the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Atmospheric implications of ocean–atmosphere physicochemical interactions
The atmosphere is the fast component of the climate which determines the meteorology, i.e., everyday whether. The ocean, on the other hand, is the slow component which regulates the climate in the long term. Detailed knowledge of the interactions between these two components is crucial in order to understand global climate phenomena.
The ocean–atmosphere interface is the largest one on our planet, occupying about 70 % of the Earth's surface. Hence, the physicochemical processes occurring at the interface can largely affect the chemical content of the ocean waters and the composition of the atmosphere.
Here, we briefly discuss the chemical composition of the sea surface microlayer (SML), emphasizing the role of surface-active compounds concentrated in the SML that influence gas exchange and modulate the production of the largest natural primary aerosols (i.e., sea spray aerosols, SSAs) across the ocean–atmosphere interface. We summarize recent research focused on multiphase and heterogeneous chemical processes, including photochemical reactions within the SML, and their impact on the formation of volatile organic compounds (VOCs), as well as subsequent effects on secondary organic aerosol (SOA) production.
Comprehensive understanding of the ocean–atmosphere physicochemical interactions is of paramount importance in order to properly address air quality and climate issues.
- Article
(3472 KB) - Full-text XML
- BibTeX
- EndNote
Oceans cover approximately 71 % (3.62×108 km2) of Earth's total surface area. Lakes account for about 3 % of the land surface (Messager et al., 2016), while rivers and streams make up roughly 0.58±0.06 % () of the nonglacial land surface (Allen and Pavelsky, 2018). During interactions between the air and water systems, substances (gases, particulate matter, precipitation) and energy (light, heat) must cross the air–water interface for transfer and exchange. Consequently, the physicochemical properties of this interface significantly influence the interactions, composition, and processes occurring between the two phases.
Since the 18th century, oceans have absorbed 20 %–40 % of anthropogenic carbon dioxide (CO2) emissions (Pereira et al., 2018) and contributed about 50 % of global oxygen (O2) production. From an atmospheric perspective, oceans regulate the budget of greenhouse gases (CO2, N2O, CH4, CH3SCH3) (Schneider-Zapp et al., 2014), while also serving as a source of many atmospheric trace species, such as aromatic hydrocarbons (Wohl et al., 2023; Rocco et al., 2021), non-methane hydrocarbons, aldehydes and ketones (Phillips et al., 2021), and other important compounds (Yang et al., 2014a, b). Thus, the physical, chemical, and biological processes at the ocean surface significantly influence the Earth's carbon cycle and atmospheric climate dynamics.
The CLAW hypothesis, an acronym derived from the initials of its four authors, was proposed by Charlson et al. (1987) and suggests that dimethyl sulfide (DMS) emissions from marine phytoplankton promote the formation of atmospheric aerosol particles and cloud condensation nuclei. This process increases cloud albedo, which in turn alters temperature and radiation, creating a feedback loop that affects DMS emissions from phytoplankton and ultimately forms a closed bioclimatic system (Charlson et al., 1987). Although this hypothesis is not entirely valid (Quinn and Bates, 2011; Woodhouse et al., 2008), it highlights the significant impact of ocean–atmosphere interactions on climate. Understanding energy and material transfer fluxes between atmospheric and aquatic systems, as well as key control processes such as biogeochemical and physical interactions and feedback mechanisms, is essential for comprehending how these coupled systems influence Earth's climate. Recent attention has focused on the physicochemical processes occurring at the atmosphere–ocean interface (Donaldson and George, 2012; Carpenter and Nightingale, 2015; Brooks and Thornton, 2018; Novak and Bertram, 2020). This area includes a thin layer of water, ranging from tens to hundreds of micrometers, known as the surface microlayer (SML).
The SML is widely distributed at the ocean surface (Knulst et al., 2003) and other water bodies (e.g., lakes, rivers, and streams). It can be subdivided into a slick and non-slick SML (Wurl et al., 2016) or even thinner layers, referred to as the surface nanolayer (Laâ et al., 2010). Owing to the inherent heterogeneity, a large variety of surface-active organic (surfactants) and inorganic substances have accumulated in the SML due to their interface affinities. Surface-active organics can also attract other more soluble organic compounds (e.g., saccharides) to the surface, leading to the so-called “co-adsorption” effect (Burrows et al., 2016; Carter-Fenk et al., 2021). The elevated concentration of these active substances establishes a unique biological, physical, and chemical milieu in contrast to the underlying water layers.
This distinctive environment facilitates the formation of peptide bonds (Griffith and Vaida, 2012), substantially reducing the physical volatility of the SML, thereby promoting dominant molecular diffusion and resulting in pronounced gradients in heat, pH, and gas concentration. Additionally, it facilitates the breakdown of energy barriers associated with chemical reactions and accelerates substance transformations. As a result, the SML forms the largest active interface on the Earth (Donaldson, 2006).
The SML is globally distributed and can remain stable at wind speeds up to 13 m s−1 (Sabbaghzadeh et al., 2017), and if disrupted, it regenerates quickly. Despite its thinness compared to the underlying water layers, its dynamic changes have a significant impact on global biogeochemical processes, including momentum and heat transfer, air–sea exchange, and aerosol production. The SML is continually consumed and replenished by biogeochemical and physical processes, maintaining a dynamic equilibrium. Supply mechanisms include atmospheric deposition and physical transport from the subsurface water column. Wind-driven convergence circulation, tidal forces, ocean shear, upwelling, and internal waves lead to localized concentrations of surface-active substances across various spatial scales (Frka et al., 2012). Removal processes involve direct injection of enriched substances into the atmosphere following bubble bursting, the chemical transformation of dissolved organic matter (DOM) upon light irradiation, or the interface oxidation processes, resulting in production of aerosols into the atmosphere. Hence, the SML holds substantial atmospheric significance. Its composition and biogeochemical transformation processes not only influence the input flux of nonbiological-source volatile organic compounds (VOCs) and aerosol particles (i.e., sea spray aerosol, SSA) to the atmosphere, but also regulate the deposition rate of trace gases on the ocean surface. Studies on the SML are crucial for understanding interactions between ocean aerosols and clouds, representing a significant area of focus in marine atmospheric chemistry. The ultimate goal is to uncover the driving forces and mechanisms behind climate change.
Currently, there is a foundational understanding of the role of the SML in ocean aerosol–cloud interactions. This review aims to revisit scientific progress concerning environmental and climate issues associated with the SML from an atmospheric chemistry perspective. Specifically, it addresses advances in research on how physical and chemical processes at the ocean surface impact the atmosphere.
The attention to the air–water interface began in the early 20th century with a series of studies on the surface tension of electrolyte solutions. At the time, a major obstacle was the absence of direct interfacial detection techniques, which restricted characterization of the interface to macroscopic experimental approaches, such as surface tension and electrostatic potential measurements (Petersen and Saykally, 2006). Consequently, theoretical models were employed to interpret experimental data and indirectly infer the behavior of inorganic ions at the interface. However, without calculations based on the atomic level, theoretical issues at the molecular level are difficult to address accurately (Jungwirth and Tobias, 2006).
Recent advances in computational capabilities and research methodologies have enabled the use of advanced surface-resolving techniques such as sum-frequency generation (Gordon et al., 2019; Seki et al., 2023), second harmonic generation, X-ray photoelectron spectroscopy (Kong et al., 2021), and molecular dynamics simulations (Petersen and Saykally, 2006). These advancements enable a more in-depth and direct exploration of the structure of the air–water interface and the specific behavior of ions at this interface in both theoretical and experimental domains. This progress has led to a paradigm shift, challenging traditional beliefs about ion behavior: certain ions are now understood to accumulate at the air–water interface (Jungwirth and Tobias, 2006). This propensity is associated with ion valence, polarity, and interactions with water molecules. Moreover, advances in technology have challenged some traditional viewpoints. For instance, recent studies using stimulated Raman three-dimensional imaging techniques have revealed the enrichment of ions on the surface of aerosol liquid films (Gong et al., 2023). These findings indicate a gradient change in the pH of deliquescent aerosol liquid films, suggesting that the method of using uniform aqueous solutions to simulate homogenous chemical processes in real aerosol liquid films may not replicate the actual chemical environment accurately. In addition to inorganic salt ions, organic molecules also exhibit a tendency to accumulate at the air–water interface (Rossignol et al., 2016) and interact with water molecules upon contact (polarization, solvation), being stored at high concentrations at the air–water interface, lowering the activation energy of reactions, and thus altering the reactivity of chemical reactions, including changes in reaction rates, product yields, and even reaction pathways. This propensity of substances to accumulate at the air–water interface is one of the key reasons why this boundary has garnered significant scientific attention.
In addition to the substance propensity at the air–water interface, the structure, composition, and behavior of water molecules at the air–water interface differ from those in the bulk phase. Petersen et al. (2004) used the Multistate Empirical Valence Bond (MS-EVB) method to predict the presence of excess protons (H3O+) at the air–water interface. However, the presence of excess charged water molecules with an odd number of hydrogen bonds has been shown at the water interface, indicating an excess negative charge on the surface (Ben-Amotz, 2022). Measurements of the electrophoretic mobility of oil droplets and bubbles in water also indicate a negatively charged interface. The simplest explanation for this phenomenon is the enrichment of hydroxide ions (OH−) at the interface and the electrostatic repulsion of hydrated hydronium ions (H3O+). However, this explanation is inconsistent with some experimental results, such as second harmonic generation and sum-frequency generation techniques, which indicate the presence of excess H3O+ at the gas–liquid interface (Petersen and Saykally, 2005). Molecular dynamics models and continuous solvent models have also shown that H3O+ is more inclined to the gas–liquid interface compared to OH−. The pH of the liquid surface may differ from that of the bulk phase, not only because of the sign of the liquid surface's electric field, but also due to the preference of OH− or H3O+ for the gas–liquid interface. Therefore, there is controversy regarding the acidity of the water interface (Saykally, 2013), as some research results indicate that the water surface is acidic (pH<4.8) (Buch et al., 2007; Mamatkulov et al., 2017), while others indicate it is alkaline (Beattie et al., 2009; Mishra et al., 2012). These differences in research results may be caused by differences in experimental methods used. Although many research results are contradictory at present, there is undoubtedly a close relationship between hydrogen bonds, charge transfer, and the formation of the air–water interface charge layer, which objectively leads to an imbalance of positive and negative ions at the water interface (Hao et al., 2022; Ben-Amotz, 2022), thereby affecting the pH of the near-surface region and consequently affecting the chemical processes at the air–water interface. It has been reported that some chemical reactions occurring at the air–water interface are accelerated (see Sect. 2.2), but also that spontaneous chemical processes occur at the air–water interface (Sect. 2.3, Lee et al., 2019b; Li et al., 2023), suggesting that the electric field existing at the air–water interface is the driving force behind spontaneous chemical processes. Some studies have observed a strong electric field at the oil–water interface of microdroplets using Raman-excited fluorescence microscopy, suggesting that this strong electric field may be caused by charge separation due to adsorbed negative ions on the surface (Xiong et al., 2020). Recently, Liu et al. (2024) detected a strong electric field at the gas–liquid interface of deliquescent nitrate aerosol microdroplets using surface-enhanced micro-Raman spectroscopy and molecular dynamics simulations, but the driving force behind this electric field remains to be discovered.
2.1 Enrichment and depletion behavior of ions and their impact on chemical processes at the air–water interface
Interest in the air–water interface was rekindled with advancements in studying halogen transformations in sea salt aerosols. Researchers realized that ion behavior at this interface may deviate from prior assumptions, particularly as some inorganic ions tend to concentrate there. Surface-exposed ions can boost gas reactivity at the interface, influencing key processes like gas absorption, halogen chemistry, and ozone (O3) depletion (George et al., 2015).
Hu et al. (1995) examined Cl2(g) and Br2(g) uptake by sodium chloride (NaCl) and iodine chloride (ICl) aerosols (120–250 µm in diameter). Their findings showed that bulk-phase reactions alone could not account for the observed absorption levels or their dependence on ion concentrations. They thus concluded that reactions at the air–water interface played a crucial role in the uptake process (Hu et al., 1995). Oum et al. (1998) and Knipping et al. (2000) investigated the reaction between hydroxyl radicals (OH) and sea salt particles; the results indicated that air–water interfacial reactions were essential to explain the observations, a process that may also occur on the ocean surface. Additionally, molecular dynamics simulations revealed that Cl− enrichment at the surface of NaCl aerosols enhanced interfacial chemical processes (Knipping et al., 2000). Field observation data indicate that detected halogen molecules (Cl2(g), Br2(g), BrCl(g)) in the marine atmosphere are correlated with O3 depletion (Spicer et al., 1998, 2002; Foster et al., 2001). Behnke et al. (1995) discovered that in the presence of O3, simulated sunlight irradiation of sea salt aerosols produces an unidentified chlorine atom precursor. Laboratory research by Laskin et al. (2003) showed that OH(g) reacts with Cl− on the surface of deliquescent NaCl aerosols to form sodium hydroxide (NaOH), increasing the alkalinity of sea salt particles and thereby enhancing the uptake of sulfur dioxide (SO2) and the formation of sulfates (Laskin et al., 2003). These studies indicate that inorganic ions enriched at the air–water interface of sea salt aerosols can profoundly impact the composition and oxidation capacity of the marine atmosphere. Since inorganic salt ions are key components of atmospheric aerosols and ocean surfaces, many studies have shown that, in addition to halogen ions directly participating in atmospheric chemical processes, the surface propensity of inorganic salt ions causes ionic strength effects that profoundly modulate multiphase reactions at the air–water interface. These processes include sulfate formation (Yu et al., 2023), O3 uptake (Mekic et al., 2018b, 2020a; Mekic and Gligorovski, 2021), and the generation and transformation of atmospheric pollutants including secondary organic aerosol (SOA; Mekic et al., 2018a, 2020c, a; Zhou et al., 2019; Wang et al., 2021; Gwendal Loisel, 2021; Pratap et al., 2021; Li et al., 2022). Although many studies have made efforts and achieved some results on this topic, most of the studies have focused on single-salt systems. A recent study investigated the interaction between ions for air–water interface propensity (Seki et al., 2023). Considering the complexity of the real atmospheric environment, extrapolating laboratory data to the atmospheric environment for evaluating environmental and climatic impacts still requires substantial effort.
2.2 Accelerated chemistry at the air–water interface
Many experimental and theoretical studies indicate that, compared to homogeneous environments, the water interface can significantly accelerate the reaction rates of certain chemical processes (Kusaka et al., 2021; Narayan et al., 2005; Klijn and Engberts, 2005; Kong and Evanseck, 2000), such as photochemistry (Kusaka et al., 2021; Gong et al., 2022), photodecomposition (Rao et al., 2023b), photosensitized reactions (Wang et al., 2024), spontaneous redox reactions (Lee et al., 2019a; Kong et al., 2021), and gas–gas reactions at the air–water interface (Liu and Abbatt, 2021).
Research into faster chemical reactions at the air–water interface draws inspiration from organic chemistry studies. Organic chemists typically avoid using water as a solvent because it can react with organic compounds, and its polarity makes it unsuitable for dissolving most nonpolar organics. As a result, aqueous solvents are generally seen as ineffective for organic reactions (Klijn and Engberts, 2005). Early studies found that some pericyclic reactions of hydrophobic organic compounds, such as the Diels–Alder cycloaddition (Breslow, 1991) and Claisen rearrangement (Gajewski, 1997), proceed faster in dilute aqueous solutions than in organic solvents or pure substances. Their acceleration may be due to the hydrophobic effect (Tian et al., 2024), which polarizes the transition state structure formed between the reactants, thus lowering the activation energy. Other factors include enhanced hydrogen bonding in the transition state, increased water cohesive energy density, and a stronger hydrophobic effect (Jung and Marcus, 2007; Kong and Evanseck, 2000; Breslow, 1991). In 2005, Barry Sharpless's group reported that the reaction rates of hydrophobic organic reactants dramatically increased under emulsion conditions (formed by rapidly stirring water-insoluble organics with water) compared to homogeneous or pure solute conditions. They concluded that both the heterogeneity and the presence of the water interface played key roles in reaction acceleration (Narayan et al., 2005; Klijn and Engberts, 2005). Despite these findings, the exact mechanism for this acceleration remains elusive. One theory suggests that about one-quarter of the OH groups of water molecules at the interface are free (unbound by hydrogen bonds). These free OH groups “extend” into the organic phase at the interface, forming hydrogen bonds with reactants in the organic phase. The transition state between the reactants is stabilized by these stronger hydrogen bonds with free OH groups, thus dramatically speeding up reactions at the “oil–water interface”(Jung and Marcus, 2007). Recent research demonstrated that photochemical reactions at the oil–water interface can be accelerated through melting point depression (Tian et al., 2024). Further, Shi et al. (2025) combined Raman spectroscopy and multivariate curve resolution to suggest that water structural disorder and enhanced electric fields at mesoscale interfaces in oil–water emulsions may contribute to accelerated chemical reactivity (Shi et al., 2025). Similar to the “oil–water interface”, some gas molecules also exhibit faster reaction rates at the air–water interface. Typically, reactions of neutral closed-shell molecules in the gas phase, while thermodynamically feasible, are slow due to high reaction barriers. In contrast, the reaction rates of the same reactants (or appropriately modified forms in the condensed phase) in multiphase environments can exceed those in the gas phase. This acceleration stems from either reduced activation energy or higher reactant concentration in the condensed phase. The formation of acid rain is a key example. The reaction between SO2(g) and hydrogen peroxide (H2O2(g)) is inefficient in the gas phase but efficient in the liquid phase. This is because the concentration of H2O2 in the atmospheric waters (H2O2(aq)) is relatively high compared to H2O2(g). Moreover, once SO2(g) dissolves in water and hydrolyzes to form , the reaction between H2O2(aq) and is highly efficient.
While previous studies have documented reaction acceleration at water interfaces and proposed mechanisms like OH group interactions with organics, other factors may also play a role. These potential contributors include reactant confinement, partial solvation, preferential orientation, droplet curvature, and surface pH variations. Despite these insights, the exact reasons for the acceleration of reactions at the water surface remain inconclusive (Ruiz-Lopez et al., 2020).
2.3 Spontaneous chemistry at the air–water interface
The thermodynamics and kinetics of many chemical reactions at interfaces differ from those in the bulk phase due to the heterogeneity of the medium at or near the interface (Zhong et al., 2019; Ruiz-Lopez et al., 2020; Kusaka et al., 2021; Wei et al., 2020). At the air–water interface, the surrounding water exerts asymmetric molecular interactions on the observed water molecules and solutes. The density of interfacial water is lower than that of bulk water, and its density fluctuations generate macroscopic capillary waves, surface roughness, and tension. These factors result in differences in surface molecular dynamics, orientation, hydrogen-bond networks, and dielectric properties compared to the bulk phase (Deal et al., 2021). When ions or surfactants adsorb at the air–water interface, they alter surface tension and surface potential, ultimately changing interfacial chemical processes (Jungwirth and Tobias, 2006; Otten et al., 2012). Recent studies report that tiny droplets (diameter 1–20 µm) spontaneously produce hydrogen peroxide on their surface, with production inversely correlated with droplet diameter. It is suggested that H2O2 forms from the combination of OH radicals generated from OH− at the droplet surface under the influence of an electric field (Lee et al., 2019b, 2020). This study has garnered significant attention because, thermodynamically, a pure water environment is unfavorable for H2O2 formation. Therefore, the study faces skepticism due to issues of reproducibility, potential contamination, and lack of a reasonable mechanistic explanation (Nguyen et al., 2023). Nevertheless, because the air–water interface is ubiquitous in the atmosphere, this spontaneous chemical process has attracted considerable attention from researchers. Following this study, a series of investigations into spontaneous chemical processes at the air–water interface have emerged. Examples include the spontaneous generation of OH radicals at the air–water interface in dark conditions (Li et al., 2023), the spontaneous conversion of I− to I and I2 at the air–water interface (Guo et al., 2023), mechanistic and quantitative studies of different inorganic salt ions on the spontaneous generation of H2O2 at the air–water interface (Angelaki et al., 2024), and the spontaneous oxidation of thiols and thioethers at the air–water interface of sea spray droplets (Rao et al., 2023a).
2.4 Molecular dynamics simulation of chemical processes at the air–water interface and their influence on the atmosphere
Due to the rapid reactions of some atmospheric species at the air–water interface, such as Criegee intermediates and SO3, it is difficult for existing experimental methods to capture their chemical reaction processes. Therefore, complementing experimental techniques, molecular dynamics simulations (such as Born–Oppenheimer molecular dynamics simulations) provide strong support for studying the fast reaction processes (picosecond scale) occurring at the air–water interface, effectively aiding in a deeper understanding of how the air–water interface influences reaction pathways, rates, and mechanisms at the molecular level (Zhong et al., 2018). For example, Born–Oppenheimer molecular dynamics simulations have shown that the rapid heterogeneous process of iodine oxidation on sea salt aerosol surfaces (picosecond scale) promotes aerosol growth (Ning et al., 2023).
In recent years, Francisco's research group (Anglada et al., 2014, 2020a; Martins-Costa et al., 2019, 2018, 2012b) has used quantum mechanics/molecular mechanics molecular dynamics (QM/MM-MD) simulations to propose that many important atmospheric species such as O3, H2O2, methylhydroperoxide, nitrogen dioxide (NO2), and HO2 radicals exhibit specific behaviors at the air–water interface, accelerating photodissociation rates, which may consequently impact the physicochemical characteristics of aerosols and the atmospheric environment. These species tend to accumulate at the air–water interface, leading to an increase in interface concentration, thus influencing interfacial chemical processes (Anglada et al., 2020b; Martins-Costa et al., 2012b). Simulation studies have found that the absorption band of O3 accumulated at the air–water interface undergoes a red shift and broadening compared to that in the gas phase, leading to an order of magnitude faster photodissociation rate of O3 at the interface than in the gas phase, resulting in a 4-order-of-magnitude increase in the rate of OH radical generation at the interface (Anglada et al., 2014). Additionally, methylhydroperoxide in the air–water interface exhibits a blue shift and broadening in its UV-visible absorption spectrum, which is attributed to hydrogen bonding between methylhydroperoxide and water molecules (Martins-Costa et al., 2017). Similarly, NO2 also exhibits accumulation behavior at the air–water interface, with broadening of its absorption band, leading to an increase in its photodissociation rate at the interface (Murdachaew et al., 2013; Martins-Costa et al., 2019). Intriguingly, the excited triplet state of SO2 () produced under sunlight irradiation can react with water molecules, leading to the formation of OH radicals and hydroxysulfinyl radical (HOSO), which is further converted to and then by the oxidation–reduction process to sulfate () (Fig. 1) (Ruiz-López et al., 2019).
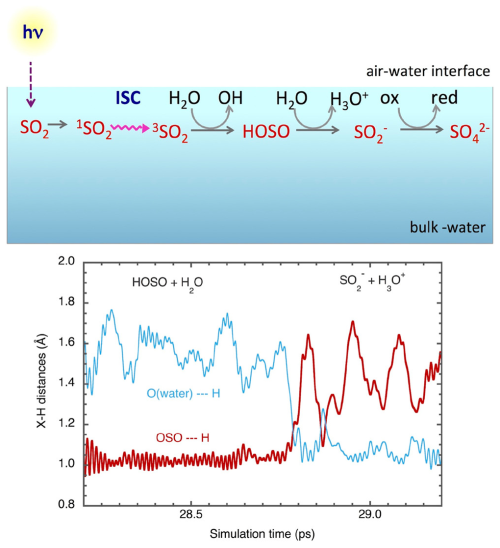
Figure 1Light-induced oxidation of SO2 is transformed to its excited triplet state (), which further promotes photosensitized chemistry, leading to the production of OH and HOSO radicals as well as sulfate. Reprinted with permission from Anglada et al. (2020b). Copyright (2020) American Chemical Society.
The importance of the formed HOSO arises from the fact that is very acidic () and undergoes fast ionic dissociation at the air–water interface. In addition, the formed ions can be further oxidized by H2O2, O3, OH, or HO2 to generate sulfuric acid (Ruiz-López et al., 2019; Anglada et al., 2020a).
Furthermore, molecular dynamics simulations have revealed that important atmospheric gases such as N2, O2, O3, OH, H2O, HO2, and H2O2 tend to accumulate at the water surface with the lowest free energy when located at the air–water interface (Vácha et al., 2004). These findings are significant not only for chemical processes occurring at aerosol surfaces but also for processes occurring at the atmosphere–ocean interface.
Although molecular dynamics theoretical studies have provided insights into how the air–water interface alters the reaction mechanisms of certain atmospheric chemical processes at the molecular level, there are still some unresolved issues. For instance, it is necessary to estimate the concentration scale of chemical processes occurring at the air–water interface to assess the significance of these specific chemical processes. Estimating the energy barriers of reaction pathways occurring at this interface is essential to determine the impact of these processes on atmospheric chemistry. Furthermore, it is essential to assess the influence of more complex compositional conditions, such as different pH values, ionic strengths, gas–liquid contact areas, and the morphology and size of hydrated aerosol particles, on the reactions at this interface.
The ocean–atmosphere interface represents the largest air–water interface on Earth. In recent years, the physicochemical processes at the ocean–atmosphere interface have received widespread attention (Donaldson and George, 2012; Carpenter and Nightingale, 2015; Brooks and Thornton, 2018; Novak and Bertram, 2020). As mentioned in the Introduction this region encompasses a thin layer of ocean surface, termed the SML. The definition of the SML commonly refers to the uppermost layer of the sea surface, typically spanning 1–1000 µm, as defined by Liss and Duce (Liss and Duce, 1997). Sampling thickness varies with methodologies and research objectives (Cunliffe and Wurl, 2015). Zhang et al. observed a “sharp change in physicochemical characteristics” at a depth of 50 µm below the ocean–atmosphere interface, proposing a refined SML thickness of 50±10 µm (Zhang et al., 2003b, a). Despite the thinness, its heterogeneity coupled with the enrichment of surface-active substances makes it pivotal in ocean–atmosphere interactions, playing a crucial role in the exchange of matter and energy between the two phases. Hence, comprehensive knowledge of the SML's characteristics is extremely important to understand the ocean–atmosphere interactions which in turn represent one of the biggest unknowns related to air quality and climate change issues.
The composition, concentration, and enrichment of the SML are variable spatiotemporally. The components mainly come from in situ biological activities, land (river) inputs (Jaffé et al., 2013; Wagner et al., 2015; Park et al., 2019), migration from underlying water column (Gašparoviæ et al., 2007), atmospheric dry and wet deposition (Milinkoviæ et al., 2022; Hunter and Liss, 1977), and sediments. In general, the SML is enriched with substances such as sugars, amino acids, proteins, lipids, and colloids (Liss and Duce, 1997; Laß et al., 2013; Laß and Friedrichs, 2011). The enrichment factor (EF) is generally used to represent the degree of enrichment of substances in the SML, as follows:
In the above equation, CSML represents the concentration of a substance in the surface microlayer, and CSSL represents the concentration of a substance in the corresponding lower layer of water in the surface microlayer. Some EFs of the SML relevant for the ocean–atmosphere interactions and implications are shown in Table 1.
Table 1Enrichment factors of various substances in the SML.
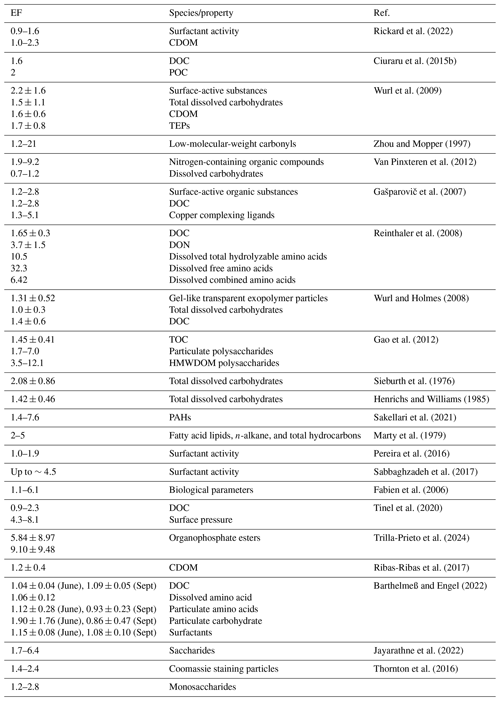
TEPs: transparent exopolymer particles; CDOM: chromophoric dissolved organic matter; POC: particulate organic carbon; DOC: dissolved organic carbon; TOC: total organic carbon; DON: dissolved organic nitrogen; HMWDOM: high-molecular-weight DOM.
Most of the substances listed in Table 1 are typically enriched in the SML through rising bubbles or diffusion. These compounds, which constitute the primary organic components of the SML, not only confer biochemical activity to the SML, but also modify its physical properties such as dampening ocean surface waves. Current research indicates that the SML significantly suppresses gas exchange between the ocean and atmosphere. Furthermore, the physicochemical processes within the SML are closely linked to the budget of marine atmospheric VOCs, as well as primary and secondary organic aerosols. Consequently, accounting for SML-specific processes is imperative when estimating the transfer rate of greenhouse gases (Pereira et al., 2018) and the marine aerosol budget.
Surfactant activity (SA) is one of the most important physicochemical parameters of the SML, determined by the surface adsorption behavior of its surfactant compounds. Surfactants, also known as surface-active substances, are compounds that can significantly reduce surface tension or interfacial tension between two liquids, liquid–gas, and liquid–solid. The surface tension of pure seawater (devoid of organic matter and particulate material) slightly varies with temperature and salinity, typically ranging from 73–75 mN m−1 (Nayar et al., 2014). In comparison to pure seawater, the surface tension of coastal sea surface decreases by 10–15 mN m−1, while that of remote ocean surfaces generally decreases by 0.5–1 mN m−1 (Frew and Nelson, 1992). Studies have reported that the SA of the SML is 0.1–1.57 mg L−1 T-X-100 (Wurl et al., 2011; Sabbaghzadeh et al., 2017). Surfactant enrichment in the SML is greater in oligotrophic regions of the ocean than in more productive waters (Wurl et al., 2011). For instance, the SA of the SML in the Northern Hemisphere is significantly higher than that in the Southern Hemisphere, with higher enrichment of surfactants in the Southern Hemisphere oceans (Sabbaghzadeh et al., 2017). This enrichment persists even at wind speeds reaching 13 m s−1 (Sabbaghzadeh et al., 2017), which suggests that the SML is stable enough to exist even at the global average wind speed of 6.6 m s−1.
Temperature is an important parameter influencing surface adsorption behavior (Pereira et al., 2018). Results from 2-year continuous observation experiments show that SA of the SML varies seasonally, displaying a quasi-sinusoidal pattern from early to late in the year. This variation corresponds to changes in phytoplankton productivity, suggesting that variations in temperature, sunlight radiation, and nutrient input (from rivers) influencing primary productivity and salinity changes may be the driving forces behind SA variations (Gas̆paroviæ and Æosoviæ, 2001). This conclusion aligns well with findings from other studies, showing that sunlight-radiation-induced degradation of large-molecule CDOM leads to an increase in SA (Rickard et al., 2022). Therefore, a deeper understanding of the photochemical processes in the SML is crucial for comprehending the transfer rates of important trace gases such as CO2, CH4, N2O, and DMS at the ocean–atmosphere interface, with significant implications for environmental climate studies (Rickard et al., 2022).
Early research on the SML primarily focused on the physical barrier effect of surfactants, suggesting that the organic film composed of surfactants would simultaneously hinder the evaporation of seawater and the transfer of atmospheric gases to the ocean (Liss and Duce, 1997; Donaldson, 2006; Rudich, 2003). Surfactants in the SML are divided into water-soluble and water-insoluble categories, among which water-soluble surfactants (such as TEPs, lipids, polysaccharides, amino acids) play a significant role in inhibiting the gas transfer rate between the ocean and the atmosphere (Bock et al., 1999; Frew et al., 1990). For instance, Tsai and Liu (2003) estimated a reduction of 20 %–50 % of the global annual net flux of CO2 by correlating surfactant abundance and distribution with local primary productivity, assuming surfactant enrichment increases alongside productivity (Tsai and Liu, 2003). However, field measurements by Wurl et al. (2011) contradicted this assumption, demonstrating that surfactant enrichment decreases with primary productivity. Their study also generated the first global maps of surfactant concentrations in the SML and their EFs by integrating experimental data on primary productivity and wind speed. These findings underscored the SML's potential global influence on air–sea gas exchange and biogeochemical cycles (Wurl et al., 2011). A more recent study by Sabbaghzadeh et al. (2017) argued that SA enrichment in the SML should be essentially decoupled from ambient wind speed. Instead, SA enrichment is more related to the SA in subsurface water (Sabbaghzadeh et al., 2017; Pereira et al., 2016). Additionally, while chlorophyll α is widely used as a proxy for primary productivity (Pereira et al., 2016), field studies have shown it to be an unreliable indicator for parameterizing the inhibitory effect of surfactants in the SML (Sabbaghzadeh et al., 2017). Table 2 summarizes the impact of surfactants on the transfer rates of CO2 at the air–water interface.
Table 2Suppression effect of surfactants on the gas transfer velocity of CO2.
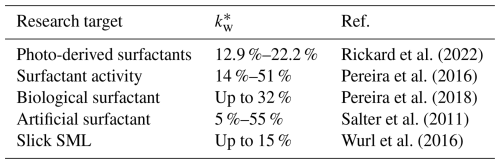
*Suppression of the gas transfer velocity.
Currently, the variations in the mass fraction of surfactants in the DOM carbon pool in the SML are believed to be the reason for the large discrepancies in the estimated values of gas transfer velocity of CO2 shown in Table 2 (Pereira et al., 2016). In addition to CO2, surfactants on the water surface also impact the gas-to-liquid phase transfer process of other gases, such as nitric acid (HNO3), ammonia(NH3), O3, and nitrogen pentoxide (N2O5), by forming a well-ordered, dense film (Clifford et al., 2007; Stemmler et al., 2008; Rouvière and Ammann, 2010; Thornton and Abbatt, 2005; Cosman and Bertram, 2008). Compared to branched-chain structures, straight-chain surfactant organic compounds exhibit the strongest inhibitory effect on gas absorption (Cosman and Bertram, 2008).
Surfactants in the SML can also physically modulate interfacial chemical reactions. For instance, an authentic SML has been shown to significantly suppress the chemical generation rate of gaseous I2(g) from O3+I2 reaction at the SML by enhancing the solubility of I2 in the SML (Schneider et al., 2022). Additionally, surfactants accumulating at the water surface can form an organic phase, enabling otherwise water-insoluble substances to dissolve into the organic layer. This enrichment elevates the organic concentration at the interface, facilitating chemical reactions that are thermodynamically or kinetically constrained in bulk aqueous phases. A notable example is the role of organic films in promoting the enrichment of polycyclic aromatic hydrocarbons (PAHs) (Donaldson, 2006), which may subsequently undergo photochemical degradation upon solar irradiation (Jiang et al., 2021; Mekic et al., 2020c). Similarly, photosensitizers like 4-carboxybenzophenone (4-CB) and imidazole-2-carboxaldehyde (IC) are “attracted” to the surface when fatty acids or fatty alcohols coat the liquid surface, thereby initiating photochemical reactions (Tinel et al., 2016). Sum-frequency generation experiments have revealed that soluble monosaccharides in solution can strongly adsorb to lipid monolayers covering the solution surface via Coulomb interactions under appropriate conditions (Burrows et al., 2016). This “co-adsorption” phenomenon is critically important for interfacial photochemical processes in the SML, as it enhances the reactivity and compositional complexity of the organic layer.
Sea spray aerosols (SSAs), also known as primary marine aerosol particles, are directly generated by wind–wave interactions and represent the largest natural source of aerosols globally (Russell et al., 2023). SSA serves as one of the primary carriers for material and energy transfer between the atmosphere and the ocean. It influences atmosphere composition through physicochemical processes, and under certain conditions, it may grow into warm and cold clouds (Fig. 2), directly or indirectly impact surface radiative balance, and profoundly affect climate change (Wang et al., 2019a; Jammoul et al., 2008; Brooks and Thornton, 2018; Demott et al., 2016; Wilson et al., 2015; Uetake et al., 2020). The current best estimate of SSA flux of 5000 Tg yr−1 can be used to calculate the SSA-related carbon flux as 35 Tg C yr−1 by approximating <1 µm SSA particles as 10 % of SSA flux with 7 % organic carbon and >1 µm particles as 90 % of SSA flux with no organic carbon (Russell et al., 2023; Tsigaridis et al., 2013). However, significant uncertainty remains in our understanding of the cloud-forming role of SSA (Quinn et al., 2017; Xu et al., 2022). This is largely due to the ambiguous link connecting organic components in the SML and the physicochemical properties of SSA and its cloud formation potentials (Russell et al., 2010), especially the prevalence and variability of the SML's direct and indirect impact on SSA composition, size distribution, and flux.
SSA forms when wind speeds exceed approximately 5 m s−1 (Quinn et al., 2015; Lewis, 2004), creating surface shear stress that breaks waves and entrains air bubbles transporting organic matter (OM) to the surface (De Leeuw et al., 2011). Rising bubbles transport OM to the surface. At the air–sea interface, bubble bursting releases SSA through two mechanisms: “film jet” (submicron particles) from bubble film rupture and “drop jet” (supermicron particles) from cavity collapse (Brooks and Thornton, 2018; Quinn et al., 2014, 2015; Prather et al., 2013). Therefore, wind speed, particularly on the sea surface (Russell et al., 2010; Liu et al., 2021a; Parungo et al., 1986), and the physicochemical properties of surface seawater, such as sea surface temperature (Liu et al., 2021a; Christiansen et al., 2019; Sellegri et al., 2006; Mårtensson et al., 2003), salinity (Mårtensson et al., 2003; Tyree et al., 2007), and SA (Cochran et al., 2016a), are considered crucial factors determining the mechanisms of SSA production.
As shown in Fig. 2, the SML is believed to play a crucial role in SSA formation (O'dowd et al., 2004; Russell et al., 2010; Wang et al., 2015), as it is inherently involved in SSA production through bubble bursting and wave breaking (Schmitt-Kopplin et al., 2012), (i) linking its organic composition to SSA's chemical makeup (Lewis et al., 2022), (ii) modulating bubble lifetime and properties (Modini et al., 2013), and (iii) influencing SSA production mechanisms, all of which are primarily results of the presence of enriched surface-active organic materials (i.e., surfactant) such as lipids and fatty acids (Mochida et al., 2002; Cochran et al., 2016b, a, 2017; Wang et al., 2015) and OM with positive buoyancy (e.g., EPSs) at the SML (Keith Bigg et al., 2004). Such an impact could further modulate ice-nucleating particle (INP) and cloud condensation nuclei (CCN) budget in the marine atmosphere. Hence, bridging the link between SML and SSA organic composition is therefore important for understanding the physicochemical properties of SSA and their subsequent environmental and climatic effects (Russell et al., 2010).
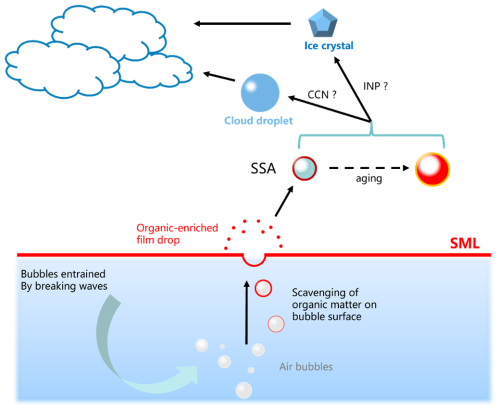
Figure 2Illustration of the role of SML in regulating SSA production and properties which could further affect its ice-nucleating and cloud condensation potentials and eventually cloud formation.
This is especially true for submicron SSA, given their dominant quantity, relatively high organic fraction, and efficient light scattering compared to supermicron SSA (O'dowd et al., 2004; Quinn et al., 2015). It has been shown that submicron SSA is predominately constituted by water-insoluble organic matter with surfactant characteristics during high biological activity periods (Facchini et al., 2008; O'dowd et al., 2004). This is evidenced by the aliphatic-rich organic-species-dominated submicron mode of SSA size distribution measured by aerosol mass spectrometry and Raman spectroscopy (Wang et al., 2015; Cochran et al., 2017) and by a relative increase in surface-active compounds in comparison with the surface water measured by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) and nuclear magnetic resonance spectroscopy (NMR; Schmitt-Kopplin et al., 2012), as well as other high-resolution mass spectrometry (Cochran et al., 2016b). The ocean-biology-associated surfactants exhibited high SA, resulting in their enrichment in the SML; thus, they provide efficient transfer such as long-chain fatty acids into film drops during the bubble bursting process at the SML (Cochran et al., 2017; Schmitt-Kopplin et al., 2012). The presence of biogenic surfactants can modulate bubble microphysics such as persistence time and bubble film cap thickness (Modini et al., 2013) and subsequent bursting process, resulting in the size distributions shifting toward smaller sizes (Sellegri et al., 2006; Tyree et al., 2007; Fuentes et al., 2010a), particle flux enhancement in the Aitken mode with suppression in other modes (Fuentes et al., 2010b; Sellegri et al., 2006), and suppression in the total number of particles produced (Modini et al., 2013). This change has also been observed during a course of 2-week phytoplankton growth in a mesocosm study (Alpert et al., 2015). However, the effect of surfactants is dependent on phytoplankton type (Fuentes et al., 2010a; Alpert et al., 2015), bubble generation method (Alpert et al., 2015), and solubility (Modini et al., 2013) and could be offset by the sea surface temperature (Sellegri et al., 2006) as observed in the annual variation of marine aerosol particle size distribution modes (O'dowd et al., 2004). The mode shifts induced by surfactants could alter the cloud-forming potentials of SSA, as its particle size distribution and the concentration of SSA are closely linked to its environmental effects (Cochran et al., 2016b; Fuentes et al., 2010b; Collins et al., 2014; Deane and Stokes, 2002; Laskina et al., 2015).
Understanding the SML's impact on SSA composition requires widespread, simultaneous measurements of both SSA and SML organic composition (Lewis et al., 2022), along with further studies on mechanisms through which SML-enriched organics adsorb onto bubble films and eventually into SSA (Burrows et al., 2014, 2016; Hasenecz et al., 2019; Carter-Fenk et al., 2021). Distinguishing “fresh” from “aged” marine aerosols using ambient measurements represents another challenge in this topic. Interferences may come from continental, anthropogenic influences and marine atmospheric aging. The recent development of an in situ primary marine aerosol particle generator (Sea Sweep and MART) (Lewis et al., 2022; Bates et al., 2012, 2020; Quinn et al., 2014) and large-scale wave tunnels (Prather et al., 2013; Sauer et al., 2022; Cochran et al., 2016b; Demott et al., 2016; Collins et al., 2014; Ault et al., 2013; Quinn et al., 2015) enable investigation of SSA's physicochemical properties and the role of environmental factors under controlled conditions without external interferences. However, Sea Sweep and MART reproduced limited size-resolved number distributions that span the full range of that produced in ambient seawater (Bates et al., 2020; Lawler et al., 2024). The complex design of a large-scale wave tunnel, along with the high requirements for operators and experimental equipment, limits its widespread application (Mayer et al., 2020), and the systematic change of the original water system (e.g., filtration of zooplankton) may introduce unknown effects (Wang et al., 2015).
The influence of the ocean on the atmosphere extends beyond heat transfer and water vapor transport. The ocean also serves as a source and sink for atmospheric aerosols and trace gases.
Over the past two decades, researchers in the field of atmospheric chemistry have conducted extensive research on chemical processes occurring at the air–water interface (George et al., 2015). Many studies have found that when certain molecules are at the gas–liquid interface, their free energy is minimized (Martins-Costa et al., 2012a, 2015; Mozgawa et al., 2014; Vácha et al., 2004). This characteristic greatly facilitates the migration, accumulation, and rapid occurrence of (photo)chemical processes at the interface. Therefore, it has been recognized that (photo)chemical processes occurring at the gas–liquid interface represent important sources and sinks for atmospheric trace gases (Rossignol et al., 2016). This chemical process is different from the previous understanding of chemistry occurring in the homogeneous phase (Ravishankara, 1997).
Compared to the scientific knowledge of atmospheric chemical processes of terrestrial VOCs, there has been a relatively limited understanding regarding the sources and sinks of marine-derived VOCs and the corresponding climate effects arising from the atmospheric physicochemical processes accompanying their life cycle in the marine boundary layer (MBL). In addition, there is a limited quantity of field measurement data and limited number of observed species derived from marine environments across both temporal and spatial scales. Therefore, the current understanding of relevant scientific issues in this field mainly stems from field observational experiments and laboratory simulation studies.
Low-molecular-weight carbonyl compounds (methanol, formaldehyde, and acetone) observed in the SML were much higher than those in SSW (Dixon et al., 2013). Their concentrations exhibited diurnal variations peaking in the afternoon, indicating that the photolytic processes at the sea surface are the source of these carbonyl compounds (Dixon et al., 2013). Schlundt et al. (2017) simultaneously measured a series of oxygenated volatile organic compounds (OVOCs) in surface seawater and marine air, finding correlations among these OVOCs, suggesting similar sources and sinks in surface seawater. A 5-year monitoring of acetone, methanol, and formaldehyde in the tropical oceanic boundary layer revealed that the tropical Atlantic region acts as a net sink for acetone but a net source for methanol and formaldehyde (Read et al., 2012). A modeling study indicated that tropical and subtropical oceans are mainly net sources of acetone, while high-latitude oceans represent sources of acetone (Wang et al., 2020a). Another modeling study suggested that the Northern Hemisphere oceans act as sinks for acetone, while the Southern Hemisphere oceans are a source of acetone (Fischer et al., 2012). Wang et al. (2019b) identified unknown sources of formaldehyde through aircraft observations, indicating that the ocean is an important source of formaldehyde in the MBL.
These observational data indicated that chemical processes occurring in the SML may be significant sources of VOCs in the MBL (Mungall et al., 2017). Currently, known sources of VOCs in the MBL cannot explain the observed concentration levels of OVOCs in airborne and seaborne observational experiments (Sinreich et al., 2010), suggesting the presence of unknown sources of OVOCs in the MBL (Singh et al., 2001; Mungall et al., 2017). There are two conjectures regarding these sources: first, these OVOCs may originate from the oxidation of non-methane hydrocarbons (NMHCs) in the atmospheric boundary layer. Due to their longer chemical lifetimes compared to NMHC precursors, they exhibit higher concentrations in the boundary layer atmosphere. Second, OVOCs may also originate from biological and chemical processes occurring in the SML, such as multiphase oxidation reactions between substances in the SML and atmospheric oxidants, or photodegradation processes of substances in the SML (Singh et al., 2001). These processes are likely driven by biological activities in the SML, although this biological driving relationship has not been fully established. This is mainly because the chemical processes occurring in the SML are currently understood primarily from results obtained by laboratory simulation experiments or theoretical model simulations (Carpenter and Nightingale, 2015). For instance, simulation study results suggested that photochemical processes occurring in the SML contribute to the formation of VOCs in the MBL at levels comparable to directly emitted VOCs from marine biological activities (Bruggemann et al., 2018). However, it remains uncertain whether these research findings can be representative of the mentioned chemical processes in the real world. On temporal and spatial scales, more extensive sampling and characterization of the SML are needed to clarify the main biogeochemical processes and influencing factors in this interface environment, especially the production, consumption mechanisms, and chemical compositions of surface-active substances. Only by deepening our understanding of this critical interface environment may we ultimately elucidate its effects on the climate.
In summary, current understanding suggests that marine VOCs primarily originate from three main processes: (i) direct emissions from marine biological activities such as DMS, isoprene, and monoterpenes released by the algae metabolism (Shaw et al., 2010; Novak et al., 2022; Halsey and Giovannoni, 2023), (ii) photochemical processes releasing OM from the SML of seawater (Carpenter and Nightingale, 2015; Novak and Bertram, 2020; Zhu and Kieber, 2018), and (iii) emissions of atmospheric trace gases from the interface oxidation processes at the ocean surface (Zhou et al., 2014; Schneider et al., 2019; Wang et al., 2023, 2022b). Compared to (i) and (ii), research on (iii) is relatively limited, and this aspect will be further elaborated in the following sections.
6.1 Photosensitized processes at the SML as a source of VOCs and SOA in the MBL
The photochemical processes occurring in the SML are one of the most significant drivers of biogeochemical processes, including the generation, degradation, and transformation of DOM. In recent years, many researchers have conducted extensive and in-depth research focusing on the following scientific question: how do the photochemical processes transform DOM into VOCs, and how does this impact the formation of SOA in the MBL?
The organic compounds that exhibit photochemical activity in the SML are referred to as chromophoric dissolved organic matter (CDOM). Upon absorption of light with sufficient energy, a given CDOM molecule gets electronically excited (electrons undergo excitation from their ground state to higher-energy unoccupied or singly occupied molecular orbitals) and goes to an excited singlet state (1CDOM*), which then reacts immediately or quickly relaxes to the lower vibrational levels. The remaining excess energy is subsequently released via mechanisms including photochemical reactions and nonreactive processes such as fluorescence, internal conversion, and intersystem crossing (ISC) where the singlet state decays to the lower-energy excited triplet state (3CDOM*). Both 1CDOM* and 3CDOM* can induce formation of hydroxyl radical (OH), singlet oxygen (1O2), superoxide (), H2O2, aqueous electron (), and organic radicals through various mechanisms (Sharpless and Blough, 2014; Mcneill and Canonica, 2016). Photosensitized reaction represents a special subset of photochemical reactions. In this process, CDOM acts as a sensitizer, and its absorption of light initiates chemical reactions involving a second species, resulting in reforming CDOM and/or generating excited or ionized states of the second species and other species. This represents a key characteristic of photosensitized processes: they enable photoreactions at wavelengths where the “target” molecule itself does not absorb light (George et al., 2015; Gomez Alvarez et al., 2012). The SML, as the top layer of the ocean surface, is directly exposed to the most intense solar radiation, experiencing intensified SA under high-intensity UV radiation, although there is still ∼10 % of the surface UVA radiation (at 360 nm) presenting at a depth of 50–70 m in most oligotrophic waters (Lee et al., 2013; Smyth, 2011). This SA, also known as surfactant activity, is positively correlated with CDOM concentration but negatively correlated with salinity (Rickard et al., 2022; Sabbaghzadeh et al., 2017; Peneziæ et al., 2023). The photochemical processes occurring in the SML not only serve as significant sources of VOCs in the MBL but also act as important transformation sites for key atmospheric trace gases such as O3, NO2, and SO2.
Nonanoic acid (NA) is the representative compound of lipid and saturated fatty acids, which are the most important biogenic DOM in the SML. When NA is present in the air or dissolved in water, its main absorption band peaks around 212 nm, while the absorption band around 272 nm is extremely weak, resulting in minimal light absorption. However, the intensity of the absorption band at 272 nm increases and extends towards 340 nm as the concentration of NA enriched in the SML rises. When reaching the monolayer concentration (∼0.6 mM), the light absorption extending to around 290–350 nm can induce electronic excitation to the excited triplet state of NA (3NA*), which can subsequently either undergo cleavage to form OH radicals and C9 acyl radicals or capture hydrogen ions from other NA molecules to generate C9 alcohol radicals and C9 carboxylic acid radicals (Rossignol et al., 2016). Figure 3 shows the NA chemistry at the air–water interface and in the bulk water.
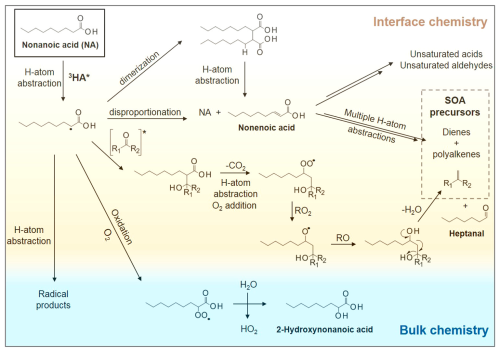
Figure 3Reaction pathways of nonanoic acid at the air–water interface and in the bulk water. Reproduced with permission from Bernard et al. (2016).
These processes initiate subsequent radical chemistry, ultimately leading to the formation of a variety of VOCs containing multiple functional groups (Rossignol et al., 2016). Although a recent study suggested an impurity contribution to UV absorption of saturated fatty acids (Saito et al., 2023), the results nevertheless support the idea that photosensitized chemistry at the ocean surface can be a source of VOCs (Schneider et al., 2024).
Currently, few proxy compounds representing CDOM species such as 4-carboxy benzophenone (4-CB) (Gomez Alvarez et al., 2012; De Laurentiis et al., 2013), imidazole-2-carboxaldehyde (IC) (Felber et al., 2020; Martins-Costa et al., 2022; Tsui et al., 2017), and humic acid (HA) are widely used in exploring the impact of photochemical processes occurring in the sea SML on the marine atmosphere (Tinel et al., 2016; Fu et al., 2015; Ciuraru et al., 2015b). Experimental findings indicated that 4-CB and IC are attracted to the liquid surface by the fatty acids or fatty alcohols (e.g., nonanoic acid and 1-octanol) that are enriched at the sea surface (Fu et al., 2015). Moreover, saccharides and polysaccharides are also attracted to the sea surface by lipids (Burrows et al., 2016). This “co-adsorption” phenomenon suggests the presence of highly active photosensitized chemical processes at the SML. The excited triplet states of the photosensitizers (e.g., 4-CB, IC) can initiate radical chemical reactions by abstracting hydrogen (H) atoms from other organic substances present at the SML, such as NA and octanol (Fu et al., 2015). After losing an H atom the NA radical can undergo reactions such as addition with oxygen molecules or radical–radical reactions, ultimately leading to the formation of VOCs with different volatilities (Tinel et al., 2016). This reaction pathway occurring at the sea surface is similar to and competitive with the H-abstraction mechanism initiated by OH radicals. Laboratory observations have shown that the photosensitized processes initiated by CDOM* can convert DOC present in the SML into VOCs containing double bonds and carbonyl groups, such as C2–C4 olefins (Riemer et al., 2000) and isoprene (Bruggemann et al., 2018). Furthermore, the oxidation of triplet-state phenol by halide anions in the SML is considered a source of marine atmospheric halogen radicals (Jammoul et al., 2009).
Humic substances (HSs) enriched at the SML can also induce similar photosensitized processes (Ciuraru et al., 2015b). The photodegradation processes of triglycerides released by phytoplankton and unsaturated fatty acids such as oleic acid and linoleic acid are considered in situ sources of HS in the ocean (Kieber et al., 1997). The photo-generation of humic-like compounds has also been observed in lake water (Carena et al., 2023). The excited state of humic-like compounds plays a crucial role in the oxidative degradation of DOM and the generation of VOCs such as isoprene (Ciuraru et al., 2015b, a), as well as small molecular carbonyl compounds like formaldehyde, acetaldehyde, and α-keto acids (Mopper et al., 1991; Kieber et al., 1990; Kieber and Mopper, 1987). Additionally, processes including the formation of low-molecular-weight carbonyl compounds (acetaldehyde, glyoxal, and methylglyoxal; Zhou and Mopper, 1997; Zhu and Kieber, 2019) are significant. Trueblood et al. (2019) compared the photochemical reactions between NA liquid films and 4-CB, humic acid, and DOM extracted from algal bloom seawater samples as photosensitizers (Trueblood et al., 2019). Although the photosensitization effect of DOM from seawater was found to be relatively low, this study provided evidence that photosensitized reactions can indeed occur in real environments (Trueblood et al., 2019).
It has been shown that pyruvic acid (PA) as a representative compound of α-dicarbonyl compounds can initiate photosensitized chemistry at the sea surface (Gomez Alvarez et al., 2012; Anglada et al., 2020a). Upon sunlight irradiation, the ketone form of PA is first excited to the singlet state and then by intersystem crossing goes to the excited triplet state (3PA*). The excited triplet state of PA can react with another PA molecule in its ground electronic state either by the abstraction of the acidic hydrogen atom via proton coupled electron transfer (Guzmán et al., 2006) or a concerted hydrogen atom abstraction (Griffith et al., 2013), which can further initiate a chain of reactions that induce decarboxylation and the formation of oligomers (Guzmán et al., 2006; Griffith et al., 2013; Eugene and Guzman, 2019; Reed Harris et al., 2017; Kappes et al., 2021). The formed oligomers at the air–water interface can further affect the VOC fluxes between the ocean and the atmosphere.
Pyruvic acid absorbs the UV fraction of sunlight with its transition band (λmax=318 nm) at the surface water (Gordon et al., 2019). More interestingly, the absorption spectrum of PA is red-shifted by 13 nm when going from dilute aqueous solution (λmax=318 nm) to a solution with ionic strength (NaCl), (I)=2.7 M (λmax=331 nm) (Mekic et al., 2019).
Considering that the pH of seawater is around 8, PA will be present as pyruvate, which is the conjugate base of pyruvic acid, arising from deprotonation of the carboxy group. Analysis by 1H NMR photolysis experiments of PA has shown that the presence of Ca2+ and Na+ in the seawater can further deprotonate PA (Luo et al., 2020).
It has been shown that charged species at the air–water interface, such as deprotonated PA, can enhance the intensity of vibrational sum frequency in the coordinated OH stretching region (∼3000–3400 cm−1). The examination of the OH stretching band provides a means to qualitatively evaluate the presence of charged species without needing to know the actual percentage of deprotonated species (Gordon et al., 2019).
Multiphase chemistry of PA indicated that the air–water interface plays a significant role in promoting chemistry not possible in either the gas or bulk aqueous phase (Kappes et al., 2021). Interestingly, PA acting as a photosensitizer may initiate cross-reactions with other organics such as glyoxal (Mekic et al., 2019) and glyoxylic acid (Xia et al., 2018), leading to formation of highly oxygenated multifunctional compounds which remain in the water or partition to the gas phase according to their physical and chemical properties (molecular mass, boiling point, etc.).
PAHs are ubiquitous compounds at the ocean surface. Although many efforts have been made to reduce the emission of PAHs, their concentrations in the aquatic environment remain high (Ravindra et al., 2008; Keyte et al., 2013). The primary emitted PAHs are deposited into the surface waters via atmospheric deposition (Ma et al., 2013). It has been shown that PAHs play an important role in the photochemical processes of the SML. Fluorene (FL), for example, can act as a photosensitizer at the sea surface while irradiated by sunlight and initiate photochemical formation of toxic compounds and a wide variety of functionalized and unsaturated organic compounds in both the aqueous phase and gas phase (Mekic et al., 2020b). The photosensitized reaction between triplet states of PAHs (pyrene, fluoranthene, and phenanthrene) and dimethyl sulfoxide (DMSO) in the sea surface layer has recently been identified as a source of organic sulfur compounds such as ethylsulfonylmethane, ethyl methanesulfonate, methanesulfonic acid, methanesulfinic acid, hydroxymethanesulfonic acid, and 2-hydroxyethanesulfonic acid in the marine atmosphere (Mekic et al., 2020c, b).
Although significant efforts have been made to explore the photochemical processes occurring in the SML, the research framework mentioned above remains relatively simplistic, primarily focusing on the photochemical characteristics of various organic surfactants, with many environmental factors impacting the reactions not thoroughly investigated. Although studies have shown a positive correlation between light intensity and VOC production rates (Alpert et al., 2017), the influence of other factors on the overall rate of photochemical reactions remains to be investigated. In order to better simulate the marine environment, researchers have attempted to increase the complexity of the research system by enhancing the complexity of photosensitizers (Trueblood et al., 2019) or introducing inorganic salt components to explore the photochemical processes in complex systems (Mekic et al., 2018a). It has been shown that the photochemical processes occurring in the SML of marine and freshwater bodies differ (Stirchak et al., 2021), and the chemical processes of organic and inorganic substances in the SML are strongly coupled and complex. For example, the ionic strength effect can quench 1[DOM] and increase the generation of steady-state 3[DOM] and 1O2 (Abdel-Shafi et al., 2001; Glover and Rosario-Ortiz, 2013), and the increase in the concentration of steady-state 3[DOM]* may be due to the ionic strength effect suppressing the consumption of 3[DOM]* through the electron transfer reaction pathway (Parker et al., 2013). Decreasing the 3[DOM]* indirectly affects the photodegradation rate of other organic substances (Grebel et al., 2012). However, this effect is selective for different types of 3[DOM]*, such as halogens affecting the photodegradation kinetics of anthracene, while having little effect on phenanthrene (Grossman et al., 2019). Additionally, the ionic strength effect has been reported to influence the photochemical generation of volatile organic sulfur compounds (Fig. 4) (Mekic et al., 2020c).
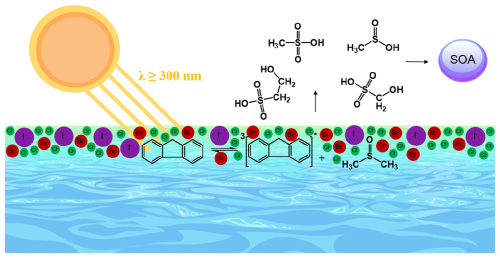
Figure 4Simplified illustration of the ionic strength effect on a photosensitized reaction between the excited triplet state of fluorene and DMSO at the air–water interface. Reprinted with permission from Mekic et al. (2020c). Copyright (2020) American Chemical Society.
Figure 4 illustrates the photosensitized reaction initiated by sunlight-activated fluorene and dimethylsulfoxide (DMSO) in the presence of halide ions Cl−, Br−, and I−. The prompt formation of gas-phase methanesulfonic acid (CH3SO3H), methanesulfinic acid (CH3SO2H), hydroxymethanesulfonic acid (CH4O4S), and 2-hydroxyethenesulfonicacid (C2H5O4SH) was observed. These compounds are typical precursors of aerosol particles, and they are commonly detected in ambient particles (Hopkins et al., 2008; Gaston et al., 2010).
Conversely, studies have reported that DOM can promote the photodegradation of nitrates to produce reactive oxygen species (Wang et al., 2020b). Therefore, investigating the behavior of photochemical processes in different salt gradients is crucial for extrapolating laboratory data to real environments.
While significant progress has been made in related studies, these research systems still diverge considerably from real SML environments. This is manifested not only in the much higher concentrations of simulated samples in the laboratory compared to real environments (Rossignol et al., 2016) but also in the significantly lower photochemical activity of real samples compared to experimental ones (Trueblood et al., 2019). For instance, recent field measurements do not confirm a photochemical production of isoprene at the SML (Kilgour et al., 2024; Kim et al., 2017) as has been shown in the laboratory measurements (Ciuraru et al., 2015a). Therefore, many efforts have to be taken to demonstrate that the chemical processes elucidated in the laboratory occur in real environments. Additionally, the complexity of the environment, such as the presence of inorganic salts and metal ions (Li et al., 2024a), may greatly influence the progress of chemical reactions, either promoting or quenching them, to the extent that the chemical processes elucidated in the laboratory may not occur in real environments, thus lacking practical significance. In order to investigate the chemical processes occurring in real SML environments, it is imperative to continuously make the research system more complex to better approximate the real environment.
6.2 Multiphase chemistry of atmospheric oxidants at the SML
In addition to being exposed to solar radiation, the SML is in direct contact with the atmosphere; this thus drives a series of complex multiphase chemical processes at the ocean–atmosphere interface (i.e., the SML) where atmospheric oxidants are deposited. Research estimates that approximately one-third of global O3 deposition occurs at the ocean surface, accounting for 600–1000 Tg O3 yr−1 (Martino et al., 2012). Another important and highly water-soluble acidic gas is SO2, which exhibits significantly higher deposition rates at the marine surface (pH≈8). Observational studies indicate that approximately 27 % of atmospheric SO2 is absorbed by the ocean (Faloona et al., 2009). Furthermore, two-layer model estimates suggest that the annual flux of SO2 deposited into the ocean is 150 Tg yr−1 (Liss and Slater, 1974), which is comparable to the global anthropogenic emissions of SO2 (95.8–119.8 Tg yr−1, Zhong et al., 2020). This highlights the critical role of the ocean in regulating the atmospheric oxidant concentration. In addition to physical factors such as wind speed, surface turbulence, and molecular diffusion, multiphase photochemical reactions at the SML play a significant role in controlling the deposition rates of O3 and SO2 (Chang et al., 2004; Liss and Slater, 1974). These processes are also major sources of VOCs and other active species in the marine atmosphere. For example, The multiphase chemical reaction between ozone (O3(g)) and iodide ions () in the SML is a primary source of gaseous iodine in the atmosphere (Reaction R1) (Schneider et al., 2023). This reaction not only accelerates the dry deposition rate of atmospheric O3 directly (Garland et al., 1980; Chang et al., 2004), but also indirectly drives atmospheric iodine chemistry, leading to new particle formation (He et al., 2023). These processes have significant implications for the atmospheric environment.
In addition to its reaction with , recent studies have demonstrated that the multiphase chemical reaction between O3 and OM (Reaction R2) in the SML exhibits reactivity comparable to Reaction R1 (Kilgour et al., 2024). Current research primarily focuses on the universality of this reaction and its VOC products. For example, Zhou et al. (2014) investigated multiphase chemical reactions between O3 and oleic acid films, along the western coast of Canada, and in the SML of the northeast Pacific, observing the formation of carbonyl-containing VOCs. Schneider et al. (2019) explored O3 multiphase chemistry with model SML samples (obtained by culturing Thalassiosira pseudonana under sterile conditions) and detected the generation of C7–C9 carbonyl-containing VOCs. Recently, our group employed liquid chromatography and mass spectrometry to identify the molecular structures of primary VOC products from O3 multiphase chemical reactions with 10 SML samples collected in the South China Sea (e.g., acetaldehyde, acetone, propionaldehyde, and C6–C9-saturated aldehydes; Wang et al., 2023), providing a robust scientific foundation for assessing the atmospheric environmental impacts of this reaction. Figure 5 shows the formation profiles of four compounds, 59, 69, 83, and 101, observed in real time during the reaction of O3 (100 ppb) with 10 SML samples collected from coastal areas and open sea in the South China Sea.
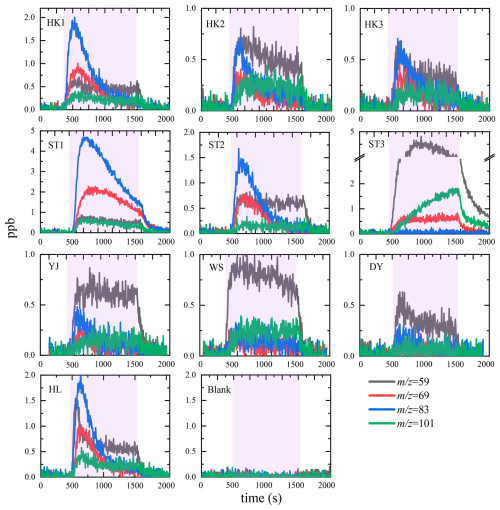
Figure 5Formation profiles of 59, 69, 83, and 101 as a function of relative time measured by PTR-ToF-MS with H3O+ as the reagent ion. The sensitivities used for converting the signals (ncps) into concentration (ppb) are acetone-based. To reduce the noise, the data have been treated with a moving average smoothing with a period of 5 s. Pink background represents the period of ozone (100 ppb) exposure on SML samples. Reprinted with permission from Wang et al. (2023). Copyright (2023) American Chemical Society.
This study aligns well with two following studies; one examined O3 multiphase chemistry in the SML from coastal waters near California, observing the formation of C5–C11 aldehyde VOCs (Kilgour et al., 2024), and another investigated O3 reactions in the Arctic Ocean SML, detecting acetaldehyde, acetone/propionaldehyde, and other aldehyde VOCs (Schneider et al., 2024). While there are minor differences among the observed VOC products, they collectively indicate that the multiphase chemical reaction between atmospheric O3 and OM in the SML is a significant driver of O3 dry deposition. Furthermore, this reaction represents an important source of carbonyl-containing VOCs in the marine atmosphere. It is worth emphasizing that our group and Schneider et al. (2024) independently observed similar nitrogen-containing VOCs (e.g., amines and nitriles) in studies of the SML from the South China Sea and the Arctic Ocean, respectively (Schneider et al., 2024; Wang et al., 2023, 2022b). These findings suggest that this reaction may also serve as a source of reactive nitrogen in the marine atmosphere. Taking one of the primary reaction products, nonanal (C9-saturated aldehyde), as an example, its reaction rate with atmospheric hydroxyl radicals (OH) is approximately an order of magnitude faster than that of DMS. Specifically, the rate constants for DMS+OH and nonanal+OH are and , respectively (Atkinson et al., 2004). Recent studies have further revealed that saturated aldehyde VOCs play a significant role in the regeneration of OH radicals (Yang et al., 2024). These findings suggest that O3 multiphase chemical reactions in the SML may significantly alter the oxidation capacity of marine atmospheres and subsequently influence aerosol formation and the evolution of atmospheric composition. However, while current research has preliminarily identified the universality of this reaction and characterized its primary VOC species and molecular structures, uncertainties remain regarding the kinetic features of VOC emissions. Consequently, there is significant uncertainty in estimating the emission fluxes of VOCs from this reaction at present.
Based on three independent studies, the annual flux of VOCs emitted into the atmosphere from the multiphase chemical reaction between O3 and OM in the SML has been estimated as follows: 45–450 Tg yr−1 (Schneider et al., 2024), 17.5–87.3 Tg yr−1 (Novak and Bertram, 2020), and 10.7–167 Tg yr−1 (Kilgour et al., 2024). While these estimates exhibit significant uncertainty, it is notable that the lower bounds of all three datasets are comparable to the annual emission flux of DMS (20.3 Tg yr−1 (Hulswar et al., 2022). This further underscores the potential for substantial impacts of this reaction on marine atmospheric chemistry. In summary, to elucidate the atmospheric chemical fate and environmental–climatic effects of these reactive VOCs, investigating the kinetic characteristics of VOC generation from this multiphase chemical reaction under different environmental conditions is essential. Such research will help better estimate the yields and production rates of VOC products, making this one of the key focuses for future study.
In addition to O3, SO2 can also undergo multiphase chemical reactions with active substances in the SML, such as fatty acids (Passananti et al., 2016; Shang et al., 2016). In marine atmospheres, SO2 primarily originates from terrestrial transport, shipping emissions, and in situ biological releases, with concentrations typically ranging from tens of parts per trillion to a few parts per billion (Nguyen et al., 1983; Kim et al., 2001; Li et al., 2024b). Among these sources, DMS and methanethiol (MeSH), released by marine phytoplankton metabolism, are the primary natural contributors of SO2 in the marine atmosphere (Novak et al., 2022).
Over the past decade, research on SO2 has predominantly focused on the formation processes and mechanisms of sulfate aerosols (Liu and Abbatt, 2021; Liu et al., 2021b). However, studies on the multiphase chemical reactions of SO2 at the ocean surface and their associated environmental effects have only recently begun to receive attention. Unlike O3, SO2 exhibits photochemical activity, adding another layer of complexity to its atmospheric chemistry.
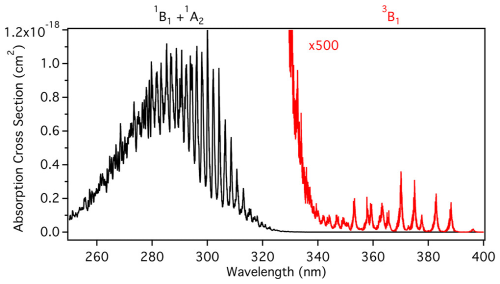
Figure 6Spectrum of SO2 showing the mixed 1B1 and 1A2 state from 250–340 nm and the forbidden 3B1 state from 240–400 nm. Reprinted with permission from Kroll et al. (2018b). Copyright (2018) American Chemical Society.
As shown in Fig. 6, SO2 absorbs radiation in the range of , causing the molecule to enter an excited singlet state (1B1+1A2) (Reaction R3). Subsequently, through intersystem crossing or collisional relaxation, it rapidly transforms into the excited triplet state (3B1). Additionally, absorption in the range of induces a forbidden spin transition, directly exciting SO2 to the triplet state (3B1) (Reaction R4). The triplet-state SO2 (3B1) has a relatively long lifetime (; Collier et al., 1970) and exhibits high reactivity, making it an active participant in the photochemical reactions of SO2 (Sidebottom et al., 1972).
SO2 in its triplet state (3B1) has been demonstrated to react with various substances, including alkanes, alkenes, and carboxylic acids (Anglada et al., 2024; Kroll et al., 2018b). Furthermore, recent theoretical calculations and experimental studies have shown that SO2 (3B1) reacts rapidly with water molecules at water surfaces to generate OH and HOSO radicals (Reaction R5) (Martins-Costa et al., 2018; Kroll et al., 2018a). These radicals can initiate further radical chemistry, leading to the oxidation of OM in the liquid phase and subsequent VOC formation. However, only a limited number of studies have explored the multiphase photochemical reactions of SO2 at the air–sea interface, highlighting the need for further research in this area.
Passananti et al. (2016) and Shang et al. (2016) investigated the multiphase photochemical reactions of SO2 on unsaturated fatty acids and long-chain olefins, which are typical active organic compounds in the SML. Their studies detected the formation of organosulfates in the liquid phase. Liang et al. (2024) explored the multiphase photochemical reactions of SO2 on biomass burning organic aerosol (BBOA), finding that the reaction between excited-state 3BBOA* and SO2 is a major source of sulfate aerosols, rather than the reaction involving SO2 in its triplet state (Reaction R5). However, these studies did not further investigate the contribution of SO2 multiphase photochemical reactions to atmospheric VOCs.
Recently, our group studied the multiphase photochemical reactions of SO2 on PAHs and PAH/dimethyl sulfoxide (DMSO) films, detecting the formation of sulfur-containing VOCs (Jiang et al., 2021). Subsequently, Deng et al. (2022) examined the multiphase photochemical reactions of SO2 on urban building surfaces and found that photolytic conversion significantly enhances VOCs production. Notably, these VOC products are like those generated by O3 reactions under simulated sunlight irradiation. This critical finding not only suggests that O3 and SO2 may share common precursor compounds in VOC formation but also implies similarities in their reaction mechanisms. For example, in the dark, both O3 and SO2 exhibit competitive effects on reactive functional groups such as the C = C bonds in unsaturated fatty acids (Passananti et al., 2016; Shang et al., 2016; Criegee, 1975). Under sunlight irradiation, SO2 in its triplet state (3B1) and its derived radicals (Reaction R5) could compete with O3 for the same reactive functional groups. Therefore, it is crucial to study and analyze O3 and SO2 as a unified system to fully understand their roles in atmospheric chemistry and VOC production.
Although the concentration of O3 in marine atmospheres is typically 1 order of magnitude higher than that of SO2 (Li et al., 2024b), and SO2 may be oxidized to sulfate by O3 in the aerosol phase (Yu et al., 2023), studies have shown that the chemical uptake reactivity of SO2 at environmental interfaces (10−6) is generally 1 order of magnitude higher than that of O3 (10−7) (Shang et al., 2016; Wang et al., 2022a; Deng et al., 2022). Additionally, Passananti et al. (2016) observed chemical uptake of SO2 and the formation of organosulfates in their studies of multiphase photochemical reactions on unsaturated fatty acids and long-chain olefins, even in the presence of O3. Similarly, Ye et al. (2018) demonstrated that when SO2 coexists with O3, it can react with Criegee intermediates derived from ozonolysis to form organosulfates and promote the generation of SOA. These findings suggest that the multiphase photochemical reactions of O3 and SO2 in the SML are not simply competitive but may involve complex synergistic or interactive mechanisms, thereby influencing the species, yields, and production rates of VOCs. Therefore, investigating the multiphase photochemical reactions of O3 and SO2 in the presence of one another, particularly focusing on the kinetic characteristics and key reaction mechanisms of VOC formation, holds significant practical importance for quantitatively describing the multiphase photochemical reactions occurring at marine–atmospheric interfaces and their associated environmental impacts.
Phytoplankton communities in seawater have long been considered the primary source of reactive surfactants, such as fatty acids. Atmospheric deposition, in contrast, is typically viewed as a minor contributor, providing only supplementary nutrients. However, recent research reveals that wildfire smoke can directly deposit substantial quantities of microbes into the SML. This microbial input can be comparable to the estimated supply from seawater and may significantly diversify the SML's microbial community (Yue et al., 2025). Critically, biomass burning also transports biologically important trace metals particularly soluble iron (SFe), a well-established growth-limiting nutrient that can alter the growth rate and composition of the phytoplankton community (Bergas-Masso et al., 2025; Guieu et al., 2005; Mahowald et al., 2018). Model estimates indicate that rising SFe deposition, driven by increasing socioeconomic activity, could boost phytoplankton productivity in the iron-limited North Atlantic by up to 20 % annually (Bergas-Masso et al., 2025). Consequently, key questions arise: how does aerosol-induced modification of SML phytoplankton communities alter the production and abundance of reactive surfactants? Furthermore, how might these changes affect multiphase chemical reactions involving surfactants and atmospheric oxidants, with implications for atmospheric chemistry?
This review demonstrates that ocean–atmosphere physicochemical interactions are an important scientific issue that remain to be further evaluated to better understand their influence on air quality and climate.
Recent research has revealed that multiphase and heterogeneous chemistry occurring at the ocean–atmosphere interface leads to the formation of numerous organic compounds, which as a function of their physical and chemical properties remain in the ocean waters or partition to the atmosphere and ultimately participate in the formation of SOA. The chemical composition of the ocean SML plays a yet unexplored but important role with respect to the multiphase and heterogeneous chemical processes. The SML consists of a plethora of organic and inorganic compounds, with concentrations reaching 1 or more orders of magnitude higher relative to the water underneath. Surfactants which mostly emerge from phytoplankton blooms are enriched at the ocean surface by physical processes such as diffusion, turbulent mixing, scavenging, and transport by bubbles, influencing the exchange of gases between the ocean and atmosphere. For example, it is still unknown if the ocean is a source or a sink of some important carbonyl compounds (e.g., acetone, acetaldehyde).
The importance of acetone in the atmosphere arises from the fact that sunlight-induced photodissociation of acetone produces peroxyacetyl radicals which can be transformed to peroxyacetylnitrates (PANs), influencing the budget of nitrogen oxides (NOx), which in turn affects the ozone concentration in the atmosphere (Singh and Hanst, 1981; Singh et al., 1994). In the upper troposphere photolysis of acetone leads to the production (30 %–40 % of the total) of OH radicals (Wang et al., 2020a). Acetaldehyde exhibits a fast rate constant with OH (), and therefore it plays an important role in the atmospheric OH budget (Atkinson et al., 1997).
Other important organic compounds enriched at the ocean surface are light-absorbing molecules, which act as photosensitizers and are capable of indirect phototransformation of the substrates of primary concern, leading to the formation of VOCs that are released in the atmosphere.
However, the majority of studies are performed under controlled laboratory conditions by using proxy compounds of photosensitizers. Therefore, it is uncertain to what degree photosensitized reactions occurring at the ocean surface can transform the organic compounds and ultimately lead to VOC formation. It is essential to increase the complexity of the studied samples and use authentic ocean SML to better understand the reaction mechanisms and ultimately the impact of the oceanic chemical processes on the VOC and SOA formation processes. For example, a recent study has shown that the excited triplet states of authentic biomass burning organic aerosols can induce multiphase reactions such as SO2 oxidation and the formation of sulfate (Liang et al., 2024).
The deposition of trace gases such as O3 and SO2 on the ocean surface and consequent chemical reactions can lead to the formation of VOCs in the atmosphere. Both oxidants react by addition to the C=C double bonds of unsaturated organic compounds enriched at the ocean surface. Intriguingly, O3 reacts by addition to the C=C during nighttime (under dark conditions) and under sunlight irradiation as well (Wang et al., 2023, 2022b), while this is not the case for SO2. The formation of charge transfer complexes may occur between SO2 and the alkenes present in the SML. As a result, the absorption spectrum of the formed charge transfer complexes may be altered, extending from 315–390 nm (Kroll et al., 2018b). During daytime under sunlight irradiation the formation of the excited triplet state of SO2 () may occur and consequently initiate photosensitized chemistry at the ocean–atmosphere interface (Kroll et al., 2018a). However, scientific knowledge of the reaction mechanisms of O3 and SO2 and their impact on VOCs is far from being understood. Laboratory and field studies are recommended using authentic ocean SML samples and a combination of trace gases in the dark and under sunlight irradiation supported by molecular dynamic simulation. Only by increasing the complexity of the research studies can we obtain relevant data to properly run the models and predict the impact of ocean–atmosphere interactions on the topical issues of the 21st century such as air quality and climate change.
The suggestions for future studies emerge from our own research plan, commenting on issues that might be important and interesting topics.
No data sets were used in this study.
YW and SG jointly formulated the main concepts presented in this paper, and both contributed to writing and editing it.
The contact author has declared that neither of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This work was supported by the Science Fund for Creative Research Groups (grant no. 42321003), the National Natural Science Foundation of China (grant nos. 42177087 and 42507139), Startup Funding of Guangdong Technion-Israel Insti- 50 tute of Technology (grant no. ST2500008), and the China Postdoctoral Science Foundation (grant no. GZB20250105).
This work was supported by the Science Fund for Creative Research Groups (grant no. 42321003), the National Natural Science Foundation of China (grant nos. 42177087 and 42507139), Startup Funding of Guangdong Technion‐Israel Institute of Technology (grant no. ST2500008), and the China Postdoctoral Science Foundation (grant no. GZB20250105).
This paper was edited by Theodora Nah and reviewed by two anonymous referees.
Abdel-Shafi, A. A., Worrall, D. R., and Wilkinson, F.: Singlet oxygen formation efficiencies following quenching of excited singlet and triplet states of aromatic hydrocarbons by molecular oxygen, J. Photoch. Photobio. A, 142, 133–143, https://doi.org/10.1016/S1010-6030(01)00507-X, 2001.
Allen, G. H. and Pavelsky, T. M.: Global extent of rivers and streams, Science, 361, 585–588, https://doi.org/10.1126/science.aat0636, 2018.
Alpert, P. A., Kilthau, W. P., Bothe, D. W., Radway, J. C., Aller, J. Y., and Knopf, D. A.: The influence of marine microbial activities on aerosol production: A laboratory mesocosm study, J. Geophys. Res.-Atmos., 120, 8841–8860, https://doi.org/10.1002/2015JD023469, 2015.
Alpert, P. A., Ciuraru, R., Rossignol, S., Passananti, M., Tinel, L., Perrier, S., Dupart, Y., Steimer, S. S., Ammann, M., Donaldson, D. J., and George, C.: Fatty Acid Surfactant Photochemistry Results in New Particle Formation, Sci. Rep.-UK, 7, 12693, https://doi.org/10.1038/s41598-017-12601-2, 2017.
Angelaki, M., Carreira Mendes Da Silva, Y., Perrier, S., and George, C.: Quantification and Mechanistic Investigation of the Spontaneous H2O2 Generation at the Interfaces of Salt-Containing Aqueous Droplets, J. Am. Chem. Soc., 146, 8327–8334, https://doi.org/10.1021/jacs.3c14040, 2024.
Anglada, J. M., Martins-Costa, M., Ruiz-López, M. F., and Francisco, J. S.: Spectroscopic signatures of ozone at the air–water interface and photochemistry implications, P. Natl. Acad. Sci. USA, 111, 11618–11623, https://doi.org/10.1073/pnas.1411727111, 2014.
Anglada, J. M., Martins-Costa, M. T. C., Francisco, J. S., and Ruiz-Lopez, M. F.: Photoinduced Oxidation Reactions at the Air-Water Interface, J. Am. Chem. Soc., 142, 16140–16155, https://doi.org/10.1021/jacs.0c06858, 2020a.
Anglada, J. M., Martins-Costa, M. T. C., Francisco, J. S., and Ruiz-López, M. F.: Photoinduced Oxidation Reactions at the Air–Water Interface, J. Am. Chem. Soc., 142, 16140–16155, https://doi.org/10.1021/jacs.0c06858, 2020b.
Anglada, J. M., Martins-Costa, M. T. C., Francisco, J. S., and Ruiz-López, M. F.: Triplet State Radical Chemistry: Significance of the Reaction of 3SO2 with HCOOH and HNO3, J. Am. Chem. Soc., 146, 14297–14306, https://doi.org/10.1021/jacs.4c03938, 2024.
Atkinson, R., Baulch, D. L., Cox, R. A., Hampson, R. F., Kerr, J. A., Rossi, M. J., and Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Supplement VI – IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry, J. Phys. Chem. Ref. Data, 26, 1329–1499, https://doi.org/10.1063/1.556010, 1997.
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., and Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I – gas phase reactions of Ox, HOx, NOx and SOx species, Atmos. Chem. Phys., 4, 1461–1738, https://doi.org/10.5194/acp-4-1461-2004, 2004.
Ault, A. P., Moffet, R. C., Baltrusaitis, J., Collins, D. B., Ruppel, M. J., Cuadra-Rodriguez, L. A., Zhao, D., Guasco, T. L., Ebben, C. J., Geiger, F. M., Bertram, T. H., Prather, K. A., and Grassian, V. H.: Size-dependent changes in sea spray aerosol composition and properties with different seawater conditions, Environ. Sci. Technol., 47, 5603–5612, https://doi.org/10.1021/es400416g, 2013.
Barthelmeß, T. and Engel, A.: How biogenic polymers control surfactant dynamics in the surface microlayer: insights from a coastal Baltic Sea study, Biogeosciences, 19, 4965–4992, https://doi.org/10.5194/bg-19-4965-2022, 2022.
Bates, T. S., Quinn, P. K., Frossard, A. A., Russell, L. M., Hakala, J., Petäjä, T., Kulmala, M., Covert, D. S., Cappa, C. D., Li, S. M., Hayden, K. L., Nuaaman, I., McLaren, R., Massoli, P., Canagaratna, M. R., Onasch, T. B., Sueper, D., Worsnop, D. R., and Keene, W. C.: Measurements of ocean derived aerosol off the coast of California, J. Geophys. Res.-Atmos., 117, n/a–n/a, https://doi.org/10.1029/2012jd017588, 2012.
Bates, T. S., Quinn, P. K., Coffman, D. J., Johnson, J. E., Upchurch, L., Saliba, G., Lewis, S., Graff, J., Russell, L. M., and Behrenfeld, M. J.: Variability in Marine Plankton Ecosystems Are Not Observed in Freshly Emitted Sea Spray Aerosol Over the North Atlantic Ocean, Geophys. Res. Lett., 47, https://doi.org/10.1029/2019gl085938, 2020.
Beattie, J. K., Djerdjev, A. M., and Warr, G. G.: The surface of neat water is basic, Faraday Discuss., 141, 31–39, https://doi.org/10.1039/B805266B, 2009.
Behnke, W., Scheer, V., and Zetzsch, C.: Production of a photolytic precursor of atomic Cl from aerosols and Cl – in the presence of O3, Kluwer Academic Publishers, Dordrecht, The Netherlands, 375–384, https://doi.org/10.1007/978-94-011-0061-8_35, 1995.
Ben-Amotz, D.: Electric buzz in a glass of pure water, Science, 376, 800–801, https://doi.org/10.1126/science.abo3398, 2022.
Bergas-Masso, E., Hamilton, D. S., Myriokefalitakis, S., Rathod, S., Gonçalves Ageitos, M., and Pérez García-Pando, C.: Future climate-driven fires may boost ocean productivity in the iron-limited North Atlantic, Nature Clim. Change, 15, 784–792, https://doi.org/10.1038/s41558-025-02356-4, 2025.
Bernard, F., Ciuraru, R., Boreave, A., and George, C.: Photosensitized Formation of Secondary Organic Aerosols above the Air/Water Interface, Environ. Sci. Technol., 50, 8678–8686, https://doi.org/10.1021/acs.est.6b03520, 2016.
Bock, E. J., Hara, T., Frew, N. M., and McGillis, W. R.: Relationship between air–sea gas transfer and short wind waves, J. Geophys. Res.-Oceans, 104, 25821–25831, https://doi.org/10.1029/1999JC900200, 1999.
Breslow, R.: Hydrophobic effects on simple organic reactions in water, Acc. Chem. Res., 24, 159–164, https://doi.org/10.1021/ar00006a001, 1991.
Brooks, S. D. and Thornton, D. C. O.: Marine Aerosols and Clouds, in: Annual Review of Marine Science, Vol 10, edited by: Carlson, C. A., and Giovannoni, S. J., Annual Review of Marine Science, Annual Reviews, Palo Alto, 289–313, https://doi.org/10.1146/annurev-marine-121916-063148, 2018.
Bruggemann, M., Hayeck, N., and George, C.: Interfacial photochemistry at the ocean surface is a global source of organic vapors and aerosols, Nat. Commun., 9, 2101, https://doi.org/10.1038/s41467-018-04528-7, 2018.
Buch, V., Milet, A., Vácha, R., Jungwirth, P., and Devlin, J. P.: Water surface is acidic, P. Natl. Acad. Sci. USA, 104, 7342–7347, https://doi.org/10.1073/pnas.0611285104, 2007.
Burrows, S. M., Ogunro, O., Frossard, A. A., Russell, L. M., Rasch, P. J., and Elliott, S. M.: A physically based framework for modeling the organic fractionation of sea spray aerosol from bubble film Langmuir equilibria, Atmos. Chem. Phys., 14, 13601–13629, https://doi.org/10.5194/acp-14-13601-2014, 2014.
Burrows, S. M., Gobrogge, E., Fu, L., Link, K., Elliott, S. M., Wang, H., and Walker, R.: OCEANFILMS-2: Representing coadsorption of saccharides in marine films and potential impacts on modeled marine aerosol chemistry, Geophys. Res. Lett., 43, 8306–8313, https://doi.org/10.1002/2016gl069070, 2016.
Carena, L., Wang, Y., Gligorovski, S., Berto, S., Mounier, S., and Vione, D.: Photoinduced production of substances with humic-like fluorescence, upon irradiation of water samples from alpine lakes, Chemosphere, 319, 137972, https://doi.org/10.1016/j.chemosphere.2023.137972, 2023.
Carpenter, L. J. and Nightingale, P. D.: Chemistry and release of gases from the surface ocean, Chem. Rev., 115, 4015–4034, https://doi.org/10.1021/cr5007123, 2015.
Carter-Fenk, K. A., Dommer, A. C., Fiamingo, M. E., Kim, J., Amaro, R. E., and Allen, H. C.: Calcium bridging drives polysaccharide co-adsorption to a proxy sea surface microlayer, Phys. Chem. Chem. Phys., 23, 16401–16416, https://doi.org/10.1039/D1CP01407B, 2021.
Chang, W., Heikes, B. G., and Lee, M.: Ozone deposition to the sea surface: chemical enhancement and wind speed dependence, Atmos. Environ., 38, 1053–1059, https://doi.org/10.1016/j.atmosenv.2003.10.050, 2004.
Charlson, R. J., Lovelock, J. E., Andreae, M. O., and Warren, S. G.: Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate, Nature, 326, 655–661, https://doi.org/10.1038/326655a0, 1987.
Christiansen, S., Salter, M. E., Gorokhova, E., Nguyen, Q. T., and Bilde, M.: Sea Spray Aerosol Formation: Laboratory Results on the Role of Air Entrainment, Water Temperature, and Phytoplankton Biomass, Environ. Sci. Technol., 53, 13107–13116, https://doi.org/10.1021/acs.est.9b04078, 2019.
Ciuraru, R., Fine, L., Pinxteren, M., D'Anna, B., Herrmann, H., and George, C.: Unravelling New Processes at Interfaces: Photochemical Isoprene Production at the Sea Surface, Environ. Sci. Technol., 49, 13199–13205, https://doi.org/10.1021/acs.est.5b02388, 2015a.
Ciuraru, R., Fine, L., van Pinxteren, M., D'Anna, B., Herrmann, H., and George, C.: Photosensitized production of functionalized and unsaturated organic compounds at the air–sea interface, Sci. Rep.-UK, 5, 12741, https://doi.org/10.1038/srep12741, 2015b.
Clifford, D., Bartels-Rausch, T., and Donaldson, D. J.: Suppression of aqueous surface hydrolysis by monolayers of short chain organic amphiphiles, Phys. Chem. Chem. Phys., 9, 1362–1369, https://doi.org/10.1039/B617079J, 2007.
Cochran, R. E., Jayarathne, T., Stone, E. A., and Grassian, V. H.: Selectivity Across the Interface: A Test of Surface Activity in the Composition of Organic-Enriched Aerosols from Bubble Bursting, J. Phys. Chem. Lett., 7, 1692–1696, https://doi.org/10.1021/acs.jpclett.6b00489, 2016a.
Cochran, R. E., Laskina, O., Jayarathne, T., Laskin, A., Laskin, J., Lin, P., Sultana, C., Lee, C., Moore, K. A., Cappa, C. D., Bertram, T. H., Prather, K. A., Grassian, V. H., and Stone, E. A.: Analysis of Organic Anionic Surfactants in Fine and Coarse Fractions of Freshly Emitted Sea Spray Aerosol, Environ. Sci. Technol., 50, 2477–2486, https://doi.org/10.1021/acs.est.5b04053, 2016b.
Cochran, R. E., Laskina, O., Trueblood, J. V., Estillore, A. D., Morris, H. S., Jayarathne, T., Sultana, C. M., Lee, C., Lin, P., Laskin, J., Laskin, A., Dowling, J. A., Qin, Z., Cappa, C. D., Bertram, T. H., Tivanski, A. V., Stone, E. A., Prather, K. A., and Grassian, V. H.: Molecular Diversity of Sea Spray Aerosol Particles: Impact of Ocean Biology on Particle Composition and Hygroscopicity, Chem, 2, 655–667, https://doi.org/10.1016/j.chempr.2017.03.007, 2017.
Collier, S. S., Morikawa, A., Slater, D. H., Calvert, J. G., Reinhardt, G., and Damon, E.: Lifetime and quenching rate constant for the lowest triplet state of sulfur dioxide, J. Am. Chem. Soc., 92, 217–218, https://doi.org/10.1021/ja00704a046, 1970.
Collins, D. B., Zhao, D. F., Ruppel, M. J., Laskina, O., Grandquist, J. R., Modini, R. L., Stokes, M. D., Russell, L. M., Bertram, T. H., Grassian, V. H., Deane, G. B., and Prather, K. A.: Direct aerosol chemical composition measurements to evaluate the physicochemical differences between controlled sea spray aerosol generation schemes, Atmos. Meas. Tech., 7, 3667–3683, https://doi.org/10.5194/amt-7-3667-2014, 2014.
Cosman, L. M. and Bertram, A. K.: Reactive Uptake of N2O5 on Aqueous H2SO4 Solutions Coated with 1-Component and 2-Component Monolayers, J. Phys. Chem. A, 112, 4625–4635, https://doi.org/10.1021/jp8005469, 2008.
Criegee, R.: Mechanism of ozonolysis, Angew. Chem. Int. Edit., 14, 745–752, https://doi.org/10.1002/anie.197507451, 1975 (in english).
Cunliffe, M. and Wurl, O.: Sampling the Sea Surface Microlayer, in: Hydrocarbon and Lipid Microbiology Protocols, edited by: McGenity, T. J., Timmis, K. N., and Nogales, B., Springer Protocols Handbooks, Springer, Berlin, Heidelberg, 255–261, https://doi.org/10.1007/8623_2015_83, 2015.
De Laurentiis, E., Maurino, V., Minero, C., Vione, D., Mailhot, G., and Brigante, M.: Could triplet-sensitised transformation of phenolic compounds represent a source of fulvic-like substances in natural waters?, Chemosphere, 90, 881–884, https://doi.org/10.1016/j.chemosphere.2012.09.031, 2013.
de Leeuw, G., Andreas, E. L., Anguelova, M. D., Fairall, C. W., Lewis, E. R., O'Dowd, C., Schulz, M., and Schwartz, S. E.: Production flux of sea spray aerosol, Rev. Geophys., 49, https://doi.org/10.1029/2010RG000349, 2011.
Deal, A. M., Rapf, R. J., and Vaida, V.: Water–Air Interfaces as Environments to Address the Water Paradox in Prebiotic Chemistry: A Physical Chemistry Perspective, J. Phys. Chem. A, 125, 4929–4942, https://doi.org/10.1021/acs.jpca.1c02864, 2021.
Deane, G. B. and Stokes, M. D.: Scale dependence of bubble creation mechanisms in breaking waves, Nature, 418, 839–844, https://doi.org/10.1038/nature00967, 2002.
DeMott, P. J., Hill, T. C., McCluskey, C. S., Prather, K. A., Collins, D. B., Sullivan, R. C., Ruppel, M. J., Mason, R. H., Irish, V. E., Lee, T., Hwang, C. Y., Rhee, T. S., Snider, J. R., McMeeking, G. R., Dhaniyala, S., Lewis, E. R., Wentzell, J. J., Abbatt, J., Lee, C., Sultana, C. M., Ault, A. P., Axson, J. L., Diaz Martinez, M., Venero, I., Santos-Figueroa, G., Stokes, M. D., Deane, G. B., Mayol-Bracero, O. L., Grassian, V. H., Bertram, T. H., Bertram, A. K., Moffett, B. F., and Franc, G. D.: Sea spray aerosol as a unique source of ice nucleating particles, P. Natl. Acad. Sci. USA, 113, 5797–5803, https://doi.org/10.1073/pnas.1514034112, 2016.
Deng, H., Lakey, P. S. J., Wang, Y., Li, P., Xu, J., Pang, H., Liu, J., Xu, X., Li, X., Wang, X., Zhang, Y., Shiraiwa, M., and Gligorovski, S.: Daytime SO2 chemistry on ubiquitous urban surfaces as a source of organic sulfur compounds in ambient air, Sci. Adv., 8, eabq6830, https://doi.org/10.1126/sciadv.abq6830, 2022.
Dixon, J. L., Beale, R., and Nightingale, P. D.: Production of methanol, acetaldehyde, and acetone in the Atlantic Ocean, Geophys. Res. Lett., 40, 4700–4705, https://doi.org/10.1002/grl.50922, 2013.
Donaldson, D. J.: The Influence of Organic Films at the Air-Aqueous Boundary on Atmospheric Processe, Chem. Rev., 106, 1445–1461, https://doi.org/10.1021/cr040367c, 2006.
Donaldson, D. J. and George, C.: Sea-Surface Chemistry and Its Impact on the Marine Boundary Layer, Environ. Sci. Technol., 46, 10385–10389, https://doi.org/10.1021/es301651m, 2012.
Eugene, A. J. and Guzman, M. I.: Production of Singlet Oxygen (1O2) during the Photochemistry of Aqueous Pyruvic Acid: The Effects of pH and Photon Flux under Steady-State O2(aq) Concentration, Environ. Sci. Technol., 53, 12425–12432, https://doi.org/10.1021/acs.est.9b03742, 2019.
Facchini, M. C., Rinaldi, M., Decesari, S., Carbone, C., Finessi, E., Mircea, M., Fuzzi, S., Ceburnis, D., Flanagan, R., Nilsson, E. D., de Leeuw, G., Martino, M., Woeltjen, J., and O'Dowd, C. D.: Primary submicron marine aerosol dominated by insoluble organic colloids and aggregates, Geophys. Res. Lett., 35, https://doi.org/10.1029/2008gl034210, 2008.
Faloona, I., Conley, S. A., Blomquist, B., Clarke, A. D., Kapustin, V., Howell, S., Lenschow, D. H., and Bandy, A. R.: Sulfur dioxide in the tropical marine boundary layer: dry deposition and heterogeneous oxidation observed during the Pacific Atmospheric Sulfur Experiment, J. Atmos. Chem., 63, 13–32, https://doi.org/10.1007/s10874-010-9155-0, 2009.
Felber, T., Schaefer, T., and Herrmann, H.: Five-Membered Heterocycles as Potential Photosensitizers in the Tropospheric Aqueous Phase: Photophysical Properties of Imidazole-2-carboxaldehyde, 2-Furaldehyde, and 2-Acetylfuran, J. Phys. Chem. A, 124, 10029–10039, https://doi.org/10.1021/acs.jpca.0c07028, 2020.
Fischer, E. V., Jacob, D. J., Millet, D. B., Yantosca, R. M., and Mao, J.: The role of the ocean in the global atmospheric budget of acetone, Geophys. Res. Lett., 39, https://doi.org/10.1029/2011gl050086, 2012.
Foster, K. L., Plastridge, R. A., Bottenheim, J. W., Shepson, P. B., Finlayson-Pitts, B. J., and Spicer, C. W.: The Role of Br2 and BrCl in Surface Ozone Destruction at Polar Sunrise, Science, 291, 471–474, https://doi.org/10.1126/science.291.5503.471, 2001.
Frew, N. M. and Nelson, R. K.: Scaling of marine microlayer film surface pressure-area isotherms using chemical attributes, J. Geophys. Res.-Oceans, 97, 5291–5300, https://doi.org/10.1029/91JC02723, 1992.
Frew, N. M., Goldman, J. C., Dennett, M. R., and Johnson, A. S.: Impact of phytoplankton-generated surfactants on air–sea gas exchange, J. Geophys. Res.-Oceans, 95, 3337–3352, https://doi.org/10.1029/JC095iC03p03337, 1990.
Frka, S., Pogorzelski, S., Kozarac, Z., and Æosoviæ, B.: Physicochemical Signatures of Natural Sea Films from Middle Adriatic Stations, J. Phys. Chem. A, 116, 6552–6559, https://doi.org/10.1021/jp212430a, 2012.
Fu, H., Ciuraru, R., Dupart, Y., Passananti, M., Tinel, L., Rossignol, S., Perrier, S., Donaldson, D. J., Chen, J., and George, C.: Photosensitized Production of Atmospherically Reactive Organic Compounds at the Air/Aqueous Interface, J. Am. Chem. Soc., 137, 8348–8351, https://doi.org/10.1021/jacs.5b04051, 2015.
Fuentes, E., Coe, H., Green, D., de Leeuw, G., and McFiggans, G.: On the impacts of phytoplankton-derived organic matter on the properties of the primary marine aerosol – Part 1: Source fluxes, Atmos. Chem. Phys., 10, 9295–9317, https://doi.org/10.5194/acp-10-9295-2010, 2010a.
Fuentes, E., Coe, H., Green, D., de Leeuw, G., and McFiggans, G.: Laboratory-generated primary marine aerosol via bubble-bursting and atomization, Atmos. Meas. Tech., 3, 141–162, https://doi.org/10.5194/amt-3-141-2010, 2010b.
Gajewski, J. J.: The Claisen Rearrangement. Response to Solvents and Substituents: The Case for Both Hydrophobic and Hydrogen Bond Acceleration in Water and for a Variable Transition State, Acc. Chem. Res., 30, 219–225, https://doi.org/10.1021/ar9600493, 1997.
Gao, Q., Leck, C., Rauschenberg, C., and Matrai, P. A.: On the chemical dynamics of extracellular polysaccharides in the high Arctic surface microlayer, Ocean Sci., 8, 401–418, https://doi.org/10.5194/os-8-401-2012, 2012.
Garland, J. A., Elzerman, A. W., and Penkett, S. A.: The mechanism for dry deposition of ozone to seawater surfaces, J. Geophys. Res.-Oceans, 85, 7488–7492, https://doi.org/10.1029/JC085iC12p07488, 1980.
Gas̆paroviæ, B., Plavšiæ, M., Æosoviæ, B., and Saliot, A.: Organic matter characterization in the sea surface microlayers in the subarctic Norwegian fjords region, Mar. Chem., 105, 1–14, https://doi.org/10.1016/j.marchem.2006.12.010, 2007.
Gas̆sparović, B. and Ćosović, B.: Distribution of surface-active substances in the northern Adriatic Sea, Mar. Chem., 75, 301–313, https://doi.org/10.1016/S0304-4203(01)00044-5, 2001.
Gaston, C. J., Pratt, K. A., Qin, X., and Prather, K. A.: Real-Time Detection and Mixing State of Methanesulfonate in Single Particles at an Inland Urban Location during a Phytoplankton Bloom, Environ. Sci. Technol., 44, 1566–1572, https://doi.org/10.1021/es902069d, 2010.
George, C., Ammann, M., D'Anna, B., Donaldson, D. J., and Nizkorodov, S. A.: Heterogeneous photochemistry in the atmosphere, Chem. Rev., 115, 4218–4258, https://doi.org/10.1021/cr500648z, 2015.
Glover, C. M. and Rosario-Ortiz, F. L.: Impact of Halides on the Photoproduction of Reactive Intermediates from Organic Matter, Environ. Sci. Technol., 47, 13949–13956, https://doi.org/10.1021/es4026886, 2013.
Gomez Alvarez, E., Wortham, H., Strekowski, R., Zetzsch, C., and Gligorovski, S.: Atmospheric Photosensitized Heterogeneous and Multiphase Reactions: From Outdoors to Indoors, Environ. Sci. Technol., 46, 1955–1963, https://doi.org/10.1021/es2019675, 2012.
Gong, C., Yuan, X., Xing, D., Zhang, D., Martins-Costa, M. T. C., Anglada, J. M., Ruiz-López, M. F., Francisco, J. S., and Zhang, X.: Fast Sulfate Formation Initiated by the Spin-Forbidden Excitation of SO2 at the Air–Water Interface, J. Am. Chem. Soc., 144, 22302–22308, https://doi.org/10.1021/jacs.2c10830, 2022.
Gong, K., Ao, J., Li, K., Liu, L., Liu, Y., Xu, G., Wang, T., Cheng, H., Wang, Z., Zhang, X., Wei, H., George, C., Mellouki, A., Herrmann, H., Wang, L., Chen, J., Ji, M., Zhang, L., and Francisco, J. S.: Imaging of pH distribution inside individual microdroplet by stimulated Raman microscopy, P. Natl. Acad. Sci. USA, 120, e2219588120, https://doi.org/10.1073/pnas.2219588120, 2023.
Gordon, B. P., Moore, F. G., Scatena, L. F., and Richmond, G. L.: On the Rise: Experimental and Computational Vibrational Sum Frequency Spectroscopy Studies of Pyruvic Acid and Its Surface-Active Oligomer Species at the Air–Water Interface, J. Phys. Chem. A, 123, 10609–10619, https://doi.org/10.1021/acs.jpca.9b08854, 2019.
Grebel, J. E., Pignatello, J. J., and Mitch, W. A.: Impact of Halide Ions on Natural Organic Matter-Sensitized Photolysis of 17â-Estradiol in Saline Waters, Environ. Sci. Technol., 46, 7128–7134, https://doi.org/10.1021/es3013613, 2012.
Griffith, E. C. and Vaida, V.: In situ observation of peptide bond formation at the water–air interface, P. Natl. Acad. Sci. USA, 109, 15697–15701, https://doi.org/10.1073/pnas.1210029109, 2012.
Griffith, E. C., Carpenter, B. K., Shoemaker, R. K., and Vaida, V.: Photochemistry of aqueous pyruvic acid, P. Natl. Acad. Sci. USA, 110, 11714–11719, https://doi.org/10.1073/pnas.1303206110, 2013.
Grossman, J. N., Kowal, S. F., Stubbs, A. D., Cawley, C. N., and Kahan, T. F.: Anthracene and Pyrene Photooxidation Kinetics in Saltwater Environments, ACS Earth and Space Chemistry, 3, 2695–2703, https://doi.org/10.1021/acsearthspacechem.9b00218, 2019.
Guieu, C., Bonnet, S., Wagener, T., and Loÿe-Pilot, M.-D.: Biomass burning as a source of dissolved iron to the open ocean?, Geophys. Res. Lett., 32, https://doi.org/10.1029/2005GL022962, 2005.
Guo, Y., Li, K., Perrier, S., An, T., Donaldson, D. J., and George, C.: Spontaneous Iodide Activation at the Air–Water Interface of Aqueous Droplets, Environ. Sci. Technol., 57, 15580–15587, https://doi.org/10.1021/acs.est.3c05777, 2023.
Guzmán, M. I., Colussi, A. J., and Hoffmann, M. R.: Photoinduced Oligomerization of Aqueous Pyruvic Acid, J. Phys. Chem. A, 110, 3619–3626, https://doi.org/10.1021/jp056097z, 2006.
Gwendal Loisel, M. M., Shiyang Liu, Wei Song, Bin Jiang, Yiqun Wang, Huifan Deng, Sasho Gligorovski: Ionic strength effect on the formation of organonitrate compounds through photochemical degradation of vanillin in liquid water of aerosols, Atmos. Environ., 246, 118140, https://doi.org/10.1016/j.atmosenv.2020.118140, 2021.
Halsey, K. H. and Giovannoni, S. J.: Biological controls on marine volatile organic compound emissions: A balancing act at the sea–air interface, Earth-Sci. Rev., 240, 104360, https://doi.org/10.1016/j.earscirev.2023.104360, 2023.
Hao, H., Leven, I., and Head-Gordon, T.: Can electric fields drive chemistry for an aqueous microdroplet?, Nat. Commun., 13, 280, https://doi.org/10.1038/s41467-021-27941-x, 2022.
Hasenecz, E. S., Kaluarachchi, C. P., Lee, H. D., Tivanski, A. V., and Stone, E. A.: Saccharide Transfer to Sea Spray Aerosol Enhanced by Surface Activity, Calcium, and Protein Interactions, ACS Earth and Space Chemistry, 3, 2539–2548, https://doi.org/10.1021/acsearthspacechem.9b00197, 2019.
He, X.-C., Simon, M., Iyer, S., Xie, H.-B., Rörup, B., Shen, J., Finkenzeller, H., Stolzenburg, D., Zhang, R., Baccarini, A., Tham, Y. J., Wang, M., Amanatidis, S., Piedehierro, A. A., Amorim, A., Baalbaki, R., Brasseur, Z., Caudillo, L., Chu, B., Dada, L., Duplissy, J., El Haddad, I., Flagan, R. C., Granzin, M., Hansel, A., Heinritzi, M., Hofbauer, V., Jokinen, T., Kemppainen, D., Kong, W., Krechmer, J., Kürten, A., Lamkaddam, H., Lopez, B., Ma, F., Mahfouz, N. G. A., Makhmutov, V., Manninen, H. E., Marie, G., Marten, R., Massabò, D., Mauldin, R. L., Mentler, B., Onnela, A., Petäjä, T., Pfeifer, J., Philippov, M., Ranjithkumar, A., Rissanen, M. P., Schobesberger, S., Scholz, W., Schulze, B., Surdu, M., Thakur, R. C., Tomé, A., Wagner, A. C., Wang, D., Wang, Y., Weber, S. K., Welti, A., Winkler, P. M., Zauner-Wieczorek, M., Baltensperger, U., Curtius, J., Kurtén, T., Worsnop, D. R., Volkamer, R., Lehtipalo, K., Kirkby, J., Donahue, N. M., Sipilä, M., and Kulmala, M.: Iodine oxoacids enhance nucleation of sulfuric acid particles in the atmosphere, Science, 382, 1308–1314, https://doi.org/10.1126/science.adh2526, 2023.
Henrichs, S. M. and Williams, P. M.: Dissolved and particulate amino acids and carbohydrates in the sea surface microlayer, Mar. Chem., 17, 141–163, https://doi.org/10.1016/0304-4203(85)90070-2, 1985.
Hopkins, R. J., Desyaterik, Y., Tivanski, A. V., Zaveri, R. A., Berkowitz, C. M., Tyliszczak, T., Gilles, M. K., and Laskin, A.: Chemical speciation of sulfur in marine cloud droplets and particles: Analysis of individual particles from the marine boundary layer over the California current, J. Geophys. Res.-Atmos., 113, https://doi.org/10.1029/2007JD008954, 2008.
Hu, J. H., Shi, Q., Davidovits, P., Worsnop, D. R., Zahniser, M. S., and Kolb, C. E.: Reactive Uptake of Cl2(g) and Br2(g) by Aqueous Surfaces as a Function of Br− and I− Ion Concentration: The Effect of Chemical Reaction at the Interface, J. Phys. Chem., 99, 8768–8776, https://doi.org/10.1021/j100021a050, 1995.
Hulswar, S., Simó, R., Galí, M., Bell, T. G., Lana, A., Inamdar, S., Halloran, P. R., Manville, G., and Mahajan, A. S.: Third revision of the global surface seawater dimethyl sulfide climatology (DMS-Rev3), Earth Syst. Sci. Data, 14, 2963–2987, https://doi.org/10.5194/essd-14-2963-2022, 2022.
Hunter, K. A. and Liss, P. S.: The input of organic material to the oceans: air–sea interactions and the organic chemical composition of the sea surface, Mar. Chem., 5, 361–379, https://doi.org/10.1016/0304-4203(77)90029-9, 1977.
Jaffé, R., Ding, Y., Niggemann, J., Vähätalo, A. V., Stubbins, A., Spencer, R. G. M., Campbell, J., and Dittmar, T.: Global Charcoal Mobilization from Soils via Dissolution and Riverine Transport to the Oceans, Science, 340, 345–347, https://doi.org/10.1126/science.1231476, 2013.
Jammoul, A., Gligorovski, S., George, C., and D'Anna, B.: Photosensitized heterogeneous chemistry of ozone on organic films, J. Phys. Chem. A, 112, 1268–1276, https://doi.org/10.1021/jp074348t, 2008.
Jammoul, A., Dumas, S., D'Anna, B., and George, C.: Photoinduced oxidation of sea salt halides by aromatic ketones: a source of halogenated radicals, Atmos. Chem. Phys., 9, 4229–4237, https://doi.org/10.5194/acp-9-4229-2009, 2009.
Jayarathne, T., Gamage, D. K., Prather, K. A., and Stone, E. A.: Enrichment of saccharides at the air–water interface: a quantitative comparison of sea surface microlayer and foam, Environ. Chem., 19, 506–516, https://doi.org/10.1071/EN22094, 2022.
Jiang, H., Carena, L., He, Y., Wang, Y., Zhou, W., Yang, L., Luan, T., Li, X., Brigante, M., Vione, D., and Gligorovski, S.: Photosensitized Degradation of DMSO Initiated by PAHs at the Air-Water Interface, as an Alternative Source of Organic Sulfur Compounds to the Atmosphere, J. Geophys. Res.-Atmos., 126, https://doi.org/10.1029/2021jd035346, 2021.
Joux, F., Agogué, H., Obernosterer, I., Dupuy, C., Reinthaler, T., Herndl, G. J., and Lebaron, P.: Microbial community structure in the sea surface microlayer at two contrasting coastal sites in the northwestern Mediterranean Sea, Aquat. Microb. Ecol., 42, 91–104, 2006.
Jung, Y. and Marcus, R. A.: On the theory of organic catalysis on water, J. Am. Chem. Soc., 129, 5492–5502, https://doi.org/10.1021/ja068120f, 2007.
Jungwirth, P. and Tobias, D. J.: Specific ion effects at the air/water interface, Chem. Rev., 106, 1259–1281, https://doi.org/10.1021/cr0403741, 2006.
Kappes, K. J., Deal, A. M., Jespersen, M. F., Blair, S. L., Doussin, J.-F., Cazaunau, M., Pangui, E., Hopper, B. N., Johnson, M. S., and Vaida, V.: Chemistry and Photochemistry of Pyruvic Acid at the Air–Water Interface, J. Phys. Chem. A, 125, 1036–1049, https://doi.org/10.1021/acs.jpca.0c09096, 2021.
Keith Bigg, E., Leck, C., and Tranvik, L.: Particulates of the surface microlayer of open water in the central Arctic Ocean in summer, Mar. Chem., 91, 131–141, https://doi.org/10.1016/j.marchem.2004.06.005, 2004.
Keyte, I. J., Harrison, R. M., and Lammel, G.: Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons – a review, Chem. Soc. Rev., 42, 9333–9391, https://doi.org/10.1039/C3CS60147A, 2013.
Kieber, D. J. and Mopper, K.: Photochemical formation of glyoxylic and pyruvic acids in seawater, Mar. Chem., 21, 135–149, https://doi.org/10.1016/0304-4203(87)90034-X, 1987.
Kieber, R. J., Zhou, X., and Mopper, K.: Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters: Fate of riverine carbon in the sea, Limnol. Oceanogr., 35, 1503–1515, https://doi.org/10.4319/lo.1990.35.7.1503, 1990.
Kieber, R. J., Hydro, L. H., and Seaton, P. J.: Photooxidation of triglycerides and fatty acids in seawater: Implication toward the formation of marine humic substances, Limnol. Oceanogr., 42, 1454–1462, https://doi.org/10.4319/lo.1997.42.6.1454, 1997.
Kilgour, D. B., Novak, G. A., Claflin, M. S., Lerner, B. M., and Bertram, T. H.: Production of oxygenated volatile organic compounds from the ozonolysis of coastal seawater, Atmos. Chem. Phys., 24, 3729–3742, https://doi.org/10.5194/acp-24-3729-2024, 2024.
Kim, B.-G., Han, J.-S., and Park, S.-U.: Transport of SO2 and aerosol over the Yellow sea, Atmos. Environ., 35, 727–737, https://doi.org/10.1016/S1352-2310(00)00344-7, 2001.
Kim, M. J., Novak, G. A., Zoerb, M. C., Yang, M., Blomquist, B. W., Huebert, B. J., Cappa, C. D., and Bertram, T. H.: Air–Sea exchange of biogenic volatile organic compounds and the impact on aerosol particle size distributions, Geophys. Res. Lett., 44, 3887–3896, https://doi.org/10.1002/2017GL072975, 2017.
Klijn, J. E. and Engberts, J.: Organic chemistry – Fast reactions “on water”, Nature, 435, 746–747, https://doi.org/10.1038/435746a, 2005.
Knipping, E. M., Lakin, M. J., Foster, K. L., Jungwirth, P., Tobias, D. J., Gerber, R. B., Dabdub, D., and Finlayson-Pitts, B. J.: Experiments and simulations of ion-enhanced interfacial chemistry on aqueous NaCl aerosols, Science, 288, 301–306, https://doi.org/10.1126/science.288.5464.301, 2000.
Knulst, J. C., Rosenberger, D., Thompson, B., and Paatero, J.: Intensive Sea Surface Microlayer Investigations of Open Leads in the Pack Ice during Arctic Ocean 2001 Expedition, Langmuir, 19, 10194–10199, https://doi.org/10.1021/la035069+, 2003.
Kong, S. and Evanseck, J. D.: Density Functional Theory Study of Aqueous-Phase Rate Acceleration and Endo/Exo Selectivity of the Butadiene and Acrolein Diels-Alder Reaction, J. Am. Chem. Soc., 122, 10418–10427, https://doi.org/10.1021/ja0010249, 2000.
Kong, X., Castarède, D., Thomson, E. S., Boucly, A., Artiglia, L., Ammann, M., Gladich, I., and Pettersson, J. B. C.: A surface-promoted redox reaction occurs spontaneously on solvating inorganic aerosol surfaces, Science, 374, 747–752, https://doi.org/10.1126/science.abc5311, 2021.
Kroll, J. A., Frandsen, B. N., Kjaergaard, H. G., and Vaida, V.: Atmospheric Hydroxyl Radical Source: Reaction of Triplet SO2 and Water, J. Phys. Chem. A, 122, 4465–4469, https://doi.org/10.1021/acs.jpca.8b03524, 2018a.
Kroll, J. A., Frandsen, B. N., Rapf, R. J., Kjaergaard, H. G., and Vaida, V.: Reactivity of Electronically Excited SO2 with Alkanes, J. Phys. Chem. A, 122, 7782–7789, https://doi.org/10.1021/acs.jpca.8b04643, 2018b.
Kusaka, R., Nihonyanagi, S., and Tahara, T.: The photochemical reaction of phenol becomes ultrafast at the air–water interface, Nat. Chem., 13, 306–311, https://doi.org/10.1038/s41557-020-00619-5, 2021.
Laâ, K., Kleber, J., and Friedrichs, G.: Vibrational sum-frequency generation as a probe for composition, chemical reactivity, and film formation dynamics of the sea surface nanolayer, Limnol. Oceanogr.-Meth., 8, 216–228, https://doi.org/10.4319/lom.2010.8.216, 2010.
Laskin, A., Gaspar, D. J., Wang, W., Hunt, S. W., Cowin, J. P., Colson, S. D., and Finlayson-Pitts, B. J.: Reactions at Interfaces As a Source of Sulfate Formation in Sea-Salt Particles, Science, 301, 340–344, https://doi.org/10.1126/science.1085374, 2003.
Laskina, O., Morris, H. S., Grandquist, J. R., Qin, Z., Stone, E. A., Tivanski, A. V., and Grassian, V. H.: Size matters in the water uptake and hygroscopic growth of atmospherically relevant multicomponent aerosol particles, J. Phys. Chem. A, 119, 4489–4497, https://doi.org/10.1021/jp510268p, 2015.
Laß, K. and Friedrichs, G.: Revealing structural properties of the marine nanolayer from vibrational sum frequency generation spectra, J. Geophys. Res.-Oceans, 116, https://doi.org/10.1029/2010JC006609, 2011.
Laß, K., Bange, H. W., and Friedrichs, G.: Seasonal signatures in SFG vibrational spectra of the sea surface nanolayer at Boknis Eck Time Series Station (SW Baltic Sea), Biogeosciences, 10, 5325–5334, https://doi.org/10.5194/bg-10-5325-2013, 2013.
Lawler, M. J., Schill, G. P., Brock, C. A., Froyd, K. D., Williamson, C., Kupc, A., and Murphy, D. M.: Sea Spray Aerosol Over the Remote Oceans Has Low Organic Content, AGU Advances, 5, e2024AV001215, https://doi.org/10.1029/2024AV001215, 2024.
Lee, J. K., Samanta, D., Nam, H. G., and Zare, R. N.: Micrometer-Sized Water Droplets Induce Spontaneous Reduction, J. Am. Chem. Soc., 141, 10585–10589, https://doi.org/10.1021/jacs.9b03227, 2019a.
Lee, J. K., Walker, K. L., Han, H. S., Kang, J., Prinz, F. B., Waymouth, R. M., Nam, H. G., and Zare, R. N.: Spontaneous generation of hydrogen peroxide from aqueous microdroplets, P. Natl. Acad. Sci. USA, 116, 19294–19298, https://doi.org/10.1073/pnas.1911883116, 2019b.
Lee, J. K., Han, H. S., Chaikasetsin, S., Marron, D. P., Waymouth, R. M., Prinz, F. B., and Zare, R. N.: Condensing water vapor to droplets generates hydrogen peroxide, P. Natl. Acad. Sci. USA, 117, 30934–30941, https://doi.org/10.1073/pnas.2020158117, 2020.
Lee, Z., Hu, C., Shang, S., Du, K., Lewis, M., Arnone, R., and Brewin, R.: Penetration of UV-visible solar radiation in the global oceans: Insights from ocean color remote sensing, J. Geophys. Res.-Oceans, 118, 4241–4255, https://doi.org/10.1002/jgrc.20308, 2013.
Lewis, E. R. and Schwartz, S. E.: Methods of Determining Size-Dependent Sea Salt Aerosol Production Fluxes, in: Sea Salt Aerosol Production: Mechanisms, Methods, Measurements and Models, 101–118, https://doi.org/10.1002/9781118666050.ch3, 2004.
Lewis, S. L., Russell, L. M., Saliba, G., Quinn, P. K., Bates, T. S., Carlson, C. A., Baetge, N., Aluwihare, L. I., Boss, E., Frossard, A. A., Bell, T. G., and Behrenfeld, M. J.: Characterization of Sea Surface Microlayer and Marine Aerosol Organic Composition Using STXM-NEXAFS Microscopy and FTIR Spectroscopy, ACS Earth and Space Chemistry, 6, 1899–1913, https://doi.org/10.1021/acsearthspacechem.2c00119, 2022.
Li, K., Guo, Y., Nizkorodov, Sergey, A., Rudich, Y., Angelaki, M., Wang, X., An, T., Perrier, S., and George, C.: Spontaneous dark formation of OH radicals at the interface of aqueous atmospheric droplets, P. Natl. Acad. Sci. USA, 120, e2220228120, https://doi.org/10.1073/pnas.2220228120, 2023.
Li, P., Pang, H., Wang, Y., Deng, H., Liu, J., Loisel, G., Jin, B., Li, X., Vione, D., and Gligorovski, S.: Inorganic Ions Enhance the Number of Product Compounds through Heterogeneous Processing of Gaseous NO2 on an Aqueous Layer of Acetosyringone, Environ. Sci. Technol., 56, 5398–5408, https://doi.org/10.1021/acs.est.1c08283, 2022.
Li, P., Mekic, M., Wang, Y., He, B., Deng, H., Xu, J., Pang, H., Jiang, B., Tang, M., Wang, X., Al-Abadleh, H. A., and Gligorovski, S.: Impact of Nitrate and Iron Ions on Uptake Coefficients and Condensed Phase Products From the Reaction of Gaseous NO2 With HULIS Proxies, J. Geophys. Res.-Atmos., 129, e2023JD039698, https://doi.org/10.1029/2023JD039698, 2024a.
Li, Y., Nie, W., Yan, C., Liu, Y., Xu, Z., Yao, X., Zhou, Y., Chi, X., and Ding, A.: Characterization of Volatile Organic Compounds Over the Eastern Seas of China in Winter, J. Geophys. Res.-Atmos., 129, e2024JD040713, https://doi.org/10.1029/2024JD040713, 2024b.
Liang, Z., Zhou, L., Chang, Y., Qin, Y., and Chan, C. K.: Biomass-burning organic aerosols as a pool of atmospheric reactive triplets to drive multiphase sulfate formation, P. Natl. Acad. Sci. USA, 121, e2416803121, https://doi.org/10.1073/pnas.2416803121, 2024.
Liss, P. S. and Duce, R. A.: The Sea Surface and Global Change, Cambridge University Press, Cambridge, https://doi.org/10.1017/CBO9780511525025, 1997.
Liss, P. S. and Slater, P. G.: Flux of Gases across the Air–Sea Interface, Nature, 247, 181–184, https://doi.org/10.1038/247181a0, 1974.
Liu, S., Liu, C. C., Froyd, K. D., Schill, G. P., Murphy, D. M., Bui, T. P., Dean-Day, J. M., Weinzierl, B., Dollner, M., Diskin, G. S., Chen, G., and Gao, R. S.: Sea spray aerosol concentration modulated by sea surface temperature, P. Natl. Acad. Sci. USA, 118, https://doi.org/10.1073/pnas.2020583118, 2021a.
Liu, T. and Abbatt, J. P. D.: Oxidation of sulfur dioxide by nitrogen dioxide accelerated at the interface of deliquesced aerosol particles, Nat. Chem., 13, 1173–1177, https://doi.org/10.1038/s41557-021-00777-0, 2021.
Liu, T., Chan, A. W. H., and Abbatt, J. P. D.: Multiphase Oxidation of Sulfur Dioxide in Aerosol Particles: Implications for Sulfate Formation in Polluted Environments, Environ. Sci. Technol., 55, 4227–4242, https://doi.org/10.1021/acs.est.0c06496, 2021b.
Liu, Y., Ge, Q., Wang, T., Zhang, R., Li, K., Gong, K., Xie, L., Wang, W., Wang, L., You, W., Ruan, X., Shi, Z., Han, J., Wang, R., Fu, H., Chen, J., Chan, C. K., and Zhang, L.: Strong electric field force at the air/water interface drives fast sulfate production in the atmosphere, Chem, 10, 330–351, https://doi.org/10.1016/j.chempr.2023.09.019, 2024.
Luo, M., Shemesh, D., Sullivan, M. N., Alves, M. R., Song, M., Gerber, R. B., and Grassian, V. H.: Impact of pH and NaCl and CaCl2 Salts on the Speciation and Photochemistry of Pyruvic Acid in the Aqueous Phase, J. Phys. Chem. A, 124, 5071–5080, https://doi.org/10.1021/acs.jpca.0c01016, 2020.
Ma, Y., Xie, Z., Yang, H., Möller, A., Halsall, C., Cai, M., Sturm, R., and Ebinghaus, R.: Deposition of polycyclic aromatic hydrocarbons in the North Pacific and the Arctic, J. Geophys. Res.-Atmos., 118, 5822–5829, https://doi.org/10.1002/jgrd.50473, 2013.
Mahowald, N. M., Hamilton, D. S., Mackey, K. R. M., Moore, J. K., Baker, A. R., Scanza, R. A., and Zhang, Y.: Aerosol trace metal leaching and impacts on marine microorganisms, Nat. Commun., 9, 2614, https://doi.org/10.1038/s41467-018-04970-7, 2018.
Mamatkulov, S. I., Allolio, C., Netz, R. R., and Bonthuis, D. J.: Orientation-Induced Adsorption of Hydrated Protons at the Air–Water Interface, Angew. Chem. Int. Edit., 56, 15846–15851, https://doi.org/10.1002/anie.201707391, 2017.
Mårtensson, E. M., Nilsson, E. D., de Leeuw, G., Cohen, L. H., and Hansson, H.-C.: Laboratory simulations and parameterization of the primary marine aerosol production, J. Geophys. Res.-Atmos., 108, https://doi.org/10.1029/2002JD002263, 2003.
Martino, M., Lézé, B., Baker, A. R., and Liss, P. S.: Chemical controls on ozone deposition to water, Geophys. Res. Lett., 39, https://doi.org/10.1029/2011GL050282, 2012.
Martins-Costa, M. T., Anglada, J. M., Francisco, J. S., and Ruiz-Lopez, M. F.: Reactivity of volatile organic compounds at the surface of a water droplet, J. Am. Chem. Soc., 134, 11821–11827, https://doi.org/10.1021/ja304971e, 2012a.
Martins-Costa, M. T. C., Anglada, J. M., Francisco, J. S., and Ruiz-Lopez, M. F.: Reactivity of Atmospherically Relevant Small Radicals at the Air–Water Interface, Angew. Chem. Int. Edit., 51, 5413–5417, https://doi.org/10.1002/anie.201200656, 2012b.
Martins-Costa, M. T. C., García-Prieto, F. F., and Ruiz-López, M. F.: Reactivity of aldehydes at the air–water interface. Insights from molecular dynamics simulations and ab initio calculations, Organic and Biomolecular Chemistry, 13, 1673–1679, https://doi.org/10.1039/C4OB02029D, 2015.
Martins-Costa, M. T. C., Anglada, J. M., Francisco, J. S., and Ruiz-López, M. F.: Impacts of cloud water droplets on the OH production rate from peroxide photolysis, Phys. Chem. Chem. Phys., 19, 31621–31627, https://doi.org/10.1039/C7CP06813A, 2017.
Martins-Costa, M. T. C., Anglada, J. M., Francisco, J. S., and Ruiz-López, M. F.: Photochemistry of SO2 at the Air–Water Interface: A Source of OH and HOSO Radicals, J. Am. Chem. Soc., 140, 12341–12344, https://doi.org/10.1021/jacs.8b07845, 2018.
Martins-Costa, M. T. C., Anglada, J. M., Francisco, J. S., and Ruiz-López, M. F.: Theoretical Investigation of the Photoexcited NO2 + H2O reaction at the Air–Water Interface and Its Atmospheric Implications, Chem.-Eur. J., 25, 13899–13904, https://doi.org/10.1002/chem.201902769, 2019.
Martins-Costa, M. T. C., Anglada, J. M., Francisco, J. S., and Ruiz-López, M. F.: Photosensitization mechanisms at the air–water interface of aqueous aerosols, Chem. Sci., 13, 2624–2631, https://doi.org/10.1039/D1SC06866K, 2022.
Marty, J. C., Saliot, A., Buat-Ménard, P., Chesselet, R., and Hunter, K. A.: Relationship between the lipid compositions of marine aerosols, the sea surface microlayer, and subsurface water, J. Geophys. Res.-Oceans, 84, 5707–5716, https://doi.org/10.1029/JC084iC09p05707, 1979.
Mayer, K. J., Sauer, J. S., Dinasquet, J., and Prather, K. A.: CAICE Studies: Insights from a Decade of Ocean–Atmosphere Experiments in the Laboratory, Acc Chem. Res., 53, 2510–2520, https://doi.org/10.1021/acs.accounts.0c00504, 2020.
McNeill, K. and Canonica, S.: Triplet state dissolved organic matter in aquatic photochemistry: reaction mechanisms, substrate scope, and photophysical properties, Environmental Science: Processes and Impacts, 18, 1381–1399, https://doi.org/10.1039/C6EM00408C, 2016.
Mekic, M. and Gligorovski, S.: Ionic strength effects on heterogeneous and multiphase chemistry: Clouds versus aerosol particles, Atmos. Environ., 244, https://doi.org/10.1016/j.atmosenv.2020.117911, 2021.
Mekic, M., Brigante, M., Vione, D., and Gligorovski, S.: Exploring the ionic strength effects on the photochemical degradation of pyruvic acid in atmospheric deliquescent aerosol particles, Atmos. Environ., 185, 237–242, https://doi.org/10.1016/j.atmosenv.2018.05.016, 2018a.
Mekic, M., Loisel, G., Zhou, W., Jiang, B., Vione, D., and Gligorovski, S.: Ionic-Strength Effects on the Reactive Uptake of Ozone on Aqueous Pyruvic Acid: Implications for Air–Sea Ozone Deposition, Environ. Sci. Technol., 52, 12306–12315, https://doi.org/10.1021/acs.est.8b03196, 2018b.
Mekic, M., Liu, J., Zhou, W., Loisel, G., Cai, J., He, T., Jiang, B., Yu, Z., Lazarou, Y. G., Li, X., Brigante, M., Vione, D., and Gligorovski, S.: Formation of highly oxygenated multifunctional compounds from cross-reactions of carbonyl compounds in the atmospheric aqueous phase, Atmos. Environ., 219, 117046, https://doi.org/10.1016/j.atmosenv.2019.117046, 2019.
Mekic, M., Wang, Y., Loisel, G., Vione, D., and Gligorovski, S.: Ionic Strength Effect Alters the Heterogeneous Ozone Oxidation of Methoxyphenols in Going from Cloud Droplets to Aerosol Deliquescent Particles, Environ. Sci. Technol., 54, 12898–12907, https://doi.org/10.1021/acs.est.0c03648, 2020a.
Mekic, M., Zeng, J., Jiang, B., Li, X., Lazarou, Y. G., Brigante, M., Herrmann, H., and Gligorovski, S.: Formation of Toxic Unsaturated Multifunctional and Organosulfur Compounds From the Photosensitized Processing of Fluorene and DMSO at the Air–Water Interface, J. Geophys. Res.-Atmos., 125, https://doi.org/10.1029/2019jd031839, 2020b.
Mekic, M., Zeng, J., Zhou, W., Loisel, G., Jin, B., Li, X., Vione, D., and Gligorovski, S.: Ionic Strength Effect on Photochemistry of Fluorene and Dimethylsulfoxide at the Air–Sea Interface: Alternative Formation Pathway of Organic Sulfur Compounds in a Marine Atmosphere, ACS Earth and Space Chemistry, 4, 1029–1038, https://doi.org/10.1021/acsearthspacechem.0c00059, 2020c.
Messager, M. L., Lehner, B., Grill, G., Nedeva, I., and Schmitt, O.: Estimating the volume and age of water stored in global lakes using a geo-statistical approach, Nat. Commun., 7, 13603, https://doi.org/10.1038/ncomms13603, 2016.
Milinkoviæ, A., Peneziæ, A., Kušan, A. C., Glušèiæ, V., Žužul, S., Skejiæ, S., Šantiæ, D., Godec, R., Pehnec, G., Omanoviæ, D., Engel, A., and Frka, S.: Variabilities of biochemical properties of the sea surface microlayer: Insights to the atmospheric deposition impacts, Sci. Total Environ., 838, 156440, https://doi.org/10.1016/j.scitotenv.2022.156440, 2022.
Mishra, H., Enami, S., Nielsen, R. J., Stewart, L. A., Hoffmann, M. R., Goddard, W. A., and Colussi, A. J.: Brønsted basicity of the air–water interface, P. Natl. Acad. Sci. USA, 109, 18679–18683, https://doi.org/10.1073/pnas.1209307109, 2012.
Mochida, M., Kitamori, Y., Kawamura, K., Nojiri, Y., and Suzuki, K.: Fatty acids in the marine atmosphere: Factors governing their concentrations and evaluation of organic films on sea-salt particles, J. Geophys. Res.-Atmos., 107, 1–10, https://doi.org/10.1029/2001JD001278, 2002.
Modini, R. L., Russell, L. M., Deane, G. B., and Stokes, M. D.: Effect of soluble surfactant on bubble persistence and bubble-produced aerosol particles, J. Geophys. Res.-Atmos., 118, 1388–1400, https://doi.org/10.1002/jgrd.50186, 2013.
Mopper, K., Zhou, X., Kieber, R. J., Kieber, D. J., Sikorski, R. J., and Jones, R. D.: Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle, Nature, 353, 60–62, https://doi.org/10.1038/353060a0, 1991.
Mozgawa, K., Mennucci, B., and Frediani, L.: Solvation at Surfaces and Interfaces: A Quantum-Mechanical/Continuum Approach Including Nonelectrostatic Contributions, J. Phys. Chem. C, 118, 4715–4725, https://doi.org/10.1021/jp4117276, 2014.
Mungall, E. L., Abbatt, J. P. D., Wentzell, J. J. B., Lee, A. K. Y., Thomas, J. L., Blais, M., Gosselin, M., Miller, L. A., Papakyriakou, T., Willis, M. D., and Liggio, J.: Microlayer source of oxygenated volatile organic compounds in the summertime marine Arctic boundary layer, P. Natl. Acad. Sci. USA, 114, 6203–6208, https://doi.org/10.1073/pnas.1620571114, 2017.
Murdachaew, G., Varner, M. E., Phillips, L. F., Finlayson-Pitts, B. J., and Gerber, R. B.: Nitrogen dioxide at the air–water interface: trapping, absorption, and solvation in the bulk and at the surface, Phys. Chem. Chem. Phys., 15, 204–212, https://doi.org/10.1039/C2CP42810E, 2013.
Narayan, S., Muldoon, J., Finn, M. G., Fokin, V. V., Kolb, H. C., and Sharpless, K. B.: “On water”: unique reactivity of organic compounds in aqueous suspension, Angew. Chem. Int. Edit., 44, 3275–3279, https://doi.org/10.1002/anie.200462883, 2005.
Nayar, K. G., Panchanathan, D., McKinley, G. H., and Lienhard, J. H., V: Surface Tension of Seawater, J. Phys. Chem. Ref. Data, 43, https://doi.org/10.1063/1.4899037, 2014.
Nguyen, B. C., Bonsang, B., and Gaudry, A.: The role of the ocean in the global atmospheric sulfur cycle, J. Geophys. Res.-Oceans, 88, 10903–10914, https://doi.org/10.1029/JC088iC15p10903, 1983.
Nguyen, D., Lyu, P., and Nguyen, S. C.: Experimental and Thermodynamic Viewpoints on Claims of a Spontaneous H2O2 Formation at the Air–Water Interface, J. Phys. Chem. B, 127, 2323–2330, https://doi.org/10.1021/acs.jpcb.2c07394, 2023.
Ning, A., Zhong, J., Li, L., Li, H., Liu, J., Liu, L., Liang, Y., Li, J., Zhang, X., Francisco, J. S., and He, H.: Chemical Implications of Rapid Reactive Absorption of I2O4 at the Air-Water Interface, J. Am. Chem. Soc., 145, 10817–10825, https://doi.org/10.1021/jacs.3c01862, 2023.
Novak, G. A. and Bertram, T. H.: Reactive VOC Production from Photochemical and Heterogeneous Reactions Occurring at the Air-Ocean Interface, Acc. Chem. Res., 53, 1014–1023, https://doi.org/10.1021/acs.accounts.0c00095, 2020.
Novak, G. A., Kilgour, D. B., Jernigan, C. M., Vermeuel, M. P., and Bertram, T. H.: Oceanic emissions of dimethyl sulfide and methanethiol and their contribution to sulfur dioxide production in the marine atmosphere, Atmos. Chem. Phys., 22, 6309–6325, https://doi.org/10.5194/acp-22-6309-2022, 2022.
O'Dowd, C. D., Facchini, M. C., Cavalli, F., Ceburnis, D., Mircea, M., Decesari, S., Fuzzi, S., Yoon, Y. J., and Putaud, J. P.: Biogenically driven organic contribution to marine aerosol, Nature, 431, 676–680, https://doi.org/10.1038/nature02959, 2004.
Otten, D. E., Shaffer, P. R., Geissler, P. L., and Saykally, R. J.: Elucidating the mechanism of selective ion adsorption to the liquid water surface, P. Natl. Acad. Sci. USA, 109, 701–705, https://doi.org/10.1073/pnas.1116169109, 2012.
Oum, K. W., Lakin, M. J., DeHaan, D. O., Brauers, T., and Finlayson-Pitts, B. J.: Formation of Molecular Chlorine from the Photolysis of Ozone and Aqueous Sea-Salt Particles, Science, 279, 74–76, https://doi.org/10.1126/science.279.5347.74, 1998.
Park, J., Dall'Osto, M., Park, K., Kim, J. H., Park, J., Park, K. T., Hwang, C. Y., Jang, G. I., Gim, Y., Kang, S., Park, S., Jin, Y. K., Yum, S. S., Simo, R., and Yoon, Y. J.: Arctic Primary Aerosol Production Strongly Influenced by Riverine Organic Matter, Environ. Sci. Technol., 53, 8621–8630, https://doi.org/10.1021/acs.est.9b03399, 2019.
Parker, K. M., Pignatello, J. J., and Mitch, W. A.: Influence of Ionic Strength on Triplet-State Natural Organic Matter Loss by Energy Transfer and Electron Transfer Pathways, Environ. Sci. Technol., 47, 10987–10994, https://doi.org/10.1021/es401900j, 2013.
Parungo, F. P., Nagamoto, C. T., Rosinski, J., and Haagenson, P. L.: A study of marine aerosols over the Pacific Ocean, J. Atmos. Chem., 4, 199–226, https://doi.org/10.1007/BF00052001, 1986.
Passananti, M., Kong, L., Shang, J., Dupart, Y., Perrier, S., Chen, J., Donaldson, D. J., and George, C.: Organosulfate Formation through the Heterogeneous Reaction of Sulfur Dioxide with Unsaturated Fatty Acids and Long-Chain Alkenes, Angew. Chem. Int. Edit., 55, 10336–10339, https://doi.org/10.1002/anie.201605266, 2016.
Peneziæ, A., Wang, X., Perrier, S., George, C., and Frka, S.: Interfacial photochemistry of marine diatom lipids: Abiotic production of volatile organic compounds and new particle formation, Chemosphere, 313, 137510, https://doi.org/10.1016/j.chemosphere.2022.137510, 2023.
Pereira, R., Schneider-Zapp, K., and Upstill-Goddard, R. C.: Surfactant control of gas transfer velocity along an offshore coastal transect: results from a laboratory gas exchange tank, Biogeosciences, 13, 3981–3989, https://doi.org/10.5194/bg-13-3981-2016, 2016.
Pereira, R., Ashton, I., Sabbaghzadeh, B., Shutler, J. D., and Upstill-Goddard, R. C.: Reduced air–sea CO2 exchange in the Atlantic Ocean due to biological surfactants, Nat. Geosci., 11, 492–496, https://doi.org/10.1038/s41561-018-0136-2, 2018.
Petersen, M. K., Iyengar, S. S., Day, T. J. F., and Voth, G. A.: The Hydrated Proton at the Water Liquid/Vapor Interface, J. Phys. Chem. B, 108, 14804–14806, https://doi.org/10.1021/jp046716o, 2004.
Petersen, P. B. and Saykally, R. J.: Evidence for an Enhanced Hydronium Concentration at the Liquid Water Surface, J. Phys. Chem. B, 109, 7976–7980, https://doi.org/10.1021/jp044479j, 2005.
Petersen, P. B. and Saykally, R. J.: On the nature of ions at the liquid water surface, Annu. Rev. Phys. Chem., 57, 333–364, https://doi.org/10.1146/annurev.physchem.57.032905.104609, 2006.
Phillips, D. P., Hopkins, F. E., Bell, T. G., Liss, P. S., Nightingale, P. D., Reeves, C. E., Wohl, C., and Yang, M.: Air–sea exchange of acetone, acetaldehyde, DMS and isoprene at a UK coastal site, Atmos. Chem. Phys., 21, 10111–10132, https://doi.org/10.5194/acp-21-10111-2021, 2021.
Pratap, V., Carlton, A. G., Christiansen, A. E., and Hennigan, C. J.: Partitioning of Ambient Organic Gases to Inorganic Salt Solutions: Influence of Salt Identity, Ionic Strength, and pH, Geophys. Res. Lett., 48, https://doi.org/10.1029/2021gl095247, 2021.
Prather, K. A., Bertram, T. H., Grassian, V. H., Deane, G. B., Stokes, M. D., Demott, P. J., Aluwihare, L. I., Palenik, B. P., Azam, F., Seinfeld, J. H., Moffet, R. C., Molina, M. J., Cappa, C. D., Geiger, F. M., Roberts, G. C., Russell, L. M., Ault, A. P., Baltrusaitis, J., Collins, D. B., Corrigan, C. E., Cuadra-Rodriguez, L. A., Ebben, C. J., Forestieri, S. D., Guasco, T. L., Hersey, S. P., Kim, M. J., Lambert, W. F., Modini, R. L., Mui, W., Pedler, B. E., Ruppel, M. J., Ryder, O. S., Schoepp, N. G., Sullivan, R. C., and Zhao, D.: Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol, P. Natl. Acad. Sci. USA, 110, 7550–7555, https://doi.org/10.1073/pnas.1300262110, 2013.
Quinn, P. K. and Bates, T. S.: The case against climate regulation via oceanic phytoplankton sulphur emissions, Nature, 480, 51–56, https://doi.org/10.1038/nature10580, 2011.
Quinn, P. K., Bates, T. S., Schulz, K. S., Coffman, D. J., Frossard, A. A., Russell, L. M., Keene, W. C., and Kieber, D. J.: Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol, Nat. Geosci., 7, 228–232, https://doi.org/10.1038/ngeo2092, 2014.
Quinn, P. K., Collins, D. B., Grassian, V. H., Prather, K. A., and Bates, T. S.: Chemistry and related properties of freshly emitted sea spray aerosol, Chem. Rev., 115, 4383–4399, https://doi.org/10.1021/cr500713g, 2015.
Quinn, P. K., Coffman, D. J., Johnson, J. E., Upchurch, L. M., and Bates, T. S.: Small fraction of marine cloud condensation nuclei made up of sea spray aerosol, Nat. Geosci., 10, 674–679, https://doi.org/10.1038/ngeo3003, 2017.
Rao, Z., Li, X., Fang, Y.-G., Francisco, J. S., Zhu, C., and Chu, C.: Spontaneous Oxidation of Thiols and Thioether at the Air–Water Interface of a Sea Spray Microdroplet, J. Am. Chem. Soc., 145, 10839–10846, https://doi.org/10.1021/jacs.3c02334, 2023a.
Rao, Z., Fang, Y.-G., Pan, Y., Yu, W., Chen, B., Francisco, J. S., Zhu, C., and Chu, C.: Accelerated Photolysis of H2O2 at the Air–Water Interface of a Microdroplet, J. Am. Chem. Soc., 145, 24717–24723, https://doi.org/10.1021/jacs.3c08101, 2023b.
Ravindra, K., Sokhi, R., and Van Grieken, R.: Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation, Atmos. Environ., 42, 2895–2921, https://doi.org/10.1016/j.atmosenv.2007.12.010, 2008.
Ravishankara, A. R.: Heterogeneous and multiphase chemistry in the troposphere, Science, 276, 1058–1065, https://doi.org/10.1126/science.276.5315.1058, 1997.
Read, K. A., Carpenter, L. J., Arnold, S. R., Beale, R., Nightingale, P. D., Hopkins, J. R., Lewis, A. C., Lee, J. D., Mendes, L., and Pickering, S. J.: Multiannual observations of acetone, methanol, and acetaldehyde in remote tropical atlantic air: implications for atmospheric OVOC budgets and oxidative capacity, Environ. Sci. Technol., 46, 11028–11039, https://doi.org/10.1021/es302082p, 2012.
Reed Harris, A. E., Pajunoja, A., Cazaunau, M., Gratien, A., Pangui, E., Monod, A., Griffith, E. C., Virtanen, A., Doussin, J.-F., and Vaida, V.: Multiphase Photochemistry of Pyruvic Acid under Atmospheric Conditions, J. Phys. Chem. A, 121, 3327–3339, https://doi.org/10.1021/acs.jpca.7b01107, 2017.
Reinthaler, T., Sintes, E., and Herndl, G. J.: Dissolved organic matter and bacterial production and respiration in the sea-surface microlayer of the open Atlantic and the western Mediterranean Sea, Limnol. Oceanogr., 53, 122–136, https://doi.org/10.4319/lo.2008.53.1.0122, 2008.
Ribas-Ribas, M., Hamizah Mustaffa, N. I., Rahlff, J., Stolle, C., and Wurl, O.: Sea Surface Scanner (S3): A Catamaran for High-Resolution Measurements of Biogeochemical Properties of the Sea Surface Microlayer, J. Atmos. Ocean. Tech., 34, 1433–1448, https://doi.org/10.1175/jtech-d-17-0017.1, 2017.
Rickard, P. C., Uher, G., and Upstill-Goddard, R. C.: Photo-Reactivity of Surfactants in the Sea–Surface Microlayer and Subsurface Water of the Tyne Estuary, UK, Geophys. Res. Lett., 49, https://doi.org/10.1029/2021gl095469, 2022.
Riemer, D. D., Milne, P. J., Zika, R. G., and Pos, W. H.: Photoproduction of nonmethane hydrocarbons (NMHCs) in seawater, Mar. Chem., 71, 177–198, https://doi.org/10.1016/S0304-4203(00)00048-7, 2000.
Rocco, M., Dunne, E., Peltola, M., Barr, N., Williams, J., Colomb, A., Safi, K., Saint-Macary, A., Marriner, A., Deppeler, S., Harnwell, J., Law, C., and Sellegri, K.: Oceanic phytoplankton are a potentially important source of benzenoids to the remote marine atmosphere, Communications Earth and Environment, 2, 175, https://doi.org/10.1038/s43247-021-00253-0, 2021.
Rossignol, S., Tinel, L., Bianco, A., Passananti, M., Brigante, M., Donaldson, D. J., and George, C.: Atmospheric photochemistry at a fatty acid-coated air–water interface, Science, 353, 699–702, https://doi.org/10.1126/science.aaf3617, 2016.
Rouvière, A. and Ammann, M.: The effect of fatty acid surfactants on the uptake of ozone to aqueous halogenide particles, Atmos. Chem. Phys., 10, 11489–11500, https://doi.org/10.5194/acp-10-11489-2010, 2010.
Rudich, Y.: Laboratory Perspectives on the Chemical Transformations of Organic Matter in Atmospheric Particles, Chem. Rev., 103, 5097–5124, https://doi.org/10.1021/cr020508f, 2003.
Ruiz-López, M. F., Martins-Costa, M. T. C., Anglada, J. M., and Francisco, J. S.: A New Mechanism of Acid Rain Generation from HOSO at the Air–Water Interface, J. Am. Chem. Soc., 141, 16564–16568, https://doi.org/10.1021/jacs.9b07912, 2019.
Ruiz-Lopez, M. F., Francisco, J. S., Martins-Costa, M. T. C., and Anglada, J. M.: Molecular reactions at aqueous interfaces, Nature Reviews Chemistry, 4, 459–475, https://doi.org/10.1038/s41570-020-0203-2, 2020.
Russell, L. M., Hawkins, L. N., Frossard, A. A., Quinn, P. K., and Bates, T. S.: Carbohydrate-like composition of submicron atmospheric particles and their production from ocean bubble bursting, P. Natl. Acad. Sci. USA, 107, 6652–6657, https://doi.org/10.1073/pnas.0908905107, 2010.
Russell, L. M., Moore, R. H., Burrows, S. M., and Quinn, P. K.: Ocean flux of salt, sulfate, and organic components to atmospheric aerosol, Earth-Sci. Rev., 239, 104364, https://doi.org/10.1016/j.earscirev.2023.104364, 2023.
Sabbaghzadeh, B., Upstill-Goddard, R. C., Beale, R., Pereira, R., and Nightingale, P. D.: The Atlantic Ocean surface microlayer from 50° N to 50° S is ubiquitously enriched in surfactants at wind speeds up to 13 m s-1, Geophys. Res. Lett., 44, 2852–2858, https://doi.org/10.1002/2017GL072988, 2017.
Saito, S., Numadate, N., Teraoka, H., Enami, S., Kobayashi, H., and Hama, T.: Impurity contribution to ultraviolet absorption of saturated fatty acids, Sci. Adv., 9, eadj6438, https://doi.org/10.1126/sciadv.adj6438, 2023.
Sakellari, A., Karavoltsos, S., Moutafis, I., Koukoulakis, K., Dassenakis, M., and Bakeas, E.: Occurrence and Distribution of Polycyclic Aromatic Hydrocarbons in the Marine Surface Microlayer of an Industrialized Coastal Area in the Eastern Mediterranean, Water, 13, https://doi.org/10.3390/w13223174, 2021.
Salter, M. E., Upstill-Goddard, R. C., Nightingale, P. D., Archer, S. D., Blomquist, B., Ho, D. T., Huebert, B., Schlosser, P., and Yang, M.: Impact of an artificial surfactant release on air–sea gas fluxes during Deep Ocean Gas Exchange Experiment II, J. Geophys. Res.-Oceans, 116, https://doi.org/10.1029/2011JC007023, 2011.
Sauer, J. S., Mayer, K. J., Lee, C., Alves, M. R., Amiri, S., Bahaveolos, C. J., Franklin, E. B., Crocker, D. R., Dang, D., Dinasquet, J., Garofalo, L. A., Kaluarachchi, C. P., Kilgour, D. B., Mael, L. E., Mitts, B. A., Moon, D. R., Moore, A. N., Morris, C. K., Mullenmeister, C. A., Ni, C. M., Pendergraft, M. A., Petras, D., Simpson, R. M. C., Smith, S., Tumminello, P. R., Walker, J. L., DeMott, P. J., Farmer, D. K., Goldstein, A. H., Grassian, V. H., Jaffe, J. S., Malfatti, F., Martz, T. R., Slade, J. H., Tivanski, A. V., Bertram, T. H., Cappa, C. D., and Prather, K. A.: The Sea Spray Chemistry and Particle Evolution study (SeaSCAPE): overview and experimental methods, Environ. Sci. Process. Impacts., 24, 290–315, https://doi.org/10.1039/d1em00260k, 2022.
Saykally, R. J.: Two sides of the acid–base story, Nat. Chem., 5, 82–84, https://doi.org/10.1038/nchem.1556, 2013.
Schlundt, C., Tegtmeier, S., Lennartz, S. T., Bracher, A., Cheah, W., Krüger, K., Quack, B., and Marandino, C. A.: Oxygenated volatile organic carbon in the western Pacific convective center: ocean cycling, air–sea gas exchange and atmospheric transport, Atmos. Chem. Phys., 17, 10837–10854, https://doi.org/10.5194/acp-17-10837-2017, 2017.
Schmitt-Kopplin, P., Liger-Belair, G., Koch, B. P., Flerus, R., Kattner, G., Harir, M., Kanawati, B., Lucio, M., Tziotis, D., Hertkorn, N., and Gebefügi, I.: Dissolved organic matter in sea spray: a transfer study from marine surface water to aerosols, Biogeosciences, 9, 1571–1582, https://doi.org/10.5194/bg-9-1571-2012, 2012.
Schneider, S., Lakey, P., Shiraiwa, M., and Abbatt, J.: Iodine Emission from the Reactive Uptake of Ozone to Simulated Seawater, Environmental Science: Processes and Impacts, https://doi.org/10.1039/d2em00111j, 2022.
Schneider, S. R., Lakey, P. S. J., Shiraiwa, M., and Abbatt, J. P. D.: Iodine emission from the reactive uptake of ozone to simulated seawater, Environmental Science: Processes and Impacts, 25, 254–263, https://doi.org/10.1039/D2EM00111J, 2023.
Schneider, S. R., Collins, D. B., Lim, C. Y., Zhu, L., and Abbatt, J. P. D.: Formation of Secondary Organic Aerosol from the Heterogeneous Oxidation by Ozone of a Phytoplankton Culture, ACS Earth and Space Chemistry, 3, 2298–2306, https://doi.org/10.1021/acsearthspacechem.9b00201, 2019.
Schneider, S. R., Collins, D. B., Boyer, M., Chang, R. Y. W., Gosselin, M., Irish, V. E., Miller, L. A., and Abbatt, J. P. D.: Abiotic Emission of Volatile Organic Compounds from the Ocean Surface: Relationship to Seawater Composition, ACS Earth and Space Chemistry, https://doi.org/10.1021/acsearthspacechem.4c00163, 2024.
Schneider-Zapp, K., Salter, M. E., and Upstill-Goddard, R. C.: An automated gas exchange tank for determining gas transfer velocities in natural seawater samples, Ocean Sci., 10, 587–600, https://doi.org/10.5194/os-10-587-2014, 2014.
Seki, T., Yu, C.-C., Chiang, K.-Y., Greco, A., Yu, X., Matsumura, F., Bonn, M., and Nagata, Y.: Ions Speciation at the Water–Air Interface, J. Am. Chem. Soc., 145, 10622–10630, https://doi.org/10.1021/jacs.3c00517, 2023.
Sellegri, K., O'Dowd, C. D., Yoon, Y. J., Jennings, S. G., and de Leeuw, G.: Surfactants and submicron sea spray generation, J. Geophys. Res.-Atmos., 111, https://doi.org/10.1029/2005JD006658, 2006.
Shang, J., Passananti, M., Dupart, Y., Ciuraru, R., Tinel, L., Rossignol, S., Perrier, S., Zhu, T., and George, C.: SO2 Uptake on Oleic Acid: A New Formation Pathway of Organosulfur Compounds in the Atmosphere, Environ. Sci. Tech. Let., 3, 67–72, https://doi.org/10.1021/acs.estlett.6b00006, 2016.
Sharpless, C. M. and Blough, N. V.: The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties, Environmental Science: Processes and Impacts, 16, 654–671, https://doi.org/10.1039/C3EM00573A, 2014.
Shaw, S. L., Gantt, B., and Meskhidze, N.: Production and Emissions of Marine Isoprene and Monoterpenes: A Review, Adv. Meteorol., 2010, 1–24, https://doi.org/10.1155/2010/408696, 2010.
Shi, L., LaCour, R. A., Qian, N., Heindel, J. P., Lang, X., Zhao, R., Head-Gordon, T., and Min, W.: Water structure and electric fields at the interface of oil droplets, Nature, 640, 87–93, https://doi.org/10.1038/s41586-025-08702-y, 2025.
Sidebottom, H. W., Badcock, C. C., Jackson, G. E., Calvert, J. G., Reinhardt, G. W., and Damon, E. K.: Photooxidation of sulfur dioxide, Environ. Sci. Technol., 6, 72–79, https://doi.org/10.1021/es60060a001, 1972.
Sieburth, J. M., Willis, P.-J., Johnson, K. M., Burney, C. M., Lavoie, D. M., Hinga, K. R., Caron, D. A., French, F. W., Johnson, P. W., and Davis, P. G.: Dissolved Organic Matter and Heterotrophic Microneuston in the Surface Microlayers of the North Atlantic, Science, 194, 1415–1418, https://doi.org/10.1126/science.194.4272.1415, 1976.
Singh, H., Chen, Y., Staudt, A., Jacob, D., Blake, D., Heikes, B., and Snow, J.: Evidence from the Pacific troposphere for large global sources of oxygenated organic compounds, Nature, 410, 1078–1081, https://doi.org/10.1038/35074067, 2001.
Singh, H. B. and Hanst, P. L.: Peroxyacetyl nitrate (PAN) in the unpolluted atmosphere – An important reservoir for nitrogen-oxides, Geophys. Res. Lett., 8, 941–944, https://doi.org/10.1029/GL008i008p00941, 1981.
Singh, H. B., Ohara, D., Herlth, D., Sachse, W., Blake, D. R., Bradshaw, J. D., Kanakidou, M., and Crutzen, P. J.: Acetone in the atmosphere – Distribution, sources, and sinks, J. Geophys. Res.-Atmos., 99, 1805–1819, https://doi.org/10.1029/93jd00764, 1994.
Sinreich, R., Coburn, S., Dix, B., and Volkamer, R.: Ship-based detection of glyoxal over the remote tropical Pacific Ocean, Atmos. Chem. Phys., 10, 11359–11371, https://doi.org/10.5194/acp-10-11359-2010, 2010.
Smyth, T. J.: Penetration of UV irradiance into the global ocean, J. Geophys. Res.-Oceans, 116, https://doi.org/10.1029/2011JC007183, 2011.
Spicer, C. W., Chapman, E. G., Finlayson-Pitts, B. J., Plastridge, R. A., Hubbe, J. M., Fast, J. D., and Berkowitz, C. M.: Unexpectedly high concentrations of molecular chlorine in coastal air, Nature, 394, 353–356, https://doi.org/10.1038/28584, 1998.
Spicer, C. W., Plastridge, R. A., Foster, K. L., Finlayson-Pitts, B. J., Bottenheim, J. W., Grannas, A. M., and Shepson, P. B.: Molecular halogens before and during ozone depletion events in the Arctic at polar sunrise: concentrations and sources, Atmos. Environ., 36, 2721–2731, https://doi.org/10.1016/S1352-2310(02)00125-5, 2002.
Stemmler, K., Vlasenko, A., Guimbaud, C., and Ammann, M.: The effect of fatty acid surfactants on the uptake of nitric acid to deliquesced NaCl aerosol, Atmos. Chem. Phys., 8, 5127–5141, https://doi.org/10.5194/acp-8-5127-2008, 2008.
Stirchak, L. T., Abis, L., Kalalian, C., George, C., and Donaldson, D. J.: Differences in Photosensitized Release of VOCs from Illuminated Seawater versus Freshwater Surfaces, ACS Earth and Space Chemistry, 5, 2233–2242, https://doi.org/10.1021/acsearthspacechem.1c00063, 2021.
Thornton, D. C. O., Brooks, S. D., and Chen, J.: Protein and Carbohydrate Exopolymer Particles in the Sea Surface Microlayer (SML), Front. Mar. Sci., 3, https://doi.org/10.3389/fmars.2016.00135, 2016.
Thornton, J. A. and Abbatt, J. P. D.: N2O5 Reaction on Submicron Sea Salt Aerosol: Kinetics, Products, and the Effect of Surface Active Organics, J. Phys. Chem. A, 109, 10004–10012, https://doi.org/10.1021/jp054183t, 2005.
Tian, Y.-M., Silva, W., Gschwind, R. M., and König, B.: Accelerated photochemical reactions at oil–water interface exploiting melting point depression, Science, 383, 750–756, https://doi.org/10.1126/science.adl3092, 2024.
Tinel, L., Rossignol, S., Bianco, A., Passananti, M., Perrier, S., Wang, X., Brigante, M., Donaldson, D. J., and George, C.: Mechanistic Insights on the Photosensitized Chemistry of a Fatty Acid at the Air/Water Interface, Environ. Sci. Technol., 50, 11041–11048, https://doi.org/10.1021/acs.est.6b03165, 2016.
Tinel, L., Adams, T. J., Hollis, L. D. J., Bridger, A. J. M., Chance, R. J., Ward, M. W., Ball, S. M., and Carpenter, L. J.: Influence of the Sea Surface Microlayer on Oceanic Iodine Emissions, Environ. Sci. Technol., 54, 13228–13237, https://doi.org/10.1021/acs.est.0c02736, 2020.
Trilla-Prieto, N., Iriarte, J., Berrojalbiz, N., Casas, G., Sobrino, C., Vila-Costa, M., Jiménez, B., and Dachs, J.: Enrichment of Organophosphate Esters in the Sea Surface Microlayer from the Atlantic and Southern Oceans, Environ. Sci. Tech. Let., 11, 1008–1015, https://doi.org/10.1021/acs.estlett.4c00636, 2024.
Trueblood, J. V., Alves, M. R., Power, D., Santander, M. V., Cochran, R. E., Prather, K. A., and Grassian, V. H.: Shedding Light on Photosensitized Reactions within Marine-Relevant Organic Thin Films, ACS Earth and Space Chemistry, 3, 1614–1623, https://doi.org/10.1021/acsearthspacechem.9b00066, 2019.
Tsai, W.-T. and Liu, K.-K.: An assessment of the effect of sea surface surfactant on global atmosphere–ocean CO2 flux, J. Geophys. Res.-Oceans, 108, https://doi.org/10.1029/2000JC000740, 2003.
Tsigaridis, K., Koch, D., and Menon, S.: Uncertainties and importance of sea spray composition on aerosol direct and indirect effects, J. Geophys. Res.-Atmos., 118, 220–235, https://doi.org/10.1029/2012JD018165, 2013.
Tsui, M. M. P., Lam, J. C. W., Ng, T. Y., Ang, P. O., Murphy, M. B., and Lam, P. K. S.: Occurrence, Distribution, and Fate of Organic UV Filters in Coral Communities, Environ. Sci. Technol., 51, 4182–4190, https://doi.org/10.1021/acs.est.6b05211, 2017.
Tyree, C. A., Hellion, V. M., Alexandrova, O. A., and Allen, J. O.: Foam droplets generated from natural and artificial seawaters, J. Geophys. Res.-Atmos., 112, https://doi.org/10.1029/2006JD007729, 2007.
Uetake, J., Hill, T. C. J., Moore, K. A., DeMott, P. J., Protat, A., and Kreidenweis, S. M.: Airborne bacteria confirm the pristine nature of the Southern Ocean boundary layer, P. Natl. Acad. Sci. USA, 117, 13275–13282, https://doi.org/10.1073/pnas.2000134117, 2020.
Vácha, R., Slavíèek, P., Mucha, M., Finlayson-Pitts, B. J., and Jungwirth, P.: Adsorption of Atmospherically Relevant Gases at the Air/Water Interface: Free Energy Profiles of Aqueous Solvation of N2, O2, O3, OH, H2O, HO2, and H2O2, J. Phys. Chem. A, 108, 11573–11579, https://doi.org/10.1021/jp046268k, 2004.
van Pinxteren, M., Müller, C., Iinuma, Y., Stolle, C., and Herrmann, H.: Chemical Characterization of Dissolved Organic Compounds from Coastal Sea Surface Microlayers (Baltic Sea, Germany), Environ. Sci. Technol., 46, 10455–10462, https://doi.org/10.1021/es204492b, 2012.
Wagner, S., Riedel, T., Niggemann, J., Vahatalo, A. V., Dittmar, T., and Jaffe, R.: Linking the Molecular Signature of Heteroatomic Dissolved Organic Matter to Watershed Characteristics in World Rivers, Environ. Sci. Technol., 49, 13798–13806, https://doi.org/10.1021/acs.est.5b00525, 2015.
Wang, K., Zhang, Y., Huang, R. J., Wang, M., Ni, H., Kampf, C. J., Cheng, Y., Bilde, M., Glasius, M., and Hoffmann, T.: Molecular Characterization and Source Identification of Atmospheric Particulate Organosulfates Using Ultrahigh Resolution Mass Spectrometry, Environ. Sci. Technol., 53, 6192–6202, https://doi.org/10.1021/acs.est.9b02628, 2019a.
Wang, S., Apel, E. C., Hornbrook, R. S., Hills, A., Emmons, L. K., Tilmes, S., Lamarque, J. F., Jimenez, J. L., Campuzano-Jost, P., Nault, B. A., Crounse, J. D., Wennberg, P. O., Ryerson, T. B., Thompson, C. R., Peischl, J., Moore, F., Nance, D., Hall, B., Elkins, J., Tanner, D., Gregory Huey, L., Hall, S. R., Ullmann, K., Orlando, J. J., Tyndall, G. S., Flocke, F. M., Ray, E., Hanisco, T. F., Wolfe, G. M., St Clair, J., Commane, R., Daube, B., Barletta, B., Blake, D. R., Weinzierl, B., Dollner, M., Conley, A., Vitt, F., Wofsy, S. C., and Riemer, D. D.: Atmospheric Acetaldehyde: Importance of Air–Sea Exchange and a Missing Source in the Remote Troposphere, Geophys. Res. Lett., 46, 5601–5613, https://doi.org/10.1029/2019GL082034, 2019b.
Wang, S. Y., Apel, E. C., Schwantes, R. H., Bates, K. H., Jacob, D. J., Fischer, E. V., Hornbrook, R. S., Hills, A. J., Emmons, L. K., Pan, L. L., Honomichl, S., Tilmes, S., Lamarque, J. F., Yang, M. X., Marandino, C. A., Saltzman, E. S., de Bruyn, W., Kameyama, S., Tanimoto, H., Omori, Y., Hall, S. R., Ullmann, K., Ryerson, T. B., Thompson, C. R., Peischl, J., Daube, B. C., Commane, R., McKain, K., Sweeney, C., Thames, A. B., Miller, D. O., Brune, W. H., Diskin, G. S., DiGangi, J. P., and Wofsy, S. C.: Global Atmospheric Budget of Acetone: Air–Sea Exchange and the Contribution to Hydroxyl Radicals, J. Geophys. Res.-Atmos., 125, https://doi.org/10.1029/2020jd032553, 2020a.
Wang, W., Liu, Y., Wang, T., Ge, Q., Li, K., Liu, J., You, W., Wang, L., Xie, L., Fu, H., Chen, J., and Zhang, L.: Significantly Accelerated Photosensitized Formation of Atmospheric Sulfate at the Air–Water Interface of Microdroplets, J. Am. Chem. Soc., https://doi.org/10.1021/jacs.3c11892, 2024.
Wang, X., Dalton, E. Z., Payne, Z. C., Perrier, S., Riva, M., Raff, J. D., and George, C.: Superoxide and Nitrous Acid Production from Nitrate Photolysis Is Enhanced by Dissolved Aliphatic Organic Matter, Environ. Sci. Tech. Let., 8, 53–58, https://doi.org/10.1021/acs.estlett.0c00806, 2020b.
Wang, X., Sultana, C. M., Trueblood, J., Hill, T. C. J., Malfatti, F., Lee, C., Laskina, O., Moore, K. A., Beall, C. M., McCluskey, C. S., Cornwell, G. C., Zhou, Y., Cox, J. L., Pendergraft, M. A., Santander, M. V., Bertram, T. H., Cappa, C. D., Azam, F., DeMott, P. J., Grassian, V. H., and Prather, K. A.: Microbial Control of Sea Spray Aerosol Composition: A Tale of Two Blooms, ACS Central Science, 1, 124–131, https://doi.org/10.1021/acscentsci.5b00148, 2015.
Wang, Y., Deng, H., Li, P., Xu, J., Jiang, B., Pang, H., and Gligorovski, S.: Molecular Characterization of the Product Compounds Formed Upon Heterogeneous Chemistry of Ozone With Riverine Surface Microlayer, J. Geophys. Res.-Atmos., 127, https://doi.org/10.1029/2022jd037182, 2022a.
Wang, Y., Mekic, M., Li, P., Deng, H., Liu, S., Jiang, B., Jin, B., Vione, D., and Gligorovski, S.: Ionic Strength Effect Triggers Brown Carbon Formation through Heterogeneous Ozone Processing of Ortho-Vanillin, Environ. Sci. Technol., 55, 4553–4564, https://doi.org/10.1021/acs.est.1c00874, 2021.
Wang, Y., Zeng, J., Wu, B., Song, W., Hu, W., Liu, J., Yang, Y., Yu, Z., Wang, X., and Gligorovski, S.: Production of Volatile Organic Compounds by Ozone Oxidation Chemistry at the South China Sea Surface Microlayer, ACS Earth and Space Chemistry, https://doi.org/10.1021/acsearthspacechem.3c00102, 2023.
Wang, Y. Q., Deng, H. F., Li, P., Xu, J. L., Loisel, G., Pang, H. W., Xu, X., Li, X., and Gligorovski, S.: Interfacial Ozone Oxidation Chemistry at a Riverine Surface Microlayer as a Source of Nitrogen Organic Compounds, Environ. Sci. Technol. Lett., 9, 493–500, https://doi.org/10.1021/acs.estlett.2c00130, 2022b.
Wei, Z., Li, Y., Cooks, R. G., and Yan, X.: Accelerated Reaction Kinetics in Microdroplets: Overview and Recent Developments, Annu. Rev. Phys. Chem., 71, 31–51, https://doi.org/10.1146/annurev-physchem-121319-110654, 2020.
Wilson, T. W., Ladino, L. A., Alpert, P. A., Breckels, M. N., Brooks, I. M., Browse, J., Burrows, S. M., Carslaw, K. S., Huffman, J. A., Judd, C., Kilthau, W. P., Mason, R. H., McFiggans, G., Miller, L. A., Najera, J. J., Polishchuk, E., Rae, S., Schiller, C. L., Si, M., Temprado, J. V., Whale, T. F., Wong, J. P., Wurl, O., Yakobi-Hancock, J. D., Abbatt, J. P., Aller, J. Y., Bertram, A. K., Knopf, D. A., and Murray, B. J.: A marine biogenic source of atmospheric ice-nucleating particles, Nature, 525, 234–238, https://doi.org/10.1038/nature14986, 2015.
Wohl, C., Li, Q., Cuevas, C. A., Fernandez, R. P., Yang, M., Saiz-Lopez, A., and Simo, R.: Marine biogenic emissions of benzene and toluene and their contribution to secondary organic aerosols over the polar oceans, Sci. Adv., 9, eadd9031, https://doi.org/10.1126/sciadv.add9031, 2023.
Woodhouse, M. T., Mann, G. W., Carslaw, K. S., and Boucher, O.: New Directions: The impact of oceanic iron fertilisation on cloud condensation nuclei, Atmos. Environ., 42, 5728–5730, https://doi.org/10.1016/j.atmosenv.2008.05.005, 2008.
Wurl, O. and Holmes, M.: The gelatinous nature of the sea-surface microlayer, Mar. Chem., 110, 89–97, https://doi.org/10.1016/j.marchem.2008.02.009, 2008.
Wurl, O., Miller, L., Röttgers, R., and Vagle, S.: The distribution and fate of surface-active substances in the sea-surface microlayer and water column, Mar. Chem., 115, 1–9, https://doi.org/10.1016/j.marchem.2009.04.007, 2009.
Wurl, O., Wurl, E., Miller, L., Johnson, K., and Vagle, S.: Formation and global distribution of sea-surface microlayers, Biogeosciences, 8, 121–135, https://doi.org/10.5194/bg-8-121-2011, 2011.
Wurl, O., Stolle, C., Van Thuoc, C., The Thu, P., and Mari, X.: Biofilm-like properties of the sea surface and predicted effects on air–sea CO2 exchange, Prog. Oceanogr., 144, 15–24, https://doi.org/10.1016/j.pocean.2016.03.002, 2016.
Xia, S.-S., Eugene, A. J., and Guzman, M. I.: Cross Photoreaction of Glyoxylic and Pyruvic Acids in Model Aqueous Aerosol, J. Phys. Chem. A, 122, 6457–6466, https://doi.org/10.1021/acs.jpca.8b05724, 2018.
Xiong, H., Lee, J. K., Zare, R. N., and Min, W.: Strong Electric Field Observed at the Interface of Aqueous Microdroplets, J. Phys. Chem. Lett., 11, 7423–7428, https://doi.org/10.1021/acs.jpclett.0c02061, 2020.
Xu, W., Ovadnevaite, J., Fossum, K. N., Lin, C., Huang, R.-J., Ceburnis, D., and O'Dowd, C.: Sea spray as an obscured source for marine cloud nuclei, Nat. Geosci., https://doi.org/10.1038/s41561-022-00917-2, 2022.
Yang, M., Blomquist, B. W., and Nightingale, P. D.: Air–sea exchange of methanol and acetone during HiWinGS: Estimation of air phase, water phase gas transfer velocities, J. Geophys. Res.-Oceans, 119, 7308–7323, https://doi.org/10.1002/2014JC010227, 2014a.
Yang, M., Beale, R., Liss, P., Johnson, M., Blomquist, B., and Nightingale, P.: Air–sea fluxes of oxygenated volatile organic compounds across the Atlantic Ocean, Atmos. Chem. Phys., 14, 7499–7517, https://doi.org/10.5194/acp-14-7499-2014, 2014b.
Yang, X., Wang, H., Lu, K., Ma, X., Tan, Z., Long, B., Chen, X., Li, C., Zhai, T., Li, Y., Qu, K., Xia, Y., Zhang, Y., Li, X., Chen, S., Dong, H., Zeng, L., and Zhang, Y.: Reactive aldehyde chemistry explains the missing source of hydroxyl radicals, Nat. Commun., 15, 1648, https://doi.org/10.1038/s41467-024-45885-w, 2024.
Ye, J., Abbatt, J. P. D., and Chan, A. W. H.: Novel pathway of SO2 oxidation in the atmosphere: reactions with monoterpene ozonolysis intermediates and secondary organic aerosol, Atmos. Chem. Phys., 18, 5549–5565, https://doi.org/10.5194/acp-18-5549-2018, 2018.
Yu, C., Liu, T., Ge, D., Nie, W., Chi, X., and Ding, A.: Ionic Strength Enhances the Multiphase Oxidation Rate of Sulfur Dioxide by Ozone in Aqueous Aerosols: Implications for Sulfate Production in the Marine Atmosphere, Environ. Sci. Technol., 57, 6609–6615, https://doi.org/10.1021/acs.est.3c00212, 2023.
Yue, S., Cheng, Y., Zheng, L., Lai, S., Wang, S., Song, T., Li, L., Li, P., Zhu, J., Li, M., Wei, L., Ma, C., Jin, R., Zhang, Y., Sun, Y., Wang, Z., Kawamura, K., Liu, C.-Q., Su, H., Andreae, M. O., and Fu, P.: Mass deposition of microbes from wildfire smoke to the sea surface microlayer, Limnol. Oceanogr., 70, 1770–1781, https://doi.org/10.1002/lno.70078, 2025.
Zhang, Z., Liu, L., Liu, C., and Cai, W.: Studies on the sea surface microlayer, J. Colloid Interf. Sci., 264, 148–159, https://doi.org/10.1016/s0021-9797(03)00390-4, 2003a.
Zhang, Z., Cai, W., Liu, L., Liu, C., and Chen, F.: Direct determination of thickness of sea surface microlayer using a pH microelectrode at original location, Science in China Series B: Chemistry, 46, 339–351, https://doi.org/10.1360/02yb0192, 2003b.
Zhong, J., Kumar, M., Francisco, J. S., and Zeng, X. C.: Insight into Chemistry on Cloud/Aerosol Water Surfaces, Acc Chem. Res., 51, 1229–1237, https://doi.org/10.1021/acs.accounts.8b00051, 2018.
Zhong, J., Kumar, M., Anglada, J. M., Martins-Costa, M. T. C., Ruiz-Lopez, M. F., Zeng, X. C., and Francisco, J. S.: Atmospheric Spectroscopy and Photochemistry at Environmental Water Interfaces, Annu. Rev. Phys. Chem., 70, 45–69, https://doi.org/10.1146/annurev-physchem-042018-052311, 2019.
Zhong, Q., Shen, H., Yun, X., Chen, Y., Ren, Y. a., Xu, H., Shen, G., Du, W., Meng, J., Li, W., Ma, J., and Tao, S.: Global Sulfur Dioxide Emissions and the Driving Forces, Environ. Sci. Technol., 54, 6508–6517, https://doi.org/10.1021/acs.est.9b07696, 2020.
Zhou, S., Gonzalez, L., Leithead, A., Finewax, Z., Thalman, R., Vlasenko, A., Vagle, S., Miller, L. A., Li, S.-M., Bureekul, S., Furutani, H., Uematsu, M., Volkamer, R., and Abbatt, J.: Formation of gas-phase carbonyls from heterogeneous oxidation of polyunsaturated fatty acids at the air–water interface and of the sea surface microlayer, Atmos. Chem. Phys., 14, 1371–1384, https://doi.org/10.5194/acp-14-1371-2014, 2014.
Zhou, W. T., Mekic, M., Liu, J. P., Loisel, G., Jin, B., Vione, D., and Gligorovski, S.: Ionic strength effects on the photochemical degradation of acetosyringone in atmospheric deliquescent aerosol particles, Atmos. Environ., 198, 83–88, https://doi.org/10.1016/j.atmosenv.2018.10.047, 2019.
Zhou, X. and Mopper, K.: Photochemical production of low-molecular-weight carbonyl compounds in seawater and surface microlayer and their air–sea exchange, Mar. Chem., 56, 201–213, https://doi.org/10.1016/S0304-4203(96)00076-X, 1997.
Zhu, Y. and Kieber, D. J.: Wavelength- and Temperature-Dependent Apparent Quantum Yields for Photochemical Production of Carbonyl Compounds in the North Pacific Ocean, Environ. Sci. Technol., 52, 1929–1939, https://doi.org/10.1021/acs.est.7b05462, 2018.
Zhu, Y. and Kieber, D. J.: Concentrations and Photochemistry of Acetaldehyde, Glyoxal, and Methylglyoxal in the Northwest Atlantic Ocean, Environ. Sci. Technol., 53, 9512–9521, https://doi.org/10.1021/acs.est.9b01631, 2019.
- Abstract
- Introduction
- Air–water interface
- Ocean–atmosphere interface
- Impact of SML on gas transfer
- Impact of SML on SSA production
- Photochemistry and multiphase chemistry at the SML
- Conclusion and outlooks
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- Air–water interface
- Ocean–atmosphere interface
- Impact of SML on gas transfer
- Impact of SML on SSA production
- Photochemistry and multiphase chemistry at the SML
- Conclusion and outlooks
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References




