the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Significant contributions of biomass burning to PM2.5-bound aromatic compounds: insights from field observations and quantum chemical calculations
Yanqin Ren
Zhenhai Wu
Fang Bi
Hong Li
Haijie Zhang
Junling Li
Rui Gao
Fangyun Long
Zhengyang Liu
Yuanyuan Ji
Polycyclic aromatic hydrocarbons (PAHs), oxygenated PAHs (OPAHs), and nitrophenols (NPs) are essential aromatic compounds that significantly affect both climate and human health. However, their sources and formation mechanisms, particularly for NPs, remain poorly understood. This study determined the concentration profiles and the main formation mechanisms of these substance classes in PM2.5 from Dongying, based on field observations and quantum chemical calculations. The daily concentrations of ∑13PAHs during heating were more than twice higher compared to those before the heating period. Benzo(b)fluoranthene was identified as the primary PAHs species. The average concentration of ∑8OPAHs reached 351 ng m−3, with significantly increased concentrations observed during the heating season (15 November–6 December 2021) wherein 1-Naphthaldehyde (1-NapA) emerged as the most prevalent OPAH species. Concentrations of ∑9NPs increased approximately 1.2 times during the heating, with 4-methyl-5-nitrocatechol (4M5NC) having the highest concentration. Positive matrix factorization (PMF) analysis identified biomass burning to be the primary source of these aromatic compounds, particularly for PAHs. Density functional theory (DFT) calculations further revealed that phenol and nitrobenzene are two main primary precursors for 4-nitrophenol, with phenol showing lower reaction barriers, and P-Cresol was identified as the primary precursor for the formation of 4M5NC. This study provides the first detailed investigation of the sources and formation mechanisms of aromatic compounds in the atmosphere of petrochemical cities, using Dongying in the Yellow River Delta as a representative case study. This investigation may provide fundamental insights and important guidance for reducing emissions of aromatic compounds in similar atmospheric environments.
- Article
(4807 KB) - Full-text XML
-
Supplement
(12890 KB) - BibTeX
- EndNote
Aromatic compounds, characterized by the presence of a benzene ring, are known for their structural stability and distinctive aromatic properties. Polycyclic aromatic hydrocarbons (PAHs), oxygenated PAHs (OPAHs), and nitrophenols (NPs) are significant aromatic compounds commonly found in atmospheric particulate matter. These compounds exerted a considerable influence on ambient air quality, climate change, and human health (Peng et al., 2023; Yhab et al., 2021; Wei et al., 2021; Lammel et al., 2020; Elzein et al., 2019).
PAHs are a class of organic compounds comprised of multiple interconnected aromatic rings, spanning a wide range of volatilities from volatile species to non-volatile high molecular weight compounds. They are ubiquitously distributed across various environmental settings. PAHs are primarily formed as byproducts of the partial combustion of carbon-rich substances, including coal, biomass, tobacco, garbage, charbroiled meat, and petroleum (del Rosario Sienra et al., 2005; Kashiwakura and Sakamoto, 2010; Chen et al., 2005; Shen et al., 2010). OPAHs are aromatic compounds with one or more carbonyl groups connected to their ring structure that further contain various quinones and ketones. OPAHs generally have lower vapor pressures upon comparison with their parent PAHs, leading to a higher propensity to be retained in the particulate phase. The direct-acting mutagen properties and the generation of reactive O2 species render some OPAHs more hazardous compared with the parent PAHs (Bandowe et al., 2010; Chung et al., 2006; Bolton et al., 2000; Pedersen et al., 2005). OPAHs are released directly via the incomplete combustion of fossil fuels, biomass, and other organic materials (Oda et al., 2001; Jakober et al., 2007) or formed secondarily through photochemical reactions of parent PAHs by atmospheric oxidants such as ozone, hydroxyl radicals, and nitrate radicals (Bandowe et al., 2014a; Wang et al., 2011; Lin et al., 2015b). Research suggests that the PAH concentrations, along with their derivatives in PM2.5, increase during winter or heating periods primarily due to biomass and coal burning (Lin et al., 2015b). Furthermore, particulate matter released from biomass burning is considered more toxic compared to that derived from other sources (Sarigiannis et al., 2015). NPs are monocyclic aromatic compounds having properties similar to benzene, including low solubility in water while high solubility in various organic solvents such as ethanol, pyridine, xylene, and chloroform. In addition to adversely affecting human health, NPs can also disrupt the equilibrium of the ecosystem, increase the risk of cancer, and disrupt plant growth (Chow et al., 2015; Liang et al., 2020; Booth et al., 2014). NPs in the atmosphere are primarily derived from primary emissions and secondary formation. Primary sources of NPs include emissions from coal combustion, vehicle exhaust, biomass combustion, and industrial and agricultural sources, similar to the sources of PAHs and OPAHs (Liang et al., 2020; Wang et al., 2017; Lu et al., 2021). Observations in the atmospheric environment indicate that secondary formation can provide more than one-third of the total NPs present (Yuan et al., 2016a). Benzene, toluene, and their derivatives are considered significant precursors in the secondary formation of NPs, although secondary NPs can also be generated through nitrification of phenols in the gas or condensation phase or by interactions between phenoxyl radicals, formed from NO2, and other aromatic compounds (Harrison et al., 2005).
In Dongying City, known for its petrochemical industry, benzene, toluene, and xylene are significant contributors to the levels of volatile organic compounds (VOCs) into the environment (Chen et al., 2020). Moreover, research has revealed that in the presence of NOx, aromatic hydrocarbons can be oxidized to yield nitroaromatic compounds, including nitrophenol, dinitrophenol, nitrocatechol, and methylnitrocatechol (Lin et al., 2015a). The primary sources of aromatic compounds in particulate matter within high-aromatic environments and the potential relationship between their secondary formation and the benzene series mentioned still require systematic investigation. Therefore, this study explored the pollution characteristics and primary sources of PAHs, OPAHs, and NPs in PM2.5 in Dongying City, and further explored the formation mechanism of typical nitrophenols based on field observations and density functional theory calculations. This study represents the first investigation regarding the sources and the forming mechanisms of aromatic compounds within the atmosphere of petrochemical cities, using Dongying in the Yellow River Delta as a representative case study.
2.1 Field observations
PM2.5 sample collection was carried out at Dongying Atmospheric Super Station (118.59° E, 37.45° N) from 27 October to 6 December 2021. The surrounding area is primarily residential and commercial, with well-developed transportation infrastructure and no significant sources of industrial pollution, making it a representative urban monitoring site (Fig. 1). A high-flow particulate sampler (TH-1000H, Wuhan Tianhong Instrument Co., LTD) was employed to obtain the samples. This instrument had a sampling flow rate equal to 1.05 m3 min−1 and a flow accuracy of ±2.5 %. It comprised a quartz microfiber filter (Quartz, 203 mm × 254 mm, Whatman, UK) with an effective rectangular dimension of 180 mm × 230 mm. Samples were collected twice daily: once during the day from 08:00 am to 07:30 pm local time (LT) and once from 08:00 pm to 07:30 am LT. Meteorological statistics, including temperature, weather conditions, humidity, wind direction and speed, were measured concurrently with each sampling.
2.2 Chemical analysis
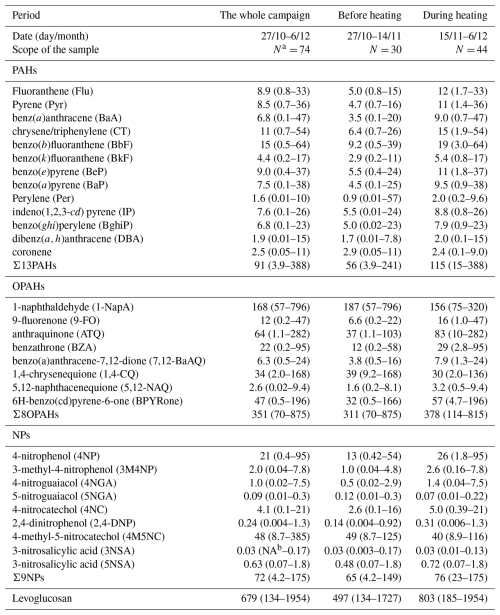
Organic species in PM2.5 were detected following a pre-treatment process that included ultrasonic extraction along with derivatization. Initially, the sampled filter was divided into four equal surface area segments, and one quarter was utilized for experimental analysis. The quarter of the sampled filter was placed into a sample bottle and then immersed in a mixture of dichloromethane and methanol solution (2:1, ) to completely submerge the filters. After that, ultrasonic extraction was performed three times, with each session lasting 15 minutes. Following the process of extraction, filtration was carried out through glass wool using a Babbitt dropper into pear-shaped flasks. The filtrates were then concentrated to a small amount through a rotary evaporator inside a vacuum. Subsequently, they were transferred to GC bottles, which were dried with a nitrogen purifier, followed by the addition of 60 µL of N, O-bis-(trimethylsilyl) trifluoroacetamide (BSTFA+TMCS, 99:1, ) and pyridine (99.5 %) (5:1, ). The bottles were then placed in an oven at 70 °C for a total time duration of 3 h to complete the derivatization process. After the reaction, the solution was cooled, and an internal standard comprised of 40 µL tridecane was added. The solution was mixed completely and then placed in the refrigerator for examination. The derivatized samples were examined using gas chromatography coupled with a mass spectroscopy detector (GC/MS: HP 7890A, HP 5975C, Agilent Co., USA). The extraction and derivatization methods described above allowed for the simultaneous measurement of the samples' polar and non-polar constituents. This study analyzed 13 different PAHs, 8 different OPAHs, and 9 different NPs, as well as some organic tracers, e.g. levoglucosan. Specific species of PAHs, OPAHs, and NPs were listed in Table S1 in the Supplement.
Inorganic ions soluble in water were quantified through an ion chromatograph (Dionex-1100). The analysis focused on eight water-soluble inorganic ions: NO, Cl−, NH, SO, Na+, Mg2+, K+, and Ca2+. The measurement of organic carbon (OC) and elemental carbon (EC) was performed through a thermal/optical carbon analyzer (DRI2015). Detailed tests for analyzing EC, OC, and inorganic ions are provided in a previous report (Ren et al., 2021).
2.3 Quantum chemical calculations
Quantum chemical calculations both in the gas phase and liquid phase were performed using the Gaussian 09 software. The geometries and frequencies for the reactant monomers, reactant complexes (RC), transition states (TS), intermediate (IM), and product complexes (PC) were performed via density functional theory (DFT) calculations at the M06-2X/6-311G(2df,2p) level of theory, which is well-established for studies of organic systems (Li and Wang, 2014; Zhao and Truhlar, 2007; Wang et al., 2025). The conductor-like polarizable continuum model (CPCM) was used for the optimization calculations to include the solvent effect in the liquid (Takano and Houk, 2005). Radicals were treated using unrestricted Hartree–Fock calculations and TS was optimized to ensure the presence of a single imaginary frequency. Intrinsic reaction coordinate (IRC) calculations were carried out to further guarantee that the TS connects the right pre- and post-complexes along the reaction coordinate (Gonzalez and Schlegel, 1989).
2.4 Quality control and quality assurance (QC & QA)
Quality control and QA procedures for sampling and laboratory analysis strictly follow the environmental monitoring technical specifications and analytical standards established by the Ministry of Environmental Protection. Periodic inspections of all analytical and testing instruments were carried out in accordance with the relevant metrological verification protocols. The quartz microfiber filter was burned inside a Muffle furnace at a temperature of 450 °C for 6 h before sampling to remove any organic contaminants. After natural cooling, it was removed and transferred to a controlled temperature and humidity box (25±1 °C, 50±5 % RH). The sampled filter was packed in a ziplock bag and stored in the refrigerator at a temperature below 0 °C until analysis. To minimize systematic errors, corresponding blank filter samples were simultaneously prepared and stored followed by analysis under the same conditions as the collected samples. Limits of detection (LOD) of the target compounds were calculated using signal-to-noise ratios of 3:1 following previous studies (Bandowe et al., 2014b; Li et al., 2016). In this work LOD are in the ranges of 0.003–0.008 ng µL−1 for PAHs, 0.007–0.37 ng µL−1 for OPAHs, and 0.0001–0.0014 ng µL−1 for NPs (Table S1). The blank sample analysis showed no serious contamination (less than 5 % of real samples) and the data reported here were all corrected according to the blanks.
3.1 Changes in meteorological conditions, gaseous pollution, and major components of PM2.5
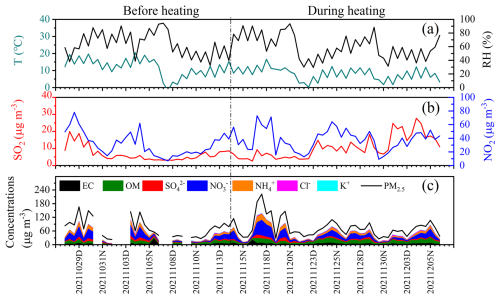
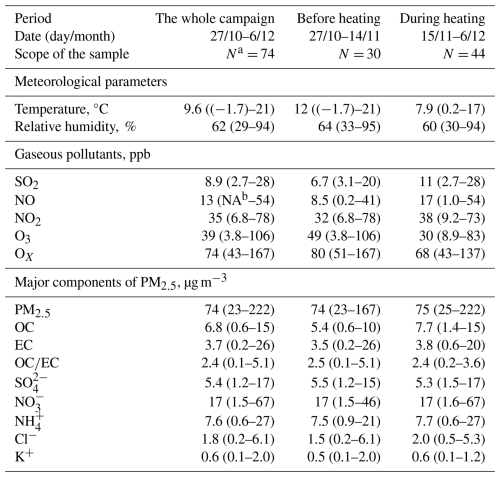
In northern cities of China, centralized heating generally starts on 15 November each year and continues until 15 March of the following year. Therefore, the entire period in this study was divided into two stages: before heating (27 October to 14 November 2021) and during heating (15 November to 6 December 2021). Figure 2 and Table 1 illustrate the temporal variations within the meteorological factors, concentrations of gaseous pollutants, as well as the primary components of PM2.5. Before heating, the relative humidity (RH) and temperature (T) were higher, averaging 64 % and 12 °C, respectively. During the heating period, the average values were found to be lower at 60 % and 7.9 °C. Overall, the average values for the entire study period were determined to be 62 % and 9.6 °C. The average concentrations of SO2 and NO during heating (11 and 17 ppb, respectively) were nearly twice that of the value observed before heating, with a slight increase in the level of NO2. These increases are primarily associated with enhanced combustion activities during the heating season, which supported by the correlation analysis (Sect. 3.3) though synergistic effects (e.g., meteorological effect) require further quantification in future studies. In contrast, the levels of O3 and OX (the sum of NO2 and O3) were significantly higher before heating, with an average value of 49 and 80 ppb, respectively, in comparison to that observed during the heating period. This variation was associated with the higher temperatures and light conditions before heating, which are conductive to photochemical reactions.
Table 1Meteorological parameter values and concentrations (mean (min–max)) of gaseous pollution and chemical components of PM2.5 during the sampling periods in Dongying.
a N: the number of samples. b NA: not available.
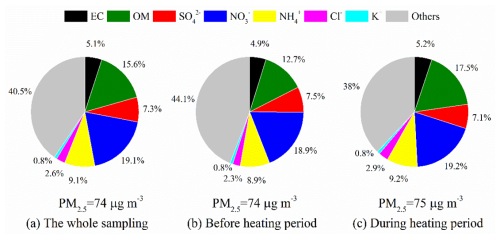
Figure 3Absolute proportion of chemical components in PM2.5 across the whole sampling period (a) and before (b) and during (c) the heating periods.
Figures 2 and 3 showed the time variations and absolute proportion of chemical components in PM2.5 before and during heating, respectively, throughout the entire sampling period. Throughout the observation period, secondary inorganic aerosols (SIA) such as SO, NO, and NH were determined as the main chemical components of PM2.5, followed by OM (1.6 times the OC) (Turpin and Lim, 2001). Before heating, the concentrations of SO, NO, and NH were found to be 5.5, 17, and 7.5 µg m−3, respectively, while during heating, they demonstrated the average values of 5.3, 17, and 7.7 µg m−3 (Table 1). The total concentrations and proportions of SIA remained relatively stable, with an average of 29.9 µg m−3 and 35 % during the whole process, respectively. Among these, NO was determined as the predominant species, constituting approximately 19 % of the total PM2.5 during the sampling period. OM comprised 12.7 % of PM2.5 before the heating period and increased significantly to 17.5 % during heating (Fig. 3b, c). Correspondingly, the average OC concentration increased from 5.4 to 7.7 µg m−3. However, the average ratio of remained relatively unchanged, with average values of 2.5 before heating and 2.4 during heating. Generally, OC is generated from both primary emissions and secondary formation, while EC is predominantly derived from primary sources (Zhang et al., 2015). The significant increase in the concentration of OC alongside a stable ratio suggests that the sources of carbonaceous aerosols in this study include both primary emissions and secondary formation processes (Zhang et al., 2015). A strong positive correlation between levoglucosan and Cl− (r=0.58–0.69) and a weaker correlation with K+ (r=0.3–0.34) were observed (Fig. 4), with both ions showing elevated concentrations during the heating period, suggests additional combustion activities, e.g. biomass burning. It is worth noting that Cl− is more robustly associated with levoglucosan, a well-established tracer for biomass burning. Thus Cl− was prioritized as the inorganic tracer for biomass burning in subsequent analyses (Sect. 3.3).
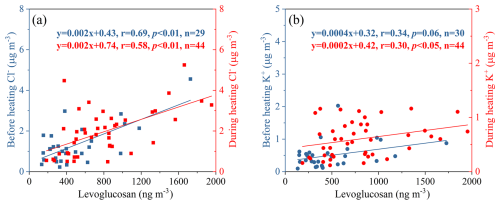
Figure 4Linear fit regressions for levoglucosan to Cl− (a) and K+ (b) before (green) and during (red) heating throughout the whole campaign (r: Pearson correlation coefficient; p: probability value, if the p value is less than 0.01, it means that there is at least a 99 % certainty of the occurrence of a certain event, and if the p value is less than 0.05 (and greater than 0.01), it means that there is at least a 95 % certainty of the occurrence of a certain event. Common standards are 0.01 and 0.05).
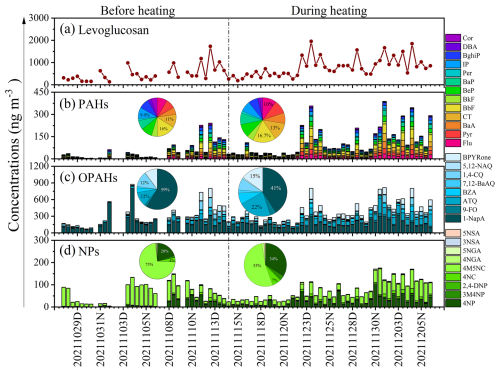
Figure 5Time series of levoglucosan (a), PAHs (b), OPAHs (c), and NPs (d) before and during heating in Dongying.
3.2 Changes in the concentration and composition of aromatic compounds
3.2.1 PAHs and OPAHs
Table 2 and Fig. 5b show that the daily concentrations of 13 different PAHs (∑13PAHs) ranged from 3.9 to 388 ng m−3, with 91 ng m−3 of overall average value throughout the sampling period. During the heating phase, concentrations averaged 115 ng m−3 (over a range of 15 to 388 ng m−3), more than twice the levels recorded before heating, which averaged 56 ng m−3 (over a range of 3.9 to 241 ng m−3). The ∑13 PAHs measured in the current research were lower in comparison to those recorded in other Chinese cities, including Xi 'an (127 ng m−3, 2016–2017) (Wang et al., 2019a) and Harbin (215 ng m−3, 2017–2018) (Ma et al., 2020). Nighttime average concentrations of PAHs (65 ng m−3 before heating and 154 ng m−3 during heating) were observed to be 1.5 to 2 times higher upon comparison with those measured during the daytime (44 ng m−3 before heating and 77 ng m−3 during heating) (Fig. 6a). The observed day-night pattern aligns with the observations obtained from coastal cities in Bohai and the Yellow Seas, as well as Jinan in Shandong Province (Chen et al., 2021; Zhang et al., 2018). This diurnal variation is strongly influenced by the emission sources (e.g. coal and biomass burning, heavy truck traffic) in addition to air temperature and boundary layer height. The concentration level of BbF in PAHs was found to be highest, accounting for 16 % of total PAHs (Fig. 5b). The dominance of BbF was found to be consistent with the previous research (Li et al., 2013, 2022; Ren et al., 2017; Bai et al., 2023). This molecular composition is mainly affected by the emission source and is influenced by the fact that low efficiency combustion activities emitting more BbF are still very common in China (Wang et al., 2009).
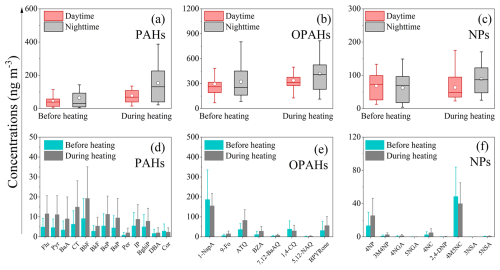
Figure 6Diurnal variation and component concentrations of PAHs (a, d), OPAHs (b, e), and NPs (c, f) before and during heating. The top and bottom of the vertical line for each box in (a)–(c) correspond to the 95th and 5th percentiles, respectively, and the top, middle, and bottom horizontal lines of the box mark the 75th, 50th, and 25th percentiles of the data range. The white dot in each box represents the mean value. The error bars in (d)–(f) indicate the standard deviation.
The average total concentration of OPAHs (∑8OPAHs) was determined to be 351 ng m−3 across all the sampling periods. Notably higher concentrations were observed during heating (average: 378 ng m−3, range: 114–812 ng m−3) compared with those before heating (average: 311 ng m−3, range: 70–875 ng m−3), though the difference did not reach statistical significance (p>0.05) (Table 2). There is a peak concentration of OPAHs during the daytime of 4 November that could be attributed to significant biomass burning activities during the nighttime of 3 November which likely enhanced both primary emissions and secondary formation processes of these compounds. Concentration of ∑8OPAHs was higher at night compared to daytime, being approximately 1.1 to 1.2 times greater both before and during the heating period (Figs. 5c and 6b). Like other pollutants, this diurnal variation is influenced not only by the boundary layer height and temperature, but also by the emission source. Among the eight OPAHs, 1-NapA was determined as the most prevalent, accounting for 59 % of ∑8OPAHs (187 ng m−3) before heating and 41 % of ∑8OPAHs (156 ng m−3) during heating (Figs. 5c and 6e). The average ∑8OPAHs concentrations in this study were significantly higher compared with those found for other Chinese urban areas, such as Guangzhou and Xi'an, with respective values of 23 and 54 ng m−3 (Ren et al., 2017). Furthermore, the ∑8OPAHs levels observed in this study exceeded those reported at various sites outside of China, such as south (traffic site, 41.8 ng m−3) (Alves et al., 2017) and central European cities (about 10 ng m−3) (Lammel et al., 2020), Thessaloniki, Greece (0.86–4.3 ng m−3) (Kitanovski et al., 2020), and Mainz, Germany (0.047–1.6 ng m−3). The significantly higher ∑8OPAHs concentrations in this study likely reflect localized emission sources, including mixed industrial-traffic-biomass burning activities, coupled with stagnant meteorological conditions during the sampling. Notably, stricter fuel quality standards and emission controls in the compared cities may also contribute to these disparities.
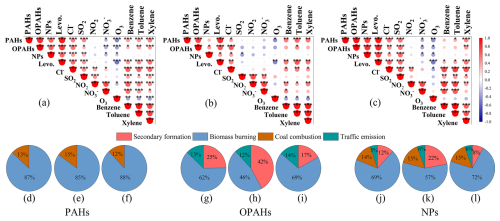
Figure 7Correlations between PAHs, OPAHs, and NPs and key gas pollutants and aerosol components throughout the whole campaign (a), before heating (b), and during heating (c) (, , ); source apportionments for PAHs (d, e, f), OPAHs (g, h, i), and NPs (j, k, l) during the whole campaign, before heating, and during heating, respectively; SF: secondary formation; FF: fossil fuel combustion; BB: biomass burning; CC: coal combustion; TE: traffic emission).
3.3 NPs
Based on the data presented in Table 2, the total concentrations of NP compounds (∑9NPs) demonstrated a mean value equal to 72 ng m−3 across the sampling period. During the heating phase, the average concentration of ∑9NPs was 76 ng m−3 (range: 23–175 ng m−3), representing a 1.2-fold increase compared with before heating (average: 65 ng m−3, range: 4.2–149 ng m−3), although this difference was not statistically significant (p>0.05). These concentrations were approximately twice those observed during autumn and winter (18 October 2017–17 January 2018) (38 ng m−3) in earlier work conducted in Beijing (Ren et al., 2024). They were also considerably higher in comparison to the values recorded in the summer (1–30 July 2017) (8.5 ng m−3) and spring (10–21 April 2017) (8.6 ng m−3) (Ren et al., 2022). Different from OPAHs and PAHs, the total concentrations of ∑9NPs did not demonstrate significant diurnal variation before heating, but a significant nighttime increase was observed during the heating period, with approximately 1.4 times higher concentrations at night compared to the levels of daytime (Figs. 5d and 6c). However, the rank order of relative abundance for the nine NP species in PM2.5 remained consistent throughout the observation period. Among all the species, 4M5NC demonstrated the highest concentration, contributing to 73 % of the total NPs before heating and 53 % during heating, followed by 4NP, comprising 20 % before heating and increased to 34 % during heating (the pie charts in Fig. 5d). The total concentration of ∑9NPs observed in the current research was generally comparable to previous measurements carried out in Beijing during winter (average: 74 ng m−3) (Li et al., 2020). Conversely, the levels were substantially higher compared to those recorded in Jinan (average: 48 ng m−3) (Wang et al., 2018), Hong Kong SAR (average: 12 ng m−3), and Xi'an (average: 17 ng m−3) (Wu et al., 2020; Chow et al., 2015). Compared to the previous measurements in other regions, the observed ∑9NPs concentrations were generally higher, such as those reported in Germany (16 ng m−3), the UK (19 ng m−3), and Belgium (32 ng m−3 in winter, 13 ng m−3 in autumn) (Teich et al., 2017; Mohr et al., 2013; Kahnt et al., 2013). The spatial differences in the above concentration levels were influenced by a combination of various factors. Besides meteorological conditions, the influence of different emission sources was very important.
3.4 Source identification and quantitative analysis
3.4.1 Primary sources of key pollutants
To better understand the primary influencing factors, Pearson correlation analyses were conducted to analyze the relationships between OPAHs, PAHs, and NPs with key PM2.5 components, gaseous pollutants, and VOC precursors. The results, presented in Fig. 7a–c, include correlations with levoglucosan, Cl−, SO2, NO2, NO, O3, benzene, toluene, and xylene. A substantially positive correlation was observed between OPAHs, PAHs, and NPs, suggesting a common source or similar formation mechanisms, particularly during the heating period. Furthermore, strong relationship were observed between these aromatic compounds and primary pollutants throughout the sampling campaign, such as levoglucosan (r= 0.90 (p<0.001), 0.77 (p<0.001), 0.61 (p<0.001) for PAHs, OPAHs, and NPs, respectively), and Cl− (r= 0.50 (p<0.001), 0.56 (p<0.001), 0.39 (p<0.001), respectively) (Fig. 7a). These relationships were strengthened during heating, especially for SO2 (r= 0.55 (p<0.001), 0.55 (p<0.001), 0.60 (p<0.001), respectively) (Fig. 7c). The data suggests that the levels of aromatic compounds were substantially affected by burning emissions throughout the sampling period which may be attributed to various sources such as biomass burning (Wang et al., 2017; Lin et al., 2017; Chow et al., 2015) and coal combustion (Lu et al., 2019). Specifically, biomass burning emerged as a major source of pollution throughout the entire campaign, while the contribution mediated by coal combustion increased remarkably during the heating period.
3.4.2 Source contribution
To further analyze the quantitative and qualitative effects of primary emissions as well as secondary formation, this study identified four categories of distinct sources during the campaign using the positive matrix factorization (PMF) model. The detailed introduction to the PMF process in the Supplement (Sect. S1), including Table S2 and Fig. S1. The first source factor, biomass burning, was recognized by the highest levoglucosan concentrations and increased particulate levels of K+ and Cl− within PM2.5 (Fig. S1a). Biomass burning emerged as the predominant source of aromatic compounds in the urban atmosphere of Dongying during the whole campaign (Fig. 7d–l), contributing 87 %, 62 %, and 69 % of the total PAHs, OPAHs, and NPs, respectively, throughout the entire campaign (Fig. 7d, g, j). Moreover, the proportional contributions of burning biomass were also higher during heating than before heating. A large number of PAHs, along with their derivatives, originate from biomass combustion, as reported by various previously published studies (Yhab et al., 2021; Wei et al., 2021; Bai et al., 2023; Li et al., 2022; Luo et al., 2021). Several previous studies have also established that NPs are directly released during biomass combustion and found emission factors over a range of 0.4 to 11.1 mg kg−1 (Iinuma et al., 2007; Wang et al., 2017). At the same time, as the most dominant species during the entire observation period, 4M5NC can also prove that biomass burning is the main source of NPs. Methyl-nitrocatechols have been proposed as tracers for processed biomass burning aerosol as they are further oxidized products of VOCs produced after biomass combustion (Iinuma et al., 2010).
The second source factor was identified as coal combustion, due to it having the highest SO2 concentration (Fig. S1b). The average contribution of coal combustion to PAHs and NPs was determined as 13 % and 14 %, respectively (Fig. 7d, j). There were no significant changes to the relative contributions of coal combustion prior to and during heating compared with those of biomass burning and traffic emission. However, the actual contribution of coal combustion source also increases along with the absolute concentrations of PAHs and NPs increasing during the heating period. Furthermore, coal combustion also proved to be an important source of PAHs based on the and ratios (Table S3) (Ohura et al., 2004; Grimmer et al., 1983). Previous research has indicated that coal combustion-related activities significantly contribute to elevated levels of particulate PAHs and NPs, particularly during the heating season (Lu et al., 2019; Wang et al., 2018; Ren et al., 2023; Cai et al., 2022; Luo et al., 2021).
In comparison to the other factor profiles, the third source factor, traffic emissions, showed a higher NO concentration loading in this factor profile (Fig. S1c). The average contribution of traffic emissions to OPAHs throughout the whole campaign was 13 %, with no substantial variation in the percentage of emissions before and during heating (14 %). There was a minimal change in the contribution of traffic emissions to NPs both before and during heating; on average, these emissions accounted for 6 % of total NPs during the campaign (Fig. 7j–l). Previous studies have identified the traffic emission as an essential source of OPAHs (Wei et al., 2021; Wang et al., 2022) and road traffic as a primary contributor to NPs (Zhang et al., 2010; Ren et al., 2022). These differences can be attributed to variations in energy use and industrial structures across different cities.
Secondary formation, the fourth factor, displayed high concentrations of O3 and a relatively higher abundance of SO, NO, and NH (Fig. S1d). Secondary formation contributed an average of 25 % of OPAHs throughout the whole campaign (Fig. 7g–i). It was the second-largest source, with a significantly higher contribution before heating (42 %) compared to during heating (17 %). Secondary formation contributed 12 % of NPs throughout the whole campaign, with higher contributions before heating (22 %) (Fig. 7j–l). The contribution of secondary formation to OPAHs and NPs before heating was about twice that of during heating, mainly because the higher temperature and stronger atmospheric oxidation before heating (Sect. 3.1) were conducive to the chemical reactions that generate these species. The vital role of secondary formation in contributing to both OPAHs and NPs agreed with recent studies (Ren et al., 2024; Ren et al., 2022). Previous modeling and field studies also identified secondary formation as a significant NP source in the atmosphere (Yuan et al., 2016b; Mayorga et al., 2021; Xie et al., 2017).
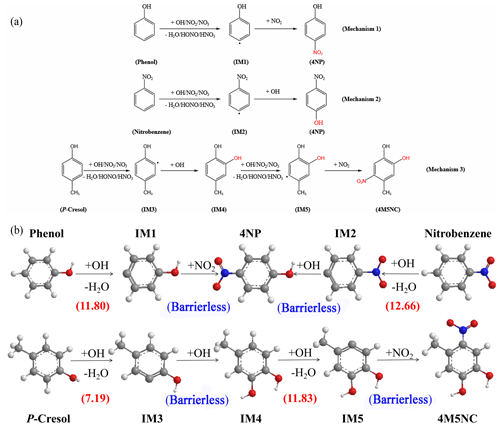
Figure 8(a) Suggested formation mechanism for 4NP and 4M5NC. (b) The proposed dominant formation mechanisms of 4NP and 4M5NC at the M06-2X/6-311G(2df,2p) level of theory. H, C, N, and O atoms are depicted as white, gray, blue, and red spheres, respectively. The reaction barrier presented in the parentheses are given in kcal mol−1.
3.5 Secondary formation mechanism based on quantum chemical calculations
Aromatic hydrocarbons are a major class of VOCs that play a vital role in secondary organic aerosols (SOA) formation, particularly in urban areas (Song et al., 2021). The present study reported a positive correlation of these aromatic compounds with the key precursors (i.e. benzene, toluene, xylene) and a negative correlation with NO2, NO and O3, which are key parameters during the reaction process (Fig. 7a–c). Therefore, these substances were primarily derived from the same source as the precursors, rather than their main products. Furthermore, considering the substantial effect of biomass combustion, these compounds are probably the secondary products formed through the oxidation process of gaseous pollutants released during the biomass burning process. Nitroaromatic hydrocarbons are critical species influenced by biomass combustion emissions where phenol, nitrobenzene, and P-Cresol are their significant precursors (Wang et al., 2020). Therefore, to better comprehend the formation mechanism of the dominant NPs species in this observation, DFT calculations at the M06-2X/6-311G(2df,2p) level of theory was carried out to explore the formation mechanism of 4NP and 4M5NC through oxidation processes of the major precursors by OH, NO2, and NO3 (Huang et al., 2010; Roman et al., 2022; Shenghur et al., 2014; Wang et al., 2019b). Electronic energies (EDFT), Gibbs free energies (GDFT) of the reactant monomers, reactant complexes (RC), transition states (TS), intermediates (IM), and product complexes (PC), with the imaginary frequency of TS at 298.15 K and 1 atm, are collected in Tables S4–S7 of the Supplement. The overall formation mechanisms, detailed in Fig. 8a, include both H-abstraction and OH/NO2 addition steps where Mechanisms 1 and 2 show the two-step formation mechanism for 4NP, while Mechanism 3 describes the four-step pathway for 4M5NC.
In the H-abstraction step, either an OH radical, NO2 or NO3 abstracts a hydrogen atom from the aromatic ring, forming an intermediate free radical (IM) and resulting in the formation of H2O, HONO or HNO3. In the OH/NO2 addition step, the IMs react with OH or NO2 without any reaction barriers. In Table S4, the calculated binding energy (E), Gibbs free energy change (ΔG) for the reactant monomers, Gibbs free energy (G), transition states (TS), reactant complexes (RC), intermediate (IM), and PCs are presented. While Fig. 8b exhibited the calculated dominant formation mechanism of 4NP and 4M5NC along with the reaction barrier in kcal mol−1.
For the formation of 4NP, two precursors, phenol, and nitrobenzene, were selected which contain three distinct types of hydrogen atoms in their aromatic rings (ortho-, meta-, and para-H). In the case where the -OH and -NO2 functional groups in 4NP are at the para-position, only the para-H in phenol and nitrobenzene was chosen for the formation of 4NP. During the daytime, when the gas-phase concentration of OH radicals is significant, phenol initially reacts with OH to yield a reactant complex RCph-OH with a ΔG of 3.16 kcal mol−1. Then RCph must overcome a reaction barrier of 8.64 kcal mol−1 to transition to the TSph-OH state, resulting in the generation of IM1, a free radical intermediate with a ΔG of −1.03 kcal mol−1. The overall H-abstraction step was slightly exothermic, with a reaction barrier of 11.80 kcal mol−1. The generated IM1 rapidly proceeds through an addition reaction with NO2 without any energy barrier, forming 4NP with a ΔG of −64.50 kcal mol−1.
During nighttime, a parallel H-abstraction step occurred where NO2 or NO3, instead of OH, abstracted a hydrogen atom from phenol. However, this step possess higher overall reaction barriers than that of OH, reaching 40.31 and 15.79 kcal mol−1 in the gas phase, respectively. Moreover, the reactions were highly endothermic with the ΔG of 36.51 and 7.42 kcal mol−1 in the gas phase, respectively, indicating that the H-abstraction step initiated by NO2 or NO3 were not feasible. Therefore, the calculations for Mechanisms 2 and 3 focused solely on the H-abstraction step initiated by OH.
For an alternative formation pathway of 4NP from nitrobenzene during the daytime, the overall H-abstraction step was slightly exothermic (−1.08 kcal mol−1) with a reaction barrier of 12.66 kcal mol−1. The generated IM2 then proceeded through a highly exothermic and barrier-free OH addition step to form 4NP. In a comparative analysis, it was possible to initiate 4NP formation with both phenol and nitrobenzene. For mechanism 1, phenol was preferred to undergo the H-abstraction reaction during the daytime and the NO2 addition reaction at nighttime. For Mechanism 2, nitrobenzene preferred to undergo both reactions involving H-abstraction and OH addition during the daytime.
The formation of 4M5NC followed a four-step mechanism with P-Cresol as the precursor. During the daytime, P-Cresol initially reacted with OH to form free radical intermediate IM3, overcoming a reaction barrier of 7.19 kcal mol−1. After that, IM3 underwent a highly exothermic OH addition step without any energy barrier to form IM4 which was then proceeded through a reaction barrier of 11.83 kcal mol−1 to produce IM5. At nighttime, the IM5 formed during the daytime quickly reacted through a highly exothermic NO2 addition step with no significant energy barrier, resulting in the formation of 4M5NC, with a ΔG of −61.14 kcal mol−1 in the gas phase and −63.47 kcal mol−1 in the liquid phase.
A comprehensive reaction mechanism (Fig. S2) of P-Cresol with OH, NO2, NO3 has been drawn to display the potentially different pathways. As can be seen, there are two parallel pathways leading to the formation of 4M5NC. One involves the initial addition of an OH radical on C2 of the benzene ring, followed by the addition of NO2 on C5 mentioned in Mechanism 3. While the other one involves the initial addition of a NO2 on C5 of the benzene ring, followed by the addition of OH on C2. Based on the calculated reaction barrier and the reactant monomer concentrations, the OH radical induced H-abstraction step possesses a much lower barrier, resulting in the following OH addition step more feasible. Hence, Mechanism 3 may be the dominant formation pathway of 4M5NC.
PAHs, OPAHs, and NACs in the PM2.5 samples were analyzed before and during heating in Dongying, a petrochemical industrial city. The concentrations of all these species were significantly greater during the heating period in comparison to those before heating. Furthermore, these compounds demonstrated higher levels at night than during the day, whereas NPs showed no notable diurnal variation. BbF, 1-NapA, and 4M5NC were found to be the most abundant species for PAHs, OPAHs, and NPs, respectively. Biomass burning emerged as the primary source of aromatic compounds, particularly during the heating period. Quantum chemical calculations revealed that the formation mechanisms of 4NP and 4M5NC involve both H-abstraction and OH/NO2 addition steps with the H-abstraction step serving as the rate-limiting step, while the OH/NO2 addition step proceeded without an energy barrier. Phenol and P-Cresol were determined as the primary precursors for the formation of 4NP and 4M5NC, respectively. This study presents the first comprehensive investigation into the sources and formation mechanisms of aromatic compounds in the atmosphere of petrochemical cities within the Yellow River Delta, with Dongying serving as a case study. It offers essential data and strategic guidance for reducing aromatic compound emissions in such urban environments.
The Data used in this study are available from the first author upon request (Yanqin Ren via renyq@craes.org.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-6975-2025-supplement.
YR, GW, and YJ designed the research; YJ, FB, and ZW collected the samples and analyzed the data; YR and HZ wrote the manuscript; JL, RG, FL, ZL, and HL contributed to the paper with useful scientific discussions and comments.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We are grateful to Xiaoyu Yan and Xurong Bai from the Chinese Research Academy of Environmental Sciences for their help in collecting samples and Yubao Chen and his colleagues from East China Normal University for providing help with analytical testing.
This research has been supported by the Key Technologies Research and Development Program (no. 2023YFC3706105), the National Research Program for Key Issues in Air Pollution Control (nos. DQGG202121, DQGG2021301), Fundamental Research Funds for Central Public Welfare Scientific Research Institutes of China (no. 2019YSKY-018), and the National Natural Science Foundation of China (nos. 42130704, 41907197, 22306179).
This paper was edited by John Liggio and reviewed by three anonymous referees.
Alves, C., Vicente, A., CustÃdio, D., Cerqueira, M., Nunes, T., Pio, C., Lucarelli, F., Calzolai, G., Nava, S., and Diapouli, E.: Polycyclic aromatic hydrocarbons and their derivatives (nitro-PAHs, oxygenated PAHs, and azaarenes) in PM2.5 from Southern European cities, Sci. Total. Environ., 595, 494–504, https://doi.org/10.1016/j.scitotenv.2017.03.256, 2017.
Bai, X., Wei, J., Ren, Y., Gao, R., Chai, F., Li, H., Xu, F., and Kong, Y.: Pollution characteristics and health risk assessment of Polycyclic aromatic hydrocarbons and Nitrated polycyclic aromatic hydrocarbons during heating season in Beijing, J. Environ. Sci., 123, 169–182, https://doi.org/10.1016/j.jes.2022.02.047, 2023.
Bandowe, B. A. M., Shukurov, N., Kersten, M., and Wilcke, W.: Polycyclic aromatic hydrocarbons (PAHs) and their oxygen-containing derivatives (OPAHs) in soils from the Angren industrial area, Uzbekistan, Environ. Pollut., 158, 2888–2899, https://doi.org/10.1016/j.envpol.2010.06.012, 2010.
Bandowe, B. A. M., Lueso, M. G., and Wilcke, W.: Oxygenated polycyclic aromatic hydrocarbons and azaarenes in urban soils: A comparison of a tropical city (Bangkok) with two temperate cities (Bratislava and Gothenburg), Chemosphere, 107, 407–414, https://doi.org/10.1016/j.chemosphere.2014.01.017, 2014a.
Bandowe, B. A. M., Meusel, H., Huang, R.-j., Ho, K., Cao, J., Hoffmann, T., and Wilcke, W.: PM 2.5-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: seasonal variation, sources and cancer risk assessment, Sci. Total. Environ., 473, 77–87, https://doi.org/10.1016/j.scitotenv.2013.11.108, 2014b.
Bolton, J. L., Trush, M. A., Penning, T. M., Dryhurst, G., and Monks, T. J.: Role of quinones in toxicology, Chem. Res. Toxicol., 13, 135–160, https://doi.org/10.1021/tx9902082, 2000.
Booth, A. M., Murphy, B., Riipinen, I., Percival, C. J., and Topping, D. O.: Connecting Bulk Viscosity Measurements to Kinetic Limitations on Attaining Equilibrium for a Model Aerosol Composition, Environ. Sci. Technol., 48, 9298–9305, https://doi.org/10.1021/es501705c, 2014.
Cai, D., Wang, X., George, C., Cheng, T., Herrmann, H., Li, X., and Chen, J.: Formation of Secondary Nitroaromatic Compounds in Polluted Urban Environments, J. Geophys. Res.-Atmos., 127, e2021JD036167, https://doi.org/10.1029/2021JD036167, 2022.
Chen, L., Liu, W., Tao, S., and Liu, W.: Spatiotemporal variations and source identification of atmospheric nitrated and oxygenated polycyclic aromatic hydrocarbons in the coastal cities of the Bohai and Yellow seas in northern China, Chemosphere, 279, 130565, https://doi.org/10.1016/j.chemosphere.2021.130565, 2021.
Chen, T., Xue, L., Zheng, P., Zhang, Y., Liu, Y., Sun, J., Han, G., Li, H., Zhang, X., Li, Y., Li, H., Dong, C., Xu, F., Zhang, Q., and Wang, W.: Volatile organic compounds and ozone air pollution in an oil production region in northern China, Atmos. Chem. Phys., 20, 7069–7086, https://doi.org/10.5194/acp-20-7069-2020, 2020.
Chen, Y., Sheng, G., Bi, X., Feng, Y., Mai, B., and Fu, J.: Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China, Environ. Sci. Technol., 39, 1861–1867, https://doi.org/10.1021/es0493650, 2005.
Chow, K. S., Huang, X. H. H., and Yu, J. Z.: Quantification of nitroaromatic compounds in atmospheric fine particulate matter in Hong Kong over 3 years: field measurement evidence for secondary formation derived from biomass burning emissions, Environ. Chem., 13, 665–673, https://doi.org/10.1071/EN15174, 2015.
Chung, M. Y., Lazaro, R. A., Lim, D., Jackson, J., Lyon, J., Rendulic, D., and Hasson, A. S.: Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts, Environ. Sci. Technol., 40, 4880–4886, https://doi.org/10.1021/es0515957, 2006.
del Rosario Sienra, M., Rosazza, N. G., and Préndez, M.: Polycyclic aromatic hydrocarbons and their molecular diagnostic ratios in urban atmospheric respirable particulate matter, Atmos. Res., 75, 267–281, https://doi.org/10.1016/j.atmosres.2005.01.003, 2005.
Elzein, A., Dunmore, R. E., Ward, M. W., Hamilton, J. F., and Lewis, A. C.: Variability of polycyclic aromatic hydrocarbons and their oxidative derivatives in wintertime Beijing, China, Atmos. Chem. Phys., 19, 8741–8758, https://doi.org/10.5194/acp-19-8741-2019, 2019.
Gonzalez, C. and Schlegel, H. B.: An improved algorithm for reaction path following, J. Chem. Phys., 90, 2154–2161, https://doi.org/10.1063/1.456010, 1989.
Grimmer, G., Jacob, J., and Noujack, K. W.: Profile of the polycyclic aromatic hydrocarbons from lubricating oils. Inventory by GC/MS – PAH in environmental materials, part 1, Fresen. Z. Anal. Chem., 314, 13–19, https://doi.org/10.1007/bf00468645, 1983.
Harrison, M. A. J., Barra, S., Borghesi, D., Vione, D., Arsene, C., and Olariu, R. I.: Nitrated phenols in the atmosphere: a review, Atmos. Environ., 39, 231–248, https://doi.org/10.1016/j.atmosenv.2004.09.044, 2005.
Huang, M., Wang, Z., Hao, L., and Zhang, W.: Theoretical investigation on the mechanism and kinetics of OH radical with ethylbenzene, Int. J. Quantum. Chem., 111, 3125–3134, https://doi.org/10.1002/qua.22751, 2010.
Iinuma, Y., Brüggemann, E., Gnauk, T., Müller, K., Andreae, M. O., Helas, G., Parmar, R., and Herrmann, H.: Source characterization of biomass burning particles: The combustion of selected European conifers, African hardwood, savanna grass, and German and Indonesian peat, J. Geophys. Res.-Atmos., 112, D08209, https://doi.org/10.1029/2006JD007120, 2007.
Iinuma, Y., Boge, O., Graefe, R., and Herrmann, H.: Methyl-Nitrocatechols: Atmospheric Tracer Compounds for Biomass Burning Secondary Organic Aerosols, Environ. Sci. Technol., 44, 8453–8459, https://doi.org/10.1021/es102938a, 2010.
Jakober, C. A., Riddle, S. G., Robert, M. A., Destaillats, H., Charles, M. J., Green, P. G., and Kleeman, M. J.: Quinone emissions from gasoline and diesel motor vehicles, Environ. Sci. Technol., 41, 4548–4554, https://doi.org/10.1021/es062967u, 2007.
Kahnt, A., Behrouzi, S., Vermeylen, R., Shalamzari, M. S., Vercauteren, J., Roekens, E., Claeys, M., and Maenhaut, W.: One-year study of nitro-organic compounds and their relation to wood burning in PM10 aerosol from a rural site in Belgium, Atmos. Environ., 81, 561–568, https://doi.org/10.1016/j.atmosenv.2013.09.041, 2013.
Kashiwakura, K. and Sakamoto, K.: Emission Characteristics and Cancer Risks of Polycyclic Aromatic Hydrocarbon Emissions from Diesel-fueled Vehicles Complying with Recent Regulations, J. Health Sci., 56, 200–207, https://doi.org/10.1248/jhs.56.200, 2010.
Kitanovski, Z., Shahpoury, P., Samara, C., Voliotis, A., and Lammel, G.: Composition and mass size distribution of nitrated and oxygenated aromatic compounds in ambient particulate matter from southern and central Europe – implications for the origin, Atmos. Chem. Phys., 20, 2471–2487, https://doi.org/10.5194/acp-20-2471-2020, 2020.
Lammel, G., Kitanovski, Z., Kukucka, P., Novák, J., and Wietzoreck, M.: Oxygenated and nitrated polycyclic aromatic hydrocarbons (OPAHs, NPAHs) in ambient air - levels, phase partitioning, mass size distributions and inhalation bioaccessibility, Environ. Sci. Technol., 54, 2615–2625, https://doi.org/10.1021/acs.est.9b06820, 2020.
Li, J., Wang, G., Ren, Y., Wang, J., Wu, C., Han, Y., Zhang, L., Cheng, C., and Meng, J.: Identification of chemical compositions and sources of atmospheric aerosols in Xi'an, inland China during two types of haze events, Sci. Total. Environ., 566, 230–237, https://doi.org/10.1016/j.scitotenv.2016.05.057, 2016.
Li, J. J., Wang, G. H., Wang, X. M., Cao, J. J., Sun, T., Cheng, C. L., Meng, J. J., Hu, T. F., and Liu, S. X.: Abundance, composition and source of atmospheric PM2.5 at a remote site in the Tibetan Plateau, China, Tellus B., 65, 20281, https://doi.org/10.3402/tellusb.v65i0.20281, 2013.
Li, X., Yang, Y., Liu, S., Zhao, Q., Wang, G., and Wang, Y.: Light absorption properties of brown carbon (BrC) in autumn and winter in Beijing: Composition, formation and contribution of nitrated aromatic compounds, Atmos. Environ., 223, 117289, https://doi.org/10.1016/j.atmosenv.2020.117289, 2020.
Li, Y. and Wang, L.: The atmospheric oxidation mechanism of 1,2,4-trimethylbenzene initiated by OH radicals, Phys. Chem. Chem. Phys., 16, 17908–17917, https://doi.org/10.1039/c4cp02027h, 2014.
Li, Y., Bai, X., Ren, Y., Gao, R., Ji, Y., Wang, Y., and Li, H.: PAHs and nitro-PAHs in urban Beijing from 2017 to 2018: Characteristics, sources, transformation mechanism and risk assessment, J. Hazard. Mater., 436, 1–11, https://doi.org/10.1016/j.jhazmat.2022.129143, 2022.
Liang, Y., Wang, X., Dong, S., Liu, Z., Mu, J., Lu, C., Zhang, J., Li, M., Xue, L., and Wang, W.: Size distributions of nitrated phenols in winter at a coastal site in north China and the impacts from primary sources and secondary formation, Chemosphere, 250, 126256, https://doi.org/10.1016/j.chemosphere.2020.126256, 2020.
Lin, P., Liu, J., Shilling, J. E., Kathmann, S. M., Laskin, J., and Laskin, A.: Molecular characterization of brown carbon (BrC) chromophores in secondary organic aerosol generated from photo-oxidation of toluene, Phys. Chem. Chem. Phys., 17, 23312–23325, https://doi.org/10.1039/c5cp02563j, 2015a.
Lin, Y., Ma, Y., Qiu, X., Li, R., Fang, Y., Wang, J., Zhu, Y., and Hu, D.: Sources, transformation, and health implications of PAHs and their nitrated, hydroxylated, and oxygenated derivatives in PM2.5 in Beijing, J. Geophys. Res.-Atmos., 120, 7219–7228, https://doi.org/10.1002/2015JD023628, 2015b.
Lin, P., Bluvshtein, N., Rudich, Y., Nizkorodov, S. A., Laskin, J., and Laskin, A.: Molecular Chemistry of Atmospheric Brown Carbon Inferred from a Nationwide Biomass Burning Event, Environ. Sci. Technol., 51, 11561–11570, https://doi.org/10.1021/acs.est.7b02276, 2017.
Lu, C., Wang, X., Li, R., Gu, R., Zhang, Y., Li, W., Gao, R., Chen, B., Xue, L., and Wang, W.: Emissions of fine particulate nitrated phenols from residential coal combustion in China, Atmos. Environ., 203, 10–17, https://doi.org/10.1016/j.atmosenv.2019.01.047, 2019.
Lu, C., Wang, X., Zhang, J., Liu, Z., Liang, Y., Dong, S., Li, M., Chen, J., Chen, H., Xie, H., Xue, L., and Wang, W.: Substantial emissions of nitrated aromatic compounds in the particle and gas phases in the waste gases from eight industries, Environ. Pollut., 283, 117132, https://doi.org/10.1016/j.envpol.2021.117132, 2021.
Luo, M., Ji, Y., Ren, Y., Gao, F., Zhang, H., Zhang, L., Yu, Y., and Li, H.: Characteristics and Health Risk Assessment of PM2.5-Bound PAHs during Heavy Air Pollution Episodes in Winter in Urban Area of Beijing, China, Atmosphere, 12, https://doi.org/10.3390/atmos12030323, 2021.
Ma, L., Li, B., Liu, Y., Sun, X., Fu, D., Sun, S., Thapa, S., Geng, J., Qi, H., Zhang, A., and Tian, C.: Characterization, sources and risk assessment of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in Harbin, a cold city in Northern China, J. Clean. Prod., 264, 121673, https://doi.org/10.1016/j.jclepro.2020.121673, 2020.
Mayorga, R. J., Zhao, Z., and Zhang, H.: Formation of secondary organic aerosol from nitrate radical oxidation of phenolic VOCs: Implications for nitration mechanisms and brown carbon formation, Atmos. Environ., 244, 117910, https://doi.org/10.1016/j.atmosenv.2020.117910, 2021.
Mohr, C., Lopez-Hilfiker, F. D., Zotter, P., Prévôt, A. S. H., Xu, L., Ng, N. L., Herndon, S. C., Williams, L. R., Franklin, J. P., Zahniser, M. S., Worsnop, D. R., Knighton, W. B., Aiken, A. C., Gorkowski, K. J., Dubey, M. K., Allan, J. D., and Thornton, J. A.: Contribution of Nitrated Phenols to Wood Burning Brown Carbon Light Absorption in Detling, United Kingdom during Winter Time, Environ. Sci. Technol., 47, 6316–6324, https://doi.org/10.1021/es400683v, 2013.
Oda, J., Nomura, S., Yasuhara, A., and Shibamoto, T.: Mobile sources of atmospheric polycyclic aromatic hydrocarbons in a roadway tunnel, Atmos. Environ., 35, 4819–4827, https://doi.org/10.1016/S1352-2310(01)00262-X, 2001.
Ohura, T., Amagai, T., Fusaya, M., and Matsushita, H.: Polycyclic aromatic hydrocarbons in indoor and outdoor environments and factors affecting their concentrations, Environ. Sci. Technol., 38, 77–83, https://doi.org/10.1021/es030512o, 2004.
Pedersen, D. U., Durant, J. L., Taghizadeh, K., Hemond, H. F., Lafleur, A. L., and Cass, G. R.: Human cell mutagens in respirable airborne particles from the northeastern United States. 2. Quantification of mutagens and other organic compounds, Environ. Sci. Technol., 39, 9547–9560, https://doi.org/10.1021/es050886c, 2005.
Peng, B., Dong, Q., Li, F., Wang, T., Qiu, X., and Zhu, T.: A systematic review of polycyclic aromatic hydrocarbon derivatives: occurrences, levels, biotransformation, exposure biomarkers, and toxicity, Environ. Sci. Technol., 57, 15314–15335, https://doi.org/10.1021/acs.est.3c03170, 2023.
Ren, Y., Zhou, B., Tao, J., Cao, J., Zhang, Z., Wu, C., Wang, J., Li, J., Zhang, L., Han, Y., Liu, L., Cao, C., and Wang, G.: Composition and size distribution of airborne particulate PAHs and oxygenated PAHs in two Chinese megacities, Atmos. Res., 183, 322–330, https://doi.org/10.1016/j.atmosres.2016.09.015, 2017.
Ren, Y., Wei, J., Wu, Z., Ji, Y., Bi, F., Gao, R., Wang, X., Wang, G., and Li, H.: Chemical components and source identification of PM2.5 in non-heating season in Beijing: The influences of biomass burning and dust, Atmos. Res., 105412, https://doi.org/10.1016/j.atmosres.2020.105412, 2021.
Ren, Y., Wei, J., Wang, G., Wu, Z., Ji, Y., and Li, H.: Evolution of aerosol chemistry in Beijing under strong influence of anthropogenic pollutants: Composition, sources, and secondary formation of fine particulate nitrated aromatic compounds, Environ. Res., 204, 111982, https://doi.org/10.1016/j.envres.2021.111982, 2022.
Ren, Y., Wang, G., Wei, J., Tao, J., Zhang, Z., and Li, H.: Contributions of primary emissions and secondary formation to nitrated aromatic compounds in themountain background region of Southeast China, Atmos. Chem. Phys., 23, 6835–6848, https://doi.org/10.5194/acp-23-6835-2023, 2023.
Ren, Y., Wu, Z., Ji, Y., Bi, F., Li, J., Zhang, H., Zhang, H., Li, H., and Wang, G.: Non-negligible secondary contribution to brown carbon in autumn and winter: inspiration from particulate nitrated and oxygenated aromatic compounds in urban Beijing, Atmos. Chem. Phys., 24, 6525–6538, https://doi.org/10.5194/acp-24-6525-2024, 2024.
Roman, C., Arsene, C., Bejan, I. G., and Olariu, R. I.: Investigations into the gas-phase photolysis and OH radical kinetics of nitrocatechols: implications of intramolecular interactions on their atmospheric behaviour, Atmos. Chem. Phys., 22, 2203–2219, https://doi.org/10.5194/acp-22-2203-2022, 2022.
Sarigiannis, D. A., Karakitsios, S. P., Dimitrios, Z., Spyridoula, N., and Marianthi, K.: Lung cancer risk from PAHs emitted from biomass combustion, Environ. Res., 137, 147–156, https://doi.org/10.1016/j.envres.2014.12.009, 2015.
Shen, G., Yang, Y., Wang, W., Tao, S., Zhu, C., Min, Y., Xue, M., Ding, J., Wang, B., and Wang, R.: Emission factors of particulate matter and elemental carbon for crop residues and coals burned in typical household stoves in China, Environ. Sci. Technol., 44, 7157–7162, https://doi.org/10.1021/es101313y, 2010.
Shenghur, A., Weber, K. H., Nguyen, N. D., Sontising, W., and Tao, F.-M.: Theoretical Study of the Hydrogen Abstraction of Substituted Phenols by Nitrogen Dioxide as a Source of HONO, J. Phys. Chem. A, 118, 11002–11014, https://doi.org/10.1021/jp508516c, 2014.
Song, M., Liu, Y., Li, X., and Lu, S.: Advances on Atmospheric Oxidation Mechanism of Typical Aromatic Hydrocarbons, Acta Chim. Sin., 79, 1214–1231, https://doi.org/10.6023/a21050224, 2021.
Takano, Y. and Houk, K. N.: Benchmarking the Conductor-like Polarizable Continuum Model (CPCM) for Aqueous Solvation Free Energies of Neutral and Ionic Organic Molecules, J. Chem. Theor. Computat., 1, 70–77, https://doi.org/10.1021/ct049977a, 2005.
Teich, M., van Pinxteren, D., Wang, M., Kecorius, S., Wang, Z., Müller, T., Močnik, G., and Herrmann, H.: Contributions of nitrated aromatic compounds to the light absorption of water-soluble and particulate brown carbon in different atmospheric environments in Germany and China, Atmos. Chem. Phys., 17, 1653–1672, https://doi.org/10.5194/acp-17-1653-2017, 2017.
Turpin, B. J. and Lim, H. J.: Species Contributions to PM2.5 Mass Concentrations: Revisiting Common Assumptions for Estimating Organic Mass, Aerosol Sci. Technol., 35, 602–610, https://doi.org/10.1080/02786820119445, 2001.
Wang, G., Kawamura, K., Xie, M., Hu, S., Gao, S., Cao, J., An, Z., and Wang, Z.: Size-distributions of n-alkanes, PAHs and hopanes and their sources in the urban, mountain and marine atmospheres over East Asia, Atmos. Chem. Phys., 9, 8869–8882, https://doi.org/10.5194/acp-9-8869-2009, 2009.
Wang, G., Zhang, S., Wu, C., Zhu, T., Xu, X., Ge, S., Sun, H., Sun, Z., Wang, J., Ji, Y., Gao, J., Ren, Y., Li, H., Zhang, F., Wang, Y., Seinfeld, J. H., and Hartmann, D.: Atmospheric sulfate aerosol formation enhanced by interfacial anions, PNAS Nexus, 4, pgaf058, https://doi.org/10.1093/pnasnexus/pgaf058, 2025.
Wang, L., Wang, X., Gu, R., Wang, H., Yao, L., Wen, L., Zhu, F., Wang, W., Xue, L., Yang, L., Lu, K., Chen, J., Wang, T., Zhang, Y., and Wang, W.: Observations of fine particulate nitrated phenols in four sites in northern China: concentrations, source apportionment, and secondary formation, Atmos. Chem. Phys., 18, 4349–4359, https://doi.org/10.5194/acp-18-4349-2018, 2018.
Wang, L., Dong, S., Liu, M., Tao, W., Xiao, B., Zhang, S., Zhang, P., and Li, X.: Polycyclic aromatic hydrocarbons in atmospheric PM2.5 and PM10 in the semi-arid city of Xi'an, Northwest China: Seasonal variations, sources, health risks, and relationships with meteorological factors, Atmos. Res., 229, 60–73, https://doi.org/10.1016/j.atmosres.2019.06.014, 2019a.
Wang, T., Zhao, J., Liu, Y., Peng, J., Wu, L., and Mao, H.: PM2.5-Bound Polycyclic Aromatic Hydrocarbons (PAHs), Nitrated PAHs (NPAHs) and Oxygenated PAHs (OPAHs) in Typical Traffic-Related Receptor Environments, J. Geophys. Res.-Atmos., 127, e2021JD035951, https://doi.org/10.1029/2021JD035951, 2022.
Wang, W., Jariyasopit, N., Schrlau, J., Jia, Y., Tao, S., Yu, T.-W., Dashwood, R. H., Zhang, W., Wang, X., and Simonich, S. L. M.: Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the Beijing Olympic Games, Environ. Sci. Technol., 45, 6887–6895, https://doi.org/10.1021/es201443z, 2011.
Wang, X., Gu, R., Wang, L., Xu, W., Zhang, Y., Chen, B., Li, W., Xue, L., Chen, J., and Wang, W.: Emissions of fine particulate nitrated phenols from the burning of five common types of biomass, Environ. Pollut., 230, 405–412, https://doi.org/10.1016/j.envpol.2017.06.072, 2017.
Wang, Y., Hu, M., Li, X., and Xu, N.: Chemical Composition, Sources and Formation Mechanisms of Particulate Brown Carbon in the Atmosphere, Prog. Chem., 32, 627–645, https://doi.org/10.7536/PC190917, 2020 (in Chinese).
Wang, Y., Hu, M., Wang, Y., Zheng, J., Shang, D., Yang, Y., Liu, Y., Li, X., Tang, R., Zhu, W., Du, Z., Wu, Y., Guo, S., Wu, Z., Lou, S., Hallquist, M., and Yu, J. Z.: The formation of nitro-aromatic compounds under high NOx and anthropogenic VOC conditions in urban Beijing, China, Atmos. Chem. Phys., 19, 7649–7665, https://doi.org/10.5194/acp-19-7649-2019, 2019b.
Wei, C., Bandowe, B. A. M., Han, Y., Cao, J., Watson,J. G., Chow, J. C., and Wilcke, W.: Polycyclic aromatic compounds (PAHs, oxygenated PAHs, nitrated PAHs, and azaarenes) in air from four climate zones of China: Occurrence, gas/particle partitioning, and health risks, Sci. Total. Environ., 786, 147234, https://doi.org/10.1016/j.scitotenv.2021.147234, 2021.
Wu, C., Wang, G., Li, J., Li, J., Cao, C., Ge, S., Xie, Y., Chen, J., Li, X., Xue, G., Wang, X., Zhao, Z., and Cao, F.: The characteristics of atmospheric brown carbon in Xi'an, inland China: sources, size distributions and optical properties, Atmos. Chem. Phys., 20, 2017–2030, https://doi.org/10.5194/acp-20-2017-2020, 2020.
Xie, M., Chen, X., Hays, M. D., Lewandowski, M., Offenberg, J. H., Kleindienst, T. E., and Holder, A. L.: Light Absorption of Secondary Organic Aerosol: Composition and Contribution of Nitroaromatic Compounds, Environ. Sci. Technol., 51, 11607–11616, https://doi.org/10.1021/acs.est.7b03263, 2017.
Yhab, D., Xxab, C., Dan, L. E., Xjab, C., Zha, B., Yca, B., Lxa, B., Mla, B., Hong, W. G., and Han, Z.: Air pollution increases human health risks of PM2.5-bound PAHs and nitro-PAHs: a case study in the Yangtze River Delta, China, Sci. Total. Environ., 770, 145402, https://doi.org/10.1016/j.scitotenv.2021.145402, 2021.
Yuan, B., Liggio, J., Wentzell, J., Li, S.-M., Stark, H., Roberts, J. M., Gilman, J., Lerner, B., Warneke, C., Li, R., Leithead, A., Osthoff, H. D., Wild, R., Brown, S. S., and de Gouw, J. A.: Secondary formation of nitrated phenols: insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014, Atmos. Chem. Phys., 16, 2139–2153, https://doi.org/10.5194/acp-16-2139-2016, 2016a.
Yuan, B., Liggio, J., Wentzell, J., Li, S.-M., Stark, H., Roberts, J. M., Gilman, J., Lerner, B., Warneke, C., Li, R., Leithead, A., Osthoff, H. D., Wild, R., Brown, S. S., and de Gouw, J. A.: Secondary formation of nitrated phenols: insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014, Atmos. Chem. Phys., 16, 2139–2153, https://doi.org/10.5194/acp-16-2139-2016, 2016b.
Zhang, J., Yang, L., Mellouki, A., Chen, J., Chen, X., Gao, Y., Jiang, P., Li, Y., Yu, H., and Wang, W.: Diurnal concentrations, sources, and cancer risk assessments of PM2.5-bound PAHs, NPAHs, and OPAHs in urban, marine and mountain environments, Chemosphere, 209, 147–155, https://doi.org/10.1016/j.chemosphere.2018.06.054, 2018.
Zhang, R., Wang, G., Guo, S., L.Zamora, M., Ying, Q., Lin, Y., Wang, W., Hu, M., and Wang, Y.: Formation of Urban Fine Particulate Matter, Chem. Rev., 115, 3803–3855, 2015.
Zhang, Y. Y., Müller, L., Winterhalter, R., Moortgat, G. K., Hoffmann, T., and Pöschl, U.: Seasonal cycle and temperature dependence of pinene oxidation products, dicarboxylic acids and nitrophenols in fine and coarse air particulate matter, Atmos. Chem. Phys., 10, 7859–7873, https://doi.org/10.5194/acp-10-7859-2010, 2010.
Zhao, Y. and Truhlar, D. G.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc., 120, 215–241, https://doi.org/10.1007/s00214-007-0310-x, 2007.