the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reaction between linear perfluoroaldehydes and hydroperoxy radical in the atmosphere: reaction mechanisms, reaction kinetics modelling, and atmospheric implications
Zegang Dong
Chaolu Xie
Linear perfluoroaldehydes are important products formed in the atmospheric oxidation of industrial fluorinated compounds. However, their atmospheric lifetimes are incompletely known. Here, we employ high level quantum chemistry methods and a dual-level strategy for kinetics to investigate the reactions of C2F5CHO and C3F7CHO with HO2. Our calculated results unveil almost equal activation enthalpies at 0 K for linear perfluoroaldehydes reaction with HO2, indicating that the carbon chain length negligibly influences reaction thermodynamics. The calculated kinetics reveal that vibrational anharmonicity enhances rate constants by a factor of 3–10, while torsional anharmonicity reduces rate constants by 34 %–55 %. Additionally, we also find that the reaction of C3F7CHO with HO2 exhibits significant pressure dependence, with transition pressures ranging from 0.026 to 2.3 bar across a temperature range of 190–350 K. Furthermore, atmospheric lifetimes of C2F5CHO and C3F7CHO are discussed based on the homogenous and heterogeneous processes. Our findings also reveal that the reactions of C2F5CHO and C3F7CHO with HO2 radicals dominate over those with OH radicals in Russia, Malaysia, and parts of Africa by the calculated results in combination with data based on global atmospheric chemical model simulations. Under nighttime conditions, HO2-initiated degradation represents a major atmospheric sink, comparable in magnitude to photolysis and Cl-initiated oxidation in gas phase, whereas hydrolysis at the air-water interface plays a critical role in the sink of linear perfluoroaldehydes. These findings establish chain-length-dependent pressure effects and conformational sampling as critical, previously unrecognized factors in kinetics calculations, providing a framework for modelling complex fluorotelomer transformations and guiding emission mitigation strategies.
- Article
(3019 KB) - Full-text XML
-
Supplement
(2012 KB) - BibTeX
- EndNote
Poly- and perfluoroalkyl substances (PFASs) are highly fluorinated compounds with long atmospheric lifetimes, which have important influences on global warming potential (GWP) and environmental health (Ackerman Grunfeld et al., 2024; Rupp et al., 2023; Sznajder-Katarzyńska et al., 2019; Wu et al., 2024). During their degradation in the atmosphere, PFASs undergo complex chemical transformations, leading to the formation of linear perfluoroaldehydes. (Alam et al.,2024; Burkholder et al., 2015; David et al., 2021; Wang et al., 2021, 2024). Linear perfluoroaldehydes (CnF2n+1CHO) are significant intermediate compounds, which belong to the aldehydes family of PFASs (Li et al., 2024; Thackray et al., 2020). Chlorofluorocarbons (CFCs) and their temporary replacements, hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), and hydrofluoroolefins (HFOs) are the important source of the linear perfluoroaldehydes (Burkholder et al., 2015; Hurley et al., 2006; Martin et al., 2005; Rand and Mabury, 2017; Wang et al., 2023; Waterland and Dobbs, 2007). For example, under low NOx conditions, the reaction of OH radicals with the potential foaming agent CF3(CF2)2CH=CH2 (HFC-1447fz) leads to the formation of C3F7CHO (Jiménez et al., 2016; Yu et al., 2024). Furthermore, the atmospheric chemical processes of linear perfluoroaldehydes are of key importance for determining the atmospheric oxidation of fluorotelomer alcohols (FTOHs) (Antiñolo et al., 2012; Hurley et al., 2004).
Linear perfluoroaldehydes were generally considered to be removed through photochemical reactions (Chiappero et al., 2006; Sellevåg et al., 2004) and free radical reactions initiated by OH and Cl radicals (Andersen et al., 2004; Chiappero et al., 2010; Wang et al., 2007). Additionally, NO3 may also contribute to their atmospheric degradation. (Burkholder et al., 2015; Ziemann and Atkinson, 2012). During the daytime, photolysis of linear perfluoroaldehydes was considered to be the dominant removal process for CnF2n+1CHO, with estimated atmospheric lifetimes ranging from hours to several days (Antiñolo et al., 2014; Chiappero et al., 2006). Antiñolo et al. (2014) reported that the photolysis lifetime of C2F5CHO is expected to be 3.5 h at 273 K, with the main degradation products of CF3CFO and COF2. In addition, previous investigations have shown that the length of the carbon chain in CnF2n+1CHO significantly affects the quantum yield of photolysis (Chiappero et al., 2006; Sellevåg et al., 2004). During the nighttime, the reactions of free radicals with CnF2n+1CHO were considered to be the major degradation pathways. However, previous studies reported relatively slow rate constants for the reaction between OH and CnF2n+1CHO (n=1–4) with the values of , , , and cm3 molecule−1 s−1, respectively, at 298 K (Andersen et al., 2004; Antiñolo et al., 2014; Solignac et al., 2007). This corresponds to a longer atmospheric lifetime >20 d for these linear perfluoroaldehydes. Moreover, the rate constant of Cl atoms with CnF2n+1CHO (n=1–4) is approximately 2×1012 cm3 molecule−1 s−1. This value is slightly faster than that of the corresponding OH radical reactions under similar conditions (Andersen et al., 2004; Sulbaek Andersen et al., 2003). The long atmospheric lifetimes of CnF2n+1CHO provide an opportunity for other atmospheric oxidation processes of CnF2n+1CHO by other atmospheric oxidants.
HO2 radicalsare of ubiquitous active species in the atmosphere with the concentration being two orders of magnitude higher than that of OH radicals (Bottorff et al., 2023; Gao et al., 2024; Albrecht et al., 2019; Zhang et al., 2019, 2024, 2022) . Moreover, previous investigations have shown that the reactions of aldehydes with HO2 affect the degradation process of aldehydes (Hermans et al., 2005; Albrecht et al., 2019; Zhou et al., 2024a). Additionally, global three-dimensional chemistry-transport model calculations suggest that the oxidation reactions of formaldehyde and acetone initiated by hydroperoxyl radical contribute to 30 % loss of formaldehyde and acetone at the tropical troposphere (Hermans et al., 2005). Nevertheless, the importance of sink pathway by HO2 is still unknown because there have not been kinetics data for linear perfluoroaldehydes with HO2 in the literature. Moreover, chain elongation may have influences on reaction kinetics due to multiple conformers. Furthermore, it is unknown for the pressure-dependent effects of larger perfluoroaldehydes with HO2. Although our previous investigations have revealed the importance of CF3CHO + HO2 in the atmosphere (Long et al., 2022), their kinetics of larger perfluoroaldehydes with HO2 are further required to investigate due to the unique features that depend on the specific reaction systems such as multi-structural anharmonicity and pressure effects in these complex systems. Additionally, it is a big challenge for addressing the larger perfluoroaldehydes with HO2 because the computational cost grows very rapidly with system size, making such calculations impractical for high-level quantum chemistry methods.
In this article, we have investigated the reactions of HO2 with linear perfluoroaldehydes CnF2n+1CHO (n=2–5), specifically focusing on C2F5CHO and C3F7CHO, referred to as Reactions (R1) and (R2) respectively. To delve into these reactions, high-level quantum chemistry calculation close to CCSDT(Q) accuracy in conjunction with dual-level strategy were performed to obtain their quantitative kinetics. Simultaneously, to provide further insight into kinetics, we detailly evaluated the impact of various parameters, including torsional anharmonicity, anharmonicity on the reaction kinetics over atmosphere-related temperatures and pressures. In addition, the chemical transformation of the formed intermediate products has been discussed in Reactions (R1) and (R2). We further estimate the enthalpies of activation at 0 K for the larger-sized reactions of longer-chain perfluoroaldehyde with HO2. Moreover, we also discuss the importance of these Reactions (R1) and (R2) by combining the calculated reaction kinetics with global atmospheric modelling. The current results not only provide a comparative analysis with the kinetics of analogous OH-initiated reactions and photolytic processes, but also extend our understanding of the role of HO2 in modulating the atmospheric lifetime of linear perfluoroaldehydes. This study not only resolves the knowledge gap regarding HO2-initiated oxidation of linear perfluoroaldehydes but also establishes a computational strategy for predicting the atmospheric fates of long-chain PFAS derivatives. Our findings provide critical insights for refining emission control strategies and mitigating the environmental persistence of these compounds.
2.1 Options for electronic structure density functionals
Our goal is to establish a precise set of electronic structure and kinetic calculation methods for the XCHO + HO2 reaction, delivering satisfactory quantitative results (Long et al., 2022). This previous study indicated that the CCSD(T)-F12a/cc-pVTZ-F12//M06-2X/MG3S theoretical methods can make good agreement with beyond-CCSD(T) results for the similar reaction of HCHO + HO2 (Long et al., 2022). Furthermore, CCSD(T)-F12a/cc-pVTZ-F12 has been shown good performance for molecules containing fluorine atoms (Dong et al., 2021; Long et al., 2022; Xia et al., 2024a). Consequently, we intend to utilize the well-validated methods in the present investigations in Reactions (R1) and (R2). Specifically, the M06-2X (Zhao and Truhlar, 2008b, a) density functional with the MG3S (Lynch et al., 2003) basis set was employed to optimize the geometries, while CCSD(T)-F12a (Adler et al., 2007; Knizia et al., 2009)/cc-pVTZ-F12 for R1 and R2 and FNO-CCSD(T)-F12 (Gyevi-Nagy et al., 2021; Taube and Bartlett, 2008)/cc-pVDZ-F12 for other CnF2n+1CHO + HO2 (n=1–5) were used to calculate single-point energies. The FNO-CCSD(T) approach that significantly improves computational efficiency with cost reduction of up to an order of magnitude was utilized to calculate larger systems. Furthermore, intrinsic reaction coordinate (IRC) calculation was done to determine the correct transition states by examining the connections of each saddle point to its corresponding minima (Hratchian and Schlegel, 2004, 2005; Kenyon, 1968).
2.2 Vibrational frequencies
We found that standard scale factor is actually not applicable for some transition states in previous investigation, so we used two scale factors (Zheng et al., 2014, 2015). The standard scale factor for M06-2X/MG3S is 0.970. Furthermore, we also calculated the specific reaction scale factors to assess the effects of anharmonicity. The reaction-specific scale factors were obtained by using the MPW1K/6-311+G(2df, 2p) electronic structure method based on the hybrid degeneracy-corrected second-order vibrational perturbation theory (HDCVPT) (Bloino et al., 2012; Kuhler et al., 1996). This is necessary and effective for eliminating the activation enthalpy error caused by the standard scale factors and the results were listed in Tables S1 and S2 in the Supplement. This was obtained by Eq. (1),
where λAnh is the ratio of anharmonic zero-point vibrational energies (ZPE) to harmonic ZPE at the MPW1K/6-311+G(2df, 2p) level. λH is 0.983 for M06-2X/MG3S to correct harmonic frequencies. The result shows that the specific reaction scale factors are 0.955 for TS1 (see Table S1) and 0.956 for TS2 (see Table S1), which is a large deviation from the standard value of 0.970; this results in a decrease in calculated enthalpies of activation of 0.72 and 0.78 for TS1 and TS2 at 0 K, respectively. In addition, multi-structural torsional anharmonicity involving reactant and transition state were all calculated using MS-T method (multi-structural method for torsional anharmonicity) (Yu et al., 2012; Zheng et al., 2011; Zheng and Truhlar, 2013).
2.3 Kinetics calculations
The dual-level strategy was utilized to compute the high-pressure limit rate constant (Long et al., 2016, 2019; Xia et al., 2024a). As shown in Eq. (2), we integrated a conventional transition-state theory rate constant predicated on higher-level (HL, CCSD(T)-F12a/cc-pVTZ-F12//M06-2X/MG3S) inputs with transmission coefficients derived from direct dynamics at a lower level (LL, M11-L/MG3S), employing a specific density functional that is chosen from the results of benchmark calculations (see Table S3). We have incorporated both a recrossing transmission coefficient and a tunneling transmission coefficient , as calculated through reaction-path variational transition state theory, with a particular emphasis on the canonical variational theory coupled with small-curvature tunneling (CVT/SCT) (Garrett and Truhlar, 1979; Liu et al., 1993; Truhlar et al., 1982). Additionally, a multi-structural transmission coefficient (FMS-T) was introduced to this framework to cancel the errors caused by the multi-structural anharmonicity, thereby advancing our approach to the DL-MS-CVT/SCT method, which provides a detailed and multifaceted treatment of the rate constant calculation, and can effectively obtain quantitative kinetics.
The pressure-dependent rate constants were done by employing the system-specific quantum Rice-Ramsperger-Kassel (SS-QRRK) theory in the temperature range of 190–350 K (Bao et al., 2016a, b; Bao and Truhlar, 2017). This method relies only on the high-pressure limiting rate constant that was calculated by the dual-level strategy. The computational details of pressure-dependent rate constants are presented in the Supplement.
2.4 Atmospheric modelling
We used GEOS-Chem 14.4.2 with a horizontal resolution of 2.0° × 2.5° to simulate space distribution of HO2 and OH at 47 vertical layers in the period from February 2018 to February 2019 (Bey et al., 2001). The time includes six months of spin-up and output per hour. GEOS-Chem is a global, three-dimensional chemical transport model associated with atmospheric composition (http://geos-chem.org, last access: 16 October 2025). Modern-Era Retrospective analysis for Research and Applications, Version 2 (MERRA-2) (Gelaro et al., 2017) was used as meteorological field data and Harmonized Emissions Component (HEMCO 3.9) was used as the source of emissions data (Lin et al., 2021). The emissions include biogenic emissions from Model of Emissions of Gases and Aerosols from Nature (MEGANv2.1) (Hu et al., 2015; McDuffie et al., 2020) and anthropogenic emissions from the global Community Emissions Data System (CEDS) (McDuffie et al., 2020) inventory. Simulation uses default full chemistry mechanism including HOx-NOx-VOC-O3-halogen chemistry, which is done by our previous investigation (Bloss et al., 2007).
2.5 Software
All density function calculation, including Zero-point energy (ZPE) correction were carried out using Gaussian 16 software package (Zhao and Truhlar, 2008b) and the single point energy calculations for CCSD(T)-F12a/cc-pVTZ-F12 and FNO-CCSD(T)-F12a/cc-pVDZ-F12 were done using Molpro 2019 (Werner et al., 2019) and MRCC code (Kállay et al., 2020, 2022). MS-T method was executed through MSTor-2023 program package (Chen et al., 2023). The rate constants were done with the KiSThelP (Canneaux et al., 2014) Polyrate 2017-C (Zheng et al., 2017) and Gaussrate 2017-B (Zheng et al., 2018).
3.1 The electronic structure of the reaction
We considered the C2F5CHO/C3F7CHO + HO2 reaction similar to the reactions of aldehydes with HO2 (Long et al., 2022). The dominant mechanism is that the hydrogen atom of HO2 is transferred to the terminal oxygen atom of , and simultaneously, the oxygen atom of HO2 is connected to the carbon atom of carbonyl group of . Figure 1 depicts ZPE corrected potential energy profile of the reaction of + HO2 at the CCSD(T)-F12a/cc-pVTZ-F12//M06-2X/MG3S level. and HO2 form reaction complexes RC1/RC2, and then pass through transition states TS1 and TS2 to form the intermediate products of C2F5CH(OH)OO (M1) and C3F7CH(OH)OO (M2), respectively. TS1 (−2.7 kcal mol−1) denotes the global minimum optimized structures of the stationary points of enthalpy of activation at 0 K; this is 0.3 and 1.6 kcal mol−1 lower than that of the CF3CHO + HO2 and CH3CHO + HO2 reactions, respectively (Long et al., 2022). This shows that the reaction of perfluoroaldehydes with HO2 may be kinetically feasible. As a comparison, the further energy profile of the C3H7CHO + HO2 reaction shows an equal enthalpy of activation of −2.7 kcal mol−1 for TS2; this is slightly lower than that of enthalpy of activation −2.4 kcal mol−1 for the reaction of CF3CHO + HO2 (Gao et al., 2024).
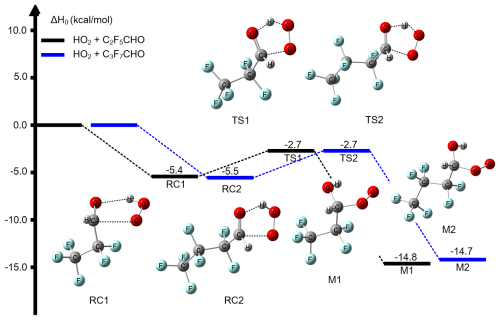
Figure 1Enthalpy profile of the HO2 addition reaction with C2F5CHO and C3F7CHO as calculated by CCSD(T)-F12a/cc-pVTZ-F12//M06-2X/MG3S level with the scale factor by the standard method at 0 K.
It is noteworthy that Fig. 1 only depicts the potential energy profile of the reaction featuring the global minimum structure. Nevertheless, the internal rotation of the C–C bond produces multiple conformers for reactants, transition states, and formed intermediate products. Their geometric configurations and energy distributions relative to the global minimum structure are presented in Fig. S1. Regarding the reactions of C2H5CHO and C3H7CHO with HO2, we have observed that as the carbon chain lengthens, the number of conformers of both reactants and transition states increases, and the energy distribution broadens. For instance, TS1 has three isomers, with an energy distribution spanning from 0 to 1.7 kcal mol−1, whereas TS2 has five isomers, and its energy distribution ranges from 0 to 1.9 kcal mol−1. In terms of geometric configurations, the low-energy isomers tend to have more linear structures, while the high-energy conformations exhibit more pronounced curling.
NO is a highly reactive gas (Lee et al., 2024). Human activities, especially agriculture and industrial processes, have led to significant NO emissions (Andersen et al., 2024; Thomson et al., 2012). Industrial activities contribute to NO levels such as fossil fuel combustion in power plants and chemical manufacturing, along with vehicle emissions. Given its prevalence from human-induced emissions, we further explore the degradation pathways of intermediate products M1 and M2 in the presence of NO. As depicted in Fig. S2, M1 and M2 undergo initial reactions with NO to yield the products C2F5CH(O)OH, C3F7CH(O)OH, and NO2, exhibiting activation enthalpies of −9.9 and −11.5 kcal mol−1 at 0 K, respectively. These results are consistent with previous studies on similar reactions involving RO2+ NO. (Berndt et al., 2015; King et al., 2001; Nie et al., 2023; Orlando et al., 2000; Vereecken and Peeters, 2009). These products then undergo unimolecular reactions to decompose into C2F5 and C3F7 radicals and formic acid in Fig. 2. Notably, the unimolecular decomposition of C2F5CH(O)OH and C3F7CH(O)OH represents the rate-determining step of the overall reaction, with corresponding activation enthalpies of 5.6 and 4.7 kcal mol−1 (0 K), respectively; this indicates that formic acid may potentially be formed via + HO2 in the presence of high concentration NO in the atmosphere. Additionally, the formed intermediate products (M1 and M2) are a typical class of RO2 radicals. In the low NOx levels, these RO2 radicals can also participate in bimolecular reactions (Ding and Long, 2022). RO2 can react with HO2, resulting in the formation of the stable product ROOH. Moreover, RO2 can react with other RO2 or R′O2 (where R′ denotes a hydrocarbon fragment) (Bottorff et al., 2023). The reaction with R′O2 frequently yields alkoxy radicals, and both of these reactions are capable of producing stable products (Goldman et al., 2021). Due to the complexity of these bimolecular reactions of the formed RO2 in the Reactions (R1) and (R2), we did not further investigate their reaction mechanisms and kinetics in the present work.
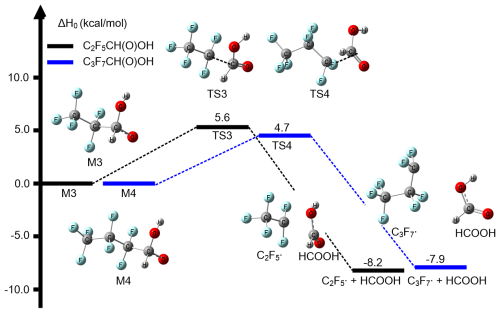
Figure 2Relative enthalpies at 0 K for the decomposition of C2F5CH(O)OH (M3) and C3F7CH(O)OH (M4) calculated by CCSD(T)-F12a/cc-pVTZ-F12//M06-2X/MG3S.
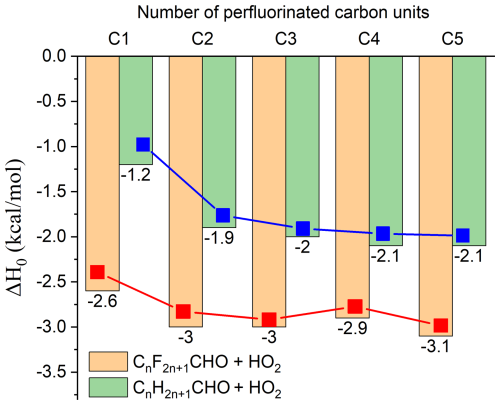
Figure 3The impacts of perfluorinated carbon length on the enthalpies of activation at 0 K in the CnH2n+1CHO/CnF2n+1CHO + HO2 reactions. The values for CnH2n+1CHO (n=1–5) + HO2 and CnF2n+1CHO + HO2 are obtained from references (Ding and Long, 2022; Gao et al., 2024) and calculated by using FNO-CCSD(T)-F12a/cc-pVDZ-F12.
We further conducted an extended study on the reactions of C4F9CHO and C5F11CHO at the FNO-CCSD(T)-F12//cc-pVDZ-F12//M06-2X/MG3S level, aiming to investigate the effects of increasing carbon chain length on the enthalpy of activation at 0 K. The calculated results show a deviation of only 0.2 kcal mol−1 in activation enthalpy at 0 K between FNO-CCSD(T)-F12//cc-pVDZ-F12 (−2.6 kcal mol−1) and CCSD(T)-F12a/cc-pVTZ-F12 (−2.4 kcal mol−1) in CF3CHO + HO2, validating the robustness of FNO-CCSD(T)-F12//cc-pVDZ-F12 for complex fluorinated systems. Data from Fig. 3 reveal an interesting phenomenon that the activation enthalpy at 0 K remains almost equal C2 (C2F5CHO) to C5 (C5F11CHO). This finding aligns with the similar trend for the reaction of CnH2n+1CHO with HO2, suggesting that the impact of carbon chain length growth on the enthalpy of activation at 0 K is quite minor (Ding and Long, 2022; Gao et al., 2024). However, the introduction of CF3 leads to a relatively lower enthalpy of activation at 0 K for the CnH2n+1CHO + HO2 reactions, primarily due to the strong electron-withdrawing ability of fluorine atoms, which can stabilize the transition state and lower the enthalpy of activation at 0 K. As the size of perfluoroaldehyde increases, the multi-structure effects caused by torsion of C-C bonds become more pronounced. The relative energy values of reactants and transition states shown in Figs. S1 and S3 (relative to the global minimum energy value, without ZPE correction) indicate that with increasing molecular size, the number of possible isomers increases, leading to a broader energy distribution. For instance, C2F5CHO exhibits three transition state conformers with energy differences spanning 0–1.7 kcal mol−1, while C3F7CHO has five conformers distributed over 0–1.8 kcal mol−1. This trend amplifies for longer chains: C5F11CHO generates 36 distinct conformers in its transition state, with energy variations extending up to 4.8 kcal mol−1. This broad energy distribution has significant implications for the thermodynamics and kinetics of the degradation process of perfluoroaldehydes, potentially increasing the diversity and complexity of reaction pathways.
3.2 Kinetics of C2F5CHO/C3F7CHO + HO2
The high-pressure limiting rate constants were calculated for the temperature range of 190-350 K, covering a wide atmospheric temperature range. For the reactions C2F5CHO + HO2 (Reaction R1) and C3F7CHO + HO2 (Reaction R2), the rate constants incorporating multi-structure anharmonicity corrections are defined as k1 andk2, respectively. According to Zheng and Truhlar (2010), the rate constants at high pressure are fitted using Eq. (3).
Table S4 lists the fitting parameters A, n, E, and T0. Here, T represents temperature in Kelvin, and R is the ideal gas constant (0.0019872 kcal mol−1 K−1). The temperature-dependent Arrhenius activation energies are determined from the fits using Eq. (4).
The high-pressure limit rate constants, incorporating multiple-structure anharmonicity torsional corrections, are illustrated in Fig. 4, with more comprehensive data provided in Tables S5–S7. Regarding the C2F5CHO + HO2 reaction, the rate constant k1 exhibits a decrease from cm3 molecule−1 s−1 at 190 K to cm3 molecule−1 s−1 at 350 K in Fig. 4 and Tables S5–S7. Similarly, the rate constant k2 for the C3F7CHO + HO2 reaction also decreases with increasing temperature. These trends are consistent with theoretical studies of non-fluorinated aldehydes such as C2H5CHO and C3H7CHO, where rate constants for reactions with HO2 were reported in the range of 10−14 to 10−13 cm3 molecule−1 s−1 at atmospheric temperatures, indicating similar reactivity between fluorinated and non-fluorinated aldehydes with HO2 (Ding and Long, 2022; Gao et al., 2024).
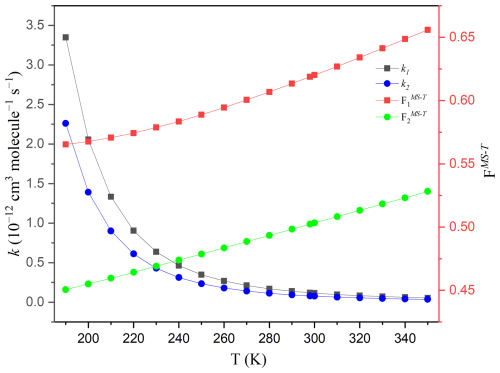
Figure 4The high-pressure limit rate constants of the reactions of C2F5CHO and C3F7CHO with HO2 at the temperature range of 190–350 K.
In addition, the effects of recrossing and multi-structural anharmonicity are quite limited, approximately ranging between 0.4 and 0.7 times. This results in the rate constants for Reactions (R1) and (R2) being 2–3 times slower than that of CF3CHO + HO2. For instance, the rate constants of C2F5CHO + HO2 and C3F7CHO + HO2 are estimated to be and cm3 molecule−1 s−1 at 298 K, respectively, which is slow by compared to cm3 molecule−1 s−1 of CF3CHO + HO2 (Long et al., 2022). Moreover, the effect of anharmonicity in vibrational-frequency scale factors on high pressure limited rate constants were further discussed. We define “f” as the ratio between the rate constant calculated using the reaction-specific vibrational-frequency scale factors and that calculated using the standard vibrational-frequency scale factors. As depicted in Fig. 5, the rate constants obtained using the reaction-specific scale factors are 3–7 and 4–10 times faster compared to those calculated using the standard scale factors. Consequently, employing reaction-specific scale factors is crucial for accurate rate calculations.
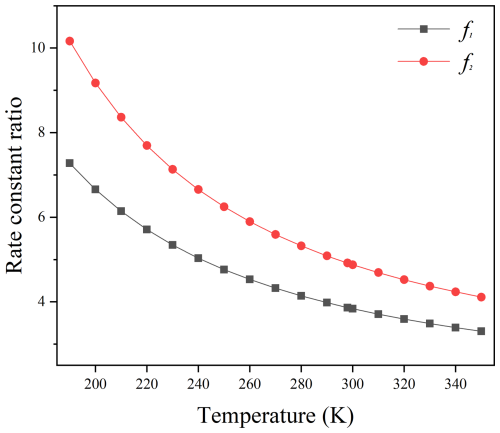
Figure 5The ratio between the rate constant calculated using the reaction-specific vibrational-frequency scale factors and the rate constant calculated using the standard vibrational-frequency scale factors, within the temperature range of 190–350 K. f1 and f2 represent the ratios for the reactions of C2F5CHO + HO2 and C3F7CHO + HO2, respectively.
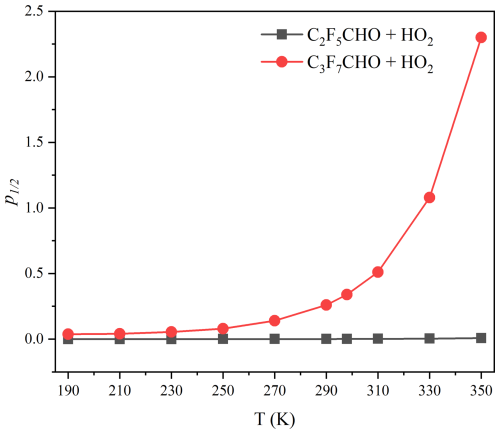
The pressure-dependent rate constants of the HO2 reaction with C2F5CHO and C3F7CHO were further calculated by using SS-QRRK method. As shown in Figs. 6–7 and Tables S8–S9, it can be observed that variations of the calculated rate constant with respect to pressure have a minimal impact on the rate constant of C2F5CHO + HO2, indicating the absence of significant pressure effects. However, significant pressure effects are observed in the C3F7CHO + HO2 reaction, particularly at temperatures above 300 K. To provide a clearer perspective, we define the transition pressure to quantify the pressure dependence. Specifically, the transition pressure is the pressure at which the pressure-dependent rate constant reaches half of its high-pressure limit. Figure 8 and Table S10 show that the transition pressure for the HO2+ C2F5CHO reaction ranging from to bar at 190–350 K, while the transition pressure ranges from to 2.3 bar at 190–350 K for the HO2+ C3F7CHO reaction. This indicates that the HO2+ C3F7CHO reaction exhibits a significant pressure dependence, and the increase in carbon chain length has a significantly affect pressure-dependent rate constants.
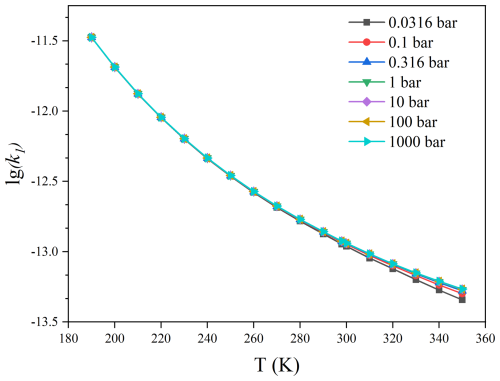
Figure 6Pressure-dependent rate constants of C2F5CHO + HO2 as functions of temperature obtained via the SS-QRRK method.
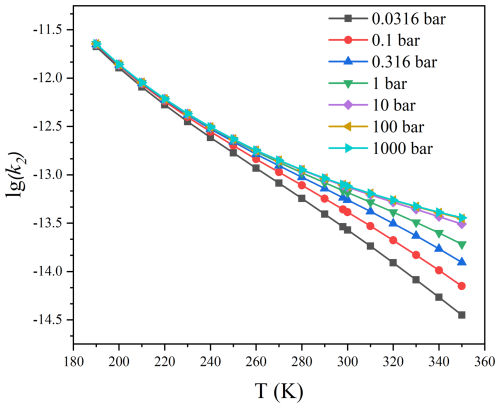
Figure 7Pressure-dependent rate constants of C3F7CHO + HO2 as functions of temperature obtained via the SS-QRRK method.
3.3 Atmospheric Implications
To provide a further insight into the atmospheric degradation pathways of linear perfluoroaldehydes, we compare the HO2-initiated linear perfluoroaldehyde reactions with the corresponding reactions with OH and Cl atom, their photolysis and hydrolysis.
We quantitatively evaluate the relative importance of OH- versus HO2-initiated degradation for C2F5CHO and C3F7CHO through rate ratios defined in Eqs. (5) and (6).
Here, k1 and k2 are the rate constants of HO2+ C2F5CHO and HO2+ C3F7CHO calculated in the present work, respectively, while kOH and kOH′ are the corresponding rate constants of OH+ C2F5CHO and OH + C3F7CHO obtained in the literature (Solignac et al., 2007; Wang et al., 2007). We calculate the rate ratios using a high OH concentration of 5×106 molecules cm−3 (Lew et al., 2020) and a typical HO2 concentration of 1.4×108 molecules cm−3 (Brasseur and Solomon, 2006). The calculated results reveal that within the temperature range of 220–320 K, the rate ratios for v1 and v2 are in the range of 79.2 to 3.76 and 45.7 to 2.43, respectively (Table 1). Therefore, the present findings indicate that HO2 initiated reactions dominate over OH initiated reactions for the degradation of C2F5CHO and C3F7CHO. We further consider the atmospheric lifetimes of C2F5CHO and C3F7CHO with respect to HO2 at 0–50 km altitude in Table 2. Rapid HO2-initiated degradation leads to short atmospheric lifetimes of ∼14.4–31.3 h for C2F5CHO and 21.6–51.8 h for C3F7CHO (Table 2), which are significantly shorter than the ∼20 d atmospheric lifetime driven by OH oxidation at below 10 km (Antiñolo et al., 2014).
Table 1Rate ratios of HO2+ C2F5CHO to OH + C2F5CHO and HO2+ C3F7CHO to OH + C3F7CHO within the Temperature Range of 240 to 350 K.
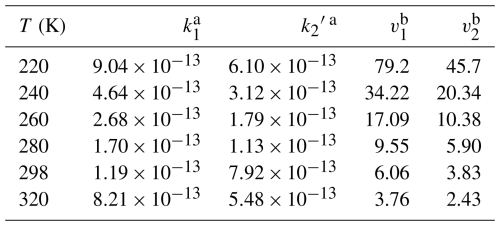
a k1 and k2′ are the rate constants of the HO2 reactions with C2F5CHO and C3F7CHO, from the literature respectively. b v1 and v2 denote the rate ratios of HO2 with C2F5CHO and C3F7CHO to OH with C2F5CHO and C3F7CHO, respectively.
Table 2Hydroperoxyl radical concentration (in molecules cm−3), rate constants (in cm3 molecule−1 s−1), and atmospheric lifetimes (in hours) with respect to bimolecular reactions as functions of altitude.
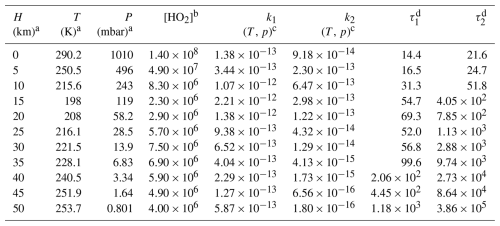
a H denotes altitude (atmospheric scale height); T denotes temperature; p denotes pressure. b Data are from Brasseur and Solomon (2006). c k1, k2 are the rate constants of the HO2 reactions with C2F5CHO and C3F7CHO, respectively. d and define the atmospheric lifetimes for HO2 reactions with C2F5CHO and C3F7CHO, respectively.
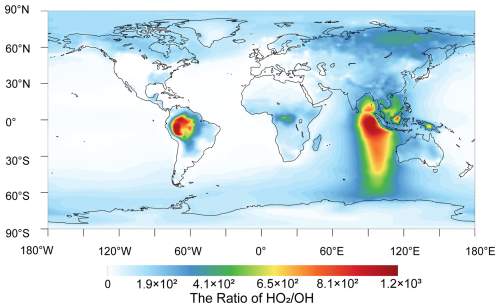
To provide further insight into the degradation of C2F5CHO and C3F7CHO under atmospheric conditions, further analysis has been done based on Geos-Chem data. GEOS-Chem simulations indicate that HO2 concentrations reach a maximum of 4.99×108 molecules cm−3 in the Amazon region, with a mean value of 9.93×107 molecules cm−3 (Long et al., 2024). In contrast, the maximum OH concentration over the Atlantic and Pacific oceans is found to be 8.03×106 molecules cm−3, with an average value of 1.06×106 molecules cm−3. (Lelieveld et al., 2016). However, the OH concentration remarkably differ from daytime to nighttime (Bey et al., 1997; Stone et al., 2012). Therefore, we consider the concentration ratio between HO2 and OH during the nighttime and daytime. During the nighttime, as shown in Fig. 9, the concentration ratio of exceeds two orders of magnitude in industrial regions such as Russia, Malaysia, and parts of Africa, with values reaching as high as 410–1200 in the Amazon. This markedly enhances the HO2-to-OH degradation rate ratios for C2F5CHO and C3F7CHO, reaching values of 88.5–259 and 56.0–164, respectively. This suggest that HO2-initiated degradation exceeds OH-initiated pathways by more than a factor of 50 during nighttime in these regions. In contrast, over oceanic regions such as the Atlantic and Pacific, the ratio falls below unity, substantially reducing the contribution of HO2 to degradation processes in these areas.
Table 3Atmospheric lifetimes (τ, hours) of C2F5CHO and C3F7CHO against major degradation pathways.
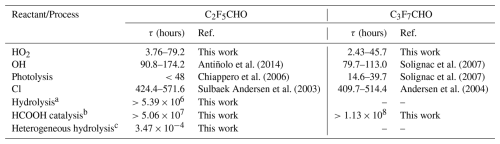
a Gas-phase hydrolysis of C2F5CHO with H2O. b HCOOH-catalyzed gas-phase hydrolysis of with H2O. c Hydrolysis of C2F5CHO with water dimer at the air-water interface.
During the nighttime, as shown in Fig. S4, the concentration ratios between HO2 and OH generally favor HO2, exhibiting maxima up to three orders of magnitude along the western coast of South America and ranging from one to two orders of magnitude in industrialized and African regions. These elevated ratios are closely associated with localized emission sources. However, During the daytime, photolysis is also an important route for removal of C2F5CHO and C3F7CHO. For C2F5CHO and C3F7CHO, photolysis represents the dominant atmospheric degradation route. C2F5CHO exhibits a high photolysis quantum yield of 0.81±0.09 at 254 nm, corresponding to an estimated atmospheric lifetime of less than 2 d in Table 3. Similarly, C3F7CHO displays a measured photolysis lifetime of 21±10 h under sunlight, confirming the efficiency of this removal mechanism (Chiappero et al., 2006; Solignac et al., 2007).
Chlorine atom reactions represent an additional potential atmospheric degradation pathway for linear perfluoroaldehydes. Kinetic measurements report rate constants of k(Cl + C2F5CHO) = (1.96±0.28) × 10−12 cm3 molecule−1 s−1 (Sulbaek Andersen et al., 2003) and k(Cl + C3F7CHO) = (2.03±0.23) × 10−12 cm3 molecule−1 s−1 (Andersen et al., 2004), which are approximately one order of magnitude higher than those for the corresponding HO2-initiated reactions. Although Cl atoms exhibit intrinsically faster reaction kinetics, the atmospheric relevance of this degradation pathway is limited by the relatively low concentrations of Cl in the troposphere. Typical Cl atom concentrations range from 1.0×104 to 3.0×105 molecules cm−3 (Chang et al., 2004; Hossaini et al., 2016; Wang et al., 2019), leading to estimated atmospheric lifetimes of approximately 400 h (∼17 d) for both C2F5CHO and C3F7CHO. This stands in sharp contrast to the significantly shorter HO2-driven lifetimes, which are typically less than 79.2 h in the lower troposphere in Table 3. The predominance of HO2-mediated degradation arises from the relatively high ambient concentrations of HO2, which compensate for its slower reaction kinetics – particularly in regions such as the Amazon, where ratios may exceed ∼103 (Li et al., 2018; Wang et al., 2019). Under such conditions, the HO2 initiated reaction rate can exceed that of Cl by 2–3 orders of magnitude. Moreover, the atmospheric relevance of Cl-initiated degradation is further constrained by its spatial heterogeneity, being primarily restricted to marine boundary layers and polluted coastal environments (Hossaini et al., 2016; Yang et al., 2022). In contrast, HO2-driven degradation is effective across continental interiors and industrialized regions.
In addition to photolysis and radical-initiated oxidation, hydrolysis constitutes another potential atmospheric sink for C2F5CHO and C3F7CHO. Taking C2F5CHO hydrolysis as an example, its gas phase hydrolysis proceeds extremely slowly, with an estimated atmospheric lifetime exceeding 5.39×106 h (Tables 3 and S11). This removal pathway is negligible, aligning with findings reported for CF3CHO (Sulbaek Andersen et al., 2006). Hydrolysis catalyzed by atmospheric acids could potentially enhance hydrolysis rates through its ability to reduce the reaction barriers (Hazra et al., 2013; Liu et al., 2021). Formic acid (HCOOH), a ubiquitous atmospheric component, forms stable complexes with water (HCOOH⋯H2O). Even at elevated concentrations of HCOOH⋯H2O complexes (e.g., 1011 molecule cm−3), the estimated hydrolysis lifetimes of C2F5CHO and C3F7CHO exceed 107 h in Table 3. These timescales remain orders of magnitude longer than those associated with HO2-initiated degradation, suggesting that the acid-catalyzed hydrolysis is insufficient to promote significant atmospheric removal of these compounds. Therefore, although acid catalysis effectively reduces the reaction barrier, its impact on the gas-phase degradation of C2F5CHO and C3F7CHO is negligible under typical tropospheric conditions.
Hydrolysis at air–water interfaces, such as those present on aerosol particles and cloud droplets, proceeds with markedly enhanced efficiency. Laboratory experiments have shown that passing gaseous CF3CHO through liquid water results in over 80 % conversion to CF3CH(OH)2 within seconds, highlighting the potential importance of heterogeneous processes in atmospheric removal pathways (Sulbaek Andersen et al., 2006). Similarly, 1H NMR measurements reveal that C2F5CHO rapidly converts to its gem-diol form, CF3CF2CH(OH)2, within 3 min upon contact with liquid water, further confirming the efficient aqueous-phase hydration of perfluoroaldehydes. (Sulbaek Andersen et al., 2006). Here, we estimate a low Gibbs free-energy barrier (ΔG=9.8 kcal mol−1) for C2F5CHO + 3H2O at air-water interfaces, proceeding via a cyclic proton-transfer mechanism by using ab initio molecular dynamics, compared to a much higher barrier of 25.5 kcal mol−1 for the corresponding gas-phase reaction (see Fig. S5a, b). More details are provided in Supplement. This results in significantly shorter atmospheric lifetimes, on the order of h. Under humid conditions, such air–water interfacial hydrolysis is likely to dominate and may effectively compete with HO2-mediated degradation pathways. Once formed, CnF2n+1CH(OH)2 reacts with OH, ultimately leading to the formation of perfluorocarboxylic acids (PFCAs). This hydrolysis-oxidation pathway represents a significant indirect source of persistent PFCAs, particularly given the ubiquity of aqueous phases in the atmosphere.
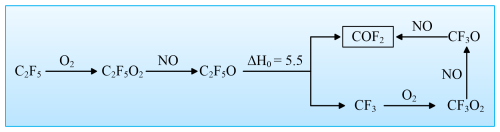
We can conclude that HO2 initiated degradation pathways dominate the gas-phase degradation of C2F5CHO and C3F7CHO. We further consider the final product in the HO2+ CnF2n+1CHO reactions. As mentioned, the HO2 reaction generates intermediate perfluoroalkyl radicals CnF2n+1, which can subsequently undergo a carbon-shortening process to form the more stable COF2, as depicted in Fig. 10. Taking the example of C2F5, the process starts with C2F5 reacting with O2 to form C2F5O2. Subsequently, C2F5O2 reacts with NO to produce C2F5O, which then undergoes C-C bond cleavage to generate CF3 and COF2. The CF3 further reacts to eventually yield COF2 through a similar reaction pathway. However, the absence of quantified rate constants for these reactions prevents a robust assessment of their global or regional impacts. A comprehensive evaluation of the role of NO would require integrating the kinetics of RO2 + NO reactions (e.g., ) into atmospheric models, which is beyond the scope of this study.
In summary, we identify that the HO2-initiated reaction represents an important atmospheric sink for linear perfluoroaldehydes in gas phase. Notably, recent studies suggest that HO2 concentrations may be elevated at air–water interfaces compared to the bulk gas phase (Angelaki et al., 2024; Li et al., 2023). Given the enhanced reactivity at the air–water interface and the complex competition between OH and HO2, interfacial HO2-driven degradation may play a more significant role than previously recognized, potentially influencing atmospheric acidity. For example, Xia et al. (2024b) recently reported both single-carbon and double-carbon scission pathways during the degradation of C7F15 on water droplet surfaces. These heterogeneous processes may contribute not only to the atmospheric removal of perfluoroaldehydes but also to the broader degradation of polyfluoroalkyl substances (PFAS). Nevertheless, further experimental and theoretical studies on reaction kinetics and mechanisms are needed to better constrain this complex chemical processes. Incorporating such processes into atmospheric models is crucial for improving the prediction of PFAS environmental fate and secondary pollution, with important implications for emission control strategies and environmental risk assessment.
In this study, we have delved into the chemical reaction kinetics of linear perfluoroaldehydes (C2F5CHO and C3F7CHO) with hydroperoxyl radicals in the gas phase using ab initio calculation methods and reaction kinetics theory. We find that the activation enthalpies for the reactions of C2F5CHO and C3F7CHO with HO2 at 0 K are both −2.7 kcal mol−1, demonstrating that carbon chain elongation in linear perfluoroaldehydes has a negligible thermodynamic influence on their enthalpies of activation at 0 K. This is further shown in C4F9CHO and C5F11CHO with HO2.
Further kinetic studies reveal that anharmonicity have a significant impact on the reaction rates, while the torsional anharmonicity, recross coefficient, and tunnelling effects contribute relatively little to the rate constants. It is particularly noteworthy that the reaction of C3F7CHO with HO2 exhibits a distinct pressure dependence, whereas the reaction of C2F5CHO with HO2 does not show such a pressure effect.
By integrating kinetics with the data based on GEOS-Chem modelling, we have identified some regions such as Russia, Malaysia, and parts of Africa, where HO2 concentration exceeds OH concentration by 2–3 orders of magnitude. Therefore, the reactions of HO2 with C2F5CHO and C3F7CHO can compete well with their corresponding reaction with OH. Specifically, the atmospheric lifetimes of C2F5CHO and C3F7CHO via HO2 are shortened to be 14.4–31.3 and 21.6–51.8 h, respectively, with orders of magnitude shorter than that of the corresponding OH-mediated pathways. In addition, photolysis, typically occurring within 48 h, represents an efficient daytime removal pathway, while heterogeneous hydrolysis proceeds rapidly at air-water interfaces with characteristic timescales of less than 1 h. Accordingly, HO2-initiated degradation should be considered a major gas-phase sink, particularly in continental source regions. Under high NOx conditions, this pathway may contribute to tropospheric HCOOH and COF2 formation.
While the present investigation establishes the HO2-mediated degradation pathway for linear perfluoroaldehydes (C2F5CHO/C3F7CHO), it simultaneously highlights critical gaps in our understanding of their atmospheric lifetimes. Notably, the current work focuses on gas-phase HO2 reactions. However, the roles of heterogeneous interfacial processes (e.g., on aerosol surfaces or cloud droplets) remain unexplored (Zhang et al., 2024). The potential for HO2-driven defluorination to generate reactive CF3 radicals, which could initiate secondary reactions (e.g., with O3 or NO2), requires systematic investigation to assess implications for atmospheric oxidizing capacity and secondary aerosol formation. Additionally, the study focuses on radical-driven pathways but acknowledges that photolysis is a competing sink for linear perfluoroaldehydes. Future work should quantify photolysis rates under stratospheric UV conditions (e.g., 200–300 nm) to reconcile discrepancies between modeled and observed atmospheric lifetimes (Thomson et al., 2025). Addressing these limitations will require integrating advanced experimental techniques (e.g., synchrotron-based photoionization mass spectrometry) with multi-scale modeling frameworks, while prioritizing under sampled environments like the upper troposphere and polar regions where HO2 reactivity anomalies could profoundly alter PFAS degradation trajectories (Alam et al., 2024; Zhou et al., 2024b). Such efforts are critical for refining environmental risk assessments of emerging HFOs and guiding the design of next-generation chemicals with minimized atmospheric persistence.
All data from this research can be obtained upon request by contacting Bo Long (wwwltcommon@sina.com) or Zegang Dong (dzegang@sina.com).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-14315-2025-supplement.
BL designed the project; ZD performed the quantum chemical calculations; CX performed the model calculations; ZD, CX, and BL analysed the data; ZD wrote the manuscript draft. ZD, CX, and BL reviewed and edited the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Also, please note that this paper has not received English language copy-editing. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This work was supported in part by the National Natural Science Foundation of China (grant nos. 42120104007 and 41775125) and by Guizhou Provincial Science and Technology Projects, China (grant nos. ZK [2022]194, CXTD [2022]001, and GCC [2023]026).
This paper was edited by Zhibin Wang and reviewed by three anonymous referees.
Ackerman Grunfeld, D., Gilbert, D., Hou, J., Jones, A. M., Lee, M. J., Kibbey, T. C. G., and O'Carroll, D. M.: Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters, Nature Geoscience, 17, 340–346, https://doi.org/10.1038/s41561-024-01402-8, 2024.
Adler, T. B., Knizia, G., and Werner, H. J.: A simple and efficient CCSD(T)-F12 approximation, Journal of Chemical Physics, 127, 221106, https://doi.org/10.1063/1.2817618, 2007.
Alam, M. S., Abbasi, A., and Chen, G.: Fate, distribution, and transport dynamics of Per- and Polyfluoroalkyl Substances (PFASs) in the environment, Journal of Environmental Management, 371, 123163, https://doi.org/10.1016/j.jenvman.2024.123163, 2024.
Albrecht, S. R., Novelli, A., Hofzumahaus, A., Kang, S., Baker, Y., Mentel, T., Wahner, A., and Fuchs, H.: Measurements of hydroperoxy radicals (HO2) at atmospheric concentrations using bromide chemical ionisation mass spectrometry, Atmos. Meas. Tech., 12, 891–902, https://doi.org/10.5194/amt-12-891-2019, 2019.
Andersen, M. P. S., Nielsen, O. J., Hurley, M. D., Ball, J. C., Wallington, T. J., Stevens, J. E., Martin, J. W., Ellis, D. A., and Mabury, S. A.: Atmospheric chemistry of n-CxF2x+1CHO (x=1, 3, 4): Reaction with Cl atoms, OH radicals and IR spectra of CxF2x+1C(O)O2NO2, Journal of Physical Chemistry A, 108, 5189–5196, https://doi.org/10.1021/jp0496598, 2004.
Andersen, S. T., McGillen, M. R., Xue, C., Seubert, T., Dewald, P., Türk, G. N. T. E., Schuladen, J., Denjean, C., Etienne, J.-C., Garrouste, O., Jamar, M., Harb, S., Cirtog, M., Michoud, V., Cazaunau, M., Bergé, A., Cantrell, C., Dusanter, S., Picquet-Varrault, B., Kukui, A., Mellouki, A., Carpenter, L. J., Lelieveld, J., and Crowley, J. N.: Measurement report: Sources, sinks, and lifetime of NOx in a suburban temperate forest at night, Atmos. Chem. Phys., 24, 11603–11618, https://doi.org/10.5194/acp-24-11603-2024, 2024.
Angelaki, M., Carreira Mendes Da Silva, Y., Perrier, S., and George, C.: Quantification and Mechanistic Investigation of the Spontaneous H2O2 Generation at the Interfaces of Salt-Containing Aqueous Droplets, Journal of the American Chemical Society, 146, 8327–8334, https://doi.org/10.1021/jacs.3c14040, 2024.
Antiñolo, M., González, S., Ballesteros, B., Albaladejo, J., and Jiménez, E.: Laboratory studies of CHF2CF2CH2OH and CF3CF2CH2OH: UV and IR absorption cross sections and OH rate coefficients between 263 and 358 K, Journal of Physical Chemistry A, 116, 6041–6050, https://doi.org/10.1021/jp2111633, 2012.
Antiñolo, M., Jiménez, E., González, S., and Albaladejo, J.: Atmospheric chemistry of CF3CF2CHO: Absorption cross sections in the UV and IR regions, photolysis at 308 nm, and gas-phase reaction with OH radicals (T=263–358 K), Journal of Physical Chemistry A, 118, 178–186, https://doi.org/10.1021/jp410283v, 2014.
Bao, J. L. and Truhlar, D. G.: Variational transition state theory: Theoretical framework and recent developments, Chemical Society Reviews, 46, 7548–7596, https://doi.org/10.1039/c7cs00602k, 2017.
Bao, J. L., Zhang, X., and Truhlar, D. G.: Predicting pressure-dependent unimolecular rate constants using variational transition state theory with multidimensional tunneling combined with system-specific quantum RRK theory: A definitive test for fluoroform dissociation, Physical Chemistry Chemical Physics, 18, 16659–16670, https://doi.org/10.1039/c6cp02765b, 2016a.
Bao, J. L., Zheng, J., and Truhlar, D. G.: Kinetics of Hydrogen Radical Reactions with Toluene Including Chemical Activation Theory Employing System-Specific Quantum RRK Theory Calibrated by Variational Transition State Theory, Journal of the American Chemical Society, 138, 2690–2704, https://doi.org/10.1021/jacs.5b11938, 2016b.
Berndt, T., Richters, S., Kaethner, R., Voigtländer, J., Stratmann, F., Sipilä, M., Kulmala, M., and Herrmann, H.: Gas-Phase Ozonolysis of Cycloalkenes: Formation of Highly Oxidized RO2 Radicals and Their Reactions with NO, NO2, SO2, and Other RO2 Radicals, Journal of Physical Chemistry A, 119, 10336–10348, https://doi.org/10.1021/acs.jpca.5b07295, 2015.
Bey, I., Aumont, B., and Toupance, G.: The nighttime production of OH radicals in the continental troposphere, Geophysical Research Letters, 24, 1067–1070, https://doi.org/10.1029/97GL00889, 1997.
Bey, I., Jacob, D. J., Yantosca, R. M., Logan, J. A., Field, B. D., Fiore, A. M., Li, Q., Liu, H. Y., Mickley, L. J., and Schultz, M. G.: Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation, Journal of Geophysical Research-Atmospheres, 106, 23073–23095, https://doi.org/10.1029/2001JD000807, 2001.
Bloino, J., Biczysko, M., and Barone, V.: General perturbative approach for spectroscopy, thermodynamics, and kinetics: Methodological background and benchmark studies, Journal of Chemical Theory and Computation, 8, 1015–1036, https://doi.org/10.1021/ct200814m, 2012.
Bloss, W. J., Lee, J. D., Heard, D. E., Salmon, R. A., Bauguitte, S. J.-B., Roscoe, H. K., and Jones, A. E.: Observations of OH and HO2 radicals in coastal Antarctica, Atmos. Chem. Phys., 7, 4171–4185, https://doi.org/10.5194/acp-7-4171-2007, 2007.
Bottorff, B., Lew, M. M., Woo, Y., Rickly, P., Rollings, M. D., Deming, B., Anderson, D. C., Wood, E., Alwe, H. D., Millet, D. B., Weinheimer, A., Tyndall, G., Ortega, J., Dusanter, S., Leonardis, T., Flynn, J., Erickson, M., Alvarez, S., Rivera-Rios, J. C., Shutter, J. D., Keutsch, F., Helmig, D., Wang, W., Allen, H. M., Slade, J. H., Shepson, P. B., Bertman, S., and Stevens, P. S.: OH, HO2, and RO2 radical chemistry in a rural forest environment: measurements, model comparisons, and evidence of a missing radical sink, Atmos. Chem. Phys., 23, 10287–10311, https://doi.org/10.5194/acp-23-10287-2023, 2023.
Brasseur, G. and Solomon, S.: Aeronomy of the middle atmosphere: chemistry and physics of the stratosphere and mesosphere, Springer Science & Business Media, 617–627 pp., https://doi.org/10.1007/1-4020-3824-0, 2006.
Burkholder, J. B., Cox, R. A., and Ravishankara, A. R.: Atmospheric Degradation of Ozone Depleting Substances, Their Substitutes, and Related Species, Chemical Reviews, 115, 3704–3759, https://doi.org/10.1021/cr5006759, 2015.
Canneaux, S., Bohr, F., and Henon, E.: KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results, Journal of Computational Chemistry, 35, 82–93, https://doi.org/10.1002/jcc.23470, 2014.
Chang, C. T., Liu, T. H., and Jeng, F. T.: Atmospheric concentrations of the Cl atom, CIO radical, and HO radical in the coastal marine boundary layer, Environmental Research, 94, 67–74, https://doi.org/10.1016/j.envres.2003.07.008, 2004.
Chen, W., Zheng, J., Bao, J. L., Truhlar, D. G., and Xu, X.: MSTor 2023: A new version of the computer code for multistructural torsional anharmonicity, now with automatic torsional identification using redundant internal coordinates, Computer Physics Communications, 288, 108740, https://doi.org/10.1016/j.cpc.2023.108740, 2023.
Chiappero, M. S., Malanca, F. E., Argüello, G. A., Wooldridge, S. T., Hurley, M. D., Ball, J. C., Wallington, T. J., Waterland, R. L., and Buck, R. C.: Atmospheric chemistry of perfluoroaldehydes (CxF2x+1CHO) and fluorotelomer aldehydes (CxF2x+1CH2CHO): Quantification of the important role, of photolysis, Journal of Physical Chemistry A, 110, 11944–11953, https://doi.org/10.1021/jp064262k, 2006.
Chiappero, M. S., Argüello, G. A., Hurley, M. D., and Wallington, T. J.: Atmospheric chemistry of n-C6F13CH2CHO: Formation from n-C6F13CH2CH2OH, kinetics, and mechanisms of reactions with chlorine atoms and OH radicals, Journal of Physical Chemistry A, 114, 6131–6137, https://doi.org/10.1021/jp101587m, 2010.
David, L. M., Barth, M., Höglund-Isaksson, L., Purohit, P., Velders, G. J. M., Glaser, S., and Ravishankara, A. R.: Trifluoroacetic acid deposition from emissions of HFO-1234yf in India, China, and the Middle East, Atmos. Chem. Phys., 21, 14833–14849, doi10.5194/acp-21-14833-2021, 2021.
Ding, D. P. and Long, B.: Reaction between propionaldehyde and hydroxyperoxy radical in the atmosphere: A reaction route for the sink of propionaldehyde and the formation of formic acid, Atmospheric Environment, 284, 119202, https://doi.org/10.1016/j.atmosenv.2022.119202, 2022.
Dong, Z. G., Xu, F., Mitchell, E., and Long, B.: Trifluoroacetaldehyde aminolysis catalyzed by a single water molecule: An important sink pathway for trifluoroacetaldehyde and a potential pathway for secondary organic aerosol growth, Atmospheric Environment, 249, 118242, https://doi.org/10.1016/j.atmosenv.2021.118242, 2021.
Gao, Q., Shen, C., Zhang, H., Long, B., and Truhlar, D. G.: Quantitative Kinetics Reveal that Reactions of HO2 are a Significant Sink for Aldehydes in the Atmosphere and may Initiate the Formation of Highly Oxygenated Molecules via Autoxidation , Physical Chemistry Chemical Physics, 26, 16160–16174, https://doi.org/10.1039/d4cp00693c, 2024.
Garrett, B. C. and Truhlar, D. G.: Criterion of minimum state density in the transition state theory of bimolecular reactions, The Journal of Chemical Physics, 70, 1593–1598, https://doi.org/10.1063/1.437698, 1979.
Gelaro, R., McCarty, W., Suárez, M. J., Todling, R., Molod, A., Takacs, L., Randles, C. A., Darmenov, A., Bosilovich, M. G., Reichle, R., Wargan, K., Coy, L., Cullather, R., Draper, C., Akella, S., Buchard, V., Conaty, A., da Silva, A. M., Gu, W., Kim, G. K., Koster, R., Lucchesi, R., Merkova, D., Nielsen, J. E., Partyka, G., Pawson, S., Putman, W., Rienecker, M., Schubert, S. D., Sienkiewicz, M., and Zhao, B.: The modern-era retrospective analysis for research and applications, version 2 (MERRA-2), Journal of Climate, 30, 5419–5454, https://doi.org/10.1175/JCLI-D-16-0758.1, 2017.
Goldman, M. J., Green, W. H., and Kroll, J. H.: Chemistry of Simple Organic Peroxy Radicals under Atmospheric through Combustion Conditions: Role of Temperature, Pressure, and NOx Level, Journal of Physical Chemistry A, 125, 10303–10314, https://doi.org/10.1021/acs.jpca.1c07203, 2021.
Gyevi-Nagy, L., Kállay, M., and Nagy, P. R.: Accurate Reduced-Cost CCSD(T) Energies: Parallel Implementation, Benchmarks, and Large-Scale Applications, Journal of Chemical Theory and Computation, 17, 860–878, https://doi.org/10.1021/acs.jctc.0c01077, 2021.
Hazra, M. K., Francisco, J. S., and Sinha, A.: Gas phase hydrolysis of formaldehyde to form methanediol: Impact of formic acid catalysis, Journal of Physical Chemistry A, 117, 11704–11710, https://doi.org/10.1021/jp4008043, 2013.
Hermans, I., Müller, J. F., Nguyen, T. L., Jacobs, P. A., and Peeters, J.: Kinetics of α-Hydroxy-alkylperoxyl radicals in oxidation processes. HO2?-Initiated oxidation of ketones/aldehydes near the tropopause, Journal of Physical Chemistry A, 109, 4303–4311, https://doi.org/10.1021/jp044080v, 2005.
Hossaini, R., Chipperfield, M. P., Saiz-Lopez, A., Fernandez, R., Monks, S., Feng, W., Brauer, P., and Von Glasow, R.: A global model of tropospheric chlorine chemistry: Organic versus inorganic sources and impact on methane oxidation, Journal of Geophysical Research, 121, 14271–14297, https://doi.org/10.1002/2016JD025756, 2016.
Hratchian, H. P. and Schlegel, H. B.: Accurate reaction paths using a Hessian based predictor-corrector integrator, Journal of Chemical Physics, 120, 9918–9924, https://doi.org/10.1063/1.1724823, 2004.
Hratchian, H. P. and Schlegel, H. B.: Using Hessian Updating To Increase the Efficiency of a Hessian Based Predictor-Corrector Reaction Path Following Method, Journal of Chemical Theory and Computation, 1, 61–69, 2005.
Hu, L., Millet, D. B., Baasandorj, M., Griffis, T. J., Turner, P., Helmig, D., Curtis, A. J., and Hueber, J.: Isoprene emissions and impacts over an ecological transition region in the U.S. Upper Midwest inferred from tall tower measurements, Journal of Geophysical Research-Atmospheres, 120, 3553–3571, https://doi.org/10.1002/2014JD022732, 2015.
Hurley, M. D., Wallington, T. J., Sulbaek Andersen, M. P., Ellis, D. A., Martin, J. W., and Mabury, S. A.: Atmospheric chemistry of fluorinated alcohols: Reaction with Cl atoms and OH radicals and atmospheric lifetimes, Journal of Physical Chemistry A, 108, 1973–1979, https://doi.org/10.1021/jp0373088, 2004.
Hurley, M. D., Ball, J. C., Wallington, T. J., Sulbaek Andersen, M. P., Nielsen, C. J., Ellis, D. A., Martin, J. W., and Mabury, S. A.: Atmospheric chemistry of n-CxF2x+1CHO (x = 1,2,3,4): Fate of n-CxF2x+1C(O) radicals, Journal of Physical Chemistry A, 110, 12443–12447, 2006.
Jiménez, E., González, S., Cazaunau, M., Chen, H., Ballesteros, B., Daële, V., Albaladejo, J., and Mellouki, A.: Atmospheric Degradation Initiated by OH Radicals of the Potential Foam Expansion Agent, CF3(CF2)2CH=CH2 (HFC-1447fz): Kinetics and Formation of Gaseous Products and Secondary Organic Aerosols, Environmental Science and Technology, 50, 1234–1242, https://doi.org/10.1021/acs.est.5b04379, 2016.
Kállay, M., Nagy, P. R., Mester, D., Rolik, Z., Samu, G., Csontos, J., Csóka, J., Szabó, P. B., Gyevi-Nagy, L., Hégely, B., Ladjánszki, I., Szegedy, L., Ladóczki, B., Petrov, K., Farkas, M., Mezei, P. D., and Ganyecz, Á.: The MRCC program system: Accurate quantum chemistry from water to proteins., The Journal of chemical physics, 152, 74107, https://doi.org/10.1063/1.5142048, 2020.
Kállay, M., Nagy, P. R., Mester, D., Gyevi-Nagy, L., Csóka, J., Szabó, P. B., Rolik, Z., Samu, G., Csontos, J., Hégely, B., Ganyecz, Á., Ladjánszki, I., Szegedy, L., Ladóczki, B., Petrov, K., Farkas, M., and Mezei, P. D.: MRCC, a quantum chemical program suite, https://mrcc.hu/ (last access: 17 October 2025), 2022.
Kenyon, R. L.: The Path of Chemical Reactions — The IRC Approach, Accounts of Chemical Research, 46, 5, https://doi.org/10.1021/cen-v046n004.p005, 1968.
King, M. D., Canosa-Mas, C. E., and Wayne, R. P.: Gas-phase reactions between RO2 and NO, HO2 or CH3O2: correlations between rate constants and the SOMO energy of the peroxy (RO2) radical, Atmospheric Environment, 35, 2081–2088, https://doi.org/10.1016/S1352-2310(00)00501-X, 2001.
Knizia, G., Adler, T. B., and Werner, H. J.: Simplified CCSD(T)-F12 methods: Theory and benchmarks, Journal of Chemical Physics, 130, 054104, https://doi.org/10.1063/1.3054300, 2009.
Kuhler, K. M., Truhlar, D. G., and Isaacson, A. D.: General method for removing resonance singularities in quantum mechanical perturbation theory, The Journal of Chemical Physics, 104, 4664–4671, https://doi.org/10.1063/1.471161, 1996.
Lee, B. H., Munger, J. W., Wofsy, S. C., Rizzo, L. V., Yoon, J. Y. S., Turner, A. J., Thornton, J. A., and Swann, A. L. S.: Sensitive Response of Atmospheric Oxidative Capacity to the Uncertainty in the Emissions of Nitric Oxide (NO) From Soils in Amazonia, Geophysical Research Letters, 51, e2023GL107214, https://doi.org/10.1029/2023GL107214, 2024.
Lelieveld, J., Gromov, S., Pozzer, A., and Taraborrelli, D.: Global tropospheric hydroxyl distribution, budget and reactivity, Atmos. Chem. Phys., 16, 12477–12493, https://doi.org/10.5194/acp-16-12477-2016, 2016.
Lew, M. M., Rickly, P. S., Bottorff, B. P., Reidy, E., Sklaveniti, S., Léonardis, T., Locoge, N., Dusanter, S., Kundu, S., Wood, E., and Stevens, P. S.: OH and HO2 radical chemistry in a midlatitude forest: measurements and model comparisons, Atmos. Chem. Phys., 20, 9209–9230, https://doi.org/10.5194/acp-20-9209-2020, 2020.
Li, K., Guo, Y., Nizkorodov, S. A., Rudich, Y., Angelaki, M., Wang, X., An, T., Perrier, S., and George, C.: Spontaneous dark formation of OH radicals at the interface of aqueous atmospheric droplets, Proceedings of the National Academy of Sciences of the United States of America, 120, e2220228120, https://doi.org/10.1073/pnas.2220228120, 2023.
Li, M., Karu, E., Brenninkmeijer, C., Fischer, H., Lelieveld, J., and Williams, J.: Tropospheric OH and stratospheric OH and Cl concentrations determined from CH4, CH3Cl, and SF6 measurements, npj Climate and Atmospheric Science, 1, 29, https://doi.org/10.1038/s41612-018-0041-9, 2018.
Li, X., Wang, Y., Cui, J., Shi, Y., and Cai, Y.: Occurrence and Fate of Per- and Polyfluoroalkyl Substances (PFAS) in Atmosphere: Size-Dependent Gas-Particle Partitioning, Precipitation Scavenging, and Amplification, Environmental Science and Technology, 58, 9283–9291, https://doi.org/10.1021/acs.est.4c00569, 2024.
Lin, H., Jacob, D. J., Lundgren, E. W., Sulprizio, M. P., Keller, C. A., Fritz, T. M., Eastham, S. D., Emmons, L. K., Campbell, P. C., Baker, B., Saylor, R. D., and Montuoro, R.: Harmonized Emissions Component (HEMCO) 3.0 as a versatile emissions component for atmospheric models: application in the GEOS-Chem, NASA GEOS, WRF-GC, CESM2, NOAA GEFS-Aerosol, and NOAA UFS models, Geosci. Model Dev., 14, 5487–5506, https://doi.org/10.5194/gmd-14-5487-2021, 2021.
Liu, J. Y., Long, Z. W., Mitchell, E., and Long, B.: New Mechanistic Pathways for the Reactions of Formaldehyde with Formic Acid Catalyzed by Sulfuric Acid and Formaldehyde with Sulfuric Acid Catalyzed by Formic Acid: Formation of Potential Secondary Organic Aerosol Precursors, ACS Earth and Space Chemistry, 5, 1363–1372, https://doi.org/10.1021/acsearthspacechem.1c00002, 2021.
Liu, Y. P., Lynch, G. C., Truong, T. N., Lu, D. hong, Truhlar, D. G., and Garrett, B. C.: Molecular Modeling of the Kinetic Isotope Effect for the [1,5] Sigmatropic Rearrangement of cis-1,3-Pentadiene, Journal of the American Chemical Society, 115, 2408–2415, https://doi.org/10.1021/ja00059a041, 1993.
Long, B., Bao, J. L., and Truhlar, D. G.: Atmospheric Chemistry of Criegee Intermediates: Unimolecular Reactions and Reactions with Water, Journal of the American Chemical Society, 138, 14409–14422, https://doi.org/10.1021/jacs.6b08655, 2016.
Long, B., Bao, J. L., and Truhlar, D. G.: Rapid unimolecular reaction of stabilized Criegee intermediates and implications for atmospheric chemistry, Nature Communications, 10, 2003, https://doi.org/10.1038/s41467-019-09948-7, 2019.
Long, B., Xia, Y., and Truhlar, D. G.: Quantitative Kinetics of HO2 Reactions with Aldehydes in the Atmosphere: High-Order Dynamic Correlation, Anharmonicity, and Falloff Effects Are All Important, Journal of the American Chemical Society, 144, 19910–19920, https://doi.org/10.1021/jacs.2c07994, 2022.
Long, B., Zhang, Y.-Q., Xie, C., Tan, X.-F., and Truhlar, D. G.: Reaction of Carbonyl Oxide with Hydroperoxymethyl Thioformate: Quantitative Kinetics and Atmospheric Implications, Research, 7, 525, https://doi.org/10.34133/research.0525, 2024.
Lynch, B. J., Zhao, Y., and Truhlar, D. G.: Effectiveness of diffuse basis functions for calculating relative energies by density functional theory, Journal of Physical Chemistry A, 107, 1384–1388, https://doi.org/10.1021/jp021590l, 2003.
Martin, J. W., Mabury, S. A., and O'Brien, P. J.: Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes, Chemico-Biological Interactions, 155, 165–180, https://doi.org/10.1016/j.cbi.2005.06.007, 2005.
McDuffie, E. E., Smith, S. J., O'Rourke, P., Tibrewal, K., Venkataraman, C., Marais, E. A., Zheng, B., Crippa, M., Brauer, M., and Martin, R. V.: A global anthropogenic emission inventory of atmospheric pollutants from sector- and fuel-specific sources (1970–2017): an application of the Community Emissions Data System (CEDS), Earth Syst. Sci. Data, 12, 3413–3442, https://doi.org/10.5194/essd-12-3413-2020, 2020.
Nie, W., Yan, C., Yang, L., Roldin, P., Liu, Y., Vogel, A. L., Molteni, U., Stolzenburg, D., Finkenzeller, H., Amorim, A., Bianchi, F., Curtius, J., Dada, L., Draper, D. C., Duplissy, J., Hansel, A., He, X. C., Hofbauer, V., Jokinen, T., Kim, C., Lehtipalo, K., Nichman, L., Mauldin, R. L., Makhmutov, V., Mentler, B., Mizelli-Ojdanic, A., Petäjä, T., Quéléver, L. L. J., Schallhart, S., Simon, M., Tauber, C., Tomé, A., Volkamer, R., Wagner, A. C., Wagner, R., Wang, M., Ye, P., Li, H., Huang, W., Qi, X., Lou, S., Liu, T., Chi, X., Dommen, J., Baltensperger, U., El Haddad, I., Kirkby, J., Worsnop, D., Kulmala, M., Donahue, N. M., Ehn, M., and Ding, A.: NO at low concentration can enhance the formation of highly oxygenated biogenic molecules in the atmosphere, Nature Communications, 14, 3347, https://doi.org/10.1038/s41467-023-39066-4, 2023.
Orlando, J. J., Iraci, L. T., and Tyndall, G. S.: Chemistry of the cyclopentoxy and cyclohexoxy radicals at subambient temperatures, Journal of Physical Chemistry A, 104, 5072–5079, https://doi.org/10.1021/jp0002648, 2000.
Rand, A. A. and Mabury, S. A.: Is there a human health risk associated with indirect exposure to perfluoroalkyl carboxylates (PFCAs)?, Toxicology, 375, 28–36, https://doi.org/10.1016/j.tox.2016.11.011, 2017.
Rupp, J., Guckert, M., Berger, U., Drost, W., Mader, A., Nödler, K., Nürenberg, G., Schulze, J., Söhlmann, R., and Reemtsma, T.: Comprehensive target analysis and TOP assay of per- and polyfluoroalkyl substances (PFAS) in wild boar livers indicate contamination hot-spots in the environment, Science of the Total Environment, 871, 162028, https://doi.org/10.1016/j.scitotenv.2023.162028, 2023.
Sellevåg, S. R., Kelly, T., Sidebottom, H., and Nielsen, C. J.: A study of the IR and UV-Vis absorption cross-sections, photolysis and OH-initiated oxidation of CF3CHO and CF3CH2CHO, Phys. Chem. Chem. Phys., 6, 1243–1252, https://doi.org/10.1039/B315941H, 2004.
Solignac, G., Mellouki, A., Le Bras, G., Yujing, M., and Sidebottom, H.: The gas phase tropospheric removal of fluoroaldehydes (CxF2x+1CHO, x=3, 4, 6), Physical Chemistry Chemical Physics, 9, 4200–4210, https://doi.org/10.1039/b614502g, 2007.
Stone, D., Whalley, L. K., and Heard, D. E.: Tropospheric OH and HO2 radicals: Field measurements and model comparisons, Chemical Society Reviews, 41, 6348–6404, https://doi.org/10.1039/c2cs35140d, 2012.
Sulbaek Andersen, M. P., Hurley, M. D., Wallington, T. J., Ball, J. C., Martin, J. W., Ellis, D. A., Mabury, S. A., and Nielsen, O. J.: Atmospheric chemistry of C2F5CHO: Reaction with Cl atoms and OH radicals, IR spectrum of C2F5C(O)O2NO2, Chemical Physics Letters, 379, 28–36, https://doi.org/10.1016/j.cplett.2003.08.004, 2003.
Sulbaek Andersen, M. P., Toft, A., Nielsen, O. J., Hurley, M. D., Wallington, T. J., Chishima, H., Tonokura, K., Mabury, S. A., Martin, J. W., and Ellis, D. A.: Atmospheric Chemistry of Perfluorinated Aldehyde Hydrates (n- CxF2x+1CH(OH)2, x=1, 3, 4): Hydration, Dehydration, and Kinetics and Mechanism of Cl Atom and OH Radical Initiated Oxidation, The Journal of Physical Chemistry A, 110, 9854–9860, https://doi.org/10.1021/jp060404z, 2006.
Sznajder-Katarzyńska, K., Surma, M., and Cieślik, I.: A Review of Perfluoroalkyl Acids (PFAAs) in terms of Sources, Applications, Human Exposure, Dietary Intake, Toxicity, Legal Regulation, and Methods of Determination, Journal of Chemistry, 2019, 2717528, https://doi.org/10.1155/2019/2717528, 2019.
Taube, A. G. and Bartlett, R. J.: Frozen natural orbital coupled-cluster theory: Forces and application to decomposition of nitroethane, Journal of Chemical Physics, 128, 164101, https://doi.org/10.1063/1.2902285, 2008.
Thackray, C. P., Selin, N. E., and Young, C. J.: A global atmospheric chemistry model for the fate and transport of PFCAs and their precursors, Environmental Science: Processes and Impacts, 22, 285–293, https://doi.org/10.1039/c9em00326f, 2020.
Thomson, A. J., Giannopoulos, G., Pretty, J., Baggs, E. M., and Richardson, D. J.: Biological sources and sinks of nitrous oxide and strategies to mitigate emissions, Philosophical Transactions of the Royal Society B, 367, 1157–1168, https://doi.org/10.1098/rstb.2011.0415, 2012.
Thomson, J. D., Campbell, J. S., Edwards, E. B., Medcraft, C., Nauta, K., Pérez-Peña, M. P., Fisher, J. A., Osborn, D. L., Kable, S. H., and Hansen, C. S.: Fluoroform (CHF3) Production from CF3CHO Photolysis and Implications for the Decomposition of Hydrofluoroolefins and Hydrochlorofluoroolefins in the Atmosphere, Journal of the American Chemical Society, 147, 33–38, https://doi.org/10.1021/jacs.4c11776, 2025.
Truhlar, D. G., Isaacson, A. D., Skodje, R. T., and Garrett, B. C.: Incorporation of quantum effects in generalized-transition-state theory, Journal of Physical Chemistry, 86, 2252–2261, https://doi.org/10.1021/j100209a021, 1982.
Vereecken, L. and Peeters, J.: Decomposition of substituted alkoxy radicals – Part I: A generalized structure-activity relationship for reaction barrier heights, Physical Chemistry Chemical Physics, 11, 9062–9074, https://doi.org/10.1039/b909712k, 2009.
Wang, Y., Liu, J., Yang, L., Zhao, X., Ji, Y. M., and Li, Z.: Theoretical studies and rate constant calculations of the reactions C2F5CHO with OH radicals and Cl atoms, Journal of Molecular Structure: THEOCHEM, 820, 26–34, https://doi.org/10.1016/j.theochem.2007.06.001, 2007.
Wang, X., Jacob, D. J., Eastham, S. D., Sulprizio, M. P., Zhu, L., Chen, Q., Alexander, B., Sherwen, T., Evans, M. J., Lee, B. H., Haskins, J. D., Lopez-Hilfiker, F. D., Thornton, J. A., Huey, G. L., and Liao, H.: The role of chlorine in global tropospheric chemistry, Atmos. Chem. Phys., 19, 3981–4003, https://doi.org/10.5194/acp-19-3981-2019, 2019.
Wang, Y., Wang, Z., Sun, M., Guo, J., and Zhang, J.: Emissions, degradation and impact of HFO-1234ze from China PU foam industry, Science of the Total Environment, 780, 146631, https://doi.org/10.1016/j.scitotenv.2021.146631, 2021.
Wang, Y., Liu, L., Qiao, X., Sun, M., Guo, J., Zhang, J., and Zhao, B.: Projections of National-Gridded Emissions of Hydrofluoroolefins (HFOs) in China, Environmental Science and Technology, 57, 8650–8659, https://doi.org/10.1021/acs.est.2c09263, 2023.
Wang, Y., Liu, L., Qiao, X., Sun, M., Guo, J., Zhao, B., and Zhang, J.: Atmospheric fate and impacts of HFO-1234yf from mobile air conditioners in East Asia, Science of the Total Environment, 916, 170137, https://doi.org/10.1016/j.scitotenv.2024.170137, 2024.
Waterland, R. L. and Dobbs, K. D.: Atmospheric chemistry of linear perfluorinated aldehydes: Dissociation kinetics of CnF2n+1CO radicals, Journal of Physical Chemistry A, 111, 2555–2562, https://doi.org/10.1021/jp067587+, 2007.
Werner, H.-J., Knowles, P. J., Knizia, G., Manby, F. R., Schütz, M., Celani, P., Györffy, W., Kats, D., Korona, T., Lindh, R., Mitrushenkov, A., Rauhut, G., Shamasundar, K. R., Adler, T. B., Amos, R. D., Bennie, S. J., Bernhardsson, A., Berning, A., Cooper, D. L., Deegan, M. J. O., Dobbyn, A. J., Eckert, F., Goll, E., Hampel, C., Hesselmann, A., Hetzer, G., Hrenar, T., Jansen, G., Köppl, C., Lee, S. J. R., Liu, Y., Lloyd, A. W., Ma, Q., Mata, R. A., May, A. J., McNicholas, S. J., Meyer, W., Miller III, T. F., Mura, M. E., Nicklass, A., O'Neill, D. P., Palmieri, P., Peng, D., Pflüger, K., Pitzer, R., Reiher, M., Shiozaki, T., Stoll, H., Stone, A. J., Tarroni, R., Thorsteinsson, T., Wang, M., and Welborn, M.: MOLPRO, version 2019.2, a package of ab initio programs, https://www.molpro.net/ (last access: 17 October 2025), 2019.
Wu, J., Zhuang, Y., Dong, B., Wang, F., Yan, Y., Zhang, D., Liu, Z., Duan, X., Bo, Y., and Peng, L.: Spatial heterogeneity of per- and polyfluoroalkyl substances caused by glacial melting in Tibetan Lake Nam Co due to global warming, Journal of Hazardous Materials, 478, 135468, https://doi.org/10.1016/j.jhazmat.2024.135468, 2024.
Xia, Y., Long, B., Liu, A., and Truhlar, D. G.: Reactions with criegee intermediates are the dominant gas-phase sink for formyl fluoride in the atmosphere, Fundamental Research, 4, 1216–1224, https://doi.org/10.1016/j.fmre.2023.02.012, 2024a.
Xia, D., Zhang, H., Ju, Y., Xie, H., Su, L., Ma, F., Jiang, J., Chen, J., and Francisco, J. S.: Spontaneous Degradation of the “Forever Chemicals” Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) on Water Droplet Surfaces, Journal of the American Chemical Society, 146, 11266–11271, https://doi.org/10.1021/jacs.4c00435, 2024b.
Yang, X., Wang, Q., Ma, N., Hu, W., Gao, Y., Huang, Z., Zheng, J., Yuan, B., Yang, N., Tao, J., Hong, J., Cheng, Y., and Su, H.: The impact of chlorine chemistry combined with heterogeneous N2O5 reactions on air quality in China, Atmos. Chem. Phys., 22, 3743–3762, https://doi.org/10.5194/acp-22-3743-2022, 2022.
Yu, T., Zheng, J., and Truhlar, D. G.: Multipath variational transition state theory: Rate constant of the 1,4-hydrogen shift isomerization of the 2-cyclohexylethyl radical, Journal of Physical Chemistry A, 116, 297–308, https://doi.org/10.1021/jp209146b, 2012.
Yu, Y., Pan, L., Sun, Q., and Wang, J.: The mechanism and kinetics of the atmospheric oxidation of CF3(CF2)2CH = CH2 (HFC-1447fz) by hydroxyl radicals: ab initio investigation, Physical Chemistry Chemical Physics, 26, 10989–10997, https://doi.org/10.1039/d3cp06149c, 2024.
Zhang, T., Zhang, Y., Wen, M., Tang, Z., Long, B., Yu, X., Zhao, C., and Wang, W.: Effects of water, ammonia and formic acid on HO2+ Cl reactions under atmospheric conditions: Competition between a stepwise route and one elementary step, RSC Advances, 9, 21544–21556, https://doi.org/10.1039/c9ra03541a, 2019.
Zhang, W., Issa, K., Tang, T., and Zhang, H.: Role of Hydroperoxyl Radicals in Heterogeneous Oxidation of Oxygenated Organic Aerosols, Environmental Science and Technology, 58, 4727–4736, https://doi.org/10.1021/acs.est.3c09024, 2024.
Zhang, Y., Cheng, Y., Zhang, T., Wang, R., Ji, J., Xia, Y., Lily, M., Wang, Z., and Muthiah, B.: A computational study of the HO2+ SO3 → HOSO2+ 3O2 reaction catalyzed by a water monomer, a water dimer and small clusters of sulfuric acid: kinetics and atmospheric implications, Physical Chemistry Chemical Physics, 24, 18205–18216, https://doi.org/10.1039/d1cp03318b, 2022.
Zhao, Y. and Truhlar, D. G.: Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions, Journal of Chemical Theory and Computation, 4, 1849–1868, https://doi.org/10.1021/ct800246v, 2008a.
Zhao, Y. and Truhlar, D. G.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06 functionals and 12 other functionals, Theoretical Chemistry Accounts, 120, 215–241, https://doi.org/10.1007/s00214-007-0401-8, 2008b.
Zheng, J. and Truhlar, D. G.: Kinetics of hydrogen-transfer isomerizations of butoxyl radicals, Physical Chemistry Chemical Physics, 12, 7782–7793, https://doi.org/10.1039/b927504e, 2010.
Zheng, J. and Truhlar, D. G.: Quantum thermochemistry: Multistructural method with torsional anharmonicity based on a coupled torsional potential, Journal of Chemical Theory and Computation, 9, 1356–1367, https://doi.org/10.1021/ct3010722, 2013.
Zheng, J., Yu, T., Papajak, E., Alecu, I. M., Mielke, S. L., and Truhlar, D. G.: Practical methods for including torsional anharmonicity in thermochemical calculations on complex molecules: The internal-coordinate multi-structural approximation, Physical Chemistry Chemical Physics, 13, 10885–10907, https://doi.org/10.1039/c0cp02644a, 2011.
Zheng, J., Meana-Pañeda, R., and Truhlar, D. G.: Prediction of Experimentally Unavailable Product Branching Ratios for Biofuel Combustion: The Role of Anharmonicity in the Reaction of Isobutanol with OH, Journal of the American Chemical Society, 136, 5150–5160, https://doi.org/10.1021/ja5011288, 2014.
Zheng, J., Oyedepo, G. A., and Truhlar, D. G.: Kinetics of the Hydrogen Abstraction Reaction From 2-Butanol by OH Radical, The Journal of Physical Chemistry A, 119, 12182–12192, https://doi.org/10.1021/acs.jpca.5b06121, 2015.
Zheng, J., Bao, J. L., Meana-Pañeda, R., Zhang, S., J.Lynch, B., Corchado, J. C., Chuang, Y., Fast, P. L., Hu, W.-P., Liu, Y.-P., Lynch, G. C., Nguyen, K. A., Jackels, C. F., Ramos, A. F., Ellingson, B. A., Melissas, V. S., Villà, J., Rossi, I., Coitiño, E. L., Pu, J., Albu, T. V., Ratkiewicz, A., Steckler, R., Garrett, B. C., Isaacson, A. D., and Truhlar, D. G.: Polyrate-version 2017-C; University of Minnesota: Minneapolis, 2017.
Zheng, J., Bao, J. L., Zhang, S., Corchado, J. C., Chuang, Y., Ellingson, B. A., and Truhlar, D. G.: Gaussrate, version 2017-B; University of Minnesota: Minneapolis, MN, https://comp.chem.umn.edu/polyrate/ (last access: 17 October 2025), 2018.
Zhou, J., Fukusaki, Y., Murano, K., Gautam, T., Bai, Y., Inomata, Y., Komatsu, H., Takeda, M., Yuan, B., Shao, M., Sakamoto, Y., and Kajii, Y.: Investigation of HO2 uptake mechanisms onto multiple-component ambient aerosols collected in summer and winter time in Yokohama, Japan, Journal of Environmental Sciences (China), 137, 18–29, https://doi.org/10.1016/j.jes.2023.02.030, 2024a.
Zhou, Y., Wang, X., Wang, C., Ji, Z., Niu, X., and Dong, H.: Fate of `forever chemicals' in the global cryosphere, Earth-Science Reviews, 259, 104973, https://doi.org/10.1016/j.earscirev.2024.104973, 2024b.
Ziemann, P. J. and Atkinson, R.: Kinetics, products, and mechanisms of secondary organic aerosol formation, Chemical Society Reviews, 41, 6582–6605, https://doi.org/10.1039/c2cs35122f, 2012.