the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Measurement report: Observational insights into the impact of dust transport on atmospheric dicarboxylic acids in ground region and free troposphere
Minxia Shen
Weining Qi
Yali Liu
Yifan Zhang
Wenting Dai
Lu Li
Xiao Guo
Yue Cao
Yingkun Jiang
Qian Wang
Shicong Li
Qiyuan Wang
Dust transport significantly affects downwind aerosol formation and regional climate, yet the evolutionary mechanisms of SOA during this process remain poorly understood. Here, we conducted vertical observations of PM2.5 and size-segregated aerosols at the foot and top of Mount Hua, focusing on C2 formation and its δ13C signatures influenced by dust transport. Under non-dust conditions, PM2.5 and diacid concentrations at the foot were 4.5 and 2.1 times higher than those at the top, indicating stronger anthropogenic influence at lower elevations. Aerosols at the top revealed enhanced photochemical aging, with higher C2 C4 (5.84 vs. 4.74), C3 C4 ratios (1.04 vs. 0.56), and more positive δ13C values (−21.5 ‰ vs. −27.6 ‰). The positive correlation of C2 with ALWC and its consistent size distribution with precursors confirm aqueous-phase oxidation as the dominant formation pathway. During dust events, although PM2.5 concentrations increased, C2 concentrations in PM2.5 decreased by 59 % at the foot and 25 % at the top. Concurrently, the δ13C values of C2 showed a positive shift, particularly at the top (from −21.5 ‰ to −13.2 ‰), suggesting that alkaline dust catalyzes the formation of 13C-enriched oxalate. Size-segregated data revealed a shift of C2 from the fine to the coarse mode, with the coarse-to-fine ratio increasing from 0.3–0.4 to 0.6–1.1. These findings demonstrate that under dust influence, the primary formation pathway of C2 shifts from aqueous-phase oxidation in fine particles to heterogeneous reactions on coarse-particle surfaces. Moreover, this shift is accompanied by a positive shift in the δ13C signature of C2 and is more pronounced at higher altitudes.
- Article
(6798 KB) - Full-text XML
-
Supplement
(1080 KB) - BibTeX
- EndNote
Dust cycling is crucial to Earth's climate system (Maher et al., 2010; Liang et al., 2022). Mineral particles from dust storms absorb and scatter solar radiation (Kumar et al., 2014), altering regional heat balance and cloud properties, which in turn affects precipitation (Mahowald et al., 2014; Kok et al., 2023; Marx et al., 2024; Xu-Yang et al., 2025). They also act as effective ice-nucleating particles (INPs) in mixed-phase clouds, regulating ice formation and the radiation budget (Fan et al., 2016; Vergara-Temprado et al., 2018; Kawai et al., 2021; Chen et al., 2024). Dust particles often contain high levels of salts, bacteria, and heavy metals, posing potential risks to human health and plant growth (Yamaguchi et al., 2016; Luo et al., 2024). Dust not only impairs air quality locally but also undergoes long-range transport, ultimately affecting both hemispheric and global climate systems (Pan et al., 2025). During transport, mineral dust may undergo heterogeneous reactions, forming secondary aerosols that aid cloud formation (Wang et al., 2020; Bikkina et al., 2023). The large surface area of these particles facilitates reactions that alter radiation transfer and photolysis rates (Sullivan and Prather, 2007).
Over the past 500 years, East Asia has been frequently hit by dust storms (Zhang et al., 2021; Wu et al., 2022). The Taklimakan and Gobi Deserts, the primary sources of East Asian dust, emit over 800 million t of dust to downwind areas annually (Sullivan et al., 2007; Wang et al., 2015; Ren et al., 2019; Zhu and Liu, 2024). Northwestern and northern China, frequently experience dusty weather due to these desert emissions (Gui et al., 2022; Liang et al., 2022). In spring 2023, Mongolia contributed over 42 % of the dust concentration in northern China (Chen et al., 2023). Although dust storms are more common in China during spring (Sun et al., 2001), a large-scale, high-intensity dust storm hit northern China on 10 January 2021. This severe dust storm, originating from southern Mongolia and western Inner Mongolia, triggered rapid air quality deterioration across downwind regions. Our synchronized field observations of PM2.5 and size-segregated aerosols at the top of Mount Hua and on the ground in the winter of 2021 successfully captured this large-scale dust event, as shown in Fig. S1 in the Supplement, extensively covering Northern China and the Guanzhong Plain. Liu et al. (2024) compared and analyzed the concentrations and size distributions of water-soluble inorganic ions during dust and non-dust periods, finding that the impact of dust on ground aerosols in the Guanzhong Plain is weaker than that in the free troposphere. Nevertheless, the specific mechanisms through which dust affects organic components, particularly secondary organic aerosol (SOA) and their precursors in the ground and troposphere remain unclear.
To investigate these processes, this study focuses on dicarboxylic acids (diacids), which serve as key tracers for SOA (Xu et al., 2022). As important components of water-soluble organic carbon, diacids are widely distributed in the atmosphere from the surface layer to the free troposphere (Fu et al., 2008; Myriokefalitakis et al., 2011). Conventional theory suggests that aqueous-phase chemical reactions occur predominantly in submicron particles containing water or cloud droplets (Lim et al., 2010; Ervens et al., 2011; Lamkaddam et al., 2021). However, field observations have reported the coexistence of oxalate and nitrate in supermicron particles during dust events (Falkovich and Schkolnik, 2004; Sullivan and Prather, 2007; Wang et al., 2015; Xu et al., 2020). To explain this, Wang et al. (2015) proposed that the reaction of nitric acid and/or nitrogen oxides with dust generates (Ca(NO3)2), which absorbs water vapor to form an aqueous phase on the dust surface. This enables the partitioning of gas-phase water-soluble organic precursors into this aqueous phase, followed by their further oxidation to form oxalic acid (C2). Research by Li et al. (2025) provides direct evidence for this mechanism, showing that aqueous nitrate coatings (Ca(NO3)2), due to their very low deliquescence relative humidity (absorbing water at atmospheric RH > 8 %), effectively promote the formation of aqueous secondary organic aerosols (aqSOA). Thus, aged dust surfaces provide critical reactive interfaces for aqSOA formation.
Tropospheric aerosols in high mountain areas are significantly influenced by long-range transport of surface pollutants, making them more representative of regional atmospheric quality. Our previous study (Shen et al., 2023) demonstrated that summer daytime valley winds on Mount Hua transport organic acids from the foot to top, thereby altering the chemical composition of the free troposphere and establishing distinct formation pathways of C2 at different altitudes. This study examines aerosol vertical distribution characteristics in winter. Low temperatures cause a significant reduction in the boundary layer height over Mount Hua, which inhibits the diffusion of local pollutants to the top. Consequently, the top remains in a free tropospheric environment where aerosols originate primarily from long-range transport from dust source regions (Liu et al., 2024). During the observational period, we documented a major dust event in which particles from dust source regions were directly transported to the top. There, they mixed with local anthropogenic pollutants, triggering complex atmospheric chemical reactions that resulted in notable vertical differences in aerosol chemical properties. However, the influence of heterogeneous reactions on dust aerosol surfaces on the generation of organic acids, particularly their role in modifying C2 formation mechanisms at different altitudes remains unclear. Therefore, using observational data from a typical dust event in winter 2021, this study focuses on examining the impacts of dust transport on the molecular distribution, particle size characteristics of diacids, and the formation mechanisms of C2. The work aims to elucidate the key role of heterogeneous chemical reactions on the surfaces of dust aerosols in the formation of SOA, providing new insights into regional atmospheric chemical processes during dust events.
2.1 Sample collection
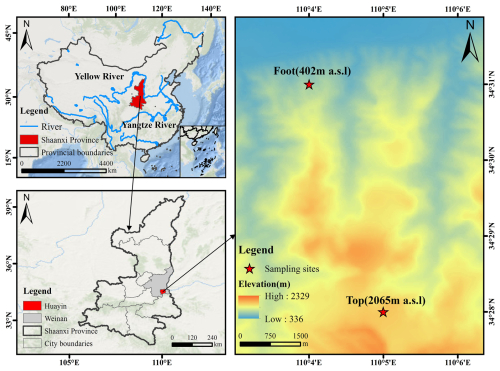
Samples were collected simultaneously at the free troposphere and the ground surface during 17 December 2020 to 12 January 2021. The sampling site at the ground surface is located on Yinquan Road, Huayin City, Weinan (34°31′ N, 110°04′ E; ∼402 m a.s.l) (referred to as “Foot”), while alpine sampling site is located at the summit of the west peak of Mount Hua (34°28′ N, 110°05′ E; ∼2065 m a.s.l.) (referred to as “Top”) (Fig. 1). PM2.5 aerosol samples was collected using medium-flow sampler (HC-1010, China Qingdao Company, China) at a flow rate of 100 L min−1 with a duration of 11 h for each sample during the day (from 08:00 to 19:00 LT) and night (from 20:00 to 07:00 LT the next day). A total of 54 samples each were collected at both the alpine region and ground. The size-segregated samples were collected for ∼71 h in each set using an Andersen multi-stage impactor (Andersen, Thermo electronic, USA) at a flow rate of 28.3 L min−1 with 9 size bins as <0.4, 0.4–0.7, 0.7–1.1, 1.1–2.1, 2.1–3.3, 3.3–4.7, 4.7–5.8, 5.8–9.0 and >9.0 µm, respectively. In a total of 9 sets of size-segregated samples were collected. All the samples were collected onto pre-combusted (450° for 6 h) quartz fiber filters produced by Whatman, UK. After sampling, the filters were stored in −18 °C until analysis.
2.2 Laboratory analysis
2.2.1 Determination of carbonaceous species and water-soluble inorganic ions
The concentrations of organic carbon (OC) and elemental carbon (EC) in PM2.5 were determined using a DRI Model 2001 carbon analyzer (Atmoslytic Inc., USA), following the IMPROVE thermal/optical reflectance (TOR) protocol (Cao et al., 2007). A 0.526 cm2 filter punch was heated stepwise in pure helium (at 120, 250, 450, and 550 °C) followed by heating in a 2 % oxygen/helium atmosphere (at 550, 700, 800 °C). The method detection limits were 0.41 µg cm−2 for OC and 0.03 µg cm−2 for EC.
Water-soluble components were extracted from a quarter of each filter using 40 mL of ultrapure water (Milli-Q, 18.2 MΩ, Merck, France) via a combined process of 1 h ultrasonication and 1 h mechanical shaking. After filtration through a 0.45 µm membrane, the extracts were preserved at 4 °C for subsequent analysis. Water-soluble inorganic ions (NH, K+, Mg2+, Ca2+, Cl−, NO, and SO) were analyzed by ion chromatography (Metrohm 940, Switzerland). Anions and cations were separated using an IonPac AS23 column and an IonPac CS12A column, with 9.0 mM Na2CO3 and 20 mM methanesulfonic acid as eluents, respectively (Zhang et al., 2011). Concurrently, water-soluble organic carbon (WSOC) was determined using a total organic carbon (TOC) analyzer (TOC-L CPH, Shimadzu, Japan) (Li et al., 2019). The detection limits for inorganic ions ranged from 0.008 to 0.022 µg m−3, while those for total carbon (TC) and inorganic carbon (IC) were 0.07 and 0.08 mg L−1, respectively.
Aerosol liquid water content (ALWC) was calculated using the ISORROPIA-II model (Fountoukis and Nenes, 2007), based on the concentrations of water-soluble inorganic ions and meteorological parameters including relative humidity (RH) and temperature (T). Meteorological data for the top and the foot were obtained from the Mount Hua Meteorological Station and the Huayin Meteorological Bureau, respectively. All statistical analyses were performed using SPSS. The data associated with this research can be accessed via the Zenodo repository (Shen et al., 2025).
2.2.2 Determination of dicarboxylic acids and related compounds
The analysis of diacids, keto-carboxylic acids, and α-dicarbonyls in PM2.5 and size-segregated aerosols was conducted based on an established derivatization method (Shen et al., 2022). A quarter of each filter was extracted by ultrasonicating in ultrapure water (Milli-Q, 18.2 MΩ, Merck, France) for three sequential 15 min intervals. To maximize the recovery of low-molecular-weight acids (e.g., C2), the extracts were alkalized to pH 8.5–9.0 with 0.1 M KOH prior to the concentration step. The aqueous extracts were concentrated to near-dryness under vacuum in a water bath maintained at 55 °C (evaporation was halted immediately after the disappearance of the last drop of solvent). The dried residues were derivatized with 14 % BF3/n-butanol at 100 °C for 1 h to convert carboxyl groups into dibutyl esters and oxo groups into dibutoxyacetals. After the reaction, the derivatives were sequentially dissolved in n-hexane, acetonitrile, and pure water, followed by triple extraction via vortex mixing to remove water-soluble inorganic substances. The aqueous lower layer was removed using a Pasteur pipette. The n-hexane layer was concentrated by rotary evaporation and N2 blow-down, reconstituted in 100 µL n-hexane, and finally analyzed by GC-FID (HP 6890, Agilent Technologies, USA) (Wang et al., 2012). The recovery of the target compounds was 83 % for C2 and ranged from 87 % to 110 % for other diacids.
Stable carbon isotopic compositions (δ13C) of C2 were determined using gas chromatography–isotope ratio mass spectrometry (GC-IRMS; Delta V Advantage, Thermo Fisher Scientific, Franklin, MA, USA) following established protocols (Kawamura and Watanabe, 2004). To ensure analytical precision (standard deviation < 0.2 ‰), derivatized samples were analyzed in triplicate. Final δ13C values of free oxalic acid were calculated via mass-balance correction to account for isotopic contributions from the BF3/n-butanol derivatizing agent.
3.1 Vertical differences in PM2.5 chemical composition at the foot and top of Mount Hua during non-dust periods
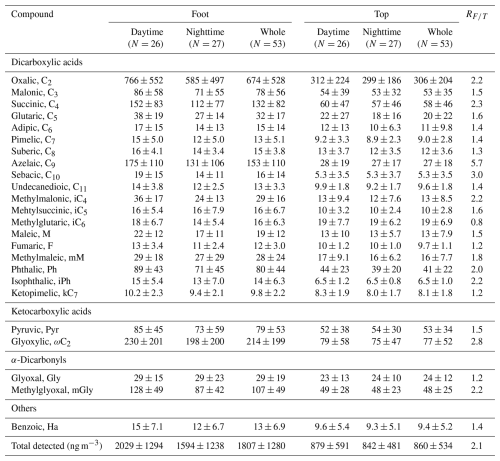
During non-dust periods, significant vertical differences were observed in the chemical composition of PM2.5 between the foot and top of Mount Hua. The average PM2.5 concentration at the foot (127±48 µg m−3) was 4.5 times higher than that at the top (28±14 µg m−3), with a mean difference of 99 µg m−3 (95 % CI: 86 to 113; t(61.05)=14.60, p<0.001) (Tables S1 and S3a in the Supplement). Carbonaceous aerosols and water-soluble ions were significantly enriched at the foot (all p<0.001; Table S3a). As key components of WSOC (Kawamura and Bikkina, 2016; Yang et al., 2020), the total concentration of diacids at the foot (1807±1280 ng m−3) was approximately 2.1 times higher than those at the top (860±534 ng m−3; Table 1), with a mean difference of 718 ng m−3 (95 % CI: 453 to 983; t(69.15)=5.40, p<0.001; Table S3b). The spatial difference was most pronounced for azelaic acid (C9), a biomarker of biomass burning (Kalogridis et al., 2018; Shen et al., 2022), with the concentration at the foot (153±110 ng m−3) being 5.7 times higher than that at the top (27±18 ng m−3; Table 1), corresponding to a mean difference of 126 ng m−3 (95 % CI: 95 to 156; t(54.77)=8.24, p<0.001; Table S3b). Consequently, the contribution of C9 to total diacids was substantially greater at the foot (8.5 %) than at the top (3.2 %) (Fig. 2b).
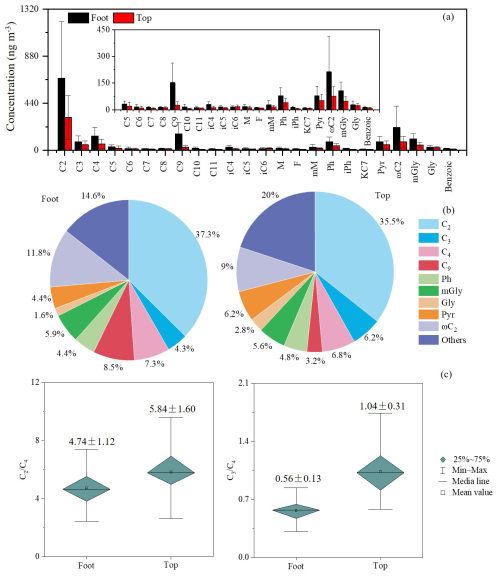
Figure 2Molecular distribution of dicarboxylic acids and related compounds (a), relative percentages of major dicarboxylic acids (b), and ratios of C2 C4 and C3 C4 (c) at the foot and top of Mount Hua during non-dust periods.
Correlation analysis of PM2.5 and its chemical components revealed no significant relationship between the two sites during winter, contrasting with the positive correlations observed in summer (Shen et al., 2023). Combined with ion composition and back-trajectory analysis from Liu et al. (2024), these findings indicate that pollutants at the top primarily originate from regional transport from the northwestern direction with minimal vertical mixing, while components at the foot of the mountain were mainly controlled by local emission sources.
Day-night differences provide further evidence supporting this conclusion. At the foot of the mountain, PM2.5, ionic components, and carbonaceous components (except for NO and NH) all showed significant day–night differences (p<0.05; Table S4a). Among the diacids, methylglyoxal (mGly) exhibited the most pronounced day–night differences (daytime: 128±49 ng m−3, 47 % higher than nighttime: 87±42 ng m−3, p<0.001; Fig. S2, Table S4b). As the terminal product of diacids photo-oxidation (Kawamura and Sakaguchi, 1999), C2 also displayed marked day–night differences (daytime: 766±552 ng m−3 vs. nighttime: 585±497 ng m−3, p=0.023), reflecting strong anthropogenic influence on ground-level photochemistry. In contrast, C2 at the top of the Mount Hua (daytime: 312±224 ng m−3 vs. nighttime: 299±186 ng m−3; p=0.941) and its precursors showed no clear day–night differences (p=0.341–0.917; Table S4c), consistent with the patterns of PM2.5 (p=0.979), OC (p=0.766), and other major components (Table S4a). Such stability is a typical characteristic of high-altitude sites located above the planetary boundary layer, primarily governed by regional transport processes (Fu et al., 2010; Li et al., 2012; Meng et al., 2014), indicating that aerosol processes in the free troposphere differ from those at ground level. In this high-altitude environment, in-cloud processes represent a key pathway for aqSOA formation. Studies have shown that C2 mainly originates from the in-cloud oxidation of precursors such as glyoxal (Gly) and isoprene (Warneck, 2003; Lim et al., 2005; Carlton et al., 2006), a mechanism supported by global model simulations (Myriokefalitakis et al., 2011). Additionally, the photochemical decomposition of C2 following its association with Fe-containing particles in clouds (Zhang et al., 2019) also contributes to the stable distribution of organic acid concentrations at high altitudes.
Aerosol aging indicators further confirmed distinct oxidation processes between different altitudes. According to existing research, succinic acid (C4) can be via hydroxylation to generate C2 and malonic acid (C3), while C3 can be further converted to C2 through intermediates such as hydroxymalonic acid or ketomalonic acid (Kawamura and Ikushima, 1993; Kunwar and Kawamura, 2014; Hoque et al., 2017). Therefore, the C2 C4 and C3 C4 ratios are widely used as effective indicators for assessing the extent of photochemical aging in organic aerosols (Kawamura and Bikkina, 2016; Meng et al., 2018; Shen et al., 2022). In this study, the C2 C4 ratio at the top of Mount Hua was 5.84±0.32, and the C3 C4 ratio was 1.04±0.08, both higher than the corresponding values at the foot (4.74±0.28 and 0.56±0.05, respectively; Fig. 2c). These results are consistent with reports from high-altitude areas such as Mount Tai (Wang et al., 2009; Meng et al., 2018) and Mount Hua (Meng et al., 2014), collectively confirming that the atmosphere undergoes more significant photochemical aging during long-range transport at high altitudes.
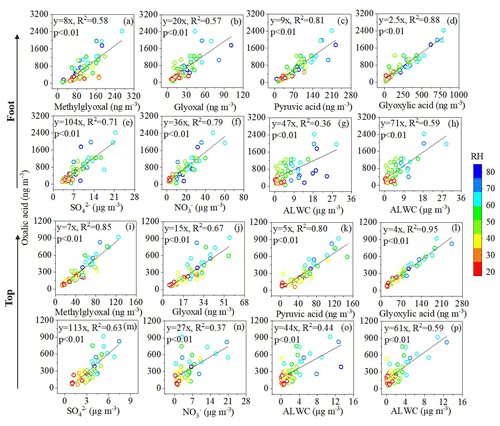
Figure 3Correlation between C2 and its key precursors, sulfate (SO), nitrate (NO), and aerosol liquid water content (ALWC) at the foot and top of Mount Hua with varying relative humidity (RH).
This study compared the correlations between C2 and its key precursors at the foot and top of Mount Hua (Fig. 3a–d and i–l), revealing differences in atmospheric oxidation pathways across altitudes. Building on this, the synergistic effects of inorganic ions and ALWC on C2 formation were investigated (Fig. 3e–h and m–p), thereby elucidating the aqueous-phase reaction mechanisms and the role of environmental factors such as humidity in C2 generation. Results showed that C2 exhibited the strongest correlation with glycolic acid (ωC2) (R2=0.88 at the foot, R2=0.95 at the top, p<0.01; Fig. 3d and l). Other key precursors included Gly (R2=0.57–0.67, p<0.01; Fig. 3b and j), mGly (R2=0.58–0.85, p<0.01; Fig. 3a and i), and pyruvic acid (Pyr: R2=0.80–0.81, p<0.01; Fig. 3c and k). These correlation characteristics confirm that aqueous-phase oxidation serves as the primary formation pathway for C2 under non-dust conditions (Deshmukh et al., 2017; Du et al., 2022). The consistently higher correlation coefficients at the top further substantiate the enhancing effect of prolonged atmospheric processes on secondary organic aerosol formation.
Contrary to previous studies (Wang et al., 2012; Meng et al., 2018), we found that C2 correlated more strongly with NO (R2=0.79, p<0.01; Fig. 3f) than with SO (R2=0.71, p<0.01; Fig. 3e) at the foot. The difference mainly stems from strong influences of local anthropogenic emissions (e.g., traffic and industrial activities) at the foot, which provide abundant NOx and lead to an increased proportion of NO in PM2.5. Since NO is more hygroscopic than SO, its elevated concentration further enhances ALWC, thereby promoting aqSOA formation and intensifying heterogeneous reaction processes (Huang et al., 2025). In contrast, at the top of Mount Hua, C2 exhibited a higher correlation with SO (R2=0.63, p<0.01; Fig. 3m) than with NO (R2=0.37, p<0.01; Fig. 3n). This phenomenon is closely related to the active in-cloud processes in the free troposphere mentioned above. SO at the top primarily originates from the oxidation of SO2 within cloud droplets (Yermakov et al., 2023), a process dominated by heterogeneous reactions (Wang et al., 2025). Meanwhile, cloud droplets also provide key reaction media for the aqueous-phase photooxidation of C2 precursors such as Gly and ωC2 (Warneck, 2003).
Table 1Average concentrations of dicarboxylic acids and related compounds in PM2.5 at the foot and top of Mount Hua during non-dust periods.
ALWC exhibited a humidity-dependent effect on C2 formation. Under RH < 75 %, ALWC was positively correlated with C2 concentration (R2=0.36–0.44, p<0.01; Fig. 3g and o), reflecting the promotion of precursor dissolution and oxidation by the expansion of the aqueous phase. However, when RH exceeded 75 %, supersaturation shifted the gas-particle partitioning equilibrium, causing C2 concentrations to decrease with increasing ALWC. After excluding high-humidity data, the correlation between ALWC and C2 significantly strengthened (R2=0.59, p<0.01; Fig. 3h and p), confirming that aqueous-phase oxidation is the primary pathway for C2 formation during non-dust periods. This finding complements the research by Yang et al. (2022) on the synergistic effects of humidity and pH, jointly revealing the complex regulatory mechanisms of C2 formation under different humidity conditions.
3.2 Impact of dust transport on PM2.5 chemical composition
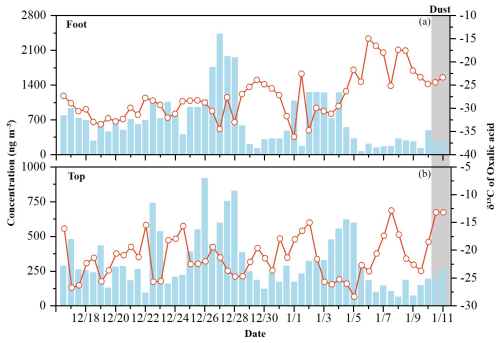
On 10 January 2021, an extensive and intense dust storm, driven by successive cold fronts and sustained high-velocity winds, swept across northern China, triggering a dramatic surge in PM2.5 concentrations (Fig. S3). At the foot of Mount Hua, PM2.5 concentrations rapidly rose from 95 to 457 µg m−3 within 24 h, reaching 3.4 times the non-dust average (127±48 µg m−3). Concurrently at the top, PM2.5 climbed from 46 to 165 µg m−3, representing a 5.9-fold rise compared to typical conditions (28±14 µg m−3). The cleaner atmospheric environment at the top amplified the relative impact of dust transport, while at the foot, existing local pollution partially masked the dust contribution. This contrast highlights the altitude-dependent response to dust events, with the top showing greater sensitivity due to its lower background pollution levels.
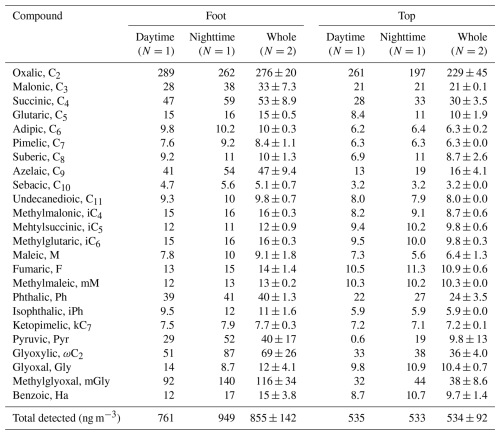
Table 2Average concentrations of dicarboxylic acids and related compounds in PM2.5 at the foot and top of Mount Hua during dust events.
HYSPLIT trajectory analysis revealed the dust originated from the Inner Mongolia-Gansu arid region and was transported along a northwest path to the study area (Fig. S4). Dust transport was closely linked to atmospheric circulation, especially in the troposphere, where changes in wind speed play a key role in dust dispersion (Yang et al., 2017). During the dust period, wind speeds at the foot increased from 2.0 to 5.3 m s−1, and high-altitude wind speeds reached 12.2 m s−1, higher than the average wind speed during non-dust periods (5.4±3.0 m s−1).
Although PM2.5 absolute concentrations rose during the dust period, component concentration changes at the two sites differed markedly. At the foot of the Mount Hua, EC concentrations remained relatively stable (4.5±2.1 during non-dust vs. 4.8±1.8 µg m−3 during dust; Table S2). OC increased from 17±8.0 to 19±4.6 µg m−3, but its mass fraction in PM2.5 decreased substantially from 13.4 % to 4.4 %, reflecting the overwhelming contribution of mineral dust. The top exhibited different characteristics, with both EC and OC concentrations doubling from 0.9 to 1.8 µg m−3 and 4.7 to 9.4 µg m−3 respectively. OC maintained a higher mass fraction of 6.5% compared to 4.4 % at the foot. These patterns indicate efficient mixing of dust with anthropogenic carbonaceous aerosols during long-range transport, coupled with more vigorous secondary formation processes in the free troposphere (Wang et al., 2023; Zheng et al., 2024).
Concentrations of mineral components like calcium and magnesium ions (Ca2+ and Mg2+) rose (foot: 1.8 to 7.7 µg m−3; top: 0.7 to 3.2 µg m−3), confirming their established role as reliable tracers of dust emissions (Li et al., 2016; Liu et al., 2024). SO concentrations also increased at both sites (foot: 5.8 to 10.0 µg m−3; top: 3.8 to 8.7 µg m−3). This increase can be attributed to both the release of inherent sulfate species in dust (e.g., CaSO4) (Wu et al., 2012) and heterogeneous reactions on dust particle surfaces, where transition metals such as Fe (III) and Mn (II) catalyze the conversion of SO2 to SO (Harris et al., 2013; Myriokefalitakis et al., 2022).
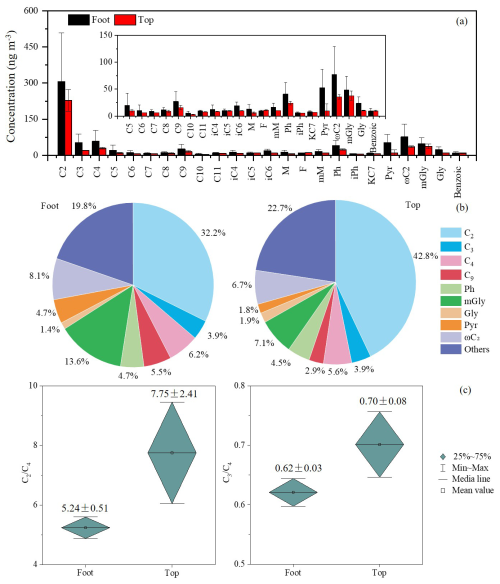
Figure 4Molecular distribution of dicarboxylic acids and related compounds (a), relative percentages of major dicarboxylic acids (b), and ratios of C2 C4 and C3 C4 (c) at the foot and top of Mount Hua during dust events.
Dust transport altered the concentrations and molecular distribution of diacids and their precursors. Although C2 remained the most concentrated acidic molecule during dust periods, its absolute concentration decreased noticeably. At the foot of Mount Hua, C2 concentrations dropped from 674±528 ng m−3 (non-dust periods) to 276±20 µg m−3 (dust periods; Table 2), decreasing by 59 %. At the top of Mount Hua, C2 concentrations decreased from 306±204 to 229±45 ng m−3 (Table 2), a reduction of 25 %. Severe ozone (O3) pollution was present in this dust storm event, and the particulate eruption promoted the generation and dispersion of O3 pollutants. O3 concentrations at the foot increased sharply from 15 to 62 µg m−3 (Fig. S3), much higher than the non-dust average of 26±19 µg m−3. The dust's extinction effect likely reduced aerosol optical thickness, enhancing surface UV radiation. Combined with a local temperature rise ( °C), this probably triggered free-radical chain reactions, promoting the heterogeneous oxidation of C2 on mineral surfaces (Usher et al., 2003; Lu et al., 2023). Notably, the proportion of C2 in total diacids exhibited contrasting trends at the two sites, decreasing from 37.3 % to 32.2 % at the foot and increasing from 35.5 % to 42.8 % at the top (Fig. 4c). This divergence could be closely related to the humidity levels at the two sites. During the dust event, the lower relative humidity at the foot (RH = 24±8.5 %) suppressed aqueous-phase oxidation, whereas the higher humidity at the top (RH = 44±11 %) favored C2 formation through such reactions. Additionally, variations in aerosol sources, transport pathways, aging processes, and potential contributions from other chemical reactions may also have influenced C2 generation.
As a major atmospheric keto acid and key precursor of C2 (Kawamura et al., 2012, 2013), ωC2 ranked second among the acids detected at both sites during non-dust periods, with concentrations of 214±199 ng m−3 at the foot and 77±52 ng m−3 (Table 1) at the top of Mount Hua. However, during dust periods, mGly became the second most abundant acid at both sites due to its enrichment on dust-particle surfaces. At the foot, mGly concentration reached 116±34 ng m−3 (Table 2; 13.6 % of total diacids), up from 5.9 % in non-dust periods. At the top, mGly concentration was 38±9 ng m−3 (Table 2; 7.1 % of total diacids) (Fig. 4b), slightly higher than the non-dust 5.6 %. Phthalic acid (Ph), a photo-oxidation product of naphthalene and other aromatic hydrocarbons, primarily originates from industrial processes and incomplete combustion of coal in heavy and diesel vehicles (Ho et al., 2006). At both foot and top of Mount Hua, the proportion of Ph remains relatively stable, accounting for approximately 4 % during both dust and non-dust periods, indicating that its sources are stable and closely related to regional industrial activities and traffic emissions. During dust periods, the C2 C4 ratios at the foot and top of Mount Hua rose to 5.24 and 7.75 (Fig. 4c), showing stronger aerosol aging. However, the C3 C4 ratio at the top of Mount Hua dropped to 0.70, which might result from the combined effects of selective adsorption of C2 onto dust particles and enhanced photolysis of C3 on mineral dust surfaces.
3.3 Size distribution characteristics of diacids and related compounds during non-dust and dust periods
During non-dust periods, the size distribution of C2 at both the foot and top of Mount Hua exhibited a distinct bimodal distribution (Fig. 5a and 5(a)), characterized by a primary peak in the fine particle mode (0.4–1.1 µm) and a secondary peak in the coarse particle mode (4.7–5.8 µm). Fine particles, with their greater specific surface area and hygroscopic nature, provide a conducive liquid-phase environment that enhances the oxidation of precursors such as mGly and Pyr, leading to C2 formation (Ervens et al., 2011; Wang et al., 2015). The high correlation between C2 and secondary inorganic ions (SO, NO, NH) (with R2 values of 0.92–0.95 at the foot and 0.48–0.92 at the top, p<0.01; as shown in Fig. 6) supports this mechanism, confirming that C2 formation during non-dust periods primarily relies on liquid-phase oxidation reactions on fine particle surfaces. The lower R2 values at the top may be due to the greater influence of long-range transport at high-altitude sites, resulting in more complex sources of precursors. C2 in the coarse particle mode likely originates from direct adsorption of biogenic emissions (such as plant waxes) or heterogeneous oxidation of gas-phase precursors on mineral dust surfaces (Wang et al., 2012).
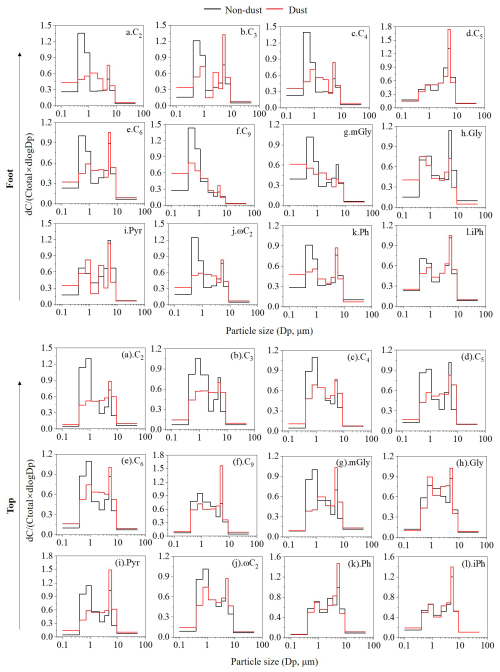
Figure 5Size distribution of dicarboxylic acids at the foot and top of Mount Hua during non-dust and dust periods.
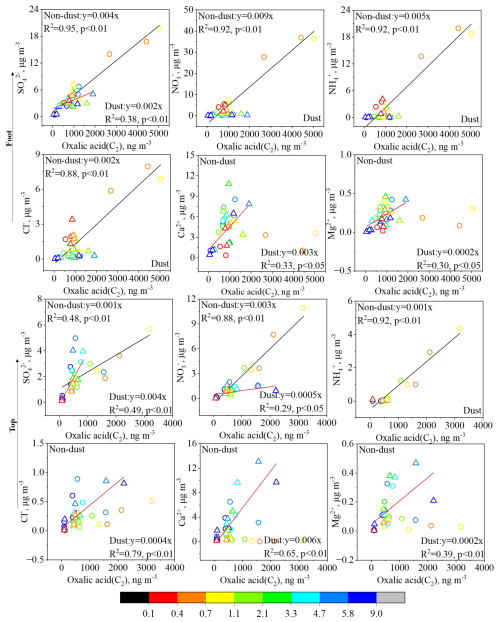
Figure 6Correlation of C2 with water-soluble ions at the foot and top of Mount Hua during non-dust and dust periods (circles in the figure represent non-dust periods, and triangles represent dust periods).
The distribution patterns of short-chain diacids, such as C3 and C4 are similar to that of C2 (Fig. 5b–c and 5(b)–(c)), with primary peaks at 0.4–1.1 µm and secondary peaks at 4.7–5.8 µm, indicating that these acids mainly come from fine particles. In contrast, glutaric (C5) acid shows distinct distribution characteristics at the two sites. At the foot, it exhibits a unimodal distribution in the coarse particles (4.7–5.8 µm) (Fig. 5d), while at the top, it displays a bimodal distribution (0.4–1.1 and 4.7–5.8 µm) (Fig. 5(d)). This significant difference in modal structure suggests that different atmospheric processes govern the behavior of C5 at different altitudes. Adipic (C6) acid shows a bimodal distribution at both sites, probably resulting from the oxidation of cyclohexene (in the fine mode) or adsorption of gas-phase precursors on coarse particle surfaces (Deshmukh et al., 2016). Azelaic (C9) acid is enriched only in fine particles at the foot, which may be closely related to the oxidation of unsaturated fatty acids emitted from biomass burning in northern regions during winter (Deshmukh et al., 2016). The coarse particle fraction of Ph likely forms through gas-phase adsorption, consistent with the strong adsorption characteristics of coarse particles reported by Kanellopoulos et al. (2021).
The particle size distributions of mGly (Fig. 5g and 5(g)) and ωC2 (Fig. 5j and 5(j)) showed remarkable consistency with that of C2 (Fig. 5a and 5(a)), providing direct evidence that aqueous-phase oxidation serves as the predominant formation pathway for C2. Pyr exhibited distinct altitudinal variation in its distribution characteristics. At the top of Mount Hua, Pyr displayed a similar size distribution pattern to C2, indicating their shared photochemical origin. In contrast, at the foot, Pyr demonstrated enrichment in coarse particles (4.7–5.8 µm), likely attributable to heterogeneous reactions of gaseous precursors from local coal combustion on mineral dust surfaces. Gly also exhibits a peak in coarse particles, likely due to its strong adsorption and chemical stability on particle surfaces. The diacids concentrations are consistently lower at the top compared to the foot, which is closely tied to substantial local emissions at the lower elevation. As shown in Fig. 6, the coal combustion and biomass burning tracer Cl− demonstrates an exceptionally strong correlation with C2 (R2=0.88, p<0.01) at the foot.
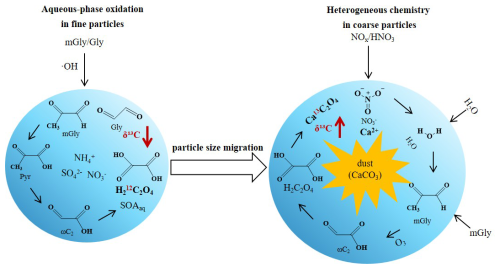
The dust transport process impacted the size distributions of diacids in aerosols (Fig. 5). At the foot of the mountain, the C2 concentration in fine particles (≤2.1 µm) decreased from 8662 to 4880 ng m−3 (a reduction of 43.7 %) during dust events, while the concentration in coarse particle (>2.1 µm) increased from 2718 to 2843 ng m−3 (an increase of 4.6 %) (Table 3). A more pronounced change was observed at the high-altitude top of Mount Hua, where the C2 concentration in coarse particles (2301 ng m−3) exceeded that in fine particles (2161 ng m−3) during dust events, indicating a shift in the dominant particle size distribution from fine to coarse modes. This shift in particle size distribution can be attributed to the formation of Ca(NO3)2 coatings resulting from the reaction between calcium carbonate and NO during dust aging (Li and Shao, 2009; Zhi et al., 2025). These hygroscopic coatings create favorable conditions for the adsorption and oxidation of gaseous organic compounds, thereby promoting the formation of SOA on the surfaces of coarse particles. Research by Li et al. (2025) further confirms that aqSOA formed on dust surfaces can effectively enhance SOA production and drive a transition in the size distribution from the submicron to the supermicron range, which is highly consistent with the observational results of this study. Size-segregated ion data (Fig. S5) provide direct evidence for the above mechanism. Ca2+ was primarily present in the coarse mode (3.3–5.8 µm), while during dust events, NO at the top migrated from the fine mode (0.4–1.1 µm) to the coarse mode (3.3–5.8 µm) and coexisted with Ca2+ in the same size range, strongly supporting the formation of Ca(NO3)2 coatings on dust particle surfaces. In contrast, at the foot of the mountain, although the concentration of NO decreased, it remained predominantly in the fine mode. This spatial difference may stem from more thorough aging and reactions of aerosols at the top due to longer transport times. Meanwhile, the foot is influenced by local pollution, resulting in higher background NO concentrations and competitive reactions with components such as SO, which may collectively delay the distinct shift of NO to the coarse mode.
Table 3Comparison of concentrations of dicarboxylic acids and related compounds in particulate matter of different particle size ranges (≤2.1 and >2.1 µm) at the foot and top of Mount Hua during non-dust and dust periods.
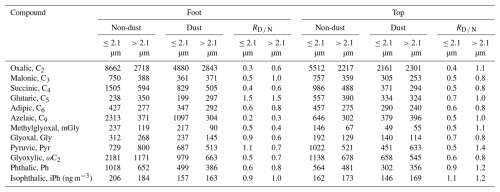
Analysis of the dust/non-dust concentration ratio () revealed values of 0.3 and 0.6 for fine and coarse particles at the foot, respectively, while these values reached 0.4 and 1.1 at the top, indicating that dust processes have more impact on the particle size distribution of diacids at high-altitude regions. Further investigations confirmed enrichment of C2 precursors (mGly, Pyr, and ωC2) in the coarse particle fraction (>2.1 µm). Observational data from the top of Mount Hua revealed that the concentration ratio () of these precursors in coarse particles reaches 0.8–1.4 during dust periods, higher than the 0.5–0.7 ratio in fine particles (≤2.1 µm), demonstrating the crucial contribution of heterogeneous oxidation on dust particle surfaces to C2 formation. Throughout dust episodes, the particle size distribution patterns of diacids (C2–C6) consistently displayed a pronounced shift from fine to coarse particles (4.7–5.8 µm) (Fig. 5). Notably, concentrations of the biomass burning tracer C9 decreased during dust episodes, while Ph and isophthalic acid (iPh) showed distinct peaks in the coarse particle mode. This phenomenon indicates that dust particles effectively scavenge gaseous pollutants through strong adsorption, thereby suppressing the formation of local fine-mode SOA. However, these gaseous precursors adsorbed onto coarse particle surfaces can still undergo heterogeneous oxidation reactions to form SOA.
During dust events, the correlation between C2 and mineral ions (Ca2+, Mg2+) showed significant enhancement (with R2 values of 0.33, 0.30; p<0.05 at the foot and 0.65, 0.39; p<0.01 at the top). This finding showed excellent agreement with the recent research results of Li et al. (2025), who similarly observed stronger correlations between Ca2+ and C2 (R2=0.46–0.95) in the coarse particle phase. This arises as organic acids like C2 in aged carbonate-containing dust particles react with carbonates to form stable salts (Ervens et al., 2011; Lim et al., 2010). This process inhibits the volatility of organic acids and stabilizes them in the coarse particle phase. Furthermore, due to differences in rock types and weathering processes, Asian dust particles inherently contain higher concentrations of alkaline metal elements (Ca and Mg) compared to dust from other regions (Yu et al., 2025). The size-resolved correlations between C2, Ca2+, and Mg2+ (Fig. 6), further support this conclusion. During dust events, the concentrations of C2, Ca2+, and Mg2+ exhibited synchronous increases with increasing particle size. In contrast, during non-dust periods, the peak concentration of C2 occurred in the fine particle size range, while mineral ion concentrations did not show corresponding increases. As described in Sect. 3.2, dust events led to reductions in C2 concentrations in PM2.5 (foot: 674±528 ng m−3 during non-dust periods vs. 276±20 ng m−3 during dust periods; top: 306±204 ng m−3 during non-dust periods vs. 229±45 ng m−3 during dust periods).
Overall, the transformation mechanisms of C2 and its precursors underwent alterations during dust events, shifting from aqueous-phase oxidation dominated in fine particles during non-dust periods to heterogeneous oxidation on coarse particle surfaces as the primary pathway during dust episodes. Regional comparative analysis further revealed that atmospheric chemical processes at the mountain foot were mainly influenced by local emission sources such as coal combustion and biomass burning, whereas the summit site more clearly reflected the complex interactions between long-range dust transport and regional atmospheric processes.
3.4 Stable carbon isotopes (δ13C) of oxalic acid
Synchronized observations at the foot and top of Mount Hua (2065 m a.s.l.) revealed an inverse correlation between C2 concentration and δ13C values in PM2.5 (Fig. 7a and b). When C2 concentration at the foot increased to 2424 ng m−3, its δ13C value decreased to −34.5 ‰, while at the top, a concentration of 917 ng m−3 corresponded to a δ13C of −24.7 ‰. Conversely, during low concentration periods, δ13C at the foot rose to −21.9 ‰ (258 ng m−3) and at the top to −17.2 ‰ (63 ng m−3). This systematic variation provides clear evidence for kinetic carbon isotope fractionation during atmospheric aqueous-phase oxidation processes. Specifically, volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOCs) with lower δ13C values preferentially react to form aqSOA, resulting in 13C-depleted products (Xu et al., 2022).
Spatially, the δ13C values of C2 in PM2.5 at the top of Mount Hua (−28.4 ‰ to −12.8 ‰, mean −21.5 ‰) were higher than those at the foot (−36.2 ‰ to −14.9 ‰, mean −27.6 ‰). This vertical gradient primarily results from long-range transport of aerosols at high altitudes coupled with deep oxidation processes. The higher C2 C4 ratio observed at Mount Hua (5.84 vs. 4.74 at the foot) indicates more pronounced atmospheric aging characteristics. This distribution pattern originates from prolonged photochemical oxidation during long-range transport, where preferential cleavage of 12C–12C bonds (due to their lower bond energy) leads to relative 13C enrichment in residual C2. In contrast, surface aerosols dominated by local fresh emissions undergo shorter oxidation periods and exhibit weaker isotope fractionation effects. Moreover, δ13C can provide insights into the sources of aerosols, Pavuluri and Kawamura (2016) found that biogenic aerosols had higher mean δ13C values (−15.8 ‰) than anthropogenic sources (−19.5 ‰). Our study shows that foot aerosols were mainly influenced by anthropogenic sources (biomass burning and coal combustion), while the top was more affected by natural sources due to richer vegetation, with long-range transport potentially weakening local isotope fractionation effects.
During dust events, C2 concentrations in PM2.5 showed decreasing trends, declining from 674±528 to 276±20 ng m−3 at the foot and from 306±204 to 229±45 ng m−3 at the top of Mount Hua. Concurrently, the δ13C values of C2 exhibited distinct positive shifts, increasing from −27.6 ‰ to −23.9 ‰ at the foot and from −21.5 ‰ to −13.2 ‰ at the top. This phenomenon reveals key chemical transformation mechanisms during dust transport: alkaline mineral surfaces promote heterogeneous catalytic oxidation of C2 precursors, with coarse-mode mineral components (Ca2+ Mg2+ etc.) preferentially combining with 13C-labeled C2 to form stable compounds like calcium oxalate. This mechanism is strongly supported by observational data during dust events, both oxalic acid and Ca2+ Mg2+ concentrations showed increases with growing particle size, while exhibiting high correlations (R2=0.30–0.65) during dust periods. Simultaneously, 12C-enriched C2 produced on fine particle surfaces moves to coarse particles through gas-particle conversion or coagulation, resulting in 13C-enriched residues remaining in fine particles. As demonstrated by the aforementioned research findings, the concentration of oxalic acid in coarse particles showed a marked increase during dust events, with this variation being particularly pronounced at the top. These two synergistic processes collectively altered both aerosol size distribution and isotopic composition characteristics.
Based on synchronous observations of PM2.5 and size-segregated aerosols from surface to mountain sites, this study reveals the influence of dust transport on the formation pathways and δ13C signature of C2 at different altitudes. The results indicate that during non-dust periods, C2 is primarily generated through aqueous-phase oxidation in fine particles, whereas during dust periods, its formation shifts to heterogeneous chemical reactions on coarse-particle surfaces (Fig. 8).
At high-altitude sites, the influence of dust on aerosols was particularly pronounced, as evidenced by enhanced aerosol aging indicators (C2 C4 ratio increased to 7.75), enriched δ13C (+8.3 ‰ compared to non-dust periods), and a distinct shift of C2 from the fine to the coarse mode. This redistribution resulted in a higher concentration of C2 in coarse particles (2301 ng m−3) than in fine particles (2161 ng m−3), producing a coarse-to-fine particle ratio of 1.1. The shift in particle size distribution was closely associated with the formation of Ca(NO3)2 coatings during dust aging. These hygroscopic coatings provide active interfaces for the adsorption and oxidation of gaseous precursors, thereby promoting the formation of C2 and other SOA on coarse particle surfaces. The migration of NO from the fine mode (0.4–1.1 µm) to the coarse mode (3.3–5.8 µm) in the presence of Ca2+ provides direct evidence for the formation of such coatings. The pronounced enrichment of δ13C further supports this reaction pathway, which is attributed to the synergistic effects of surface-catalyzed oxidation (favoring 13C retention) and metal-oxalate complexation. In contrast, aerosols at the low-altitude site were dominated by local anthropogenic emissions, exhibiting a lower degree of aging, as reflected by higher C2 concentrations (674±528 ng m−3) and lower δ13C values (−27.6 ‰). The higher background concentration of NO and competitive reactions with components such as SO collectively delayed the shift of NO to the coarse mode.
This study clarifies how dust alters the formation pathways, particle-size distribution, and δ13C signature of C2, highlighting the significant altitudinal variation of these effects. Our findings provide critical insights into the altitude-dependent transformation of SOA during dust transport, thereby enhancing the understanding of mountain atmospheric chemistry and regional climate effects. Limited by field observations, the microkinetic parameters of the aforementioned surface reactions remain unquantified. Future work should integrate laboratory experiments with model simulations to fully elucidate the microscopic mechanisms and regional climate impacts of this heterogeneous process.
The data in this study are available at https://doi.org/10.5281/zenodo.15788834 (Shen et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-16147-2025-supplement.
JL conceived and designed the study. MS conducted the literature search, performed sample and data analysis, and wrote the manuscript. JL and QiyW contributed to manuscript revision. YL, YZ, WD, LL, XG, YC, YJ, QiaW and SL collected particulate samples and supervised the experiments. All authors provided critical feedback on the manuscript and approved the final version.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The authors extend their gratitude to all colleagues and students whose contributions were instrumental to this work. Jianjun Li specifically wishes to acknowledge the personal support received from the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
This research has been supported by the National Natural Science Foundation of China (grant no. 42407156), the State Key Laboratory of Loess and Quaternary Geology (grant no. SKLLOG2307), the Natural Science Basic Research Program of Shaanxi Province (grant no. 2025JC-YBQN-450), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (grant no. 2020407).
This paper was edited by James Allan and reviewed by two anonymous referees.
Bikkina, P., Bikkina, S., and Kawamura, K.: Role of aerosol liquid water content on the production of dicarboxylic acids in the dust-laden air masses over the Arabian Sea: Implications for heterogeneous chemistry, Atmos. Res., 289, 106743, https://doi.org/10.1016/j.atmosres.2023.106743, 2023.
Cao, J. J., Lee, S. C., Chow, J. C., Watson, J. G., Ho, K. F., Zhang, R. J., Jin, Z. D., Shen, Z. X., Chen, G. C., Kang, Y. M., Zou, S. C., Zhang, L. Z., Qi, S. H., Dai, M. H., Cheng, Y. and Hu. K.: Spatial and seasonal distributions of carbonaceous aerosols over China, J. Geophys. Res.-Atmos., 112, D22S11, https://doi.org/10.1029/2006JD008205, 2007.
Carlton, A. G., Turpin, B. J., Lim, H. J., Altieri, K. E., and Seitzinger, S.: Link between isoprene and secondary organic aerosol (SOA): Pyruvic acid oxidation yields low volatility organic acids in clouds, Geophy. Res. Lett., 33, L06822, https://doi.org/10.1029/2005GL025374, 2006.
Chen, J. C., Xu, J. Z., Wu, Z. J., Meng, X. X. Y., Yu, Y., Ginoux, P., DeMott, P. J., Xu, R., Zhai, L. X., Yan, Y. F., Zhao, C. F., Li, S. M., Zhu, T., and Hu, M.: Decreased dust particles amplify the cloud cooling effect by regulating cloud ice formation over the Tibetan Plateau, Sci. Adv., 10, eado0885, https://doi.org/10.1126/sciadv.ado0885, 2024.
Chen, S. Y., Zhao, D., Huang, J. P., He, J. Q., Chen, Y., Chen, J. Y., Bi, H. R., Lou, G. T., Du, S. K., Zhang, Y., and Yang, F.: Mongolia contributed more than 42 % of the dust concentrations in Northern China in March and April 2023, Adv. Atmos. Sci., 40, 1549–1557, https://doi.org/10.1007/s00376-023-3062-1, 2023.
Deshmukh, D. K., Kawamura, K., and Deb, M. K.: Dicarboxylic acids, ω-oxocarboxylic acids, α-dicarbonyls, WSOC, OC, EC, and inorganic ions in wintertime size-segregated aerosols from central India: Sources and formation processes, Chemosphere, 161, 27–42, https://doi.org/10.1016/j.chemosphere.2016.06.107, 2016.
Deshmukh, D. K., Kawamura, K., Deb, M. K., and Boreddy, S. K. R.: Sources and formation processes of water-soluble dicarboxylic acids, ω-oxocarboxylic acids, α-dicarbonyls, and major ions in summer aerosols from eastern central India, J. Geophys. Res.-Atmos., 122, 3630–3652, https://doi.org/10.1002/2016JD026246, 2017.
Du, W., Ding, Z. J., Lei, Y. L., Zhang, S., Wu, C., Zhang, F., Wang, F. L., Lv, S. J., Liu, X. D., Meng, J. J., and Wang, G. H.: Atmospheric fine particulate dicarboxylic acids and related SOA in winter at the background site of Yangtze River Delta: Implication for the long-distance transport of solid fuels burning, Atmos. Environ., 289, 11932, https://doi.org/10.1016/j.atmosenv.2022.119320, 2022.
Ervens, B., Turpin, B. J., and Weber, R. J.: Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): a review of laboratory, field and model studies, Atmos. Chem. Phys., 11, 11069–11102, https://doi.org/10.5194/acp-11-11069-2011, 2011.
Falkovich, A. H. and Schkolnik, G.: Adsorption of organic compounds pertinent to urban environmentsonto mineral dust particles, J. Geophys. Res.-Atmos., 109, D02208, https://doi.org/10.1029/2003JD003919, 2004.
Fan, J. Y., Wang, Y., Rosenfeld, D., and Liu, X. H.: Review of aerosol–cloud interactions: Mechanisms, significance, and challenges, J. Atmos. Sci., 73, 4221–4252, https://doi.org/10.1175/JAS-D-16-0037.1, 2016.
Fountoukis, C. and Nenes, A.: ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-NH-Na+-SO-NO-Cl−-H2O aerosols, Atmos. Chem. Phys., 7, 4639–4659, https://doi.org/10.5194/acp-7-4639-2007, 2007.
Fu, P. Q., Kawamura K., Kanaya, Y., and Wang, Z. F.: Contributions of biogenic volatile organic compounds to the formation of secondary organic aerosols over Mt. Tai, Central East China, Atmos. Environ., 44, 4817–4826, https://doi.org/10.1016/j.atmosenv.2010.08.040, 2010.
Fu, T. M., Jacob, D. J., Wittrock, F., Burrows, J. P., Vrekoussis, M., and Henze, D. K.: Global budgets of atmospheric glyoxal and methylglyoxal, and implications for formation of secondary organic aerosols, J. Geophys. Res.-Atmos., 113, D15303, https://doi.org/10.1029/2007JD009505, 2008.
Gui, K., Yao, W. R., Che, H. Z., An, L. C., Zheng, Y., Li, L., Zhao, H. J., Zhang, L., Zhong, J. T., Wang, Y. Q., and Zhang, X. Y.: Record-breaking dust loading during two mega dust storm events over northern China in March 2021: aerosol optical and radiative properties and meteorological drivers, Atmos. Chem. Phys., 22, 7905–7932, https://doi.org/10.5194/acp-22-7905-2022, 2022.
Harris, E., Sinha, B., van Pinxteren, D., Tilgner, A., Fomba, K. W., Schneider, J., Roth, A., Gnauk, T., Fahlbusch, B., Mertes, S., Lee, T., Collett, J., Foley, S., Borrmann, S., Hoppe, P., and Herrmann, H.: Enhanced role of transition metal ion catalysis during in-cloud oxidation of SO2, Science, 340, 727–730, https://doi.org/10.1126/science.1230911, 2023.
Ho, K. F., Lee, S. C., Cao, J. J., Kawamura, K., Watanabe, T., Cheng, Y., and Chow, J. C.: Dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban roadside area of Hong Kong, Atmos. Environ., 40, 3030–3040, https://doi.org/10.1016/j.atmosenv.2005.11.069, 2006.
Hoque, M. M. M., Kawamura, K., and Uematsu, M.: Spatio temporal distributions of dicarboxylic acids, ω-oxocarboxylic acids, pyruvic acid, α-dicarbonyls and fatty acids in the marine aerosols from the North and South Pacific, Atmos. Res., 185, 158–168, https://doi.org/10.1016/j.atmosres.2016.10.022, 2017.
Huang, R. J., Li, Y. J., Chen, Q., Zhang, Y. L., Lin, C. S., Chan, C. K., Yu, J. Z., Gouw, J., Tong, S. R., Jiang, J. K., Wang, W. G., Ding, X., Wang, X. M., Ge, M. F., Zhou, W. J., Worsnop, D., Boy, M., Bilde, M., Dusek, U., Carlton, A. G., Hoffmann, T., McNeill, V. F., and Glasius M.: Secondary organic aerosol in urban China: A distinct chemical regime for air pollution studies, Science, 389, eadq2840, https://doi.org/10.1126/science.adq2840, 2025.
Kalogridis, A. C., Popovicheva, O. B., Engling, G., Diapouli, E., Kawamura, K., Tachibana, E., Ono, K., Kozlov, V. S., and Eleftheriadis. K.: Smoke aerosol chemistry and aging of Siberian biomass burning emissions in a large aerosol chamber, Atmos. Environ., 185, 15–28, https://doi.org/10.1016/j.atmosenv.2018.04.033, 2018.
Kanellopoulos, P. G., Chrysochou, E., Koukoulakis, K., and Bakeas E.: Secondary organic aerosol markers and related polar organic compounds in summer aerosols from a sub-urban site in Athens: Size distributions, diurnal trends and source apportionment, Atmos. Pollut. Res., 12, 1–13, https://doi.org/10.1016/j.apr.2021.02.013, 2021.
Kawai, K., Matsui, H., and Tobo, Y.: High potential of Asian dust to act as ice nucleating particles in mixed-phase clouds simulated with a global aerosol-climate model, J. Geophys. Res.-Atmos., 126, e2020JD034263, https://doi.org/10.1029/2020JD034263, 2021.
Kawamura, K. and Bikkina, S.: A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation, Atmos. Res., 170, 140–160, https://doi.org/10.1016/j.atmosres.2015.11.018, 2016.
Kawamura, K. and Ikushima, K.: Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere, Environ. Sci. Technol., 27, 2227–2235, https://doi.org/10.1021/es00047a033, 1993.
Kawamura, K. and Sakaguchi, F.: Molecular distributions of water soluble dicarboxylic acids in marine aerosols over the Pacific Ocean including tropics, J. Geophys. Res.-Atmos., 104, 3501–3509, https://doi.org/10.1029/1998JD100041, 1999.
Kawamura, K. and Watanabe, T.: Determination of stable carbon isotopic compositions of low molecular weight dicarboxylic acids and ketocarboxylic acids in atmospheric aerosol and snow samples, Anal. Chem., 76, 5762–5768, https://doi.org/10.1021/ac049491m, 2004.
Kawamura, K., Ono, K., Tachibana, E., Charriére, B., and Sempéré, R.: Distributions of low molecular weight dicarboxylic acids, ketoacids and α-dicarbonyls in the marine aerosols collected over the Arctic Ocean during late summer, Biogeosciences, 9, 4725–4737, https://doi.org/10.5194/bg-9-4725-2012, 2012.
Kawamura, K., Tachibana, E., Okuzawa, K., Aggarwal, S. G., Kanaya, Y., and Wang, Z. F.: High abundances of water-soluble dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in the mountaintop aerosols over the North China Plain during wheat burning season, Atmos. Chem. Phys., 13, 8285–8302, https://doi.org/10.5194/acp-13-8285-2013, 2013.
Kok, J. F., Storelvmo, T., Karydis, V. A., Adebiyi, A. A., Mahowald, N. M., Evan, A. T., He, C. L., and Leung, D. M.: Mineral dust aerosol impacts on global climate and climate change, Nat. Rev. Earth Environ., 4, 71–86, https://doi.org/10.1038/s43017-022-00379-5, 2023.
Kumar, R., Barth, M. C., Madronich, S., Naja, M., Carmichael, G. R., Pfister, G. G., Knote, C., Brasseur, G. P., Ojha, N., and Sarangi, T.: Effects of dust aerosols on tropospheric chemistry during a typical pre-monsoon season dust storm in northern India, Atmos. Chem. Phys., 14, 6813–6834, https://doi.org/10.5194/acp-14-6813-2014, 2014.
Kunwar, B. and Kawamura, K.: Seasonal distributions and sources of low molecular weight dicarboxylic acids, v-oxocarboxylic acids, pyruvic acid, a-dicarbonyls and fatty acids in ambient aerosols from subtropical Okinawa in the western Pacific Rim, Environ. Chem., 11, 673–689, https://doi.org/10.1071/EN14097, 2014.
Lamkaddam, H., Dommen, J., Ranjithkumar, A., Gordon, H., Wehrle, G., Krechmer, J., Majluf, F., Salionov, D., Schmale, J., Bjelić, S., Carslaw, K. S., Haddad, I. E., and Baltensperger, U.: Large contribution to secondary organic aerosolfrom isoprene cloud chemistry, Sci. Adv., 7, eabe2952, https://doi.org/10.1126/sciadv.abe2952, 2021.
Li, J. J., Wang, G. H., Zhou, B. H., Cheng, C. L., Cao, J. J., Shen, Z. X., and An, Z. S.: Airborne particulate organics at the summit (2060 m, a.s.l.) of Mt. Hua in central China during winter: Implications for biofuel and coal combustion, Atmos. Res., 106, 108–119, https://doi.org/10.1016/j.atmosres.2011.11.012, 2012.
Li, J. J., Wang, G. H., Ren, Y. Q., Wang, J. Y., Wu, C., Han, Y., Zhang, L., Cheng, C. L., and Meng, J. J.: Identification of chemical compositions and sources of atmospheric aerosols in Xi'an, inland China during two types of haze events, Sci. Total Environ., 566–567, 230–237, https://doi.org/10.1016/j.scitotenv.2016.05.057, 2016.
Li, J. J., Wang, G. H., Zhang, Q., Li, J., Wu, C., Jiang, W. Q., Zhu, T., and Zeng, L. M.: Molecular characteristics and diurnal variations of organic aerosols at a rural site in the North China Plain with implications for the influence of regional biomass burning, Atmos. Chem. Phys., 19, 10481–10496, https://doi.org/10.5194/acp-19-10481-2019, 2019.
Li, W. J. and Shao, L. Y.: Observation of nitrate coatings on atmospheric mineral dust particles, Atmos. Chem. Phys., 9, 1863–1871, https://doi.org/10.5194/acp-9-1863-2009, 2009.
Li, W. J., Ito, A., Wang, G. C., Zhi, M. K., Xu, L., Yuan, Q., Zhang, J., Liu, L., Wu, F., Laskin, A., Zhang, D. Z., Zhang, X.Y., Zhu, T., Chen, J. M., Mihalopoulos, N., Bougiatioti, A., Kanakidou, M., Wang, G. H., Hu, H. L., Zhao, Y., and Shi, Z. B.: Aqueous-phase secondary organic aerosol formation on mineral dust, Natl. Sci. Rev., nwaf221, https://doi.org/10.1093/nsr/nwaf221, 2025.
Liang, P., Chen, B., Yang, X. P., Liu, Q. Q., Li, A. R., Mackenzie, L., and Zhang, D. G.: Revealing the dust transport processes of the 2021 mega dust storm event in northern China, Sci. Bull., 67, 21–24, https://doi.org/10.1016/j.scib.2021.08.014, 2022.
Lim, H. J., Carlton, A. G., and Turpin, B. J.: Isoprene forms secondary organic aerosol through cloud processing: model simulations, Environ. Sci. Technol., 39, 4441–4446, https://doi.org/10.1021/es048039h, 2005.
Lim, Y. B., Tan, Y., Perri, M. J., Seitzinger, S. P., and Turpin, B. J.: Aqueous chemistry and its role in secondary organic aerosol (SOA) formation, Atmos. Chem. Phys., 10, 10521–10539, https://doi.org/10.5194/acp-10-10521-2010, 2010.
Liu, H. J., Feng, Q., Huang, Y., Wu, F., Liu, Y. L., Shen, M. X., Guo, X., Dai, W. T., Qi, W. N., Zhang, Y. F., Li, L., Wang, Q. Y., Zhou, B. H., and Li, J. J.: Composition and size distribution of wintertime inorganic aerosols at ground and alpine regions of northwest China, Chin. Chem. Lett., 35, 109636, https://doi.org/10.1016/j.cclet.2024.109636, 2024.
Lu, D., Li, H., Tian, M. K., Wang, G. C., Qin, X. F., Zhao, N., Huo, J. T., Yang, F., Lin, Y. F., Chen, J., Fu, Q. Y., Duan, Y. S., Dong, X.Y., Deng, C. R., Abdullaev, S. F., and Huang, K.: Secondary aerosol formation during a special dust transport event: impacts from unusually enhanced ozone and dust backflows over the ocean, Atmos. Chem. Phys., 23, 13853–13868, https://doi.org/10.5194/acp-23-13853-2023, 2023.
Luo, Y. Y., Yao, Q., Ding, P., Hou, M., Deng, F. C., Wang, Y. B., Ding, C., Li, X., Wang, D. C., Sun, Z. k., Tang, S., Mao, Y. X., and Yao, X. Y.: Health impacts of an extreme dust event: a case and risk assessment study on airborne bacteria in Beijing, China, Environ. Sci. Eur., 36, 41, https://doi.org/10.1186/s12302-024-00858-0, 2024.
Maher, B. A., Prospero, J. M., Mackie, D., Gaiero, D., Hesse, P. P., and Balkanski, Y.: Global connections between aeolian dust, climate and ocean biogeochemistry at the present day and at the last glacial maximum, Earth Sci. Rev., 99(1–2), 61–97, https://doi.org/10.1016/j.earscirev.2009.12.001, 2010.
Mahowald, N., Albani, S., Kok, J. F., Engelstaeder, S., Scanza, R., Ward, D. S., and Flanner, M. G.: The size distribution of desert dust aerosols and its impact on the Earth system, Aeolian Res., 15, 53–71, https://doi.org/10.1016/j.aeolia.2013.09.002, 2014.
Marx, S. K., Hooper, J., Irino, T., Stromsoe, N., Saunders, K. M., Seki, O., Dosseto, A., Johansen, A., Hua, Q., Dux, F., Jacobsen, G., and Zawadzki, A.: Atmospheric particulates over the northwestern Pacific during the late Holocene: Volcanism, dust, and human perturbation, Sci. Adv., 10, eadn3311, https://doi.org/10.1126/sciadv.adn3311, 2024.
Meng, J. J., Wang, G. H., Li, J. J., Cheng, C. L., Ren, Y. Q., Huang, Y., Cheng, Y. B., Cao, J. J., and Zhang, T.: Seasonal characteristics of oxalic acid and related SOA in the free troposphere of Mt. Hua, central China: Implications for sources and for mation mechanisms, Sci. Total Environ., 493, 1088–1097, https://doi.org/10.1016/j.scitotenv.2014.04.086, 2014.
Meng, J. J., Wang, G. H., Hou, Z. F, Liu, X. D, Wei, B. J, Wu, C., Cao, C., Wang, J. Y., Li, J. J., Cao, J. J., Zhang, E., Dong, J., Liu, J. Z, Ge, S. S., and Xie, Y. N.: Molecular distribution and stable carbon isotopic compositions of dicarboxylic acids and related SOA from biogenic sources in the summertime atmosphere of Mt. Tai in the North China Plain, Atmos. Chem. Phys., 18, 15069–15086, https://doi.org/10.5194/acp-18-15069-2018, 2018.
Myriokefalitakis, S., Tsigaridis, K., Mihalopoulos, N., Sciare, J., Nenes, A., Kawamura, K., Segers, A., and Kanakidou, M.: In-cloud oxalate formation in the global troposphere: a 3-D modeling study, Atmos. Chem. Phys., 11, 5761–5782, https://doi.org/10.5194/acp-11-5761-2011, 2011.
Myriokefalitakis, S., Bergas-Massó, E., Gonçalves-Ageitos, M., García-Pando, C. P., Noije, T., Sager, P. L., Ito, A., Athanasopoulou, E., Nenes, A., Kanakidou, M., Krol, M. C., and Gerasopoulos, E.: Multiphase processes in the EC-Earth model and their relevance to the atmospheric oxalate, sulfate, and iron cycles, Geosci. Model Dev., 15, 3079–3120, https://doi.org/10.5194/gmd-15-3079-2022, 2022.
Pan, H. Z., Hu, Z. Y., Feng, T. C., Huang, Z. W., Liu, Q. T., and Feng, G. L.: Distribution characteristics and air-quality effect of intercontinental transport dust: An unexpected dust storm case study in China, Atmos. Environ., 350, 121177, https://doi.org/10.1016/j.atmosenv.2025.121177, 2025.
Pavuluri, C. M. and Kawamura, K.: Enrichment of 13C in diacids and related compounds during photochemical processing of aqueous aerosols: New proxy for organic aerosols aging, Sci. Rep., 6, 36467, https://doi.org/10.1038/srep36467, 2016.
Ren, Y. Q., Wang, G. H., Li, J. J., Wu, C., Cao, C., Li, J., Wang, J. Y., Ge, S. S., Xie, Y. N., Li, X. R., Meng, F. and Li, H.: Evolution of aerosol chemistry in Xi'an during the spring dust storm periods: Implications for heterogeneous formation of secondary organic aerosols on the dust surface, Chemosphere, 215, 413–421. https://doi.org/10.1016/j.chemosphere.2018.10.064, 2019.
Shen, M. X., Ho, K. F., Dai, W. T., Liu, S. X., Zhang, T., Wang, Q. Y., Meng, J. J., Chow, J. C., Watson, J. G., Cao, J. J., and Li, J. J.: Distribution and stable carbon isotopic composition of dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in fresh and aged biomass burning aerosols, Atmos. Chem. Phys., 22, 7489–7504, https://doi.org/10.5194/acp-22-7489-2022, 2022.
Shen, M. X., Qi, W. N., Guo, X., Dai, W. T., Wang, Q. Y., Liu, Y. L., Zhang, Y. F., Cao, Y., Chen, Y. K., Li, L., Liu, H. J., Cao, J. J., and Li, J. J.: Influence of vertical transport on chemical evolution of dicarboxylic acids and related secondary organic aerosol from surface emission to the top of Mount Hua, Northwest China, Sci. Total Environ., 858, 159892, https://doi.org/10.1016/j.scitotenv.2022.159892, 2023.
Shen, M. X., Qi, W. N., Liu, Y. L., Zhang, Y. F., Dai, W. T., Li, L., Guo, X., Cao, Y., Jiang, Y. K., Wang, Q., Li, S. C., Wang, Q. Y., and Li, J. J: Measurement report: Observational insights into the impact of dust transport on atmospheric dicarboxylic acids in ground region and free troposphere, Zenodo [data set], https://doi.org/10.5281/zenodo.15788834, 2025.
Sullivan, R. C. and Prather, K. A.: Investigations of the diurnal cycle and mixing state of oxalic acid in individual particles in Asian aerosol outflow, Environ. Sci. Technol., 41, 8062–8069, https://doi.org/10.1021/es071134g, 2007.
Sullivan, R. C., Guazzotti1, S. A., Sodeman, D. A., and Prather, K. A.: Direct observations of the atmospheric processing of Asian mineral dust, Atmos. Chem. Phys., 7, 1213–1236, https://doi.org/10.5194/acp-7-1213-2007, 2007.
Sun, J. M., Zhang, M. Y., and Liu, T. S.: Spatial and temporal characteristics of dust storms in China and its surrounding regions, 1960–1999: Relations to source area and climate, J. Geophys. Res.-Atmos., 106, 10325–10333, https://doi.org/10.1029/2000JD900665, 2001.
Usher, C. R., Michel, A. E., and Vicki, H.: Grassian Reactions on Mineral Dust, Chem. Rev., 103, 4883–4939, https://doi.org/10.1021/cr020657y, 2003.
Vergara-Temprado, J., Miltenberger, A. K., Furtado, K., Grosvenor, D. P., Shipway, B. J., Hill, A. A., Wilkinson, J. M., Field, P. R., Murray, B. J., and Carslaw, K. S.: Strong control of southern ocean cloud reflectivity by ice-nucleating particles, P. Natl. Acad. Sci. USA, 115, 2687–2692, https://doi.org/10.1073/pnas.1721627115, 2018.
Wang, G. H., Kawamura, K., Umemoto, N., Xie, M. J, Hu, S. Y., and Wang, Z. F.: Water-soluble organic compounds in PM2.5 and size-segregated aerosols over Mount Tai in North China Plain, J. Geophys. Res.-Atmos., 114, D19208, https://doi.org/10.1029/2008jd011390, 2009.
Wang, G. H., Kawamura, K., Cheng, C. L., Li, J. J., Cao, J. J., Zhang, R. J., Zhang, T., Liu, S. X., and Zhao, Z. Z.: Molecular distribution and stable carbon isotopic composition of dicarboxylic acids, ketocarboxylic acids, and α-dicarbonyls in size-resolved atmospheric particles from Xi'an city, China, Environ. Sci. Technol., 46, 4783–4791, https://doi.org/10.1021/es204322c, 2012.
Wang, G. H., Cheng, C. L., Meng, J. J., Huang, Y., Li, J. J., and Ren, Y. Q.: Field observation on secondary organic aerosols during Asian dust storm periods: Formation mechanism of oxalic acid and related compounds on dust surface, Atmos. Environ., 113, 169–176, https://doi.org/10.1016/j.atmosenv.2015.05.013, 2015.
Wang, G. H., Zhang, S., Wu, C., Zhu, T., Xu, X. B., Ge, S. S., Sun, H. T., Sun, Z. R., Wang, J. X., Ji, Y. M., Gao, J., Ren, Y. Q., Li, H., Zhang, F., Wang, Y., and Seinfeld, J. H.: Atmospheric sulfate aerosol formation enhanced by interfacial anions, PNAS Nexus, 4, pgaf058, https://doi.org/10.1093/pnasnexus/pgaf058, 2025.
Wang, Y. D., Zhou, L., Wang, W. G., and Ge, M. F.: Heterogeneous uptake of formic acid and acetic acid on mineral dust and coal fly ash, ACS Earth Space Chem., 4, 202–210, https://doi.org/10.1021/acsearthspacechem.9b00263, 2020.
Wang, Z., Shi, C., Zhang, H., Chen, Y. J., Chi, X. Y., Xia, C., Wang, S. Y., Zhu, Y. Z., Zhang, K., Chen, X. T., Xing, C., and Liu, C.: Measurement report: Dust and anthropogenic aerosols' vertical distributions over northern China dense aerosols gathered at the top of the mixing layer, Atmos. Chem. Phys., 23, 14271–14292, https://doi.org/10.5194/acp-23-14271-2023, 2023.
Warneck, P.: In-cloud chemistry opens pathway to the formation of oxalic acid in the marine atmosphere, Atmos. Environ., 37, 2423–2427, https://doi.org/10.1016/S1352-2310(03)00136-5, 2003.
Wu, C. L., Lin, Z. H., Shao, Y. P., Liu, X. H., and Li, Y.: Drivers of recent decline in dust activity over East Asia, Nat. Commun., 13, 7105, https://doi.org/10.1038/s41467-022-34823-3, 2022.
Wu, F., Zhang, D. Z., Cao, J. J., Xu, H. M., and An, Z. S.: Soil-derived sulfate in atmospheric dust particles at Taklimakan desert, Geophys. Res. Lett., 39, L24803, https://doi.org/10.1029/2012GL054406, 2012.
Xu, B. Q., Zhang, G., Gustafsson, Ö., Kawamura, K., Li, J., Andersson, A., Bikkina, S., Kunwar, B., Pokhrel, A., Zhong, G. C., Zhao, S. Z., Li, J., Huang, C., Cheng, Z. N., Zhu, S. Y., Peng, P. A., and Sheng, G. Y.: Large contribution of fossil-derived components to aqueous secondary organic aerosols in China, Nat. Commun., 13, 5115, https://doi.org/10.1038/s41467-022-32863-3, 2022.
Xu, W. Y., Kuang, Y., Liang, L. L., He, Y., Cheng, H. B., Bian, Y. X., Tao, J. C., Zhang, G., Zhao, P. S., Ma, N., Zhao, H. R., Zhou, G. S., Su, H., Cheng, Y. F., Xu, X. B., Shao, M., amd Sun, Y. L.: Dust-dominated coarse particles as a medium for rapid secondary organic and inorganic aerosol formation in highly polluted air, Environ. Sci. Technol., 54, 15710–15721, https://doi.org/10.1021/acs.est.0c07243, 2020.
Xu-Yang, Y. J. J., Skonieczny, C., Ayrault, S., Barbier, J., Bizeul, R., Bryskere, O., Chaboche, P., Chalaux-Clergue, T., Corcho-Alvarado, J. A., Foucher, A., Karsenti, A., Leblanc, M., Orizaola, G., Plautre, A., Röllin, S., Taraconat, N., Tenaud, N., Valdés, A. E., Dulac, F., and Evrard, O.: Radioactive contamination transported to Western Europe with Saharan dust, Sci. Adv., 11, eadr9192, https://doi.org/10.1126/sciadv.adr9192, 2025.
Yamaguchi, N., Baba, T., Ichijo, T., Himezawa, Y., Enoki, K., Saraya, M., Li, P., and Nasu, M.: Abundance and community structure of bacteria on Asian dust particles collected in Beijing, China, during the Asian Dust Season, Biol. Pharm. Bull., 39, 68–77, https://doi.org/10.1248/bpb.b15-00573, 2016.
Yang, C., Zhou, S. X., Zhang, C. Y., Yu, M. Y., Cao, F., and Zhang, Y. L.: Atmospheric chemistry of oxalate: Insight into the role of relative humidity and aerosol acidity from high-resolution observation, J. Geophys. Res.-Atmos., 127, e2021JD035364, https://doi.org/10.1029/2021JD035364, 2022.
Yang, J., Zhao, W. Y., Wei, L. F., Zhang, Q., Zhao, Y., Hu, W., Wu, L. B., Li, X. D., Pavuluri, C. M., Pan, X. L., Sun, Y. L., Wang, Z. F., Liu, C. Q., Kawamura, K., and Fu, P. Q.: Molecular and spatial distributions of dicarboxylic acids, oxocarboxylic acids, and-dicarbonyls in marine aerosols from the South China Sea to the eastern Indian Ocean, Atmos. Chem. Phys., 20, 6841–6860, https://doi.org/10.5194/acp-20-6841-2020, 2020.
Yang, Y., Russell, L. M., Lou, S. J., Liao, H., Guo, J. P., Liu, Y., Singh, B., and Ghan, S. J.: Dust-wind interactions can intensify aerosol pollution over eastern China, Nat. Commun., 8, 15333, https://doi.org/10.1038/ncomms15333, 2017.
Yermakov, A. N., Aloyan, A. E., Arutyunyan, V. O., and Pronchev, G. B.: On the mechanism of sulfur dioxide oxidation in cloud drops, Izv. Atmos. Ocean. Phys., 59, 538–547, https://doi.org/10.1134/S0001433823050055, 2023.
Yu, Z. C., Song, R. Y., Wang, Q. H., Quan, J. Y., Zhang, H. Y., Fu, J. J., and Jiang, G. B.: Research progress of atmospheric chemical process of airborne dust particles, Environ. Chem., 44, 854–867, https://doi.org/10.7524/j.issn.0254-6108.2024051303, 2025.
Zhang, G. H., Lin, Q. H., Peng, L., Yang, Y. X., Jiang, F., Liu, F. X., Song, W., Chen, D. H., Cai, Z., Bi, X. H., Miller, M., Tang, M. J., Huang, W. L., Wang, X. M., Peng, P. A., and Sheng, G. Y.: Oxalate formation enhanced by Fe-containing particles and environmental implications, Environ. Sci. Technol., 53, 1269–1277, https://doi.org/10.1021/acs.est.8b05280, 2019.
Zhang, S., Xu, H., Lan, J. H., Goldsmith, Y., Torfstein, A., Zhang, G. L., Zhang, J., Song, Y. P., Zhou, K., Tan, L.C., Xu, S., Xu, X. M., and Enzel, Y.: Dust storms in northern China during the last 500 years, Sci. China Earth Sci., 64, 813–824, https://doi.org/10.1007/s11430-020-9730-2, 2021.
Zhang, T., Cao, J. J., Tie, X. X., Shen, Z. X., Liu, S. X., Ding, H., Han, Y. M., Wang, G. H., Ho, K. F., Qiang, J., and Li, W. T.: Water-soluble ions in atmospheric aerosols measured in Xi'an, China: Seasonal variations and sources, Atmos. Res., 102, 110–119, https://doi.org/10.1016/j.atmosres.2011.06.014, 2011.
Zheng, F. X., Li, J. W., Hua, C. J., Xie, J. L., Zhang, Y. S., Li, L. Y., Shen, S. N., Hakala, S., Yan, C., Feng, Z. M., Fan, X. L., Bianchi, F., Petäjä, T., Kerminen, V., Kulmala, M., Xia, M., Zha, Q., Du, W., Daellenbach, K. R., Cai, J., and Liu, Y. C.: Dust event identification and characterization with one-year online observations in Beijing, Sci. Total Environ., 956, 177296, https://doi.org/10.1016/j.scitotenv.2024.177296, 2024.
Zhi, M. K., Wang, G. C., Xu, L., Li, K. l., Nie, W., Niu, H. Y., Shao, L. Y., Liu, Z. R., Yi, Z. W., Wang, Y. T., Shi, Z. B., Ito, A., Zhai, S. X., and Li, W. J.: How acid iron dissolution in aged dust particles responds to the buffering capacity of carbonate minerals during Asian dust storms, Environ. Sci. Technol., 59, 6167–6178, https://doi.org/10.1021/acs.est.4c12370, 2025.
Zhu, Q. Z. and Liu, Y. J.: The dominant factor in extreme dust events over the Gobi Desert is shifting from extreme winds to extreme droughts, npj Clim. Atmos. Sci., 7, 141, https://doi.org/10.1038/s41612-024-00689-z, 2024.