the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Formation drivers and photochemical effects of ClNO2 in a coastal city of Southeast China
Gaojie Chen
Xiaolong Fan
Yee Jun Tham
Ziyi Lin
Xiaoting Ji
Lingling Xu
Baoye Hu
Nitryl chloride (ClNO2) is an important precursor of chlorine (Cl) radical, significantly affecting ozone (O3) formation and photochemical oxidation. However, the key drivers of ClNO2 production are not fully understood. In this study, the field observations of ClNO2 and related parameters were conducted in a coastal city of Southeast China during the autumn of 2022, combining with machine learning and model simulations to elucidate its key influencing factors and atmospheric impacts. Elevated concentrations of ClNO2 (>500 ppt) were notably observed during nighttime in late autumn, accompanied by increased levels of dinitrogen pentoxide (N2O5) and nitrate (). Nighttime concentrations of ClNO2 peaked at 3.4 ppb, while its daytime levels remained significant, reaching up to 100 ppt and sustaining at approximately 40 ppt at noon. Machine learning and field observations identified nighttime N2O5 heterogeneous uptake as the predominant pathway for ClNO2 production, whereas photolysis may contribute to its daytime generation. Additionally, ambient temperature (T) and relative humidity (RH) emerged as primary meteorological factors affecting ClNO2 formation, mainly through their effects on thermal equilibrium and N2O5 hydrolysis processes, respectively. Ultraviolet (UV) radiation was found to play a dual role in ClNO2 concentrations around noon. Box model simulations showed that, under high-ClNO2 conditions, the rates of alkane oxidation by Cl radical in the early morning exceeded those by OH radical. Consequently, volatile organic compound (VOC) oxidation by Cl radical contributed ∼19 % to ROx production rates, thereby significantly impacting O3 formation and atmospheric oxidation capacity. This research enriched the understanding of ClNO2 generation and loss pathways, providing valuable insights for the regulation of photochemical pollution in coastal regions.
- Article
(5642 KB) - Full-text XML
-
Supplement
(1317 KB) - BibTeX
- EndNote
Chlorine (Cl) radical, as an important atmospheric oxidant, can react with volatile organic compounds (VOCs) to affect ROx (including OH, HO2, and RO2) radicals and ozone (O3) formation (Yi et al., 2023), thereby perturbing atmospheric chemical components and air quality (Peng et al., 2021; Li et al., 2020). The reaction rates between Cl radical and some alkanes are several orders of magnitude faster than those involving OH radical (Atkinson et al., 2006). Furthermore, the related studies indicated that the production rates of Cl radical in the early morning could significantly exceed the production rates of OH radical formed via O3 photolysis (Phillips et al., 2012; Tham et al., 2016), thereby enhancing the atmospheric oxidation capacity (AOC).
Nitryl chloride (ClNO2) is one of the major Cl radical precursors in the tropospheric atmosphere (Thornton et al., 2010; Xue et al., 2015; Liu et al., 2017). It is mainly generated by the heterogeneous uptake of dinitrogen pentoxide (N2O5) on chloride-containing aerosols (Finlayson-Pitts et al., 1989; Thornton et al., 2010), among which N2O5 is produced through the equilibrium reaction with nitrogen dioxide (NO2) and nitrate (NO3) radical. Since Osthoff et al. (2008) first detected over 1 ppb of ClNO2 in the urban outflows of America (Osthoff et al., 2008), significant production of ClNO2 has been widely observed in the polluted coastal and inland areas with abundant anthropogenic emissions and chloride sources, with concentrations ranging from tens of parts per thousand (ppt) to several parts per billion (ppb) (Riedel et al., 2012; Mielke et al., 2013, 2011; Phillips et al., 2012; Bannan et al., 2015; Wang et al., 2016, 2022; Xia et al., 2020, 2021; Yun et al., 2018; Li et al., 2023). For the diurnal profile of ClNO2, its concentrations generally peaked and accumulated at midnight, then rapidly decreased to low levels due to strong photolysis after sunrise (Ma et al., 2023; Mielke et al., 2011; Xia et al., 2020). However, elevated daytime concentrations of ClNO2 have been observed in field studies, mainly attributed to reduced photolysis rates under heavy cloud or fog cover and to contributions from horizontal and vertical transport (Tham et al., 2016; Xia et al., 2021; Jeong et al., 2019; Mielke et al., 2013; Bannan et al., 2015). Notably, the recent laboratory research demonstrated that nitrate () photolysis can generate ClNO2 alongside Cl2 (Dalton et al., 2023), yet this mechanism has not been confirmed under real atmospheric conditions.
At present, the observation studies of ClNO2 focused on investigating its influencing factors, such as the N2O5 uptake coefficient and the production yield of ClNO2 (Thornton et al., 2003; Tham et al., 2018). The field and laboratory studies have indicated that ClNO2 production was mainly affected by ambient temperature (T), relative humidity (RH), and particle components (e g., chloride (Cl−), , and liquid water content) (Bertram and Thornton, 2009; Wang et al., 2023, 2020). In addition to influencing factors, the photochemical effects of ClNO2 photolysis have been extensively evaluated (Xue et al., 2015; Xia et al., 2021; Tham et al., 2016). Cl radical released by ClNO2 photolysis will oxidize VOCs to promote the formation of RO2 radical and O3, greatly compensating for the underestimation of RO2 radical and O3 generation in model simulations (Peng et al., 2021; Ma et al., 2023). The field measurements of ClNO2 have been conducted in different atmospheric environments, while the key drivers of ClNO2 chemistry were still not well recognized. Moreover, it is pertinent to explore whether there are additional and unrecognized sources of ClNO2 beyond its heterogeneous generation from N2O5.
In this study, the comprehensive measurements of ClNO2 and related parameters were conducted in a coastal city of Southeast China during the autumn of 2022. Field observations, combined with a machine learning model, were used to reveal the key driving factors of ClNO2 formation. Furthermore, we further investigated the potential mechanisms driving daytime ClNO2 generation. Additionally, we also assessed the photochemical impacts of ClNO2 based on a box model. Overall, this study underscored the important role of in the ClNO2 chemistry.
2.1 Field measurements
The intensive field measurements of ClNO2, related precursors, and meteorological parameters from 9 October–5 December 2022 were performed at an urban site (Institute of Urban Environment, Chinese Academy of Sciences) in a coastal city (Xiamen) of Southeast China (Fig. S1 in the Supplement). Here, ClNO2, N2O5, gaseous pollutants (volatile organic compounds (VOCs), NOx, SO2, CO, and O3), aerosol mass concentrations, ionic components, size distribution, and meteorological factors were simultaneously detected. Meanwhile, an iodide-adduct time-of-flight chemical ionization mass spectrometer (I−-ToF-CIMS) was used to measure ClNO2 and N2O5. The principles and settings of the I−-ToF-CIMS were similar to previous studies (Ma et al., 2023; Yan et al., 2023). Detailed descriptions of this observation site and these instruments have been provided in previous work (Chen et al., 2024; Hu et al., 2022; see Text S1 and Table S1 in the Supplement). For the calibrations of ClNO2 and N2O5, ClNO2 was produced by passing Cl2 (6 ppm in N2) through a moist mixture of sodium nitrite (NaNO2) and sodium chloride (NaCl) (Thaler et al., 2011; Wang et al., 2022), and N2O5 was synthesized by the reactions of O3 and excessive NO2 (Tham et al., 2016; Wang et al., 2016). The dependences of ClNO2 and N2O5 sensitivities on relative humidity are presented in Fig. S2 in the Supplement. The uncertainties of the ClNO2 and N2O5 measurements were estimated to be ∼15 % and 12 %, respectively. The details of ClNO2 and N2O5 calibrations and uncertainty analysis are displayed in Text S2 in the Supplement.
2.2 Machine learning model
Here, the extreme gradient boosting (XGBoost) model coupling with the Shapely additive explanations (SHAP) model (the XGBoost–SHAP model) was used to identify the key influencing factors of ClNO2 formation. Meanwhile, the XGBoost model was applied to establish the predictive model of ClNO2 based on the observed data of gaseous precursors and meteorological factors; the SHAP model was employed to evaluate the importance of each feature affecting the simulated concentrations of ClNO2. The SHAP model is an interpretability tool designed to analyse the contributions of individual features to model predictions. It employs an additive explanatory framework that regards all features as contributors, drawing inspiration from cooperative game theory. For each predicted instance, SHAP assigns a Shapley value, representing the cumulative contribution of each feature. Positive SHAP values indicate that a feature increases the model's predicted outcome, signifying a positive contribution. Conversely, negative SHAP values suggest that the feature reduces the predicted value, reflecting a negative contribution. The absolute value of the SHAP score reflects the magnitude of the contribution, regardless of direction, offering insight into the overall importance of the feature. The true value, on the other hand, reveals the direction of the contribution (positive or negative), facilitating a clearer understanding of the relationship between the feature and the prediction. Besides, the partial dependence plot (PDP) analysis offers a visual representation of the marginal effect that the factors have on the model's predicted outcome. It is based on the principle of stabilizing the values of non-target features, and it systematically altered the target feature's values according to the model's algorithmic framework to derive the predicted values.
ClNO2 concentrations served as the dependent variable, with trace gases (SO2, CO, NO2, NO, O3, and N2O5), PM2.5 and its inorganic compositions (, , , and Cl−), and meteorological parameters (T, RH, UV, wind speed (WS), wind direction (WD), and boundary layer height (BLH)) acting as independent variables. The simulated ClNO2 concentrations by the XGBoost model were highly similar to the observed values (R2=0.91), indicating the good performance of the XGBoost model (Fig. S3 in the Supplement). Detailed introductions and settings of the XGBoost–SHAP model are provided in Text S3 in the Supplement.
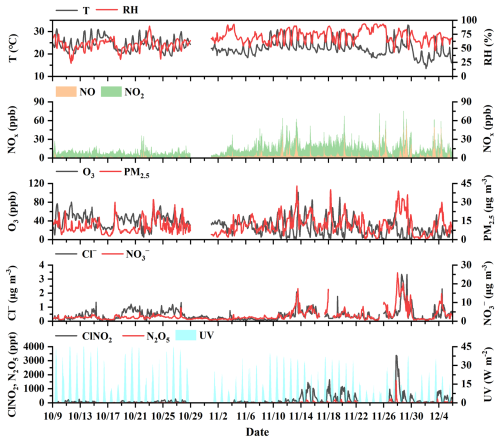
Figure 1The time series of ClNO2, related precursors, and meteorological parameters during the autumn observation period.
2.3 The box model
The observation-based model (OBM) was utilized to assess the impacts of ClNO2 on photochemically atmospheric oxidation. As delineated in earlier studies (Xue et al., 2015; Tham et al., 2016; Xia et al., 2021; Peng et al., 2021, 2022), the Master Chemical Mechanism (MCM; version 3.3.1) was adopted, and established chlorine chemistry mechanisms have been integrated. The Tropospheric Ultraviolet and Visible (TUV) radiation model was used to calculate ClNO2 photolysis rates (JClNO2) under clear-sky conditions. The simulated JClNO2 values were then scaled based on field-measured JNO2 values. A thorough exposition of the box model configuration can be found in our previous publications (Liu et al., 2022b, a) and in Text S4 in the Supplement. Observation data, including ClNO2, VOCs, HCHO, HONO, CO, O3, NO, NO2, and SO2, along with meteorological factors as constraint, were input into the box model at an hourly resolution (Table S2 in the Supplement). Two scenarios were examined: one representing observation-average conditions from 9 October–5 December and the other reflecting a high-ClNO2 case observed on 28 November.
This study focused on elucidating the influence of ClNO2 on the formation of ROx radical and O3. The O3 production rate minus the O3 loss rate was used to calculate the net O3 production rate (Eqs. S1–S3 in the Supplement). The AOC is calculated by the sum of the rates of CH4, CO, and VOCs oxidized by atmospheric oxidants (O3, OH, Cl, and NO3 radical) (Eq. S4 in the Supplement) (Xue et al., 2015; Yi et al., 2023). Both scenarios were evaluated with and without including ClNO2 inputs to assess its impacts on these processes.
3.1 Overview of observations
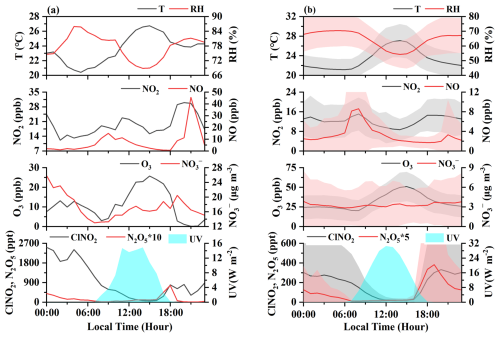
Figure 1 displays the time series of ClNO2, N2O5, and related parameters, including O3, NOx, PM2.5, Cl−, , and meteorological parameters, during the autumn observation period. Our observation shows a decline in T and UV values from October to November, with average RH values increasing from ∼60 % in October to ∼70 % in November (excluding rainy days). During the entire measurement period, ClNO2 concentrations exhibited significant variability, with elevated levels (>500 ppt) frequently observed in late autumn, particularly after 10 November. The elevation of ClNO2 concentrations coincided with increased levels of N2O5 and during late autumn. The concentrations of ClNO2 at our study site reached several parts per billion (ppb), compared with previous field measurements conducted at urban, suburban, rural, background, and mountain sites (Table S3 in the Supplement), indicating its widespread presence in diverse atmospheric environments. The highest concentrations of ClNO2 were detected during the night of 27 November, with a maximum hourly average of 3.4 ppb. Peak concentrations of N2O5 and were also observed on that night (Fig. 1). On the evening of 27 November, N2O5 concentrations rapidly decreased after 07:00 p.m. (local time, LT), while ClNO2 and concentrations significantly increased, reflecting fast N2O5 heterogeneous hydrolysis and effective formation of ClNO2. Notably, on the following day (28 November) (Fig. 2a), ClNO2 concentrations sustained above 100 ppt around noon, partially related to weakened UV values () under heavy fog and cloud cover, with the RH values of ∼70 % at that time. Similar research in California has shown ClNO2 concentrations exceeding 100 ppt 4 h after sunrise due to reduced photolysis (Mielke et al., 2013).
The average diurnal changes in ClNO2 and related parameters during the entire measurement campaign are depicted in Fig. 2b. As expected, ClNO2 exhibited a distinct diurnal variation, peaking and accumulating after sunset and decreasing in the early morning. However, ClNO2 concentrations remained ∼40 ppt around noon, which is different to some other studies, in which ClNO2 concentrations decreased to near the detection limit around midday (Wang et al., 2022; Niu et al., 2022). A similar observation in North China declared ClNO2 concentrations above 60 ppt in the afternoon (Liu et al., 2017). Previous studies have indicated that abundant ClNO2 may be transported from the upper atmosphere or air mass, contributing to the elevated ClNO2 concentrations in the early morning (Tham et al., 2016; Xia et al., 2021; Jeong et al., 2019). However, the explanations for the concentrations of ClNO2 around noon remained elusive.
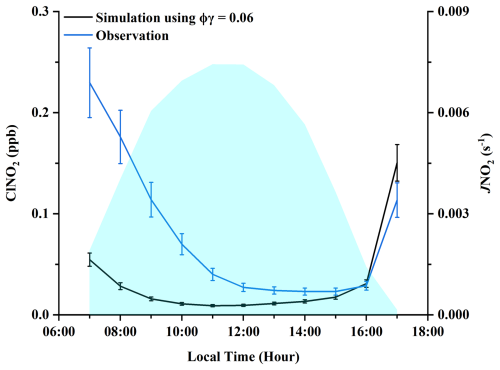
To evaluate the contribution of the heterogeneous N2O5 uptake to daytime ClNO2 levels, we calculated ClNO2 production using a box model, considering (1) the contribution of heterogeneous N2O5 uptake to ClNO2 production and (2) ClNO2 loss via photolysis, aerosol uptake, and reaction with OH (Text S5 and S6 in the Supplement). We used a γ(N2O5) value of 0.06, a ϕ(ClNO2) value of 1.0, and a γ(ClNO2) value of 0.006 in our calculations, which represent upper-end estimates based on previous field studies (Mcduffie et al., 2018a, b; Tham et al., 2016). However, as shown in Fig. 3, the simulated daytime ClNO2 concentrations were lower than the observed values. Therefore, we believe that the observed daytime ClNO2 levels, particularly around noon, cannot be adequately explained by heterogeneous N2O5 uptake alone, suggesting the presence of additional sources contributing to the formation of daytime ClNO2.
3.2 Key drivers of ClNO2 formation
The XGBoost–SHAP model was employed to investigate the major drivers of ClNO2 production during the whole observation period. The average absolute SHAP value of each feature was ranked to determine the key drivers of ClNO2 formation, with larger SHAP values suggesting greater contributions (Fig. 4a). Additionally, features with positive SHAP values (depicted as red points) indicate that higher values of those features positively affect ClNO2 concentrations and vice versa (Fig. 4b). Overall, N2O5, , T, RH, and UV were the most important features affecting ClNO2 concentrations. Notably, these factors exhibited varied behaviours between daytime and nighttime periods.
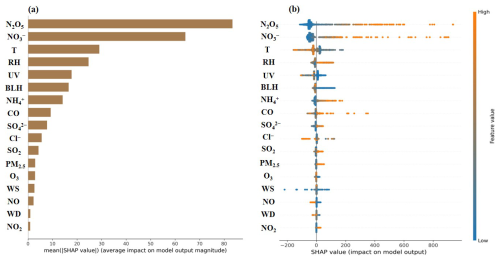
Figure 4Relative importance of each feature to ClNO2 using XGBoost–SHAP during the autumn observation period. The mean absolute SHAP value (a) and a summary plot of SHAP values of each feature (b).
In our study, N2O5 was identified as the most important influencing factor, consistent with its role in ClNO2 formation through heterogeneous uptake processes (Thornton et al., 2010; Finlayson-Pitts et al., 1989). After sunset, ClNO2 concentrations markedly increased due to active nighttime N2O5 chemistry, while this heterogeneous uptake process was hindered after sunrise as N2O5 concentrations decreased significantly (Fig. 1) (Niu et al., 2022; Wang et al., 2020; Tan et al., 2022). Indeed, the concentrations of ClNO2 were evidently increased when N2O5 concentrations exceeded ∼13 ppt, predominantly during the nighttime (Fig. 5a). Conversely, in northern Europe, the ClNO2 concentrations were mainly controlled by O3 and NO2, rather than by the heterogeneous uptake of N2O5 (Sommariva et al., 2018). In Heshan of South China, chloride and PM2.5 were the major factors affecting ClNO2 formation (Wang et al., 2022). Differently, the relative importance of derived from the XGBoost–SHAP result indicated that elevated ClNO2 concentrations were associated with high concentrations of besides N2O5. According to Fig. 5b, high concentrations () are accompanied by the elevation of ClNO2, especially its concentrations reaching 6.2 µg m−3. Previous studies suggested that increased concentrations of decreased γ(N2O5), which would limit the production of ClNO2 (Wahner et al., 1998; Mentel et al., 1999; Bertram and Thornton, 2009). As depicted in Fig. S4 in the Supplement, the dependence of γ(N2O5) on concentrations follows the nitrate suppression effect. Therefore, the importance of nighttime for ClNO2 levels is that they are co-products from the processes of N2O5 heterogeneous uptake. As shown in Fig. 1, compared to low- conditions, ClNO2 production was enhanced in high- conditions. Especially in late autumn, increased aerosol abundances and N2O5 levels enhanced N2O5 uptake, further promoting ClNO2 and production. Considering the limited contribution of N2O5 hydrolysis to daytime levels (Yan et al., 2023; Zang et al., 2022; Chen et al., 2020), the impact of high concentrations on daytime ClNO2 concentrations warrants further analysis.
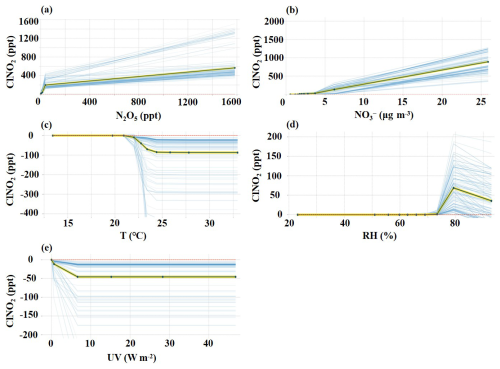
Figure 5Isolation plots of PDP for N2O5 (a), (b), T (c), RH (d), and UV (e). The average variations in simulated ClNO2 with changes in factors are indicated by the splines with yellow and black curves, and blue curves present all situations during the whole observation period.
The simulated concentrations of ClNO2, based on the XGBoost–SHAP model, were significantly elevated when concentrations were higher than 3.7 µg m−3 (Fig. 5b). Consequently, the average daily concentrations of were classified as high () and low () to further elucidate the impacts of on the formation of ClNO2. Figure 6 presents the diurnal variations in the relative importance of factors based on the SHAP values under high and low concentrations. Unexpectedly, daytime was the dominant influencing factor for daytime ClNO2 (Fig. 6a). High concentrations of daytime positively affected the daytime concentrations of ClNO2, independent of N2O5 uptake processes. As depicted in Fig. 6a, daytime N2O5 did not promote the elevation of daytime ClNO2. Negative SHAP values for N2O5 during the daytime indicate that the contribution of N2O5 chemistry to daytime ClNO2 levels was limited. Therefore, it is very likely that high concentrations of daytime participated in daytime ClNO2 production. A recent study suggested that nitrate photolysis produced ClNO2 in addition to Cl2 (Dalton et al., 2023), but this has not been verified by field observations. Figure 7 shows that daytime ClNO2 concentrations correlated well (R=0.62) with the product of a proxy of photolysis ) on aerosol surfaces, implying that the photolysis of likely resulted in the daytime formation of ClNO2 at our study site. Furthermore, high concentrations of and Cl−, along with large values of Sa (Figs. 7a–c and S5 in the Supplement) in the daytime accelerated photolysis, promoting the formation of ClNO2, while ClNO2 concentrations exhibited a weak correlation with JNO2. It should be emphasized that the weak correlation between JNO2 and ClNO2 concentrations does not deny the potential contribution of nitrate photolysis, which could be explained by the fact that ClNO2 concentrations are affected by both its production and loss processes. Specifically, photolysis rates exert dual effects on daytime ClNO2 concentrations: positive effects through photochemical production pathways and negative effects through direct ClNO2 photolytic loss. Given the short daytime lifetime of ClNO2, we calculated the missing ClNO2 production rate (production rate minus loss rate) to assess the contributions from unknown sources (Text S6). The production rates of unknown sources showed a good correlation with JNO2 (R=0.41) (Fig. S6 in the Supplement), indicating that photochemical processes may enhance ClNO2 production. Notably, the strong correlation between the observed concentrations of ClNO2 and the photolysis proxy () has revealed the possibility of the contribution of photolysis to the unknown daytime ClNO2 source.
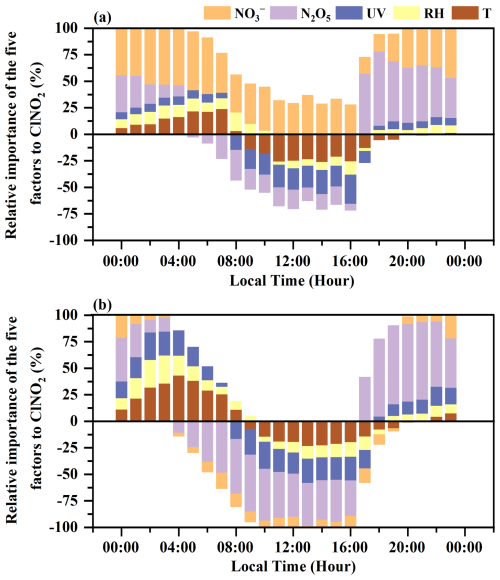
Figure 6The diurnal variations in the relative importance of factors to ClNO2 based on the SHAP values under the high () (a) and low () (b) ClNO2 concentrations.
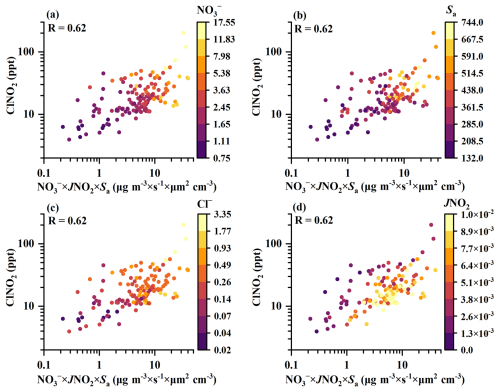
Figure 7The relationship of daytime ClNO2 concentrations (12:00–15:00 local time) and a proxy of nitrate () photolysis (). The colours of the dots represent (a), Sa (b), Cl− (c), and JNO2 (d) amounts.
In terms of meteorological factors, UV, T, and RH were the major influencing factors. The photolysis was the most important sink of ClNO2 in the daytime, leading to a rapid reduction in ClNO2 concentrations, particularly in the early morning (Fig. 5e and Fig. 6). However, it is crucial to understand the dual role of photolysis intensity in determining daytime ClNO2 levels. As mentioned before, photolysis can contribute to the generation of ClNO2 by promoting photolysis, while also causing the rapid decomposition of ClNO2. As reported in California (Mielke et al., 2013), reduced photolysis rates even increased daytime ClNO2 levels by decreasing ClNO2 loss through photolysis. The impact of ambient temperature on ClNO2 was probably reflected in its thermal equilibrium with N2O5. Elevated daytime ambient temperature suppressed the formation of N2O5, resulting in low N2O5 concentrations, which further limited the contribution of heterogeneous N2O5 uptake to daytime ClNO2 generation (Figs. 5c and 6). During the whole observation period from October to November, the drop in ambient temperature facilitated ClNO2 production by decreasing the thermal decomposition process. Increased RH values provided favourable conditions for the nighttime N2O5 hydrolysis reactions, thereby affecting ClNO2 production (Figs. 5d and 6), while high RH (>80 %) also weakened the generation of ClNO2. Notably, Cl− was not the most important factor of ClNO2 formation at our study site (Fig. 4), likely attributed to the abundant chlorine source in coastal regions (Peng et al., 2022).
3.3 Impact of ClNO2 photolysis on ROx budget
The photochemical effects of ClNO2 were evaluated under the observation-average conditions and the high-ClNO2 case based on the box model. The largest Cl production rates (P(Cl)) contributed by ClNO2 photolysis were 0.05 ppb h−1 for the observation-average conditions, which were lower than 0.19 ppb h−1 for the high-ClNO2 case. The difference led to variable levels of atmospheric oxidation capacity induced by Cl radical. Cl radical released via the photolysis of ClNO2 initiated the oxidation of VOCs. Among VOC groups (including alkanes, alkenes, alkynes, aromatics, and oxygenated volatile organic compounds (OVOCs)), Cl radical primarily oxidized alkanes (∼65.0 %), followed by OVOCs (∼12.7 %) for both the observation-average conditions and the high-ClNO2 case (Fig. 8a and b). The contributions of Cl radical and other atmospheric oxidants (including OH radical and O3) to daytime VOC oxidation were also compared (Fig. 8c, d and Table 1). In our study, the oxidation of alkanes by Cl radical for the observation-average conditions was about 11.7 %, which increased by 44.8 % for the high-ClNO2 case and was higher than that in London (Bannan et al., 2015), Weybourne (Bannan et al., 2017), Boston (Rutherford et al., 1995), and LA (Fraser et al., 1997) and lower than that in Hong Kong (Xue et al., 2015). It should be noted that the rates of Cl radical reacting with alkanes even exceeded those of OH radical in the early morning for the high-ClNO2 case. The largest rates of alkanes oxidized by Cl radical were approximately twice as high as those of OH radical at 10:00 a.m. (LT) (Fig. 8e and f), highlighting that the photochemical effects of Cl radical released via ClNO2 photolysis were particularly important for VOC oxidation during the morning hours at our study site.
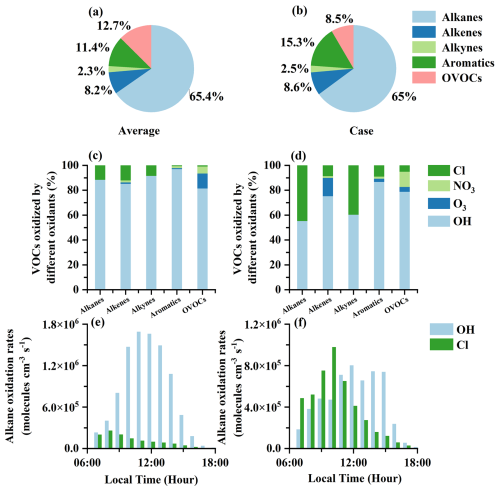
Figure 8The impacts of Cl radials released by ClNO2 photolysis and other atmospheric oxidants (including OH, NO3, and O3) on VOC oxidation under the observation-average conditions and high-ClNO2 case, respectively. The contributions of different VOC groups oxidized by Cl radical during the observation average (a). The contributions of different VOC groups oxidized by Cl radical during the case (b). The contributions of different atmospheric oxidants (including OH, Cl, NO3, and O3) to VOC groups during the observation average (c). The contributions of different atmospheric oxidants (including OH, Cl, NO3, and O3) to VOC groups during the case (d). Comparisons of alkane oxidation rates () by OH and Cl radical during the observation average (e). Comparisons of alkane oxidation rates by OH and Cl radical () during the case (f).
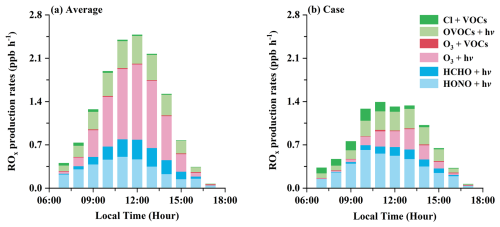
Figure 9The contributions of different production pathways to ROx production rates under the observation-average conditions (a) and high-ClNO2 case (b).
Table 1Relative importance of Cl, OH, and O3 to the daytime oxidation of VOC groups (including alkanes, alkenes, alkynes, aromatics, and OVOCs) around the world (Xue et al., 2015; Bannan et al., 2015, 2017; Rutherford et al., 1995; Fraser et al., 1997).
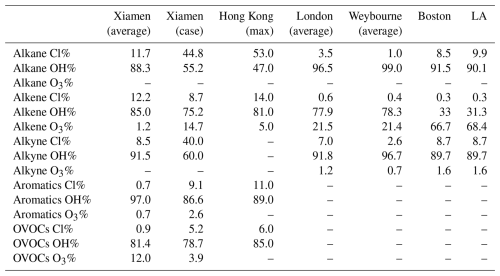
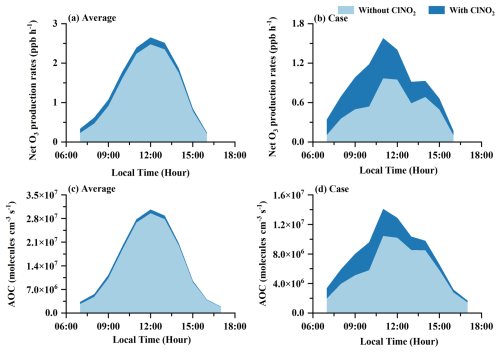
Figure 10The impacts of Cl radials released by ClNO2 photolysis on net O3 production rates and the AOC levels under the observation-average conditions (a, c) and high-ClNO2 case (b, d).
Table 2The impacts of ClNO2 photolysis on ROx (OH, HO2, and RO2) levels, P(ROx), and P(O3) around the world (Xia et al., 2021; Wang et al., 2022, 2016; Tham et al., 2016; Xue et al., 2015; Bannan et al., 2017; Jeong et al., 2019).
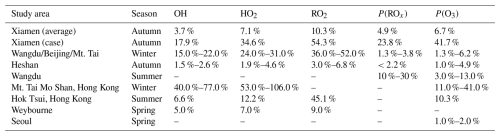
The oxidation of VOCs by Cl radical further affects the generation of ROx () radicals. The ROx radical production rates for the high-ClNO2 case were evidently lower than those under the observation-average conditions, primarily due to reduced photolysis rates on that day. However, the total ROx radical production rates averagely increased by 23.8 % with ClNO2 photolysis for the high-ClNO2 case, higher than a 4.9 % increase for the observation-average conditions (Fig. S7 in the Supplement). For the observation-average conditions, O3 (32.7 %), HONO (31.7 %), and OVOC (21.6 %) photolysis were the most significant contributors to ROx radical production in the early morning (07:00–10:00 a.m. (LT)), with VOC oxidation by Cl radical contributing only 3.7 % (Fig. 9a). However, for the high-ClNO2 case, VOC oxidation induced by Cl radical in the early morning accounted for 19.1 % of ROx radical production, which was higher than O3 (7.4 %) and HCHO (4.1 %) photolysis, close to OVOC (19.0 %) photolysis (Fig. 9b). The contributions of ClNO2 photolysis to the ROx radical production rates in our study were larger than previous results observed in autumn in Heshan (Wang et al., 2022) and North China (Xia et al., 2021), similar to those in summer in Wangdu (Tham et al., 2016). Thus, the concentrations of OH, HO2, and RO2 radicals in the box model with ClNO2 inputs averagely increased by 17.9 %, 34.6 %, and 54.3 % for the high-ClNO2 case, higher than the increases of 3.7 %, 7.1 %, and 10.3 % contributed by the observation-average conditions, respectively (Fig. S8 in the Supplement). The uplift in the concentrations of ROx radicals also accelerated the generation of O3. The increase in the net O3 production rates (P(O3)) for the observation-average conditions reached 0.13 ppb h−1 (15.8 %) in the daytime on average (Fig. 10a), while larger elevations in the net P(O3) were observed for the high-ClNO2 case (Fig. 10b), with a maximum of 0.64 ppb h−1 (120 %) at 10:00 a.m. (LT). As a result, increased ROx radical and O3 greatly enhanced the atmospheric oxidation capacity (Fig. 10c and d), especially for the high-ClNO2 case (up to 65 %).
Table 2 summarizes the impacts of ClNO2 photolysis on ROx radical and O3 production in our study and previous observations around the world (Xia et al., 2021; Wang et al., 2022, 2016; Tham et al., 2016; Xue et al., 2015; Bannan et al., 2017; Jeong et al., 2019), indicating that the photochemical impacts of ClNO2 were variable in different atmospheric environments. At our study site, the effects of ClNO2 photolysis on ROx radical production were important, especially in the early morning. The enhanced ROx radical production induced by ClNO2 photolysis accelerated the chemical generation of O3. Primary ROx radical production rates (including O3, HONO, HCHO, OVOCs, and ClNO2) were regarded as one of the most important parameters to O3 formation (Lu et al., 2023). Therefore, the considerable contribution of ClNO2 photolysis to primary ROx radical production in the early morning may bring new challenges for O3 alleviation.
In conclusion, we present 2 months of field measurements in the coastal area of Southeast China during the autumn, coupled with machine learning and model simulations, providing new insights into ClNO2 chemistry. Our observation shows that the increase in the concentrations of ClNO2 was accompanied by elevated concentrations of N2O5 and , low values of T and UV, and high values of RH. The nighttime heterogeneous uptake of N2O5 was identified as the major source of ClNO2, while photolysis served as a potential daytime ClNO2 source. Cl radical released by ClNO2 photolysis after sunrise had important photochemical effects in the early morning. The photolysis of high ClNO2 concentrations resulted in net O3 production rates and atmospheric oxidation capacity levels increasing by 120 % and 65 %, respectively. Our results enhanced the understanding of ClNO2 chemistry in coastal regions, calling for more observations and laboratory research to fully reveal its exact role in different atmospheric environments.
Data are available upon request to Jinsheng Chen (jschen@iue.ac.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-7815-2025-supplement.
JC provided funding support for field measurements, designed the study, and revised the article. GC designed the study, analysed the data, and wrote the article. HW helped perform the calibrations and revised the article. XF revised the article. XF, HW, YJT, ZL, XJ, LX, and BH contributed to discussions about the article.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors acknowledge the National Natural Science Foundation of China, the Science and Technology Department of Fujian Province, the Center for Excellence in Regional Atmospheric Environment Project, the Xiamen Atmospheric Environment Observation and Research Station of Fujian Province, and the Fujian Key Laboratory of Atmospheric Ozone Pollution Prevention (Institute of Urban Environment, Chinese Academy of Sciences).
This work was funded by the National Natural Science Foundation of China (grant no. U22A20578, 42305102, and 42277091), the Science and Technology Department of Fujian Province (grant no. 2022L3025), the National Key Research and Development Program (grant no. 2022YFC3700304), the STS Plan Supporting Project of the Chinese Academy of Sciences in Fujian Province (grant no. 2023T3013), the Fujian Provincial Environmental Protection Science and Technology Plan Projects (grant no. 2023R004), and the Xiamen Atmospheric Environment Observation and Research Station of Fujian Province. Yee Jun Tham acknowledges the funding support from the Guangdong Basic and Applied Basic Research Foundation (grant no. 2022A1515010852) and the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (23hytd002).
This paper was edited by Lea Hildebrandt Ruiz and reviewed by three anonymous referees.
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., Troe, J., and IUPAC Subcommittee: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II – gas phase reactions of organic species, Atmos. Chem. Phys., 6, 3625–4055, https://doi.org/10.5194/acp-6-3625-2006, 2006.
Bannan, T. J., Booth, A. M., Bacak, A., Muller, J. B. A., Leather, K. E., Le Breton, M., Jones, B., Young, D., Coe, H., Allan, J., Visser, S., Slowik, J. G., Furger, M., Prévôt, A. S. H., Lee, J., Dunmore, R. E., Hopkins, J. R., Hamilton, J. F., Lewis, A. C., Whalley, L. K., Sharp, T., Stone, D., Heard, D. E., Fleming, Z. L., Leigh, R., Shallcross, D. E., and Percival, C. J.: The first UK measurements of nitryl chloride using a chemical ionization mass spectrometer in central London in the summer of 2012, and an investigation of the role of Cl atom oxidation, J. Geophys. Res.-Atmos., 120, 5638–5657, https://doi.org/10.1002/2014jd022629, 2015.
Bannan, T. J., Bacak, A., Le Breton, M., Flynn, M., Ouyang, B., McLeod, M., Jones, R., Malkin, T. L., Whalley, L. K., Heard, D. E., Bandy, B., Khan, M. A. H., Shallcross, D. E., and Percival, C. J.: Ground and Airborne U. K. Measurements of Nitryl Chloride: An Investigation of the Role of Cl Atom Oxidation at Weybourne Atmospheric Observatory, J. Geophys. Res.-Atmos., 122, 11154–111165, https://doi.org/10.1002/2017jd026624, 2017.
Bertram, T. H. and Thornton, J. A.: Toward a general parameterization of N2O5 reactivity on aqueous particles: the competing effects of particle liquid water, nitrate and chloride, Atmos. Chem. Phys., 9, 8351–8363, https://doi.org/10.5194/acp-9-8351-2009, 2009.
Chen, G., Ji, X., Chen, J., Xu, L., Hu, B., Lin, Z., Fan, X., Li, M., Hong, Y., and Chen, J.: Photochemical pollution during summertime in a coastal city of Southeast China: Ozone formation and influencing factors, Atmos. Res., 301, 107270, https://doi.org/10.1016/j.atmosres.2024.107270, 2024.
Chen, X., Wang, H., Lu, K., Li, C., Zhai, T., Tan, Z., Ma, X., Yang, X., Liu, Y., Chen, S., Dong, H., Li, X., Wu, Z., Hu, M., Zeng, L., and Zhang, Y.: Field Determination of Nitrate Formation Pathway in Winter Beijing, Environ. Sci. Technol., 54, 9243–9253, https://doi.org/10.1021/acs.est.0c00972, 2020.
Dalton, E. Z., Hoffmann, E. H., Schaefer, T., Tilgner, A., Herrmann, H., and Raff, J. D.: Daytime Atmospheric Halogen Cycling through Aqueous-Phase Oxygen Atom Chemistry, J. Am. Chem. Soc., 145, 15652–15657, https://doi.org/10.1021/jacs.3c03112, 2023.
Finlayson-Pitts, B. J., Ezell, M. J., and Pitts, J. N.: Formation of chemically active chlorine compounds by reactions of atmospheric NaCl particles with gaseous N2O5 and ClONO2, Nature, 337, 241–244, https://doi.org/10.1038/337241a0, 1989.
Fraser, M. P., Cass, G. R., Simoneit, B. R., and Rasmussen, R.: Air quality model evaluation data for organics. 4. C2–C36 non-aromatic hydrocarbons, Environ. Sci. Technol., 31, 2356–2367, https://doi.org/10.1021/es960980g, 1997.
Hu, B., Duan, J., Hong, Y., Xu, L., Li, M., Bian, Y., Qin, M., Fang, W., Xie, P., and Chen, J.: Exploration of the atmospheric chemistry of nitrous acid in a coastal city of southeastern China: results from measurements across four seasons, Atmos. Chem. Phys., 22, 371–393, https://doi.org/10.5194/acp-22-371-2022, 2022.
Jeong, D., Seco, R., Gu, D., Lee, Y., Nault, B. A., Knote, C. J., Mcgee, T., Sullivan, J. T., Jimenez, J. L., Campuzano-Jost, P., Blake, D. R., Sanchez, D., Guenther, A. B., Tanner, D., Huey, L. G., Long, R., Anderson, B. E., Hall, S. R., Ullmann, K., Shin, H., Herndon, S. C., Lee, Y., Kim, D., Ahn, J., and Kim, S.: Integration of airborne and ground observations of nitryl chloride in the Seoul metropolitan area and the implications on regional oxidation capacity during KORUS-AQ 2016, Atmos. Chem. Phys., 19, 12779–12795, https://doi.org/10.5194/acp-19-12779-2019, 2019.
Li, F., Huang, D. D., Nie, W., Tham, Y. J., Lou, S., Li, Y., Tian, L., Liu, Y., Zhou, M., and Wang, H.: Observation of nitrogen oxide-influenced chlorine chemistry and source analysis of Cl2 in the Yangtze River Delta, China, Atmos. Environ., 306, 119829, https://doi.org/10.1016/j.atmosenv.2023.119829, 2023.
Li, Q., Badia, A., Wang, T., Sarwar, G., Fu, X., Zhang, L., Zhang, Q., Fung, J., Cuevas, C. A., Wang, S., Zhou, B., and Saiz-Lopez, A.: Potential Effect of Halogens on Atmospheric Oxidation and Air Quality in China, J. Geophys. Res.-Atmos., 125, e2019JD032058, https://doi.org/10.1029/2019JD032058, 2020.
Liu, T., Chen, G., Chen, J., Xu, L., Li, M., Hong, Y., Chen, Y., Ji, X., Yang, C., Chen, Y., Huang, W., Huang, Q., and Wang, H.: Seasonal characteristics of atmospheric peroxyacetyl nitrate (PAN) in a coastal city of Southeast China: Explanatory factors and photochemical effects, Atmos. Chem. Phys., 22, 4339–4353, https://doi.org/10.5194/acp-22-4339-2022, 2022a.
Liu, T., Hong, Y., Li, M., Xu, L., Chen, J., Bian, Y., Yang, C., Dan, Y., Zhang, Y., Xue, L., Zhao, M., Huang, Z., and Wang, H.: Atmospheric oxidation capacity and ozone pollution mechanism in a coastal city of southeastern China: analysis of a typical photochemical episode by an observation-based model, Atmos. Chem. Phys., 22, 2173–2190, https://doi.org/10.5194/acp-22-2173-2022, 2022b.
Liu, X., Qu, H., Huey, L. G., Wang, Y., Sjostedt, S., Zeng, L., Lu, K., Wu, Y., Hu, M., Shao, M., Zhu, T., and Zhang, Y.: High Levels of Daytime Molecular Chlorine and Nitryl Chloride at a Rural Site on the North China Plain, Environ. Sci. Technol., 51, 9588–9595, https://doi.org/10.1021/acs.est.7b03039, 2017.
Lu, K., Zhou, H., Lee, J., Nelson, B., and Zhang, Y.: Ozone mitigations beyond the control of nitrogen oxides and volatile organic compounds, Sci. Bull., 68, 1989–1992, https://doi.org/10.1016/j.scib.2023.07.051, 2023.
Ma, W., Chen, X., Xia, M., Liu, Y., Wang, Y., Zhang, Y., Zheng, F., Zhan, J., Hua, C., and Wang, Z.: Reactive Chlorine Species Advancing the Atmospheric Oxidation Capacities of Inland Urban Environments, Environ. Sci. Technol., 57, 14638–14647, https://doi.org/10.1021/acs.est.3c05169, 2023.
McDuffie, E. E., Fibiger, D. L., Dubé, W. P., Lopez Hilfiker, F., Lee, B. H., Jaeglé, L., Guo, H., Weber, R. J., Reeves, J. M., Weinheimer, A. J., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Dibb, J. E., Veres, P., Ebben, C., Sparks, T. L., Wooldridge, P. J., Cohen, R. C., Campos, T., Hall, S. R., Ullmann, K., Roberts, J. M., Thornton, J. A., and Brown, S. S.: ClNO2 Yields From Aircraft Measurements During the 2015 WINTER Campaign and Critical Evaluation of the Current Parameterization, J. Geophys. Res.-Atmos., 123, 12994–13015, https://doi.org/10.1029/2018JD029358, 2018a.
McDuffie, E. E., Fibiger, D. L., Dubé, W. P., Lopez-Hilfiker, F., Lee, B. H., Thornton, J. A., Shah, V., Jaeglé, L., Guo, H., Weber, R. J., Michael Reeves, J., Weinheimer, A. J., Schroder, J. C., Campuzano-Jost, P., Jimenez, J. L., Dibb, J. E., Veres, P., Ebben, C., Sparks, T. L., Wooldridge, P. J., Cohen, R. C., Hornbrook, R. S., Apel, E. C., Campos, T., Hall, S. R., Ullmann, K., and Brown, S. S.: Heterogeneous N2O5 Uptake During Winter: Aircraft Measurements During the 2015 WINTER Campaign and Critical Evaluation of Current Parameterizations, J. Geophys. Res.-Atmos., 123, 4345–4372, https://doi.org/10.1002/2018JD028336, 2018b.
Mentel, T. F., Sohn, M., and Wahner, A. J. P. C. C. P.: Nitrate effect in the heterogeneous hydrolysis of dinitrogen pentoxide on aqueous aerosols, Phys. Chem. Chem. Phys., 1, 5451–5457, https://doi.org/10.1039/A905338g, 1999.
Mielke, L. H., Furgeson, A., and Osthoff, H. D.: Observation of ClNO2 in a Mid-Continental Urban Environment, Environ. Sci. Technol., 45, 8889–8896, https://doi.org/10.1021/es201955u, 2011.
Mielke, L. H., Stutz, J., Tsai, C., Hurlock, S. C., Roberts, J. M., Veres, P. R., Froyd, K. D., Hayes, P. L., Cubison, M. J., Jimenez, J. L., Washenfelder, R. A., Young, C. J., Gilman, J. B., Gouw, J. A., Flynn, J. H., Grossberg, N., Lefer, B. L., Liu, J., Weber, R. J., and Osthoff, H. D.: Heterogeneous formation of nitryl chloride and its role as a nocturnal NOx reservoir species during CalNex-LA 2010, J. Geophys. Res.-Atmos., 118, 10638–10652, https://doi.org/10.1002/jgrd.50783, 2013.
Niu, Y.-B., Zhu, B., He, L.-Y., Wang, Z., Lin, X.-Y., Tang, M.-X., and Huang, X.-F.: Fast Nocturnal Heterogeneous Chemistry in a Coastal Background Atmosphere and Its Implications for Daytime Photochemistry, J. Geophys. Res.-Atmos., 127, e2022JD036716, https://doi.org/10.1029/2022JD036716, 2022.
Osthoff, H. D., Roberts, J. M., Ravishankara, A. R., Williams, E. J., Lerner, B. M., Sommariva, R., Bates, T. S., Coffman, D., Quinn, P. K., Dibb, J. E., Stark, H., Burkholder, J. B., Talukdar, R. K., Meagher, J., Fehsenfeld, F. C., and Brown, S. S.: High levels of nitryl chloride in the polluted subtropical marine boundary layer, Nat. Geosci., 1, 324–328, https://doi.org/10.1038/ngeo177, 2008.
Peng, X., Wang, W., Xia, M., Chen, H., Ravishankara, A. R., Li, Q., Saiz-Lopez, A., Liu, P., Zhang, F., Zhang, C., Xue, L., Wang, X., George, C., Wang, J., Mu, Y., Chen, J., and Wang, T.: An unexpected large continental source of reactive bromine and chlorine with significant impact on wintertime air quality, Natl. Sci. Rev., 8, nwaa304, https://doi.org/10.1093/nsr/nwaa304, 2021.
Peng, X., Wang, T., Wang, W., Ravishankara, A., George, C., Xia, M., Cai, M., Li, Q., Salvador, C. M., and Lau, C.: Photodissociation of particulate nitrate as a source of daytime tropospheric Cl2, Nat. Commun., 13, 1–10, https://doi.org/10.1038/s41467-022-28383-9, 2022.
Phillips, G. J., Tang, M. J., Thieser, J., Brickwedde, B., Schuster, G., Bohn, B., Lelieveld, J., and Crowley, J. N.: Significant concentrations of nitryl chloride observed in rural continental Europe associated with the influence of sea salt chloride and anthropogenic emissions, Geophys. Res. Lett., 39, L10811, https://doi.org/10.1029/2012gl051912, 2012.
Riedel, T. P., Bertram, T. H., Crisp, T. A., Williams, E. J., Lerner, B. M., Vlasenko, A., Li, S. M., Gilman, J., de Gouw, J., Bon, D. M., Wagner, N. L., Brown, S. S., and Thornton, J. A.: Nitryl chloride and molecular chlorine in the coastal marine boundary layer, Environ. Sci. Technol., 46, 10463–10470, https://doi.org/10.1021/es204632r, 2012.
Rutherford, J. A., Koehl, W. J., Benson, J. D., Burns, V. R., Hochhauser, A. M., Knepper, J. C., Leppard, W. R., Painter, L. J., Rapp, L. A., and Rippon, B.: Effects of Gasoline Properties on Emissions of Current and Future Vehicles-T50, T90, and Sulfur Effects-Auto/Oil Air Quality Improvement Research Program, SAE Technical Paper, 0148-7191, https://doi.org/10.4271/952510, 1995.
Sommariva, R., Hollis, L. D. J., Sherwen, T., Baker, A. R., Ball, S. M., Bandy, B. J., Bell, T. G., Chowdhury, M. N., Cordell, R. L., Evans, M. J., Lee, J. D., Reed, C., Reeves, C. E., Roberts, J. M., Yang, M., and Monks, P. S.: Seasonal and geographical variability of nitryl chloride and its precursors in Northern Europe, Atmos. Sci. Lett., 19, e844, https://doi.org/10.1002/asl.844, 2018.
Tan, Z., Fuchs, H., Hofzumahaus, A., Bloss, W. J., Bohn, B., Cho, C., Hohaus, T., Holland, F., Lakshmisha, C., Liu, L., Monks, P. S., Novelli, A., Niether, D., Rohrer, F., Tillmann, R., Valkenburg, T. S. E., Vardhan, V., Kiendler-Scharr, A., Wahner, A., and Sommariva, R.: Seasonal variation in nitryl chloride and its relation to gas-phase precursors during the JULIAC campaign in Germany, Atmos. Chem. Phys., 22, 13137–13152, https://doi.org/10.5194/acp-22-13137-2022, 2022.
Thaler, R. D., Mielke, L. H., and Osthoff, H. D.: Quantification of nitryl chloride at part per trillion mixing ratios by thermal dissociation cavity ring-down spectroscopy, Anal. Chem., 83, 2761–2766, https://doi.org/10.1021/ac200055z, 2011.
Tham, Y. J., Wang, Z., Li, Q., Wang, W., Wang, X., Lu, K., Ma, N., Yan, C., Kecorius, S., Wiedensohler, A., Zhang, Y., and Wang, T.: Heterogeneous N2O5 uptake coefficient and production yield of ClNO2 in polluted northern China: roles of aerosol water content and chemical composition, Atmos. Chem. Phys., 18, 13155–13171, https://doi.org/10.5194/acp-18-13155-2018, 2018.
Tham, Y. J., Wang, Z., Li, Q., Yun, H., Wang, W., Wang, X., Xue, L., Lu, K., Ma, N., Bohn, B., Li, X., Kecorius, S., Größ, J., Shao, M., Wiedensohler, A., Zhang, Y., and Wang, T.: Significant concentrations of nitryl chloride sustained in the morning: investigations of the causes and impacts on ozone production in a polluted region of northern China, Atmos. Chem. Phys., 16, 14959–14977, https://doi.org/10.5194/acp-16-14959-2016, 2016.
Thornton, J. A., Braban, C. F., and Abbatt, J. P.: N2O5 hydrolysis on sub-micron organic aerosols: the effect of relative humidity, particle phase, and particle size, Phys. Chem. Chem. Phys., 5, 4593–4603, https://doi.org/10.1039/B307498F, 2003.
Thornton, J. A., Kercher, J. P., Riedel, T. P., Wagner, N. L., Cozic, J., Holloway, J. S., Dubé, W. P., Wolfe, G. M., Quinn, P. K., Middlebrook, A. M., Alexander, B., and Brown, S. S.: A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry, Nature, 464, 271–274, https://doi.org/10.1038/nature08905, 2010.
Wahner, A., Mentel, T. F., Sohn, M., and Stier, J.: Heterogeneous reaction of N2O5 on sodium nitrate aerosol, J. Geophys. Res.-Atmos., 103, 31103–31112, https://doi.org/10.1029/1998JD100022, 1998.
Wang, H., Chen, X., Lu, K., Tan, Z., Ma, X., Wu, Z., Li, X., Liu, Y., Shang, D., Wu, Y., Zeng, L., Hu, M., Schmitt, S., Kiendler-Scharr, A., Wahner, A., and Zhang, Y.: Wintertime N2O5 uptake coefficients over the North China Plain, Sci. Bull., 65, 765–774, https://doi.org/10.1016/j.scib.2020.02.006, 2020.
Wang, H., Yuan, B., Zheng, E., Zhang, X., Wang, J., Lu, K., Ye, C., Yang, L., Huang, S., Hu, W., Yang, S., Peng, Y., Qi, J., Wang, S., He, X., Chen, Y., Li, T., Wang, W., Huangfu, Y., Li, X., Cai, M., Wang, X., and Shao, M.: Formation and impacts of nitryl chloride in Pearl River Delta, Atmos. Chem. Phys., 22, 14837–14858, https://doi.org/10.5194/acp-22-14837-2022, 2022.
Wang, H., Wang, H., Lu, X., Lu, K., Zhang, L., Tham, Y. J., Shi, Z., Aikin, K., Fan, S., Brown, S. S., and Zhang, Y.: Increased night-time oxidation over China despite widespread decrease across the globe, Nat. Geosci., 16, 217–223, https://doi.org/10.1038/s41561-022-01122-x, 2023.
Wang, T., Tham, Y. J., Xue, L., Li, Q., Zha, Q., Wang, Z., Poon, S. C. N., Dubé, W. P., Blake, D. R., Louie, P. K. K., Luk, C. W. Y., Tsui, W., and Brown, S. S.: Observations of nitryl chloride and modeling its source and effect on ozone in the planetary boundary layer of southern China, J. Geophys. Res.-Atmos., 121, 2476–2489, https://doi.org/10.1002/2015JD024556, 2016.
Xia, M., Peng, X., Wang, W., Yu, C., Sun, P., Li, Y., Liu, Y., Xu, Z., Wang, Z., Xu, Z., Nie, W., Ding, A., and Wang, T.: Significant production of ClNO2 and possible source of Cl2 from N2O5 uptake at a suburban site in eastern China, Atmos. Chem. Phys., 20, 6147–6158, https://doi.org/10.5194/acp-20-6147-2020, 2020.
Xia, M., Peng, X., Wang, W., Yu, C., Wang, Z., Tham, Y. J., Chen, J., Chen, H., Mu, Y., Zhang, C., Liu, P., Xue, L., Wang, X., Gao, J., Li, H., and Wang, T.: Winter ClNO2 formation in the region of fresh anthropogenic emissions: seasonal variability and insights into daytime peaks in northern China, Atmos. Chem. Phys., 21, 15985–16000, https://doi.org/10.5194/acp-21-15985-2021, 2021.
Xue, L. K., Saunders, S. M., Wang, T., Gao, R., Wang, X. F., Zhang, Q. Z., and Wang, W. X.: Development of a chlorine chemistry module for the Master Chemical Mechanism, Geosci. Model Dev., 8, 3151–3162, https://doi.org/10.5194/gmd-8-3151-2015, 2015.
Yan, C., Tham, Y. J., Nie, W., Xia, M., Wang, H., Guo, Y., Ma, W., Zhan, J., Hua, C., and Li, Y.: Increasing contribution of nighttime nitrogen chemistry to wintertime haze formation in Beijing observed during COVID-19 lockdowns, Nat. Geosci., 16, 975–981, https://doi.org/10.1038/s41561-023-01285-1, 2023.
Yi, X., Sarwar, G., Bian, J., Huang, L., Li, Q., Jiang, S., Liu, H., Wang, Y., Chen, H., and Wang, T.: Significant Impact of Reactive Chlorine on Complex Air Pollution Over the Yangtze River Delta Region, China, J. Geophys. Res.-Atmos., 128, e2023JD038898, https://doi.org/10.1029/2023JD038898, 2023.
Yun, H., Wang, T., Wang, W., Tham, Y. J., Li, Q., Wang, Z., and Poon, S. C. N.: Nighttime NOx loss and ClNO2 formation in the residual layer of a polluted region: Insights from field measurements and an iterative box model, Sci. Total Environ., 622–623, 727–734, https://doi.org/10.1016/j.scitotenv.2017.11.352, 2018.
Zang, H., Zhao, Y., Huo, J., Zhao, Q., Fu, Q., Duan, Y., Shao, J., Huang, C., An, J., Xue, L., Li, Z., Li, C., and Xiao, H.: High atmospheric oxidation capacity drives wintertime nitrate pollution in the eastern Yangtze River Delta of China, Atmos. Chem. Phys., 22, 4355–4374, https://doi.org/10.5194/acp-22-4355-2022, 2022.