the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Complex plant-derived organic aerosol as ice-nucleating particles – more than the sums of their parts?
Isabelle Steinke
Naruki Hiranuma
Roger Funk
Kristina Höhler
Nadine Tüllmann
Nsikanabasi Silas Umo
Peter G. Weidler
Thomas Leisner
Quantifying the impact of complex organic particles on the formation of ice crystals in clouds remains challenging, mostly due to the vast number of different sources ranging from sea spray to agricultural areas. In particular, there are many open questions regarding the ice nucleation properties of organic particles released from terrestrial sources such as decaying plant material.
In this work, we present results from laboratory studies investigating the immersion freezing properties of individual organic compounds commonly found in plant tissue and complex organic aerosol particles from vegetated environments, without specifically investigating the contribution from biological particles, which may contribute to the overall ice nucleation efficiency observed at high temperatures. To characterize the ice nucleation properties of plant-related aerosol samples for temperatures between 242 and 267 K, we used the Aerosol Interaction and Dynamics in the Atmosphere (AIDA) cloud chamber and the Ice Nucleation SpEctrometer of the Karlsruhe Institute of Technology (INSEKT), which is a droplet freezing assay. Individual plant components (polysaccharides, lignin, soy and rice protein) were mostly less ice active, or similarly ice active, compared to microcrystalline cellulose, which has been suggested by recent studies to be a proxy for quantifying the primary cloud ice formation caused by particles originating from vegetation. In contrast, samples from ambient sources with a complex organic matter composition (agricultural soils and leaf litter) were either similarly ice active or up to 2 orders of magnitude more ice active than cellulose. Of all individual organic plant components, only carnauba wax (i.e., lipids) showed a similarly high ice nucleation activity as that of the samples from vegetated environments over a temperature range between 245 and 252 K. Hence, based on our experimental results, we suggest considering cellulose as being representative for the average ice nucleation activity of plant-derived particles, whereas lignin and plant proteins tend to provide a lower limit. In contrast, complex biogenic particles may exhibit ice nucleation activities which are up to 2 orders of magnitude higher than observed for cellulose, making ambient plant-derived particles a potentially important contributor to the population of ice-nucleating particles in the troposphere, even though major uncertainties regarding their transport to cloud altitude remain.
- Article
(1128 KB) - Full-text XML
-
Supplement
(1857 KB) - BibTeX
- EndNote
Ice formation in the atmosphere has a significant influence on the microphysical and radiative properties of clouds. At temperatures above 235 K, atmospheric aerosol particles may act as ice-nucleating particles (INPs; Pruppacher and Klett, 2010; Vali et al., 2015). In mixed-phase clouds, immersion freezing is often the dominant ice nucleation mode (Hande and Hoose, 2017). Immersion freezing refers to a solid particle initiating ice formation inside a supercooled cloud droplet.
Over the past decades, many different particle types initiating freezing in mixed-phase clouds have been studied extensively (Hoose and Möhler, 2012; Murray et al., 2012; Kanji et al., 2017). Mineral dust particles emitted from desert areas have been identified as ubiquitous INPs which initiate ice nucleation in clouds over a wide range of temperature and humidity conditions (Boose et al., 2016; Ullrich et al., 2017). Cloud-level concentrations of potentially very ice-active primary biological aerosol particles (Hoose and Möhler, 2012) are much lower than background concentrations of mineral dust, with differences of up to 8 orders of magnitude in some cases (Hummel et al., 2018). Nevertheless, several laboratory studies, remote sensing measurements and studies characterizing ice crystal residuals have found evidence for the potential impact of these particles and more numerous nanoscale fragments on ice formation in mixed-phase clouds (e.g., Möhler et al., 2007; Pratt et al., 2009; Kanitz et al., 2011; O'Sullivan et al., 2015). Also, recent studies indicate a missing source of INPs beyond mineral dust, with biological particles from terrestrial environments being a likely candidate for initiating freezing in shallow mixed-phase clouds (O'Sullivan et al., 2018). One of the potential sources for these terrestrial INPs is agricultural areas, which may contribute between 7 % and 75 % to the regional dust burden (Ginoux et al., 2012) due to emissions driven by wind erosion and land management activities such as tilling and harvesting (Hoffmann et al. 2008; Funk et al., 2008; Iturri et al., 2017). Vegetated areas are another source for complex organic aerosol particles associated with leaf detritus (Coz et al., 2010).
One of the characteristics of biological INPs is that they include a vast variety of different particle types, ranging from primary biological particles, such as bacteria, fungi and pollen, to complex organic particles carrying different ice-nucleating agents and originating from biogenic sources (Schnell and Vali, 1973; Hoose and Möhler, 2012; Murray et al., 2012; Augustin et al., 2013; O'Sullivan et al., 2014; Tobo et al., 2014; Conen et al., 2016; Steinke et al., 2016). An example of complex organic particles is agricultural soil dust particles for which the observed high ice nucleation efficiency can be linked to microbiological activity and the presence of organic macromolecules (O'Sullivan et al., 2014; Tobo et al., 2014; Hill et al., 2016; Steinke et al., 2016; Suski et al., 2018). The expression of bacterial and fungal ice-active proteins is highly variable because environmental stress (e.g., a change in temperature) can change the structure of the ice-nucleating proteins, resulting in a loss of functionality (Pummer et al., 2012). In contrast, some of the organic macromolecules found in agricultural soils are very inert as they are able to withstand physical and chemical treatments, e.g., with heat or exposure to enzymes (Hill et al., 2016). With decaying plant material being one of the sources of these macromolecules (Hill et al., 2016), the need arises to better characterize the ice nucleation properties of plant-derived particles and their individual organic components.
Lignin and polysaccharides are integral components of plant cell structures and contribute up to 50 % to plant debris (Williams and Gray, 1974). Proteinaceous components of leaf litter (e.g., enzymes, storage proteins or structure proteins) vary considerably but have been found to account for up to 15 % (Williams and Gray, 1974). Lipids contribute up to 10 % to dry leaf mass (Graça et al., 2005). Note that only 50 % of the organic matter is accessible through chemical degradative techniques which inadvertently impact the structure of the extracted organic matter (Kögel-Knabner, 2002).
In this study, we investigate the immersion freezing properties of commercially available plant-derived organic compounds such as lignin, polysaccharides, plant wax and plant proteins – which are the main components of decaying plant material – as well as ambient bulk samples rich in plant material. We used commercially available organic compounds as analogues for plant-derived organics. Note that many of the extraction methods for organic matter may cause significant changes in the physicochemical properties of the extracted organic compounds (Kögel-Knabner, 2002). Experiments were conducted at the Aerosol Interactions and Dynamics in the Atmosphere (AIDA) cloud chamber and complemented by drop freezing assay studies using the Ice Nucleation SpEctrometer of the Karlsruhe Institute of Technology (INSEKT). From our experimental results, we derived temperature-dependent parameterizations based on the ice nucleation active surface site (INAS) densities concept (Connolly et al., 2009; Niemand et al., 2012). These parameterizations were then used to estimate upper limits for ambient INP concentrations for complex organic aerosols from vegetated environments.
2.1 Samples
In Table 1 we describe the samples used in this study, which include commercially available plant-derived organic compounds and bulk samples from vegetated environments.
Note that the agricultural dust from harvesting machines (bulk sample) contains roughly 90 % of biological material, e.g., partially intact plant cells and similar particles (Fig. S1 in the Supplement). The soil dust sample from Wyoming has been investigated in a recent study by Tobo et al. (2014), who found that organics contribute significantly to the ice nucleation efficiency observed for size-selected particles (d=600 nm). Representative microscopy images of all other samples used in this study are shown in the Supplement (Fig. S2).
2.2 AIDA immersion freezing experiments
Immersion freezing initiated by plant-related particles was investigated in the AIDA cloud chamber (Karlsruhe Institute of Technology, Germany). The AIDA cloud chamber consists of a cylindrical aluminum vessel (volume 84 m3) which is enclosed by a thermally insulated box. The ascent of cloud parcels is simulated by lowering the pressure from ambient levels (about 1000 hPa) to around 800 mbar resulting in a decrease in the temperature and an increase in the relative humidity.
A fan at the bottom of the AIDA chamber ensures homogeneous mixing (also with regard to temperature and humidity) across the whole chamber volume, except for transition zones near the chamber walls. The overall uncertainty of the mean gas temperature is about K (Möhler et al., 2006). The absolute water vapor partial pressure is measured with a tunable diode laser instrument and converted into humidity values by leveraging the saturation pressure formulation given in the review by Murphy and Koop (2005). The relative humidity values can be measured with an accuracy of % (Fahey et al., 2014).
Particle background concentrations within the cloud chamber are typically below 0.1 cm−3. For the immersion freezing experiments presented in this work, aerosol samples were injected into the cloud chamber by using a rotating brush generator (RBG 1000; Palas GmbH) for dry dispersion. Additionally, impactor stages were used to eliminate particles larger than 3–5 µm. The aerosol size distribution at the beginning of each experimental run was measured by combining data from an aerodynamic particle sizer (APS; model 3321, TSI Incorporated) and a scanning mobility particle sizer (SMPS; model 3076, TSI Incorporated). The combined aerosol size distributions are used to estimate the available aerosol surface area based on volume-equivalent sphere diameters; this then results in an estimate of the geometric surface area.
Upon reaching water saturation during an expansion experiment, aerosol particles within the cloud chamber are activated to droplets and may freeze subsequently. Ice crystal number concentrations are measured with two optical particle counters (white light aerosol spectrometer, namely WELAS1 and WELAS2; series 2300 and 2500; Palas GmbH) with size ranges of 0.7–46 and 5–240 µm in optical particle diameter, respectively (Wagner and Möhler, 2013). Ice crystals are discriminated from droplets by choosing a size threshold which is evaluated individually for each experiment.
2.3 Droplet freezing assay studies
To investigate the freezing of suspensions created with the bulk samples and hence to account for freezing caused by particles larger than 5 µm, a droplet freezing technique was employed. The Ice Nucleation SpEctrometer of the Karlsruhe Institute of Technology (INSEKT) setup (Schiebel, 2017) is based on the droplet freezing assay originally developed at Colorado State University (Hill et al., 2014).
Suspensions were created from bulk samples, combining 2 mg of material with 20 mL of deionized water (resistivity about 18 MΩ), which were passed through a filter with a pore diameter of 0.1 µm (Whatman Puradisc 25). Suspensions were shaken by hand (about 1 min), and the suspension tube was then submerged in an ultrasonic bath (5 min) to promote dispersion of the particles. In addition to the original suspensions, we also created suspensions with a dilution factor of 15 and 225 by adding filtered deionized water in proportion. Original and diluted suspensions were partitioned into 192 wells (aliquot volume – 50 µL) of a sterile polypropylene polymerase chain reaction (PCR) tray, with 32 wells set aside for blank measurements, i.e., freezing of particle-free filtered deionized water. These blank measurements are used for determining the background, which is then subtracted from the observed freezing curves. In this study, droplet freezing was measured at a cooling rate of 0.33 K min−1. Cooling is achieved by flowing chilled ethanol through a custom-made aluminum block which encloses the bottom part of the PCR tray. The overall temperature uncertainty is K (Schiebel, 2017).
2.4 Ice nucleation active surface site densities
For all experiments, the ice nucleation efficiency was quantified by calculating the ice nucleation active surface site (INAS) density ns. The ns values were derived by scaling the observed ice crystal number concentration nice with the available aerosol surface Aaer (Connolly et al., 2009; Niemand et al., 2012). Exemplary size distributions for leaf litter and lignin are shown in Fig. S3.
For the cloud chamber experiments, the aerosol surface Aaer (µm2 cm−3) was calculated from the APS and SMPS size distribution data using volume-equivalent sphere diameters (Möhler et al., 2006). In this study, it was assumed that all aerosol particles were activated to droplets upon reaching water saturation. Hence, the full aerosol surface area was considered to be available for immersion freezing. The ice crystal number concentration nice was derived from particle size distributions measured with the optical particle counters, WELAS1 and WELAS2, in conjunction with a size threshold above which particles are counted as ice crystals. Based on the measurement uncertainties of the observed ice crystal concentration, , and the aerosol surface area concentration, , the resulting uncertainty of the INAS density is (Ullrich et al., 2017).
For the droplet freezing studies, the INAS density values were derived from normalizing the cumulative INP concentrations nice with the specific aerosol surface Aaer (m2 g−1) derived from Brunauer–Emmett–Teller (BET) surface measurements. For our INAS density uncertainty analysis, we considered only the uncertainty of the cumulative INP concentrations which is based on statistics. Confidence intervals (at 95 %) have been estimated according to the improved Wald interval, which implicitly assumes a normal approximation for binomially distributed measurement errors (Agresti and Coull, 1998). Hence, in our INAS density analysis, we neglected the uncertainties of the BET surface measurements which are, in most cases, considerably smaller (i.e., ) than the previously described statistical uncertainties of the cumulative INP concentrations (Hiranuma et al., 2015a). Another source of uncertainty – which is considerably more difficult to quantify – was the contribution of larger particles. These larger particles may sediment quickly within the suspension and were probably underrepresented in the sampled aliquots. Thus, the particle surface area available for freezing was most likely overestimated in some cases. However, to fully understand this effect, more studies are needed. Additionally, suspending particles in water may lead to the desorption and potential redistribution of soluble material. This change in soluble material could also lead to differences in the observed ice nucleation properties when comparing cloud chamber experiments with droplet freezing studies.
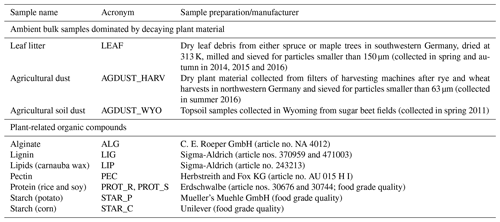
In Fig. 1 we present results from AIDA cloud chamber experiments with commercially available plant-related organic compounds and natural samples (see Table 1). For comparison, we show the ice nucleation activity of microcrystalline cellulose (Hiranuma et al., 2015b), which is a prevalent natural polymer derived from plant fragments, leaf litter, wood fiber, nonwood fiber and/or even microbes (Quiroz-Castañeda and Folch-Mallol, 2013; Vlachou et al., 2018). We also show the ice nucleation efficiency of agricultural soil dusts investigated in a study by Steinke et al. (2016) and an estimate for leaf litter from a study by Schnell and Vali (1973). The ice nucleation activity of each sample is expressed as the INAS density ns.
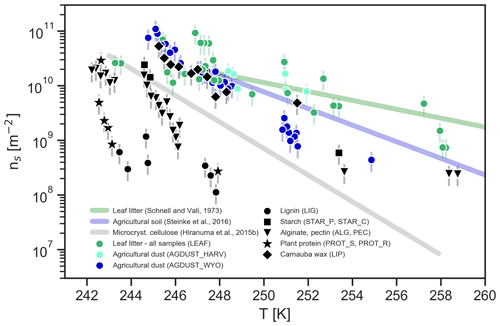
Figure 1Immersion freezing results for plant-related organic compounds compared to ambient samples. Ice nucleation efficiency is expressed as INAS density values based on AIDA cloud chamber experiments.
Figure 1 shows that the observed ice nucleation efficiencies of most individual plant-related organic compounds tend to be lower in comparison to samples from natural environments. However, there is a large spread in INAS density values when comparing different plant-related organic compounds. Particularly noticeable is the low ice nucleation efficiency observed for plant protein and for which freezing was observed only below 248 K. In this study, we tested two different types of plant proteins (PROT_R and PROT_SOY), derived from soy or rice (not differentiated in Fig. 1). Only lignin (LIG) shows an ice nucleation activity as low as the plant protein samples. Alginate, pectin and starch (which mainly consist of highly complex polysaccharides) are similarly as ice active as microcrystalline cellulose (Hiranuma et al., 2015b) and desert dusts (Ullrich et al., 2017 – not shown in Fig. 1). Above 250 K, the complex polysaccharides investigated in this study (ALG, PEC, STAR_P and STAR_C) tend to be more ice active than cellulose. Our data also indicate that the temperature dependence of the polysaccharides investigated in this study is possibly less pronounced than for cellulose. Note that this finding is based only on a few data points due to the low observed ice nucleation efficiency above 252 K.
Of all plant-related compounds, carnauba wax (LIP) shows the highest ice nucleation efficiency, which is comparable to decaying leaves and two agricultural samples, i.e., dust from a sugar beet field (AGDUST_WYO) and material collected from harvesting machines (AGDUST_HARV). Carnauba wax is a mixture of hydrocarbons, aliphatic esters and fatty alcohols (Vandenburg and Wilder, 1970), with an average chain length of 50 carbon atoms (Basson and Reynhardt, 1988). Crystalline fatty alcohols (C16–C18) have been highlighted recently in a study by DeMott et al. (2018) with regard to their ability to nucleate ice at 261 K via condensation freezing. Based on theoretical considerations, hydrocarbons with long chains are potentially very good at initiating ice formation (Qiu et al., 2017), but conclusive experimental evidence is still missing. Hence, these theoretical considerations might provide an explanation for the high ice nucleation ability of carnauba wax.
For samples like the agricultural soil dusts and the leaf litter investigated in this study, some studies (e.g., Schnell and Vali, 1973; Steinke et al., 2016) have found similarly high ice nucleation efficiencies.
In contrast, at 258 K, leaf litter from the Arctic consisting of birch and grass leaves (Conen et al., 2016) has been observed to show relatively low ice nucleation efficiencies compared to leaf litter in our study – based on AIDA results and similar efficiencies when comparing against our droplet freezing assay.
Figure 2Immersion freezing results for selected plant-related samples and illite by comparing INSEKT-derived INAS density values to results from AIDA experiments (Fig. 1). Ice nucleation efficiency is expressed as INAS density values based on INSEKT droplet freezing experiments and specific surface areas indicated in the legend.
Hence, the high INAS density values observed in our cloud chamber studies can be interpreted as the upper limits for the ice nucleation efficiency of ambient plant-related aerosol particles. Note that for our leaf litter samples we did not differentiate between samples collected at different points in time or for different species. Due to the high variability, it was not possible to clearly derive a seasonal trend from the observed ice nucleation efficiencies.
In Fig. 2, we show INSEKT-derived INAS density values for selected samples investigated in the previously described AIDA cloud chamber studies. For every sample at least two experimental runs were conducted, using freshly prepared suspensions for each run. The PROT_S sample was investigated to establish the lower boundary of ice nucleation activity observed for plant components whereas the AGDUST_HARV and the LEAF samples were used to represent ambient samples. Note that for the droplet freezing experiments, the INAS densities are evaluated based on the specific surface areas derived from BET measurements rather than the geometric surface areas which were used for analyzing the AIDA experiments. The droplet freezing experiments are complementary to the cloud chamber studies as they provide insights regarding the freezing properties of the bulk material, in particular with regard to including particles larger than 5 µm which are largely eliminated by the impactor stages in our AIDA experiments. Also, observing the freezing of bulk suspensions allows for quantifying the immersion freezing efficiencies at a lower supercooling which are more difficult to quantify in AIDA cloud chamber studies. For leaf litter we observe that INAS density values agree well between the INSEKT and AIDA experiments. Similarly for plant protein (PROT_S), the agreement is reasonable. For AGDUST_HARV, there is a difference of approximately more than 1 order of magnitude, which is possibly caused by larger particles being undersampled due to sedimentation within the suspensions.
Figure 2 shows that the hierarchy in ice nucleation activities is similar to that observed in the AIDA cloud chamber experiments, with leaf litter and agricultural dust being the most ice-active samples. The steep onset of ice nucleation observed for the agricultural dust at 267 K suggests a contribution from biological particles (Suski et al., 2018). In contrast, the reasons for the steep onset observed for the leaf litter sample are a bit more unclear as most studies investigating primary biological particles have observed freezing onsets and high ice nucleation efficiency already at temperatures above 260 K (see references in Hoose and Möhler, 2012). However, one recent study has found indications for macromolecules associated with microbial activity being ice active at about 258 K (O'Sullivan et al., 2015). Soy protein particles initiate ice formation at higher temperatures (i.e., already below 258 K) than observed in AIDA cloud chamber experiments, but the overall ice nucleation efficiency is still lower than for the complex organic samples from natural environments. Unfortunately, it was not possible to reliably determine INAS density values for carnauba wax (LIP) due to its very low dispersibility. Figure 2 also shows the INAS density values observed for illite as a proxy for freezing induced by mineral dust.
In conclusion, the results from the droplet freezing studies confirm the trend observed in our AIDA cloud chamber experiments, with particles from vegetated and agricultural environments being highly ice active, whereas individual organic compounds tend to be lower in their ice nucleation efficiencies. It should be noted that the organic compounds investigated in this study may not fully represent the complexity of real organic compounds in plants which often include mixtures, e.g., ligno-polysaccharide complexes with unknown chemical structures (Kögel-Knabner, 2002). At temperatures above 260 K, the gap between individual plant-related compounds and particles from natural environments may be attributed to primary biological particles (e.g., fungi and bacteria) according to our droplet freezing measurements of harvesting dust. For example, ice nucleation efficiencies observed for particles generated from leaf litter fall within the lower range of values observed for bacteria (Hoose and Möhler, 2012).
There are, however, also differences between the ice nucleation efficiencies derived from AIDA cloud chamber experiments and droplet freezing studies, which are strongly dependent on the aerosol type. Some of these differences might be explained by differences in the evaluation of the INAS density values, which are either related to the geometric surface or the specific surface area. For illite, normalizing by BET surface area results in INAS density values which are 1 order of magnitude lower compared to values derived by using geometric surface estimates (Hiranuma et al., 2015a). Also, for some samples there are possibly differences in the effective size distribution due to agglomeration or low dispersibility in the suspensions. In contrast, the dry dispersion method (i.e., the rotating brush generator) is more likely to encourage the disaggregation of particle agglomerates. Similar differences regarding the freezing of aqueous suspensions in comparison to dry dispersion experiments have been observed in other studies as well (Hiranuma et al., 2015a, 2019).
Our experimental results suggest that the main components of decaying plant material (i.e., cellulose and lignin) are not very good predictors of ice nucleation by ambient plant-related particles. However, the INAS density values observed for leaf litter and agricultural dust may help to constrain the upper limits of their respective ambient INP concentrations. The INAS density values for leaf litter and agricultural dust can be described by temperature-dependent functions, with the following:
and
Note that these functions are only valid within certain temperature ranges, i.e., and , with all temperatures given in kelvin. Equations (1) and (2) have been derived from the cloud chamber experiments exclusively and are represented in Fig. 2. Note that, based on our droplet freezing experiments, both of these aerosol types may have relatively sharp ice nucleation onsets at 257 K (leaf litter) and 267 K (agricultural dusts).
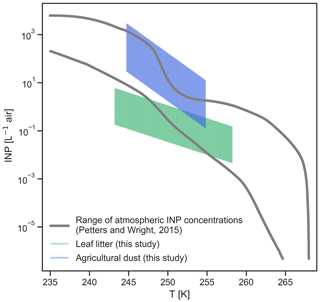
Figure 3Comparison between atmospheric INP concentrations (Petters and Wright, 2015) and estimates for INPs from leaf litter and agricultural dust based on AIDA cloud chamber experiments.
Figure 3 shows a comparison between ambient INP concentrations derived from precipitation samples from several sites in the USA and Europe (Petters and Wright, 2015) and estimates for INP concentrations from leaf litter (Eq. 1) and agricultural dust (Eq. 2). Note that ambient INP measurements may scatter significantly more than found in the study by Petters and Wright (2015), with deviations of up to 4 orders of magnitude between different studies (Kanji et al., 2017).
Ground-based measurements for leaf litter concentrations range between 30 ng m−3 and 1 µg m−3 (Hildemann et al., 1996; Sánchez-Ochoa et al., 2007). Sánchez-Ochoa et al. (2007) use cellulose found in aerosol particles as a proxy for plant debris concentrations, relying on observations at six European sites for a time span of 2 years, and with two of the sites being located on mountains. Hildemann et al. (1996) used higher alkanes (e.g., occurring in plant waxes) to fingerprint plant debris in aerosol particles sampled in the greater Los Angeles area. For agricultural dust, ground-based concentration varies between <10 and 100 µg m−3, with up to 800 µg m−3 observed occasionally for very strong wind erosion events (Gillette et al., 1978; Sharratt et al., 2007; Hoffmann and Funk, 2015). Annually averaged boundary layer concentrations for desert dust vary between 0.1 and 30 µg m−3 (Ginoux et al., 2001), which is comparable to the aforementioned concentrations of complex organic particles. Anthropogenic dust sources contribute roughly 25 % to the global dust burden, with regional variations ranging from 7 % to 75 % (Ginoux et al., 2012). In areas with intense agricultural land use, e.g., in eastern North America, India, eastern China and Europe, anthropogenic dust emissions contribute generally to more than 60 % of the total dust burden (Huang et al., 2015). Note, however, that there is a substantial uncertainty regarding the number and size of particles emitted from agricultural activities and their transport to cloud altitudes and the resulting atmospheric lifetime. This uncertainty is rooted in a lack of emission flux data above 5–10 m, which is the height at which dust fluxes from agricultural areas are commonly observed (e.g., in the study by Zobeck and Van Pelt, 2006). Using Eqs. (1) and (2) and assuming an aerosol surface area of 1 and 36 m2 g−1 as measured by BET analysis for leaf litter and soil dust, respectively, we can derive order of magnitude estimates for the expected atmospheric INP contributions from leaf litter and agricultural dust. In Fig. 3, we have scaled down agricultural dust INPs by a factor of 100 and leaf litter INPs by a factor of 10 to at least partially account for transport losses. Scaling factors are derived from model results presented in Hoose et al. (2010), using vertical profiles for desert dusts and biological particles as rough proxies for the samples investigated in this study.
The estimates presented in this study should be considered as the upper limits, with emission fluxes of organic particles acting as INPs being poorly constrained, and more detailed modeling case studies are needed. We find that plant-derived organic INPs from leaf litter and agricultural areas are within the same order of magnitude as INP concentrations derived from precipitation measurements and field campaigns (Petters and Wright, 2015; Kanji et al., 2017). This finding further emphasizes the potential of plant-related sources contributing to ambient INPs.
Complex organic particles are emitted from terrestrial sources, with wind erosion, soil cultivation and harvesting crops as potential main drivers for emissions of organic matter associated with plant debris and decomposed residues (Funk et al., 2008; Hoffmann et al., 2008; Coz et al., 2010; Ginoux et al., 2012). These sources are becoming increasingly important in the global view as climate change, soil degradation and excessive land use will promote dust emissions from agriculturally used areas. In this study, we investigated the immersion freezing properties of plant-related organic particles and samples from vegetated environments. We used a combination of the AIDA cloud chamber and INSEKT droplet freezing experiments to cover a temperature range between 242 and 267 K. Our experiments show that the samples with a complex organic composition are equally as ice active as (or more ice active than) individual plant-related compounds. Lignin and plant protein samples are inefficient INPs, whereas starches, alginate and pectin show moderate to high ice nucleation efficiencies. Surprisingly, carnauba wax – which is a mixture of aliphatic esters and fatty acids – shows the highest ice nucleation activity of all the organic compounds investigated in this study. INP estimates based on our cloud chamber experiments lend themselves to the hypothesis that aerosolized particles from leaf litter and agricultural areas are potentially important contributors to atmospheric INPs. However, the high ice nucleation efficiency of these particles could not be fully explained by the ice nucleation activity of individual organic compounds commonly found in plant tissue, potentially indicating a contribution from primary biological particles or organics associated with microbial activity. Thus, further future studies are indeed demanded and warranted.
All data in this manuscript will be made available as part of a KITopen data repository.
The supplement related to this article is available online at: https://doi.org/10.5194/acp-20-11387-2020-supplement.
IS and NH designed and conducted the experiments, with contributions from KH, OM and NSU. PGW conducted the BET surface measurements, and NT provided the SEM images. IS and NH analyzed the data. IS prepared the paper with input from all coauthors.
The authors declare that they have no conflict of interest.
Hinrich Grothe (Vienna University of Technology) is acknowledged for having provided several organic samples (lignin, carnauba wax, pectin, alginate and starches) investigated in this study.
The IMK–AAF technicians team (Georg Scheurig, Rainer Buschbacher, Tomasz Chudy, Olga Dombrowski and Steffen Vogt) is acknowledged for their continued support in ensuring the smooth operation of the AIDA cloud chamber.
This study was conducted with financial support from the
Carl Zeiss Foundation and the German Science Foundation (DFG) through the
research unit INUIT (grant nos. FOR 1525 and MO668/4-2). Isabelle Steinke was also funded in
part by the US Department of Energy (DOE; BER) through the Early Career
Program. Nsikanabasi Silas Umo was funded by the Alexander von Humboldt Foundation, Germany (grant no. 1188375). Some microscopy research and sample
precharacterizations were performed in the Environmental Molecular Sciences
Laboratory (under the user proposal 49077), which is a DOE Office of Science User
Facilities, sponsored by the Office of Biological and Environmental Research,
and located at the Pacific Northwest National Laboratory.
The article processing charges for this open access
publication were covered by a Research
Centre of the Helmholtz Association.
This paper was edited by Hinrich Grothe and reviewed by two anonymous referees.
Agresti, A. and Coull, B. A.: Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions, Am. Stat., 52, 119–126, https://doi.org/10.1080/00031305.1998.10480550, 1998.
Augustin, S., Wex, H., Niedermeier, D., Pummer, B., Grothe, H., Hartmann, S., Tomsche, L., Clauss, T., Voigtländer, J., Ignatius, K., and Stratmann, F.: Immersion freezing of birch pollen washing water, Atmos. Chem. Phys., 13, 10989–11003, https://doi.org/10.5194/acp-13-10989-2013, 2013.
Basson, I. and Reynhardt, E. C.: An investigation of the structures and molecular dynamics of natural waxes. II. Carnauba wax, J. Phys. D Appl. Phys., 21, 1429–1433, https://doi.org/10.1088/0022-3727/21/9/017, 1988.
Boose, Y., Welti, A., Atkinson, J., Ramelli, F., Danielczok, A., Bingemer, H. G., Plötze, M., Sierau, B., Kanji, Z. A., and Lohmann, U.: Heterogeneous ice nucleation on dust particles sourced from nine deserts worldwide – Part 1: Immersion freezing, Atmos. Chem. Phys., 16, 15075–15095, https://doi.org/10.5194/acp-16-15075-2016, 2016.
Conen, F., Stopelli, E., and Zimmermann, L.: Clues that decaying leaves enrich Arctic air with ice nucleating particles, Atmos. Environ., 129, 91–94, https://doi.org/10.1016/j.atmosenv.2016.01.027, 2016.
Connolly, P. J., Möhler, O., Field, P. R., Saathoff, H., Burgess, R., Choularton, T., and Gallagher, M.: Studies of heterogeneous freezing by three different desert dust samples, Atmos. Chem. Phys., 9, 2805–2824, https://doi.org/10.5194/acp-9-2805-2009, 2009.
Coz, E., Artíñano, B., Clark, L. M., Hernandez, M., Robinson, A. L., Casuccio, G. S., Lersch, T. L., and Pandis, S. N.: Characterization of Fine Primary Biogenic Organic Aerosol in an Urban Area in the Northeastern United States, Atmos Environ , 44, 3952–3962, https://doi.org/10.1016/j.atmosenv.2010.07.007, 2010.
DeMott, P. J., Mason, R. H., McCluskey, C. S., Hill, T. C. J., Perkins, R. J., Desyaterik, Y., Bertram, A. K., Trueblood, J. V., Grassian, V. H., Qiu, Y., Molinero, V., Tobo, Y., Sultana, C. M., Lee, C. T., and Prather, K. A.: Ice nucleation by particles containing long-chain fatty acids of relevance to freezing by sea spray aerosols, Environ. Sci.-Proc. Imp., 20, 1559–1569, https://doi.org/10.1039/c8em00386f, 2018.
Fahey, D. W., Gao, R.-S., Möhler, O., Saathoff, H., Schiller, C., Ebert, V., Krämer, M., Peter, T., Amarouche, N., Avallone, L. M., Bauer, R., Bozóki, Z., Christensen, L. E., Davis, S. M., Durry, G., Dyroff, C., Herman, R. L., Hunsmann, S., Khaykin, S. M., Mackrodt, P., Meyer, J., Smith, J. B., Spelten, N., Troy, R. F., Vömel, H., Wagner, S., and Wienhold, F. G.: The AquaVIT-1 intercomparison of atmospheric water vapor measurement techniques, Atmos. Meas. Tech., 7, 3177–3213, https://doi.org/10.5194/amt-7-3177-2014, 2014.
Funk, R., Reuter, H. I., Hoffmann, C., Engel, W., and Öttl, D.: Effect of moisture on fine dust emission from tillage operations on agricultural soils, Earth Surf. Process. Land., 33, 1851–1863, https://doi.org/10.1002/esp.1737, 2008.
Gillette, D. A., Clayton, R. N., Mayeda, T. K., Jackson, M. L., and Sridhar, K.: Tropospheric Aerosols from Some Major Dust Storms of the Southwestern United States, J. Appl. Meteorol., 17, 832–845, https://doi.org/10.1175/1520-0450(1978)017<0832:TAFSMD>2.0.CO;2, 1978.
Ginoux, P., Chin, M., Tegen, I., Prospero, J. M., Holben, B., Dubovik, O., and Lin, S.-J.: Sources and distributions of dust aerosols simulated with the GOCART model, J. Geophys. Res.-Atmos., 106, 20255–20273, https://doi.org/10.1029/2000jd000053, 2001.
Ginoux, P., Prospero, J. M., Gill, T. E., Hsu, N. C., and Zhao, M.: Global-scale attribution of anthropogenic and natural dust sources and their emission rates based on MODIS Deep Blue aerosol products, Rev. Geophys., 50, RG3005, https://doi.org/10.1029/2012RG000388, 2012.
Graça, M. A. S., Bärlocher, F., and Gessner, M. O.: Methods to study litter decomposition: a practical guide, Springer, Dordrecht, 2005.
Hande, L. B. and Hoose, C.: Partitioning the primary ice formation modes in large eddy simulations of mixed-phase clouds, Atmos. Chem. Phys., 17, 14105–14118, https://doi.org/10.5194/acp-17-14105-2017, 2017.
Hildemann, L. M., Rogge, W. F., Cass, G. R., Mazurek, M. A., and Simoneit, B. R. T.: Contribution of primary aerosol emissions from vegetation-derived sources to fine particle concentrations in Los Angeles, J. Geophys. Res.-Atmos., 101, 19541–19549, https://doi.org/10.1029/95JD02136, 1996.
Hill, T. C. J., Moffett, B. F., DeMott, P. J., Georgakopoulos, D. G., Stump, W. L., and Franc, G. D.: Measurement of Ice Nucleation-Active Bacteria on Plants and in Precipitation by Quantitative PCR, Appl. Environ. Microb., 80, 1256–1267, https://doi.org/10.1128/aem.02967-13, 2014.
Hill, T. C. J., DeMott, P. J., Tobo, Y., Fröhlich-Nowoisky, J., Moffett, B. F., Franc, G. D., and Kreidenweis, S. M.: Sources of organic ice nucleating particles in soils, Atmos. Chem. Phys., 16, 7195–7211, https://doi.org/10.5194/acp-16-7195-2016, 2016.
Hiranuma, N., Augustin-Bauditz, S., Bingemer, H., Budke, C., Curtius, J., Danielczok, A., Diehl, K., Dreischmeier, K., Ebert, M., Frank, F., Hoffmann, N., Kandler, K., Kiselev, A., Koop, T., Leisner, T., Möhler, O., Nillius, B., Peckhaus, A., Rose, D., Weinbruch, S., Wex, H., Boose, Y., DeMott, P. J., Hader, J. D., Hill, T. C. J., Kanji, Z. A., Kulkarni, G., Levin, E. J. T., McCluskey, C. S., Murakami, M., Murray, B. J., Niedermeier, D., Petters, M. D., O'Sullivan, D., Saito, A., Schill, G. P., Tajiri, T., Tolbert, M. A., Welti, A., Whale, T. F., Wright, T. P., and Yamashita, K.: A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: a comparison of 17 ice nucleation measurement techniques, Atmos. Chem. Phys., 15, 2489–2518, https://doi.org/10.5194/acp-15-2489-2015, 2015a.
Hiranuma, N., Möhler, O., Yamashita, K., Tajiri, T., Saito, A., Kiselev, A., Hoffmann, N., Hoose, C., Jantsch, E., Koop, T., and Murakami, M.: Ice nucleation by cellulose and its potential contribution to ice formation in clouds, Nat. Geosci., 8, 273–277, https://doi.org/10.1038/ngeo2374, 2015b.
Hiranuma, N., Adachi, K., Bell, D. M., Belosi, F., Beydoun, H., Bhaduri, B., Bingemer, H., Budke, C., Clemen, H.-C., Conen, F., Cory, K. M., Curtius, J., DeMott, P. J., Eppers, O., Grawe, S., Hartmann, S., Hoffmann, N., Höhler, K., Jantsch, E., Kiselev, A., Koop, T., Kulkarni, G., Mayer, A., Murakami, M., Murray, B. J., Nicosia, A., Petters, M. D., Piazza, M., Polen, M., Reicher, N., Rudich, Y., Saito, A., Santachiara, G., Schiebel, T., Schill, G. P., Schneider, J., Segev, L., Stopelli, E., Sullivan, R. C., Suski, K., Szakáll, M., Tajiri, T., Taylor, H., Tobo, Y., Ullrich, R., Weber, D., Wex, H., Whale, T. F., Whiteside, C. L., Yamashita, K., Zelenyuk, A., and Möhler, O.: A comprehensive characterization of ice nucleation by three different types of cellulose particles immersed in water, Atmos. Chem. Phys., 19, 4823–4849, https://doi.org/10.5194/acp-19-4823-2019, 2019.
Hoffmann, C. and Funk, R.: Diurnal changes of PM10-emission from arable soils in NE-Germany, Aeolian Res., 17, 117–127, https://doi.org/10.1016/j.aeolia.2015.03.002, 2015.
Hoffmann, C., Funk, R., Li, Y., and Sommer, M.: Effect of grazing on wind driven carbon and nitrogen ratios in the grasslands of Inner Mongolia, Catena, 75, 182–190, https://doi.org/10.1016/j.catena.2008.06.003, 2008.
Hoose, C. and Möhler, O.: Heterogeneous ice nucleation on atmospheric aerosols: a review of results from laboratory experiments, Atmos. Chem. Phys., 12, 9817–9854, https://doi.org/10.5194/acp-12-9817-2012, 2012.
Hoose, C., Kristjánsson, J. E., Chen, J., and Hazra, A.: A Classical-Theory-Based Parameterization of Heterogeneous Ice Nucleation by Mineral Dust, Soot, and Biological Particles in a Global Climate Model, J. Atmos. Sci., 67, 2483–2503, https://doi.org/10.1175/2010JAS3425.1, 2010
Huang, J. P., Liu, J. J., Chen, B., and Nasiri, S. L.: Detection of anthropogenic dust using CALIPSO lidar measurements, Atmos. Chem. Phys., 15, 11653–11665, https://doi.org/10.5194/acp-15-11653-2015, 2015.
Hummel, M., Hoose, C., Pummer, B., Schaupp, C., Fröhlich-Nowoisky, J., and Möhler, O.: Simulating the influence of primary biological aerosol particles on clouds by heterogeneous ice nucleation, Atmos. Chem. Phys., 18, 15437–15450, https://doi.org/10.5194/acp-18-15437-2018, 2018.
Iturri, L. A., Funk, R., Leue, M., Sommer, M., and Buschiazzo, D. E.: Wind sorting affects differently the organo-mineral composition of saltating and particulate materials in contrasting texture agricultural soils, Aeolian Res., 28, 39–49, https://doi.org/10.1016/j.aeolia.2017.07.005, 2017.
Kanitz, T., Seifert, P., Ansmann, A., Engelmann, R., Althausen, D., Casiccia, C., and Rohwer, E. G.: Contrasting the impact of aerosols at northern and southern midlatitudes on heterogeneous ice formation, Geophys. Res. Lett., 38, L17802, https://doi.org/10.1029/2011gl048532, 2011.
Kanji, Z. A., Ladino, L. A., Wex, H., Boose, Y., Burkert-Kohn, M., Cziczo, D. J., and Krämer, M.: Overview of Ice Nucleating Particles, Meteor. Mon., 58, 1.1–1.33, https://doi.org/10.1175/amsmonographs-d-16-0006.1, 2017.
Kögel-Knabner, I.: The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter, Soil Biol. Biochem., 34, 139–162, https://doi.org/10.1016/S0038-0717(01)00158-4, 2002.
Möhler, O., Field, P. R., Connolly, P., Benz, S., Saathoff, H., Schnaiter, M., Wagner, R., Cotton, R., Krämer, M., Mangold, A., and Heymsfield, A. J.: Efficiency of the deposition mode ice nucleation on mineral dust particles, Atmos. Chem. Phys., 6, 3007–3021, https://doi.org/10.5194/acp-6-3007-2006, 2006.
Möhler, O., DeMott, P. J., Vali, G., and Levin, Z.: Microbiology and atmospheric processes: the role of biological particles in cloud physics, Biogeosciences, 4, 1059–1071, https://doi.org/10.5194/bg-4-1059-2007, 2007.
Murphy, D. M. and Koop, T.: Review of the vapour pressures of ice and supercooled water for atmospheric applications, Q. J. Roy. Meteor. Soc., 131, 1539–1565, https://doi.org/10.1256/qj.04.94, 2005.
Murray, B. J., O'Sullivan, D., Atkinson, J. D., and Webb, M. E.: Ice nucleation by particles immersed in supercooled cloud droplets, Chem. Soc. Rev., 41, 6519–6554, https://doi.org/10.1039/c2cs35200a, 2012.
Niemand, M., Möhler, O., Vogel, B., Vogel, H., Hoose, C., Connolly, P., Klein, H., Bingemer, H., DeMott, P., Skrotzki, J., and Leisner, T.: A Particle-Surface-Area-Based Parameterization of Immersion Freezing on Desert Dust Particles, J. Atmos. Sci., 69, 3077–3092, https://doi.org/10.1175/jas-d-11-0249.1, 2012.
O'Sullivan, D., Murray, B. J., Malkin, T. L., Whale, T. F., Umo, N. S., Atkinson, J. D., Price, H. C., Baustian, K. J., Browse, J., and Webb, M. E.: Ice nucleation by fertile soil dusts: relative importance of mineral and biogenic components, Atmos. Chem. Phys., 14, 1853–1867, https://doi.org/10.5194/acp-14-1853-2014, 2014.
O'Sullivan, D., Adams, M. P., Tarn, M. D., Harrison, A. D., Vergara-Temprado, J., Porter, G. C. E., Holden, M. A., Sanchez-Marroquin, A., Carotenuto, F., Whale, T. F., McQuaid, J. B., Walshaw, R., Hedges, D. H. P., Burke, I. T., Cui, Z., and Murray, B. J.: Contributions of biogenic material to the atmospheric ice-nucleating particle population in North Western Europe, Sci. Rep., 8, 13821, https://doi.org/10.1038/s41598-018-31981-7, 2018.
Petters, M. D. and Wright, T. P.: Revisiting ice nucleation from precipitation samples, Geophys. Res. Lett., 42, 8758–8766, https://doi.org/10.1002/2015GL065733, 2015.
Pratt, K. A., DeMott, P. J., French, J. R., Wang, Z., Westphal, D. L., Heymsfield, A. J., Twohy, C. H., Prenni, A. J., and Prather, K. A.: In situ detection of biological particles in cloud ice-crystals, Nat. Geosci., 2, 398–401, https://doi.org/10.1038/ngeo521, 2009.
Pruppacher, H. R. and Klett, J. D.: Microphysics of clouds and precipitation, 2nd Edn., Atmospheric and Oceanographic Sciences Library, Springer, Dordrecht, 2010.
Pummer, B. G., Bauer, H., Bernardi, J., Bleicher, S., and Grothe, H.: Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen, Atmos. Chem. Phys., 12, 2541–2550, https://doi.org/10.5194/acp-12-2541-2012, 2012.
Qiu, Y., Odendahl, N., Hudait, A., Mason, R., Bertram, A. K., Paesani, F., DeMott, P. J., and Molinero, V.: Ice Nucleation Efficiency of Hydroxylated Organic Surfaces Is Controlled by Their Structural Fluctuations and Mismatch to Ice, J. Am. Chem. Soc., 139, 3052–3064, https://doi.org/10.1021/jacs.6b12210, 2017.
Quiroz-Castañeda, R. E. and Folch-Mallol, J. L.: Hydrolysis of Biomass Mediated by Cellulases for the Production of Sugars, in: Sustainable Degradation of Lignocellulosic Biomass – Techniques, Applications and Commercialization, edited by: Chandel, A. and Silvério da Silva, S., InTechOpen, London, UK, 2013.
Sánchez-Ochoa, A., Kasper-Giebl, A., Puxbaum, H., Gelencser, A., Legrand, M., and Pio, C.: Concentration of atmospheric cellulose: A proxy for plant debris across a west-east transect over Europe, J. Geophys. Res.-Atmos., 112, D23S08, https://doi.org/10.1029/2006JD008180, 2007.
Schiebel, T.: Ice Nucleation Activity of Soil Dust Aerosols, PhD dissertation, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2017.
Schnell, R. C. and Vali, G.: Atmospheric Ice Nuclei from Decomposing Vegetation, Nature, 236, 163–165, https://doi.org/10.1038/236163a0, 1972.
Schnell, R. C. and Vali, G.: World-wide Source of Leaf-derived Freezing Nuclei, Nature, 246, 212–213, https://doi.org/10.1038/246212a0, 1973.
Sharratt, B., Feng, G., and Wendling, L.: Loss of soil and PM10 from agricultural fields associated with high winds on the Columbia Plateau, Earth Surf. Process. Land., 32, 621–630, https://doi.org/10.1002/esp.1425, 2007.
Steinke, I., Funk, R., Busse, J., Iturri, A., Kirchen, S., Leue, M., Möhler, O., Schwartz, T., Schnaiter, M., Sierau, B., Toprak, E., Ullrich, R., Ulrich, A., Hoose, C., and Leisner, T.: Ice nucleation activity of agricultural soil dust aerosols from Mongolia, Argentina, and Germany, J. Geophys. Res.-Atmos., 121, 13559–13576, https://doi.org/10.1002/2016jd025160, 2016.
Suski, K. J., Hill, T. C. J., Levin, E. J. T., Miller, A., DeMott, P. J., and Kreidenweis, S. M.: Agricultural harvesting emissions of ice-nucleating particles, Atmos. Chem. Phys., 18, 13755–13771, https://doi.org/10.5194/acp-18-13755-2018, 2018.
Tobo, Y., DeMott, P. J., Hill, T. C. J., Prenni, A. J., Swoboda-Colberg, N. G., Franc, G. D., and Kreidenweis, S. M.: Organic matter matters for ice nuclei of agricultural soil origin, Atmos. Chem. Phys., 14, 8521–8531, https://doi.org/10.5194/acp-14-8521-2014, 2014.
Ullrich, R., Hoose, C., Möhler, O., Niemand, M., Wagner, R., Höhler, K., Hiranuma, N., Saathoff, H., and Leisner, T.: A New Ice Nucleation Active Site Parameterization for Desert Dust and Soot, J. Atmos. Sci., 74, 699–717, https://doi.org/10.1175/jas-d-16-0074.1, 2017.
Vali, G., DeMott, P. J., Möhler, O., and Whale, T. F.: Technical Note: A proposal for ice nucleation terminology, Atmos. Chem. Phys., 15, 10263–10270, https://doi.org/10.5194/acp-15-10263-2015, 2015.
Vandenburg, L. E. and Wilder, E. A.: The structural constituents of carnauba wax, J. Am. Oil Chem. Soc., 47, 514–518, https://doi.org/10.1007/bf02639240, 1970.
Vlachou, A., Daellenbach, K. R., Bozzetti, C., Chazeau, B., Salazar, G. A., Szidat, S., Jaffrezo, J.-L., Hueglin, C., Baltensperger, U., Haddad, I. E., and Prévôt, A. S. H.: Advanced source apportionment of carbonaceous aerosols by coupling offline AMS and radiocarbon size-segregated measurements over a nearly 2-year period, Atmos. Chem. Phys., 18, 6187–6206, https://doi.org/10.5194/acp-18-6187-2018, 2018.
Wagner, R. and Möhler, O.: Heterogeneous ice nucleation ability of crystalline sodium chloride dihydrate particles, J. Geophys. Res.-Atmos., 118, 4610–4622, https://doi.org/10.1002/jgrd.50325, 2013.
Williams, S. T. and Gray, T. R. G.: Decomposition of litter on the soil surface, in: Biology of Plant Litter Decomposition, edited by: Dickinson, C. H. and Pugh, G. J. F., Academic Press, London, 611–632, 1974.
Zobeck, T. M. and Van Pelt, R. S.: Wind-Induced Dust Generation and Transport Mechanics on a Bare Agricultural Field, J. Hazard. Mater., 132, 26–38, https://doi.org/10.1016/j.jhazmat.2005.11.090, 2006.