remote oxygenated group appears to reduce the value of the rate coefficient slightly, compared with that of the generic alkene or dialkene rate coefficient. In the cases of a series of cis-hex-3-enyl esters (i.e. cis-) the rate coefficient is reported to depend systematically on the size of the remote R group, rather than displaying a consistent influence of the -OC(=O)- substructure itself (Zhang et al., 2018). Within this series, the rate coefficient for the largest compound (with R=n-C3H7) agrees well with the reference rate coefficient, whereas that for the smallest (with R = H) is about a factor of 3 lower than the reference rate coefficient. Clearly, further information is required for unsaturated compounds possessing remote oxygenated substituents before refined estimation methods can be developed that take account of this type of effect.
The preferred data include a rate coefficient for methyl chavicol (1-prop-2-enyl-4-methoxy-benzene), which contains a remote methoxy group as part of a methoxyphenyl substituent at the carbon atom α to the alkene double bond. The rate coefficient, reported by Gai et al. (2013), is well described by the generic rate coefficient kA1O3 (Table 1). This suggests that the aromatic substituent at the α carbon atom has no effect on the rate coefficient. However, further data on alk-2-enyl substituted aromatic compounds are ideally required to confirm this.
4.3 Vinylic oxygenated compounds
When the oxygenated group (including -C(=O)H, -C(=O)- and -C(=O)O-) is a substituent of the C=C group itself (i.e. vinylic oxygenates), the data indicate that the rate coefficients are much less sensitive to the presence of other alkyl groups attached to the C=C group (and in some cases actually decrease upon additional substitution). In contrast, the data for some classes clearly show a greater influence of substituent size. It is therefore not possible to treat these compounds using modifications to the SAR methods presented for alkenes in Sect. 3, and it is necessary to assign generic rate coefficients for addition of O3 to a series of vinylic oxygenate structures. Three categories of vinylic oxygenate structure are considered, namely vinyl aldehydes and ketones (Table 6), vinyl esters and acids (Table 7) and vinyl ethers (Table 8).
Table 6Reference rate coefficients for O3 addition to acyclic vinyl aldehyde and ketone structures.a
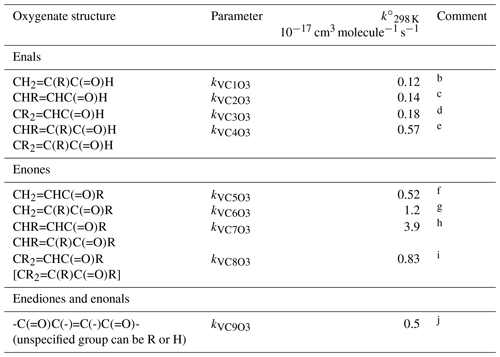
a Determined from data for the compound or sets of
compounds identified in subsequent comments. Rate coefficients are also
default values for related compounds possessing other remote oxygenated
substituents. The values of should be
used in Eq. (5), with the globally optimized value of αS=0.19.
b Based on preferred data for methacrolein.
c Based on data for trans-but-2-enal,
trans-pent-2-enal, trans-hex-2-enal,
trans-hept-2-enal, trans-oct-2-enal and
trans-non-2-enal.
d Based on data for 3-methyl-2-butenal.
e Based on data for trans-2-methyl but-2-enal and
2-methyl pent-2-enal.
f Based on data for methyl vinyl ketone and pent-1-en-3-one.
g Based on data for 3-methyl-3-buten-2-one.
h Based on data for pent-3-en-2-one, hex-4-en-3-one and
3-methyl-pent-3-en-2-one.
i Based on data for 4-methyl-pent-3-en-2-one. Also inferred to
apply to .
j Based on data for 4-oxo-pent-2-enal and cis- and trans-3-hexen-2,5-dione. Value assumed to apply to all corresponding vinylic keto-aldehydes, dialdehydes and diketones, except for the unique case when all unspecified groups are H, i.e. butenedial.
Table 7Reference rate coefficients for O3 addition to acyclic vinylic esters and acidsa.
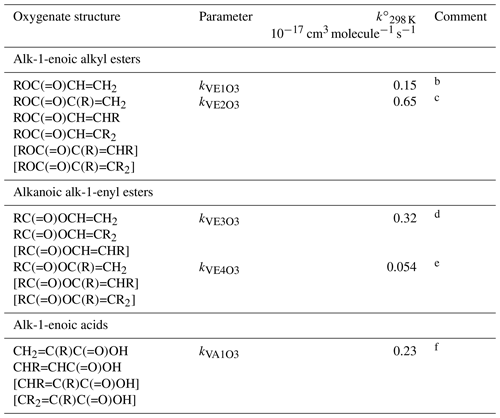
a Determined from data for the compound or sets of
compounds identified in subsequent comments. Rate coefficients are also
default values for related compounds possessing other remote oxygenated
substituents. Data for peroxymethacryloyl nitrate (MPAN) suggest that
parameters for alk-1-enoic alkyl esters can reasonably be applied to
corresponding unsaturated PANs and, by inference, peracids. The values of
should be used in Eq. (5), with the
globally optimized value of αS=0.19.
b Based on preferred data for methyl and n-butyl acrylate.
c Based on data for methyl, ethyl, n-propyl, i-propyl,
n-butyl and i-butyl methacrylate, ethyl crotonate and ethyl 3,3-dimethyl
acrylate. Also inferred to apply to and
.
d Based on data for vinyl acetate, vinyl propionate and
2-methylpropenyl acetate. Also inferred to apply to .
e Based on data for i-propenyl acetate. Also inferred to apply
to and .
f Based on data for methacrylic acid and trans-pent-2-enoic acid. Also inferred to apply to bracketed structures shown.
Table 8Reference rate coefficients for O3 addition to vinylic ethersa.
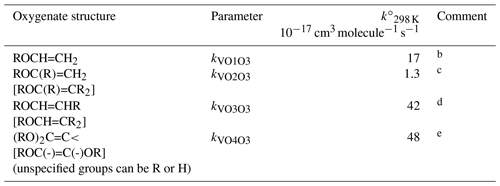
a Determined from data for the compound or sets of
compounds identified in subsequent comments. Rate coefficients are also
default values for related compounds possessing other remote oxygenated
substituents. The values of should be used in
Eq. (5), with the globally optimized value of αS=0.19.
b Based on preferred data for ethyl, n-propyl, n-butyl and
i-butyl vinyl ether. Also consistent with data for ethylene glycol vinyl
ether and ethylene glycol divinyl ether, but underestimates rate coefficient
for t-butyl vinyl ether and overestimates rate coefficient for diethylene
glycol divinyl ether.
c Based on preferred data for 2-methoxypropene and
2-ethoxypropene.
d Approximately based on lower-limit rate coefficient for ethyl
n-propenyl ether. Also inferred to apply to bracketed structures shown.
e Based on data for 1,1-dimethoxyethene and assumed to apply to all other 1,1- and 1,2-dialkoxyalkenes.
The influence of substituent group size is clearly apparent in the data for vinyl aldehydes and ketones, vinyl ethers and alk-1-enoic acid alkyl esters, for example as discussed by Ren et al. (2019) for the esters. Accordingly, the following expression is used to describe the 298 K data,
where and αs are constants. ni is the number of carbon atoms in the ith substituent group, where each relevant substituent group is represented by “R” in the structures shown in Tables 6–8. therefore quantifies the rate coefficient when each R group in the given structure is CH3, and is the incremental increase for each additional carbon in any substituent. As defined, therefore, the same incremental increase is assumed to apply to each R group in the given structure, although the trends in the preferred data are generally based on information for particular R groups. Additional data are therefore required to test this assumption. It was also found that there was only marginal benefit in using independent values of αs for the different vinylic oxygenate categories, based on the data currently available. A single category-independent value of αs=0.19 was therefore optimized for simplicity. The calculated and observed rate coefficients are compared on the scatter plot in Fig. 5.
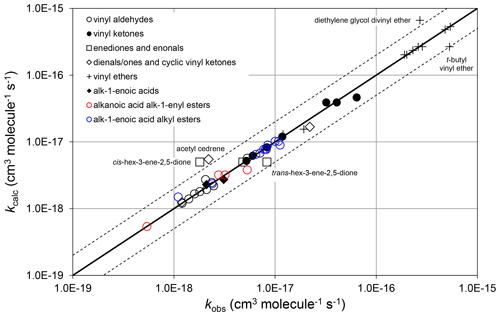
Figure 5Scatter plot of observed and calculated values of k at 298 K for the reactions of O3 with vinylic oxygenated compounds (see Sect. 4.3). The broken lines show the factor-of-2 range.
There is only limited information available on the temperature dependences of these reactions. Where data are available (e.g. for methacrolein and methyl vinyl ketone), the data suggest that the temperature dependence can reasonably be represented by , with and ), and this approach is adopted in the present work.
There are very limited data for conjugated dialkenes containing vinylic oxygenated substituents and for cyclic vinylic oxygenates, and it is not possible to propose SAR methods for most oxygenated groups at the present time. The data include rate coefficients for some relevant conjugated dienals/dienones and cyclic vinyl ketones (hexa-2,4-diendial, cyclohex-2-en-1-one, β-ionone and acetyl-cedrene). In contrast to the compounds discussed above, the rate coefficients for these species are all reasonably well described by applying the appropriate conjugated dialkene or alkene rate coefficient determined by the methods presented in Sect. 3, reduced by a factor of 50 for each alkyl group replaced by a -C(=O)H or -C(=O)- group. This assumption is provisionally applied in the current work, although further data are clearly required. There are also limited data for some furans and dihydrofurans. The rate coefficients for these species are influenced by the compounds being aromatic (in the cases of the furans) and also by ring strain effects, and it is difficult to extend the methods developed here for unsaturated ethers to cover these species. The methods presented here are therefore not applicable to heterocyclic compounds with endocyclic double bonds, such as furans and dihydrofurans.
There are no data for compounds containing a number of vinylic oxygenated functional groups (e.g. -ONO2 and -OOH), although such compounds are not expected to be prevalent in atmospheric chemistry. Data for the reactions of O3 with vinylic alcohols are very limited, because kinetics studies tend to be complicated by keto–enol tautomerism. A recent theoretical study of the reaction of O3 with 4-hydroxy-pent-3-en-2-one (Ji et al., 2020), the enolic tautomer of pentane-2,4-dione (acetyl acetone), suggests that the presence of the hydroxy group has a limited effect, the reported rate coefficient at 298 K ( ) being comparable with kVC7O3. However, it is noted that this is more than an order of magnitude greater than the laboratory determination of Zhou et al. (2008) for the reaction of O3 with the tautomeric mixture of pentane-2,4-dione and 4-hydroxy-pent-3-en-2-one. Further information is clearly required to allow the effects of vinylic hydroxy groups to be defined with confidence. Until then, they are provisionally assumed to have the same influence as vinylic H atoms in the present work.
4.4 Combinations of groups
Data for compounds containing two different vinylic oxygenated substituents listed in Tables 6–8 are limited to 4-methoxy-but-3-ene-2-one. This therefore falls into both of the and ROCH=CHR generic structure categories, and the rate coefficients for these categories, kVC7O3 and kVO3O3, differ by an order of magnitude. The rate coefficient for 4-methoxy-but-3-ene-2-one ( ) is actually a factor of three lower than that for the less reactive category (kVC7O3). Based on this, it is tentatively suggested that the estimated rate coefficient for compounds containing two different vinylic oxygenated substituents should be based on the less reactive category.
Data for compounds containing both vinylic and allylic oxygenated groups are limited to 2-methyl-4-nitrooxy-cis-2-buten-1-al, which contains a vinyl -C(=O)H group and an allyl -ONO2 group. In this case, the rate coefficient ( ) is in reasonable agreement with that of the relevant vinylic category, kVC4O3 (Table 6), suggesting that the allyl -ONO2 group has almost no additional deactivating effect in this case. This is consistent with the relative insensitivity of the vinylic rate coefficients to the presence of additional substituents, and it is therefore tentatively suggested that the appropriate rate coefficient in Tables 6–8 can be applied, with no additional effect from an allylic substituent in relevant cases (i.e. the factors in Table 2 are only applied with rate coefficients derived from those shown in Tables 1 and 4).
It is well established that the addition of O3 to a C=C bond leads to initial formation of a primary ozonide (POZ), or 1,2,3-trioxolane product (e.g. Calvert et al., 2000; Johnson and Marston, 2008). In compounds with multiple C=C bonds, the SAR methods developed here define kcalc in terms of a summation of the various alkene and/or conjugated dialkene structures within the poly-unsaturated compound, and therefore also provide the basis for estimating branching ratios for the formation of the various isomeric POZs (e.g. see calculations B4, B5 and C2 in the Supplement). Using β-ocimene as an example, the present SARs provide a value of at 298 K, which agrees well with the preferred value of . The component rate coefficients also indicate that the reaction is expected to occur predominantly (85 %) at the isolated C=C bond, leading to the formation of POZ1, as shown in the schematic in Fig. 6. This conclusion is also supported by comparison of kobs with that reported for the reaction of O3 with the β-ocimene oxidation product 4-methyl-hexa-3,5-dienal, which retains the conjugated dialkene structure (Baker et al., 2004).
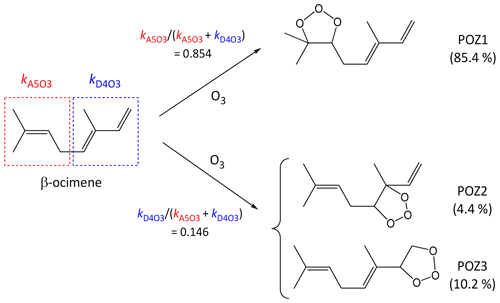
Figure 6Schematic of the concerted addition of O3 to β-ocimene to form primary ozonides (POZs), showing the calculated contributions of the different pathways at 298 K, based on the reported estimation methods (see Sect. 5).
The addition of O3 to conjugated dialkene structures leads to the formation of either of two primary ozonides, as shown in Fig. 6 for the example of the minor channel of β-ocimene ozonolysis (POZ2 and POZ3). In the cases of symmetrically substituted conjugated dialkene structures (i.e. , , and , where “R” represents any alkyl group or remotely substituted oxygenated group) it is reasonable to assume that the addition of O3 occurs equally at the two possible sites. There has only been limited information reported on the products of the reactions of O3 with unsymmetrically substituted conjugated dialkenes. Most of this information relates to the reaction of O3 with isoprene (), but with selected product yields reported for subsequently formed carbonyl compounds in a few other cases (Lewin et al., 2001). In the case of isoprene, product information indicates that the addition of O3 occurs significantly at both sites, but with about 60 % at the less substituted -CH=CH2 bond (e.g. Aschmann and Atkinson, 1994; Nguyen et al., 2016), and the same branching ratio can therefore reasonably be assigned to structures in general. The information for other conjugated dialkenes also suggests preferential addition of O3 at the less substituted C=C bond (Lewin et al., 2001; Mackenzie-Rae et al., 2016). On this basis, it is tentatively assumed that 60 % of addition occurs at a less substituted C=C that contains one fewer alkyl substituents (e.g. as in or ), 70 % at a less substituted C=C bond that contains two fewer alkyl substituents (e.g. as in or ) and 80 % at a less substituted C=C bond that contains three fewer alkyl substituents (i.e. as in alone). Clearly, further information is required to allow these addition ratios to be assigned with greater certainty. In the absence of reported mechanistic data, the same rules are also applied to conjugated dialkene structures with vinylic or allylic oxygenated groups.
Updated and extended SAR methods have been developed to estimate rate coefficients for the reactions of O3 with unsaturated organic species. The group contribution methods were optimized using a database including a set of preferred rate coefficients for 221 species. The overall performance of the SARs in determining log k298 K is now summarized.
The distribution of errors (log kcalc∕kobs), the root-mean-squared error (RMSE), the mean absolute error (MAE) and the mean bias error (MBE) were examined to assess the overall reliability of the SAR. The RMSE, MAE and MBE are here defined as
where n is the number of species in the dataset. A total of 198 of the 221 species in the database contributed to the statistical analysis. Six species could not be included, because only upper- or lower-limit recommendations are available. In addition, the SAR methods do not currently include styrenes, heterocyclic species and conjugated poly-alkenes (11 species), and the smallest species in some homologous series (i.e. ethene, buta-1,3-diene, acrolein, butenedial and acrylic acid) are not covered by the SAR categories because the double bonds do not contain (additional) organic substituents (see Sects. 3 and 4). Finally, because of the factor-of-60 difference between kcalc and kobs, 3,4-diethylhex-2-ene was also excluded from the statistical analysis as an outlier (see Sect. 3.1). However, it is emphasized that there is no firm basis for believing that the reported rate coefficient for 3,4-diethylhex-2-ene (Grosjean and Grosjean, 1996) is any less reliable than many other rate coefficients in the database. Given the substantial disagreement between kcalc and kobs, confirmatory measurements of that rate coefficient, and data for other similar branched alkenes, would clearly be valuable to help test and refine the methods presented here.
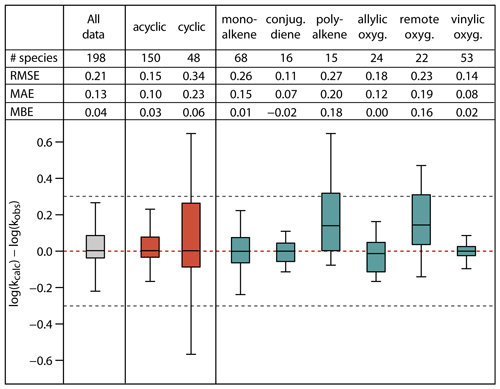
Figure 7Root-mean-square error (RMSE), mean absolute error (MAE), mean bias error (MBE) and box plot for the error distribution in the estimated log k298 K values for the full set and subsets of the unsaturated species in the database. The bottom and the top of the boxes are the 25th (Q1) and 75th percentiles (Q3); the black band is the median value. The whiskers extend to the most extreme data point, which is no more than 1.5× (Q3–Q1) from the box. The black dotted lines correspond to agreement within a factor 2.
Figure 7 summarizes the statistics for the full set of 198 species, for acyclic and cyclic species collectively, and for various alkene and unsaturated oxygenate subsets. With the exception of the poly-alkene and remotely substituted oxygenate subsets, the calculated log k298 K for all categories shows no significant bias, with MBE at or below 0.06 log units and with median values of the error distributions close to 0. Overall, the SAR methods overestimate k298 K for poly-alkenes and remotely substituted oxygenates by a factor of about 1.5. This is likely due to a number of contributory factors that are not fully accounted for in the SAR methods, including effects of remote substituents on double bond reactivity (e.g. see Sect. 4.2) and possible systematic ring strain effects in cyclic poly-alkenes that are incompatible with the factors derived from simpler compounds (see Sects. 3.2 and 3.4).
The RMSE for the various alkene and unsaturated oxygenate subsets covers the range from 0.11 to 0.27 log units; i.e. the relative errors for the calculated k298 K lie in the range 29–86 %. Of these, the poly-alkene and remotely substituted oxygenate subsets again have values towards the high end of the range (0.27 and 0.23, respectively). The RMSE for the mono-alkene subset is also elevated (0.26), this being mainly due to the influence of polycyclic species on the overall statistics. Accordingly, the RMSE of cyclic species collectively (0.34) is substantially higher than that for acyclic species (0.15), corresponding to relative errors for the calculated k298 K of about 120 % and 40 %, respectively. The large errors for cyclic species result from the difficulties in accounting fully for ring strain and steric effects in polycyclic alkenes and cyclic poly-alkenes, as also illustrated in Fig. 2. Finally, for the full database, the SARs give fairly reliable k298K estimates, with a MAE of 0.13 and a RMSE of 0.21, corresponding to an overall agreement of the calculated k298K within about 60 %. Although this level of agreement is considered reasonable, it is noted that the methods generally do not perform as well as those for the reactions of OH with alkenes and unsaturated organic oxygenates (Jenkin et al., 2018a). This may be explained by the O3 reaction being a concerted process, which is more influenced by orientational effects, ring strain and steric hindrance than the OH reaction (e.g. see Johnson et al., 2000), and therefore less easy to represent with a practical SAR. As discussed in Sects. 3 and 4, and highlighted by Vereecken et al. (2018), additional kinetics studies would be highly valuable for some classes of alkene and unsaturated oxygenate to help the SAR methods to be further assessed and refined, including data for multifunctional oxygenated species in particular.
All relevant data and supporting information have been provided in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/acp-20-12921-2020-supplement.
All authors defined the scope of the work. MEJ developed and extended the SAR methods and drafted the manuscript, which were both reviewed by all co-authors. RV and BA tested the SAR methods in GECKO-A and carried out the statistical analysis in Sect. 6.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Simulation chambers as tools in atmospheric research (AMT/ACP/GMD inter-journal SI)”. It is not associated with a conference.
An anonymous referee is gratefully acknowledged for helpful review comments, and we also thank Bill Carter (University of California, Riverside) for providing comments and feedback on the application of the SAR methods.
This research has been supported by the European Commission (EUROCHAMP-2020 (grant no. 730997)), and as part of the MAGNIFY project by the Natural Environment Research Council (grant no. NE/M013448/1), the Agence Nationale de la Recherche (grant no. ANR-14-CE01-0010) and the National Centre for Atmospheric Science (Composition Directorate).
This paper was edited by Christian George and reviewed by two anonymous referees.
Aschmann, S. M. and Atkinson, R.: Formation yields of methyl vinyl ketone and methacrolein from the gas-phase reaction of O3 with isoprene, Environ. Sci. Technol.,28, 1539–1542, 1994.
Atkinson, R. and Arey, J.: Atmospheric degradation of volatile organic compounds, Chem. Rev., 103, 4605–4638, 2003.
Aumont, B., Szopa, S., and Madronich, S.: Modelling the evolution of organic carbon during its gas-phase tropospheric oxidation: development of an explicit model based on a self generating approach, Atmos. Chem. Phys., 5, 2497–2517, https://doi.org/10.5194/acp-5-2497-2005, 2005.
Baker, J., Arey, J., and Atkinson, R.: Kinetics of the gas-phase reactions of OH radicals, NO3 radicals and O3 with three C7-carbonyls formed from the atmospheric reactions of myrcene, ocimene and terpinolene, J. Atmos. Chem., 48, 241–260, 2004.
Calvert, J. G., Atkinson, R., Kerr, J. A., Madronich, S., Moortgat, G. K., Wallington, T. J., and Yarwood, G.: The mechanisms of atmospheric oxidation of the alkenes, Oxford University Press, Oxford, ISBN 978-0-19513177-2, 2000.
Calvert, J. G., Mellouki, A., Orlando, J. J., Pilling, M. J., and Wallington, T. J.: The mechanisms of atmospheric oxidation of the oxygenates, Oxford University Press, Oxford, ISBN 978-0-19976707-6, 2011.
Calvert, J. G., Orlando, J. J., Stockwell, W. R., and Wallington, T. J.: The mechanisms of reactions influencing atmospheric ozone, Oxford University Press, Oxford, ISBN 978-0-19023302-0, 2015.
Chhantyal-Pun, R., Rotavera, B., McGillen, M. R., Khan, M. A. H., Eskola, A. J., Caravan, R. L., Blacker, L., Tew, D. P., Osborn, D. L., Percival, C. J., Shallcross, D. E., and Orr-Ewing, A. J.: Criegee intermediate reactions with carboxylic acids: a potential source of secondary organic aerosol in the atmosphere, ACS Earth Space Chem., 2, 833–842, 2018.
Cox, R. A., Ammann, M., Crowley, J. N., Herrmann, H., Jenkin, M. E., McNeill, V. F., Mellouki, A., Troe, J., and Wallington, T. J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume VII – Criegee intermediates, Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2020-472, in review, 2020.
Criegee, R.: Mechanism of Ozonolysis, Angew. Chem. Int. Edit., 14, 745–752, https://doi.org/10.1002/anie.197507451, 1975.
Ehn, M., Thornton, J. A., Kleist, E., Sipilä, M., Junninen, H., Pullinen, I., Springer, M., Rubach, F., Tillmann, R., Lee, B., Lopez-Hilfiker, F., Andres, S., Acir, I. H., Rissanen, M. P., Jokinen, T., Schobesberger, S., Kangasluoma, J., Kontkanen, J., Nieminen, T., Kurteìn, T., Nielsen, L. B., Jørgensen, S., Kjaergaard, H. G., Canagaratna, M., Dal Maso, M., Berndt, T., Petäjä, T., Wahner, A., Kerminen, V. M., Kulmala, M., Worsnop, D. R., Wildt, J., and Mentel, T. F.: A large source of low-volatility secondary organic aerosol, Nature, 506, 476–479, https://doi.org/10.1038/nature13032, 2014.
Gai, Y., Wang, W., Ge, M., Kjaergaard, H. G., Jørgensen, S., and Du, L.: Methyl chavicol reactions with ozone, OH and NO3 radicals: rate constants and gas-phase products, Atmos. Environ., 77, 696–702, 2013.
Grosjean, E. and Grosjean, D.: Rate constants for the gas phase reaction of ozone with 1,1-disubstituted alkenes, Int. J. Chem. Kinet., 28, 911–918, 1996.
Hallquist, M., Wenger, J. C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., Dommen, J., Donahue, N. M., George, C., Goldstein, A. H., Hamilton, J. F., Herrmann, H., Hoffmann, T., Iinuma, Y., Jang, M., Jenkin, M. E., Jimenez, J. L., Kiendler-Scharr, A., Maenhaut, W., McFiggans, G., Mentel, Th. F., Monod, A., Prévôt, A. S. H., Seinfeld, J. H., Surratt, J. D., Szmigielski, R., and Wildt, J.: The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 9, 5155–5236, https://doi.org/10.5194/acp-9-5155-2009, 2009.
Heaton, K. J., Dreyfus, M. A., Wang, S., and Johnston, M. V.: Oligomers in the early stage of biogenic secondary organic aerosol formation and growth, Environ. Sci. Technol., 41, 6129– 6136, https://doi.org/10.1021/es070314n, 2007.
Jenkin, M. E., Saunders, S. M., and Pilling, M. J.: The tropospheric degradation of volatile organic compounds: a protocol for mechanism development, Atmos. Environ., 31, 81–104, 1997.
Jenkin, M. E., Valorso, R., Aumont, B., Rickard, A. R., and Wallington, T. J.: Estimation of rate coefficients and branching ratios for gas-phase reactions of OH with aliphatic organic compounds for use in automated mechanism construction, Atmos. Chem. Phys., 18, 9297–9328, https://doi.org/10.5194/acp-18-9297-2018, 2018a.
Jenkin, M. E., Valorso, R., Aumont, B., Rickard, A. R., and Wallington, T. J.: Estimation of rate coefficients and branching ratios for gas-phase reactions of OH with aromatic organic compounds for use in automated mechanism construction, Atmos. Chem. Phys., 18, 9329–9349, https://doi.org/10.5194/acp-18-9329-2018, 2018b.
Jenkin, M. E., Valorso, R., Aumont, B., and Rickard, A. R.: Estimation of rate coefficients and branching ratios for reactions of organic peroxy radicals for use in automated mechanism construction, Atmos. Chem. Phys., 19, 7691–7717, https://doi.org/10.5194/acp-19-7691-2019, 2019.
Ji, Y., Qin, D., Zheng, J., Shi, Q., Wang, J., Lin, Q., Chen, J., Gao, Y., Li, G., and An, T.: Mechanism of the atmospheric chemical transformation of acetylacetone and its implications in night-time second organic aerosol formation, Sci. Total Environ., 720, 137610, https://doi.org/10.1016/j.scitotenv.2020.137610, 2020.
Johnson, D. and Marston, G.: The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere, Chem. Soc. Rev., 37, 699–716, 2008.
Johnson, D., Rickard, A. R., McGill, C. D., and Marston, G.: The influence of orbital asymmetry on the kinetics of the gas-phase reactions of ozone with unsaturated compounds, Phys. Chem. Chem. Phys., 2, 323–328, 2000.
Jokinen, T., Berndt, T., Makkonen, R., Kerminen, V.-M., Junninen, H., Paasonen, P., Stratmann, F., Herrmann, H., Guenther, A. B., Worsnop, D. R., Kulmala, M., Ehn, M., and Sipilä, M.: Production of extremely low volatile organic compounds from biogenic emissions: Measured yields and atmospheric implications, P. Natl. Acad. Sci. USA, 112, 7123–7128, https://doi.org/10.1073/pnas.1423977112, 2015.
Kalalian, C., El Dib, G., Singh, H. J., Rao, P. K., Roth, E., and Chakir, A.: Temperature dependent kinetic study of the gas phase reaction of ozone with 1-penten-3-ol, cis-2-penten-1-ol and trans-3-hexen-1-ol: Experimental and theoretical data, Atmos. Environ., 223, 117306, 2020.
Lewin, A. G., Johnson, D., Price, D. W., and Marston, G.: Aspects of the kinetics and mechanism of the gas-phase reactions of ozone with conjugated dienes, Phys. Chem. Chem. Phys., 3, 1253–1261, 2001.
Mackenzie-Rae, F. A., Karton, A., and Saunders, S. M.: Computational investigation into the gas-phase ozonolysis of the conjugated monoterpene α-phellandrene, Phys. Chem. Chem. Phys., 18, 27991–28002, 2016.
Mackenzie-Rae, F. A., Wallis, H. J., Rickard, A. R., Pereira, K. L., Saunders, S. M., Wang, X., and Hamilton, J. F.: Ozonolysis of α-phellandrene – Part 2: Compositional analysis of secondary organic aerosol highlights the role of stabilised Criegee intermediates, Atmos. Chem. Phys., 18, 4673–4693, https://doi.org/10.5194/acp-18-4673-2018, 2018.
Mason, S. A., Arey, J., and Atkinson, R.: Rate constants for the gas-phase reactions of NO3 radicals and O3 with C6−C14 1-alkenes and 2-methyl-1-alkenes at 296±2 K, J. Phys. Chem. A, 113, 5649–5656, 2009.
McGillen, M. R., Carey, T. J., Archibald, A. T., Wenger, J. C., Shallcross, D. E., and Percival, C. J.: Structure–activity relationship (SAR) for the gas-phase ozonolysis of aliphatic alkenes and dialkenes, Phys. Chem. Chem. Phys., 10, 1757–1768, 2008.
Mouchel-Vallon, C., Newland, M. J., Valorso, R., Camredon, M., Aumont, B., Vereecken, L., Jenkin, M. E., and Rickard, A. R.: Estimation of mechanistic parameters in the gas-phase reactions of ozone with alkenes for use in automated mechanism construction, in preparation, 2020.
Nguyen, T. B., Tyndall, G. S., Crounse, J. D., Teng, A. P., Bates, K. H., Schwantes, R. H., Coggon, M. M., Zhang, L., Feiner, P., Milller, D. O., Skog, K. M., Rivera-Rios, J. C., Dorris, M., Olson, K. F., Koss, A., Wild, R. J., Brown, S. S., Goldstein, A. H., de Gouw, J. A., Brune, W. H., Keutsch, F. N., Seinfeld, J. H., and Wennberg, P. O.: Atmospheric fates of Criegee intermediates in the ozonolysis of isoprene, Phys. Chem. Chem. Phys., 18, 10241–10254, 2016.
Ren, Y., Cai, M., Daële, V., and Mellouki, A.: Rate coefficients for the reactions of OH radical and ozone with a series of unsaturated esters, Atmos. Environ., 200, 243–253, 2019.
Sakamoto, Y., Inomata, S., and Hirokawa, J.: Oligomerization reaction of the Criegee intermediate leads to secondary organic aerosol formation in ethylene ozonolysis, J. Phys. Chem. A, 117, 12912–12921, https://doi.org/10.1021/jp408672m, 2013.
Saunders, S. M., Jenkin, M. E., Derwent, R. G., and Pilling, M. J.: Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 3, 161–180, https://doi.org/10.5194/acp-3-161-2003, 2003.
Sindelarova, K., Granier, C., Bouarar, I., Guenther, A., Tilmes, S., Stavrakou, T., Müller, J.-F., Kuhn, U., Stefani, P., and Knorr, W.: Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years, Atmos. Chem. Phys., 14, 9317–9341, https://doi.org/10.5194/acp-14-9317-2014, 2014.
Vereecken, L., Aumont, B., Barnes, I., Bozzelli, J. W., Goldman, M. J., Green, W. H., Madronich, S., McGillen, M. R., Mellouki, A., Orlando, J. J., Picquet-Varrault, B., Rickard, A. R., Stockwell, W. R., Wallington, T. J., and Carter, W. P. L.: Perspective on mechanism development and structure-activity relationships for gas-phase atmospheric chemistry, Int. J. Chem. Kinet., 50, 435–469, 2018.
Zhang, Q., Lin, X., Gai, Y., Ma, Q., Zhao, W., Fang, B., Long, B., and Zhang, W.: Kinetic and mechanistic study on gas phase reactions of ozone with a series of cis-3-hexenyl esters, RSC Adv., 8, 4230–4238, https://doi.org/10.1039/c7ra13369c, 2018.
Zhao, Y., Wingen, L. M., Perraud, V., Greaves, J., and Finlayson- Pitts, B. J.: Role of the reaction of stabilized Criegee intermediates with peroxy radicals in particle formation and growth in air, Phys. Chem. Chem. Phys., 17, 12500–12514, https://doi.org/10.1039/c5cp01171j, 2015.
Zhou, S., Barnes, I., Zhu, T., Bejan, I., Albu, M., and Benter, T.: Atmospheric chemistry of acetylacetone, Environ. Sci. Technol. 42, 7905–7910, 2008.





