the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The critical role of volatile organic compound emissions in nitrate formation in Lhasa, Tibetan Plateau: insights from oxygen isotope anomaly measurements
Xueqin Zheng
Nima Chuduo
Bian Ba
Pengfei Yu
Phu Drolgar
Fang Cao
Yanlin Zhang
Atmospheric particulate nitrate aerosol (), produced via the oxidation of nitrogen oxides (), plays an important role in atmospheric chemistry and air quality, yet its formation mechanism remains poorly constrained in the plateau region. In this study, we report for the first time the yearly variation in the signatures of the stable oxygen isotope anomaly () in collected in the urban Lhasa (3650 meters above sea level (m a.s.l.)), on the Tibetan Plateau, China. Our results show that NO2+OH was the largest contributor to formation (46±26 %), followed by NO3+volatile organic compound (VOC) (26±18 %) and N2O5+H2O (28±11 %) using the Bayesian Isotope Mixture Model. Notably, there were significant differences in the NO2+OH, NO3+VOC, and N2O5+H2O pathways between spring and the other three seasons (T test, p<0.05). Using the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) dispersion model, we highlighted the influence of VOC emissions from regions such as Afghanistan and northern India, which enhanced concentrations in Lhasa during spring. Furthermore, the diurnal distribution of oxidation pathways varied distinctly across seasons, suggesting that these differences in pathways were attributed to aerosol liquid water content (ALWC), VOC concentrations, and the atmospheric lifetime of .
- Article
(8026 KB) - Full-text XML
-
Supplement
(1996 KB) - BibTeX
- EndNote
Nitrate aerosol () is a key component regulating the mass concentration of atmospheric fine particulate matter (PM2.5), which is highly related to air quality (Colmer et al., 2020), public health (Zhang et al., 2019, 2017; Geng et al., 2021), and the climate system (Clark and Tilman, 2008). Globally, the mass contribution of in PM2.5 is in the range of 5 %–30 % (Huang et al., 2014; Xu et al., 2019; Salameh et al., 2015; Espina-Martin et al., 2024; Bell et al., 2007; Sun et al., 2022), depending on the locations and the severities of air pollution. For example, it was reported that accounted for 22 %, 27 %, and 26 % of PM2.5 in megacities in China (Zong et al., 2020), Europe (Espina-Martin et al., 2024), and the US (Sun et al., 2022), respectively. In addition, some studies found that the contribution of would increase by 3–8 times with the occurrence of the particle-derived haze pollution (Ge et al., 2024; Song et al., 2019; Yin et al., 2022; Walters et al., 2024).
It is well known that atmospheric is formed by the oxidation of nitrogen oxides () with different oxidants such as O3, OH, and RO2 (Text S1 in the Supplement). In general, atmospheric chemical transportation models are employed to depict the detailed oxidation pathways of formation. However, there remains considerable uncertainty in modeling the contribution of individual oxidation pathways to formation, particularly the N2O5+H2O pathway, due to the wide variability of key parameters such as the N2O5 uptake coefficient, which has been shown to vary significantly depending on aerosol composition, relative humidity, and temperature. For example, it was reported that the predicted N2O5 uptake to formation in Beijing, as estimated using WRF-Chem, ranged from 5 % to 21 % (Su et al., 2017). Higher contributions between 66 % and 85 % have been observed when applying the CMAQ model in Beijing (Qiu et al., 2019). Therefore, the application of alternative techniques is crucial for providing more reliable estimates and enhancing our understanding of formation mechanisms, in addition to the insights gained from atmospheric chemical transportation models.
Stable oxygen isotope anomaly () is recognized as a powerful tool to track the formation pathways of atmospheric (Zhang et al., 2024; Feng et al., 2023). This is because the oxygen atoms in the terminal positions of O3 exhibit an elevated Δ17O () (Vicars and Savarino, 2014), whereas the Δ17O values of other atmospheric oxidants (e.g., H2O, OH, and RO2) that can be incorporated into are very close to 0 ‰. (Dubey et al., 1997; Barkan and Luz, 2003; Alexander et al., 2020) Therefore, serves as a unique tracer of O3 involvement in its formation pathways, offering valuable insights into the relative contributions of individual reactions. In recent years, the use of to elucidate formation has garnered considerable attention. Walters et al. (2024) reported that the major formation pathways of annual HNO3 production in the northeastern US were NO2+OH (46 %), N2O5 uptake (34 %), and organic nitrate hydrolysis (12 %), with notable seasonal variability. Additionally, Zhang et al. (2022) observed that the contribution of nocturnal chemistry to formation increased at night, peaking at 72 % around midnight. In contrast, the contribution of NO2+OH rose with sunrise, reaching its highest fraction (48 %) around noon. However, nearly all current Δ17O-related observations have been conducted in the plain cities, with little attention given to plateau cities, where atmospheric conditions generally suffer from distinct energy consumption patterns and unique climatic factors (e.g., intense solar radiation). In this study, we present detailed results from comprehensive field observations conducted in Lhasa (3650 meters above sea level, m a.s.l.), one of the highest cities in the world, located on the Tibetan Plateau, China. For the first time, we quantify the relative contribution of three oxidation pathways to formation in Lhasa on the basis of ambient measurements for Δ17O signatures in .
2.1 Sampling campaign
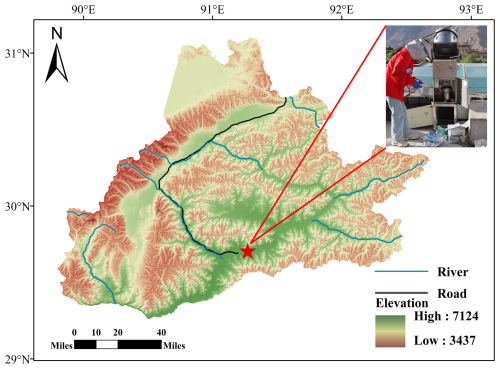
PM2.5 samples were collected on the roof of a building (∼ 15 m above ground) at the Meteorological Bureau of Lhasa (91.08° E, 29.40° N; Fig. 1) in China. Lhasa, the capital of the Tibet Autonomous Region, is a rapidly developing city with a population of ∼950 000 and an urban area of ∼30 000 km2 (Office of the Leading Group, 2025). The sampling site is surrounded by mixed land use, including residential areas, government offices, religious temples, and commercial zones, with minimal heavy industry. The strong solar radiation and large diurnal temperature variations in this sampling site can lead to pronounced changes in the boundary layer height, which in turn significantly influence vertical mixing and the transport of air pollutants.
The sampling campaign was conducted from June 2022 to July 2023 using a high-volume PM2.5 sampler, which operated at a flow rate of 1.0 m3 min−1. Samples were collected once a week, with each sampling session lasting 48 h, except during intensive sampling periods in the summer (30 June–14 July 2022) and winter (28 January–7 February 2023). During these intensive periods, each sample was collected for 12 h, from 8:00 to 20:00 LT and from 20:00 to 8:00 LT on the following day. During the autumn of 2022, Lhasa experienced intermittent COVID-19 control measures, including restricted movement, reduced traffic activity, and temporary lockdowns in urban areas (Lhasa Municipal People's Government, 2025). Before sampling, all quartz filters (, i.e., 20.3 cm×25.4 cm, Pallflex) were calcined in a muffle furnace at 450 °C for 6 h to prevent impurities from contaminating the collected PM2.5 samples. After sampling, the samples were collected and stored in a freezer at −20 °C.
2.2 Measurements of water-soluble ions and isotopes
Water-soluble ions were measured by ion chromatography (Dionex ICS-5000, Thermo Scientific Inc.) (Chen et al., 2022). In brief, a part of the filter membranes (4.54 cm2) was cut using a 17 mm diameter punch and placed in a 15 mL centrifuge tube with 10 mL of 18.2 MΩ ultrapure water. The tube was then subjected to ultrasonic treatment in an ice-water bath for 30 min to prevent ion volatilization. The extract was filtered through a 0.22 µm filter into a 30 mL sample bottle. This process was repeated with an additional 10 mL of water to ensure full extraction. The final extract was analyzed by ion chromatography. The method detection limits (MDLs) for Cl−, , , Na+, , K+, Mg2+, and Ca2+ were 0.001, 0.001, 0.003, 0.02, 0.01, 0.02, 0.006, and 0.02 mg L−1, respectively.
Stable oxygen isotopes (δ17O, δ18O, Δ17O, and ) of were determined using an isotope ratio mass spectrometer (MAT253, Thermo Fisher Scientific, USA) at Nanjing University of Information Science and Technology (Fan et al., 2021; Zhang et al., 2022). Briefly, from filter extractions (containing at least 0.8 µg N) was converted into gaseous N2O using the bacterial denitrifier method. N2O was then further thermally decomposed into O2 and N2 in a gold tube heated to 800 °C. The produced O2 was analyzed for oxygen isotopes by an isotope ratio mass spectrometer. The duplicate analysis showed that the errors were within 1.32 % for .
2.3 Primary data sources
Meteorological parameters, including ambient temperature (T), relative humidity (RH), rainfall, radiation, wind direction (WD), and wind speed (WS) during the sampling campaign, were obtained from the Meteorological Bureau of Lhasa. Additionally, NO2 and O3 during the sampling campaign were downloaded from the National Meteorological Information Center (https://air.cnemc.cn:18007/, last access: 21 December 2023).
2.4 Evaluation of oxidation pathways
In our study, we aimed to quantify the relative contribution of different oxidation pathways to production based on . Due to the low Cl− concentrations observed in Lhasa, the formation pathways considered in this study were limited to NO2+OH, NO3+volatile organic compound (VOC), and N2O5+H2O. Although NO3+VOC is generally considered a minor pathway in continental regions (Alexander et al., 2009), we included it because elevated VOC concentrations were observed at our sampling site in Lhasa, influenced by both biomass burning (e.g., incense burning) and anthropogenic sources (e.g., vehicle emissions) (Tang et al., 2022). The relative contributions of the three pathways were determined using a Δ17O-based mass balance approach (Michalski et al., 2003), as shown in Eqs. (1) and (2):
where value is the Δ17O value of in PM2.5. The (, , and correspond to the Δ17O values from NO2+OH, NO3+VOC, and N2O5+H2O, respectively. The Δ17O values for each pathway were calculated using Eqs. (3)–(5) (Savarino et al., 2016; Alexander et al., 2009):
Previous studies have demonstrated a linear correlation between Δ17O−O3 and with Δ17O−O3 values ranging from 20 % to 40 % in tropospheric O3 (Vicars and Savarino, 2014; Ishino et al., 2017). The equations are shown as follows (Vicars et al., 2012):
Based on previous observations of tropospheric O3, the average value was approximately 39 ‰. The α value represents the proportional contribution of O3 to the NO oxidation pathway and can be estimated using the following Eqs. (7) (Alexander et al., 2009). When NOx is in photochemical steady state, Δ17O−NO2 can be represented using the following Eq. (10):
where T represents the ambient temperature (K) (Kunasek et al., 2008). The HO2 mixing ratios were estimated using empirical equations in the absence of direct HO2 observations (Kanaya et al., 2007). Due to the lower temperatures in Lhasa during non-summer seasons, HO2 concentrations were assessed using a formula derived from winter conditions.
Winter
Summer
2.5 Stable isotope analysis in the R (SIAR) model
In this study, stable isotope analysis in the R (SIAR) model was employed to estimate the relative contributions of three main pathways to (Parnell et al., 2010). The SIAR model is well-suited for analyzing multiple formation pathways, as it effectively incorporates uncertainties and parameter variability, leading to more reliable estimates. Specifically, this model allows for a detailed analysis of oxygen isotope (Δ17O), enabling accurate modeling of formation pathways based on oxygen isotope measurements. The SIAR model is a Bayesian mixture model, mathematically formulated as follows:
where Xi is the observed Δ17O value for sample i (), and pj is the proportional contribution of each formation pathway j to the sample i. fij is the Δ17O value of formation pathway j for sample i and follows a normal distribution with mean (μj) and variance (). Within the Bayesian framework, prior distributions are assigned to each pj, and these are updated with the observed data Xi to obtain posterior distributions, allowing for inference of the proportional contributions pj of each pathway.
2.6 Aerosol liquid water content (ALWC) and the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT)
To evaluate the influence of ALWC on formation, ALWC was calculated using the ISORROPIA II model developed by Fountoukis and Nenes (Fountoukis and Nenes, 2007). The ISORROPIA II model includes two modes: the forward mode, which requires the concentrations of both particulate and gaseous pollutants as inputs, and the reverse mode, which only requires the concentrations of particulate pollutants. The model computes the ALWC in both modes based on particulate pollutant concentrations (e.g., , Na+, Ca2+, K+, and Mg2+), as well as ambient RH and T. In this study, the reverse mode was employed due to the lack of gaseous pollutant concentration observations.
Additionally, the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model was utilized to compute 72 h back trajectories during the sampling campaign. HYSPLIT, developed by the National Oceanic and Atmospheric Administration Air Resources Laboratory, is available on their website (https://www.ready.noaa.gov/HYSPLIT.php, last access: 1 December 2024). This model has been widely used for simulating the transport and dispersion trajectories of pollutants such as PM2.5, VOC, O3, and NOx, among others (He et al., 2022; Zhao et al., 2015; Cao et al., 2023). Backward trajectories for each sampling day were calculated at an altitude of 3650 m using meteorological data from the Global Data Assimilation System (GDAS), available through the US Air Resources Laboratory (https://www.ready.noaa.gov/data/archives/gdas1/, last access: 15 December 2023).
3.1 Overview of the meteorological parameters in Lhasa during the sampling campaign
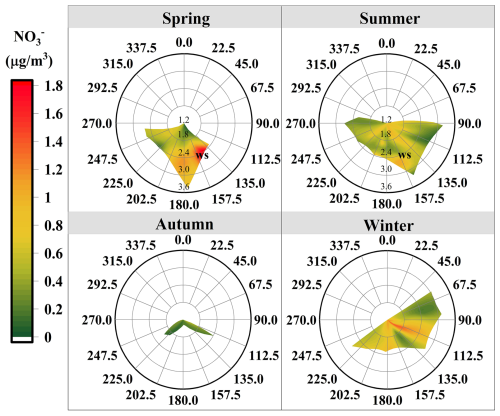
Figure 2a presents the daily variations in meteorological parameters, including T, RH, rainfall, and solar radiation. During the sampling campaign, the annual average T was 11.5 °C, ranging from −2.83 to 24.2 °C. The highest average T was observed in summer (19.7 °C), while the lowest (3.11 °C) was recorded in winter. RH varied between 6.67 % and 66.8 %, with the lowest average RH occurring in winter (17.1 %) and the highest in summer (35.6 %). The near-surface layer of Lhasa is influenced by a thermal low-pressure system, and the southwest monsoon, active between June and September, transports moisture-laden air from the Indian Ocean, resulting in increased rainfall during summer. Solar radiation intensity exhibited a seasonal trend consistent with those of T and RH, peaking in summer (394 W m−2) and reaching its lowest levels in winter (220 W m−2). The dominant WD was southeast in spring and southwest in the other three seasons (Fig. 3). WS was highest in spring and lowest in autumn.
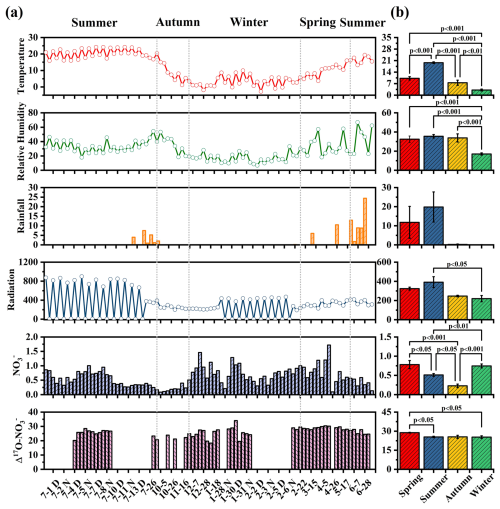
Figure 2(a) shows the time series of temperature (°C), relative humidity (%), rainfall (mm), radiation (W m−2), concentration (µg m−3), and (‰) from June 2022 to July 2023. (b) shows the average values at different seasons with their statistical significance.
3.2 concentration
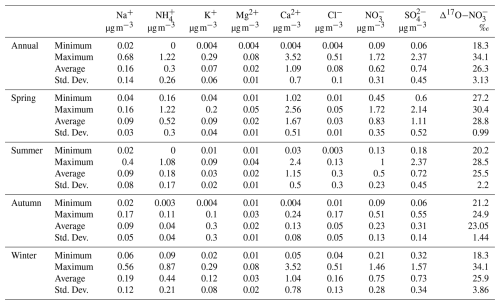
mass concentrations ranged from 0.10 to 1.72 µg m−3, with an average value of . concentrations exhibited distinct seasonal patterns. As shown in Fig. S1 in the Supplement, the equivalent concentrations of [] were considerably higher than those of [], indicating that was insufficient to fully neutralize . This suggests that a portion of may have existed in other forms, such as KNO3 and Ca(NO3)2. This inference is supported by the strong positive correlations between and K+ (r=0.64, p<0.1) and Ca2+ (r=0.43, p<0.01), especially in spring, as shown in Fig. S2. In contrast, showed relatively weak negative correlations with T (, p<0.01) and RH (, p<0.1), indicating that under the specific atmospheric conditions in Lhasa, meteorological parameters might not be the dominant factors controlling the gas-particle partitioning of . The maximum monthly average values of concentration occurred in spring () with the instantaneous maximum reaching 1.72 µg m−3, whereas the lowest was recorded in autumn () with an instantaneous minimum of only 0.09 µg m−3 (Table 1). The elevated concentrations in spring could be attributed to biomass burning from South and Southeast Asia (Figs. S3 and S4). The strong correlation between and K+ in spring further supports this explanation.
In spring, high concentrations were associated with weak southeasterly winds () in the bivariate polar plot, suggesting probable impacts from local emissions (Fig. 3). The southeasterly sector of the sampling site includes residential areas, agricultural land, and major transportation routes, which are potential NOx sources. In spring, intensified agricultural activities (e.g., fertilization, biomass burning) might increase NOx emissions. Meanwhile, low wind speeds likely limit atmospheric dispersion, promoting the local accumulation of precursors and enhancing production. During the rainy summer, shorter lifetimes indicated a weak influence from regional transport, with a more pronounced contribution from local emissions. In autumn, concentrations were relatively low, which coincided with strict local COVID-19 restrictions in Lhasa. These measures significantly reduced human activity and traffic, leading to suppressed local emissions. Despite low wind speeds typically favoring pollutant accumulation, concentrations remained low, suggesting that both reduced local sources and seasonal meteorological conditions constrained production. Nevertheless, the persistence of measurable under such stagnant conditions also implied a potential contribution from regional transport during this period. In winter, elevated concentrations under low wind speeds () emphasized the significant contribution of local emissions. These findings underscored that both regional transport and local emissions were important contributors to concentrations in Lhasa. Furthermore, based on our day-night sampling scheme, no nychthemeral (day–night) differences in concentrations were detected (Table S1 in the Supplement). A similar day-night pattern of concentrations has also been observed in Beijing (Luo et al., 2020).
3.3 Oxygen isotopes of
To explore the three major oxidation pathways of formation, 53 samples representing varying concentrations across different seasons were selected for oxygen isotope measurements (Fig. 2b). The values ranged from 18.3‰ to 34.1‰, with an average of 26.3±3.13 ‰, which is slightly lower than the global average of 28.6±4.5 ‰ simulated by the Global Chemical Transport Model (Alexander et al., 2020). As shown in Table S2, the observed values in this study were similar to most mid- and low-latitude regions, but lower than those in polar regions (∼32 ‰). As listed in Table S1, the average values in spring, summer, autumn, and winter were 28.8±8.0 ‰, 25.5±2.20 ‰, 25.6±1.35 ‰, and 25.9±3.56 ‰, respectively. The differences in values between spring and summer, as well as between spring and winter, were statistically significant (p<0.05). The elevated values in spring could be attributed to a higher proportion of nocturnal pathways that enrich values, such as NO3+VOC and N2O5+H2O pathways. In contrast, the lower values in the other three seasons suggested a greater production of formation via the NO2+OH pathway, leading to more negative values. Diurnal variation in values also differed across seasons (Fig. S5). In summer, the average of values during the day (25.3±2.39 ‰) was lower than at night (26.7±1.03 ‰). Conversely, in winter, the average of values during the day (28.0±3.79 ‰) was significantly higher than at night (24.4±3.85 ‰). Similar diurnal patterns, with higher daytime values and lower nighttime values, have also been observed in winter in the US (Vicars et al., 2013) and other cities in China (He et al., 2018).
4.1 A comparison of oxidation pathways in Lhasa with other megacities in plain regions
Typically, observations of and estimated α (the proportion of O3 oxidation in NO2 production rate) values are employed to quantify the contributions of major oxidation pathways in conjunction with a Bayesian model. The α value ranged from 0.63 to 0.93, with an average of 0.83±0.06, suggesting the significance of O3 participation in NO oxidation during the sampling campaign. On the other hand, our α values were lower than those (0.85–1) for other midlatitude regions (Alexander et al., 2009). The α values are influenced by the relative amount of O3, HO2, and RO2 in NOx cycling. Due to the generally high O3 concentrations (O3>50 ppb) observed in Lhasa, nearly all α values exceeded 0.8 (Fig. S6). To evaluate the impact of key parameters on the estimated contributions of different formation pathways, we conducted a sensitivity analysis by assuming the α values and Δ17O value of the terminal oxygen atoms of O3 (). As listed in Table S3, the assumptions of α and had an impact on the formation mechanisms. When was fixed at 39 ‰, increasing α from 0.7 to 0.9 led to a notable increase in the relative contribution of the NO2+OH pathway from 25 % to 46 %, while that of the NO3+VOC pathway decreased from 46 % to 25 %. The N2O5+H2O pathway remained nearly constant, with contributions ranging between 28 % and 29 %, indicating that this pathway is relatively insensitive to changes in α values. Similarly, when α was varied within a reasonable range (0.68–0.93), increasing the value from 37 ‰ to 39 ‰ led to an increase in the NO2+OH contribution from 37 % to 46 % and a corresponding decrease in the NO3+VOC contribution from 35 % to 26 %. Again, the N2O5+H2O contribution remained stable at ∼28 %. These results suggest that the estimated contributions of NO2+OH and NO3+VOC pathways are sensitive to assumptions about α and , whereas the contribution of the N2O5+H2O pathway is relatively robust under the tested conditions. Because Lhasa is characterized by relatively high VOC concentrations and is generally close to 39 ‰, we consider our parameter assumptions reasonable for further estimating formation pathways for each sample.
On average, the relative contributions of NO2+OH (), NO3+VOC (), and N2O5+H2O () to formation in Lhasa during the sampling campaign were 46±26 %, 26±19 %, and 28±11 %, respectively. To better understand the characteristics of formation mechanisms in Lhasa, we performed a detailed comparison across China for the relative contributions of key oxidation pathways using the Δ17O methodology (Fig. 4). Overall, similar to most Chinese cities, formation in Lhasa was predominantly driven by the NO2+OH pathway, exhibiting distinct seasonal and regional variations. In particular, the average values were generally several times higher in spring in Lhasa than in other urban cities. Compared to rural/remote areas, the average values showed higher fractions in Lhasa, revealing the influence of anthropogenic emissions, i.e., vehicle exhaust and heating, on formation. In Lhasa, the capital of Tibet, field measurements over different years showed a substantial increase in VOC concentrations in urban areas of the Tibetan Plateau, comparable to those in North China (Tang et al., 2022), revealing the importance of the active NO3+VOC pathway for pollution formation in Lhasa. In fact, recent studies have recognized NO3+VOC as a major formation mechanism for production. For instance, Fan et al. (2021) found that the values in Beijing increased from 17 % in summer to 32 % in winter based on measurements. He et al. (2018) estimated the and values and found that they were in the range of 16 %–56 %, underscoring the significant roles of these pathways during haze events in Beijing. Similarly, Feng et al. (2023) also reported that the values were up to 49.6 % in winter in northern China. In Guangzhou, Wang et al. (2023) noted that the average value was at 488 m (25 %), which was higher than that at the ground (12 %). Furthermore, Li et al. (2022) reported that the values increased from 5 % in urban to 13.5 % in rural regions in Northeast China. Although the specific nighttime RO2 production mechanism in Lhasa remains unclear, studies in other cities have demonstrated that the NO3+VOC pathway was the dominant channel for nighttime RO2 production (Fisher et al., 2016), which in turn led to the formation of alkyl and multifunctional nitrates (RONO2) and eventually . In such cases, the RO2 concentration is expected to be correlated with NO3 radical production, which depends on the reaction rate of O3 and NO2 (Brown and Stutz, 2012). Given the relatively high nighttime O3 concentrations in Lhasa, it is plausible that O3-driven nighttime NO3 chemistry plays an important role, thereby enhancing NO3+VOC derived from RO2 production and formation. Global modeling studies also support the significance of this pathway. For instance, Alexander et al. (2020) reported that the NO3+VOC pathway via the RONO2 mechanism accounted for 3 % of global formation on average. The relatively high values observed in Lhasa are broadly consistent with these findings, especially under conditions of high VOC concentrations and strong nighttime oxidant levels.
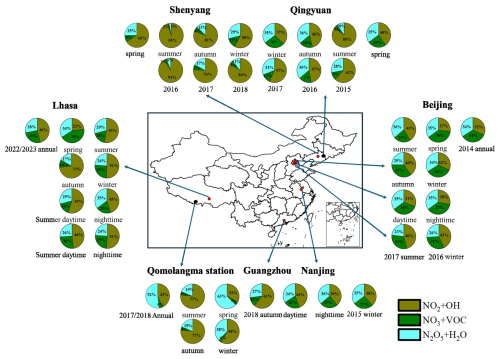
Figure 4Summary of the relative contributions of key oxidation pathways using the Δ17O methodology across China (data given in Table S4). Colors for the study labels indicate the type of sampling location: urban areas (red) and rural/remote areas (black). The pie charts show the relative contribution of different pathways to formation: ( deep yellow), (deep green), and (light blue).
4.2 Seasonal and diurnal variations of oxidation pathways
Figure S7 illustrates the seasonal variations in the relative contributions of the three main oxidation pathways to formation. When comparing different seasons, the values were lower (p<0.01) in spring (22.6 %) than in winter (50.8 %), summer (52.9 %), and autumn (73.2 %). The dominance of the NO2+OH pathway in autumn is consistent with observations at Mount Everest during the autumn season of 2017 and 2018, suggesting that formation on the Tibetan Plateau in autumn may be mainly driven by the NO2+OH pathway (Lin et al., 2021; Wang et al., 2020b).
A significant increase in the values was observed in spring (p<0.05). First, O3 and NO2 are precursors of NO3. In this work, the highest concentrations of O3 were found in spring (), likely leading to elevated NO3 concentrations. Additionally, the low T and reduced OH radical concentrations in spring facilitate the reaction of NO2 and O3 to synthesize NO3. This might be an appropriate reason for the values in spring. High-altitude locations such as Nepal (5079 m a.s.l.) and Qomolangma Station (4300 m a.s.l.) have experienced stratospheric ozone intrusions, especially in spring and winter, as reported in previous studies (Zhang et al., 2025, 2022; Cristofanelli et al., 2010; Morin et al., 2007; Lin et al., 2016; Yin et al., 2017; Wang et al., 2020b). Notably, such intrusions in spring may elevate tropospheric O3 levels in Lhasa, resulting in a mixture of tropospheric and stratospheric O3 that enhances production. Second, previous studies have indicated that the Afghanistan–Pakistan–Tajikistan region, the Indo-Gangetic Plain, and the Meghalaya–Myanmar region could transport industrial VOC emissions to various zones in Tibet from west to east. Additionally, agricultural areas in northern India could contribute biomass burning-related VOC emissions to the middle-northern and eastern regions of Tibet (Li et al., 2017). During our sampling campaign, South and Southeast Asia air clusters were notably prevalent in the springtime, coinciding with intensive fire spots observed in Afghanistan, Pakistan, India, Nepal, and Bhutan (Figs. S3 and S4). These observations, combined with the prevailing South and Southeast Asia air mass trajectories in spring, strongly suggest that long-range transported VOCs from South Asia were delivered to Lhasa and likely participated in local production via the NO3+VOC pathway. Moreover, recent studies have shown that ambient VOC concentrations in the urban areas on the Qinghai–Tibet Plateau were comparable to those in the North China Plain (Tang et al., 2022). The input of VOC emissions through long-range transport might further elevate VOC concentrations, thereby promoting formation via the NO3+VOC pathway and contributing to the enhanced values observed in spring. While VOCs appear to play a dominant role in the process, it should be noted that other nitrogen species (e.g., NO, NO2) associated with biomass burning emissions may also be transported over long distances and influence formation in Lhasa. These co-transported nitrogen compounds, although not directly quantified in this study, could further contribute to production in spring. Taken together, these findings provide strong evidence that long-range transport of biomass burning emissions, particularly from South Asia, can substantially influence springtime formation in Lhasa.
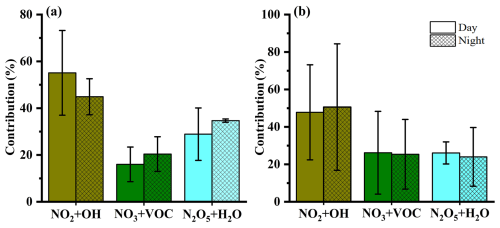
Figure 5The relative contributions (mean ± SD values) of NO2+OH, NO3+VOC, and N2O5+H2O to formation during the day and night in (a) summer and (b) winter in Lhasa during the sampling campaign.
Similarly, the values exhibited their highest contributions during spring, with significant seasonal differences (p<0.05) except when compared to summer (p>0.05). Typically, high RH enhances formation via the N2O5+H2O pathway. However, studies have revealed that during sandstorm events, a significantly large N2O5 uptake coefficient was observed on urban aerosols in spring (Xia et al., 2019). In this study, the mean Ca2+ concentration in PM2.5 was found to be the highest in spring, suggesting a possible role of dust in facilitating N2O5 uptake. Additionally, the N2O5+H2O pathway has been reported to be promoted by elevated concentrations (Lin et al., 2021), which were also highest in spring. Therefore, the increased values during spring might be attributed to the combined effects of lower RH, elevated Ca2+ levels, and high concentrations.
Interestingly, distinct diurnal patterns of oxidation pathways were observed during the sampling campaign (Fig. 5). In summer, the NO2+OH pathway showed a significantly higher contribution during the daytime (55.1 %) compared to nighttime (44.9 %), which is attributed to increased OH radical synthesis during longer days and higher temperatures in Lhasa (Rohrer and Berresheim, 2006). A previous study indicated that lower NO2 and higher O3 concentrations enhance the relative contribution of the OH pathway to formation (Wang et al., 2019). Additionally, the concentration of ALWC (the detailed information is given in Text S3) was higher at night than during the day in summer, favoring formation through nocturnal formation. In winter, , , and were similar during both day and night. Typically, photolytic destruction and chemical reactions with NO are rapid sinks during the daytime, with lifetimes generally less than 5 s, resulting in extremely low concentrations. Similarly, the atmospheric lifetime of N2O5 under sunlight is also very short (Wang et al., 2018). Thus, daytime NO3 and N2O5 chemistry is often considered negligible. However, a recent study revealed that a non-negligible amount of NO3 radicals can persist during the daytime in cold months, owing to the limited solar radiation (Hellén et al., 2018). Wang et al. (2020a) found that the daytime production rate of NO3 can be substantial due to elevated concentrations of O3 and NO2, suggesting that the mixing ratios of NO3 and N2O5 during the day may not be negligible. Furthermore, in winter, lower temperatures and elevated NO2 concentrations facilitate a quasi-steady-state equilibrium between NO3 and N2O5, slowing the overall reactivity of the precursors (Brown et al., 2003). This equilibrium condition minimizes diurnal fluctuations in precursor concentrations, resulting in relatively stable nocturnal and daytime formation pathways, including NO3+VOC and N2O5+H2O. Nevertheless, we acknowledge that the exact role of daytime NO3N2O5 chemistry remains uncertain in Lhasa and should be further assessed using concurrent field observations or chemical transport models. Moreover, when interpreting the diurnal differences in values, the atmospheric lifetime of must be considered. Given that the atmospheric lifetime of is generally more than 12 h, each sample might reflect both daytime and nighttime production impacting values (Park et al., 2004; Vicars et al., 2013).
4.3 Integrated analysis of oxidation pathways in Lhasa
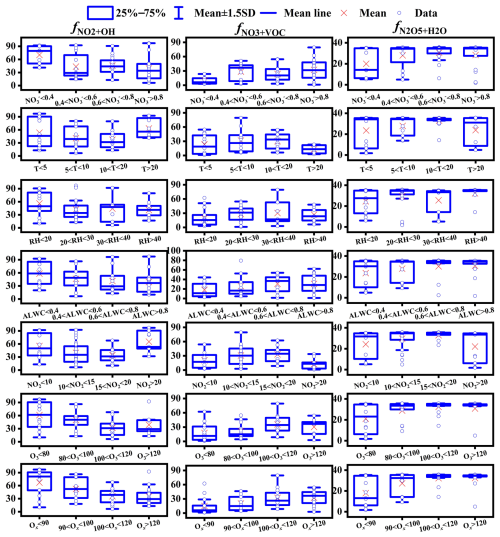
As shown in Fig. S8, the NO3+VOC pathway emerged as the major contributor to formation during periods of high spikes. To elucidate the formation pathways under different concentrations, samples were categorized into different concentration ranges (Fig. 6). We found that the values increased and values decreased with the concentrations increasing. Although recent field radical measurements in urban sites in China found that OH and HO2 radicals during haze periods were comparable to those on clean days (Slater et al., 2020; Yang et al., 2021), our results suggested that the NO3+VOC pathway still played an important role in production under high concentrations in Lhasa, possibly due to enhanced VOC emissions. In addition to concentration effects, meteorological factors typically also regulate the oxidation pathways. Typically, high T promotes the formation via values (Han et al., 2015). However, our study revealed that the relationship between T and values did not consistently show a positive trend. Further analysis indicated that NO2 and O3 concentrations were negatively correlated, with lower NO2 concentrations paired with elevated O3 levels (Fig. S9). values reached their minimum when NO2 was between 15 and 20 µg m−3 and O3 was within 100–120 µg m−3. Although OH radicals exhibit a higher oxidation potential (2.8 V) than O3 (2.07 V), their atmospheric availability is much lower than that of O3 (Carslaw et al., 1999; Dubey et al., 1997). Therefore, NO2 at lower concentrations is more likely to be oxidized by OH than by O3, even though O3 concentrations were high. With increasing NO2 concentrations, the availability of OH radicals for oxidizing NO2 became lower, resulting in a relatively higher proportion of NO2 being oxidized by O3 although O3 concentrations were low. However, when the concentration of O3 was below 20 µg m−3, O3 concentrations were not sufficient to oxidize NO2 due to the higher NO2 concentrations and OH radicals for oxidizing NO2 would redominate. These observations underscore that in high-altitude urban environments like Lhasa, OH effectiveness is more important for oxidation pathways than that of O3. Additionally, we identified an intriguing positive correlation between the atmospheric oxidizing capacity () and values. values were lowest when Ox was less than 90 µg m−3, corresponding to a maximum contribution from the NO2+OH pathway. This suggests that Ox is more indicative of the pathways of formation in the atmosphere compared to either NO2 or O3 alone. Typically, high RH and ALWC were also positively correlated with . However, RH was associated with variable contributions from the N2O5+H2O pathway in our study, while increasing ALWC significantly enhanced this pathway, indicating ALWC as a more reliable indicator of formation.
4.4 Implications
The oxidation pathways of in Lhasa, China, were constrained using a full year of measurements from 2022 to 2023. Based on seasonal data, we observed a significant increase in the relative contribution of the NO3+VOC to formation during spring. Furthermore, the diurnal distribution of oxidation pathways varied distinctly across seasons. To better understand the factors influencing these pathways, we integrated meteorological conditions, NOx precursors, and ALWC for a more comprehensive analysis of formation. The results revealed that Ox and ALWC were more reliable indicators of oxidation pathways than meteorological factors. Notably, Lhasa's unique high-altitude environment, such as strong solar radiation, persistently high O3, and elevated VOC, promotes active NO3+VOC chemistry, especially in spring. Atmospheric ALWC is primarily produced by hygroscopic aerosols such as , , and Cl−. Therefore, in addition to controlling NO2, O3, and VOC, reducing these hygroscopic aerosols is crucial for effective PM2.5 pollution control.
Although this study provides valuable insights into formation mechanisms in Lhasa, we must acknowledge the associated uncertainties due to the lack of comprehensive observational constraints in Lhasa. Specifically, the limited understanding of local RO2 concentrations led us to adopt empirical parameterizations and refer to measurements from other regions, which inevitably introduced uncertainty into the pathway apportionment. In addition, the absence of direct observations of nighttime NO emissions and the NO2–NO isotope exchange processes in this region further complicates the interpretation of diurnal variations in formation pathways. To improve the robustness of Δ17O-based pathway analysis, future studies should consider synchronous measurements of both NO2 and isotopes.
All data are presented in the main text and/or the Supplement. For additional data, please contact the corresponding author (liu.junwen@jnu.edu.cn).
The supplement related to this article is available online at https://doi.org/10.5194/acp-25-12451-2025-supplement.
JL designed, conceived, and led the research. XZ performed the data analysis and drafted the manuscript. JL, XZ, NC, and BB planned and carried out the measurements. NC, BB, and PD were responsible for measuring the meteorological parameters. JL and PY secured funding for the continuous aerosol sampling and analysis. FC and YZ provided expertise on isotope analysis methods. JL offered guidance on data analysis, and all authors contributed to revising the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This study was supported by the Natural Science Foundation of Xizang Autonomous Region (XZ202401ZR0067), Guangdong Basic and Applied Basic Research Foundation (2024B1515040026), the second Tibetan Plateau Scientific Expedition and Research Program (20190ZKK0604), and the Guangdong Provincial General Colleges and Universities Innovation Team Project (Natural Science) (2024KCXTD004).
This paper was edited by Lisa Whalley and reviewed by two anonymous referees.
Alexander, B., Hastings, M. G., Allman, D. J., Dachs, J., Thornton, J. A., and Kunasek, S. A.: Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (Δ17O) of atmospheric nitrate, Atmos. Chem. Phys., 9, 5043–5056, https://doi.org/10.5194/acp-9-5043-2009, 2009.
Alexander, B., Sherwen, T., Holmes, C. D., Fisher, J. A., Chen, Q., Evans, M. J., and Kasibhatla, P.: Global inorganic nitrate production mechanisms: comparison of a global model with nitrate isotope observations, Atmos. Chem. Phys., 20, 3859–3877, https://doi.org/10.5194/acp-20-3859-2020, 2020.
Barkan, E. and Luz, B.: High-precision measurements of 17O16O and 18O16O of O2 and O2 Ar−1 ratio in air, Rapid Commun. Mass Sp., 17, 2809–2814, https://doi.org/10.1002/rcm.1267, 2003.
Bell, M. L., Dominici, F., Ebisu, K., Zeger, S. L., and Samet, J. M.: Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies, Environ. Health Persp., 115, 989–995, https://doi.org/10.1289/ehp.9621, 2007.
Brown, S. S. and Stutz, J.: Nighttime radical observations and chemistry, Chem. Soc. Rev., 41, 6405–6447, https://doi.org/10.1039/C2CS35181A, 2012.
Brown, S. S., Stark, H., and Ravishankara, A.: Applicability of the steady state approximation to the interpretation of atmospheric observations of NO3 and N2O5, J. Geophys. Res.-Atmos., 108, https://doi.org/10.1029/2011JD016544, 2003.
Cao, X., Xing, Q., Hu, S., Xu, W., Xie, R., Xian, A., Xie, W., Yang, Z., and Wu, X.: Characterization, reactivity, source apportionment, and potential source areas of ambient volatile organic compounds in a typical tropical city, J. Environ. Sci., 123, 417–429, https://doi.org/10.1016/j.jes.2022.08.005, 2023.
Carslaw, N., Creasey, D. J., Heard, D. E., Lewis, A. C., McQuaid, J. B., Pilling, M. J., Monks, P. S., Bandy, B. J., and Penkett, S. A.: Modeling OH, HO2, and RO2 radicals in the marine boundary layer: 1. Model construction and comparison with field measurements, J. Geophys. Res.-Atmos., 104, 30241–30255, https://doi.org/10.1029/1999JD900783, 1999.
Chen, Z., Pei, C., Liu, J., Zhang, X., Ding, P., Dang, L., Zong, Z., Jiang, F., Wu, L., and Sun, X.: Non-agricultural source dominates the ammonium aerosol in the largest city of South China based on the vertical δ15N measurements, Sci. Total Environ., 848, 157750, https://doi.org/10.1016/j.scitotenv.2022.157750, 2022.
Clark, C. M. and Tilman, D.: Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands, Nature, 451, 712–715, https://doi.org/10.1038/nature06503, 2008.
Colmer, J., Hardman, I., Shimshack, J., and Voorheis, J.: Disparities in PM2.5 air pollution in the United States, Science, 369, 575–578, https://doi.org/10.1126/science.aaz9353, 2020.
Cristofanelli, P., Bracci, A., Sprenger, M., Marinoni, A., Bonafè, U., Calzolari, F., Duchi, R., Laj, P., Pichon, J. M., Roccato, F., Venzac, H., Vuillermoz, E., and Bonasoni, P.: Tropospheric ozone variations at the Nepal Climate Observatory-Pyramid (Himalayas, 5079 m a.s.l.) and influence of deep stratospheric intrusion events, Atmos. Chem. Phys., 10, 6537–6549, https://doi.org/10.5194/acp-10-6537-2010, 2010.
Dubey, M. K., Mohrschladt, R., Donahue, N. M., and Anderson, J. G.: Isotope specific kinetics of hydroxyl radical (OH) with water (H2O): Testing models of reactivity and atmospheric fractionation, J. Phys. Chem. A, 101, 1494–1500, https://doi.org/doi/abs/10.1021/jp962332p, 1997.
Espina-Martin, P., Perdrix, E., Alleman, L., and Coddeville, P.: Origins of the seasonal variability of PM2.5 sources in a rural site in Northern France, Atmos. Environ., 120660, https://doi.org/10.1016/j.atmosenv.2024.120660, 2024.
Fan, M.-Y., Zhang, Y.-L., Lin, Y.-C., Hong, Y., Zhao, Z.-Y., Xie, F., Du, W., Cao, F., Sun, Y., and Fu, P.: Important role of NO3 radical to nitrate formation aloft in urban Beijing: Insights from triple oxygen isotopes measured at the tower, Environ. Sci. Technol., 56, 6870–6879, https://doi.org/10.1021/acs.est.1c02843, 2021.
Feng, X., Chen, Y., Chen, S., Peng, Y., Liu, Z., Jiang, M., Feng, Y., Wang, L., Li, L., and Chen, J.: Dominant Contribution of NO3 Radical to Formation during Heavy Haze Episodes: Insights from High-Time Resolution of Dual Isotopes Δ17O and δ18O, Environ. Sci. Technol., 57, 20726–20735, https://doi.org/10.1021/acs.est.3c07590, 2023.
Fisher, J. A., Jacob, D. J., Travis, K. R., Kim, P. S., Marais, E. A., Chan Miller, C., Yu, K., Zhu, L., Yantosca, R. M., Sulprizio, M. P., Mao, J., Wennberg, P. O., Crounse, J. D., Teng, A. P., Nguyen, T. B., St. Clair, J. M., Cohen, R. C., Romer, P., Nault, B. A., Wooldridge, P. J., Jimenez, J. L., Campuzano-Jost, P., Day, D. A., Hu, W., Shepson, P. B., Xiong, F., Blake, D. R., Goldstein, A. H., Misztal, P. K., Hanisco, T. F., Wolfe, G. M., Ryerson, T. B., Wisthaler, A., and Mikoviny, T.: Organic nitrate chemistry and its implications for nitrogen budgets in an isoprene- and monoterpene-rich atmosphere: constraints from aircraft (SEAC4RS) and ground-based (SOAS) observations in the Southeast US, Atmos. Chem. Phys., 16, 5969–5991, https://doi.org/10.5194/acp-16-5969-2016, 2016.
Fountoukis, C. and Nenes, A.: ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+––Na+–––Cl−–H2O aerosols, Atmos. Chem. Phys., 7, 4639–4659, https://doi.org/10.5194/acp-7-4639-2007, 2007.
Ge, S., Su, J., Zhao, P., Li, J., Liu, S., Qiu, Y., Pu, W., and Ma, Z.: Characteristics of PM2.5 hygroscopicity and the influences of water-soluble ions during haze events in Beijing, Atmos. Environ., 322, 120382, https://doi.org/10.1016/j.atmosenv.2024.120382, 2024.
Geng, G., Zheng, Y., Zhang, Q., Xue, T., Zhao, H., Tong, D., Zheng, B., Li, M., Liu, F., and Hong, C.: Drivers of PM2.5 air pollution deaths in China 2002–2017, Nat. Geosci., 14, 645–650, https://doi.org//10.1038/s41561-021-00792-3, 2021.
Han, T., Liu, X., Zhang, Y., Qu, Y., Zeng, L., Hu, M., and Zhu, T.: Role of secondary aerosols in haze formation in summer in the Megacity Beijing, J. Environ. Sci., 31, 51–60, https://doi.org/10.1016/j.jes.2014.08.026, 2015.
He, P., Xie, Z., Chi, X., Yu, X., Fan, S., Kang, H., Liu, C., and Zhan, H.: Atmospheric Δ17O () reveals nocturnal chemistry dominates nitrate production in Beijing haze, Atmos. Chem. Phys., 18, 14465–14476, https://doi.org/10.5194/acp-18-14465-2018, 2018.
He, S., Huang, M., Zheng, L., Chang, M., Chen, W., Xie, Q., and Wang, X.: Seasonal variation of transport pathways and potential source areas at high inorganic nitrogen wet deposition sites in southern China, J. Environ. Sci., 114, 444–453, https://doi.org/10.1016/j.jes.2021.12.024, 2022.
Hellén, H., Praplan, A. P., Tykkä, T., Ylivinkka, I., Vakkari, V., Bäck, J., Petäjä, T., Kulmala, M., and Hakola, H.: Long-term measurements of volatile organic compounds highlight the importance of sesquiterpenes for the atmospheric chemistry of a boreal forest, Atmos. Chem. Phys., 18, 13839–13863, https://doi.org/10.5194/acp-18-13839-2018, 2018.
Huang, R.-J., Zhang, Y., Bozzetti, C., Ho, K.-F., Cao, J.-J., Han, Y., Daellenbach, K. R., Slowik, J. G., Platt, S. M., and Canonaco, F.: High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 514, 218–222, https://doi.org/10.1038/nature13774, 2014.
Ishino, S., Hattori, S., Savarino, J., Jourdain, B., Preunkert, S., Legrand, M., Caillon, N., Barbero, A., Kuribayashi, K., and Yoshida, N.: Seasonal variations of triple oxygen isotopic compositions of atmospheric sulfate, nitrate, and ozone at Dumont d'Urville, coastal Antarctica, Atmos. Chem. Phys., 17, 3713–3727, https://doi.org/10.5194/acp-17-3713-2017, 2017.
Kanaya, Y., Cao, R., Akimoto, H., Fukuda, M., Komazaki, Y., Yokouchi, Y., Koike, M., Tanimoto, H., Takegawa, N., and Kondo, Y.: Urban photochemistry in central Tokyo: 1. Observed and modeled OH and HO2 radical concentrations during the winter and summer of 2004, J. Geophys. Res.-Atmos., 112, https://doi.org/10.1029/2007JD008670, 2007.
Kunasek, S., Alexander, B., Steig, E., Hastings, M., Gleason, D., and Jarvis, J.: Measurements and modeling of Δ17O of nitrate in snowpits from Summit, Greenland, J. Geophys. Res.-Atmos., 113, https://doi.org/10.1029/2008JD010103, 2008.
Lhasa Municipal People's Government: Overview of Lhasa, https://www.lasa.gov.cn/lasa/yxls/yx.shtml, last access: 30 June 2025.
Li, H., He, Q., Song, Q., Chen, L., Song, Y., Wang, Y., Lin, K., Xu, Z., and Shao, M.: Diagnosing Tibetan pollutant sources via volatile organic compound observations, Atmos. Environ., 166, 244–254, https://doi.org/10.1016/j.atmosenv.2017.07.031, 2017.
Li, Z., Walters, W. W., Hastings, M. G., Song, L., Huang, S., Zhu, F., Liu, D., Shi, G., Li, Y., and Fang, Y.: Atmospheric nitrate formation pathways in urban and rural atmosphere of Northeast China: Implications for complicated anthropogenic effects, Environ. Pollut., 296, 118752, https://doi.org/10.1016/j.envpol.2021.118752, 2022.
Lin, M., Zhang, Z., Su, L., Hill-Falkenthal, J., Priyadarshi, A., Zhang, Q., Zhang, G., Kang, S., Chan, C. Y., and Thiemens, M. H.: Resolving the impact of stratosphere-to-troposphere transport on the sulfur cycle and surface ozone over the Tibetan Plateau using a cosmogenic 35S tracer, J. Geophys. Res.-Atmos., 121, 439–456, https://doi.org/10.1002/2015JD023801, 2016.
Lin, Y.-C., Zhang, Y.-L., Yu, M., Fan, M.-Y., Xie, F., Zhang, W.-Q., Wu, G., Cong, Z., and Michalski, G.: Formation mechanisms and source apportionments of airborne nitrate aerosols at a Himalayan-Tibetan Plateau site: Insights from nitrogen and oxygen isotopic compositions, Environ. Sci. Technol., 55, 12261–12271, https://doi.org/10.1021/acs.est.1c03957, 2021.
Luo, L., Kao, S., Wu, Y., Zhang, X., Lin, H., Zhang, R., and Xiao, H.: Stable oxygen isotope constraints on nitrate formation in Beijing in springtime, Environ. Pollut., 263, 114515, https://doi.org/10.1016/j.envpol.2020.114515, 2020.
Michalski, G., Scott, Z., Kabiling, M., and Thiemens, M. H.: First measurements and modeling of Δ17O in atmospheric nitrate, Geophys. Res. Lett., 30, https://doi.org/10.1029/2003GL017015, 2003.
Morin, S., Savarino, J., Bekki, S., Gong, S., and Bottenheim, J. W.: Signature of Arctic surface ozone depletion events in the isotope anomaly (Δ17O) of atmospheric nitrate, Atmos. Chem. Phys., 7, 1451–1469, https://doi.org/10.5194/acp-7-1451-2007, 2007.
Office of the Leading Group: Lhasa reported 31 new local COVID-19 cases yesterday, with 14 medium-risk areas adjusted, https://xinwen.bjd.com.cn/content/s6340d130e4b0b60bbc5d4ecd.html, last access: 1 July 2025.
Park, R. J., Jacob, D. J., Field, B. D., Yantosca, R. M., and Chin, M.: Natural and transboundary pollution influences on sulfate-nitrate-ammonium aerosols in the United States: Implications for policy, J. Geophys. Res.-Atmos., 109, https://doi.org/10.1029/2003JD004473, 2004.
Parnell, A. C., Inger, R., Bearhop, S., and Jackson, A. L.: Source partitioning using stable isotopes: coping with too much variation, PLoS One, 5, e9672, https://doi.org/10.1371/journal.pone.0009672, 2010.
Qiu, X., Ying, Q., Wang, S., Duan, L., Zhao, J., Xing, J., Ding, D., Sun, Y., Liu, B., Shi, A., Yan, X., Xu, Q., and Hao, J.: Modeling the impact of heterogeneous reactions of chlorine on summertime nitrate formation in Beijing, China, Atmos. Chem. Phys., 19, 6737–6747, https://doi.org/10.5194/acp-19-6737-2019, 2019.
Rohrer, F. and Berresheim, H.: Strong correlation between levels of tropospheric hydroxyl radicals and solar ultraviolet radiation, Nature, 442, 184–187, https://doi.org/10.1038/nature04924, 2006.
Salameh, D., Detournay, A., Pey, J., Pérez, N., Liguori, F., Saraga, D., Bove, M. C., Brotto, P., Cassola, F., and Massabò, D.: PM2.5 chemical composition in five European Mediterranean cities: A 1 year study, Atmos. Res., 155, 102–117, https://doi.org/10.1016/j.atmosres.2014.12.001, 2015.
Savarino, J., Vicars, W. C., Legrand, M., Preunkert, S., Jourdain, B., Frey, M. M., Kukui, A., Caillon, N., and Gil Roca, J.: Oxygen isotope mass balance of atmospheric nitrate at Dome C, East Antarctica, during the OPALE campaign, Atmos. Chem. Phys., 16, 2659–2673, https://doi.org/10.5194/acp-16-2659-2016, 2016.
Slater, E. J., Whalley, L. K., Woodward-Massey, R., Ye, C., Lee, J. D., Squires, F., Hopkins, J. R., Dunmore, R. E., Shaw, M., Hamilton, J. F., Lewis, A. C., Crilley, L. R., Kramer, L., Bloss, W., Vu, T., Sun, Y., Xu, W., Yue, S., Ren, L., Acton, W. J. F., Hewitt, C. N., Wang, X., Fu, P., and Heard, D. E.: Elevated levels of OH observed in haze events during wintertime in central Beijing, Atmos. Chem. Phys., 20, 14847–14871, https://doi.org/10.5194/acp-20-14847-2020, 2020.
Song, W., Wang, Y.-L., Yang, W., Sun, X.-C., Tong, Y.-D., Wang, X.-M., Liu, C.-Q., Bai, Z.-P., and Liu, X.-Y.: Isotopic evaluation on relative contributions of major NOx sources to nitrate of PM2.5 in Beijing, Environ. Pollut., 248, 183–190, https://doi.org/10.1016/j.envpol.2019.01.081, 2019.
Su, X., Tie, X., Li, G., Cao, J., Huang, R., Feng, T., Long, X., and Xu, R.: Effect of hydrolysis of N2O5 on nitrate and ammonium formation in Beijing China: WRF-Chem model simulation, Sci. Total Environ., 579, 221–229, https://doi.org/10.1016/j.scitotenv.2016.11.125, 2017.
Sun, P., Farley, R. N., Li, L., Srivastava, D., Niedek, C. R., Li, J., Wang, N., Cappa, C. D., Pusede, S. E., and Yu, Z.: PM2.5 composition and sources in the San Joaquin Valley of California: A long-term study using ToF-ACSM with the capture vaporizer, Environ. Pollut., 292, 118254, https://doi.org/10.1016/j.envpol.2021.118254, 2022.
Tang, G., Yao, D., Kang, Y., Liu, Y., Liu, Y., Wang, Y., Bai, Z., Sun, J., Cong, Z., Xin, J., Liu, Z., Zhu, Z., Geng, Y., Wang, L., Li, T., Li, X., Bian, J., and Wang, Y.: The urgent need to control volatile organic compound pollution over the Qinghai-Tibet Plateau, iScience, 25, 105688, https://doi.org/10.1016/j.isci.2022.105688, 2022.
Vicars, W., Morin, S., Savarino, J., Wagner, N., Erbland, J., Vince, E., Martins, J., Lerner, B., Quinn, P., and Coffman, D.: Spatial and diurnal variability in reactive nitrogen oxide chemistry as reflected in the isotopic composition of atmospheric nitrate: Results from the CalNex 2010 field study, J. Geophys. Res.-Atmos., 118, 10567–510588, https://doi.org/10.1002/jgrd.50680, 2013.
Vicars, W. C. and Savarino, J.: Quantitative constraints on the 17O-excess (Δ17O) signature of surface ozone: Ambient measurements from 50 N to 50 S using the nitrite-coated filter technique, Geochim. Cosmochim. Ac., 135, 270–287, https://doi.org/10.1016/j.gca.2014.03.023, 2014.
Vicars, W. C., Bhattacharya, S., Erbland, J., and Savarino, J.: Measurement of the 17O-excess (Δ17O) of tropospheric ozone using a nitrite-coated filter, Rapid Commun. Mass Sp., 26, 1219–1231, https://doi.org/10.1002/rcm.6218, 2012.
Walters, W. W., Pye, H. O., Kim, H., and Hastings, M. G.: Modeling the Oxygen Isotope Anomaly (Δ17O) of Reactive Nitrogen in the Community Multiscale Air Quality Model: Insights into Nitrogen Oxide Chemistry in the Northeastern United States, ACS ES&T Air, https://doi.org/10.1021/acsestair.3c00056, 2024.
Wang, H., Lu, K., Guo, S., Wu, Z., Shang, D., Tan, Z., Wang, Y., Le Breton, M., Lou, S., Tang, M., Wu, Y., Zhu, W., Zheng, J., Zeng, L., Hallquist, M., Hu, M., and Zhang, Y.: Efficient N2O5 uptake and NO3 oxidation in the outflow of urban Beijing, Atmos. Chem. Phys., 18, 9705–9721, https://doi.org/10.5194/acp-18-9705-2018, 2018.
Wang, H., Chen, X., Lu, K., Hu, R., Li, Z., Wang, H., Ma, X., Yang, X., Chen, S., Dong, H., Liu, Y., Fang, X., Zeng, L., Hu, M., and Zhang, Y.: NO3 and N2O5 chemistry at a suburban site during the EXPLORE-YRD campaign in 2018, Atmos. Environ., 224, 117180, https://doi.org/10.1016/j.atmosenv.2019.117180, 2020a.
Wang, K., Hattori, S., Kang, S., Lin, M., and Yoshida, N.: Isotopic constraints on the formation pathways and sources of atmospheric nitrate in the Mt. Everest region, Environ. Pollut., 267, 115274, https://doi.org/10.1016/j.envpol.2020.115274, 2020b.
Wang, Y., Liu, J., Jiang, F., Chen, Z., Wu, L., Zhou, S., Pei, C., Kuang, Y., Cao, F., and Zhang, Y.: Vertical measurements of stable nitrogen and oxygen isotope composition of fine particulate nitrate aerosol in Guangzhou city: Source apportionment and oxidation pathway, Sci. Total Environ., 865, 161239, https://doi.org/10.1016/j.scitotenv.2022.161239, 2023.
Wang, Y. L., Song, W., Yang, W., Sun, X. C., Tong, Y. D., Wang, X. M., Liu, C. Q., Bai, Z. P., and Liu, X. Y.: Influences of atmospheric pollution on the contributions of major oxidation pathways to PM2.5 nitrate formation in Beijing, J. Geophys. Res.-Atmos., 124, 4174–4185, https://doi.org/10.1029/2019JD030284, 2019.
Xia, M., Wang, W., Wang, Z., Gao, J., Li, H., Liang, Y., Yu, C., Zhang, Y., Wang, P., Zhang, Y., Bi, F., Cheng, X., and Wang, T.: Heterogeneous Uptake of N2O5 in Sand Dust and Urban Aerosols Observed during the Dry Season in Beijing, Atmosphere, 10, 204, https://doi.org/10.3390/atmos10040204, 2019.
Xu, Q., Wang, S., Jiang, J., Bhattarai, N., Li, X., Chang, X., Qiu, X., Zheng, M., Hua, Y., and Hao, J.: Nitrate dominates the chemical composition of PM2.5 during haze event in Beijing, China, Sci. Total Environ., 689, 1293–1303, https://doi.org/10.1016/j.scitotenv.2019.06.294, 2019.
Yang, X., Lu, K., Ma, X., Liu, Y., Wang, H., Hu, R., Li, X., Lou, S., Chen, S., and Dong, H.: Observations and modeling of OH and HO2 radicals in Chengdu, China in summer 2019, Sci. Total Environ., 772, 144829, https://doi.org/10.1016/j.scitotenv.2020.144829, 2021.
Yin, M., Guan, H., Luo, L., Xiao, H., and Zhang, Z.: Using nitrogen and oxygen stable isotopes to analyze the major NOx sources to nitrate of PM2.5 in Lanzhou, northwest China, in winter-spring periods, Atmos. Environ., 276, 119036, https://doi.org/10.1016/j.atmosenv.2022.119036, 2022.
Yin, X., Kang, S., de Foy, B., Cong, Z., Luo, J., Zhang, L., Ma, Y., Zhang, G., Rupakheti, D., and Zhang, Q.: Surface ozone at Nam Co in the inland Tibetan Plateau: variation, synthesis comparison and regional representativeness, Atmos. Chem. Phys., 17, 11293–11311, https://doi.org/10.5194/acp-17-11293-2017, 2017.
Zhang, Q., Jiang, X., Tong, D., Davis, S. J., Zhao, H., Geng, G., Feng, T., Zheng, B., Lu, Z., Streets, D. G., Ni, R., Brauer, M., van Donkelaar, A., Martin, R. V., Huo, H., Liu, Z., Pan, D., Kan, H., Yan, Y., Lin, J., He, K., and Guan, D.: Transboundary health impacts of transported global air pollution and international trade, Nature, 543, 705–709, https://doi.org/10.1038/nature21712, 2017.
Zhang, Q., Zheng, Y., Tong, D., Shao, M., Wang, S., Zhang, Y., Xu, X., Wang, J., He, H., and Liu, W.: Drivers of improved PM2.5 air quality in China from 2013 to 2017, P. Natl. Acad. Sci. USA, 116, 24463–24469, https://doi.org/10.1073/pnas.1907956116, 2019.
Zhang, Y., Zhao, T., Ning, G., Xu, X., Chen, Z., Jia, M., Sun, X., Shu, Z., Lu, Z., and Liu, J.: A unique mechanism of ozone surges jointly triggered by deep stratospheric intrusions and the Tibetan Plateau topographic forcing, Geophys. Res. Lett., 52, e2024GL114207, https://doi.org/10.1029/2024GL114207, 2025.
Zhang, Y.-L., Zhang, W., Fan, M.-Y., Li, J., Fang, H., Cao, F., Lin, Y.-C., Wilkins, B. P., Liu, X., and Bao, M.: A diurnal story of in urban Nanjing and its implication for nitrate aerosol formation, npj Climate and Atmospheric Science, 5, 50, https://doi.org/10.1038/s41612-022-00273-3, 2022.
Zhang, Z., Jiang, Z., Zhou, T., and Geng, L.: Reconciling Modeled and Observed in Beijing Winter Haze With Heterogeneous Chlorine Chemistry, J. Geophys. Res.-Atmos., 129, e2023JD039740, https://doi.org/10.1029/2023JD039740, 2024.
Zhao, M., Huang, Z., Qiao, T., Zhang, Y., Xiu, G., and Yu, J.: Chemical characterization, the transport pathways and potential sources of PM2.5 in Shanghai: Seasonal variations, Atmos. Res., 158, 66–78, https://doi.org/10.1016/j.atmosres.2015.02.003, 2015.
Zong, Z., Tan, Y., Wang, X., Tian, C., Li, J., Fang, Y., Chen, Y., Cui, S., and Zhang, G.: Dual-modelling-based source apportionment of NOx in five Chinese megacities: Providing the isotopic footprint from 2013 to 2014, Environ. Int., 137, 105592, https://doi.org/10.1016/j.envint.2020.105592, 2020.